Insights into the Corrosion Inhibition Performance of Plant Extracts of Different Genera in the Asteraceae Family for Q235 Steel in H2SO4 Medium
Abstract
1. Introduction
2. Results and Discussion
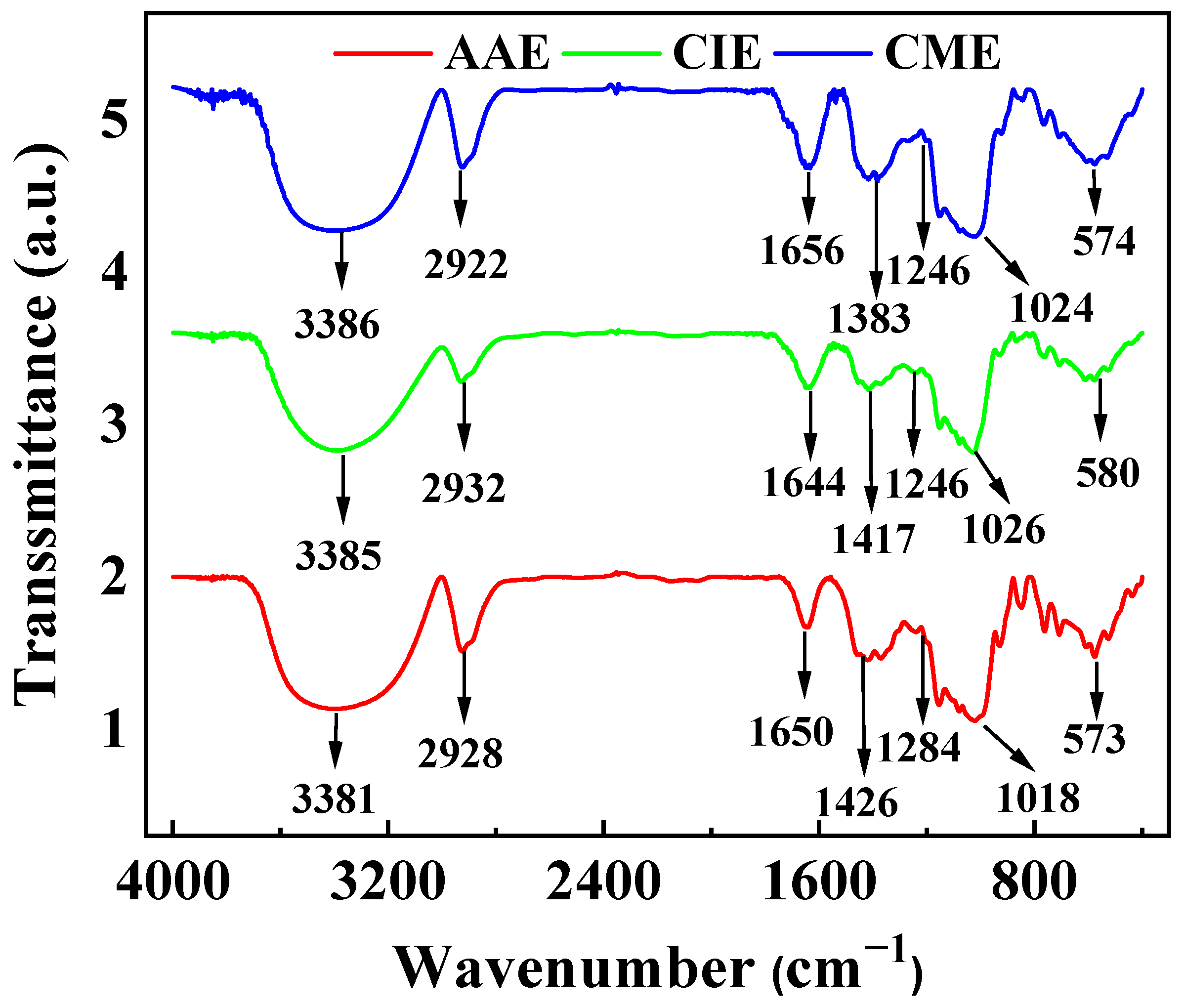
2.1. Characterizations of AAE, CIE, and CME
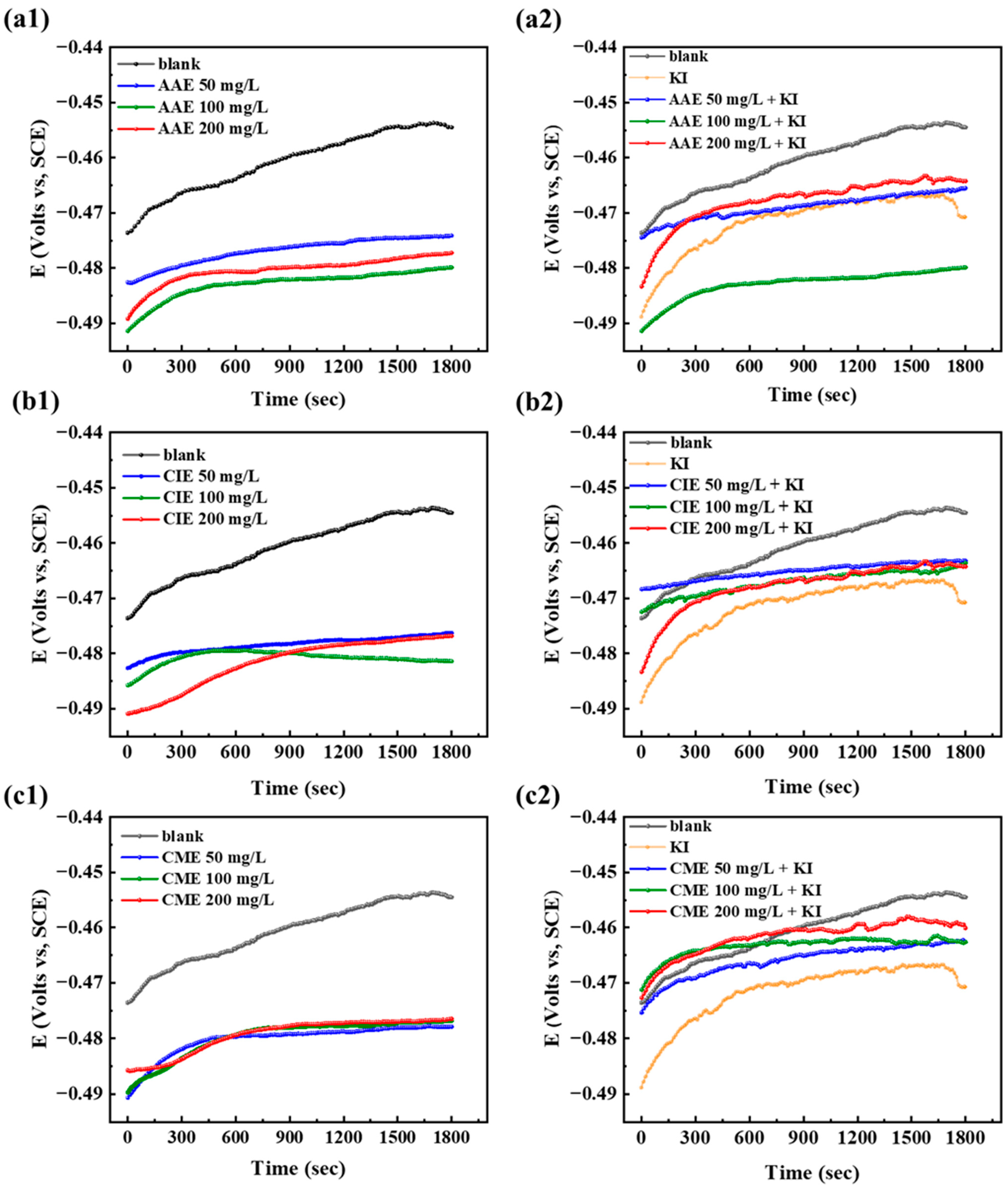
2.2. Open Circuit Potential (OCP) Curves
2.3. Potentiodynamic Polarization (PDP) Curves
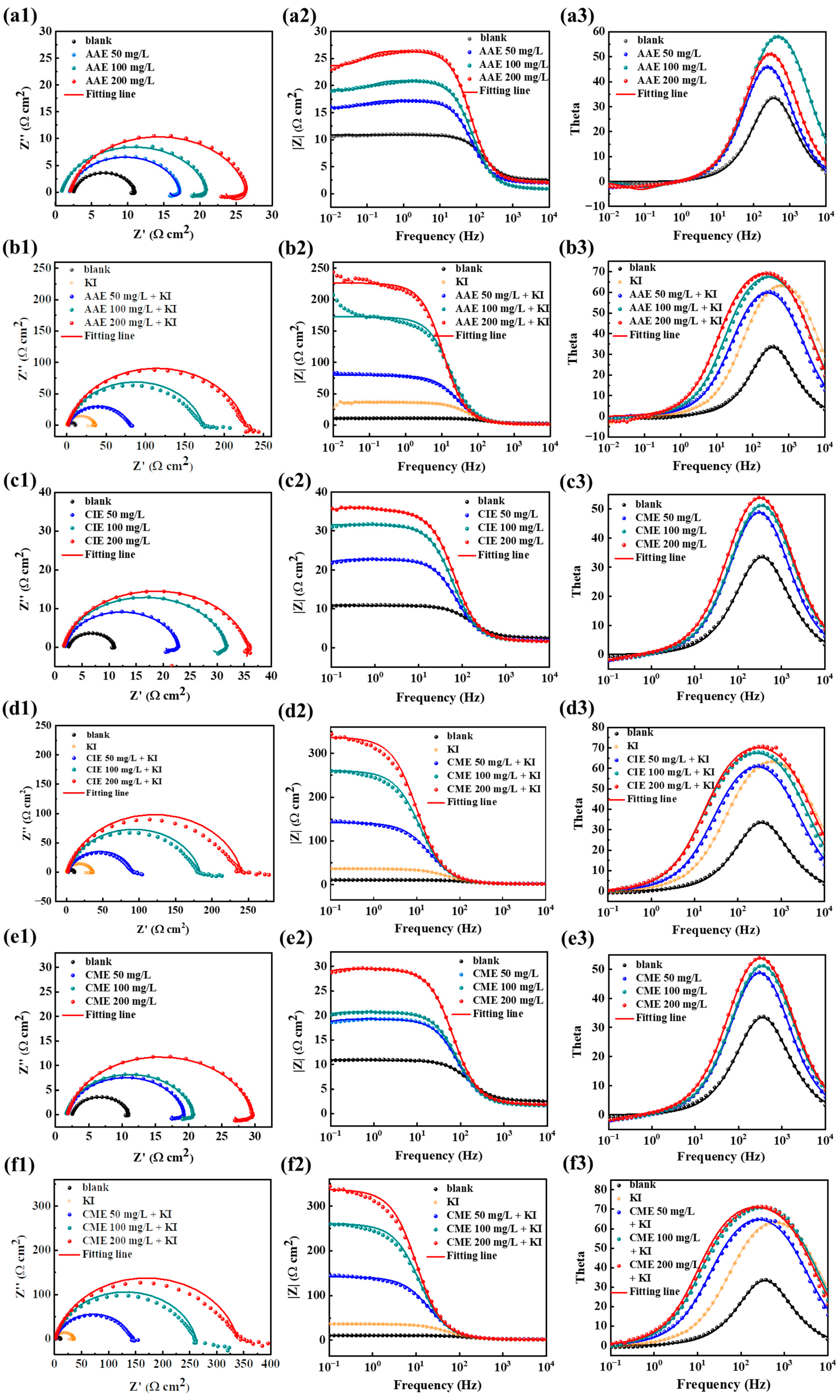
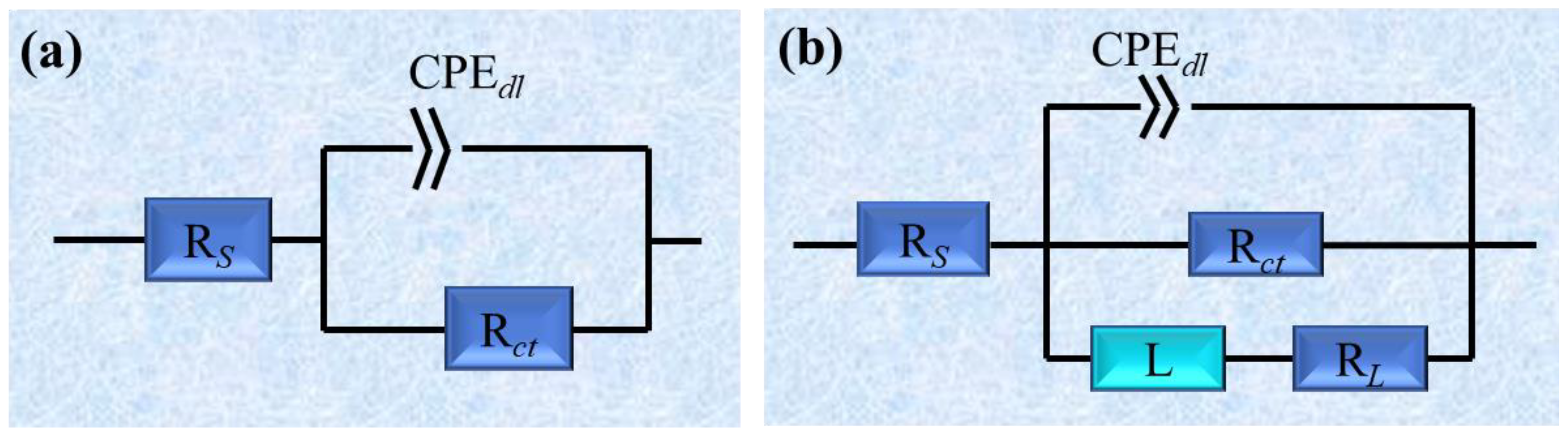
2.4. Electrochemical Impedance Spectroscopy (EIS) Curves
2.5. Long-Term Corrosion Inhibition Performance
2.6. XPS Results
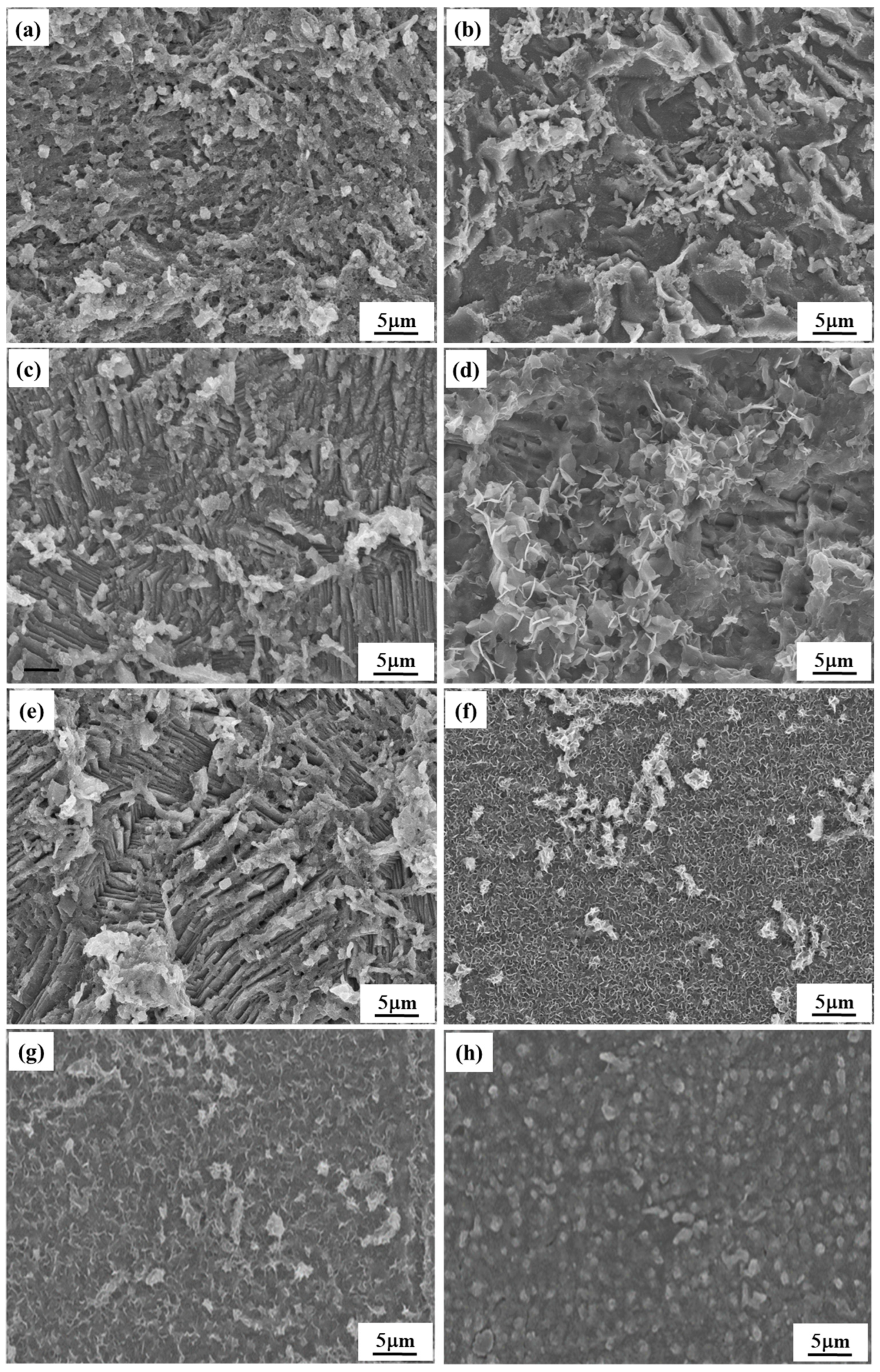
2.7. SEM Observations
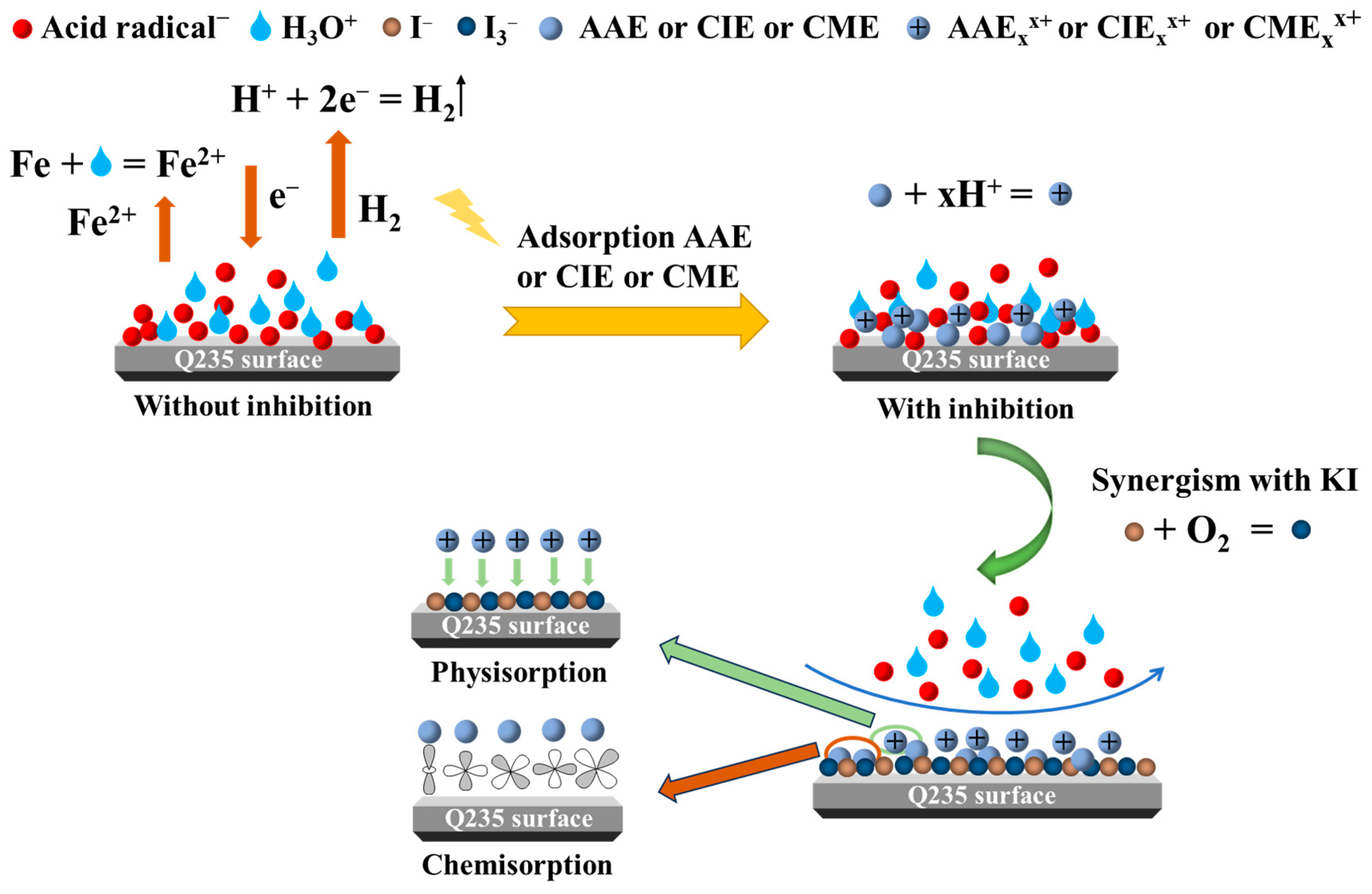
