1. Introduction
Inflammatory bowel diseases (IBDs)—including Crohn’s disease (CD) and ulcerative colitis (UC)—are chronic inflammatory diseases of the gastrointestinal tract with a high burden for both patients and society [
1]. There is no curative treatment for IBDs, primarily due to their complex and largely unknown etiology, involving interactions between environmental factors, genetics, diet, the intestinal microbiota, and the immune system [
2].
Current assessment methods, primarily based on endoscopy, are considered to be the gold standard for evaluating mucosal activity; however, they are invasive and costly, thus limiting their routine application [
3]. This has prompted an increasing interest in non-invasive biomarkers, which may facilitate patient monitoring and improve quality of life [
4,
5,
6].
Extracellular vesicles (EVs) are membrane-bound particles secreted by cells, which play a crucial role in intercellular communication and can encapsulate various biomolecules, including proteins that contribute to disease pathogenesis [
7]. These diverse contents endow EVs with biomarker potential for disease diagnosis, prognosis, and monitoring [
8]. Despite the gastrointestinal tract being a primary source of human EVs, their specific roles in IBD pathogenesis and correlation with disease types remain poorly characterized. Preliminary studies suggest that EVs modulate immune responses through proteins involved in macrophage activation [
9,
10].
EVs containing Annexin A1 activate wound repair pathways in mice, and their levels are elevated in the sera of active IBD patients compared to healthy controls (HCs) [
11]. This suggests that they could be useful in disease monitoring and as potential therapeutic targets. Additionally, EVs derived from inflamed intestinal sites in IBD patients exhibit distinct protein profiles; EVs from patients with severe inflammation contain increased levels of inflammatory cytokines, while those from patients in endoscopic remission have cytokine levels akin to those from HCs [
12]. Notably, these active IBD-derived EVs exert pro-inflammatory effects on colonic epithelial cells in vitro.
The present study analyzed the proteome composition of serum EVs from IBD (CD and UC) patients, with and without intestinal inflammation, and HCs. The aim was to perform a comprehensive proteomic characterization of EVs to discover potential new biomarkers and identify molecular pathways involved in IBD pathogenesis. The focus on serum EVs aimed to overcome the limitations of traditional serum proteomic analyses, which often hinder the identification of low-abundance proteins that could play crucial roles in IBD pathogenesis.
3. Discussion
This study provides insights into the profile of circulating EVs in IBD patients, suggesting their potential to reflect disease condition and distinguish between active and quiescent sates. Hence, these results have a great impact, not just in unravelling the basis underlying IBD’s pathogenesis, but in identifying proteins with potential utility as novel biomarkers to aid in the diagnosis and/or monitoring of IBD in the absence of an invasive colonoscopy.
Despite advancements in the clinical management of IBD patients, there is still a lack of useful non-invasive biomarkers that can reliably facilitate the diagnosis and monitoring of disease progression and treatment response. EVs have emerged as key mediators of intercellular communication, carrying biomolecules that influence inflammation and immune responses in the gastrointestinal tract. Evidence suggests that the protein composition of EVs can reflect the inflammatory state of the intestine [
13]. In this context, previous studies have pointed to the potential of EVs as non-invasive biomarkers for IBD. One study identified the proteasome subunit alpha type 7 in salivary EVs as a promising candidate to differentiate IBD patients from HCs [
14]. However, that study did not establish a correlation between biomarker expression and disease activity, nor could it effectively compare CD and UC due to its limited sample size. Another study analyzed serum exosomes in IBD patients compared to HCs and in a mouse model of acute colitis [
15]. Pregnancy zone protein, known for its immunosuppressive properties, was significantly increased in serum exosomes both in IBD and in mouse colitis. However, like the previous studies, this research did not distinguish between CD and UC patients, and the small sample size limited the findings. Collectively, these studies highlight the premise of EVs as biomarkers for IBD while emphasizing the need for larger, more comprehensive investigations to clarify their clinical utility.
