Evaluation of the Antioxidant Properties and Bioactivity of Koroneiki and Athinolia Olive Varieties Using In Vitro Cell-Free and Cell-Based Assays
Abstract
1. Introduction
2. Results
2.1. Determination of the Antioxidant, Reducing, and Antigenotoxic Properties of Koroneiki and Athinolia Olive Samples in Cell-Free Systems
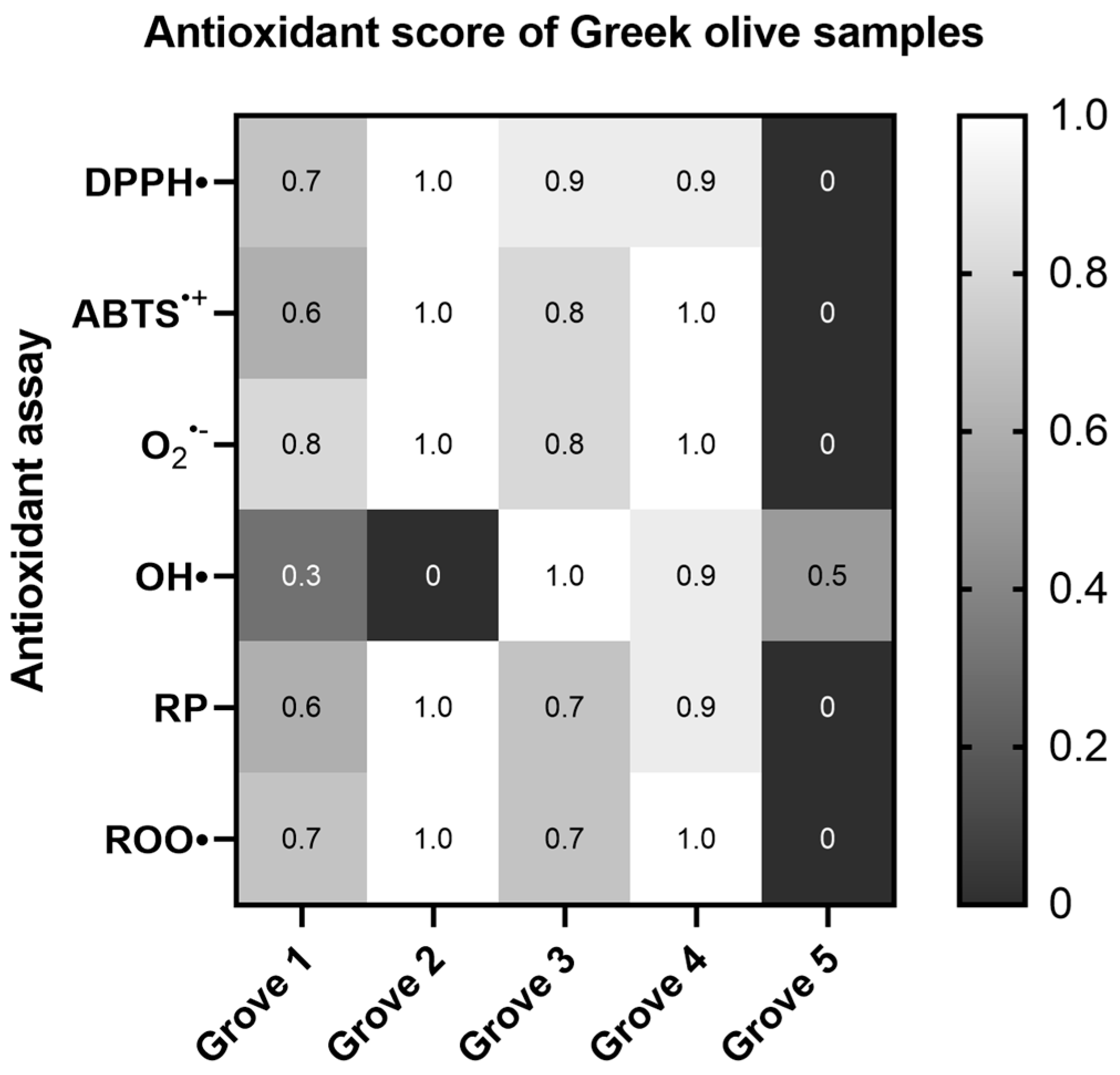
2.1.1. Overall Comparison
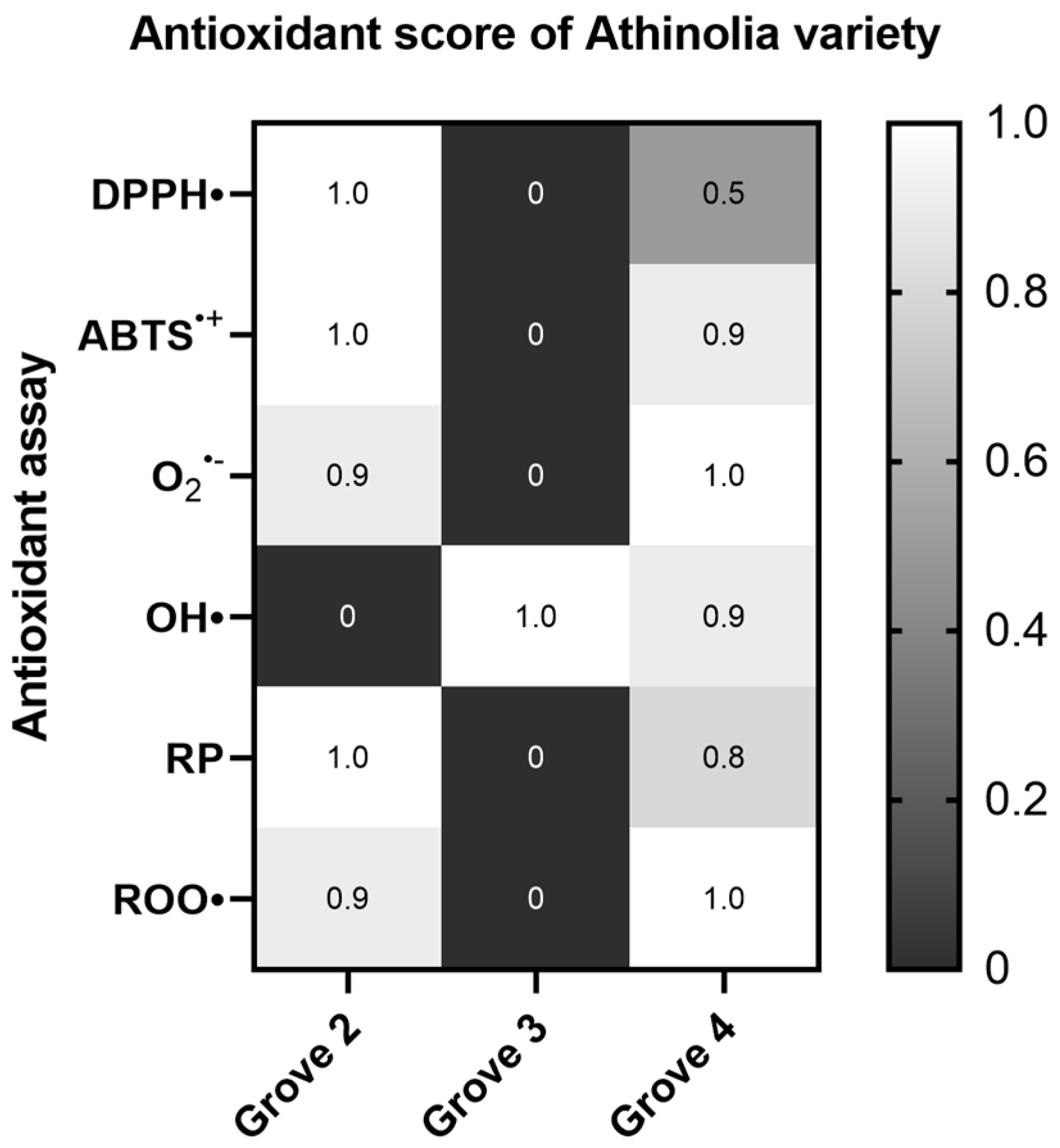
2.1.2. Athinolia Variety
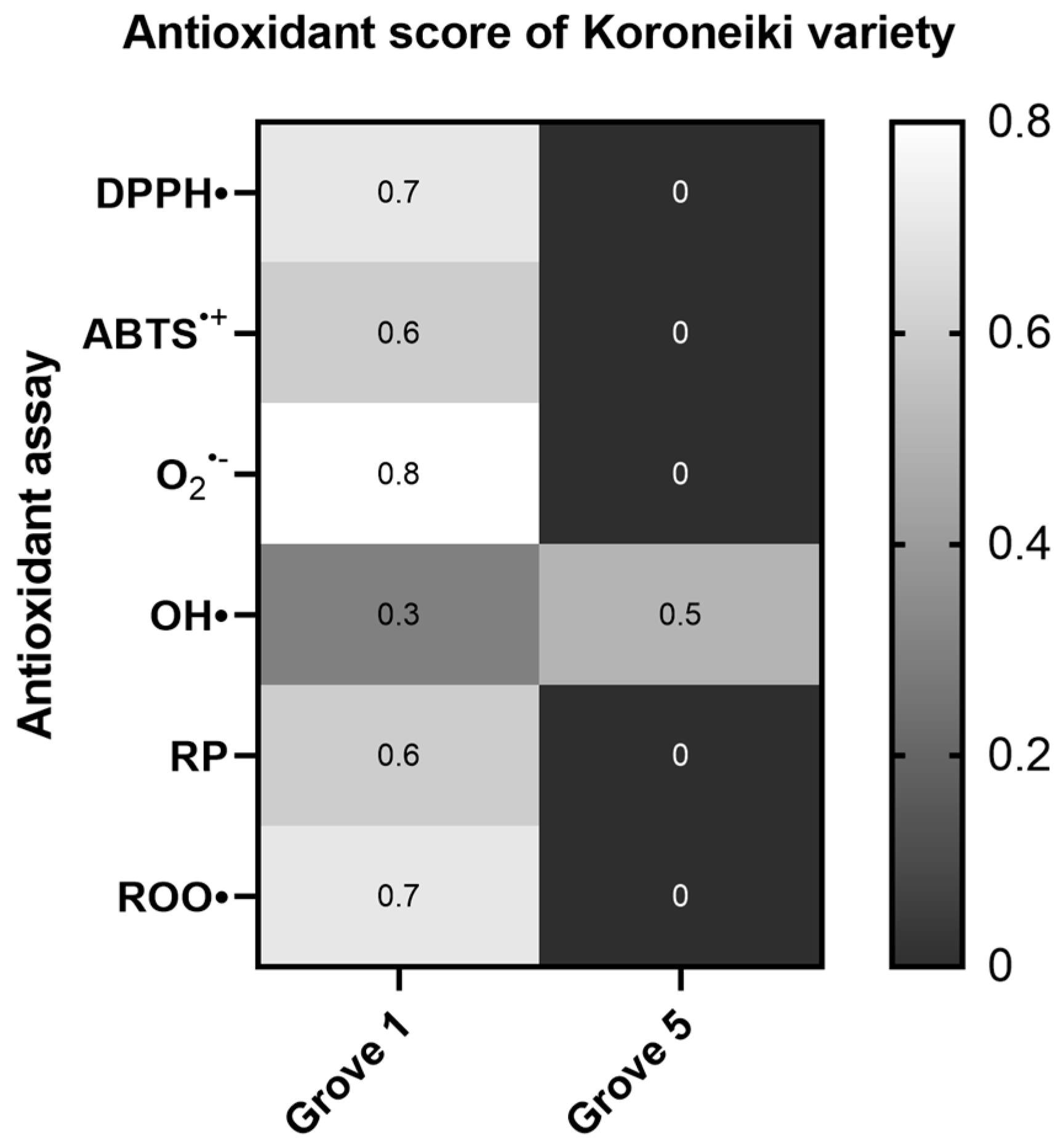
2.1.3. Koroneiki Variety
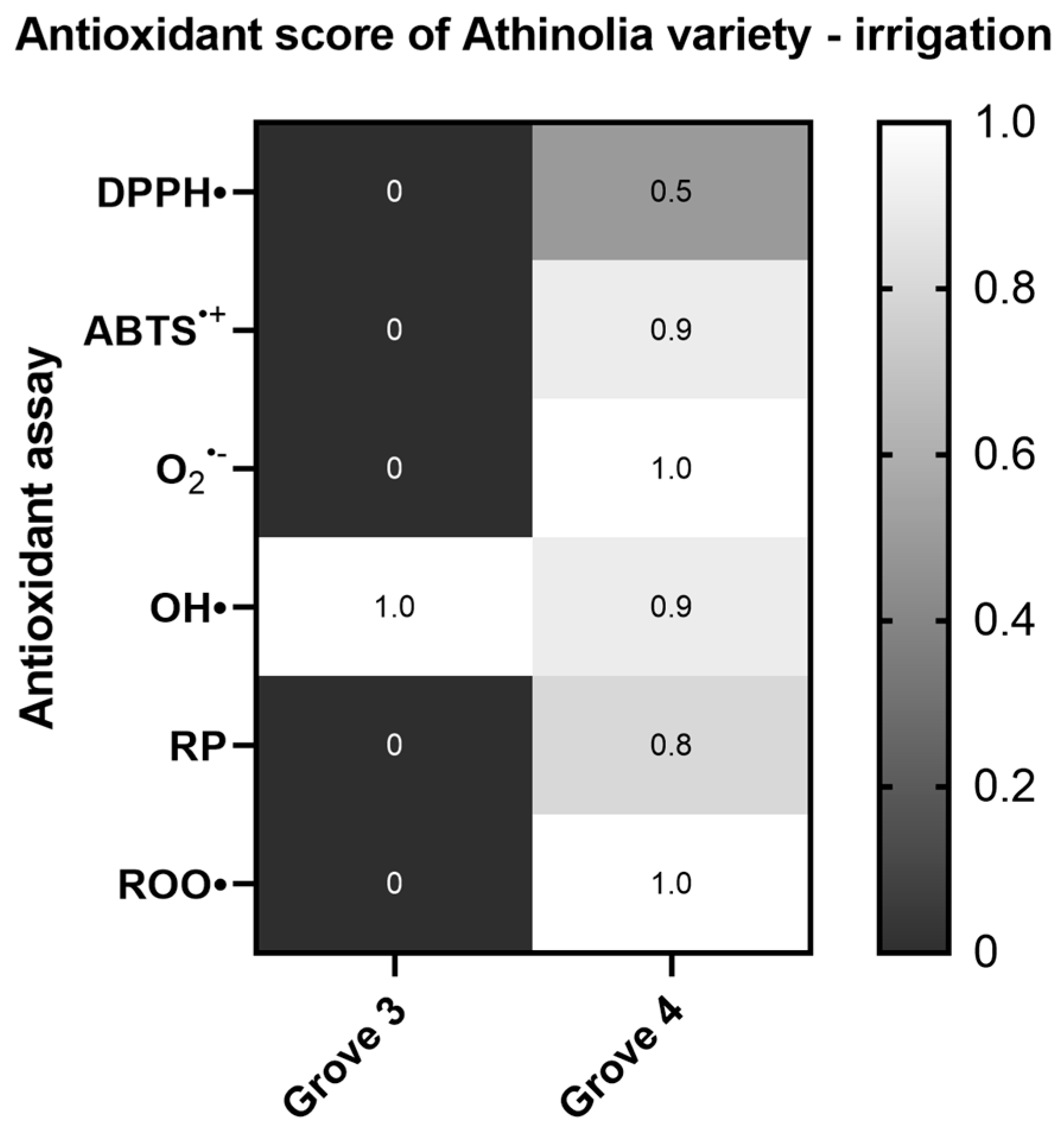
2.1.4. Effect of Irrigation
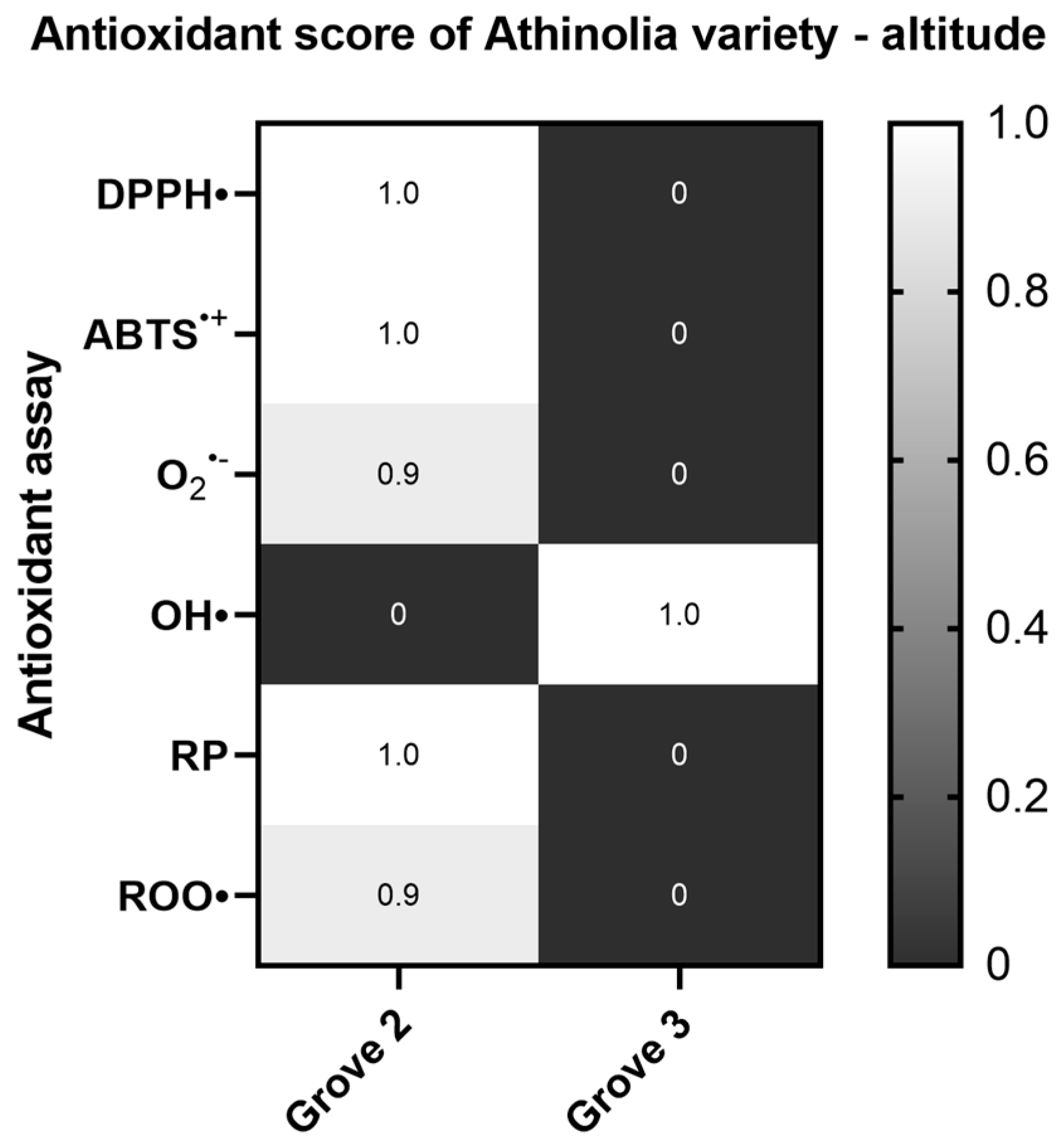
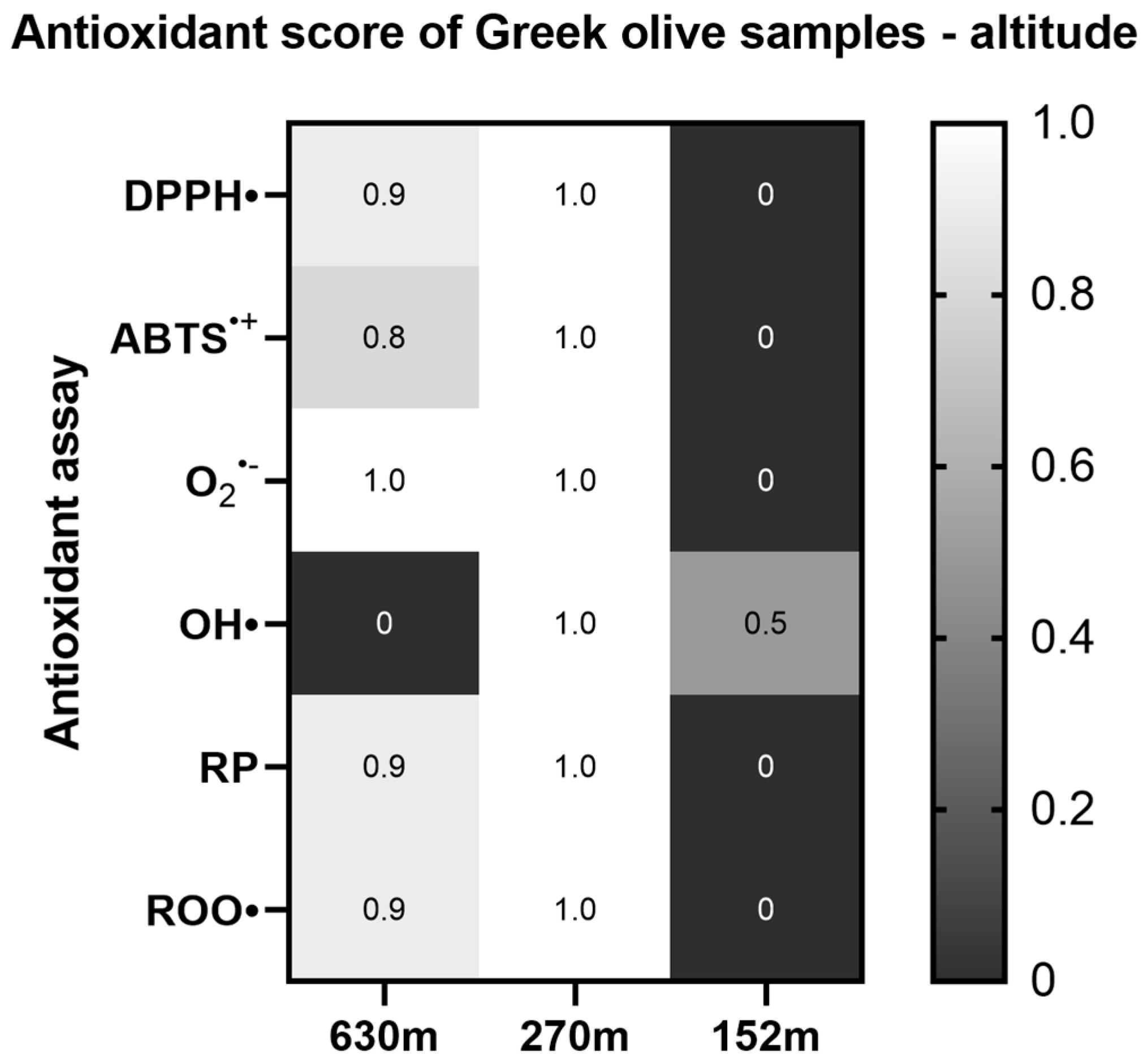
2.1.5. Altitude
2.2. Determination of the Effects of Koroneiki and Athinolia Olive Samples on Redox Biomarkers in Cell-Based Systems
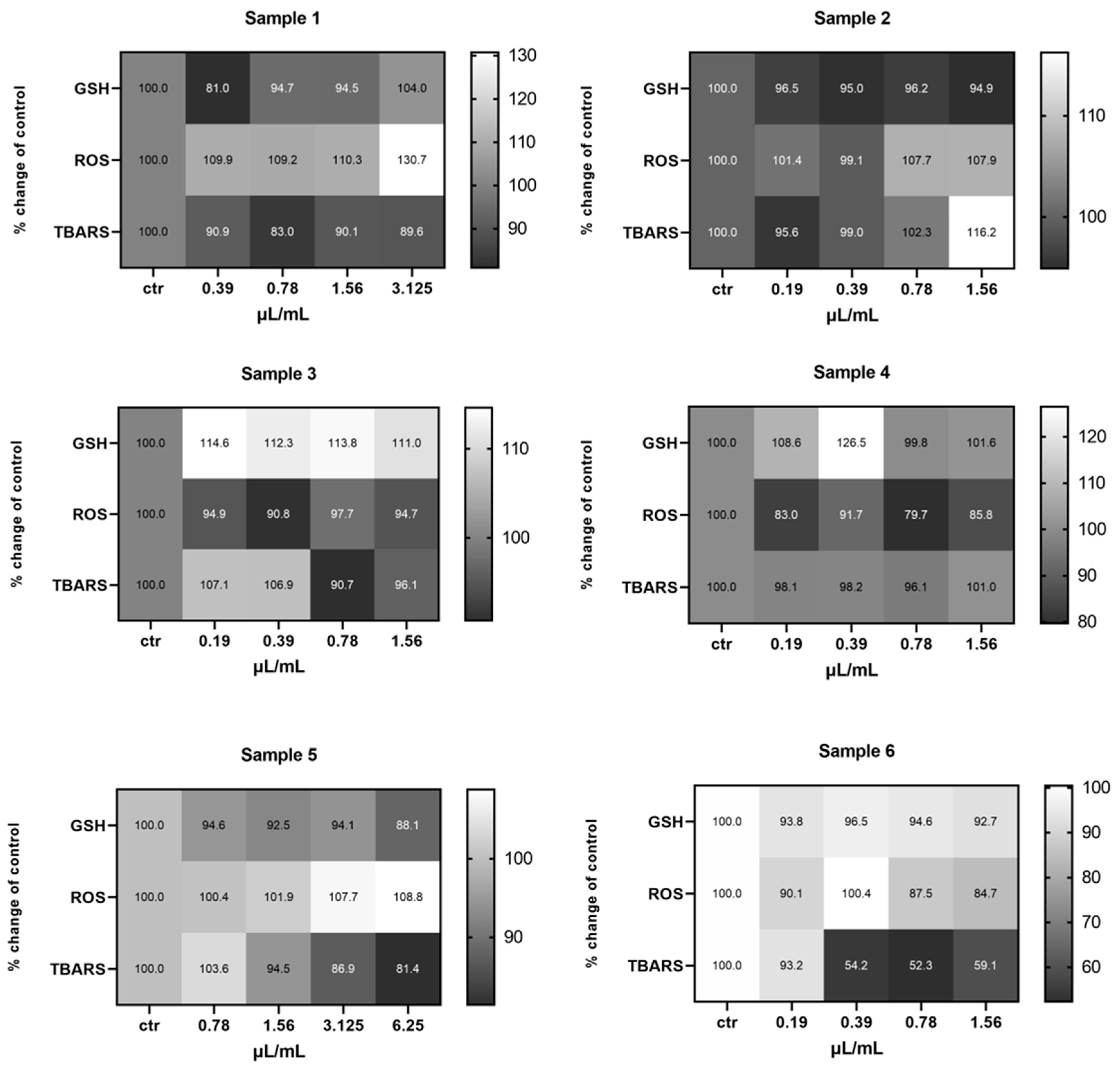
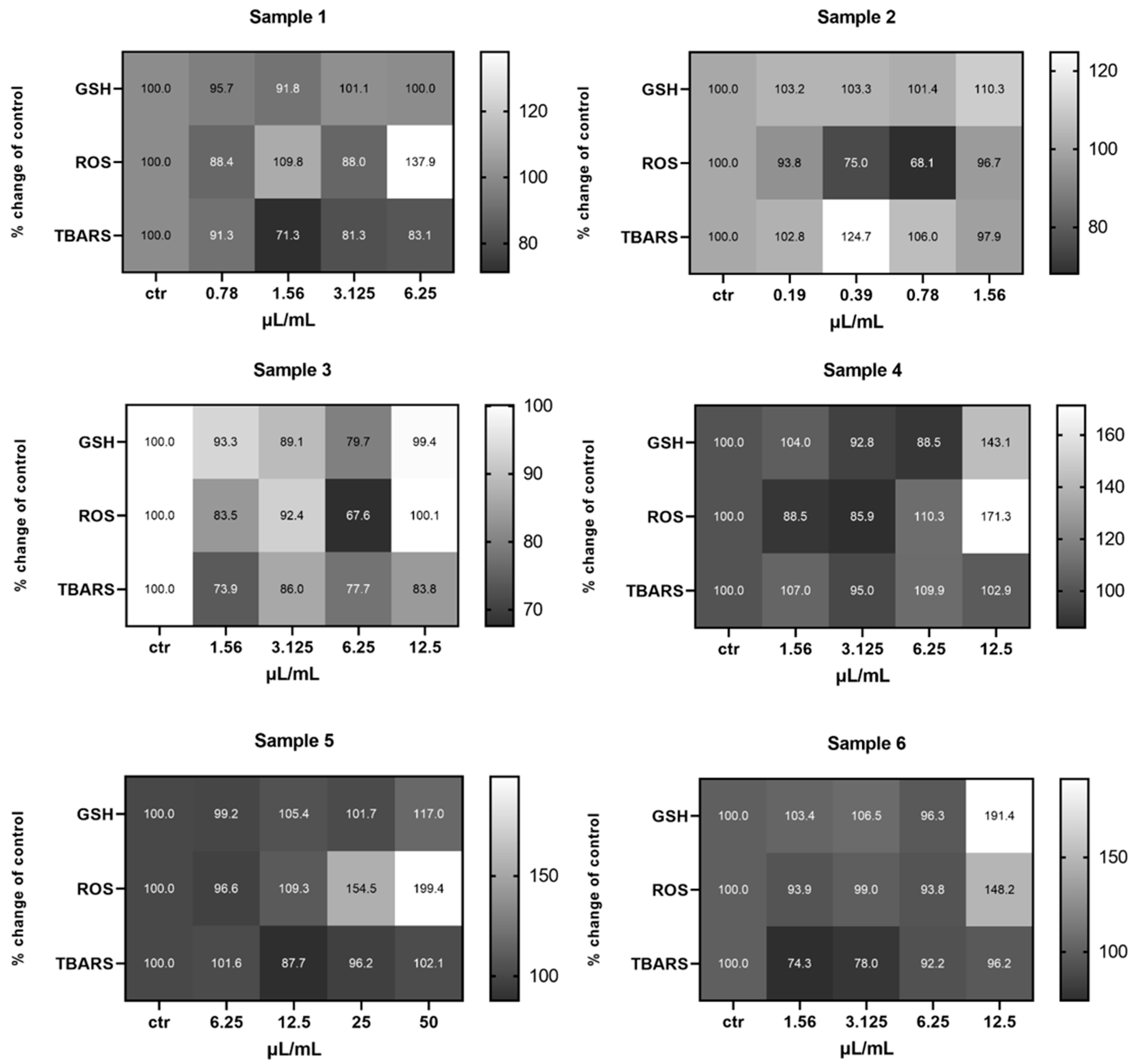
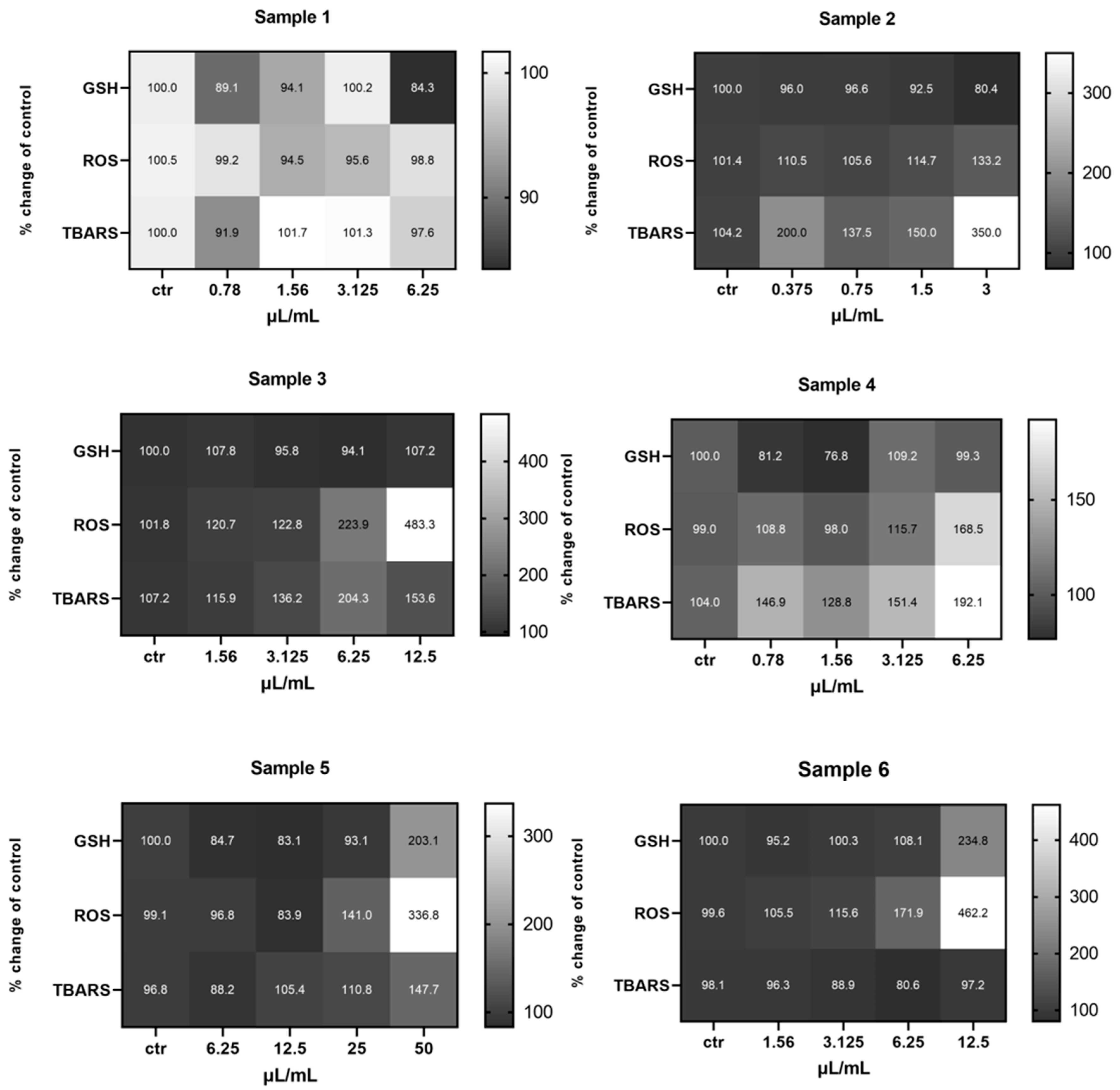
2.2.1. EA.hy926 Cells
2.2.2. HepG2 Cells
2.2.3. MKN45 Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Olive Samples
4.3. Sample Preparation
4.4. Assessment of Antioxidant Properties In Vitro Cell-Free Systems
4.4.1. DPPH• Radical Scavenging Assay
4.4.2. ABTS•+ Radical Scavenging Assay
4.4.3. Superoxide (O2•−) Radical Assay
