1. Introduction
Retroviruses such as HIV-1 and HIV-2 are responsible for causing AIDS in humans [
1]. Despite being distinct types, they share commonalities in replication mechanisms, modes of transmission, and clinical manifestations [
2]. Of the two, around 630,000 AIDS-related fatalities occurred in 2024, with an estimated 40.8 million individuals living with HIV-1 globally. This represents a minor decline in mortality as compared to 2020, although overall prevalence is still rising [
3]. Notably, more than 80% of adult HIV-1 infections are acquired through mucosal exposure, with sexual contact being the predominant route [
4]. As the virus continues to spread, there is an urgent need for scientific research to develop a more potent vaccine. However, the virus’s wide genetic diversity, which results in several subtypes, makes vaccine development difficult [
5]. The viral Env glycoproteins gp120 and gp41 are particularly variable and act as the key part of the virus to infect host cells. These glycoproteins facilitate viral ingress by mediating the virus attachment to the host T cells [
6]. Among them, gp120 is critically involved in recognizing host receptors by binding to CD4 on T cells [
7]. One proposed approach to prevent infection involves neutralizing the attachment between gp120 and the CD4 receptor, thereby inhibiting viral entry into CD4
+ cells [
8]. In this context, the generation of neutralizing Abs has emerged as a promising avenue, as these Abs can target the gp120-CD4 interface and compete with CD4 for binding [
9]. Experimental studies have demonstrated that such Abs can effectively neutralize gp120, reinforcing its value as a prime target for the potent and effective HIV-1 vaccine development candidates [
4].
Nef is one of the first proteins translated after HIV-1 enters the host cell. It typically consists of 200 to 215 amino acids, with the 206-amino-acid variant being the most prevalent. Nef is structurally composed of a flexible C-terminal length, a central core domain, and an N-terminal membrane-anchoring region. People with Nef-deficient HIV-1 strains have reported a noticeable slowdown in the course of their illness [
10]. This highlights Nef as a key pathogenic element, notable for its diverse functions and numerous cellular interaction partners [
11]. Its most well-characterized functions include enhancing viral infectivity, interfering with host cell signaling and activation, and downregulating CD4, CD8, CD3, and MHC-I molecules [
12]. These multifaceted actions of Nef contribute significantly to HIV-1 pathogenesis and efficient viral replication. Additionally, Nef exhibits sequence variability across different HIV-1 isolates and undergoes frequent mutations, enabling immune evasion [
13]. The importance of Nef in viral replication, immune modulation, and pathogenicity emphasizes its value in vaccine development. As a key element recognized by cellular immunity, the recognition and depiction of T cell epitopes within Nef offer promising opportunities for the development of potent therapeutic and preventive approaches [
14].
A distinct group of people living in a community with HIV, known as elite controllers, possess the remarkable ability to persist symptom-free and maintain elevated CD4
+ T cell levels over an extended duration without the aid of antiretroviral therapy (ART) [
15]. Studies have shown that these individuals exhibit increased populations of CTLs capable of producing interferon-gamma (IFN-γ), which promotes Th1-mediated immune feedback, suggesting its involvement in effective HIV-1 control [
16]. Proliferation of CTLs has been demonstrated to significantly assist in the suppression of HIV infection [
17]. Despite extensive efforts, traditional vaccine development approaches have largely failed to produce a successful HIV-1 vaccine. In contrast, a novel and promising avenue involves therapeutic immunization of people with an existing HIV-1 infection, which has shown potential in delaying or preventing progression to AIDS [
18].
A therapeutic HIV-1 vaccine aims to trigger highly robust and comprehensive immune feedback, particularly those directed at conserved regions of the virus rather than those typically elicited during natural infection. However, the successful progress of such vaccines has been hindered by issues such as inefficient delivery systems and inadequately optimized immunogen designs [
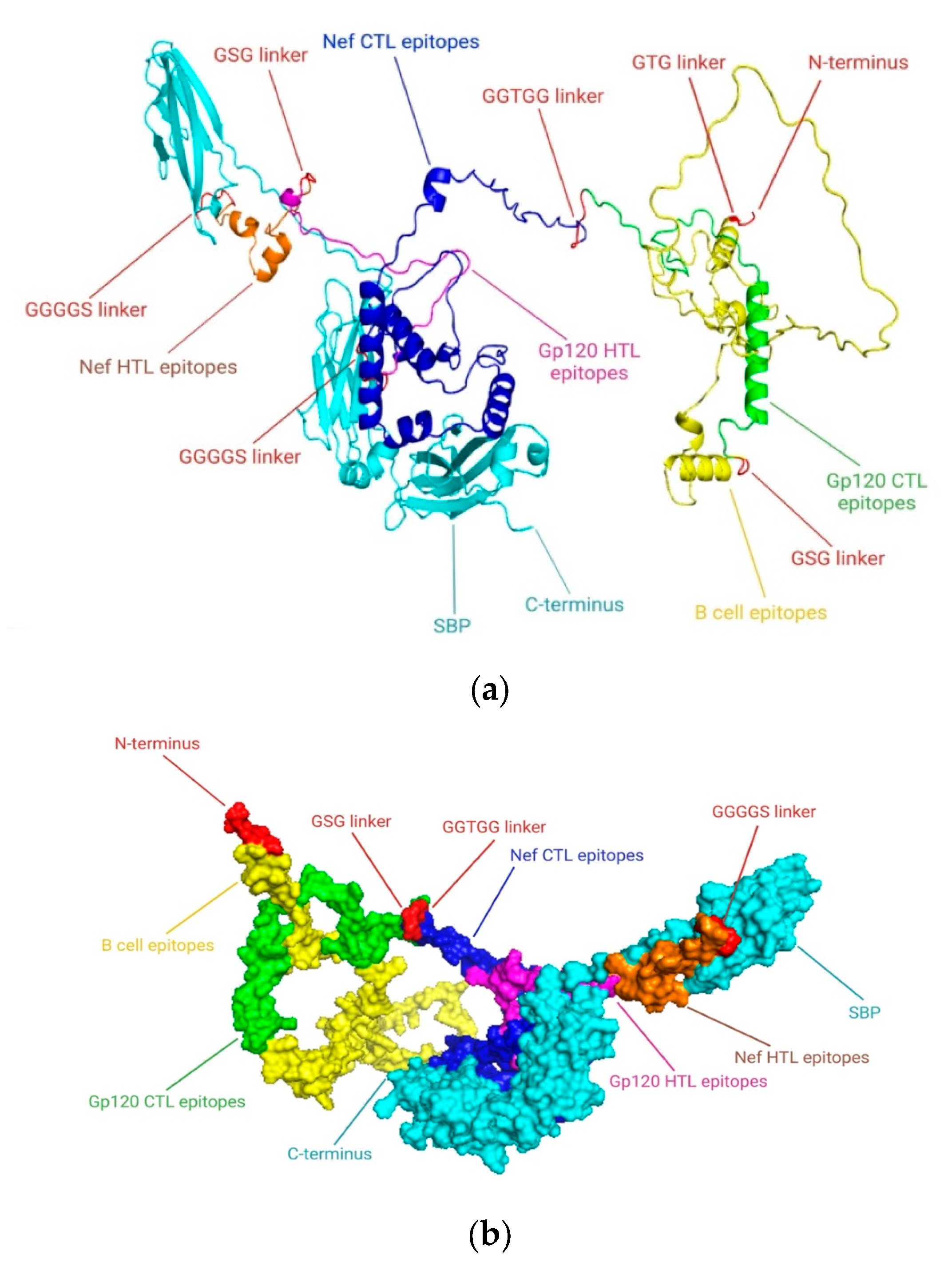
19]. An emerging and promising approach in this area involves constructing simulated multi-epitope Ags. These are engineered by selecting epitopes from common viral immunogens and incorporating a broad spectrum of protective, immunoregulatory T cell epitopes (
Figure 1). The goal is to induce potent and targeted immune feedback capable of effectively controlling HIV-1 infection [
20]. To subdue initial restrictions and enhance the efficacy of vaccines, researchers are turning to computational tools. In silico techniques enable the identification of potentially immunogenic peptide sequences (epitopes) from linear protein sequences. Additionally, MD simulations are used to assess the binding affinities and determine epitope MHC complexes, helping to distinguish the most promising epitope candidates [
20]. Rodríguez-Fonseca and colleagues used three-dimensional (3D) models of the gp120 protein to study dendrimer-G4-PAMAM-peptide complexes. Sole peptides or the peptide-dendrimer formulated mixtures were intravenously administered to female BALB/c mice in the current experiment. Their findings demonstrated that the peptides induced immune feedback at both systemic and mucosal regions. Notably, the serum and nasal secretions resulted in elevated levels of IgG and IgA Abs after dendrimer-peptide formulated vaccine administration [
21]. As the gp120 protein has a pivotal role in viral attachment to its host receptor and pathogenicity so we selected this Ag as a principal model in our HIV-1 vaccine development program. This study aimed to develop a co-epitope vaccine capable of eliciting strong T cell–mediated immunity, with emphasis on CTLs and HTLs. Using computational biology methods, we identified and validated conserved antigenic epitopes from the gp120 and Nef proteins of HIV-1, selected for their potential to activate both B- and T-cell responses. These epitopes were combined into a final vaccine construct using suitable linkers and the SBP adjuvant to enhance cellular immune activation.