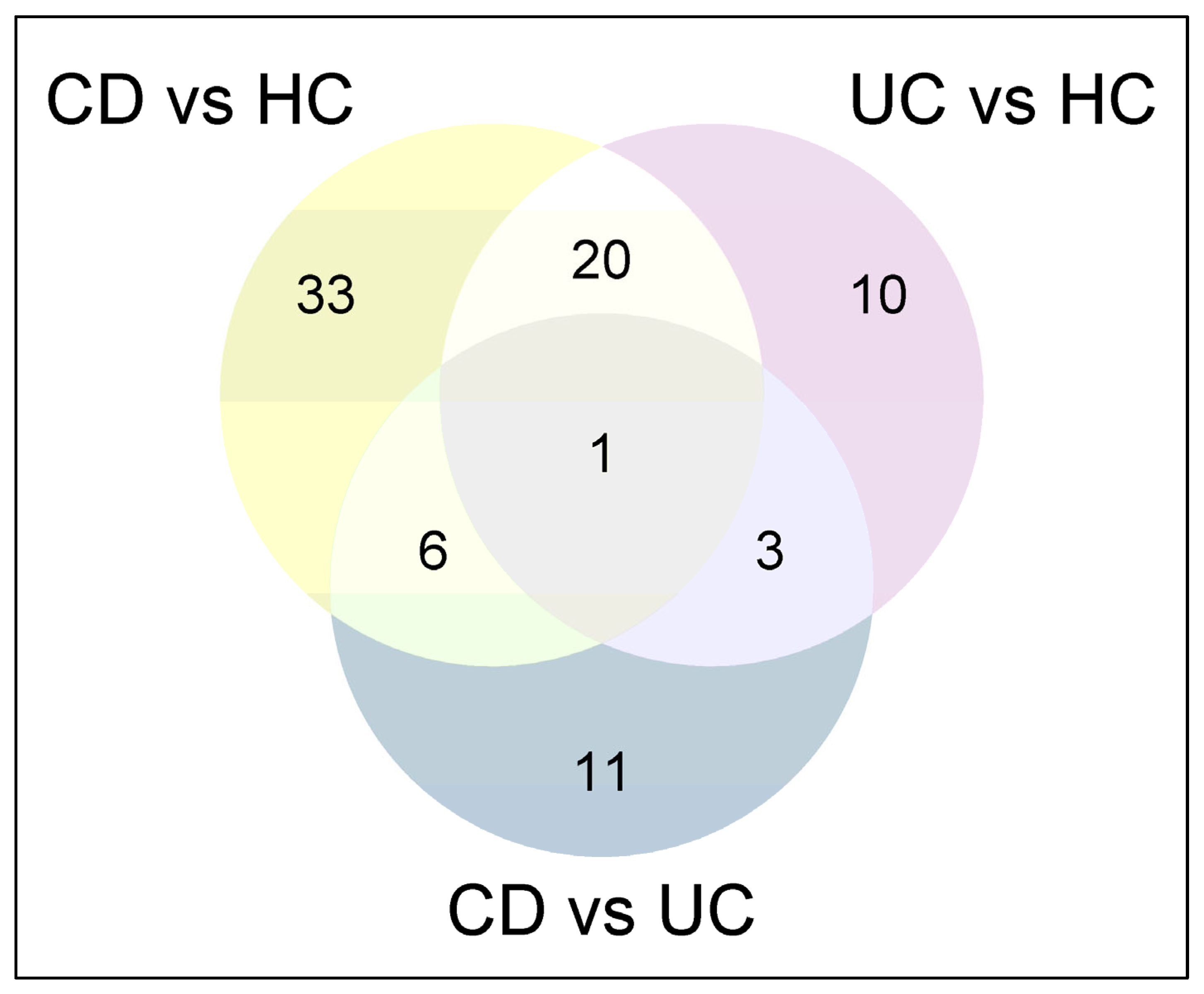
This study is unique as it is the first to isolate serum EVs from IBD patients and HCs specifically distinguishing, among IBD patients, between CD and UC and between patients with and without intestinal inflammation. By analyzing EVs’ protein profiles, we identified potential biomarkers associated with IBD presence and disease activity. Our findings are consistent with those of previous studies that have demonstrated that EVs carry biomolecules reflective of inflammatory processes in the gut. Notably, we observed significant differences in the proteomic composition of EVs between active disease in CD and UC compared to HCs, which points to the potential value of EVs as non-invasive biomarkers for disease activity.