2.8. Corrosion Inhibition Mechanism of AAE, CIE, and CME
3. Experiment
3.1. Material Preparations
3.2. Electrochemical Tests
3.3. Surface Characterization
4. Conclusions
- (1)
- The characteristic peaks indicated that the primary constituents of flavonoids, saponins, and polysaccharide organic compounds in the three extracts of AAE, CIE, and CME were identical, which confirmed the similarity of the main components among the three extracts from different genera of composite plants.
- (2)
- The presence of AAE, CIE, CME, or KI alone mainly acted as a cathodic inhibitor, while the combination of the three extracts with KI as a mixed inhibitor further enhanced the inhibition of the cathodic reaction and anodic reaction. Additionally, when AAE, CIE, and CME were combined with KI, the capacitive reactance arc radius, impedance mode, and phase angle range exhibited significantly greater values compared to their individual usage.
- (3)
- AAE, CIE, CME, and KI exhibited moderate inhibitory effects on Q235 steel in 0.5 M of H2SO4. The combination of AAE, CIE, and CME with KI demonstrated a significant synergistic effect, resulting in inhibition efficiencies up to 96.29%, 96.50%, and 97.52%, respectively. Furthermore, the combination of the three extracts with KI displayed excellent long-term sustained release properties by maintaining high levels for 48 h, achieving the highest corrosion inhibition efficiency after 4 h of 98.64%, 97.65%, and 99.06%, respectively.