3. Discussion
Computational biology has become a key player in the development of vaccines, particularly in predicting immunogenic peptides that aid in the development of both safe and effective vaccines. By using peptide prediction tools, it is possible to reduce the high costs and minimize adverse side effects typically connected with vaccines derived from attenuated pathogens, whether live or inactive [
31]. Even while antiretroviral treatments have made great progress in treating AIDS, an efficient HIV-1 vaccine is still desperately needed to combat this persistent worldwide health emergency [
32]. Unfortunately, because they mainly targeted a limited range of HIV genotypes, the majority of prior vaccine candidates were ineffective against the quickly evolving HIV [
33]. According to studies on the in silico-designed multiple-epitope EP HIV-1090 vaccine, these vaccines’ capacity to prevent HIV-1 is severely hampered by their inability to provoke robust CTL and HTL responses [
34]. Additionally, studies using different multiple-epitope vaccinations on BALB/c mice demonstrated that they are unable to produce broadly neutralizing Abs [
35]. The vaccine’s poor effectiveness is also a result of improper cytokine activation and an inability to elicit innate immune responses. Since CTL-mediated immunity is essential for controlling viral infections, a successful HIV-1 vaccine must elicit strong immunological responses, especially by boosting both CTL and HTL responses. Furthermore, HTL-mediated immunity is necessary to support the formation of Abs and to foster a functional CD8
+ CTL response, both of which contribute to viral control and viral load reduction [
36]. When combined with in vivo research, novel immunoinformatic techniques, especially those aimed at identifying multi-functional T cell epitopes, might greatly enhance the design of HIV-1 immunogens [
37]. Vaccines based on several epitopes can induce robust HTL and cellular responses. The techniques used in these investigations have opened the door for the development of a vaccine with various epitopes that can stimulate the generation of cytokines like IFN-γ, produce broadly neutralizing Abs, and interact with TLRs to stimulate the optimal innate immune responses. By adding SBP to the vaccine C-terminal, this strategy further improved the immunological response. In silico strategies have become increasingly pivotal in the design of co-epitope vaccines, encouraged by the promising outcomes of numerous studies. Notably, the study demonstrated that a multiepitope construct comprising Nef, Rev, Gp160, and P24 epitopes elicited strong cellular and humoral immune responses in mice. Heterologous prime-boost regimens significantly enhanced IFN-γ, Granzyme B, and IgG2a/IgG2b levels, suggesting their promise as a multiepitope-based HIV-1 vaccine candidate [
38]. Similarly, an in silico study identified a truncated p24-Nef fusion protein enriched with conserved CTL epitopes and strong MHC binding, achieving >70% predicted population coverage. These findings support its potential as a promising therapeutic HIV-1 vaccine candidate [
39]. A recent study demonstrated that full-length CD40L and IFN-γ, when fused with Nef, enhanced immune responses more effectively than multiepitope constructs. Our results focusing on Nef and gp120 epitopes similarly emphasize the importance of optimized epitope selection, suggesting that combining potent epitopes with strong adjuvant strategies could further improve HIV-1 vaccine design [
40]. Similar studies have proposed multiepitope vaccine constructs, where the adjuvant-containing HIV-1b showed enhanced immunogenicity and stability. Our findings on gp120 and Nef epitopes support this strategy, emphasizing the role of conserved, immunogenic epitopes in developing effective HIV-1 vaccines [
41]. Similarly, a multiple-epitope vaccine that targets HIV-1 specifically has been developed, which is comparable to what we are currently studying [
42].
A vaccine targeting HIV-1 was designed using an immunoinformatic method, paying special emphasis to the Env glycoprotein gp120, which promotes virulence and attachment to the host receptor CD4. Nef also contributes significantly to HIV-1 pathogenesis by promoting viral replication. Since surface glycoproteins constitute the primary site of contact with the host immune system and are widely expressed on viruses, they have long been thought to be excellent candidates for vaccine development. This work used a new and effective computational biology-based technology to develop a co-epitope-based vaccine that targets different strains of HIV-1 by utilizing large amounts of genomic data. The main objective of this effort was to produce a vaccine that could lower the global burden linked to HIV-1, given the effective potential of computational approaches in vaccine development. The consensus sequence served as the foundation for the construction of the 3D structure and anticipated epitopes. While ElliProt was used to provide non-linear predictions, linear epitope predictions for MHC-I and MHC-II binding sites produced a variety of peptide options. Furthermore, the identification of epitopes that may be recognized by Abs was aided by the 3D visualization of gp120 and Nef. A QSAR model was used to estimate IC50 values in order to evaluate peptide affinity for MHC; lower IC50 values indicate better binding affinity which helps with peptide classification.
Initiating a strong immune response to defend the host from viral infections depends on T cells, particularly HTLs and CTLs. However, compared to vaccines that activate both CTLs and HTLs, those that only target CTL responses have shown decreased efficacy. In light of their unique potential, co-epitope vaccines have a number of advantages over prevalent and single-epitope vaccines. (i) To ensure a varied immune response, a variety of T cell receptors (TCRs) from various MHC class I and II molecules are capable of recognizing comprehensive self and foreign epitopes. (ii) A thorough and well-coordinated defense can be produced by the overlapping CTL, HTL, and B cell epitopes, which can simultaneously elicit humoral and cellular immune feedback. (iii) Adding an adjuvant to the vaccine formulation increases immunogenicity and promotes long-term immunological feedback. (iv) The development of vaccines is made simpler by avoiding issues related to pathogen culture and in vitro Ag expression. To further maximize immunological responses, T cell epitopes that may bind different MHC-I and class II molecules were chosen using the IEDB server. T cell epitopes with a higher binding potential and lower IC50 values were selectively targeted by this technique. Antigenic B cell epitopes, on the other hand, interact directly with B cell receptors (BCRs) to start the development of Abs that are confined to particular epitopes. The IEDB server was used to determine if B cell epitopes were linear or continuous. Based on positive results from the initial screening, 122 T cell and B cell epitopes were finalized. The most promising epitopes, which revealed potent antigenic qualities and were devoid of allergens, poisons, and human proteome homologies were found through additional screening rounds. HTL epitopes’ capacity to trigger cytokine reactions, including IFN-γ, IL-4, and IL-10, was also assessed. To enhance the vaccine Ag uptake potential, the SBP was conjugated at the C-terminal. The peptides GTG, GSG, GGTGG, and GGGGS were used to link the epitopes in order to coordinate the vaccine stability and structure. The vaccine immunogenicity, antigenicity, and durability were all enhanced by the conjugation of SBP and the linkers.
It has been demonstrated that longer peptides are more successful than shorter ones at eliciting immunological responses [
43]. The ability to develop co-epitope constructions that contain both T- and B cell epitopes, to be efficiently exposed and rectified in order to elicit the intended immune response is still somewhat unknown. Accurately anticipating TAP transport and proteasomal cleavage is essential for improving the effectiveness of multiple-epitope-based vaccines. All of the finalized epitopes including those for CD8
+ and CD4
+ T cells are probably attainable for immunological activation during Ag processing and presentation by capable APCs were assessed from the NetCTL 1.2 server. To make sure these epitopes are chosen appropriately for vaccine development, it is crucial to evaluate the immune responses they elicit. An effective, quick, and economical substitute for in vitro and in vivo immunological evaluation is the use of in silico cytokine prediction algorithms, since specific cytokines can be produced by specific residues and motifs within epitopes [
44]. IFN-γ is a cytokine that has antiviral, immune-regulatory, and antitumor effects that is essential to both innate and adaptive immunity. Its release plays an important function in lowering the HIV-1 viral load and is essential for the Th1 response [
45]. We evaluated the potential of specific HTL epitopes to produce IFN-γ and other cytokines in this investigation. The IFNepitope server was used to anticipate which peptides will attach to MHC-II molecules and produce the secretion of IFN-γ. Based on their anticipated SVM scores, the majority of our chosen HTL epitopes had a strong ability to stimulate IFN-γ and cytokine production. IFN-γ production is intimately associated with HIV-specific T cell immunogenicity and Th1 response activation [
46]. According to certain research, IL-10 also has anti-HIV effects by preventing the secretion of inflammatory cytokines [
47]. Moreover, studies indicate that T cells that induce IL-10 may aid in lowering HIV replication in expectant mothers [
48].
Vaccine immunogens must account for the diversity of HLA tissue types as well as the extensive antigenic variation in HIV-1. In our investigation, we selected peptides containing a range of epitopes with diverse HLA binding specificities to enhance population coverage. MHC-II epitope coverage was 73.35%, whereas MHC-I epitope population coverage was 90.76%. Furthermore, the co-epitope structures exposed a significantly increasing population coverage in areas with high HIV-1 prevalence. The M group of HIV-1 subtypes exhibited a 75% conservation score for the final epitopes. The high conservation across these subtypes decreased the possibility of viral immune evasion, offering broader protection. We used the Vaxigen, AllerTOP v.2.0, and ToxinPred services to evaluate the toxicity, allergenicity, and antigenicity of the final HIV-1 gp120-Nef vaccine design and the anticipated epitopes, respectively. The outcomes verified that the vaccine was non-toxic, non-allergenic, and antigenic. The ProtParam tool from the ExPASy server was used to analyze the physicochemical properties of each predicted epitope as well as the entire vaccine construct. The vaccine exhibited adequate stability with a high pI value of 8.87. The predicted half-life was more than 10 h in a prokaryotic E. coli system and 30 h in mammalian reticulocytes, indicating its potential for stable and efficient production. Additionally, a GRAVY value of −0.646, which is linked to greater water solubility and is also confirmed by predictions from the SolPro server, revealed the vaccine’s hydrophilic character.
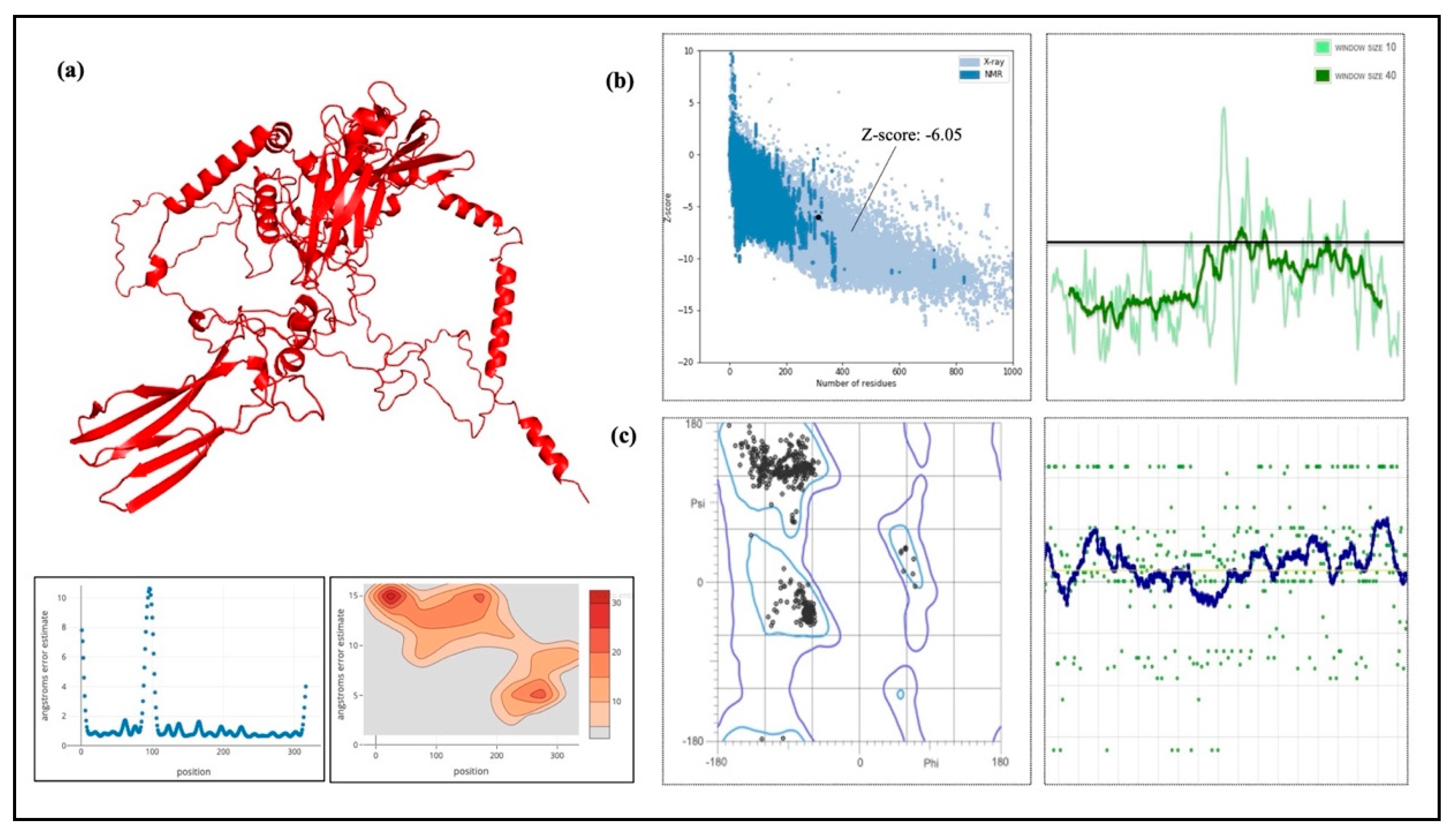
To confirm the protein closely resembled its original structure, the formulated vaccine 3D structure was first modeled using the RoseTTAFold online server and then improved using the GalaxyRefine server. Our results showed that the quality and accuracy of the projected 3D structures increased when the vaccine build was enhanced. When the 3D and improved structure of the formulated vaccine was evaluated, the results were further verified using the Ramachandran plot and the Z-score analysis. This research supported the vaccine’s potential efficacy by confirming that it assumed a structurally appropriate conformation. The vaccine Z-score was −6.05, which is within the permissible range for protein crystal structures confirmed by experiment. Crucially, the Ramachandran plot favorable areas had the largest concentration of amino acids [
49]. Furthermore, we conducted protein-protein docking studies between TLRs and the projected constructions. Through pathogen detection and adaptive immune response, TLRs are essential for innate immune system activation [
50]. In particular, TLR3 is in charge of activating DCs in response to HIV-1, whereas TLR2 and TLR4 identify viral structural proteins and start the induction of inflammatory cytokines [
51].