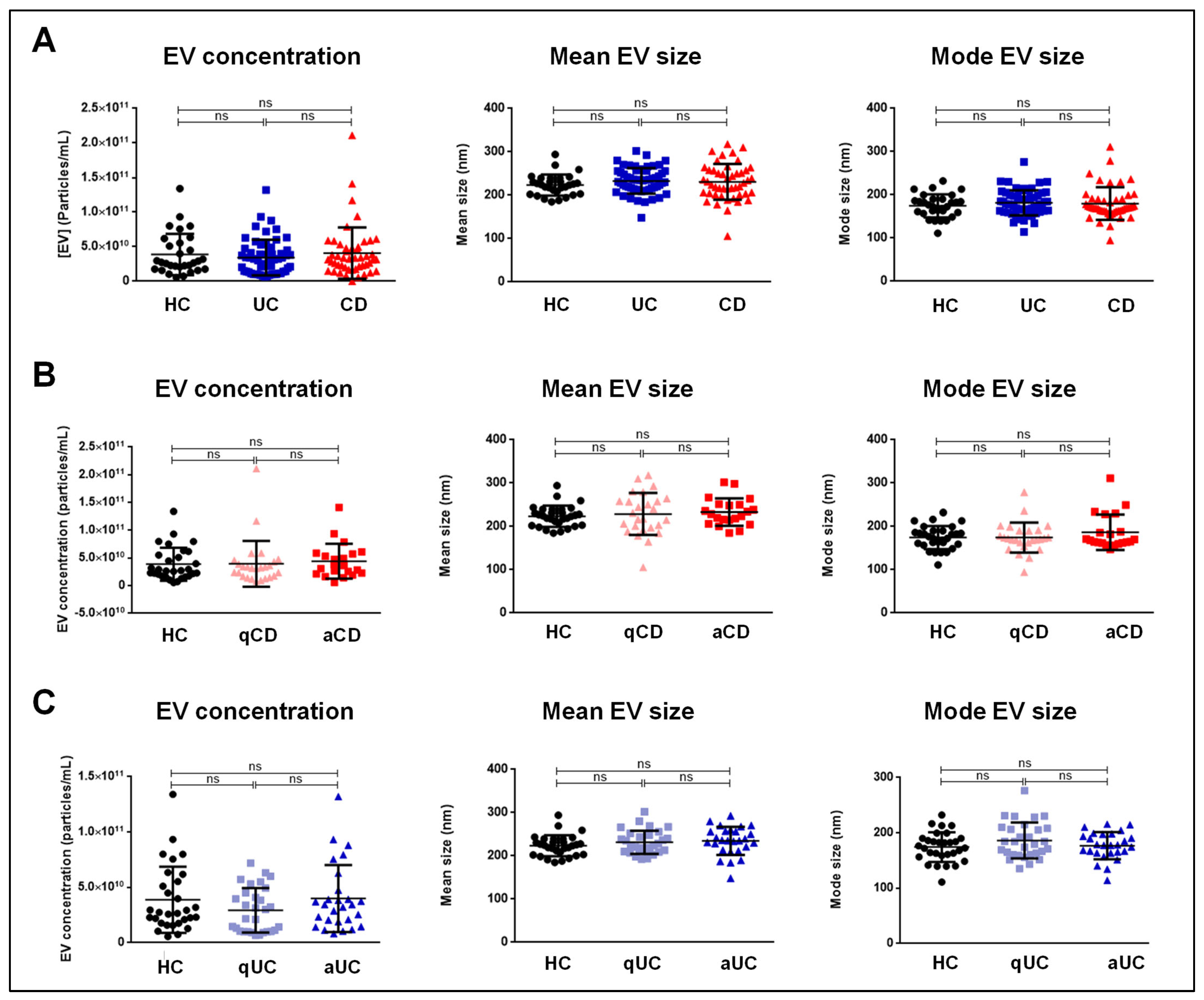
Our findings revealed no significant differences in EV size or concentration across the different groups, including comparisons between the active and quiescent phases of the disease. These data suggest that the total number of EVs does not vary significantly between IBD patients and HCs. Therefore, functional changes in EV composition, rather than their quantity, may better reflect disease activity.