- (4)
- The XPS and SEM images confirmed the effective adsorption of AAE, CIE, and CME on the Q235 steel surface, resulting in the formation of a protective film. The combined synergistic effect of AAE, CIE, and CME with KI significantly retarded the corrosion process on the Q235 steel surface.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palumbo, G.; Święch, D.; Górny, M. Guar Gum as an Eco-Friendly Corrosion Inhibitor for N80 Carbon Steel under Sweet Environment in Saline Solution: Electrochemical, Surface, and Spectroscopic Studies. Int. J. Mol. Sci. 2023, 24, 12269. [Google Scholar] [CrossRef] [PubMed]
- Robles, E.C.; Olivares-Xometl, O.; Likhanova, N.V.; Arellanes-Lozada, P.; Lijanova, I.V.; Díaz-Jiménez, V. Synthesis of Ammonium-Based ILs with Different Lengths of Aliphatic Chains and Organic Halogen-Free Anions as Corrosion Inhibitors of API X52 Steel. Int. J. Mol. Sci. 2023, 24, 7613. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.S.; Guo, Z.Y.; Zhi, H.; Qiang, Y.J.; Liu, X.Y.; Zhang, Y.; Wan, Y.; Xiang, T.F.; Li, X.H. Experimental and computational exploration of the biodegradable platanus acerifolia leaf extract against mild steel corrosion in hydrochloric acid. J. Mater. Res. Technol. 2024, 30, 7830–7842. [Google Scholar] [CrossRef]
- Gómez-Sánchez, G.; Olivares-Xometl, O.; Arellanes-Lozada, P.; Likhanova, N.V.; Lijanova, I.V.; Arriola-Morales, J.; Díaz-Jiménez, V.; López-Rodríguez, J. Temperature Effect on the Corrosion Inhibition of Carbon Steel by Polymeric Ionic Liquids in Acid Medium. Int. J. Mol. Sci. 2023, 24, 6291. [Google Scholar] [CrossRef] [PubMed]
- El-Lateef, H.M.; El-Dabea, T.; Khalaf, M.M.; Abu-Dief, A.M. Innovation of Imine Metal Chelates as Corrosion Inhibitors at Different Media: A Collective Study. Int. J. Mol. Sci. 2022, 23, 9360. [Google Scholar] [CrossRef]
- Hrimla, M.; Bahsis, L.; Laamari, M.R.; Julve, M.; Stiriba, S.E. An Overview on the Performance of 1,2,3-Triazole Derivatives as Corrosion Inhibitors for Metal Surfaces. Int. J. Mol. Sci. 2022, 23, 16. [Google Scholar] [CrossRef]
- Zeng, C.; Zhou, Z.; Mai, W.J.; Chen, Q.H.; He, J.; Liao, B.K. Exploration on the corrosion inhibition performance of Salvia miltiorrhiza extract as a green corrosion inhibitor for Q235 steel in HCl environment. J. Mater. Res. Technol. 2024, 32, 3857–3870. [Google Scholar] [CrossRef]
- Li, Y.F.; Li, Z.Q.; Ma, T.; Zeng, L.; Chen, H.X.; Lei, X.X.; Ma, K.; Zhang, Z.H.; Ding, Y.W.; Han, J.T. Corrosion mitigation of peanut shell extract as a novel effective corrosion inhibitor for carbon steel in sulfuric acid medium. J. Mater. Res. Technol. 2023, 27, 4706–4722. [Google Scholar] [CrossRef]
- Paiva, V.M.; Oliveira, S.M.D.; Almeida, C.M.D.S.D.; Araujo, J.R.D.; Archanjo, B.S.; D’Elia, E. Pumpkin (Cucurbita maxima) seed-derived nitrogen, phosphorus, and sulfur carbon quantum dot as an inhibitor of corrosion for mild steel in HCl solution. J. Mater. Res. Technol. 2024, 28, 2504–2515. [Google Scholar] [CrossRef]
- Junaedi, S.; Al-Amiery, A.A.; Kadihum, A.; Kadhum, A.A.H.; Mohamad, A.B. Inhibition Effects of a Synthesized Novel 4-Aminoantipyrine Derivative on the Corrosion of Mild Steel in Hydrochloric Acid Solution together with Quantum Chemical Studies. Int. J. Mol. Sci. 2013, 14, 11915–11928. [Google Scholar] [CrossRef] [PubMed]
- Tokuad, S.; Muto, I.; Sugawara, Y.; Takahashi, M.; Matsumoto, M.; Hara, N. Micro-electrochemical investigation on the role of Mg in sacrificial corrosion protection of 55mass%Al-Zn-Mg coated steel. Corros. Sci. 2017, 129, 126–135. [Google Scholar] [CrossRef]
- Ebenso, E.E.; Isabirye, D.A.; Eddy, N.O. Adsorption and Quantum Chemical Studies on the Inhibition Potentials of Some Thiosemicarbazides for the Corrosion of Mild Steel in Acidic Medium. Int. J. Mol. Sci. 2010, 11, 2473–2498. [Google Scholar] [CrossRef]
- Birbilis, N.; Choudhary, S.; Scully, J.R.; Taheir, M.L. A perspective on corrosion of multi-principal element alloys. npj Mater. Degrad. 2021, 5, 14. [Google Scholar] [CrossRef]
- Yao, X.; Qiang, Y.; Guo, L.; Xu, Q.; Wen, L.; Jin, Y. Renewable low-cost brassica rapa subsp. Extract for protection of Q235 steel in H2SO4 medium: Experimental and modeling studies. J. Ind. Eng. Chem. 2022, 114, 427–437. [Google Scholar] [CrossRef]
- Kaur, J.; Daksh, N.; Saxena, A. Corrosion Inhibition Applications of Natural and Eco-Friendly Corrosion Inhibitors on Steel in the Acidic Environment: An Overview. Arab. J. Sci. Eng. 2022, 47, 57–74. [Google Scholar] [CrossRef]
- Panchal, J.; Shah, D.; Patel, R.; Shah, S.; Prajapati, M.; Shah, M. Comprehensive Review and Critical Data Analysis on Corrosion and Emphasizing on Green Eco-friendly Corrosion Inhibitors for Oil and Gas Industries. J. Bio Tribo Corros. 2021, 7, 107. [Google Scholar] [CrossRef]
- Faraji, M.; Pezzato, L.; Yazdanpanah, A.; Nardi, G.; Esmailzadeh, M.; Calliari, I. Effect of Natural Inhibitors on the Corrosion Properties of Grade 2 Titanium Alloy. Materials 2024, 17, 5202. [Google Scholar] [CrossRef]
- Kemel, M. Eco-friendly corrosion inhibition of steel using phenolic compounds from Cynara syriaca. J. Ind. Eng. Chem. 2024, 140, 617–630. [Google Scholar] [CrossRef]
- Liao, B.K.; Quan, R.X.; Feng, P.X.; Wang, H.; Wang, W.; Niu, L. Carbon steel anticorrosion performance and mechanism of sodium lignosulfonate. Rare Met. 2024, 43, 356–365. [Google Scholar] [CrossRef]
- Liao, B.K.; Ma, S.Q.; Zhang, S.Y.; Li, X.X.; Quan, R.X.; Wan, S.; Guo, X.P. Fructus cannabis protein extract powder as a green and high effective corrosion inhibitor for Q235 carbon steel in 1 M HCl solution. Int. J. Biol. Macromol. 2023, 239, 124358. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Z.; Cao, J.; Gao, L.M.; Chen, Q.H.; Yang, J.J.; Liao, B.K.; Guo, X.P. Improving the discharge performance of aqueous Mg-air battery using dicarboxylic acid additives. Mater. Res. Bull. 2025, 182, 113160. [Google Scholar] [CrossRef]
