Anti-Inflammatory and Anti-Fibrotic Effects of a Mixture of Polyphenols Extracted from “Navelina” Orange in Human Hepa-RG and LX-2 Cells Mediated by Cannabinoid Receptor 2
Abstract
1. Introduction
2. Results
2.1. Analysis of OE Characteristics
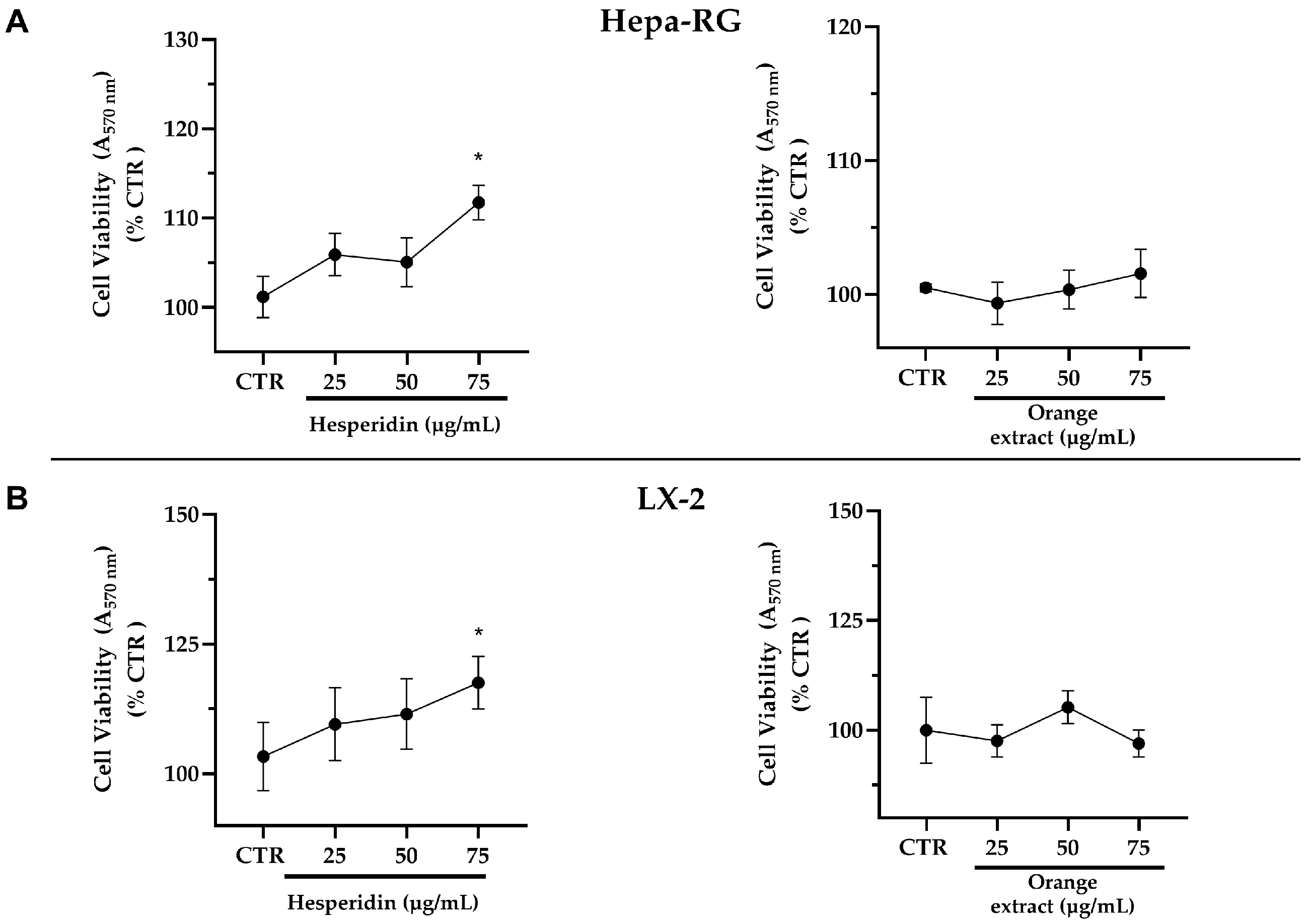
2.2. Effects of Increasing Concentrations of HE or OE on the Viability of Hepa-RG and LX-2 Liver Cells
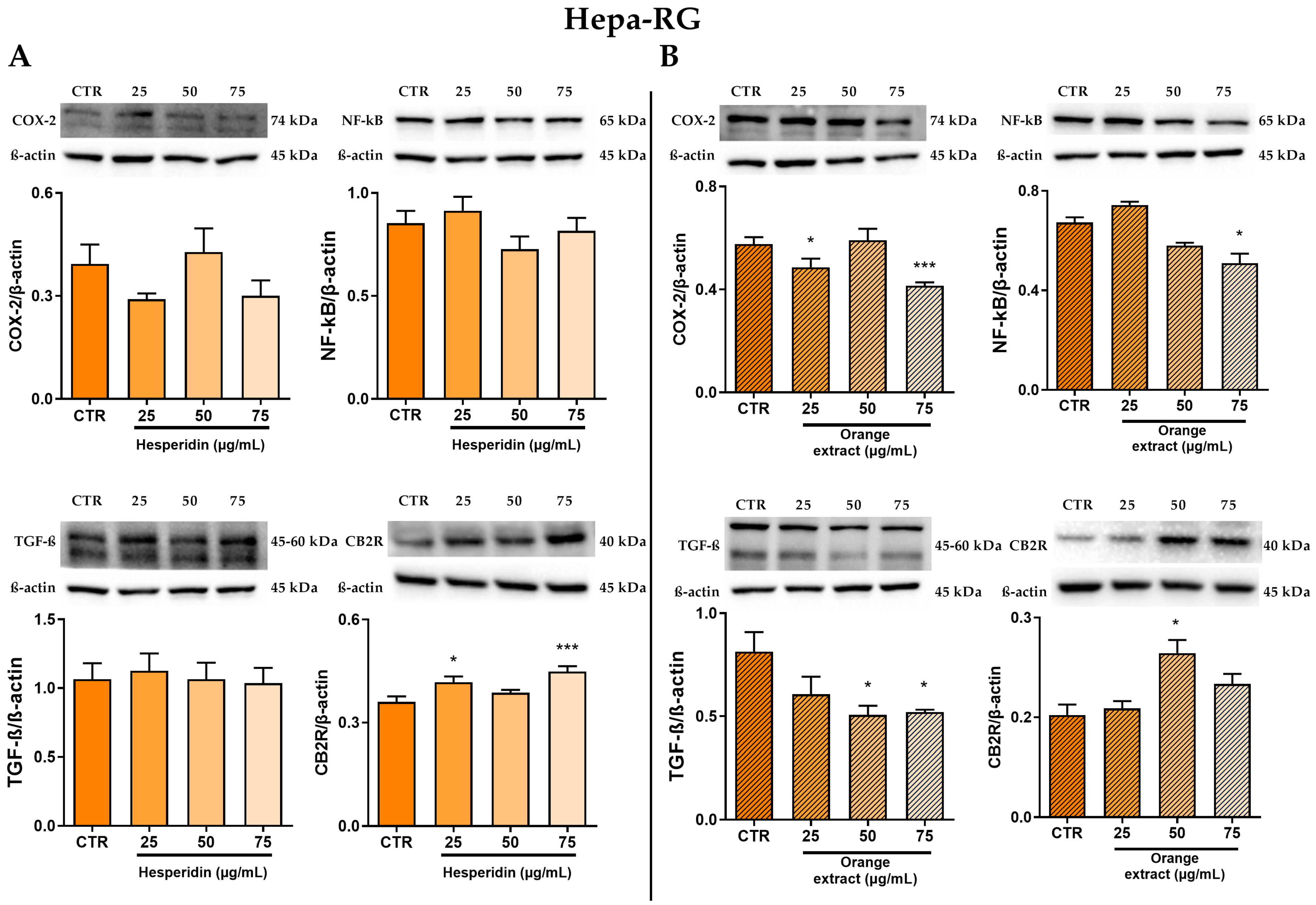
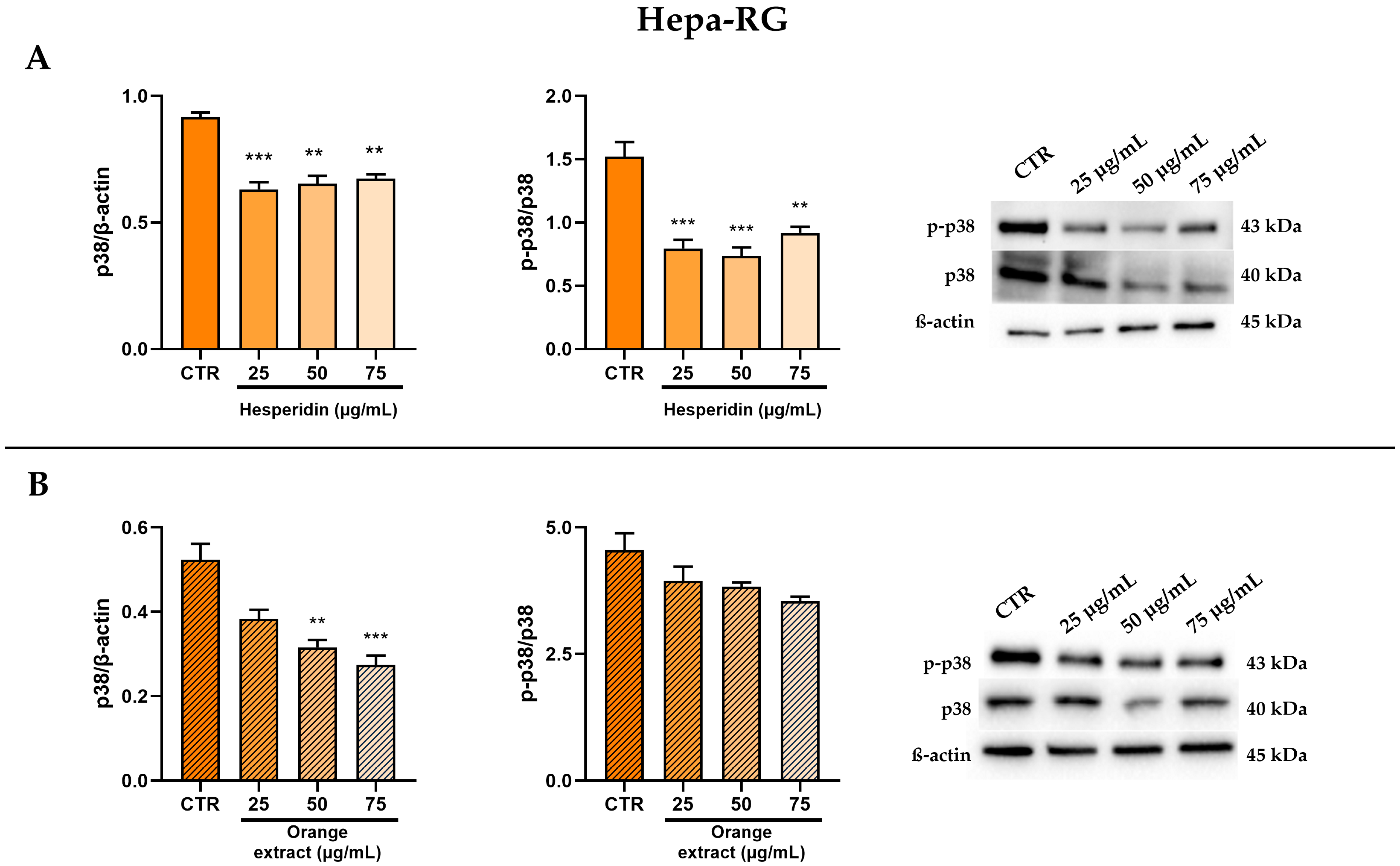
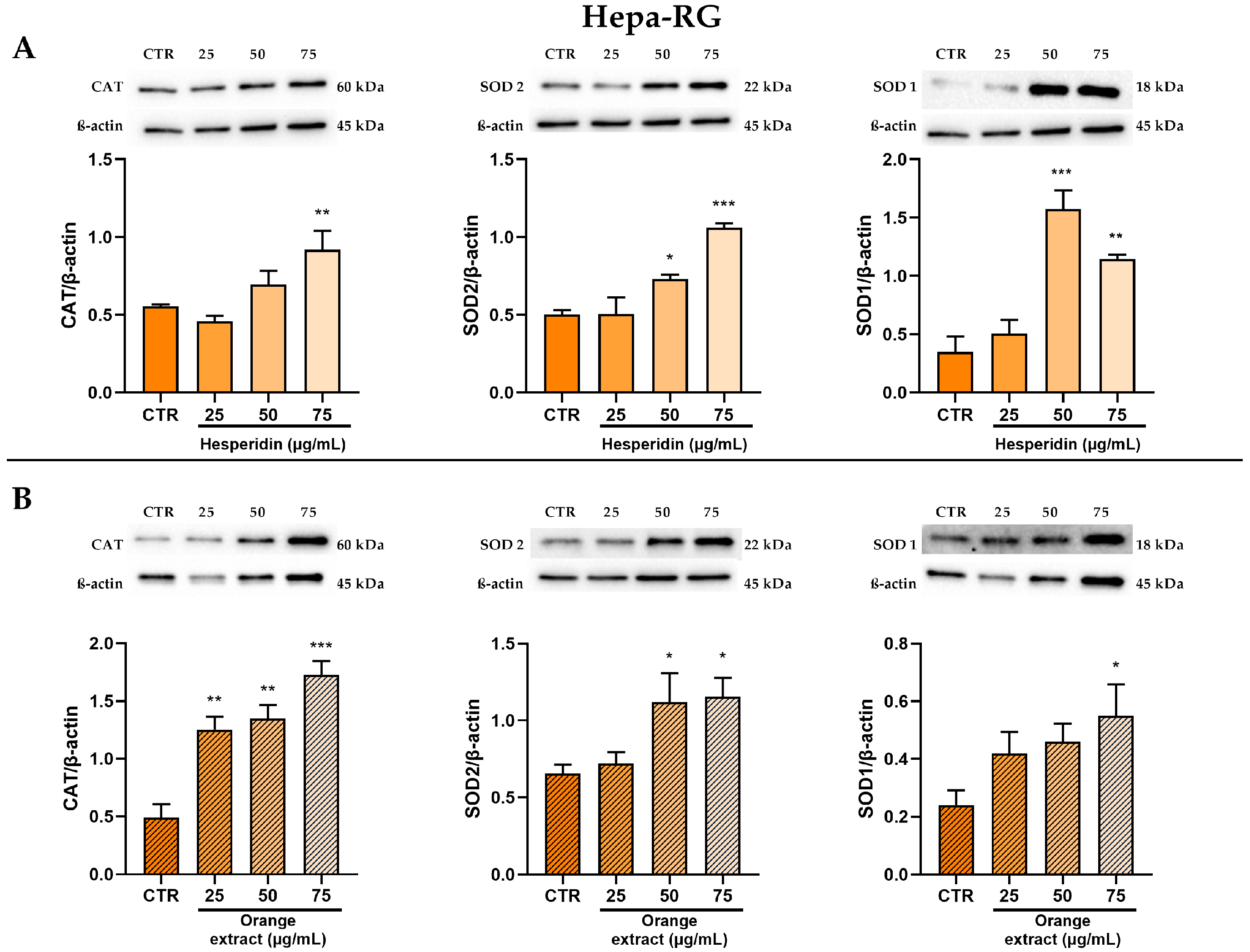
2.3. Anti-Inflammatory and Anti-Fibrotic Effects in Hepa-RG Cells Treated with HE or OE
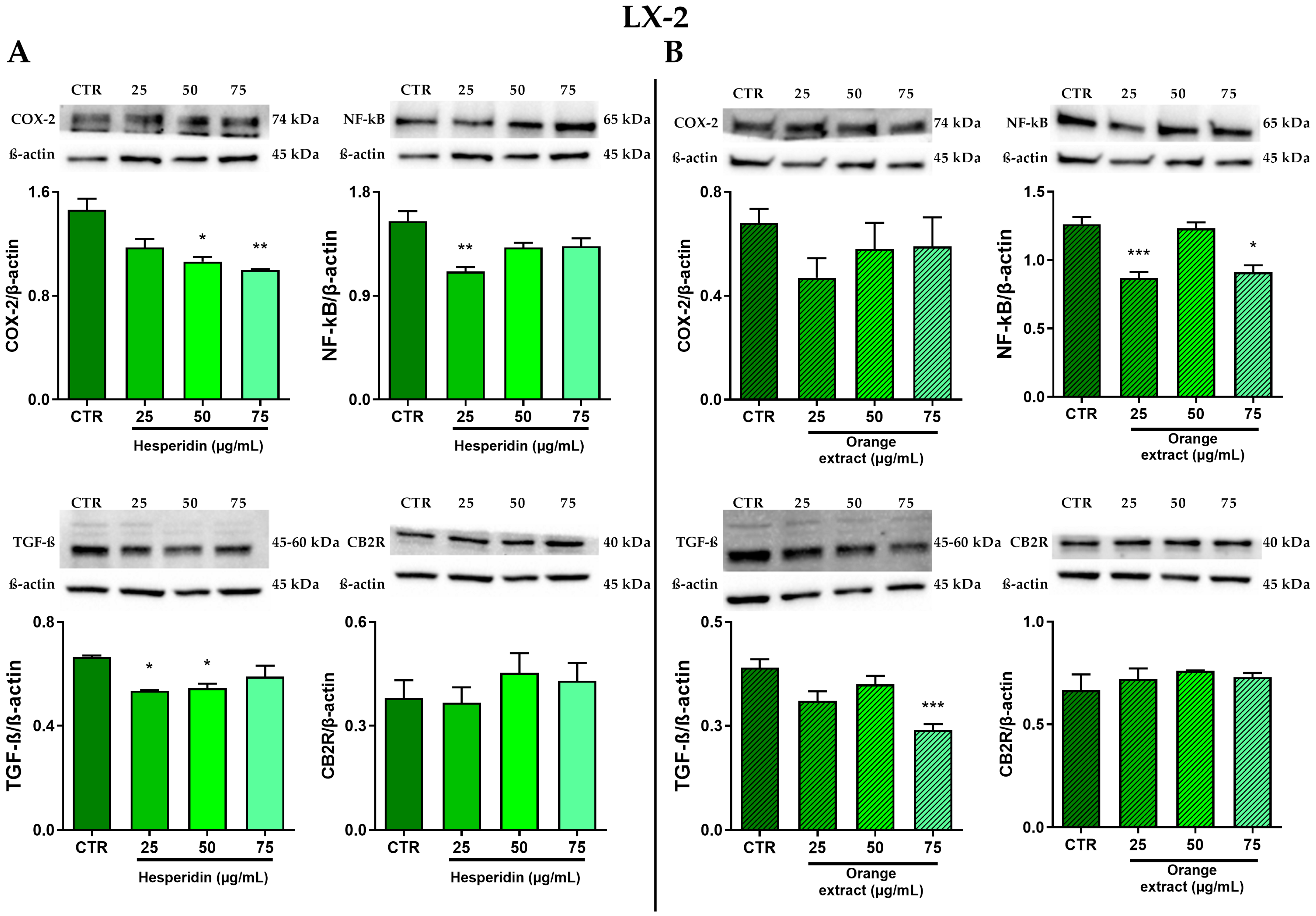
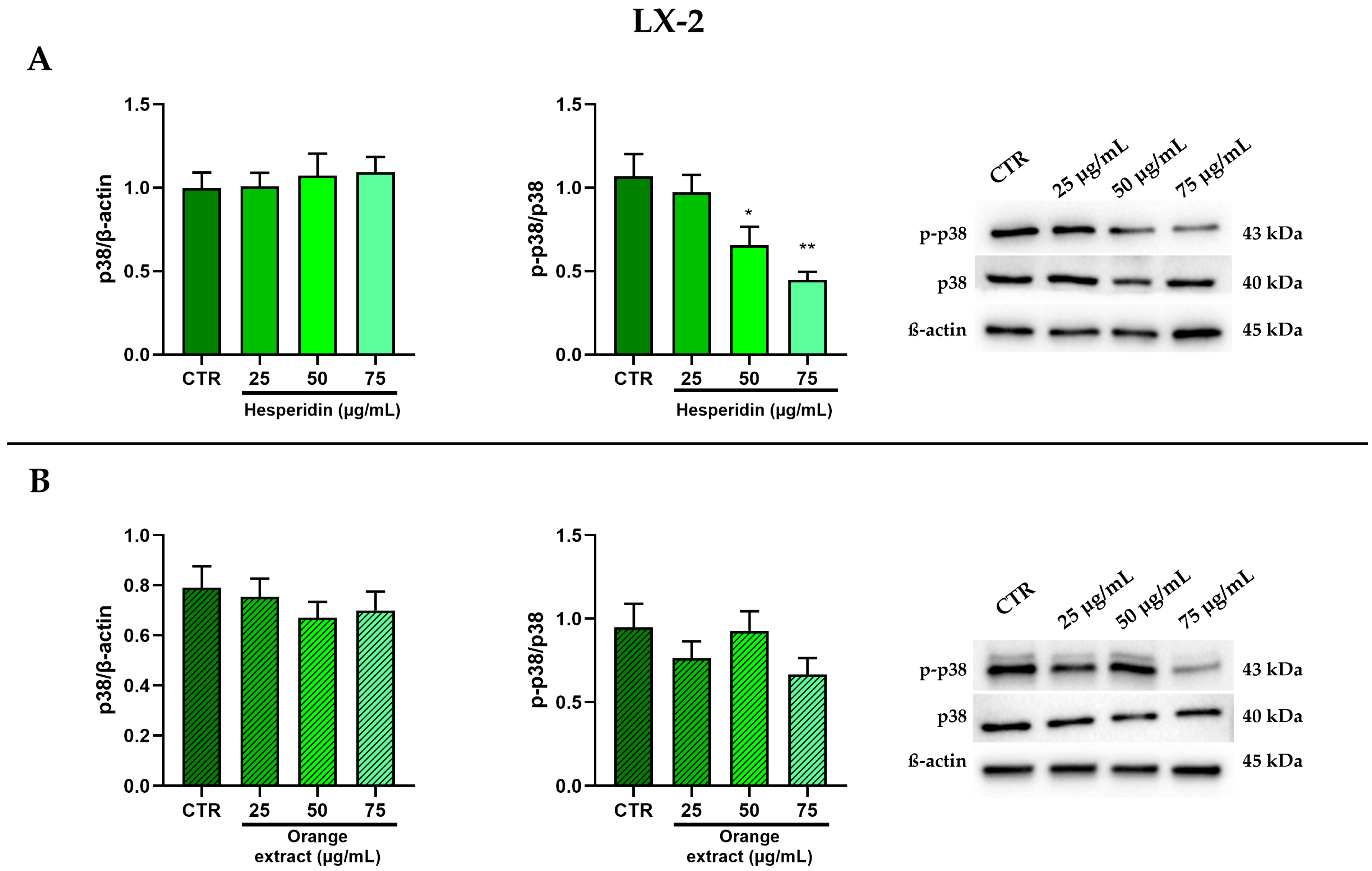
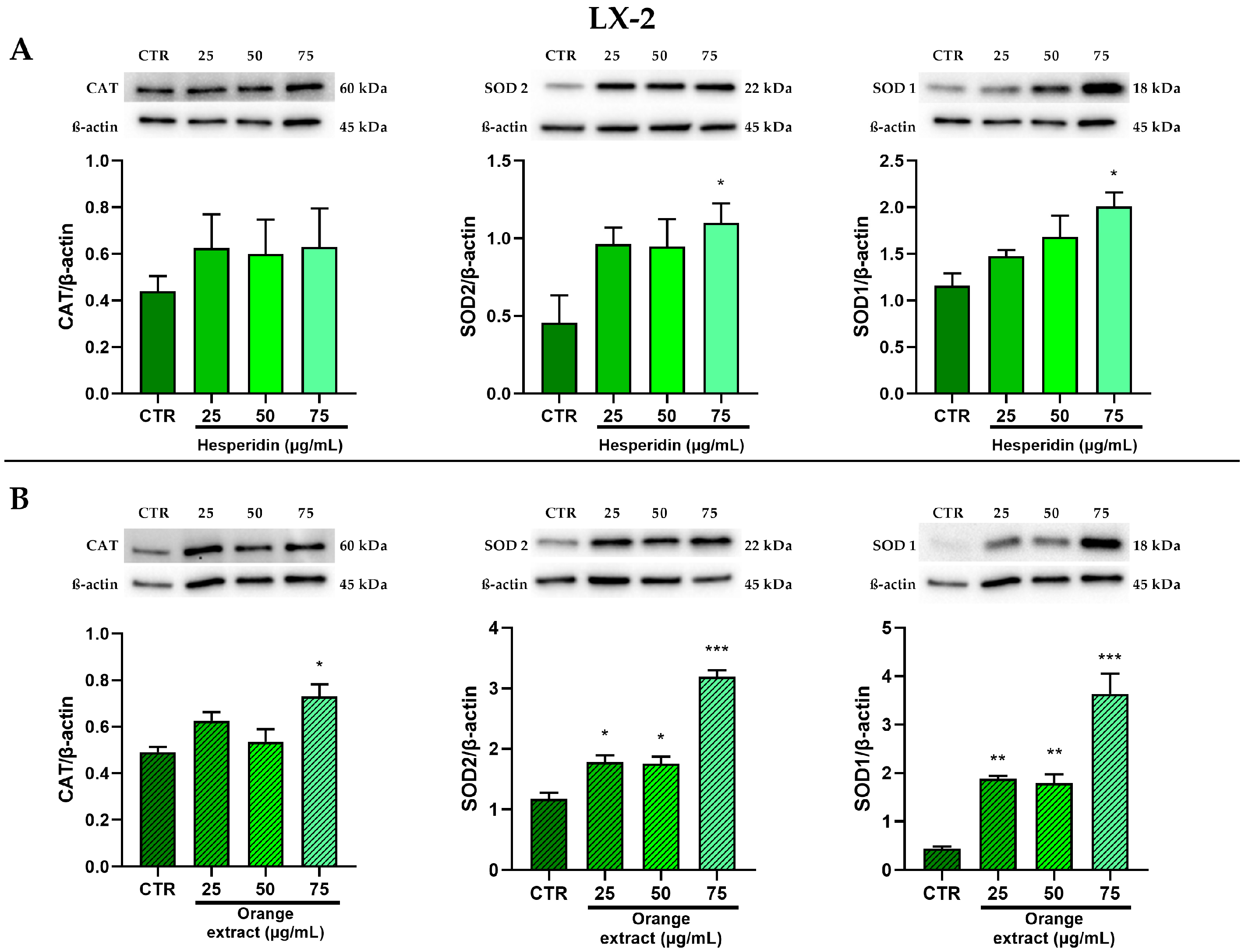
2.4. Anti-Inflammatory and Anti-Fibrotic Effects in LX-2 Cells Treated with HE or OE
2.5. Anti-Oxidant Effects of HE or OE Treatments
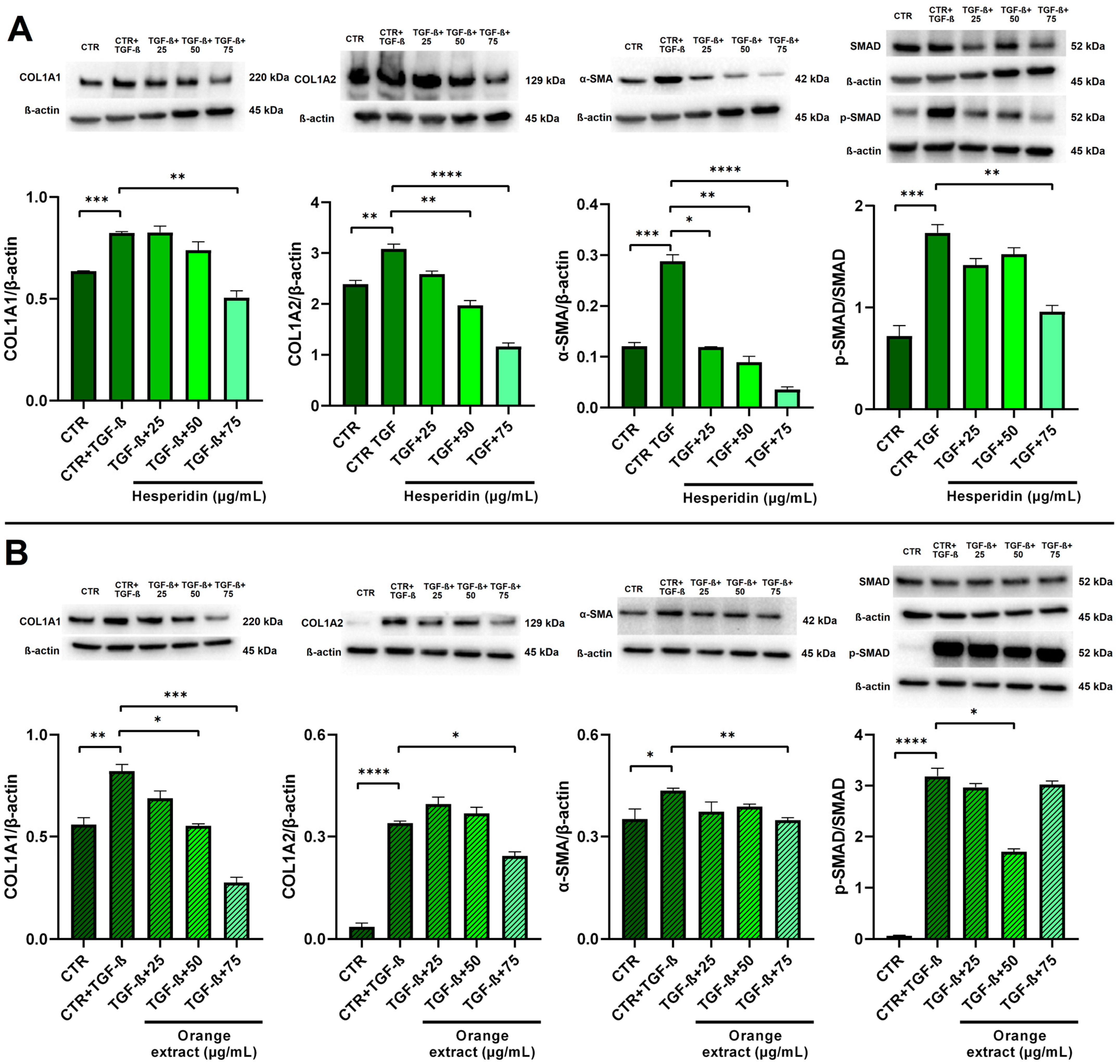
2.6. Anti-Fibrotic Effects of HE and OE Treatments in LX-2-Activated Cells
3. Discussion
4. Materials and Methods
4.1. Preparation of the Extract of OE and Determination of the Total Phenolic Content
4.2. Cell Lines, Culture Media and Treatments
4.3. Viability Assay and Western Blot Analysis
4.4. Extraction of Lipids for Fatty Acid Analysis by Gas Chromatography
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seminara, S.; Bennici, S.; Di Guardo, M.; Caruso, M.; Gentile, A.; La Malfa, S.; Distefano, G. Sweet Orange: Evolution, Characterization, Varieties, and Breeding Perspectives. Agriculture 2023, 13, 264. [Google Scholar] [CrossRef]
- Mamma, D.; Christakopoulos, P. Biotransformation of Citrus By-Products into Value Added Products. Waste Biomass Valorization 2014, 5, 529–549. [Google Scholar] [CrossRef]
- Barrales, F.M.; Silveira, P.; de Paula Menezes Barbosa, P.; Ruviaro, A.R.; Paulino, B.N.; Pastore, G.M.; Macedo, G.A.; Martinez, J. Recovery of Phenolic Compounds from Citrus By-Products Using Pressurized Liquids—An Application to Orange Peel. Food Bioprod. Process. 2018, 112, 9–21. [Google Scholar] [CrossRef]
- del Carmen Razola-Díaz, M.; Guerra-Hernández, E.J.; García-Villanova, B.; Verardo, V. Recent Developments in Extraction and Encapsulation Techniques of Orange Essential Oil. Food Chem. 2021, 354, 129575. [Google Scholar] [CrossRef] [PubMed]
- Romero-Lopez, M.R.; Osorio-Diaz, P.; Bello-Perez, L.A.; Tovar, J.; Bernardino-Nicanor, A. Fiber Concentrate from Orange (Citrus sinensis L.) Bagase: Characterization and Application as Bakery Product Ingredient. Int. J. Mol. Sci. 2011, 12, 2174–2186. [Google Scholar] [CrossRef]
- Šafranko, S.; Šubarić, D.; Jerković, I.; Jokić, S. Citrus By-Products as a Valuable Source of Biologically Active Compounds with Promising Pharmaceutical, Biological and Biomedical Potential. Pharmaceuticals 2023, 16, 1081. [Google Scholar] [CrossRef]