There is a strong association between disease activity and the differential abundance of specific proteins, underscoring distinct molecular alterations in aCD and aUC compared to HCs. These differences suggest that the molecular alterations accompanying disease exacerbations differ between CD and UC. These findings are relevant, as they suggest that targeted therapeutic strategies could be designed based on these specific proteomic signatures, potentially leading to more personalized treatment approaches.
Additionally, we identified two distinct subsets of protein biomarkers capable of distinguishing CD and UC from HCs, which deserve further validation in an independent cohort. The first subset showed the highest discriminative power, emerging as the most reliable set of markers. However, the second subset demonstrated moderate accuracy and exhibited slightly lower discriminative capability. Despite this, these proteins may still hold significant potential for disease characterization.
Functional enrichment analysis of the differential-abundance proteins in serum EVs has provided valuable insights into the biological processes associated with disease activity in IBD patients. Differential serum EV proteins in aCD were enriched in biological processes such as protein localization to CENP-A-containing chromatin and megakaryocyte differentiation. These processes, which are fundamental for genetic maintenance [
16] and the formation of cells involved in hemostasis [
17], have not been previously associated with IBD. Notably, the molecular functions of these proteins are enriched in components like the complement component C1q complex, which plays a pivotal role in immune regulation, and in binding activities associated with hemoglobin and haptoglobin, indicating systemic effects of inflammation.
In aUC, differential serum EV proteins were involved in processes such as the negative regulation of hydrogen peroxide catabolic processes, epidermis development, and opsonization, suggesting altered oxidative stress responses and immune modulation during active disease [
18]. Previous studies have shown that hydrogen peroxide levels are increased in the non-inflamed colonic epithelium of UC patients, evidencing the role of hydrogen peroxide in both the pathogenesis and relapse of this debilitating form of IBD [
19,
20]. Additionally, genetically engineered mice that are unable to neutralize colonic hydrogen peroxide [glutathione (GSH) peroxidase-knockout mice] develop colitis analogous to human UC [
21]. This indicates that hydroxide peroxide generated in colonic epithelial cells can diffuse extracellularly and initiate colonic inflammation. At the molecular level, significant functions such as choline binding, hemoglobin binding, and antioxidant activity suggest potential dysregulation of oxidative pathways that may be directly associated with disease activity in aUC.
Although the findings described herein pointing to candidate biomarkers for IBD are promising, their preliminary nature is the main limitation of our study. Future studies in larger and independent cohorts of patients will be necessary to validate these biomarkers and evaluate their predictive ability for disease activity. Moreover, further functional studies thoroughly elucidating the biological role of the differential-abundance serum EV proteins in the pathogenesis of IBD will be critical for advancing our understanding of these diseases.
This study has several strengths. It is the first study to comprehensively characterize the proteomic profile of serum-derived EVs in CD and UC, in both active and quiescent disease states, and HCs, with the aim of identifying proteins associated with disease activity. Furthermore, the inclusion of a large, well-defined cohort of 150 participants (30 aCD, 30 qCD, 30 aUC, 30 qUC, and 30 HCs) provided a robust dataset for analysis, significantly enhancing this study’s statistical power and serving as a starting point for future validation studies.