4.4.4. Hydroxyl Radical (OH•) Scavenging Assay
4.4.5. Reducing Power Assay
4.4.6. Peroxyl Radical (ROO•)-Induced Plasmid DNA Relaxation Assay
4.5. Assessment of Bioactivity In Vitro Cell-Based Systems
4.5.1. Cell Cultures
4.5.2. Cell Viability Assay
4.5.3. Determination of Reduced Glutathione (GSH) and Reactive Oxygen Species (ROS) Levels by Flow Cytometry
4.5.4. Determination of Thiobarbituric Acid Reactive Substances (TBARS) Levels by Spectrophotometry
4.5.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Green, P.S. A Revision of Olea L. (Oleaceae). Kew Bull. 2002, 57, 91. [Google Scholar] [CrossRef]
- Rallo, L.; Díez, C.M.; Morales-Sillero, A.; Miho, H.; Priego-Capote, F.; Rallo, P. Quality of olives: A focus on agricultural preharvest factors. Sci. Hortic. 2018, 233, 491–509. [Google Scholar] [CrossRef]
- Gaforio, J.J.; Visioli, F.; Alarcón-de-la-Lastra, C.; Castañer, O.; Delgado-Rodríguez, M.; Fitó, M.; Hernández, A.F.; Huertas, J.R.; Martínez-González, M.A.; Menendez, J.A.; et al. Virgin Olive Oil and Health: Summary of the III International Conference on Virgin Olive Oil and Health Consensus Report, JAEN (Spain) 2018. Nutrients 2019, 11, 2039. [Google Scholar] [CrossRef]