- Sha, S.P.; Modak, D.; Sarkar, S.; Roy, S.K.; Sah, S.P.; Ghatani, K.; Bhattacharjee, S. Fruit Waste: A Current Perspective for the Sustainable Production of Pharmacological, Nutraceutical, and Bioactive Resources. Front. Microbiol. 2023, 14, 1260071. [Google Scholar] [CrossRef]
- Gil-Chávez, G.J.; Villa, J.A.; Ayala-Zavala, J.F.; Heredia, J.B.; Sepulveda, D.; Yahia, E.M.; González-Aguilar, G.A. Technologies for Extraction and Production of Bioactive Compounds to Be Used as Nutraceuticals and Food Ingredients: An Overview. Compr. Rev. Food Sci. Food Saf. 2013, 12, 5–23. [Google Scholar] [CrossRef]
- Tirkey, N.; Pilkhwal, S.; Kuhad, A.; Chopra, K. Hasperidin, a Citrus Bioflavonoid, Decreases the Oxidative Stress Produced by Carbon Tetrachloride in Rat Liver and Kidney. BMC Pharmacol. 2005, 5, 2. [Google Scholar] [CrossRef]
- Banjarnahor, S.D.S.; Artanti, N. Antioxidant Properties of Flavonoids. Med. J. Indones. 2014, 23, 239–244. [Google Scholar] [CrossRef]
- Ciumărnean, L.; Milaciu, M.V.; Runcan, O.; Vesa, S.C.; Răchisan, A.L.; Negrean, V.; Perné, M.G.; Donca, V.I.; Alexescu, T.G.; Para, I.; et al. The Effects of Flavonoids in Cardiovascular Diseases. Molecules 2020, 25, 4320. [Google Scholar] [CrossRef] [PubMed]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-Inflammatory Effects of Flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef] [PubMed]
- Abotaleb, M.; Samuel, S.M.; Varghese, E.; Varghese, S.; Kubatka, P.; Liskova, A.; Büsselberg, D. Flavonoids in Cancer and Apoptosis. Cancers 2019, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, J.A.; Gallego, P.; Grande, L. Role of Inflammatory Response in Liver Diseases: Therapeutic Strategies. World J. Hepatol. 2018, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M. Hematological Alterations in Diabetic Rats—Role of Adipocytokines and Effect of Citrus Flavonoids. EXCLI J. 2013, 12, 647–657. [Google Scholar]
- Mahmoud, A.M.; Ashour, M.B.; Abdel-Moneim, A.; Ahmed, O.M. Hesperidin and Naringin Attenuate Hyperglycemia-Mediated Oxidative Stress and Proinflammatory Cytokine Production in High Fat Fed/Streptozotocin-Induced Type 2 Diabetic Rats. J. Diabetes Complicat. 2012, 26, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Vabeiryureilai, M.; Lalrinzuali, K. Determination of Anti-Inflammatory and Analgesic Activities of a Citrus Bioflavanoid, Hesperidin in Mice. Immunochem. Immunopathol. 2015, 1, 1000107. [Google Scholar] [CrossRef]
- Sakata, K.; Hirose, Y.; Qiao, Z.; Tanaka, T.; Mori, H. Inhibition of Inducible Isoforms of Cyclooxygenase and Nitric Oxide Synthase by Flavonoid Hesperidin in Mouse Macrophage Cell Line. Cancer Lett. 2003, 199, 139–145. [Google Scholar] [CrossRef]