4. Material and Methods
4.1. Study Design
A total of 150 participants were included in the study: 30 quiescent CD patients (qCD), 30 active CD patients (aCD), 30 quiescent UC patients (qUC), 30 active UC patients (aUC), and 30 HCs. The demographic and clinical characteristics of the study population are shown in
Table 10. Serum samples from subjects who met the inclusion criteria were provided by the Gastrointestinal Biologic Samples Collection of Dr. Javier P. Gisbert (Reg. C.0003482). The Ethics Committee of Hospital Universitario de La Princesa approved the study protocol.
4.2. Subject Recruitment
IBD patients (CD or UC) with active or inactive endoscopic disease, undergoing a colonoscopy indicated by medical criteria, were considered. The CD patients had luminal disease (CD patients with perianal disease were not included in the study). Only patients with complete ileocolonoscopy were considered. Patients were classified into active or quiescent IBD according to endoscopic findings. HCs included subjects undergoing colonoscopy for colorectal cancer surveillance, changes in bowel habit, or rectal bleeding. Only individuals with a macroscopically and histologically normal intestine and no evidence of disease were selected. The exclusion criteria for both groups included pregnancy, active infection, neoplasia, or any chronic condition that could affect the results.
4.3. Data Collection
The variables included in the database were IBD type (location and behavior), age at IBD diagnosis, time of disease evolution, smoking habit, surgical interventions due to IBD, IBD treatment, and clinical and endoscopic disease activity.
4.4. Endoscopic IBD Activity
Experienced gastroenterologists performed the ileocolonoscopies and graded the findings according to the Simple Endoscopic Score index for CD (SES-CD) and the Mayo endoscopic sub-score for UC. An SES-CD score between 0 and 2 was considered to represent inactive CD. Regarding UC activity, Mayo endoscopic sub-scores of 0 and 1 were considered to represent inactive UC.