- Loumou, A.; Giourga, C. Olive groves: “The life and identity of the Mediterranean”. Agric. Hum. Values 2003, 20, 87–95. [Google Scholar] [CrossRef]
- Sofo, A.; Manfreda, S.; Dichio, B.; Fiorentino, M.; Xiloyannis, C. The olive tree: A paradigm for drought tolerance in Medi-terranean climates. Hydrol. Earth Syst. Sci. 2008, 12, 293–301. [Google Scholar] [CrossRef]
- Nikoloudakis, N.; Banilas, G.; Gazis, F.; Hatzopoulos, P.; Metzidakis, J. Discrimination and Genetic Diversity among Cultivated Olives of Greece Using RAPD Markers. J. sAm. Soc. Hortic. Sci. 2003, 128, 741–746. [Google Scholar] [CrossRef]
- Bartolini, G.; Prevost, G.; Messeri, C.; Carignani, G. Olive cultivar names and synonyms and collections detected in a literature review. Acta Hortic. 1999, 159–162. [Google Scholar] [CrossRef]
- Barazani, O.; Westberg, E.; Hanin, N.; Dag, A.; Kerem, Z.; Tugendhaft, Y.; Hmidat, M.; Hijawi, T.; Kadereit, J.W. A comparative analysis of genetic variation in rootstocks and scions of old olive trees—A window into the history of olive cultivation practices and past genetic variation. BMC Plant Biol. 2014, 14, 146. [Google Scholar] [CrossRef]