- Hirata, A.; Murakami, Y.; Shoji, M.; Kadoma, Y.; Fujisawa, S. Kinetics of Radical-Scavenging Activity of Hesperetin and Hesperidin and Their Inhibitory Activity on COX-2 Expression. Anticancer. Res. 2005, 25, 3367–3374. [Google Scholar] [PubMed]
- Kang, S.R.; Il Park, K.; Park, H.S.; Lee, D.H.; Kim, J.A.; Nagappan, A.; Kim, E.H.; Lee, W.S.; Shin, S.C.; Park, M.K.; et al. Anti-Inflammatory Effect of Flavonoids Isolated from Korea Citrus aurantium L. on Lipopolysaccharide-Induced Mouse Macrophage RAW 264.7 Cells by Blocking of Nuclear Factor-Kappa B (NF-ΚB) and Mitogen-Activated Protein Kinase (MAPK) Signalling Pathways. Food Chem. 2011, 129, 1721–1728. [Google Scholar] [CrossRef]
- Al-Rikabi, R.; Al-Shmgani, H.; Dewir, Y.H.; El-Hendawy, S. In Vivo and In Vitro Evaluation of the Protective Effects of Hesperidin in Lipopolysaccharide-Induced Inflammation and Cytotoxicity of Cell. Molecules 2020, 25, 478. [Google Scholar] [CrossRef]
- Garg, A.; Garg, S.; Zaneveld, L.J.D.; Singla, A.K. Chemistry and Pharmacology of the Citrus Bioflavonoid Hesperidin. Phytother. Res. 2001, 15, 655–669. [Google Scholar] [CrossRef]
- Wilmsen, P.K.; Spada, D.S.; Salvador, M. Antioxidant Activity of the Flavonoid Hesperidin in Chemical and Biological Systems. J. Agric. Food Chem. 2005, 53, 4757–4761. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First Line Defence Antioxidants-Superoxide Dismutase (SOD), Catalase (CAT) and Glutathione Peroxidase (GPX): Their Fundamental Role in the Entire Antioxidant Defence Grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Tsang, C.K.; Liu, Y.; Thomas, J.; Zhang, Y.; Zheng, X.F.S. Superoxide Dismutase 1 Acts as a Nuclear Transcription Factor to Regulate Oxidative Stress Resistance. Nat. Commun. 2014, 5, 3446. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Alrumaihi, F.; Sarwar, T.; Babiker, A.Y.; Khan, A.A.; Prabhu, S.V.; Rahmani, A.H. Exploring Therapeutic Potential of Catalase: Strategies in Disease Prevention and Management. Biomolecules 2024, 14, 697. [Google Scholar] [CrossRef]
- Notarnicola, M.; Tutino, V.; De Nunzio, V.; Cisternino, A.M.; Cofano, M.; Donghia, R.; Giannuzzi, V.; Zappimbulso, M.; Milella, R.A.; Giannelli, G.; et al. Daily Orange Consumption Reduces Hepatic Steatosis Prevalence in Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease: Exploratory Outcomes of a Randomized Clinical Trial. Nutrients 2024, 16, 3191. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Kim, J.K. The Role of Cannabidiol in Liver Disease: A Systemic Review. Int. J. Mol. Sci. 2024, 25, 2370. [Google Scholar] [CrossRef] [PubMed]
- Mallat, A.; Teixeira-Clerc, F.; Lotersztajn, S. Cannabinoid Signaling and Liver Therapeutics. J. Hepatol. 2013, 59, 891–896. [Google Scholar] [CrossRef]
- Mallat, A.; Lotersztajn, S. Endocannabinoids and Liver Disease. I. Endocannabinoids and Their Receptors in the Liver. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 294, G9–G12. [Google Scholar] [CrossRef]