4.5. Serum EV Isolation
Serum samples (1 mL aliquots) were thawed at room temperature and processed using a series of centrifugations at 4 °C. First, serum was centrifuged to remove small cell debris, followed by ultracentrifugation at 100,000 × g for 75 min to pellet the EVs. The EV pellet was washed and subjected to a second ultracentrifugation under the same conditions. The final EV pellet was resuspended in 20 µL of phosphate-buffered saline (PBS) for immediate use or stored at −80 °C for further experiments.
4.6. Transmission Electron Microscopy (TEM)
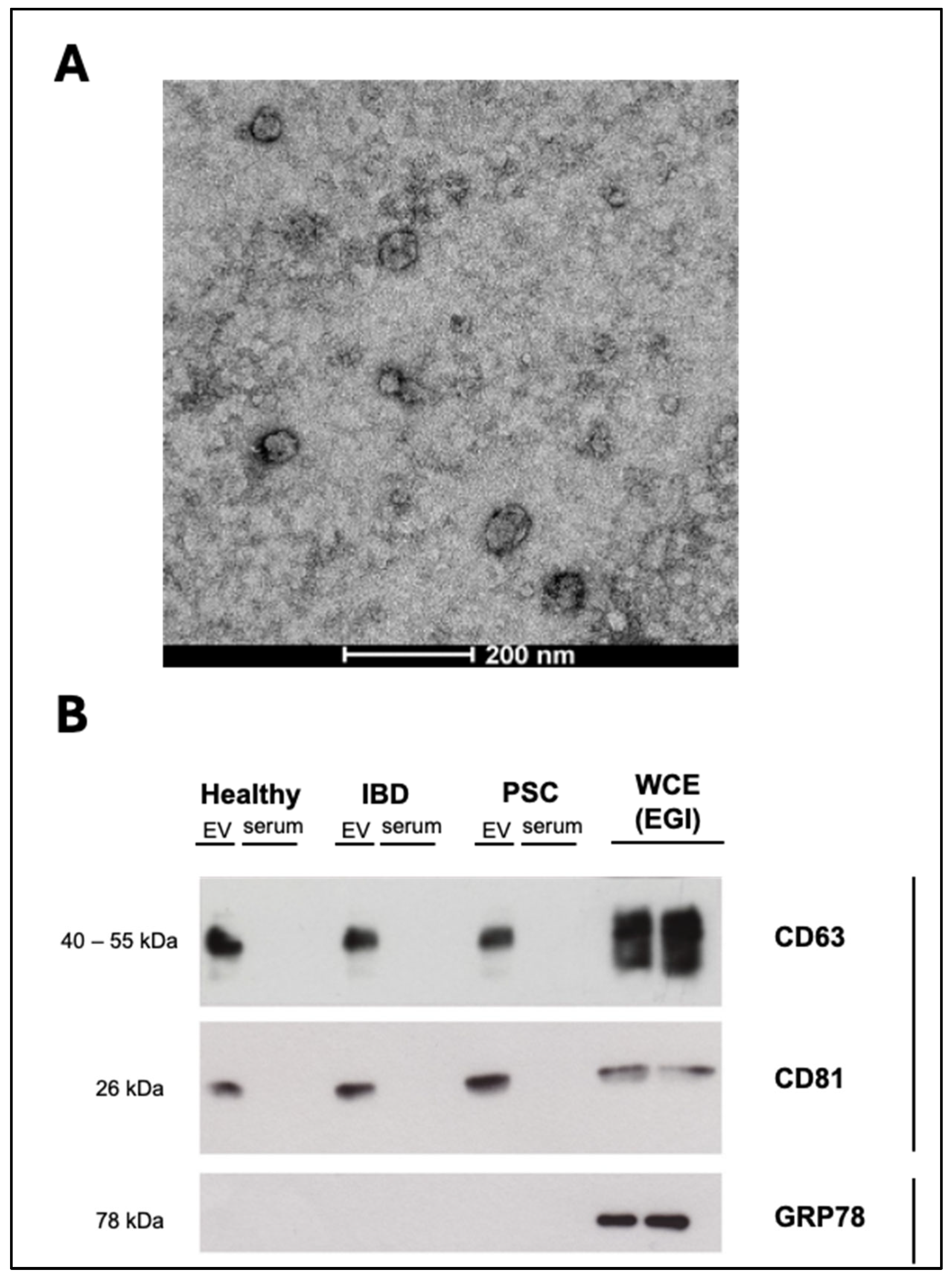
For the characterization of EVs, PBS-resuspended EV isolates were negatively stained and evaluated by TEM. EV samples were directly adsorbed onto a glow-discharged (60 seg low discharging using a PELCO easy-glow device) carbon-coated copper grid (300 mesh). Afterwards, the grids were fixed with 2% paraformaldehyde (PFA) in 0.2 M PBS (pH 7.4) for 20 min and washed with distilled water. Then, contrast staining was performed by incubating the grids with 4% uranyl acetate at 4 °C for 15 min. TEM images were obtained by using a TECNAI G2 20 C-TWIN high-resolution transmission electron microscope (FEI, Donostia-San Sebastian, Spain), at an acceleration voltage of 200 kV.
4.7. Distribution and Concentration of EVs
The size distribution and concentration of the EVs were measured by nanoparticle tracking analysis (NTA) using a NanoSight LM10 system (Malvern, UK). Post-acquisition settings for NTA were standardized for all samples. Each video was analyzed to determine the mode vesicle size and EV concentration.
4.8. Western Blot Analysis
Protein expression in the EV samples was analyzed by immunoblotting. Positive (CD63 and CD8) and negative (GRP78) EV markers were evaluated.