- Barazani, O.; Keren-Keiserman, A.; Westberg, E.; Hanin, N.; Dag, A.; Ben-Ari, G.; Fragman-Sapir, O.; Tugendhaft, Y.; Kerem, Z.; Kadereit, J.W. Genetic variation of naturally growing olive trees in Israel: From abandoned groves to feral and wild? BMC Plant Biol. 2016, 16, 261. [Google Scholar] [CrossRef]
- Riley, F. Olive oil production on bronze age Crete: Nutritional properties, processing methods and storage life of Minoan olive oil. Oxf. J. Archaeol. 2002, 21, 63–75. [Google Scholar] [CrossRef]
- Theodosi, S.; Kosma, I.S.; Badeka, A.V. Quality characteristics of Koroneiki olive oil from Zakynthos island (Greece) and differentiation depending on the altitude level. Eur. Food Res. Technol. 2021, 247, 1235–1248. [Google Scholar] [CrossRef]
- Nenadis, N.; Mastralexi, A.; Tsimidou, M.Z. Physicochemical Characteristics and Antioxidant Potential of the Greek PDO and PGI Virgin Olive Oils (VOOs). Eur. J. Lipid Sci. Technol. 2018, 121, 1800172. [Google Scholar] [CrossRef]
- Hatzopoulos, P.; Banilas, G.; Giannoulia, K.; Gazis, F.; Nikoloudakis, N.; Milioni, D.; Haralampidis, K. Breeding, Molecular Markers and Molecular Biology of the Olive Tree. Available online: https://www.semanticscholar.org/paper/Breeding%2C-molecular-markers-and-molecular-biology-Hatzopoulos-Banilas/f8de98691ddf208654019a5146c289e8efb5cea8 (accessed on 10 January 2025).