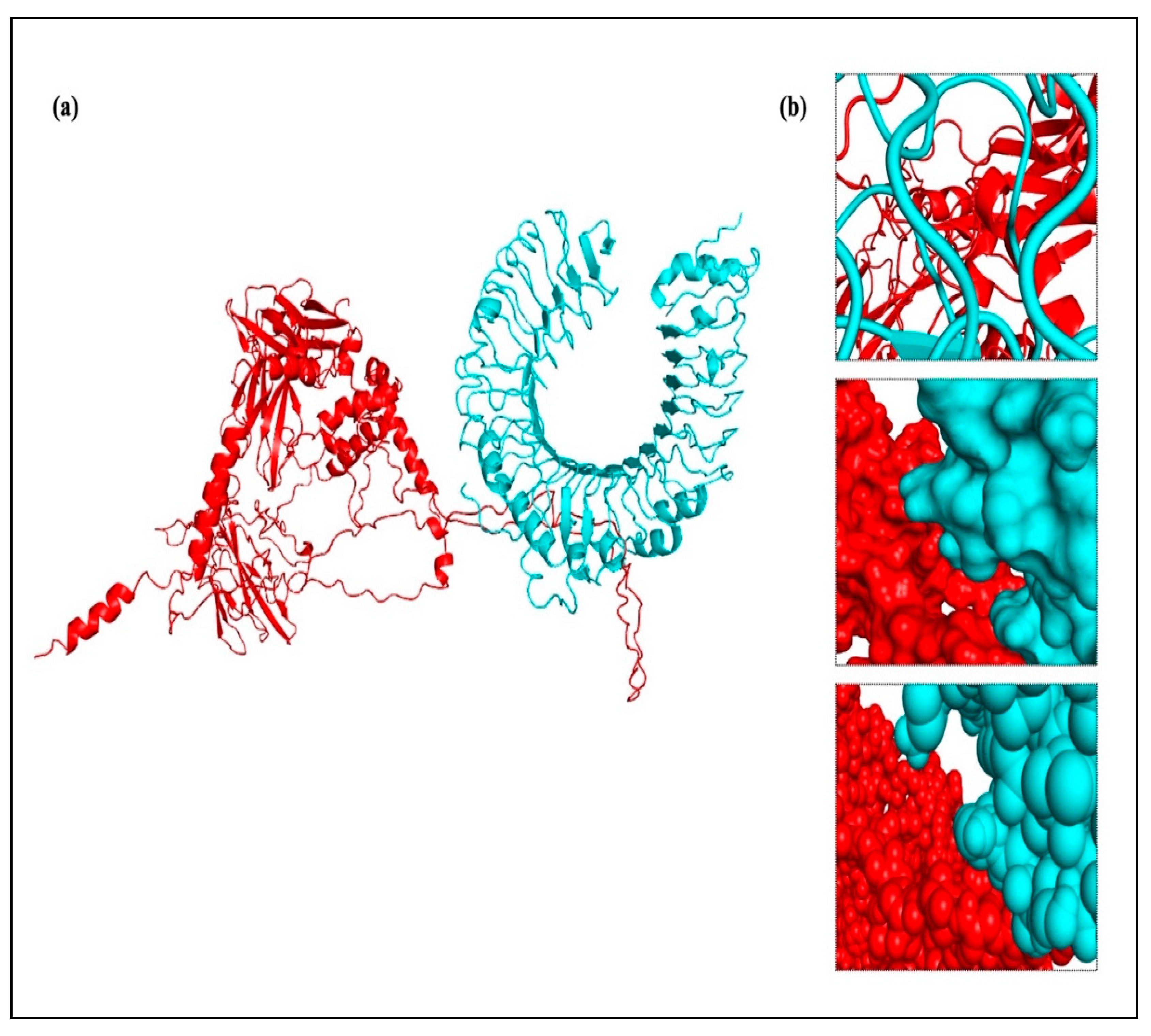
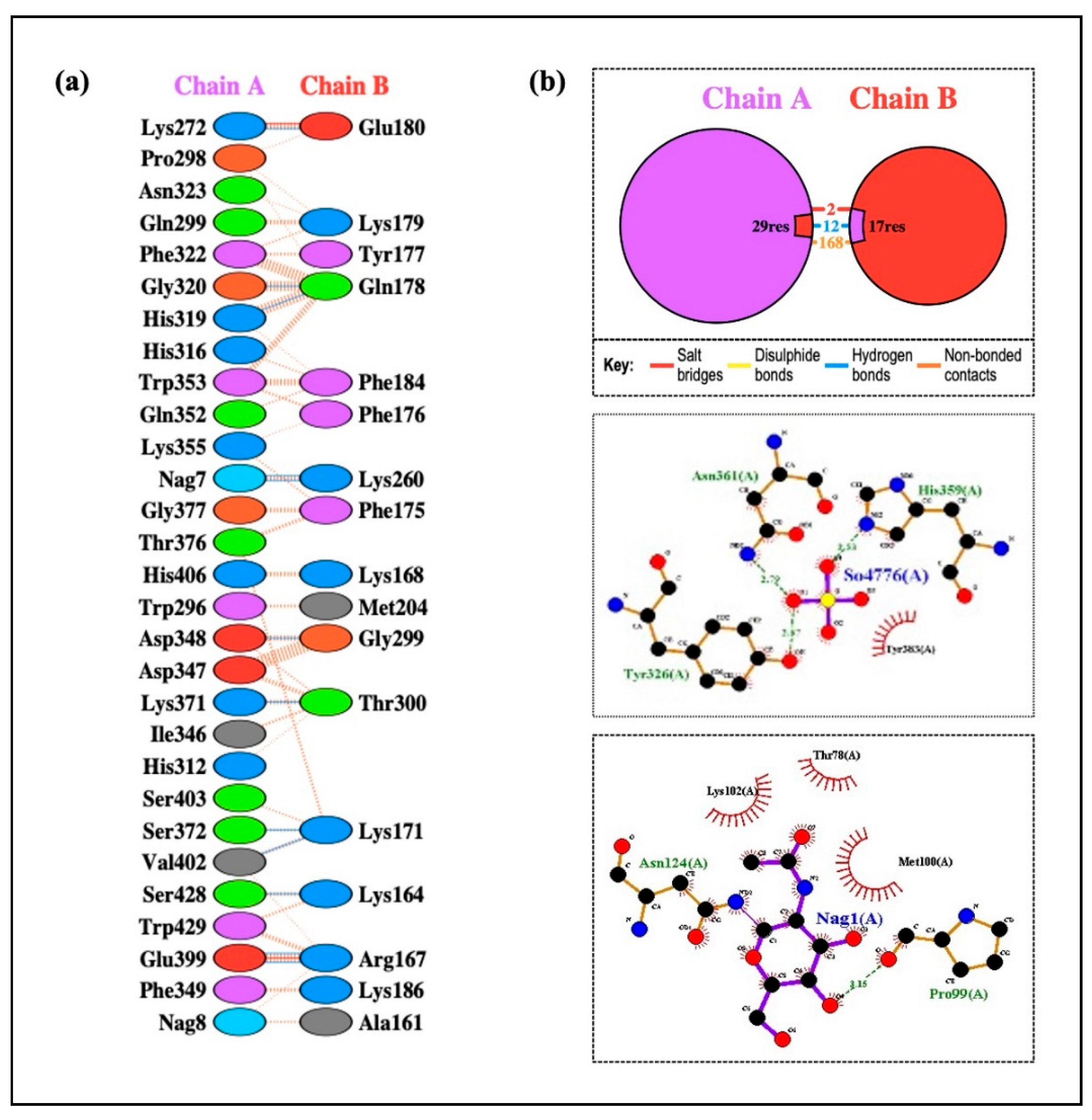
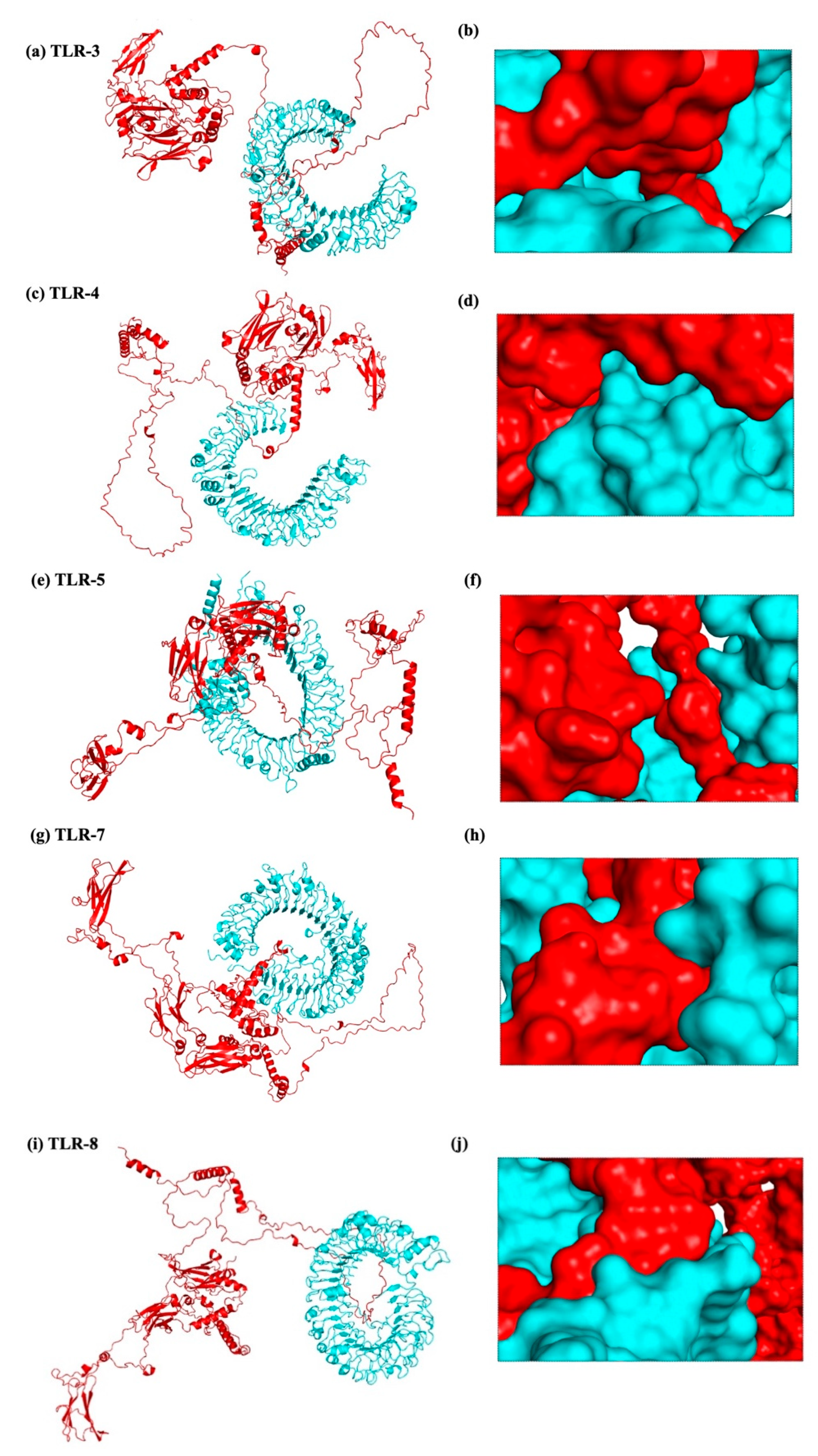
According to the methodology section, we used an in silico experiment to determine the interaction between the vaccine construct and a number of TLRs in our investigation. Low energy values and a high binding potential between the vaccine construct and the TLRs were found in the results. Different patterns of contacts between interchain residues in the TLR-vaccine complexes were revealed by contact map analysis. Interestingly, there were more interchain interactions between different protein domains in the TLR2-vaccine complex. Three distinct sites on the contact map exposed interchain interactions between various domains in the TLR3 and TLR5 vaccine complexes. In the TLR4-vaccine complex, fewer interchain interactions were seen, indicating a weaker TLR-vaccine chain binding. Whereas the TLR8-vaccine complex exposed contact between several vaccine chain residues and the TLR chain, the TLR7-vaccine complex only showed interactions between residues that were another. These outcomes propose that the developed vaccine designs may activate downstream signaling pathways and engage TLRs, resulting in the generation of pro-inflammatory cytokines that may take part in the fight against HIV-1 infection. Both humoral and cellular immune feedback should be elicited by a strong HIV-1 therapeutic vaccine. Inflammatory gene transcription is triggered by a TRIF-dependent signaling cascade that is initiated by TLR-3 activation [
