Impact of Larval Sertraline Exposure on Alternative Splicing in Neural Tissue of Adult Drosophila melanogaster
Abstract
1. Introduction
2. Results
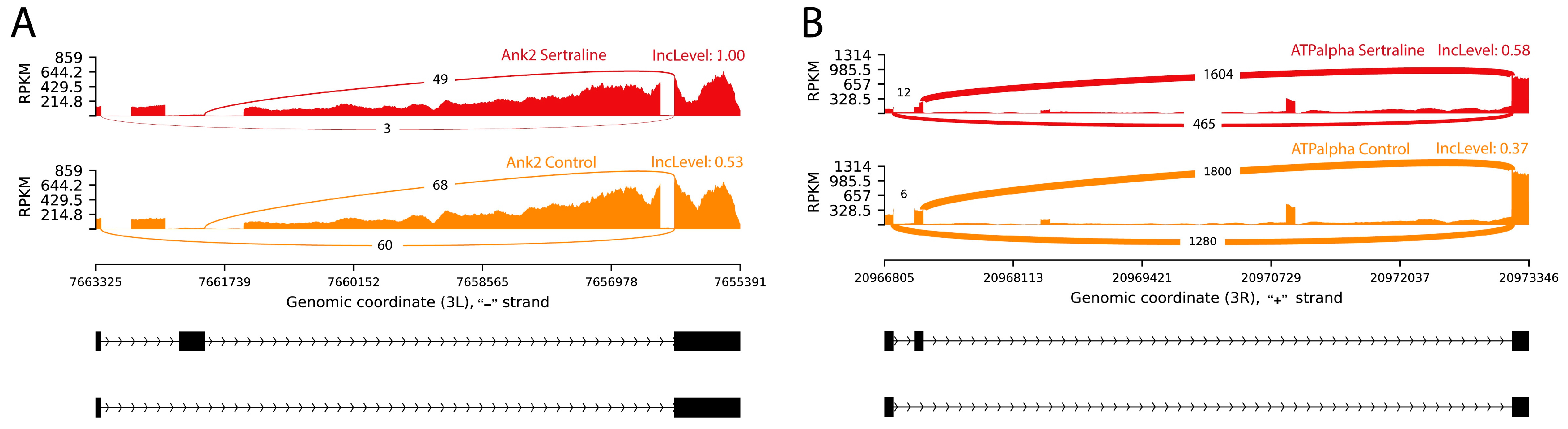
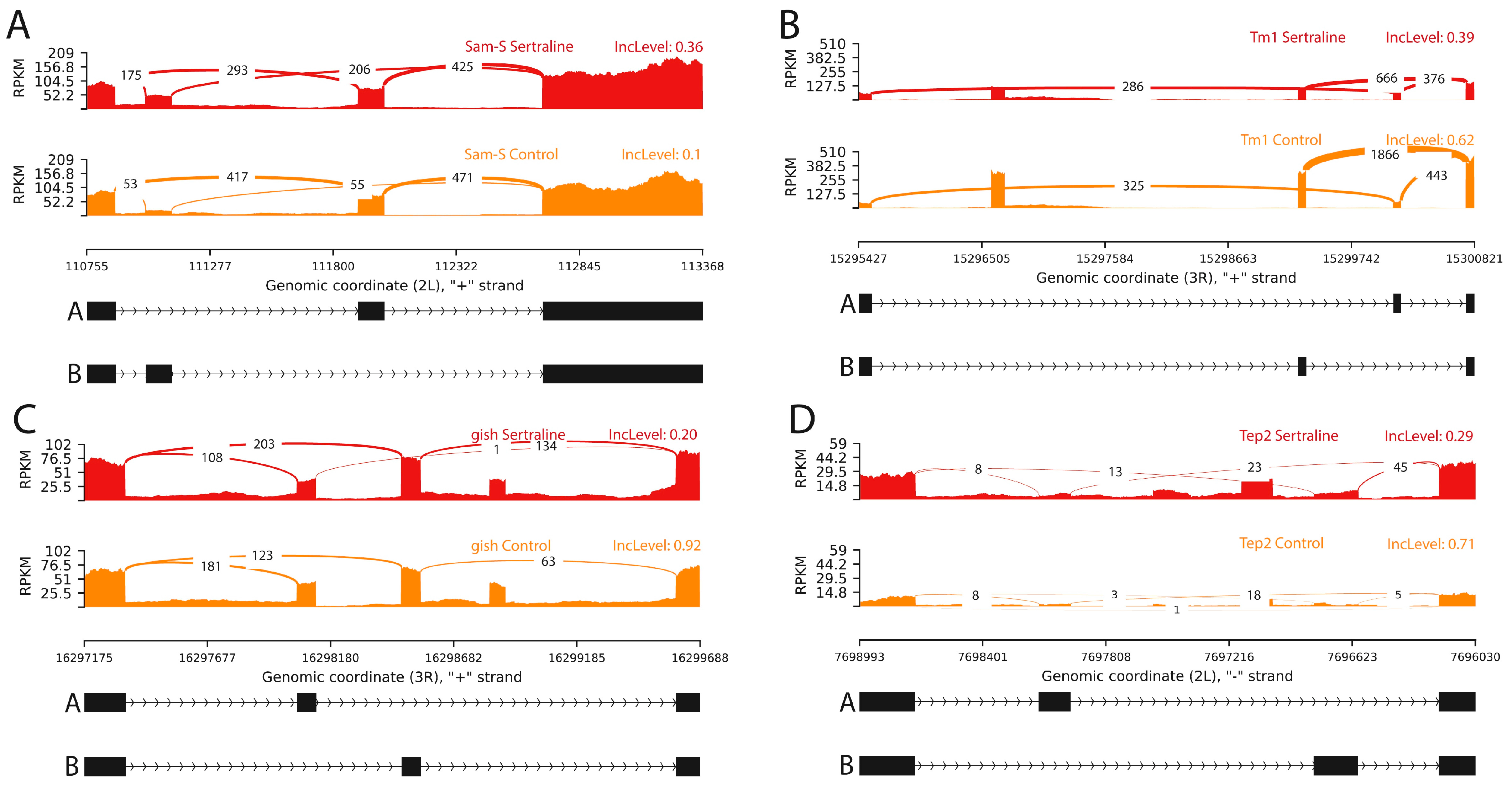
2.1. Splicing Variants by Exon Inclusion and Exclusion
2.2. Splicing Variants by Intron Retention
2.3. Splicing Variants by Mutually Exclusive Exons
3. Discussion
4. Materials and Methods
4.1. Experimental Treatment
4.2. Neural Tissue Isolation Procedure
4.3. RNA Isolation Techniques
4.4. Bioinformatic Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khushboo Siddiqi, N.J.; de Lourdes Pereira, M.; Sharma, B. Neuroanatomical, Biochemical, and Functional Modifications in Brain Induced by Treatment with Antidepressants. Mol. Neurobiol. 2022, 59, 3564–3584. [Google Scholar] [CrossRef] [PubMed]
- Tseilikman, V.E.; Tseilikman, O.B.; Karpenko, M.N.; Traktirov, D.S.; Obukhova, D.A.; Shatilov, V.A.; Zhukov, M.S.; Manuilov, G.V.; Yegorov, O.N.; Aristov, M.R.; et al. Unraveling the Serotonergic Mechanism of Stress-Related Anxiety: Focus on Co-Treatment with Resveratrol and Selective Serotonin Reuptake Inhibitors. Biomedicines 2024, 12, 2455. [Google Scholar] [CrossRef]
- Roberts, C.; Sahakian, B.J.; Robbins, T.W. Psychological mechanisms and functions of 5-HT and SSRIs in potential therapeutic change: Lessons from the serotonergic modulation of action selection, learning, affect, and social cognition. Neurosci. Biobehav. Rev. 2020, 119, 138–167. [Google Scholar] [CrossRef] [PubMed]
- Huddart, R.; Hicks, J.K.; Ramsey, L.B.; Strawn, J.R.; Smith, D.M.; Bobonis Babilonia, M.; Altman, R.B.; Klein, T.E. PharmGKB summary: Sertraline pathway, pharmacokinetics. Pharmacogenet. Genom. 2020, 30, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Duarte, D.; Vale, N. Antidepressant Drug Sertraline against Human Cancer Cells. Biomolecules 2022, 12, 1513. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Gu, X.; Chen, H.; Zeng, Q.; Mao, Z.; Jin, M.; Li, H.; Ge, Y.; Zha, J.; Martyniuk, C.J. Transcriptome profiling reveals toxicity mechanisms following sertraline exposure in the brain of juvenile zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2022, 242, 113936. [Google Scholar] [CrossRef] [PubMed]
- Marasco, L.E.; Kornblihtt, A.R. The physiology of alternative splicing. Nat. Rev. Mol. Cell Biol. 2023, 24, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Meldolesi, J. Alternative splicing by NOVA factors: From gene expression to cell physiology and pathology. Int. J. Mol. Sci. 2020, 21, 3941. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yang, X.; Ren, Z.; Chen, C.; Liu, C. Alternative splicing in mouse brains affected by psychological stress is enriched in the signaling, neural transmission and blood-brain barrier pathways. Mol. Psychiatry 2023, 28, 4707–4718. [Google Scholar] [CrossRef]