- Julien, B.; Grenard, P.; Teixeira-Clerc, F.; Van Nhieu, J.T.; Li, L.; Karsak, M.; Zimmer, A.; Mallat, A.; Lotersztajn, S. Antifibrogenic Role of the Cannabinoid Receptor CB2 in the Liver. Gastroenterology 2005, 128, 742–755. [Google Scholar] [CrossRef]
- Kaminska, B. MAPK Signalling Pathways as Molecular Targets for Anti-Inflammatory Therapy—From Molecular Mechanisms to Therapeutic Benefits. Biochim. Biophys. Acta (BBA)—Proteins Proteom. 2005, 1754, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Fan, T.; Xiao, C.; Tian, H.; Zheng, Y.; Li, C.; He, J. TGF-β Signaling in Health, Disease and Therapeutics. Signal Transduct. Target. Ther. 2024, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Pellicoro, A.; Ramachandran, P.; Iredale, J.P.; Fallowfield, J.A. Liver Fibrosis and Repair: Immune Regulation of Wound Healing in a Solid Organ. Nat. Rev. Immunol. 2014, 14, 181–194. [Google Scholar] [CrossRef]
- Canbay, A.; Taimr, P.; Torok, N.; Higuchi, H.; Friedman, S.; Gores, G.J. Apoptotic Body Engulfment by a Human Stellate Cell Line Is Profibrogenic. Lab. Investig. 2003, 83, 655–663. [Google Scholar] [CrossRef]
- Luedde, T.; Schwabe, R.F. NF-ΚB in the Liver-Linking Injury, Fibrosis and Hepatocellular Carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 108–118. [Google Scholar] [CrossRef]
- Zhan, S.S.; Jiang, J.X.; Wu, J.; Halsted, C.; Friedman, S.L.; Zern, M.A.; Torok, N.J. Phagocytosis of Apoptotic Bodies by Hepatic Stellate Cells Induces NADPH Oxidase and Is Associated with Liver Fibrosis In Vivo. Hepatology 2006, 43, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Seki, E.; Schwabe, R.F. Hepatic Inflammation and Fibrosis: Functional Links and Key Pathways. Hepatology 2015, 61, 1066–1079. [Google Scholar] [CrossRef]
- Li, S.; Tan, H.Y.; Wang, N.; Cheung, F.; Hong, M.; Feng, Y. The Potential and Action Mechanism of Polyphenols in the Treatment of Liver Diseases. Oxid. Med. Cell Longev. 2018, 2018, 8394818. [Google Scholar] [CrossRef]
- Kim, J.M.; Jung, I.A.; Kim, J.M.; Choi, M.-H.; Yang, J.H. Anti-Inflammatory Effect of Cinnamomum Japonicum Siebold’s Leaf through the Inhibition of P38/JNK/AP-1 Signaling. Pharmaceuticals 2023, 16, 1402. [Google Scholar] [CrossRef]
- Marquardt, J.U.; Gomez-Quiroz, L.; Arreguin Camacho, L.O.; Pinna, F.; Lee, Y.-H.; Kitade, M.; Domínguez, M.P.; Castven, D.; Breuhahn, K.; Conner, E.A.; et al. Curcumin Effectively Inhibits Oncogenic NF-ΚB Signaling and Restrains Stemness Features in Liver Cancer. J. Hepatol. 2015, 63, 661–669. [Google Scholar] [CrossRef] [PubMed]
- ZHANG, H.; SUN, Q.; XU, T.; HONG, L.; FU, R.; WU, J.; DING, J. Resveratrol Attenuates the Progress of Liver Fibrosis via the Akt/Nuclear Factor-ΚB Pathways. Mol. Med. Rep. 2016, 13, 224–230. [Google Scholar] [CrossRef]