- Zhang, M.Q.; Guo, L.; Zhu, M.Y.; Wang, K.; Zhang, R.H.; He, Z.Y.; Lin, Y.H.; Leng, S.L.; Anadebe, V.C.; Zheng, X.W. Akebia trifoliate koiaz peels extract as environmentally benign corrosion inhibitor for mild steel in HCl solutions: Integrated experimental and theoretical investigations. J. Ind. Eng. Chem. 2021, 101, 227–236. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, C.; Xu, S.Y.; Xiang, B.; Marzouki, R. Combining electrochemical, surface topography analysis, and theoretical alculation methods to insight into the anti-corrosion property of Syzygium samarangense leaf extract. J. Ind. Eng. Chem. 2021, 102, 302–311. [Google Scholar] [CrossRef]
- Dar, M.A. A review: Plant extracts and oils as corrosion inhibitors in aggressive media. Ind. Lubr. Tribol. 2011, 63, 227–233. [Google Scholar] [CrossRef]
- Luo, Z.G.; Zhang, Y.; Wang, H.; Wan, S.; Song, L.F.; Liao, B.K.; Guo, X.P. Modified nano-lignin as a novel biomass-derived corrosion inhibitor for enhanced corrosion resistance of carbon steel. Corros. Sci. 2024, 227, 111705. [Google Scholar] [CrossRef]
- Supardi, J.; Rizal, S.; Ali, N.; Fonna, S.; Ikramullah, I.; Arifin, A.K. Investigating an environmentally in aqueous solution of potacium chloride. J. Mater. Res. Technol. 2024, 29, 303–310. [Google Scholar] [CrossRef]
- Zhou, Z.Y.; Min, X.H.; Wan, S.; Liu, J.H.; Liao, B.K.; Guo, X.P. A novel green corrosion inhibitor extracted from waste feverfew root for carbon steel in H2SO4 solution. Results Eng. 2023, 17, 100971. [Google Scholar] [CrossRef]
- Liao, B.K.; Luo, Z.G.; Wan, S.; Chen, L.J. Insight into the anti-corrosion performance of Acanthopanax senticosus leaf extract as eco-friendly corrosion inhibitor for carbon steel in acidic medium. J. Ind. Eng. Chem. 2023, 117, 238–246. [Google Scholar] [CrossRef]
- Wan, S.; Zhang, T.; Chen, H.; Liao, B.K.; Guo, X.P. Kapok leaves extract and synergistic iodide as novel effective corrosion inhibitors for Q235 carbon steel in H2SO4 medium. Ind. Crops Prod. 2022, 178, 114649. [Google Scholar] [CrossRef]
- Gapsari, F.; Darmadi, D.B.; Setyarini, P.H.; Wijaya, H.; Madurani, K.A.; Juliano, H.; Sulaiman, A.M.; Hidayatullah, S.; Tanji, A.; Hermawan, H. Analysis of corrosion inhibition of Kleinhovia hospita plant extract aided by quantification of hydrogen evolution using a GLCM/SVM method. Int. J. Hydrogen Energy 2023, 48, 15392–15405. [Google Scholar] [CrossRef]
- Golshani, Z.; Moezabad, H.C.; Amiri, M.; Sajadi, G.S.; Naghizade, R.; Hosseini, S.M.A. Effect of Thyme extract as an ecofriendly inhibitor for corrosion of mild steel in acidic media. Mater. Corros. 2022, 73, 460–469. [Google Scholar] [CrossRef]
- Berrissoul, A.; Ouarhach, A.; Benhiba, F.; Romane, A.; Zarrouk, A.; Guenbour, A.; Dikici, B.; Dafali, A. Evaluation of Lavandula mairei extract as green inhibitor for mild steel corrosion in 1 M HCl solution. Experimental and theoretical approach. J. Mol. Liq. 2020, 313, 113493. [Google Scholar] [CrossRef]
- Asadi, N.; Ramezanzadeh, M.; Bahlakeh, G.; Ramezanzadeh, B. Utilizing Lemon Balm extract as an effective green corrosion inhibitor for mild steel in 1M HCl solution: A detailed experimental, molecular dynamics, Monte Carlo and quantum mechanics study. J. Taiwan Inst. Chem. Eng. 2019, 95, 252–272. [Google Scholar] [CrossRef]
- Fernine, Y.; Salim, R.; Arrousse, N.; Haldhar, R.; El Hajjaji, F.; Kim, S.C.; Ebn Touhami, M.; Taleb, M. Anti-corrosion performance of Ocimum basilicum seed extract as environmental friendly inhibitors for mild steel in HCl solution: Evaluations of electrochemical, EDX, DFT and Monte Carlo. J. Mol. Liq. 2022, 355, 118677. [Google Scholar] [CrossRef]
- Dehghani, A.; Ramezanzadeh, B. Rosemary extract inhibitive behavior against mild steel corrosion in tempered 1 M HCl media. Ind. Crops Prod. 2023, 193, 116183. [Google Scholar] [CrossRef]
- Al-Senani, G.M.; Alshabanat, M. Study the Corrosion Inhibition of Carbon Steel in 1 M HCl Using Extracts of Date Palm Waste. Int. J. Electrochem. Sci. 2018, 13, 3777–3788. [Google Scholar] [CrossRef]
- Adam, M.R.; Rahim, A.A.; Shah, A.M. Synergy between iodide ions and mangrove tannins as inhibitors of mild steel corrosion. Ann. For. Sci. 2015, 72, 9–15. [Google Scholar] [CrossRef]
- Haque, J.; Verma, C.; Srivastava, V.; Waw, N. Corrosion inhibition of mild steel in 1M HCl using environmentally benign Thevetia peruviana flower extracts. Sustain. Chem. Pharm. 2021, 19, 100354. [Google Scholar] [CrossRef]
- Ofuyekpone, O.D.; Utu, O.G.; Onyekpe, B.O.; Adediran, A.A.; Oki, M. Evaluation of protection performances of Stylosanthes gracilis extract against 304L corrosion in H2SO4 solution. Case Stud. Chem. Environ. Eng. 2020, 2, 100058. [Google Scholar] [CrossRef]
- Anadebe, V.C.; Onukwuli, O.D.; Omotioma, M.; Okafor, N.A. Experimental, theoretical modeling and optimization of inhibition efficiency of pigeon pea leaf extract as anti-corrosion agent of mild steel in acid environment. Mater. Chem. Phys. 2019, 233, 120–132. [Google Scholar] [CrossRef]
- Srivastava, M.; Tiwari, P.; Srivastava, S.K.; Kumar, A.; Ji, G.; Prakas, R. Low cost aqueous extract of Pisum sativum peels for inhibition of mild steel corrosion. J. Mol. Liq. 2018, 254, 357–368. [Google Scholar] [CrossRef]
- Omran, M.A.; Fawzy, M.; Mahmoud, A.E.; Abdullatef, O.A. Optimization of mild steel corrosion inhibition by water hyacinth and common reed extracts in acid media using factorial experimental design. Green Chem. Lett. Rev. 2022, 15, 214–230. [Google Scholar] [CrossRef]
- Nazari, A.; Ramezanzadeh, B.; Guo, L.; Dehghani, A. Application of green active bio-molecules from the aquatic extract of Mint leaves for steel corrosion control in hydrochloric acid (1M) solution: Surface, electrochemical, and theoretical explorations. Colloids Surface. A. 2023, 656, 130540. [Google Scholar] [CrossRef]