- Verma, P.; Shakya, M. Transcriptomics and sequencing analysis of gene expression profiling for major depressive disorder. Indian J. Psychiatry 2021, 63, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A.; Krick, K.; Karisetty, B.C.; Armour, E.M.; Heller, E.A.; Elefant, F. Tip60’s Novel RNA-Binding Function Modulates Alternative Splicing of Pre-mRNA Targets Implicated in Alzheimer’s Disease. J. Neurosci. 2023, 43, 2398–2423. [Google Scholar] [CrossRef]
- Kumari, A.; Sedehizadeh, S.; Brook, J.D.; Kozlowski, P.; Wojciechowska, M. Differential fates of introns in gene expression due to global alternative splicing. Hum. Genet. 2022, 141, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Pang, T.L.; Ding, Z.; Liang, S.B.; Li, L.; Zhang, B.; Zhang, Y.; Fan, Y.J.; Xu, Y.Z. Comprehensive Identification and Alternative Splicing of Microexons in Drosophila. Front. Genet. 2021, 12, 642602. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xuan, J.; Couch, L.; Iyer, A.; Wu, Y.; Li, Q.Z.; Guo, L. Sertraline induces endoplasmic reticulum stress in hepatic cells. Toxicology 2014, 322, 78–88. [Google Scholar] [CrossRef]
- Firouzabadi, D.; Firouzabadi, N.; Kalani, K.; Zomorrodian, K.; Tehrani, E.S. Response to sertraline is influenced by GNβ3 gene G-350A variant in patients with major depressive disorder. Eur. J. Clin. Pharmacol. 2019, 75, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Hortsch, M.; Paisley, K.L.; Tian, M.Z.; Qian, M.; Bouley, M.; Chandler, R. The axonal localization of large Drosophila ankyrin2 protein isoforms is essential for neuronal functionality. Mol. Cell. Neurosci. 2002, 20, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Bham, K.; Senapati, S. Human ankyrins and their contribution to disease biology: An update. J. Biosci. 2020, 45, 146. [Google Scholar] [CrossRef]
- Hopitzan, A.A.; Baines, A.J.; Kordeli, E. Molecular evolution of ankyrin: Gain of function in vertebrates by acquisition of an obscurin/titin-binding-related domain. Mol. Biol. Evol. 2006, 23, 46–55. [Google Scholar] [CrossRef]
- Palladino, M.J.; Bower, J.E.; Kreber, R.; Ganetzky, B. Neural dysfunction and neurodegeneration in Drosophila Na+/K+ ATPase alpha subunit mutants. J. Neurosci. 2003, 23, 1276–1286. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, U.; Dasgupta, A.; Rout, J.K.; Singh, O.P. Effects of lithium therapy on Na+-K+-ATPase activity and lipid peroxidation in bipolar disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2012, 37, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Texada, M.J.; Simonette, R.A.; Deery, W.J.; Beckingham, K.M. Tropomyosin is an interaction partner of the Drosophila coiled coil protein Yuri Gagarin. Exp. Cell Res. 2011, 317, 474–487. [Google Scholar] [CrossRef] [PubMed]