- Meng, X.; Ma, Y.; Li, K.; Ji, M.; Lin, L.; Xiao, X.; Zhao, Y.; Su, G. Effect and Mechanism of Apple Polyphenols in Regulating Intestinal Flora and Inhibiting the TLR4/NF-ΚB/TGF-β Signaling Pathway to Alleviate Alcoholic Liver Fibrosis. Plant Foods Human. Nutr. 2024, 79, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Pang, H.; Chen, Y.; Huang, L.; Liu, H.; Zheng, Y.; Sun, C.; Zhang, G.; Wang, G. Anti-Inflammatory Effect of a Polyphenol-Enriched Fraction from Acalypha wilkesiana on Lipopolysaccharide-Stimulated RAW 264.7 Macrophages and Acetaminophen-Induced Liver Injury in Mice. Oxid. Med. Cell Longev. 2018, 2018, 7858094. [Google Scholar] [CrossRef]
- Kao, E.-S.; Hsu, J.-D.; Wang, C.-J.; Yang, S.-H.; Cheng, S.-Y.; Lee, H.-J. Polyphenols Extracted from Hibiscus sabdariffa L. Inhibited Lipopolysaccharide-Induced Inflammation by Improving Antioxidative Conditions and Regulating Cyclooxygenase-2 Expression. Biosci. Biotechnol. Biochem. 2009, 73, 385–390. [Google Scholar] [CrossRef]
- Tejada, S.; Pinya, S.; Martorell, M.; Capó, X.; Tur, J.A.; Pons, A.; Sureda, A. Potential Anti-Inflammatory Effects of Hesperidin from the Genus Citrus. Curr. Med. Chem. 2019, 25, 4929–4945. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Cheng, X.; Zeng, F.; Xu, Q.; Hou, L. The Role of TGF-β/SMAD Signaling in Hepatocellular Carcinoma: From Mechanism to Therapy and Prognosis. Int. J. Biol. Sci. 2024, 20, 1436–1451. [Google Scholar] [CrossRef] [PubMed]
- Fabregat, I.; Caballero-Díaz, D. Transforming Growth Factor-β-Induced Cell Plasticity in Liver Fibrosis and Hepatocarcinogenesis. Front. Oncol. 2018, 8, 357. [Google Scholar] [CrossRef] [PubMed]
- Dewidar, B.; Soukupova, J.; Fabregat, I.; Dooley, S. TGF-β in Hepatic Stellate Cell Activation and Liver Fibrogenesis: Updated. Curr. Pathobiol. Rep. 2015, 3, 291–305. [Google Scholar] [CrossRef]
- Tsuchida, T.; Friedman, S.L. Mechanisms of Hepatic Stellate Cell Activation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 397–411. [Google Scholar] [CrossRef]
- Louvet, A.; Teixeira-Clerc, F.; Chobert, M.-N.; Deveaux, V.; Pavoine, C.; Zimmer, A.; Pecker, F.; Mallat, A.; Lotersztajn, S. Cannabinoid CB2 Receptors Protect against Alcoholic Liver Disease by Regulating Kupffer Cell Polarization in Mice. Hepatology 2011, 54, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Argenziano, M.; Pota, V.; Di Paola, A.; Tortora, C.; Marrapodi, M.M.; Giliberti, G.; Roberti, D.; Pace, M.C.; Rossi, F. CB2 Receptor as Emerging Anti-Inflammatory Target in Duchenne Muscular Dystrophy. Int. J. Mol. Sci. 2023, 24, 3345. [Google Scholar] [CrossRef] [PubMed]