4.9. Mass Spectrometry Analysis and Protein Identification
The samples were processed with an extraction buffer (7 M urea, 2 M thiourea, 4% CHAPS, and 100 mM DTT) and incubated for 30 min with agitation. Next, Filter-Aided Sample Preparation (FASP) was performed for the digestion of proteins. After digestion, peptides were recovered from the filter units and subjected to ethyl acetate. After careful removal of the last upper ethyl acetate layer, the samples were speed-vacuumed in an RVC2 25 speedvac concentrator (Christ). The samples were further desalted using stage-tip C18 microcolumns (Zip-tip, Millipore, Burlington, MA, USA) and resuspended in 0.1% FA prior to mass spectrometry analysis.
Peptide separation was conducted in a nanoACQUITY UPLC system (Waters, Milford, MA, USA) coupled with an LTQ Orbitrap XL (Thermo Electron, Waltham, MA, USA) and/or Synapt G2 Si (Waters) mass spectrometer. A linear gradient of 3–50% acetonitrile over 120 min was used for peptide elution. Peptides were identified using Mascot v2.1 (Matrix Science) through Proteome Discoverer 1.4 (Thermo Electron), with carbamidomethylation of cysteines as a fixed modification and oxidation of methionines as a variable modification. Searches were conducted against the Uniprot/Swissprot database, with a decoy search to estimate the false discovery rate (FDR). Only peptides with an FDR < 1% were selected.
For label-free differential protein expression analysis, Progenesis LC-MS (Nonlinear Dynamics) was used. Raw files were imported from both Orbitrap and Synapt runs, with one run designated as the reference for aligning precursor masses in all other samples. The raw abundances of each feature were automatically normalized against the reference run and logarithmized. Samples were grouped according to the comparisons being performed. A peak list containing the information of these significantly different features was generated and exported to the Mascot search engine (Matrix Science Ltd., Boston, MA, USA) for the identification of peptides. The list of identified peptides was imported in Progenesis LC-MS, and the previously quantified features were matched to the corresponding peptides. Only proteins with at least two quantified non-conflicting peptides were selected. Proteins with a p-value < 0.05 and ratio > 1.5 were considered to be significantly deregulated.
4.10. Functional Analysis
Gene Ontology (GO) enrichment analysis was carried out using the Database for Annotation, Visualization, and Integrated Discovery (DAVID) online tool (
http://david.abcc.ncifcrf.gov/summary.jsp, accessed on 25 December 2024). DAVID is a GO term annotation and enrichment analysis tool used to highlight the most relevant GO terms associated with a given gene list. Fisher’s exact test was used to determine whether the proportion of genes considered to belong to certain GO terms or categories differed significantly between the dataset and the background. Biological process, molecular function, and cellular component categories were assessed, and only GO terms enriched with an FDR < 5% were considered for comparison and discussion.
4.11. Statistical Analysis
Quantitative variables are presented as means and standard deviations. Categorical variables are presented as numbers of events and percentages. The Kolmogorov–Smirnov test was used to determine the normality of data distribution for quantitative variables. Differences in this type of variable between groups were analyzed by Student’s t test or the Wilcoxon rank-sum test according to whether the variables were normally distributed or not. For categorical variables, differences between groups were assessed using the chi-squared test. Differences were considered significant when p < 0.05.
For biomarker discovery, the dataset with all of the variables from the individual patients was analyzed by Anaxomics Biotech (
http://www.anaxomics.com, accessed on 25 December 2024) using a data mining approach, employing the algorithm Balanced_Accuracy [
22]. Cross-validated balanced accuracy (ACC) was used as a classifier optimization measure together with the cross-validated
p-value. In order to prioritize the generalization capability of the conclusion, a K-fold validation analysis was performed, yielding cross-validated quality measures (such as accuracy) for each biomarker [
23].
Functional and pathway enrichment analysis was conducted using DAVID to perform GO enrichment analysis using SRplot [
24].