Brain–Bone Axis in Physiological and Pathological Conditions
Abstract
1. Introduction
2. Brain–Bone Axis and Bone Metabolism Key Players
3. Brain and Bone: Similarities in Physiological and Pathological Conditions
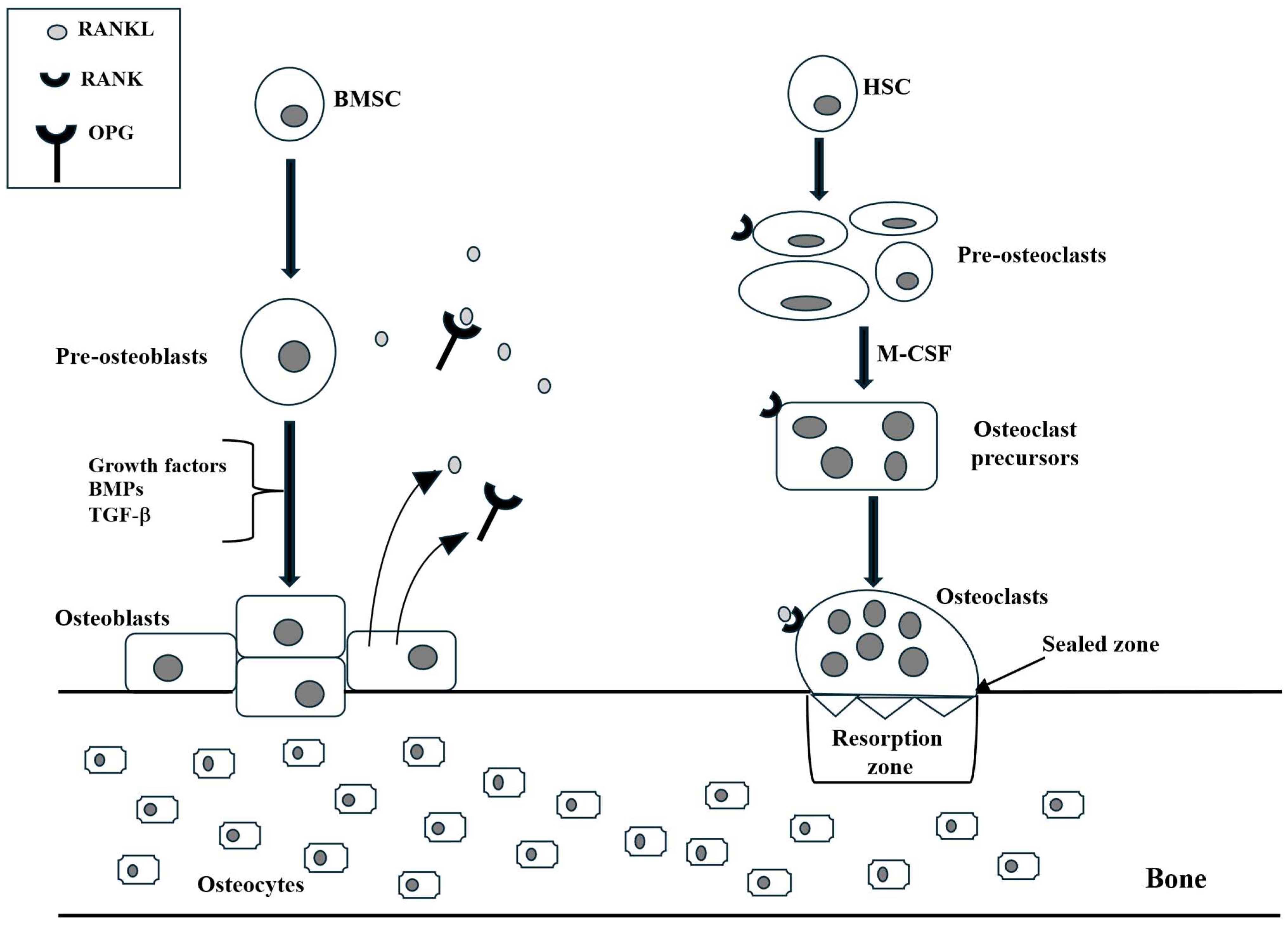
3.1. Osteoblasts
3.2. Osteoclasts
3.3. Osteocytes and Neurons Share Morphological Characteristics
4. Regulators of Bone Metabolism—Neurotransmitters and Hormones
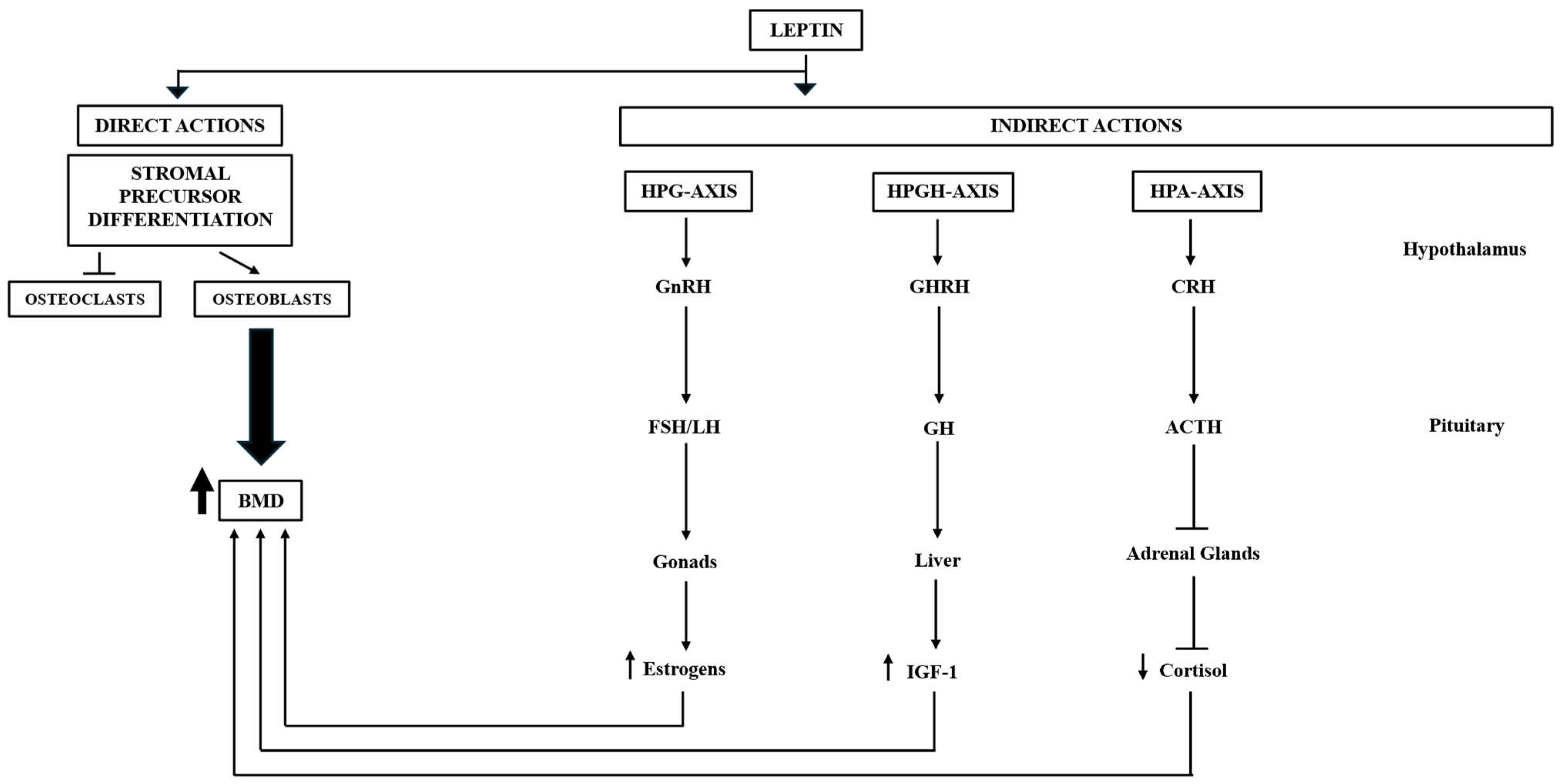