- Trebicka, J.; Racz, I.; Siegmund, S.V.; Cara, E.; Granzow, M.; Schierwagen, R.; Klein, S.; Wojtalla, A.; Hennenberg, M.; Huss, S.; et al. Role of Cannabinoid Receptors in Alcoholic Hepatic Injury: Steatosis and Fibrogenesis Are Increased in CB2 Receptor-Deficient Mice and Decreased in CB1 Receptor Knockouts. Liver Int. 2011, 31, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.Y.; Alexa, K.; Cortes, M.; Schatzman-Bone, S.; Kim, A.J.; Mukhopadhyay, B.; Cinar, R.; Kunos, G.; North, T.E.; Goessling, W. Cannabinoid Receptor Signaling Regulates Liver Development and Metabolism. Development 2016, 143, 609–622. [Google Scholar] [CrossRef] [PubMed]
- Scherer, T.; Buettner, C. The Dysregulation of the Endocannabinoid System in Diabesity—A Tricky Problem. J. Mol. Med. 2009, 87, 663–668. [Google Scholar] [CrossRef]
- Scandiffio, R.; Bonzano, S.; Cottone, E.; Shrestha, S.; Bossi, S.; De Marchis, S.; Maffei, M.E.; Bovolin, P. Beta-Caryophyllene Modifies Intracellular Lipid Composition in a Cell Model of Hepatic Steatosis by Acting through CB2 and PPAR Receptors. Int. J. Mol. Sci. 2023, 24, 6060. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; Silvestri, C. Lifestyle and Metabolic Syndrome: Contribution of the Endocannabinoidome. Nutrients 2019, 11, 1956. [Google Scholar] [CrossRef] [PubMed]
- Dasram, M.H.; Walker, R.B.; Khamanga, S.M. Recent Advances in Endocannabinoid System Targeting for Improved Specificity: Strategic Approaches to Targeted Drug Delivery. Int. J. Mol. Sci. 2022, 23, 13223. [Google Scholar] [CrossRef]
- Samat, A.; Tomlinson, B.; Taheri, S.; Thomas, G. Rimonabant for the Treatment of Obesity. Recent. Pat. Cardiovasc. Drug Discov. 2008, 3, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Westenberger, G.; Sellers, J.; Fernando, S.; Junkins, S.; Han, S.M.; Min, K.; Lawan, A. Function of Mitogen-Activated Protein Kinases in Hepatic Inflammation. J. Cell Signal 2021, 2, 172–180. [Google Scholar]
- Elsharkawy, A.M.; Mann, D.A. Nuclear Factor-ΚB and the Hepatic Inflammation-Fibrosis-Cancer Axis. Hepatology 2007, 46, 590–597. [Google Scholar] [CrossRef]
- Singer, C.A.; Baker, K.J.; McCaffrey, A.; AuCoin, D.P.; Dechert, M.A.; Gerthoffer, W.T. P38 MAPK and NF-ΚB Mediate COX-2 Expression in Human Airway Myocytes. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2003, 285, L1087–L1098. [Google Scholar] [CrossRef] [PubMed]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxid. Med. Cell Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef] [PubMed]
- Altomare, A.; Baron, G.; Gianazza, E.; Banfi, C.; Carini, M.; Aldini, G. Lipid Peroxidation Derived Reactive Carbonyl Species in Free and Conjugated Forms as an Index of Lipid Peroxidation: Limits and Perspectives. Redox Biol. 2021, 42, 101899. [Google Scholar] [CrossRef]