- Alaneme, K.K.; Olusegun, S.J.; Alo, A.W. Corrosion inhibitory properties of elephant grass (Pennisetum purpureum) extract: Effect on mild steel corrosion in 1M HCl solution. Alex. Eng. J. 2016, 55, 1069–1076. [Google Scholar] [CrossRef]
- Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B.; Ramezanzadeh, M. Aloysia citrodora leaves extract corrosion retardation effect on mild-steel in acidic solution: Molecular/atomic scales and electrochemical explorations. J. Mol. Liq. 2020, 310, 113221. [Google Scholar] [CrossRef]
- Meng, S.G.; Liu, Z.N.; Zhao, X.Q.; Fan, B.M.; Liu, H.; Guo, M.; Hao, H. Efficient corrosion inhibition by sugarcane purple rind extract for carbon steel in HCl solution: Mechanism analyses by experimental and in silico insights. RSC Adv. 2021, 11, 31693–31711. [Google Scholar] [CrossRef]
- Odewunmi, N.A.; Umoren, S.A.; Gasem, Z.M.; Ganiyu, S.A.; Muhammad, Q. L-Citrulline: An active corrosion inhibitor component of watermelon rind extract for mild steel in HCl medium. J. Taiwan Inst. Chem. Eng. 2015, 51, 177–185. [Google Scholar] [CrossRef]
- Feng, L.J.; Zhang, S.S.; Hao, L.; Du, H.C.; Pan, R.K.; Haung, G.F. Cucumber (Cucumis sativus L.) Leaf Extract as a Green Corrosion Inhibitor for Carbon Steel in Acidic Solution: Electrochemical, Functional and Molecular Analysis. Molecules 2022, 27, 3826. [Google Scholar] [CrossRef]
- Ogunleye, O.O.; Arinkoola, A.O.; Eletta, O.A.; Agbede, O.O.; Osho, Y.A.; Morakinyo, A.F. Green corrosion inhibition and adsorption characteristics of Luffa cylindrica leaf extract on mild steel in hydrochloric acid environment. Heliyon 2020, 6, e03205. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B.; Ramezanzadeh, M. A combined experimental and theoretical study of green corrosion inhibition of mild steel in HCl solution by aqueous Citrullus lanatus fruit (CLF) extract. J. Mol. Liq. 2019, 279, 603–624. [Google Scholar] [CrossRef]
- Haldhar, R.; Prasad, D.; Kamboj, D.; Kaya, S.; Dagdag, O.; Guo, L. Corrosion inhibition, surface adsorption and computational studies of Momordica charantia extract: A sustainable and green approach. SN Appl. Sci. 2021, 3, 25. [Google Scholar] [CrossRef]
- Divya, P.; Subhashini, S.; Prithiba, A.; Rajalakshmi, R. Tithonia Diversifolia flower Extract as green Corrosion Inhibitor for Mild Steel in Acid Medium. Mater. Today Proc. 2019, 18, 1581–1591. [Google Scholar] [CrossRef]
- Pal, S.; Lgaz, H.; Tiwari, P.; Chung, I.M.; Ji, G.; Prakash, R. Experimental and theoretical investigation of aqueous and methanolic extracts of Prunus dulcis peels as green corrosion inhibitors of mild steel in aggressive chloride media. J. Mol. Liq. 2019, 276, 347–361. [Google Scholar] [CrossRef]
- Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B.; Ramezanzadeh, M. Applying detailed molecular/atomic level simulation studies and electrochemical explorations of the green inhibiting molecules adsorption at the interface of the acid solution-steel substrate. J. Mol. Liq. 2020, 299, 112220. [Google Scholar] [CrossRef]
- Zheng, X.W.; Gong, M.; Li, Q.; Huo, L. Corrosion inhibition of mild steel in sulfuric acid solution by loquat (Eriobotrya japonica Lindl.) leaves extract. Sci. Rep. 2018, 9, 9140. [Google Scholar] [CrossRef]
- Umoren, S.; Obot, I.B.; Gasem, Z.; Odewunmi, N.A. Experimental and Theoretical Studies of Red Apple Fruit Extract as Green Corrosion Inhibitor for Mild Steel in HCl Solution. J. Dis. Sci. Technol. 2015, 36, 789–802. [Google Scholar] [CrossRef]
- Wang, Q.H.; Liu, L.; Zhang, Q.; Wu, X.D.; Zheng, H.H.; Gao, P.; Zeng, G.M.; Yan, Z.T.; Sun, Y.; Li, X.M. Insight into the anti-corrosion performance of Artemisia argyi leaves extract as eco-friendly corrosion inhibitor for carbon steel in HCl medium. Sustain. Chem. Pharm. 2022, 27, 100710. [Google Scholar] [CrossRef]
- Zhang, H.B.; Ni, Z.H.; Wu, H.T.; Xu, Z.D.; Zhang, W.Y.; Huang, H.X.; Zhou, Q.; Yue, X.G.; Bao, J.K.; Li, X.M. Corrosion Inhibition of Carbon Steel in Hydrochloric Acid by Chrysanthemum Indicum Extract. Int. J. Electrochem. Sci. 2020, 15, 5487–5499. [Google Scholar] [CrossRef]
- Mourya, P.; Banerjee, S.; Singh, M.M. Corrosion inhibition of mild steel in acidic solution by Tagetes erecta (Marigold flower) extract as a green inhibitor. Corros. Sci. 2014, 85, 352–363. [Google Scholar] [CrossRef]
- Kalkhambkar, A.G.; Rajappa, S.K.; Manjanna, J.; Malimath, G.H. Saussurea obvallatta leaves extract as a potential eco-friendly corrosion inhibitor for mild steel in 1 M HCl. Inorg. Chem. Commun. 2022, 143, 109799. [Google Scholar] [CrossRef]
- Hassannejad, H.; Nouri, A. Sunflower seed hull extract as a novel green corrosion inhibitor for mild steel in HCl solution. J. Mol. Liq. 2018, 254, 377–382. [Google Scholar] [CrossRef]
- Kouache, A.; Khelifa, A.; Boutoumi, H.; Moulay, S.; Feghoul, A.; Idir, B.; Aoudj, S. Experimental and theoretical studies of Inula viscosa extract as a novel eco-friendly corrosion inhibitor for carbon steel in 1 M HCl. J. Adhes. Sci. Technol. 2022, 36, 988–1016. [Google Scholar] [CrossRef]
- Wang, Y.B.; Li, L.J.; He, J.B.; Sum, B.J. Extract of Silybum marianum (L.) Gaertn Leaves as a Novel Green Corrosion Inhibitor for Carbon Steel in Acidic Solution. Materials 2024, 17, 4794. [Google Scholar] [CrossRef]
- Beniaich, G.; Beniken, M.; Salim, R.; Arrousse, N.; Ech-chibi, E.; Rais, Z.; Sadiq, A.; Nafidi, H.A.; Bin Jardan, Y.A.; Bourhia, M.; et al. Anticorrosive Effects of Essential Oils Obtained from White Wormwood and Arar Plants. Separations 2023, 10, 396. [Google Scholar] [CrossRef]
- Cang, H.; Shi, W.Y.; Shao, J.L.; Xu, Q. Study of Stevia rebaudiana Leaves as Green Corrosion Inhibitor for Mild Steel in Sulphuric Acid by Electrochemical Techniques. Int. J. Electrochem. Sci. 2012, 7, 3726–3736. [Google Scholar] [CrossRef]
- Krysa, M.; Szymańska-Chargot, M.; Zdunek, A. FT-IR and FT-Raman fingerprints of flavonoids—A review. Food. Chem. 2022, 393, 133430. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.; Yin, J.Y.; Nie, S.P.; Xie, M.Y. Applications of infrared spectroscopy in polysaccharide structural analysis: Progress, challenge and perspective. Food. Chem. X. 2021, 12, 100168. [Google Scholar] [CrossRef] [PubMed]