52]. This activation promotes the development of DCs into potent immunostimulatory cells capable of cross-priming T cells. TLR-3 signaling is required for DC activation in HIV-1 infection [
53]. Conversely, TLR-4 stimulation causes IL-6 to be produced, which has the potential to reactivate latent HIV-1 [
54]. TLR-10 has also been connected to increased HIV-1 infection [
55].
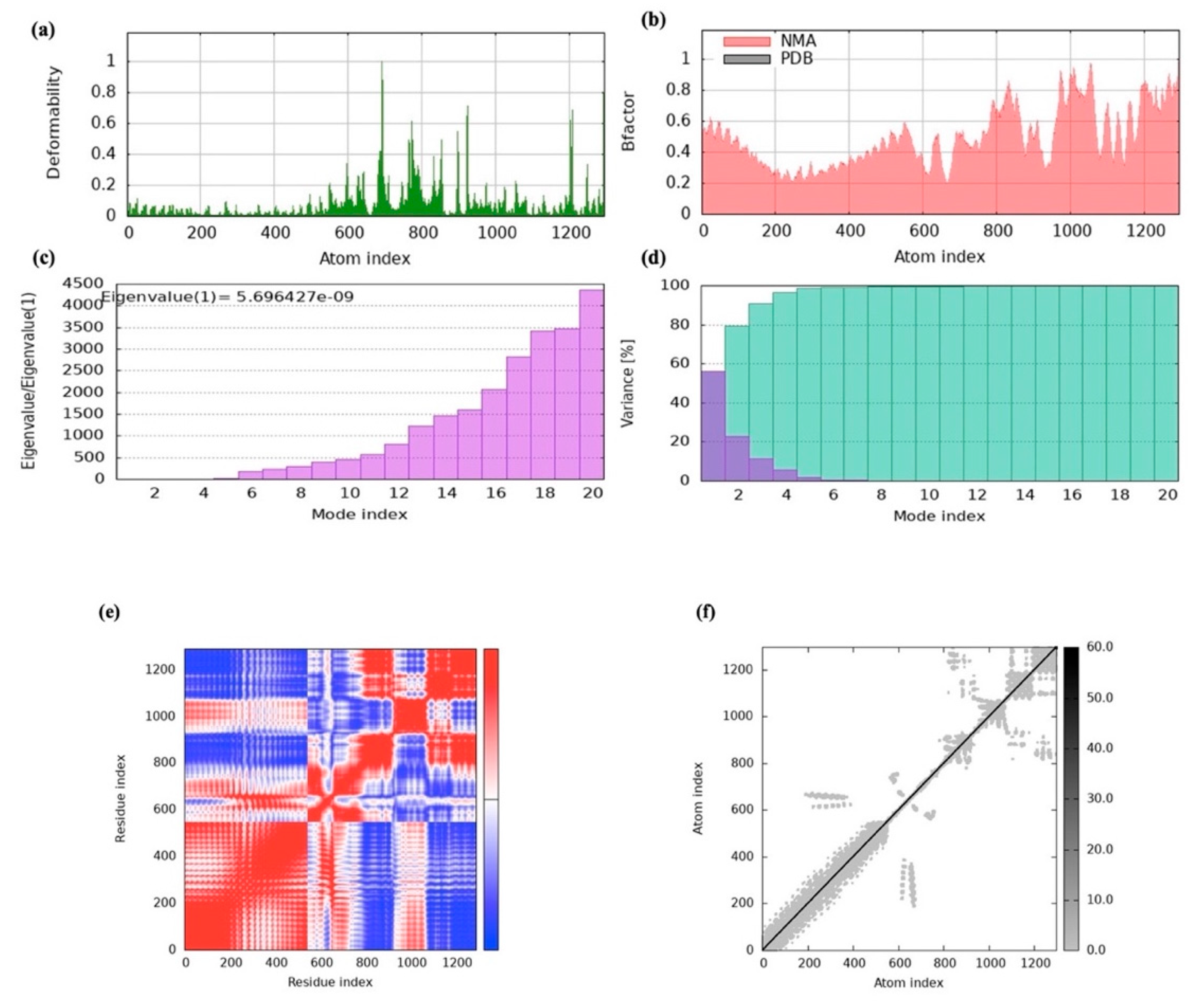
To evaluate the vaccine-receptor complex’s equilibrium and binding efficiency, MD simulations were used. The pathway by which the vaccine and TLRs interact over time was shown by these simulations. The findings indicated a high binding affinity since the vaccine design efficiently occupied the TLRs with little energy. Furthermore, iMODS normal-mode analysis exposed that the vaccine complex had high eigenvalues, which suggested a lower degree of deformability. This implies that the vaccine’s structure is comparatively inflexible and impervious to fluctuation in shape. This conclusion was corroborated by the deformability graph, which showed that the vaccine is probably going to keep its stability and structural integrity. Furthermore, useful details regarding the stability and conformational changes in the physiological system were provided by atomistic simulations. By analyzing multiple indicators derived from the MD simulation trajectory data, we were able to examine the stability and conformational changes in the vaccine–receptor complex in greater detail [
56]. Our comprehension of the vaccine construct’s dynamic behavior and structural characteristics in combination with TLRs is improved by these simulations.