- Texada, M.J.; Simonette, R.A.; Johnson, C.B.; Deery, W.J.; Beckingham, K.M. Yuri gagarin is required for actin, tubulin and basal body functions in Drosophila spermatogenesis. J. Cell Sci. 2008, 121, 1926–1936. [Google Scholar] [CrossRef] [PubMed]
- Kracklauer, M.P.; Wiora, H.M.; Deery, W.J.; Chen, X.; Bolival, B.; Romanowicz, D.; Simonette, R.A.; Fuller, M.T.; Fischer, J.A.; Beckingham, K.M. The Drosophila SUN protein Spag4 cooperates with the coiled-coil protein Yuri Gagarin to maintain association of the basal body and spermatid nucleus. J. Cell Sci. 2010, 123, 2763–2772. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, J.D.; Texada, M.J.; Munjaal, R.; Baker, D.A.; Beckingham, K.M. Gravitaxis in Drosophila melanogaster: A forward genetic screen. Genes Brain Behav. 2006, 5, 222–239. [Google Scholar] [CrossRef]
- Sinclair, D.A.R.; Syrzycka, M.; Macauley, M.S.; Rastgardani, T.; Komljenovic, I.; Vocadlo, D.J.; Brock, H.W.; Honda, B.M. Drosophila O-GlcNAc transferase (OGT) is encoded by the Polycomb group (PcG) gene, super sex combs (sxc). Proc. Natl. Acad. Sci. USA 2009, 106, 13427–13432. [Google Scholar] [CrossRef]
- Almannai, M.; Marafi, D.; El-Hattab, A.W. WIPI proteins: Biological functions and related syndromes. Front. Mol. Neurosci. 2022, 15, 1011918. [Google Scholar] [CrossRef] [PubMed]
- Jelani, M.; Dooley, H.C.; Gubas, A.; Mohamoud, H.S.A.; Khan, M.T.M.; Ali, Z.; Kang, C.; Rahim, F.; Jan, A.; Vadgama, N.; et al. A mutation in the major autophagy gene, WIPI2, associated with global developmental abnormalities. Brain 2019, 142, 1242–1254. [Google Scholar] [CrossRef] [PubMed]
- Stavoe, A.K.H.; Holzbaur, E.L.F. Neuronal autophagy declines substantially with age and is rescued by overexpression of WIPI2. Autophagy 2020, 16, 371–372. [Google Scholar] [CrossRef]
- Gupta, A.R.; Pirruccello, M.; Cheng, F.; Kang, H.J.; Fernandez, T.V.; Baskin, J.M.; Choi, M.; Liu, L.; Ercan-Sencicek, A.G.; Murdoch, J.D.; et al. Rare deleterious mutations of the gene EFR3A in autism spectrum disorders. Mol. Autism 2014, 5, 31. [Google Scholar] [CrossRef]
- Wei, X.; Wang, J.; Yang, E.; Zhang, Y.; Qian, Q.; Li, X.; Huang, F.; Sun, B. Efr3b is essential for social recognition by modulating the excitability of CA2 pyramidal neurons. Proc. Natl. Acad. Sci. USA 2024, 121, e2314557121. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.; Lee, J.H.; Choi, B.; Won, S.Y.; Cho, K.S. Genetic dissection of Alzheimer’s disease using Drosophila models. Int. J. Mol. Sci. 2020, 21, 884. [Google Scholar] [CrossRef]