- Shahini, M.H.; Ramezanzadeh, M.; Ramezanzadeh, B. Effective steel alloy surface protection from HCl attacks using Nepeta Pogonesperma plant stems extract. Colloids Surf. A 2022, 634, 127990. [Google Scholar] [CrossRef]
- Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B.; Ramezanzadeh, M. Experimental complemented with microscopic (electronic/atomic)-level modeling explorations of Laurus nobilis extract as green inhibitor for carbon steel in acidic solution. J. Ind. Eng. Chem. 2020, 84, 52–71. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, F.; Liu, Y.K.; Huang, H.; Tang, Z.B.; Wang, J.B.; Li, X.F.; Wu, M.Z.; Wang, T.G.; Zheng, J.; et al. Effect of plasma treatment on nucleation of Au nanoparticles. J. Mater. Res. Technol. 2022, 20, 2235–2246. [Google Scholar] [CrossRef]
- Chen, Q.H.; Zhou, Z.Y.; Feng, M.T.; He, J.H.; Xu, Y.Q.; Liao, B.K. Unraveling the inhibitive performance and adsorption behavior of expired compound glycyrrhizin tablets as an eco-friendly corrosion inhibitor for copper in acidic medium. J. Taiwan Inst. Chem. Eng. 2025, 168, 105913. [Google Scholar] [CrossRef]
- Du, P.F.; Deng, S.D.; Du, G.B.; Shao, D.D.; Xu, D.K.; Li, X.H. Synergistic inhibition effect of Mikania micrantha extract with potassium iodide on the corrosion of cold rolled steel in methanesulfonic acid solution. Corros. Sci. 2023, 220, 111296. [Google Scholar] [CrossRef]
- Motamedi, M.; Ramezanzadzh, B.; Mandavian, M. Corrosion inhibition properties of a green hybrid pigment based on Pr-Urtica Dioica plant extract. J. Ind. Eng. Chem. 2018, 66, 116–125. [Google Scholar] [CrossRef]
- Gonzalez-Canche, N.G.; Flores-Johnson, E.A.; Cortes, P.; Carrillo, J.G. Evaluation of surface treatments on 5052-H32 aluminum alloy for enhancing the interfacial adhesion of thermoplastic-based fiber metal laminates. Int. J. Adhes. Adhes. 2018, 88, 90–99. [Google Scholar] [CrossRef]
- Abeng, F.E.; Ita, B.I.; Ikpi, M.E.; Chukwuike, V.I.; Ikeuba, A.I.; Edim, M.M.; Chidiebere, M.A.; Thakur, A.; Anadebe, V.C. Millettia aboensis leaves extract as eco-friendly corrosion inhibitor for mild steel in acidizing solution: From experimental to molecular level prediction. Results Eng. 2024, 24, 102950. [Google Scholar] [CrossRef]
- Zhu, J.H.; Lin, B.L.; Duan, T.H.; Lin, H.Q.; Zhang, G.Y.; Zhou, X.X.; Xu, Y.Y. Zea mays bracts extract as an eco-friendly corrosion inhibitor for steel in HCl pickling solution: Experimental and simulation studies. Arab. J. Chem. 2024, 17, 105895. [Google Scholar] [CrossRef]
- Kowsari, E.; Payami, M.; Amini, R.; Ramezanzadeh, B.; Javanbakht, M. Task-specific ionic liquid as a new green inhibitor of mild steel corrosion. Appl. Surf. Sci. 2014, 289, 478–486. [Google Scholar] [CrossRef]
- Zheng, T.Y.; Liu, J.Y.; Wang, M.H.; Liu, Q.; Wang, J.; Chong, Y.; Jia, G.X. Synergistic corrosion inhibition effects of quaternary ammonium salt cationic surfactants and thiourea on Q235 steel in sulfuric acid: Experimental and theoretical research. Corros. Sci. 2022, 199, 110199. [Google Scholar] [CrossRef]
- Finsgar, M. Surface analysis of the 2-mercaptobenzothiazole corrosion inhibitor on 6082 aluminum alloy using ToF-SIMS and XPS. Anal. Methods 2020, 12, 456–465. [Google Scholar] [CrossRef]
- Qiang, Y.J.; Guo, L.; Li, H.; Lan, X.J. Fabrication of environmentally friendly Losartan potassium film for corrosion inhibition of mild steel in HCl medium. Chem. Eng. J. 2021, 406, 126863. [Google Scholar] [CrossRef]
- Wang, H.; Deng, S.; Du, G.; Li, X. Synergistic mixture of Eupatorium adenophora spreng leaves extract and KI as a novel green inhibitor for steel corrosion in 5.0 M H3PO4. J. Mater. Res. Technol. 2023, 23, 5082–5104. [Google Scholar] [CrossRef]
- Teng, X.Y.; Deng, S.D.; Li, X.H. Synergistic corrosion inhibition of rubber seed extract with KI on cold rolled steel in sulfuric acid solution. J. Taiwan Inst. Chem. Eng. 2024, 161, 105564. [Google Scholar] [CrossRef]
- Du, P.F.; Yang, H.F.; Deng, S.D.; Li, X.H. Synergistic inhibition of Mikania micrantha extract with iodide ion on the corrosion of cold rolled steel in trichloroacetic acid medium. J. Ind. Eng. Chem. 2024, 139, 358–377. [Google Scholar] [CrossRef]
- Wang, Y.; Qiang, Y.J.; Zhi, H.; Ran, B.Y.; Zhang, D.W. Evaluating the synergistic effect of maple leaves extract and iodide ions on corrosion inhibition of Q235 steel in H2SO4 solution. J. Ind. Eng. Chem. 2023, 117, 422–433. [Google Scholar] [CrossRef]
- Qiu, L.; Li, X.H.; Xu, D.K.; Shao, D.D.; Du, G.B.; Deng, S.D. Comparison of the corrosion inhibition property on cold rolled steel in sulfuric acid media between reflux and ultrasound extracts from rapeseed meal. Ind. Crops Prod. 2024, 216, 118809. [Google Scholar] [CrossRef]
- Alhaidar, B.; Reeshah, F.; Jammoal, Y. The Possibility of Using Barley Grains Extract as an Acidic Corrosion Inhibitor for Carbon Steel. Baghdad Sci. J. 2024, 21, 1306–1317. [Google Scholar] [CrossRef]
- Zhu, L.; Fan, J.; Huang, H.; Guo, L.; Zhu, M.; Zheng, X.; Obot, I.B. Inhibitive effect of different solvent fractions of bamboo shoots extract on the corrosion of mild steel in 0.5 mol/L H2SO4 solution. J. Mol. Struct. 2021, 1243, 130852. [Google Scholar] [CrossRef]
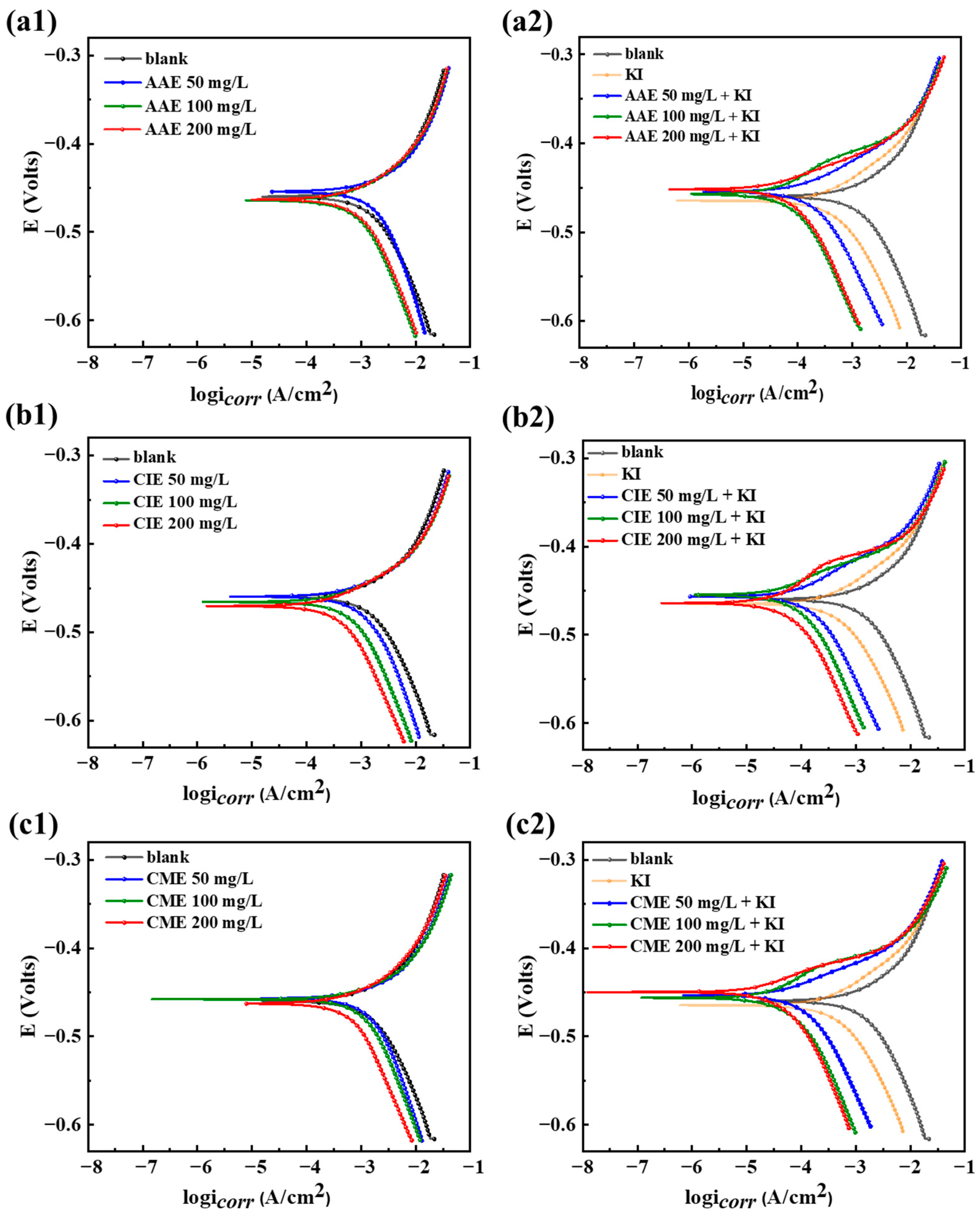


| Concentration (mg/L) | Ecorr (V) | icorr (mA·cm−2) | η (%) | |
|---|---|---|---|---|
| blank | -- | −0.46 | 1.21 ± 0.06 | -- |
| KI | -- | −0.47 | 0.32 ± 0.009 | 73.30 ± 0.15 |
| AAE | 50 | −0.45 | 0.782 ± 0.025 | 35.87 ± 0.59 |
| 100 | −0.46 | 0.63 ± 0.032 | 47.77 ± 0.10 | |
| 200 | −0.46 | 0.67 ± 0.027 | 44.63 ± 0.25 | |
| AAE + 60 mg/L of KI | 50 | −0.45 | 0.12 ± 0.0054 | 90.25 ± 0.21 |
| 100 | −0.46 | 0.08 ± 0.0013 | 93.69 ± 0.32 | |
| 200 | −0.45 | 0.04 ± 0.0025 | 96.69 ± 0.01 | |