- Kalogeropoulos, N.; Tsimidou, M.Z. Antioxidants in Greek Virgin Olive Oils. Antioxidants 2014, 3, 387–413. [Google Scholar] [CrossRef]
- Banilas, G.; Korkas, E.; Kaldis, P.; Hatzopoulos, P. Olive and grapevine biodiversity in Greece and Cyprus—A review. In Sustainable Agriculture Reviews; Lichtfouse, E., Ed.; Springer: Dordrecht, The Netherlands, 2009; Volume 2, pp. 401–428. [Google Scholar]
- Korifi, R.; Le Dréau, Y.; Molinet, J.; Artaud, J.; Dupuy, N. Composition and authentication of virgin olive oil from French PDO regions by chemometric treatment of Raman spectra. J. Raman Spectrosc. 2011, 42, 1540–1547. [Google Scholar] [CrossRef]
- Moriana, A.; Orgaz, F.; Pastor, M.; Fereres, E. Yield Responses of a Mature Olive Orchard to Water Deficits. J. Am. Soc. Hortic. Sci. 2003, 128, 425–431. [Google Scholar] [CrossRef]
- Costagli, G.; Gucci, R.; Rapoport, H. Growth and development of fruits of olive ‘Frantoio’ under irrigated and rainfed conditions. J. Hortic. Sci. Biotechnol. 2003, 78, 119–124. [Google Scholar] [CrossRef]
- D’Andria, R.; Lavini, A.; Morelli, G.; Patumi, M.; Terenziani, S.; Calandrelli, D.; Fragnito, F. Effects of water regimes on ®ve pickling and double aptitude olive cultivars (Olea europaea L.). J. Hortic. Sci. Biotechnol. 2004, 79, 18–25. [Google Scholar] [CrossRef]
- Lozano-Castellón, J.; López-Yerena, A.; Rinaldi De Alvarenga, J.F.; Romero Del Castillo-Alba, J.; Vallverdú-Queralt, A.; Escribano-Ferrer, E.; Lamuela-Raventós, R.M. Health-promoting properties of oleocanthal and oleacein: Two secoiridoids from extra-virgin olive oil. Crit. Rev. Food Sci. Nutr. 2020, 60, 2532–2548. [Google Scholar] [CrossRef]
- Yubero-Serrano, E.M.; Lopez-Moreno, J.; Gomez-Delgado, F.; Lopez-Miranda, J. Extra virgin olive oil: More than a healthy fat. Eur. J. Clin. Nutr. 2019, 72, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Gomes, L.; Leitão, F.; Coelho, A.V.; Boas, L.V. Phenolic Compounds and Antioxidant Activity of Olea europaea L. Fruits and Leaves. Food Sci. Technol. Int. 2006, 12, 385–395. [Google Scholar] [CrossRef]
- Boskou, D.; Blekas, G.; Tsimidou, M. Phenolic Compounds in Olive Oil and Olives. Available online: https://www.researchgate.net/publication/259560918_Phenolic_compounds_in_olive_oil_and_olives_D_Boskou_G_Blekas_and_M_Tsimidou (accessed on 10 January 2025).
- Arslan, D.; Özcan, M.M. Drying of tomato slices: Changes in drying kinetics, mineral contents, antioxidant activity and color parameters Secado de rodajas de tomate: Cambios en cinéticos del secado, contenido en minerales, actividad antioxidante y parámetros de color. CyTA-J. Food 2011, 9, 229–236. [Google Scholar] [CrossRef]
- El Riachy, M.; Priego-Capote, F.; León, L.; Rallo, L.; Luque de Castro, M.D. Hydrophilic antioxidants of virgin olive oil. Part 2: Biosynthesis and biotransformation of phenolic compounds in virgin olive oil as affected by agronomic and processing factors. Eur. J. Lipid Sci. Technol. 2011, 113, 692–707. [Google Scholar] [CrossRef]
- Alagna, F.; Mariotti, R.; Panara, F.; Caporali, S.; Urbani, S.; Veneziani, G.; Esposto, S.; Taticchi, A.; Rosati, A.; Rao, R.; et al. Olive phenolic compounds: Metabolic and transcriptional profiling during fruit development. BMC Plant Biol. 2012, 12, 162. [Google Scholar] [CrossRef] [PubMed]
- Medina, E.; Morales-Sillero, A.; Ramírez, E.M.; Rallo, P.; Brenes, M.; Romero, C. New genotypes of table olives: Profile of bioactive compounds. Int. J. Food Sci. Technol. 2012, 47, 2334–2341. [Google Scholar] [CrossRef]
- Alexandraki, V.; Georgalaki, M.; Papadimitriou, K.; Anastasiou, R.; Zoumpopoulou, G.; Chatzipavlidis, I.; Papadelli, M.; Vallis, N.; Moschochoritis, K.; Tsakalidou, E. Determination of triterpenic acids in natural and alkaline-treated Greek table olives throughout the fermentation process. LWT 2014, 58, 609–613. [Google Scholar] [CrossRef]
- Horiuchi, K.; Shiota, S.; Hatano, T.; Yoshida, T.; Kuroda, T.; Tsuchiya, T. Antimicrobial Activity of Oleanolic Acid from Salvia officinalis and Related Compounds on Vancomycin-Resistant Enterococci (VRE). Biol. Pharm. Bull. 2007, 30, 1147–1149. [Google Scholar] [CrossRef]
- Montilla, M.P.; Agil, A.; Navarro, M.C.; Jiménez, M.I.; García-Granados, A.; Parra, A.; Cabo, M.M. Antioxidant Activity of Maslinic Acid, a Triterpene Derivative Obtained from Olea europaea. Planta Medica 2003, 69, 472–474. [Google Scholar] [CrossRef]
- Servili, M.; Sordini, B.; Esposto, S.; Taticchi, A.; Urbani, S.; Sebastiani, L. Metabolomics of Olive Fruit: A Focus on the Secondary Metabolites. In The Olive Tree Genome; Springer: Berlin/Heidelberg, Germany, 2016; pp. 123–139. [Google Scholar]
- Veskoukis, A.S.; Kerasioti, E.; Priftis, A.; Kouka, P.; Spanidis, Y.; Makri, S.; Kouretas, D. A battery of translational biomarkers for the assessment of the in vitro and in vivo antioxidant action of plant polyphenolic compounds: The biomarker issue. Curr. Opin. Toxicol. 2018, 13, 99–109. [Google Scholar] [CrossRef]
- Kouka, P.; Priftis, A.; Stagos, D.; Angelis, A.; Stathopoulos, P.; Xinos, N.; Skaltsounis, A.-L.; Mamoulakis, C.; Tsatsakis, A.M.; Spandidos, D.A.; et al. Assessment of the antioxidant activity of an olive oil total polyphenolic fraction and hydroxytyrosol from a Greek Olea europea variety in endothelial cells and myoblasts. Int. J. Mol. Med. 2017, 40, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Orozco, R.; Roca, M.; Gandul-Rojas, B.; Gallardo-Guerrero, L. DPPH-scavenging capacity of chloroplastic pigments and phenolic compounds of olive fruits (cv. Arbequina) during ripening. J. Food Compos. Anal. 2011, 24, 858–864. [Google Scholar] [CrossRef]
- Piscopo, A.; De Bruno, A.; Zappia, A.; Poiana, M. Antioxidant activity of dried green olives (Carolea cv.). LWT 2014, 58, 49–54. [Google Scholar] [CrossRef]
- Dias, M.C.; Santos, C.; Silva, S.; Pinto, D.C.G.A.; Silva, A.M.S. Physiological and Metabolite Reconfiguration of Olea europaea to Cope and Recover from a Heat or High UV–B Shock. J. Agric. Food Chem. 2020, 68, 11339–11349. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.C.; Pinto, D.C.; Correia, C.; Moutinho-Pereira, J.; Oliveira, H.; Freitas, H.; Silva, A.M.; Santos, C. UV-B radiation modulates physiology and lipophilic metabolite profile in Olea europaea. J. Plant Physiol. 2018, 222, 39–50. [Google Scholar] [CrossRef]
- Ben Brahim, S.; Kelebek, H.; Ammar, S.; Abichou, M.; Bouaziz, M. LC–MS phenolic profiling combined with multivariate analysis as an approach for the characterization of extra virgin olive oils of four rare Tunisian cultivars during ripening. Food Chem. 2017, 229, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Dağdelen, A.; Tümen, G.; Özcan, M.M.; Dündar, E. Phenolics profiles of olive fruits (Olea europaea L.) and oils from Ayvalık, Domat and Gemlik varieties at different ripening stages. Food Chem. 2013, 136, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Borges, T.H.; López, L.C.; Pereira, J.A.; Cabrera–Vique, C.; Seiquer, I. Comparative analysis of minor bioactive constituents (CoQ10, tocopherols and phenolic compounds) in Arbequina extra virgin olive oils from Brazil and Spain. J. Food Compos. Anal. 2017, 63, 47–54. [Google Scholar] [CrossRef]