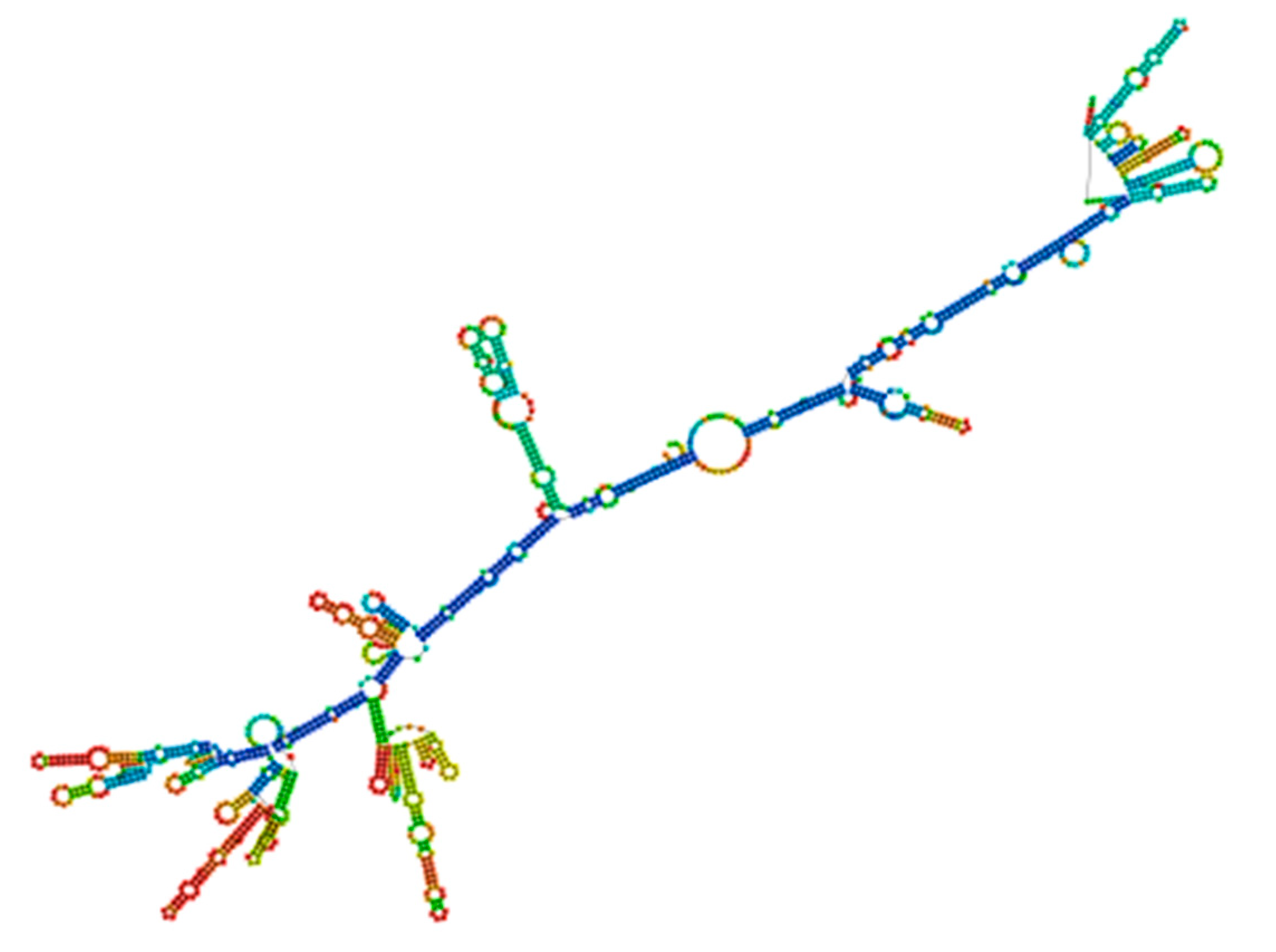
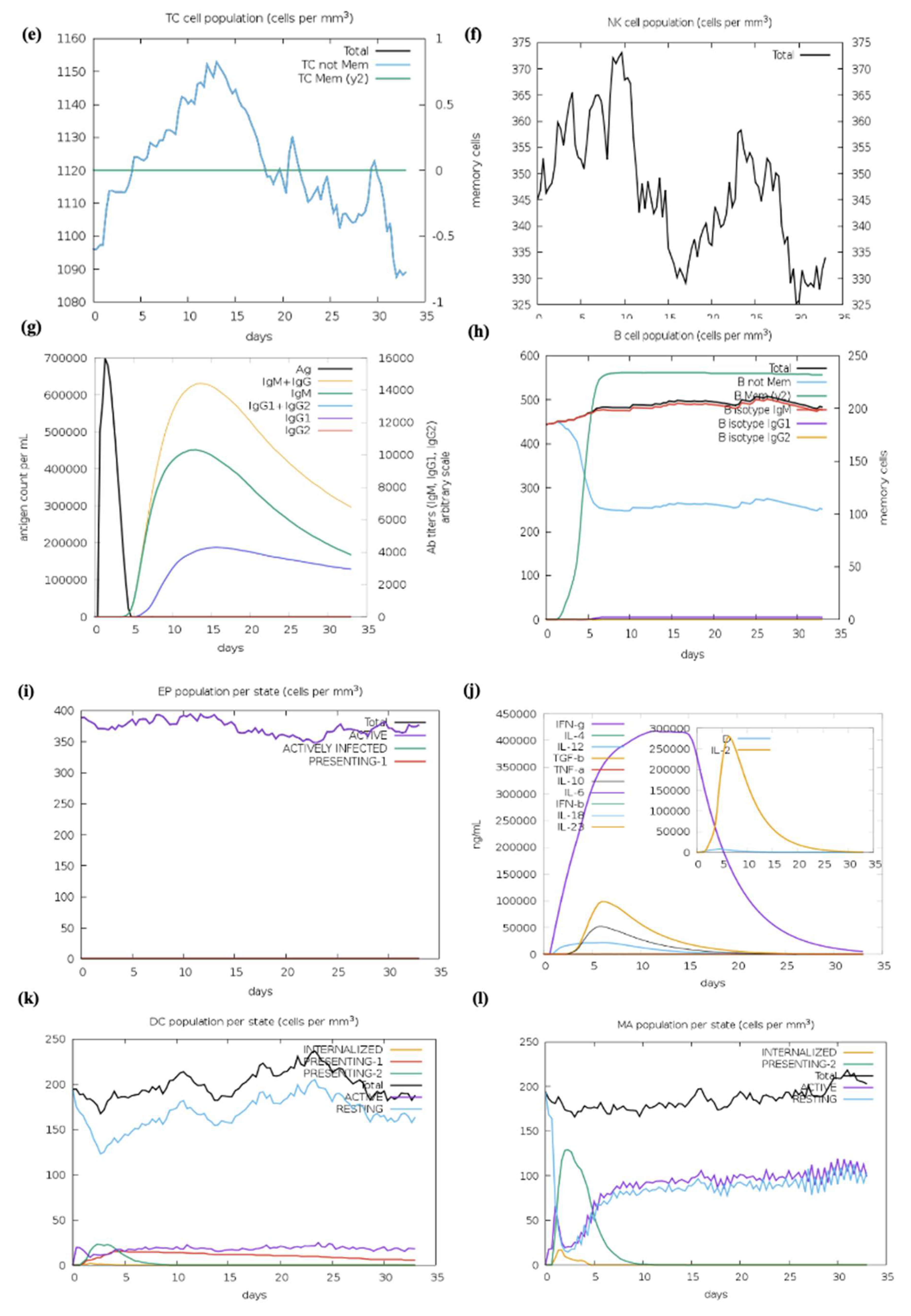
We optimized the vaccine mRNA using the JCAT tool and the E. coli K-12 strain as the cell culture system. This platform was used to evaluate the translation efficiency of the final vaccine design. The outcomes showed a CAI of 0.95 and a GC content of 54%, both of which are considered optimal as a CAI score greater than 0.60 and a GC percentage of 30% to 70% are generally regarded as desirable. The pET plasmid’s codon optimization process involved the use of NcoI and XhoI restriction enzymes to cleave the N- and C-termini, respectively. The insertion of a HisTag into the plasmid enables efficient post-translational purification of the vaccine. Moreover, the secondary structure of the final vaccine construct was evaluated using the RNAfold software (version 2.1.0), which revealed a minimum free energy score of −806.89 kcal/mol, suggesting improved stability for the vaccine in the body. For the vaccine to work effectively, its stability is essential. However, promising results were obtained from in silico evaluation of the host immune feedback to the developed co-epitope vaccine. Both B and T cell activation were anticipated by the C-ImmSim algorithm, resulting in immunological memory that lasts a lifetime. Strong protection against the virus is indicated by the induction of IgG1 and IgG2 Abs, which suggests the activation of Th1 and Th2 responses against HIV-1 Ags. Furthermore, the ICM server data demonstrated that a rise in CTL levels was correlated with an increase in Th1 cell numbers. The vaccine design which contains immunogenic B and T cell epitopes from gp120-Nef, appears to have significant potential for developing a co-epitope vaccine against HIV-1 according to these results taken together. However, additional in vitro and in vivo research is necessary to completely assess our vaccine potential.
4. Materials and Methods
4.1. Exploration of Protein Sequences
The GenBank database server was used for the sequence retrieval for the identification of gp120 and Nef proteins associated with HIV-1. The STRAP program was utilized to conduct multiple sequence alignment to analyze different variable and conserved regions within the gp120 and Nef proteins. A consensus sequence was then generated using the MUSCLE server.
Table 1 mentions all the computational tools used in this study.
4.2. Epitope Prediction for B Cells
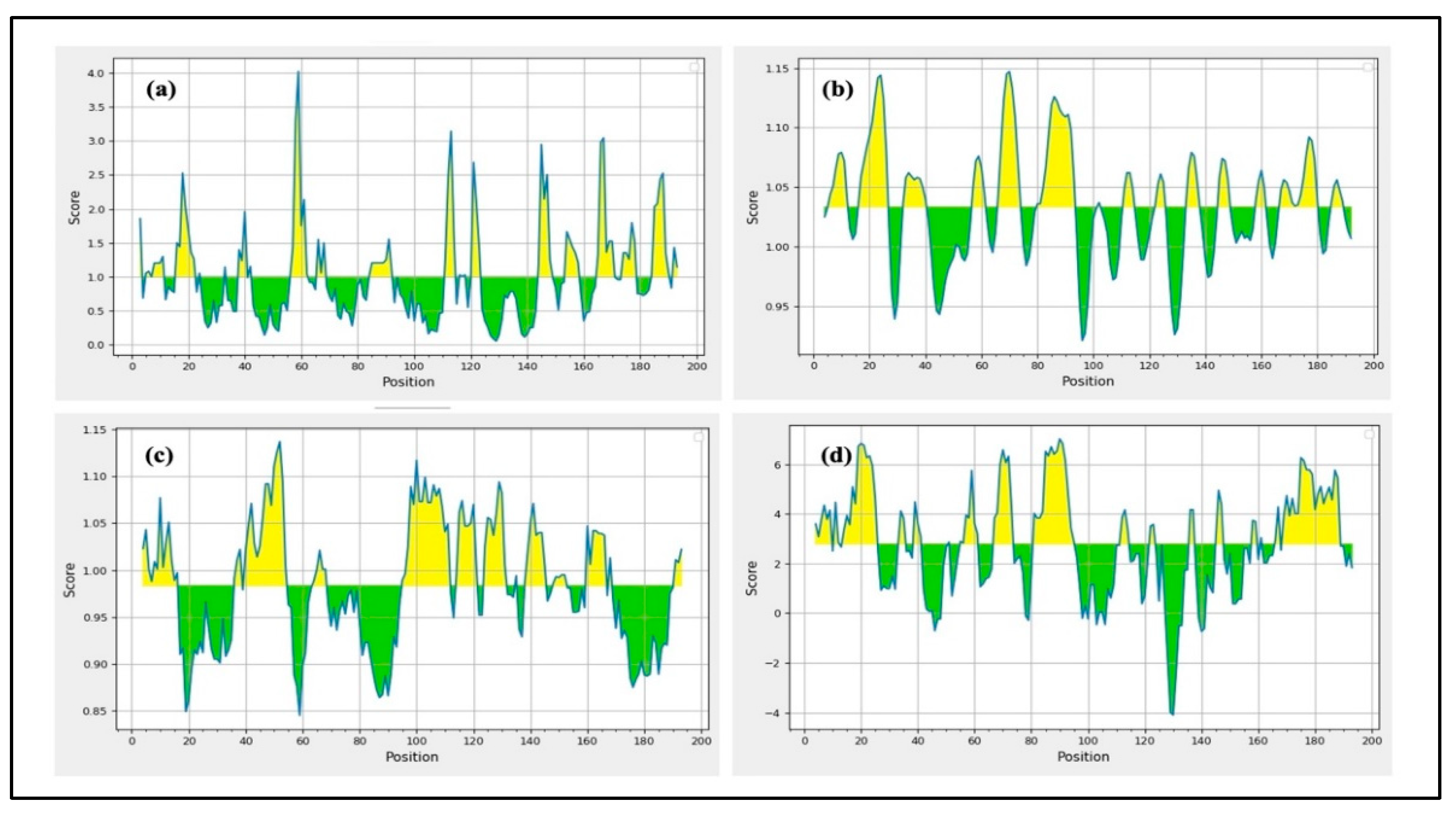
Two computational tools, such as BepiPred-2.0 and the BepiPred Linear Epitope Prediction tool accessible through the IEDB resource, were used in this investigation to assess linear B cell epitopes within gp120 and Nef proteins. During analysis, both tools were utilized with their default threshold values [
57,
58]. The random forest machine learning technique used by BepiPred-2.0 was trained on structural data from known Ab-antigen complexes. This technique combines sequence-based prediction improved by 3D structural information with an extensive dataset of linear epitopes from IEDB.