- Tutino, V.; Gigante, I.; Scavo, M.P.; Refolo, M.G.; De Nunzio, V.; Milella, R.A.; Caruso, M.G.; Notarnicola, M. Stearoyl-CoA Desaturase-1 Enzyme Inhibition by Grape Skin Extracts Affects Membrane Fluidity in Human Colon Cancer Cell Lines. Nutrients 2020, 12, 693. [Google Scholar] [CrossRef] [PubMed]
- Tutino, V.; De Nunzio, V.; Donghia, R.; Aloisio Caruso, E.; Cisternino, A.M.; Iacovazzi, P.A.; Mastrosimini, A.M.; Fernandez, E.A.; Giannuzzi, V.; Notarnicola, M. Significant Increase in Oxidative Stress Indices in Erythrocyte Membranes of Obese Patients with Metabolically-Associated Fatty Liver Disease. J. Pers. Med. 2024, 14, 315. [Google Scholar] [CrossRef]
- Milella, R.A.; De Rosso, M.; Gasparro, M.; Gigante, I.; Debiase, G.; Forleo, L.R.; Marsico, A.D.; Perniola, R.; Tutino, V.; Notarnicola, M.; et al. Correlation between Antioxidant and Anticancer Activity and Phenolic Profile of New Apulian Table Grape Genotypes (V. vinifera L.). Front. Plant Sci. 2023, 13, 1064023. [Google Scholar] [CrossRef] [PubMed]
- Milella, R.A.; Basile, T.; Alba, V.; Gasparro, M.; Giannandrea, M.A.; Debiase, G.; Genghi, R.; Antonacci, D. Optimized Ultrasonic-Assisted Extraction of Phenolic Antioxidants from Grape (Vitis vinifera L.) Skin Using Response Surface Methodology. J. Food Sci. Technol. 2019, 56, 4417–4428. [Google Scholar] [CrossRef] [PubMed]
- Tutino, V.; Gigante, I.; Milella, R.A.; De Nunzio, V.; Flamini, R.; De Rosso, M.; Scavo, M.P.; Depalo, N.; Fanizza, E.; Caruso, M.G.; et al. Flavonoid and Non-Flavonoid Compounds of Autumn Royal and Egnatia Grape Skin Extracts Affect Membrane PUFA’s Profile and Cell Morphology in Human Colon Cancer Cell Lines. Molecules 2020, 25, 3352. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]


| A Hepa-RG Fatty Acids (%) | HE | OE | ||||||
| CTR | 25 µg/mL | 50 µg/mL | 75 µg/mL | CTR | 25 µg/mL | 50 µg/mL | 75 µg/mL | |
| n-6 PUFAs | ||||||||
| LA | 3.83 ± 0.06 | 3.66 ± 0.18 | 3.53 ± 0.20 | 3.66 ± 0.19 | 2.97 ± 0.06 | 3.07 ± 0.05 | 2.92 ± 0.14 | 2.59 ± 0.06 * |
| DGLA | 1.02 ± 0.06 | 0.97 ± 0.05 | 1.03 ± 0.03 | 0.93 ± 0.06 | 0.79 ± 0.10 | 0.62 ± 0.09 | 0.61 ± 0.03 | 0.52 ± 0.07 |
| AA | 5.38 ± 0.28 | 4.65 ± 0.17 | 5.06 ± 0.12 | 4.68 ± 0.25 | 5.45 ± 0.12 | 5.65 ± 0.32 | 4.59 ± 0.08 * | 4.80 ± 0.17 |
| n-3 PUFAs | ||||||||
| EPA | 1.01 ± 0.04 | 1.13 ± 0.07 | 1.18 ± 0.09 | 1.02 ± 0.04 | 0.86 ± 0.05 | 0.69 ± 0.1 | 0.67 ± 0.01 | 0.67 ± 0.02 |
| DHA | 2.86 ± 0.19 | 2.8 ± 0.16 | 2.52 ± 0.11 | 2.72 ± 0.14 | 3.06 ± 0.12 | 2.61 ± 0.3 | 2.69 ± 0.09 | 2.57 ± 0.11 |
| Total fatty acids | ||||||||
| SFAs | 56.98 ± 0.61 | 55.92 ± 1.83 | 57.67 ± 0.44 | 58.24 ± 1.93 | 47.41 ± 5.28 | 48.27 ± 4.1 | 48.95 ± 1.26 | 49.12 ± 2.2 |
| MUFAs | 26.03 ± 0.12 | 27.59 ± 1.4 | 25.39 ± 0.26 | 25.61 ± 1.59 | 26.9 ± 0.36 | 28.07 ± 1.18 | 26.4 ± 0.58 | 25.64 ± 1.29 |
| PUFAs | 16.99 ± 0.70 | 16.49 ± 0.43 | 16.95 ± 0.35 | 16.15 ± 0.4 | 16.42 ± 0.44 | 15.31 ± 1.18 | 14.27 ± 0.18 | 13.56 ± 0.52 * |