- Kalogiouri, N.P.; Aalizadeh, R.; Thomaidis, N.S. Application of an advanced and wide scope non-target screening workflow with LC-ESI-QTOF-MS and chemometrics for the classification of the Greek olive oil varieties. Food Chem. 2018, 256, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Artajo, L.; Romero, M.; Tovar, M.; Motilva, M. Effect of irrigation applied to olive trees (Olea europaea L.) on phenolic compound transfer during olive oil extraction. Eur. J. Lipid Sci. Technol. 2006, 108, 19–27. [Google Scholar] [CrossRef]
- Miho, H.; Moral, J.; Barranco, D.; Ledesma-Escobar, C.; Priego-Capote, F.; Díez, C. Influence of genetic and interannual factors on the phenolic profiles of virgin olive oils. Food Chem. 2020, 342, 128357. [Google Scholar] [CrossRef]
- Mousavi, S.; Stanzione, V.; Mariotti, R.; Mastio, V.; Azariadis, A.; Passeri, V.; Valeri, M.C.; Baldoni, L.; Bufacchi, M. Bioactive Compound Profiling of Olive Fruit: The Contribution of Genotype. Antioxidants 2022, 11, 672. [Google Scholar] [CrossRef]
- El Riachy, M.; Priego-Capote, F.; León, L.; de Castro, M.D.L.; Rallo, L. Virgin olive oil phenolic profile and variability in progenies from olive crosses. J. Sci. Food Agric. 2012, 92, 2524–2533. [Google Scholar] [CrossRef]
- de la Rosa, R.; Arias-Calderón, R.; Velasco, L.; León, L. Early selection for oil quality components in olive breeding progenies. Eur. J. Lipid Sci. Technol. 2015, 118, 1160–1167. [Google Scholar] [CrossRef]
- Loizidou, M.; Giannakopoulos, C.; Bindi, M.; Moustakas, K. Climate change impacts and adaptation options in the Medi-terranean basin. Reg. Environ. Change 2016, 16, 1859–1861. [Google Scholar] [CrossRef]
- Chaves, M.M.; Marôco, J.P.; Pereira, J.S. Understanding plant responses to drought—From genes to the whole plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef]
- Araújo, M.; Prada, J.; Mariz-Ponte, N.; Santos, C.; Pereira, J.A.; Pinto, D.C.G.A.; Silva, A.M.S.; Dias, M.C. Antioxidant Adjustments of Olive Trees (Olea europaea) under Field Stress Conditions. Plants 2021, 10, 684. [Google Scholar] [CrossRef] [PubMed]
- Uslu, N.; Özcan, M.M. The effect of irrigation and harvest time on bioactive properties of olive fruits issued from some olive varieties grown in Mediterranean region. Eur. Food Res. Technol. 2020, 246, 2587–2599. [Google Scholar] [CrossRef]
- García-Garví, J.M.; Noguera-Artiaga, L.; Hernández, F.; Pérez-López, A.J.; Burgos-Hernández, A.; Carbonell-Barrachina, Á.A. Quality of Olive Oil Obtained by Regulated Deficit Irrigation. Horticulturae 2023, 9, 557. [Google Scholar] [CrossRef]
- Ouni, Y.; Taamalli, A.; Gómez-Caravaca, A.M.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Zarrouk, M. Characterisation and quantification of phenolic compounds of extra-virgin olive oils according to their geographical origin by a rapid and resolutive LC–ESI-TOF MS method. Food Chem. 2011, 127, 1263–1267. [Google Scholar] [CrossRef] [PubMed]
- Bendini, A.; Cerretani, L.; Carrasco-Pancorbo, A.; Gómez-Caravaca, A.M.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Lercker, G.; Simal-Gandara, J. Phenolic Molecules in Virgin Olive Oils: A Survey of Their Sensory Properties, Health Effects, Antioxidant Activity and Analytical Methods. An Overview of the Last Decade Alessandra. Molecules 2007, 12, 1679–1719. [Google Scholar] [CrossRef]
- Stefanoudaki, E.; Williams, M.; Chartzoulakis, K.; Harwood, J. Effect of Irrigation on Quality Attributes of Olive Oil. J. Agric. Food Chem. 2009, 57, 7048–7055. [Google Scholar] [CrossRef] [PubMed]
- Manolikaki, I.; Digalaki, N.; Psarras, G.; Tzerakis, C.; Sergentani, C.; Papamanolioudaki, A.; Tul, S.; Koubouris, G. Seasonal Variation of Leaf Ca, Fe, and Mn Concentration in Six Olive Varieties. Int. J. Plant Biol. 2022, 13, 95–105. [Google Scholar] [CrossRef]
- Romero, M.P.; Girona, J.; Motilva, M.J.; Tovar, M.J.; Alegre, S. Influence of regulated deficit irrigation strategies applied to olive trees (Arbequina cultivar) on oil yield and oil composition during the fruit ripening period. J. Sci. Food Agric. 2000, 80, 2037–2043. [Google Scholar] [CrossRef]
- Tovar, M.J.; Motilva, M.J.; Romero, M.P. Changes in the Phenolic Composition of Virgin Olive Oil from Young Trees (Olea europaea L. cv. Arbequina) Grown under Linear Irrigation Strategies. J. Agric. Food Chem. 2001, 49, 5502–5508. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, F.; Basile, B.; Morelli, G.; D’andria, R.; Tonutti, P. Effects of irrigation on fruit ripening behavior and metabolic changes in olive. Sci. Hortic. 2012, 144, 201–207. [Google Scholar] [CrossRef]
- Mougiou, N.; Baalbaki, B.; Doupis, G.; Kavroulakis, N.; Poulios, S.; Vlachonasios, K.E.; Koubouris, G.C. The Effect of Low Temperature on Physiological, Biochemical and Flowering Functions of Olive Tree in Relation to Genotype. Sustainability 2020, 12, 10065. [Google Scholar] [CrossRef]
- Patumi, M.; D’Andria, R.; Marsilio, V.; Fontanazza, G.; Morelli, G.; Lanza, B. Olive and olive oil quality after intensive monocone olive growing (Olea europaea L., cv. Kalamata) in different irrigation regimes. Food Chem. 2002, 77, 27–34. [Google Scholar] [CrossRef]
- Gómez-Rico, A.; Salvador, M.D.; Moriana, A.; Pérez, D.; Olmedilla, N.; Ribas, F.; Fregapane, G. Influence of different irrigation strategies in a traditional Cornicabra cv. olive orchard on virgin olive oil composition and quality. Food Chem. 2007, 100, 568–578. [Google Scholar] [CrossRef]
- Rinaldi, R.; Amodio, M.; Colelli, G.; Nanos, G.; Pliakoni, E. Effect of deficit irrigation on fruit and oil quality of ’konservolea’ olives. Acta Hortic. 2011, 445–451. [Google Scholar] [CrossRef]
- Servili, M.; Esposto, S.; Lodolini, E.; Selvaggini, R.; Taticchi, A.; Urbani, S. Irrigation effects on quality, phenolic composition, and selected volatiles of virgin olive oils cv. Leccino. J. Agric. Food Chem. 2007, 55, 6609–6618. [Google Scholar] [CrossRef]
- Petridis, A.; Therios, I.; Samouris, G.; Koundouras, S.; Giannakoula, A. Effect of water deficit on leaf phenolic composition, gas exchange, oxidative damage and antioxidant activity of four Greek olive (Olea europaea L.) cultivars. Plant Physiol. Biochem. 2012, 60, 1–11. [Google Scholar] [CrossRef]
- Mousa, Y.M.; Gerasopoulos, D.; Metzidakis, I.; Kiritsakis, A. Effect of Altitude on Fruit and Oil Quality Characteristics of ‘Mastoides’ Olives. J. Sci. Food Agric. 1996, 71, 345–350. [Google Scholar] [CrossRef]
- Kalogiouri, N.P.; Kritikou, E.; Martakos, I.C.; Lazarou, C.; Pentogennis, M.; Thomaidis, N.S. Characterization of the Phenolic Fingerprint of Kolovi Extra Virgin Olive Oils from Lesvos with Regard to Altitude and Farming System Analyzed by UHPLC-QTOF-MS. Molecules 2021, 26, 5634. [Google Scholar] [CrossRef] [PubMed]