To increase confidence in the predicted epitopes, the results were further endorsed by employing the iBCE-EL platform. iBCE-EL is an online tool that identifies linear B cell epitopes by combining various input features such as dipeptide composition, physicochemical properties, and machine learning algorithms, including extremely randomized trees and gradient boosting methods. It generates both classification and probability scores for each peptide sequence submitted [
59].
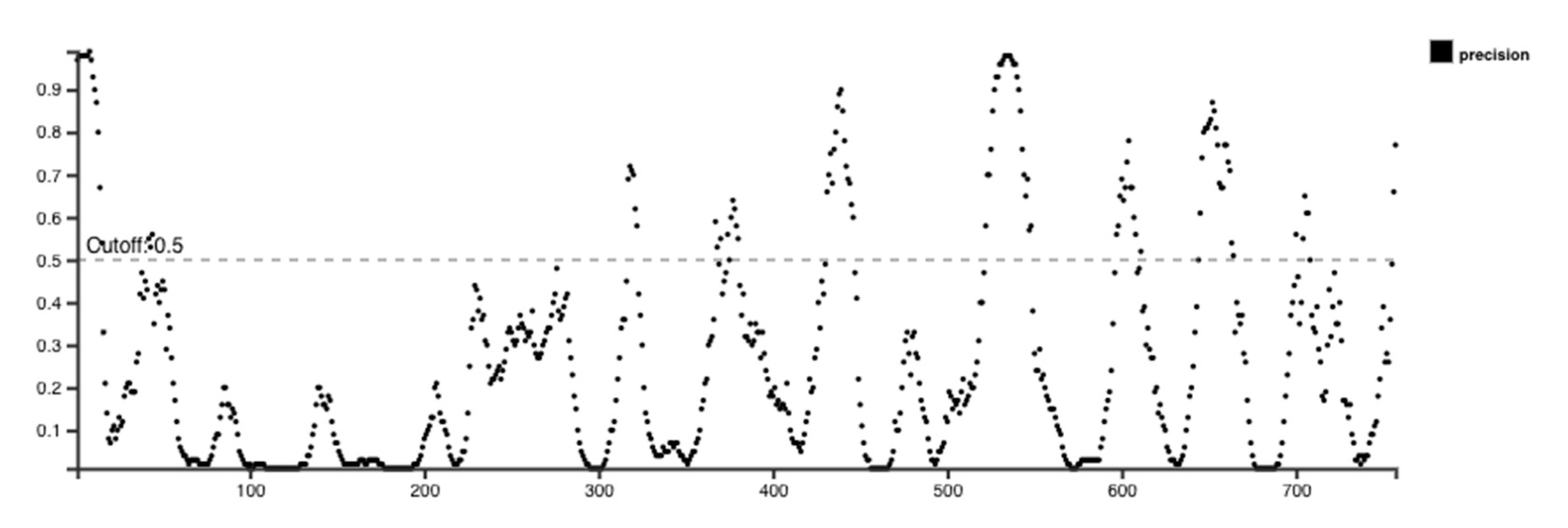
B cell epitopes are in large numbers and belong to the discontinuous category, which reveals that amino acid residues in these epitopes are far distant in their linear sequence yet physically nearby in the protein’s 3D structure. The ElliPro program was assessed to anticipate conformational type epitopes in the final vaccine design 3D model. The ElliPro analyzes the protein’s structural geometry and has demonstrated high accuracy with an AUC score of 0.732, ranking it among the most effective tools for identifying conformational Ab-binding sites.
4.3. CTL Epitope Prediction
The IEBD analysis library assembled the NN-align platform was used to analyze the epitopes to find MHC-I-binding alleles, including both common and uncommon variants. Peptide lengths ranging from 8 to 12 residues and an IC50 threshold value of less than 100 were among the characteristics used in the analysis for MHC-I-binding potential. IC50 values in the peptides found less than 50 nM were regarded as having strong binding affinity, while moderate affinity was considered among those below 500 nM, and weak affinity was considered among those below 5000 nM. Consequently, a stronger binding affinity is indicated by a smaller IC50. Furthermore, several characteristics of the selected epitopes, such as processing scores, proteasomal cleavage locations, TAP values, and MHC-I-binding tendency, were predicted using the IEDB server. The NN-align algorithm was employed for the determination of all these parameters.
4.4. Evaluation of MHC-I Immunogenicity
The IEDB MHC-I immunogenicity prediction tool was used to assess MHC peptides that might be immunogenic within the infected host cell. The server’s default parameters were employed to evaluate the chosen epitopes. In order to proceed with a further experimental plan, only highly immunogenic peptides were selected from the predicted epitope list.
4.5. HTL Epitope
HTLs are key players in the immune feedback function by recognizing the Ags and modulating the immune system to activate B cells as well as CTLs. MHC-II T cell epitopes were evaluated using the Immune Epitope Database website, and percentile rank and MHC binding affinity parameters were utilized to predict epitopes. The IEDB-recommended conjunctional approach used techniques like NetMHCIIpan, Sturniolo, CombLib, and SMM-align to identify HTL epitopes [
59]. The selection of final epitopes was considered on their IC
50 score lower than 500 nM, their ability to elicit IFN-γ release, their propensity to show emerging characteristics, and their binding scores (lower scores indicate stronger binding affinity).
4.6. Selection of Promising Epitopes
Based on knowledge gained from earlier studies, the thresholds for epitope selection were established. Finding a compromise between assuring specificity and optimizing accuracy to locate immunogenic epitopes was the aim. The selection of the final epitopes was based on different numbers of immunogenic tests as well as their physicochemical properties. This dataset is primarily focused on HIV-1 since it offers a thorough compilation of immunological and non-immunogenic epitopes after a meta-analysis of studies with an emphasis on MHC-I epitopes.
4.7. Proteasomal Cleavage/TAP Transport
The process of predicting protease cleavage, as well as transporter-associated antigen processing (TAP), was performed via the NetCTL 1.2 method. Through a series of algorithms, the computer evaluates the efficacy of MHC-I binding, TAP transport, and proteasomal C-terminal cleavage. The predictions were performed using the default parameters with the TAP transport ability set at 0.05 in weight and the C-terminal cleavage assigned a weight of 0.15.
4.8. Prediction of IFN-γ Cytokine Inducer
Epitopes with the capability of eliciting the cytokine IFN-γ were predicted via the online server. A dominant Th1 immune response cytokine for antiviral activity in both innate and adaptive immune systems is IFN-γ. Amongst others, this method predicts MHC-II binding peptides which can boost the production of IFN-γ by CD4+ T cells. The server parameters were then changed to select the SVM-based method, so that IFN-γ production is favored over the production of other cytokines.
4.9. Population Coverage Assessment
The coverage of particular epitopes in the population was ascertained using the population coverage tool available on the IEDB website. The world’s population was evaluated using this tool. The optimal epitopes for different human leukocyte antigen (HLA) bindings can be chosen with the use of population coverage estimation. In order to offset the burden of MHC limitation in T cell responses, different ethnic groups may carry epitopes with higher HLA binding affinities; hence, those groups may have different frequencies. In this study, the HLA binding alleles for MHC-I and MHC-II were assessed for HLA binding using the gp120 with Nef proteins. Additionally, the peptide sequences from several HIV-1 subtypes in group M were examined using the IEDB epitope conservation analysis program in order to find conserved cross-reactive epitopes.
4.10. Antigenicity, Allergenicity, and Solubility Prediction
The antigenicity of the finished vaccine construct and its components was evaluated using the AntigenPro and VaxiJen 2.0 servers. VaxiJen depends on the primary amino acid characteristics and the auto cross-covariance (ACC) transformation, which converts protein sequences to fixed vectors [
60]. AntigenPro, in contrast, is a predictor of protein antigenicity pathogen-independent sequence [
61]. To evaluate the allergenicity of the vaccine and its constituent parts, AllergenFP 1.0 and AllerTOP 2.0 were applied. AllergenFP classifies allergens using a binary classifier and blends non-allergic proteins to the set delimited by five E-descriptors. The ACC transformation translates these descriptors to fixed vectors [
62]. E-descriptors and the ACC transformation are also utilized by AllerTOP. The Protein-Sol and SolPro tools assessed the solubility of the components of the vaccine. SolPro uses support vector machines (SVM) to predict protein solubility with an accuracy of approximately 74% based on tenfold cross-validation. Protein-Sol, however, predicts
E. coli soluble proteins, drawing information from a system devoid of cells [
63].
4.11. Toxicity and Physicochemical Characteristics Assessment
The final vaccine construct toxicity and its ingredients were analyzed using the ToxinPred program. ToxinPred uses an SVM model to categorize proteins according to their characteristics as either hazardous or non-toxic [
64]. Furthermore, the vaccine and its constituent physicochemical attributes were assessed with the ExPASy ProtParam tool, which analyzes the charge, hydropathicity, half-life, instability index, theoretical isoelectric point (pI), and molecular weight [
65,
66].