| B LX-2 Fatty Acids (%) | HE | OE | ||||||
| CTR | 25 µg/mL | 50 µg/mL | 75 µg/mL | CTR | 25 µg/mL | 50 µg/mL | 75 µg/mL | |
| n-6 PUFAs | ||||||||
| LA | 2.92 ± 0.04 | 2.49 ± 0.18 | 2.51 ± 0.20 | 2.74 ± 0.04 | 2.28 ± 0.09 | 2.29 ± 0.23 | 2.36 ± 0.2 | 2.53 ± 0.21 |
| DGLA | 1.14 ± 0.04 | 1.11 ± 0.01 | 1.11 ± 0.03 | 0.99 ± 0.10 | 1.07 ± 0.08 | 0.96 ± 0.17 | 0.90 ± 0.13 | 0.90 ± 0.19 |
| AA | 7.29 ± 0.13 | 7.25 ± 0.10 | 6.99 ± 0.02 | 6.45 ± 0.23 ** | 7.40 ± 0.16 | 6.16 ± 0.88 | 5.69 ± 0.75 | 5.44 ± 1.19 |
| n-3 PUFAs | ||||||||
| EPA | 0.79 ± 0.07 | 0.68 ± 0.03 | 0.58 ± 0.04 | 0.66 ± 0.04 | 0.55 ± 0.08 | 0.61 ± 0.13 | 0.52 ± 0.08 | 0.59 ± 0.19 |
| DHA | 4.31 ± 0.14 | 4.12 ± 0.12 | 4.05 ± 0.05 | 4.01 ± 0.19 | 4.50 ± 0.18 | 3.69 ± 0.59 | 3.57 ± 0.41 | 2.70 ± 0.12 |
| Total fatty acids | ||||||||
| SFAs | 47.41 ± 1.44 | 47.26 ± 1.19 | 48.39 ± 0.60 | 49.55 ± 0.10 | 47.51 ± 1.11 | 53.44 ± 6.11 | 57.14 ± 5.23 | 59.45 ± 5.96 * |
| MUFAs | 33.91 ± 1.05 | 34.43 ± 0.99 | 33.82 ± 0.76 | 31.99 ± 0.19 | 33.79 ± 0.76 | 31.14 ± 3.99 | 28.01 ± 3.64 | 26.84 ± 3.87 |
| PUFAs | 18.68 ± 0.40 | 18.31 ± 0.24 | 17.79 ± 0.35 | 18.46 ± 0.16 | 18.69 ± 0.47 | 15.42 ± 2.15 | 14.86 ± 1.6 | 13.72 ± 2.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saponara, I.; Aloisio Caruso, E.; Cofano, M.; De Nunzio, V.; Pinto, G.; Centonze, M.; Notarnicola, M. Anti-Inflammatory and Anti-Fibrotic Effects of a Mixture of Polyphenols Extracted from “Navelina” Orange in Human Hepa-RG and LX-2 Cells Mediated by Cannabinoid Receptor 2. Int. J. Mol. Sci. 2025, 26, 512. https://doi.org/10.3390/ijms26020512
Saponara I, Aloisio Caruso E, Cofano M, De Nunzio V, Pinto G, Centonze M, Notarnicola M. Anti-Inflammatory and Anti-Fibrotic Effects of a Mixture of Polyphenols Extracted from “Navelina” Orange in Human Hepa-RG and LX-2 Cells Mediated by Cannabinoid Receptor 2. International Journal of Molecular Sciences. 2025; 26(2):512. https://doi.org/10.3390/ijms26020512
Chicago/Turabian StyleSaponara, Ilenia, Emanuela Aloisio Caruso, Miriam Cofano, Valentina De Nunzio, Giuliano Pinto, Matteo Centonze, and Maria Notarnicola. 2025. "Anti-Inflammatory and Anti-Fibrotic Effects of a Mixture of Polyphenols Extracted from “Navelina” Orange in Human Hepa-RG and LX-2 Cells Mediated by Cannabinoid Receptor 2" International Journal of Molecular Sciences 26, no. 2: 512. https://doi.org/10.3390/ijms26020512
APA StyleSaponara, I., Aloisio Caruso, E., Cofano, M., De Nunzio, V., Pinto, G., Centonze, M., & Notarnicola, M. (2025). Anti-Inflammatory and Anti-Fibrotic Effects of a Mixture of Polyphenols Extracted from “Navelina” Orange in Human Hepa-RG and LX-2 Cells Mediated by Cannabinoid Receptor 2. International Journal of Molecular Sciences, 26(2), 512. https://doi.org/10.3390/ijms26020512