- Zakraoui, M.; Hannachi, H.; Pasković, I.; Vidović, N.; Pasković, M.P.; Palčić, I.; Major, N.; Ban, S.G.; Hamrouni, L. Effect of Geographical Location on the Phenolic and Mineral Composition of Chetoui Olive Leaves. Foods 2023, 12, 2565. [Google Scholar] [CrossRef]
- Ouni, Y. The phenolic compounds and compositional quality of Chétoui virgin olive oil: Effect of altitude. Afr. J. Biotechnol. 2012, 11. [Google Scholar] [CrossRef]
- Dabbou, S.; Issaoui, M.; Esposto, S.; Sifi, S.; Taticchi, A.; Servili, M.; Montedoro, G.F.; Hammami, M. Cultivar and growing area effects on minor compounds of olive oil from autochthonous and European introduced cultivars in Tunisia. J. Sci. Food Agric. 2009, 89, 1314–1325. [Google Scholar] [CrossRef]
- Špika, M.J.; Perica, S.; Žanetić, M.; Škevin, D. Virgin Olive Oil Phenols, Fatty Acid Composition and Sensory Profile: Can Cultivar Overpower Environmental and Ripening Effect? Antioxidants 2021, 10, 689. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.; de la Rosa, R.; Moukhli, A.; El Riachy, M.; Mariotti, R.; Torres, M.; Pierantozzi, P.; Stanzione, V.; Mastio, V.; Zaher, H.; et al. Plasticity of fruit and oil traits in olive among different environments. Sci. Rep. 2019, 9, 16968. [Google Scholar] [CrossRef]
- Ahn, K.; Pan, S.; Beningo, K.; Hupe, D. A permanent human cell line (EA.hy926) preserves the characteristics of endothelin converting enzyme from primary human umbilical vein endothelial cells. Life Sci. 1995, 56, 2331–2341. [Google Scholar] [CrossRef]
- Skaperda, Z.; Tekos, F.; Makri, S.; Angelakis, C.; Vassi, E.; Vardakas, P.; Patouna, A.; Terizi, K.; Kyriazi, D.; Kouretas, D. A novel combined bioactivity/chemoactivity holistic approach for the evaluation of dietary supplements. Food Chem. Toxicol. 2021, 152, 112159. [Google Scholar] [CrossRef] [PubMed]
- Donato, M.; Tolosa, L.; Gómez-Lechón, M.J. Culture and Functional Characterization of Human Hepatoma HepG2 Cells. In Protocols in In Vitro Hepatocyte Research; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2014; Volume 1250, pp. 77–93. [Google Scholar]
- Montoya, T.; Sánchez-Hidalgo, M.; Castejón, M.L.; Rosillo, M.; González-Benjumea, A.; Alarcón-De-La-Lastra, C. Dietary Oleocanthal Supplementation Prevents Inflammation and Oxidative Stress in Collagen-Induced Arthritis in Mice. Antioxidants 2021, 10, 650. [Google Scholar] [CrossRef]
- Hashim, Y.Z.H.-Y.; Worthington, J.; Allsopp, P.; Ternan, N.G.; Brown, E.M.; McCann, M.J.; Rowland, I.R.; Esposto, S.; Servili, M.; Gill, C.I.R. Virgin olive oil phenolics extract inhibit invasion of HT115 human colon cancer cells in vitro and in vivo. Food Funct. 2014, 5, 1513–1519. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Lim, D.Y.; Jung, J.I.; Cho, H.J.; Park, S.Y.; Kwon, G.T.; Kang, Y.-H.; Lee, K.W.; Choi, M.-S.; Park, J.H.Y. Dietary oleuropein inhibits tumor angiogenesis and lymphangiogenesis in the B16F10 melanoma allograft model: A mechanism for the suppression of high-fat diet-induced solid tumor growth and lymph node metastasis. Oncotarget 2017, 8, 32027–32042. [Google Scholar] [CrossRef]
- Calahorra, J.; Martínez-Lara, E.; De Dios, C.; Siles, E. Hypoxia modulates the antioxidant effect of hydroxytyrosol in MCF-7 breast cancer cells. PLoS ONE 2018, 13, e0203892. [Google Scholar] [CrossRef] [PubMed]
- Zubair, H.; Bhardwaj, A.; Ahmad, A.; Srivastava, S.K.; Khan, M.A.; Patel, G.K.; Singh, S.; Singh, A.P. Hydroxytyrosol Induces Apoptosis and Cell Cycle Arrest and Suppresses Multiple Oncogenic Signaling Pathways in Prostate Cancer Cells. Nutr. Cancer 2017, 69, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Diez-Bello, R.; Jardin, I.; Lopez, J.; El Haouari, M.; Ortega-Vidal, J.; Altarejos, J.; Salido, G.; Salido, S.; Rosado, J. (−)-Oleocanthal inhibits proliferation and migration by modulating Ca2+ entry through TRPC6 in breast cancer cells. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2018, 1866, 474–485. [Google Scholar] [CrossRef] [PubMed]
- León-González, A.J.; Auger, C.; Schini-Kerth, V.B. Pro-oxidant activity of polyphenols and its implication on cancer chemoprevention and chemotherapy. Biochem. Pharmacol. 2015, 98, 371–380. [Google Scholar] [CrossRef]
- Kouka, P.; Tekos, F.; Valta, K.; Mavros, P.; Veskoukis, A.S.; Angelis, A.; Skaltsounis, A.-L.; Kouretas, D. Olive tree blossom polyphenolic extracts exert antioxidant and antimutagenic activities in vitro and in various cell lines. Oncol. Rep. 2019, 42, 2814–2825. [Google Scholar] [CrossRef]
- Corona, G.; Deiana, M.; Incani, A.; Vauzour, D.; Dessì, M.A.; Spencer, J.P.E. Hydroxytyrosol inhibits the proliferation of human colon adenocarcinoma cells through inhibition of ERK1/2 and cyclin D1. Mol. Nutr. Food Res. 2009, 53, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Kourti, M.; Alvanou, M.V.; Skaperda, Z.; Tekos, F.; Papaefstathiou, G.; Stathopoulos, P.; Kouretas, D. Antioxidant and DNA-Protective Activity of an Extract Originated from Kalamon Olives Debittering. Antioxidants 2023, 12, 333. [Google Scholar] [CrossRef] [PubMed]
- Masella, R.; Varì, R.; D’Archivio, M.; Di Benedetto, R.; Scazzocchio, B.; Giovannini, C.; Matarrese, P.; Malorni, W. Extra Virgin Olive Oil Biophenols Inhibit Cell-Mediated Oxidation of LDL by Increasing the mRNA Transcription of Glutathione-Related Enzymes. J. Nutr. 2004, 134, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Goya, L.; Mateos, R.; Bravo, L. Effect of the olive oil phenol hydroxytyrosol on human hepatoma HepG2 cells. Eur. J. Nutr. 2007, 46, 70–78. [Google Scholar] [CrossRef]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary Polyphenols and the Prevention of Diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef] [PubMed]
- Hashim, Y.Z.; Rowland, I.R.; McGlynn, H.; Servili, M.; Selvaggini, R.; Taticchi, A.; Esposto, S.; Montedoro, G.; Kaisalo, L.; Wähälä, K.; et al. Inhibitory effects of olive oil phenolics on invasion in human colon adenocarcinoma cells in vitro. Int. J. Cancer 2007, 122, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Amiri-Nowdijeh, A.; Moosavi, M.A.; Hosseinzadeh, S.; Soleimani, M.; Sabooni, F.; Hosseini-Mazinani, M. Anti-oxidant and Selective Anti-proliferative Effects of the Total Cornicabra Olive Polyphenols on Human Gastric MKN45 Cells. Iran. J. Biotechnol. 2019, 17, 37–44. [Google Scholar] [CrossRef]
- Peyrol, J.; Riva, C.; Amiot, M.J. Hydroxytyrosol in the Prevention of the Metabolic Syndrome and Related Disorders. Nutrients 2017, 9, 306. [Google Scholar] [CrossRef] [PubMed]
- Marković, A.K.; Torić, J.; Barbarić, M.; Brala, C.J. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef] [PubMed]
- Enrique. Cadenas and Lester. Packer. “Handbook of Antioxidants.” 2002, p. 712. Available online: https://dokumen.pub/handbook-of-antioxidants-oxidative-stress-and-disease-2nbsped-9780824705473-0824705475.html (accessed on 10 January 2025).