| CIE | 50 | −0.46 | 0.64 ± 0.03 | 47.02 ± 0.17 |
| 100 | −0.47 | 0.44 ± 0.011 | 63.55 ± 0.05 | |
| 200 | −0.47 | 0.32± 0.0076 | 73.55 ± 0.09 | |
| CIE + 60 mg/L of KI | 50 | −0.46 | 0.15 ± 0.0016 | 87.93 ± 0.36 |
| 100 | −0.45 | 0.07 ± 0.001 | 94.36 ± 0.16 | |
| 200 | −0.46 | 0.05 ± 0.0008 | 95.74 ± 0.11 | |
| CME | 50 | −0.46 | 1.11 ± 0.0016 | 8.26 ± 0.23 |
| 100 | −0.46 | 0.69 ± 0.002 | 43.22 ± 0.39 | |
| 200 | −0.46 | 0.49 ± 0.01 | 59.42 ± 0.06 | |
| CME + 60 mg/L of KI | 50 | −0.45 | 0.06 ± 0.0002 | 94.97 ± 0.06 |
| 100 | −0.46 | 0.05 ± 0.0045 | 95.67 ± 0.17 | |
| 200 | −0.45 | 0.02 ± 0.006 | 98.26 ± 0.06 |
| Concentration (mg/L) | RS (Ω cm2) | CPEdl (μF cm−2) | n | RL (Ω cm2) | L (µH cm2) | Rct (Ω cm2) | ηEIS (%) | |
|---|---|---|---|---|---|---|---|---|
| blank | -- | 2.53 ± 0.27 | 2.31 × 10−4 | 0.90 | -- | -- | 8.36 ± 0.43 | -- |
| KI | -- | 0.60 ± 0.12 | 1.81 × 10−4 | 0.86 | -- | -- | 35.27 ± 6.84 | 76.30 ± 1.17 |
| AAE | 50 | 1.98 ± 0.2 | 2.54 × 10−4 | 0.90 | 152.62 ± 13.54 | 319.91 | 15.30 ± 2.17 | 45.36 ± 1.53 |
| 100 | 0.85 ± 0.03 | 1.82 × 10−4 | 0.89 | 200.21 ± 10.81 | 257.16 | 20.16 ± 2.62 | 58.53 ± 0.99 | |
| 200 | 2.06 ± 0.22 | 1.75 × 10−4 | 0.90 | 184.86 ± 25.82 | 400.03 | 24.42 ± 2.89 | 65.77 ± 0.70 | |
| AAE + KI 60 mg/L | 50 | 1.82 ± 0.14 | 1.68 × 10−4 | 0.84 | -- | -- | 77.84 ± 8.92 | 89.26 ± 0.21 |
| 100 | 1.48 ± 0.08 | 1.19 × 10−4 | 0.86 | -- | -- | 171.53 ± 30.18 | 95.13 ± 0.20 | |
| 200 | 1.37 ± 0.15 | 9.14 × 10−5 | 0.86 | -- | -- | 225.60 ± 48.34 | 96.29 ± 0.22 | |
| CIE | 50 | 1.85 ± 0.26 | 1.75 × 10−4 | 0.91 | 171.45 ± 19.25 | 490.80 | 20.96 ± 2.87 | 60.11 ± 1.04 |
| 100 | 1.63 ± 0.07 | 1.54 × 10−4 | 0.90 | 295.80 ± 35.11 | 2192.00 | 30.08 ± 5.68 | 72.21 ± 1.31 | |
| 200 | 1.60 ± 0.33 | 1.33 × 10−4 | 0.90 | 133.96 ± 21.08 | 1419.36 | 34.06 ± 6.33 | 75.46 ± 1.13 | |
| CIE + KI 60 mg/L | 50 | 2.02 ± 0.11 | 1.31 × 10−4 | 0.85 | -- | -- | 88.93 ± 12.09 | 90.60 ± 0.24 |
| 100 | 1.58 ± 0.18 | 1.00 × 10−4 | 0.86 | -- | -- | 182.46 ± 34.23 | 95.41 ± 0.20 | |
| 200 | 1.51 ± 0.13 | 9.97 × 10−5 | 0.88 | -- | -- | 239.50 ± 63.71 | 96.50 ± 0.31 | |
| CME | 50 | 1.86 ± 0.41 | 1.93 × 10−4 | 0.91 | 144.62 ± 10.12 | 399.31 | 17.46 ± 2.26 | 52.12 ± 1.14 |
| 100 | 1.62 ± 0.24 | 1.87 × 10−4 | 0.90 | 184.34 ± 19.8 | 588.83 | 19.14 ± 2.88 | 56.32 ± 1.36 | |
| 200 | 1.83 ± 0.31 | 1.63 × 10−4 | 0.89 | 252.10 ± 26.4 | 885.06 | 27.82 ± 4.11 | 69.95 ± 0.90 | |
| CME + KI 60 mg/L | 50 | 1.83 ± 0.16 | 1.13 × 10−4 | 0.86 | -- | -- | 140.72 ± 29.63 | 94.06 ± 0.34 |
| 100 | 1.25 ± 0.06 | 8.87 × 10−5 | 0.87 | -- | -- | 260.44 ± 45.15 | 96.79 ± 0.13 | |
| 200 | 1.54 ± 0.13 | 7.80 × 10−5 | 0.87 | -- | -- | 336.54 ± 58.48 | 97.52 ± 0.10 |
| Time (h) | Rs (Ω cm2) | CPEdl (µF cm−2) | n | Rct (Ω cm2) | ηEIS (%) | |
|---|---|---|---|---|---|---|
| blank | -- | 2.53 ± 0.27 | 2.31 × 10−4 | 0.90 | 8.36 ± 0.43 | -- |
| KI | -- | 0.60 ± 0.12 | 1.81 × 10−4 | 0.86 | 35.27 ± 6.84 | 76.30 ± 1.17 |
| AAE 200 mg/L + KI 60 mg/L | 1 | 1.25 ± 0.11 | 6.64 × 10−5 | 0.88 | 370.83 ± 58.54 | 97.75 ± 0.08 |
| 2 | 1.25 ± 0.15 | 5.03 × 10−5 | 0.90 | 536.12 ± 84.98 | 98.44 ± 0.05 | |
| 4 | 1.32 ± 0.09 | 4.36 × 10−5 | 0.90 | 613.14 ± 91.51 | 98.64 ± 0.05 | |
| 8 | 1.31 ± 0.13 | 4.63 × 10−5 | 0.91 | 557.76 ± 63.40 | 98.50 ± 0.03 | |
| 16 | 1.03 ± 0.04 | 8.63 × 10−5 | 0.82 | 273.33 ± 40.26 | 96.94 ± 0.09 | |
| 24 | 0.92 ± 0.05 | 1.19 × 10−4 | 0.69 | 169.71 ± 20.04 | 95.07 ± 0.10 | |
| 48 | 1.70 ± 0.21 | 1.44 × 10−4 | 0.64 | 116.90 ± 10.80 | 92.85 ± 0.10 | |
| CIE 200 mg/L + KI 60 mg/L | 1 | 1.83 ± 0.26 | 1.05 × 10−4 | 0.88 | 180.26 ± 26.88 | 95.36 ± 0.14 |
| 2 | 1.87 ± 0.20 | 6.92 × 10−5 | 0.89 | 299.93 ± 58.92 | 97.21 ± 0.14 | |
| 4 | 1.87 ± 0.17 | 6.75 × 10−5 | 0.89 | 355.70 ± 65.69 | 97.65 ± 0.11 | |
| 8 | 1.86 ± 0.24 | 6.88 × 10−5 | 0.88 | 340.39 ± 79.61 | 97.54 ± 0.17 | |
| 16 | 1.80 ± 0.20 | 7.84 × 10−5 | 0.82 | 297.71 ± 60.08 | 97.19 ± 0.15 | |
| 24 | 1.79 ± 0.18 | 1.10 × 10−4 | 0.81 | 236.50 ± 52.80 | 96.47 ± 0.23 | |
| 48 | 0.57 ± 0.05 | 1.62 × 10−4 | 0.84 | 80.27 ± 16.26 | 89.59 ± 0.56 | |
| CME 200 mg/L + KI 60 mg/L | 1 | 1.89 ± 0.22 | 6.32 × 10−5 | 0.89 | 535.76 ± 101.03 | 98.44 ± 0.07 |
| 2 | 1.85 ± 0.24 | 3.97 × 10−5 | 0.90 | 796.04 ± 104.89 | 98.95 ± 0.03 | |
| 4 | 1.84 ± 0.13 | 3.27 × 10−5 | 0.90 | 893.13 ± 113.25 | 99.06 ± 0.02 | |
| 8 | 1.85 ± 0.17 | 4.11 × 10−5 | 0.89 | 739.92 ± 108.40 | 98.87 ± 0.03 | |
| 16 | 1.56 ± 0.19 | 6.62 × 10−5 | 0.84 | 444.41 ± 80.25 | 98.12 ± 0.08 | |
| 24 | 1.28 ± 0.10 | 1.12 × 10−4 | 0.79 | 223.46 ± 42.26 | 96.26 ± 0.18 | |
| 48 | 0.59 ± 0.05 | 1.24 × 10−4 | 0.75 | 120.60 ± 15.84 | 93.07 ± 0.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, T.-S.; Mai, W.-J.; Li, H.-Z.; Wei, B.-X.; Xu, Y.-Q.; Liao, B.-K. Insights into the Corrosion Inhibition Performance of Plant Extracts of Different Genera in the Asteraceae Family for Q235 Steel in H2SO4 Medium. Int. J. Mol. Sci. 2025, 26, 561. https://doi.org/10.3390/ijms26020561
Chu T-S, Mai W-J, Li H-Z, Wei B-X, Xu Y-Q, Liao B-K. Insights into the Corrosion Inhibition Performance of Plant Extracts of Different Genera in the Asteraceae Family for Q235 Steel in H2SO4 Medium. International Journal of Molecular Sciences. 2025; 26(2):561. https://doi.org/10.3390/ijms26020561
Chicago/Turabian StyleChu, Tian-Shu, Wen-Jie Mai, Hui-Zhen Li, Bo-Xin Wei, Yu-Qing Xu, and Bo-Kai Liao. 2025. "Insights into the Corrosion Inhibition Performance of Plant Extracts of Different Genera in the Asteraceae Family for Q235 Steel in H2SO4 Medium" International Journal of Molecular Sciences 26, no. 2: 561. https://doi.org/10.3390/ijms26020561
APA StyleChu, T.-S., Mai, W.-J., Li, H.-Z., Wei, B.-X., Xu, Y.-Q., & Liao, B.-K. (2025). Insights into the Corrosion Inhibition Performance of Plant Extracts of Different Genera in the Asteraceae Family for Q235 Steel in H2SO4 Medium. International Journal of Molecular Sciences, 26(2), 561. https://doi.org/10.3390/ijms26020561