4.12. Epitope Hydropathy Analysis
As HIV-1 vaccines need to be displayed on the protein surface for immune response activation, all epitopes must be hydrophilic. Evaluating the hydrophobicity of epitopes required for computing the GRAVY (Grand Average of hydropathy) score, which was performed using the ProtParam service, was one of the steps. Each protein’s total hydropathy values are added up, and the result is divided by the total number of amino acid residues to determine the GRAVY score. A hydrophobic protein is indicated by a positive GRAVY score, whereas the existence of hydrophilic areas is shown by a negative score.
4.13. Cluster Analysis of MHC-Restricted Alleles
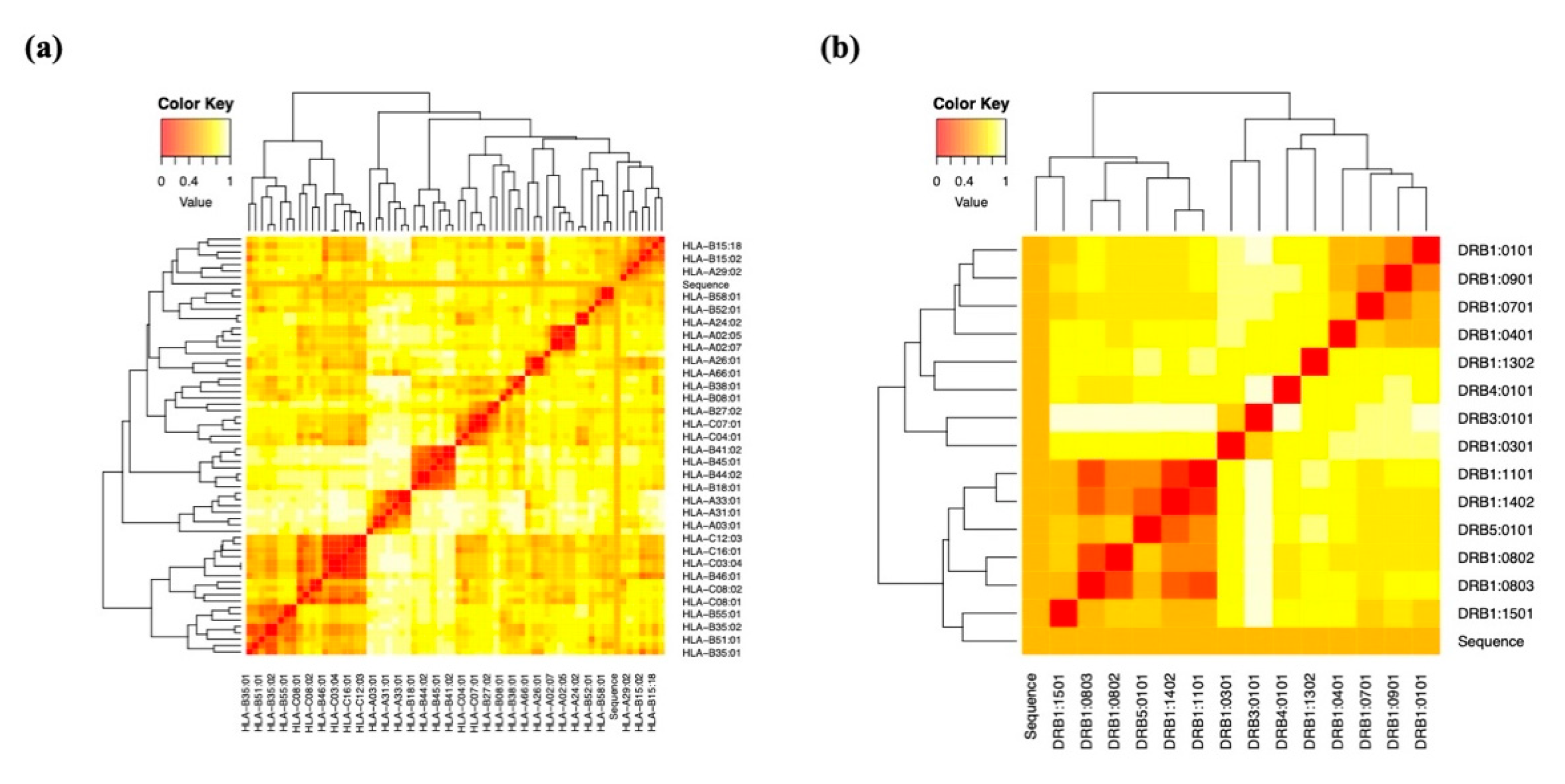
We developed a set of epitopes for presentation on MHC class I/II molecules using the online IEDB. We also used the MHCcluster v2.0 server to further validate these predictions, which revealed more proof. This site uses a static heat map to illustrate the connection between peptides and HLA function [
67].
4.14. Design of Co-Epitope Subunit Vaccine
The most promising epitopes were carefully analyzed, which included enhanced antigenic potential, non-toxicity, non-allergenicity, no homology to the human proteome, and conservation among specific strains. HTL epitopes that could elicit cytokine responses were selected for vaccine formulation after their capacity to trigger cytokine production was assessed. Potential epitopes were preferred for the construction of a co-epitope final vaccine design that targets HIV-1 after fulfilling the requirements and passing the aforementioned analysis. The final potential epitopes from B cells, HTL, and CTL included in the vaccine design and conjugated with the SBP sequence using various linkers at the C-terminal. Phage display screening was used in our lab to identify the SBP. A human liver cDNA expression library was screened against HBsAg to identify this 344-amino-acid protein. A GGGGS linker connected the SBP peptide to the epitopes, and its conjugation boosted the immunological response that the vaccine elicited.
4.15. Evaluation of the Vaccine Structure Physicochemical Characteristics
Assuring high antigenicity is essential to vaccine production because it guarantees that the vaccine will be recognized by the host immune system, following immune cell stimulation and subsequent immunological responses [
68]. Allergenicity testing is necessary to avoid allergic reactions in the host. Additionally, a deeper analysis of the vaccine physicochemical characteristics is important to guarantee its efficacy and safety. The VaxiJen v2.0 tool, with a highly reliable threshold of 0.4, was used for antigenicity evaluation of the final vaccine sequence. AlgPred and AllerTop v2.0 were also used to assess the vaccine’s allergenicity. AlgPred compares frequent epitopes in protein regions using different approaches to predict antigenic and possible allergenic potential. After an allergenicity screen was performed using the MEME/MAST motif prediction tool from AllerTop v2.0, physicochemical properties like pI value, half-life, GRAVY index, etc., were predicted using the ProtParam tool. Conversely, the SCRATCH protein predictors SOLpro tool was used to forecast the vaccine construct solubility while maintaining the default parameters. The Protein-Sol tool was used to cross-verify the results. Solubility is crucial to ensuring that the vaccine remains soluble when administered to the host. If a vaccine collects into insoluble particles, it may lose its effectiveness. Protein-Sol predicts solubility using a fast sequence-based algorithm, while SolPro uses an SVM-based method. These resources offer trustworthy approximations of protein sequence solubility.
4.16. Prediction of Vaccine Secondary and Tertiary Structures
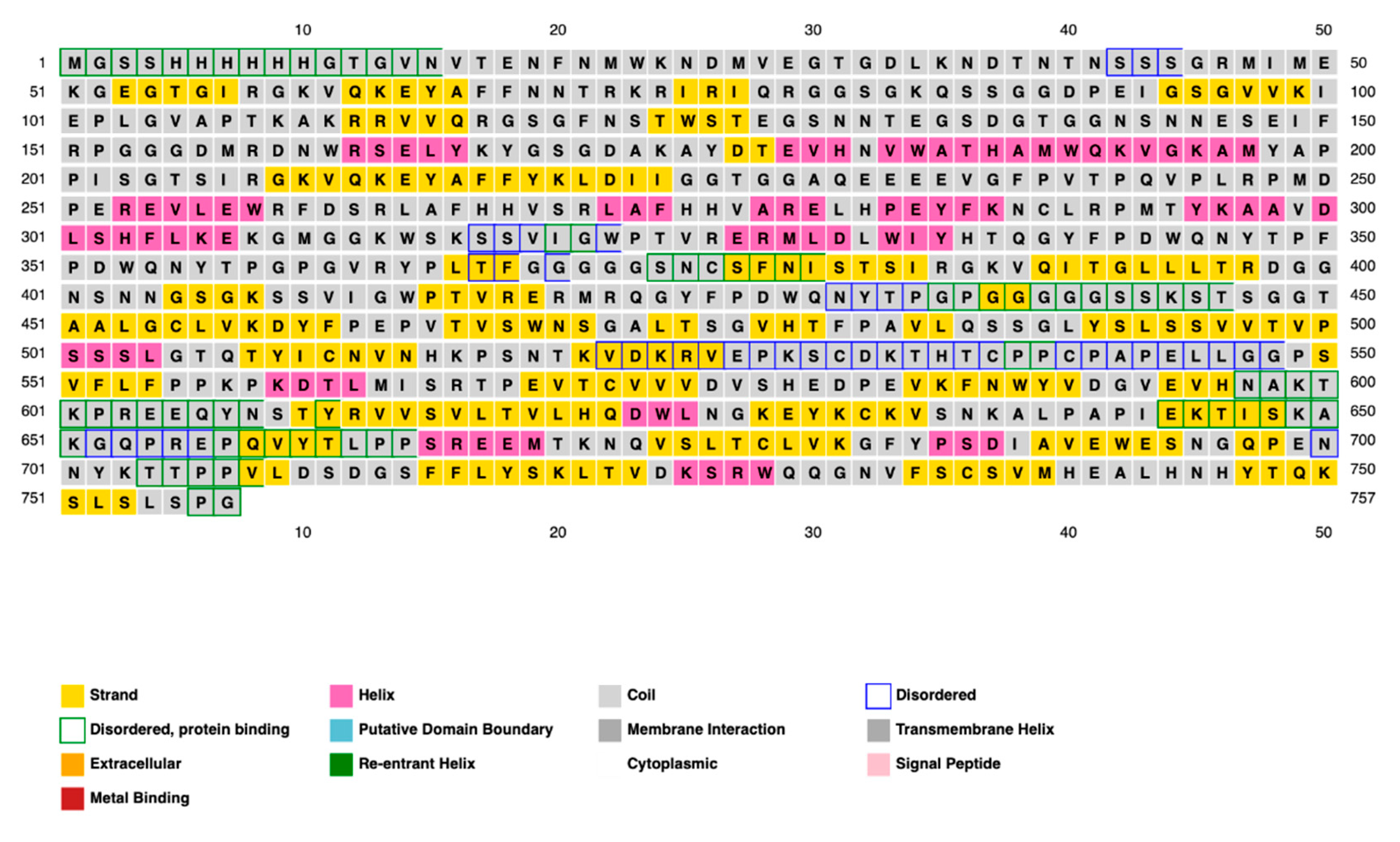
The structural conformation of a protein largely determines its function; therefore, both secondary and tertiary structures should be carefully considered in vaccine design. Following antigenicity and allergenicity testing, the online PRISPRED application was sectionalized for secondary structure analysis of the vaccine while maintaining all default values. Secondary structural features, such as folds, helices, transmembrane topology, and domain recognition, are analyzed using PRISPRED [
68]. To provide an additional layer of explanation for these findings, additional 2D structural studies were performed utilizing the SOPMA and Phyre2 servers for comparison and validation. We constructed a 3D model for the vaccine using the RoseTTAFold (BOINC version 7.6.22) program. RoseTTAFold combines a protein sequence pattern, amino acid interactions between proteins, and possible 3D structures into a neural network-based “three-track” algorithm. This thorough approach makes it possible to comprehend a protein’s folded structure and chemical makeup from all angles. RoseTTAFold has shown remarkable accuracy levels that are on par with AlphaFold from DeepMind. The technology provides a comprehensive understanding of a protein’s structure by allowing information to move between one-, two-, and 3D levels [
69].