- Nordgren, K.K.S.; Peng, Y.; Pelleymounter, L.L.; Moon, I.; Abo, R.; Feng, Q.; Eckloff, B.; Yee, V.C.; Wieben, E.; Weinshilboum, R.M. Methionine adenosyltransferase 2A/2B and methylation: Gene sequence variation and functional genomics. Drug Metab. Dispos. 2011, 39, 2135–2147. [Google Scholar] [CrossRef]
- Marjon, K.; Kalev, P.; Marks, K. Cancer Dependencies: PRMT5 and MAT2A in MTAP/p16-Deleted Cancers. Annu. Rev. Cancer Biol. 2021, 5, 371–390. [Google Scholar] [CrossRef]
- Obata, F.; Miura, M. Enhancing S-adenosyl-methionine catabolism extends Drosophila lifespan. Nat. Commun. 2015, 6, 8332. [Google Scholar] [CrossRef] [PubMed]
- Watabe, E.; Togo-Ohno, M.; Ishigami, Y.; Wani, S.; Hirota, K.; Kimura-Asami, M.; Hasan, S.; Takei, S.; Fukamizu, A.; Suzuki, Y.; et al. m6A-mediated alternative splicing coupled with nonsense-mediated mRNA decay regulates SAM synthetase homeostasis. EMBO J. 2021, 40, e106434. [Google Scholar] [CrossRef] [PubMed]
- Brettle, M.; Patel, S.; Fath, T. Tropomyosins in the healthy and diseased nervous system. Brain Res. Bull. 2016, 126, 311–323. [Google Scholar] [CrossRef]
- Gray, K.T.; Kostyukova, A.S.; Fath, T. Actin regulation by tropomodulin and tropomyosin in neuronal morphogenesis and function. Mol. Cell. Neurosci. 2017, 84, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Tobacman, L.S. Cooperative binding of tropomyosin to actin. Adv. Exp. Med. Biol. 2008, 644, 85–94. [Google Scholar] [CrossRef]
- Stefen, H.; Suchowerska, A.K.; Chen, B.J.; Brettle, M.; Kuschelewski, J.; Gunning, P.W.; Janitz, M.; Fath, T. Tropomyosin isoforms have specific effects on the transcriptome of undifferentiated and differentiated B35 neuroblastoma cells. FEBS Open Bio 2018, 8, 570–583. [Google Scholar] [CrossRef] [PubMed]
- Blandin, S.; Levashina, E.A. Thioester-containing proteins and insect immunity. Mol. Immunol. 2004, 40, 903–908. [Google Scholar] [CrossRef]
- Shokal, U.; Kopydlowski, H.; Harsh, S.; Eleftherianos, I. Thioester-containing proteins 2 and 4 affect the metabolic activity and inflammation response in Drosophila. Infect. Immun. 2018, 86. [Google Scholar] [CrossRef] [PubMed]
- Signor, S.; Nuzhdin, S. Dynamic changes in gene expression and alternative splicing mediate the response to acute alcohol exposure in Drosophila melanogaster. Heredity 2018, 121, 342–360. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Song, L.; Zhao, J.; Qiu, L.; Gao, Y.; Song, X.; Li, L.; Zhang, Y.; Zhang, L. The genomic structure, alternative splicing and immune response of Chlamys farreri thioester-containing protein. Dev. Comp. Immunol. 2009, 33, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Alhadab, A.A.; Brundage, R.C. Population Pharmacokinetics of Sertraline in Healthy Subjects: A Model-Based Meta-analysis. AAPS J. 2020, 22, 73. [Google Scholar] [CrossRef] [PubMed]