- Xynos, N.; Papaefstathiou, G.; Psychis, M.; Argyropoulou, A.; Aligiannis, N.; Skaltsounis, A.-L. Development of a green extraction procedure with super/subcritical fluids to produce extracts enriched in oleuropein from olive leaves. J. Supercrit. Fluids 2012, 67, 89–93. [Google Scholar] [CrossRef]
- Bast, A.; Haenen, G.R. Ten misconceptions about antioxidants. Trends Pharmacol. Sci. 2013, 34, 430–436. [Google Scholar] [CrossRef]
- Mokhtari, K.; Rufino-Palomares, E.E.; Pérez-Jiménez, A.; Reyes-Zurita, F.J.; Figuera, C.; García-Salguero, L.; Medina, P.P.; Peragón, J.; Lupiáñez, J.A. Maslinic Acid, a Triterpene from Olive, Affects the Antioxidant and Mitochondrial Status of B16F10 Melanoma Cells Grown under Stressful Conditions. Evidence-Based Complement. Altern. Med. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Castellano, J.M.; Guinda, A.; Delgado, T.; Rada, M.; Cayuela, J.A. Biochemical Basis of the Antidiabetic Activity of Oleanolic Acid and Related Pentacyclic Triterpenes. Diabetes 2013, 62, 1791–1799. [Google Scholar] [CrossRef]
- Du, Y.; Ko, K.M. Oleanolic Acid Protects against Myocardial Ischemia-Reperfusion Injury by Enhancing Mitochondrial Antioxidant Mechanism Mediated by Glutathione and α-Tocopherol in Rats. Planta Medica 2005, 72, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Li, Q.; Li, Y.; Liu, Z.; Fan, Y.; Liu, Z.; Zhao, H.; Li, J.; Han, Z. Antidiabetic and antioxidant effects of oleanolic acid from Ligustrum lucidum Ait in alloxan-induced diabetic rats. Phytotherapy Res. 2009, 23, 1257–1262. [Google Scholar] [CrossRef]
- Cheng, K.; Liu, J.; Sun, H.; Xie, J. Synthesis of Oleanolic Acid Dimers as Inhibitors of Glycogen Phosphorylase. Chem. Biodivers. 2010, 7, 690–697. [Google Scholar] [CrossRef]
- Dharmappa, K.K.; Kumar, R.V.; Nataraju, A.; Mohamed, R.; Shivaprasad, H.V.; Vishwanath, B.S. Anti-Inflammatory Activity of Oleanolic Acid by Inhibition of Secretory Phospholipase A2. Planta Medica 2008, 75, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.C.; Cui, J.Y.; Klaassen, C.D. Beneficial Role of Nrf2 in Regulating NADPH Generation and Consumption. Toxicol. Sci. 2011, 123, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Bigagli, E.; Cinci, L.; Paccosi, S.; Parenti, A.; D’Ambrosio, M.; Luceri, C. Nutritionally relevant concentrations of resveratrol and hydroxytyrosol mitigate oxidative burst of human granulocytes and monocytes and the production of pro-inflammatory mediators in LPS-stimulated RAW 264.7 macrophages. Int. Immunopharmacol. 2017, 43, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Di Benedetto, R.; Varì, R.; Scazzocchio, B.; Filesi, C.; Santangelo, C.; Giovannini, C.; Matarrese, P.; D’Archivio, M.; Masella, R. Tyrosol, the major extra virgin olive oil compound, restored intracellular antioxidant defences in spite of its weak antioxidative effectiveness. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Mohan, V.; Das, S.; Rao, S.B.S. Hydroxytyrosol, a dietary phenolic compound forestalls the toxic effects of methylmercury-induced toxicity in IMR-32 human neuroblastoma cells. Environ. Toxicol. 2015, 31, 1264–1275. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ramiro, I.; Martín, M.; Ramos, S.; Bravo, L.; Goya, L. Olive oil hydroxytyrosol reduces toxicity evoked by acrylamide in human Caco-2 cells by preventing oxidative stress. Toxicology 2011, 288, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Martín, M.A.; Ramos, S.; Granado-Serrano, A.B.; Rodríguez-Ramiro, I.; Trujillo, M.; Bravo, L.; Goya, L. Hydroxytyrosol induces antioxidant/detoxificant enzymes and Nrf2 translocation via extracellular regulated kinases and phosphatidylinositol-3-kinase/protein kinase B pathways in HepG2 cells. Mol. Nutr. Food Res. 2010, 54, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Stearns, M.E.; Amatangelo, M.D.; Varma, D.; Sell, C.; Goodyear, S.M. Combination Therapy with Epigallocatechin-3-Gallate and Doxorubicin in Human Prostate Tumor Modeling Studies. Am. J. Pathol. 2010, 177, 3169–3179. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, R.; Sepporta, M.V.; Mazza, T.; Rosignoli, P.; Fuccelli, R.; De Bartolomeo, A.; Crescimanno, M.; Taticchi, A.; Esposto, S.; Servili, M.; et al. Influence of Cultivar and Concentration of Selected Phenolic Constituents on the in Vitro Chemiopreventive Potential of Olive Oil Extracts. J. Agric. Food Chem. 2011, 59, 8167–8174. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Cano, A.; Hernández-Ruíz, J.; García-Cánovas, F.; Acosta, M.; Arnao, M.B. An end-point method for estimation of the total antioxidant activity in plant material. Phytochem. Anal. 1998, 9, 196–202. [Google Scholar] [CrossRef]
- Gülçin, I.; Küfrevioǧlu, İ.; Oktay, M.; Büyükokuroǧlu, M.E. Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica L.). J. Ethnopharmacol. 2003, 90, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.-K.; Osawa, T.; Kawakishi, S. Hydroxyl Radical-scavenging Effects of Spices and Scavengers from Brown Mustard (Brassica nigra). Biosci. Biotechnol. Biochem. 1997, 61, 118–123. [Google Scholar] [CrossRef]
- Priftis, A.; Mitsiou, D.; Halabalaki, M.; Ntasi, G.; Stagos, D.; Skaltsounis, L.A.; Kouretas, D. Roasting has a distinct effect on the antimutagenic activity of coffee varieties. Mutat. Res. Toxicol. Environ. Mutagen. 2018, 829–830, 33–42. [Google Scholar] [CrossRef]
- Yen, G.C.; Der Duh, P. Scavenging Effect of Methanolic Extracts of Peanut Hulls on Free-Radical and Active-Oxygen Species. J. Agric. Food Chem. 1994, 42, 629–632. [Google Scholar] [CrossRef]
- Paul, T.; Young, M.J.; Hill, I.E.; Ingold, K.U. Strand Cleavage of Supercoiled DNA by Water-Soluble Peroxyl Radicals. The Overlooked Importance of Peroxyl Radical Charge. Biochemistry 2000, 39, 4129–4135. [Google Scholar] [CrossRef] [PubMed]
- Bal-Price, A.; Coecke, S. Guidance on Good Cell Culture Practice (GCCP). Neuromethods 2011, 56, 1–25. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Vardakas, P.; Skaperda, Z.; Tekos, F.; Trompeta, A.-F.; Tsatsakis, A.; Charitidis, C.A.; Kouretas, D. An integrated approach for assessing the in vitro and in vivo redox-related effects of nanomaterials. Environ. Res. 2021, 197, 111083. [Google Scholar] [CrossRef] [PubMed]
- Keles, M.; Taysi, S.; Sen, N.; Aksoy, H.; Akçay, F. Effect of Corticosteroid Therapy on Serum and CSF Malondialdehyde and Antioxidant Proteins in Multiple Sclerosis. Can. J. Neurol. Sci./J. Can. Sci. Neurol. 2001, 28, 141–143. [Google Scholar] [CrossRef]

| Sample | Varietal | Irrigation | Altitude (m) | |
|---|---|---|---|---|
| Koroneiki | Athinolia | |||
| Grove 1 | √ | - | 580 | |
| Grove 2 | √ | - | 630 | |
| Grove 3 | √ | - | 270 | |
| Grove 4 | √ | √ | 270 | |
| Grove 5 | √ | √ | 152 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gkasdrogka, M.; Tekos, F.; Skaperda, Z.; Vardakas, P.; Kouretas, D. Evaluation of the Antioxidant Properties and Bioactivity of Koroneiki and Athinolia Olive Varieties Using In Vitro Cell-Free and Cell-Based Assays. Int. J. Mol. Sci. 2025, 26, 743. https://doi.org/10.3390/ijms26020743
Gkasdrogka M, Tekos F, Skaperda Z, Vardakas P, Kouretas D. Evaluation of the Antioxidant Properties and Bioactivity of Koroneiki and Athinolia Olive Varieties Using In Vitro Cell-Free and Cell-Based Assays. International Journal of Molecular Sciences. 2025; 26(2):743. https://doi.org/10.3390/ijms26020743
Chicago/Turabian StyleGkasdrogka, Maria, Fotios Tekos, Zoi Skaperda, Periklis Vardakas, and Demetrios Kouretas. 2025. "Evaluation of the Antioxidant Properties and Bioactivity of Koroneiki and Athinolia Olive Varieties Using In Vitro Cell-Free and Cell-Based Assays" International Journal of Molecular Sciences 26, no. 2: 743. https://doi.org/10.3390/ijms26020743
APA StyleGkasdrogka, M., Tekos, F., Skaperda, Z., Vardakas, P., & Kouretas, D. (2025). Evaluation of the Antioxidant Properties and Bioactivity of Koroneiki and Athinolia Olive Varieties Using In Vitro Cell-Free and Cell-Based Assays. International Journal of Molecular Sciences, 26(2), 743. https://doi.org/10.3390/ijms26020743