4.17. Validation and Improvement of the 3D Vaccine Structure
When designing vaccines, computer-based techniques are essential, especially when there is a lack of experimental data. In biomedical applications, the development of 3D models is a crucial first step, but it is not always enough to guarantee accuracy; experimental data is necessary for confirmation. It is feasible to enhance the quality of initially created models by using 3D structure refinement, which fixes local mistakes while maintaining the important structure of the vaccine. The GalaxyRefine tool was used to enhance the vaccine’s 3D structure. This server enhances structural quality through dynamic simulation and refinement using a CASP10-tested technique [
70]. It is still difficult to refine 3D vaccine models consistently and precisely, particularly when working with high-resolution data. The PROCHECK server was used to develop Ramachandran plots in order to verify the vaccine design’s accuracy [
71]. With the van der Waals radius of side chains taken into account, these figures evaluate the permitted and prohibited dihedral angles (psi and phi) of the amino acid organizations. Furthermore, the ProSA-web application was utilized, which computes a Z-score for protein structure validation using statistical techniques. We could evaluate the consistency and quality of the vaccine’s design by comparing the Z-score, which represents the standard of the protein composition and with the Z-scores from the PDB database for experimentally determined protein structures [
72].
4.18. Vaccine Disulfide Engineering Evaluation
Vaccine design undoubtedly encompasses the enhancement of the stability of vaccines by way of numerous molecular interactions. Disulphide by Design 2 v12.2 was used to analyze disulfide regions in the vaccine construct. This tool works to locate expected sites within a protein structure where disulfide bonds can be formed. Based on the 5th Cbeta-Cbeta distance, the server computationally predicts protein structures to precisely determine the χ3 torsion angle and uses this as a geometric framework constructed from naturally occurring disulfide bonds [
73].
4.19. Vaccine Construct Docking with TLRs
Drug design and the study of protein–protein interactions both strongly rely on docking. It entails using protein-protein docking to combine distinct protein structures in order to anticipate the structure of a complex. The final vaccine structure was docked with multiple TLRs in this study, including TLR-2, 3, 4, 5, and 8 (PDB IDs: 2Z7X, 1ZIW, 3FXI, 3J0A, and 3W3G), to perform protein-protein docking. PyMOL v2.3.4 was used to increase the 3D architectures of the vaccine complex and TLRs for energy minimization. Several online docking technologies were employed to evaluate vaccine-receptor interactions in order to enhance prediction quality. Before docking, water molecules were eliminated from the PDB structures of HIV-1 gp120-Nef, TLRs, and the vaccine. During the docking study, the Chimera v1.13.1 tool was employed to assess the 3D configuration of the formulated vaccine structure. The HADDOCK server was used for the assessment of bonding areas between the vaccine construct and TLR2. The HADDOCK study chose the best-ranked complexes based on the lowest mean RMSD and the lowest intermolecular binding energy, which represent the complete HIV-1 gp120-Nef-TLR interaction.
ClusPro 2.0 was used to sort the clusters of docked complexes in the second docking phase with an emphasis on the lowest and center energy values [
74]. The default docking parameters were used for the MM-GBSA (Molecular Mechanics/Generalized Born Surface Area) study. MD simulation was employed to predict the reciprocity between the TLRs and the vaccine construct in greater detail following successful docking. The docked complexes were visualized using the Discovery Studio Visualizer. Furthermore, the protein–protein interactions within the complexes were examined using PDBsum [
75]. The LigPlot
+ v.2.2.4 software was used to evaluate H-band formation. The CB-Dock2 web server was utilized to identify potential ligand-binding pockets in the designed protein structure. The server detected and ranked cavities based on their volume and spatial properties. CB-Dock2 provided detailed information on the volume, coordinates (x, y, z), and size of each pocket, facilitating the selection of suitable binding sites for subsequent molecular docking studies.
4.20. Vaccine-Receptor Complex MD Simulation
MD simulations with iMOD were used to explore transition paths between two homologous structures. The server performs normal mode analysis (NMA) to calculate essential protein stability characteristics through its internal computation process. Protein stability can be assessed through key components, which include the elastic network model together with the covariance matrix and eigenvalues, as well as B-factor values and the main-chain deformability plot. This inquiry provides a better understanding of protein stability together with its structural dynamics [
76].
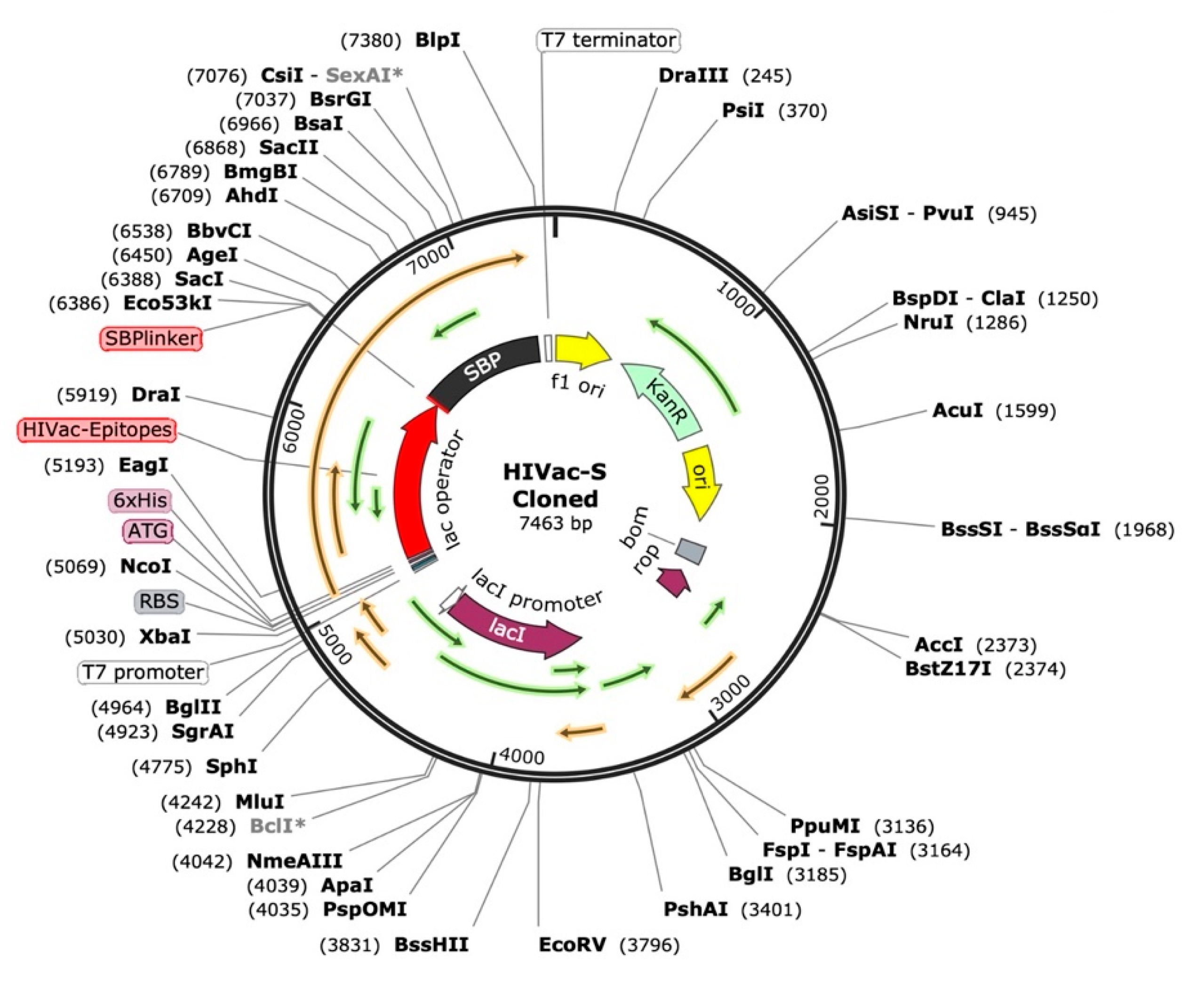
4.21. Vaccine Construct Codon Optimization and In Silico Cloning
For the peptide vaccine transcription to produce an appropriate DNA sequence, the targeted protein was reverse-translated. The target protein’s efficient expression in the selected organism was subsequently ensured by optimizing the DNA sequence for codon use. The Java Codon Adaptation Tool conducted the process of codon adaptation for the recommended vaccination protein through its service [
77]. The selected host organism for this study consists of the
E. coli K-12 bacterial strain. During the adaptation step, we excluded prokaryotic ribosome-binding regions as well as rho-independent transcription terminators and
NcoI and
XhoI restriction enzyme cleavage regions. We employed the NcoI and XhoI restriction regions to integrate the optimized DNA sequence into the pET-28a vector conjugated with HisTag. The HisTag functioned to facilitate protein solubilization and purification during the affinity-based process [
77,
78].
4.22. Vaccine Constructs Immune Simulation
The forecasted immune response to the computationally designed vaccine construct was assessed through the C-ImmSim server. The server combines agent-based simulation with machine learning algorithms to generate immune response predictions through the position-specific scoring matrix (PSSM). The simulation ran through 1050 steps to replicate the interval of four weeks between vaccine doses. The simulation process treated both injections through a virtual eight-hour period to match the real-time sequence of events. The analysis preserved all default parameter values throughout its execution.