- Lan, H.W.; Lu, Y.N.; Zhao, X.D.; Jin, G.N.; Lu, J.M.; Jin, C.H.; Ma, J.; Jin, X.; Xu, X.; Piao, L.X. New role of sertraline against Toxoplasma gondii-induced depression-like behaviours in mice. Parasite Immunol. 2021, 43, e12893. [Google Scholar] [CrossRef]
- West, E.G.; Sellers, D.J.; Chess-Williams, R.; McDermott, C. The anxiolytic sertraline reduces the impact of psychological stress on bladder function in mice. Life Sci. 2021, 278, 119598. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- Shen, S.; Park, J.W.; Lu, Z.X.; Lin, L.; Henry, M.D.; Wu, Y.N.; Zhou, Q.; Xing, Y. rMATS: Robust and flexible detection of differential alternative splicing from replicate RNA-Seq data. Proc. Natl. Acad. Sci. USA 2014, 111, E5595–E5601. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos-Cruz, L.F.; Campos-Aguilar, M.; Castañeda-Partida, L.; Sigrist-Flores, S.C.; Heres-Pulido, M.E.; Dueñas-García, I.E.; Piedra-Ibarra, E.; Jiménez-Flores, R.; Ponciano-Gómez, A. Impact of Larval Sertraline Exposure on Alternative Splicing in Neural Tissue of Adult Drosophila melanogaster. Int. J. Mol. Sci. 2025, 26, 563. https://doi.org/10.3390/ijms26020563
Santos-Cruz LF, Campos-Aguilar M, Castañeda-Partida L, Sigrist-Flores SC, Heres-Pulido ME, Dueñas-García IE, Piedra-Ibarra E, Jiménez-Flores R, Ponciano-Gómez A. Impact of Larval Sertraline Exposure on Alternative Splicing in Neural Tissue of Adult Drosophila melanogaster. International Journal of Molecular Sciences. 2025; 26(2):563. https://doi.org/10.3390/ijms26020563
Chicago/Turabian StyleSantos-Cruz, Luis Felipe, Myriam Campos-Aguilar, Laura Castañeda-Partida, Santiago Cristobal Sigrist-Flores, María Eugenia Heres-Pulido, Irma Elena Dueñas-García, Elías Piedra-Ibarra, Rafael Jiménez-Flores, and Alberto Ponciano-Gómez. 2025. "Impact of Larval Sertraline Exposure on Alternative Splicing in Neural Tissue of Adult Drosophila melanogaster" International Journal of Molecular Sciences 26, no. 2: 563. https://doi.org/10.3390/ijms26020563
APA StyleSantos-Cruz, L. F., Campos-Aguilar, M., Castañeda-Partida, L., Sigrist-Flores, S. C., Heres-Pulido, M. E., Dueñas-García, I. E., Piedra-Ibarra, E., Jiménez-Flores, R., & Ponciano-Gómez, A. (2025). Impact of Larval Sertraline Exposure on Alternative Splicing in Neural Tissue of Adult Drosophila melanogaster. International Journal of Molecular Sciences, 26(2), 563. https://doi.org/10.3390/ijms26020563