4.1. Leptin
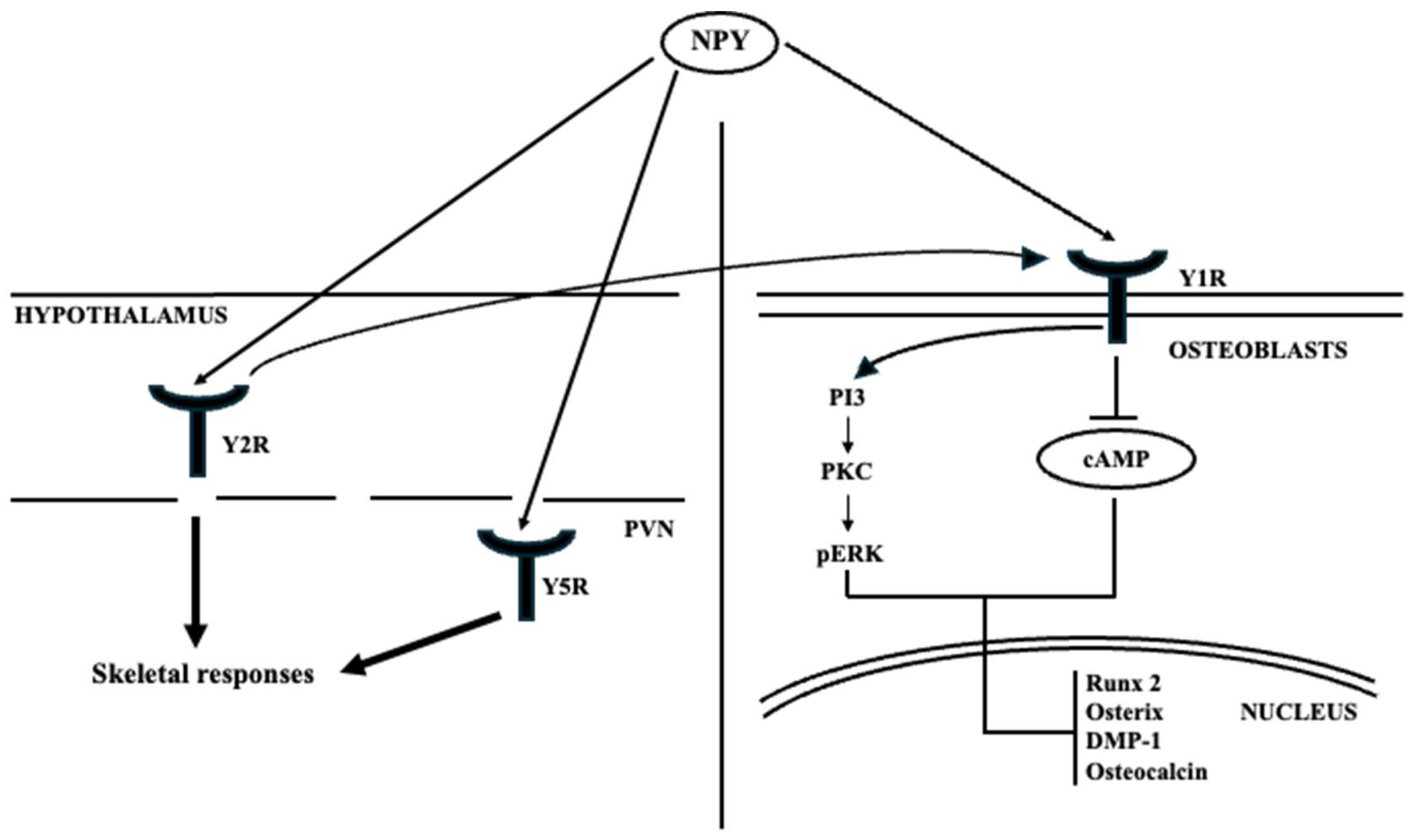
4.2. Neuropeptide Y
4.3. Semaphorins
4.4. Dopamine
4.5. Serotonin
4.6. Norepinephrine
4.7. Estrogens
4.8. Parathormone
4.9. Osteocalcin
5. Other Regulatory Mechanisms
5.1. RhoA/ROCK
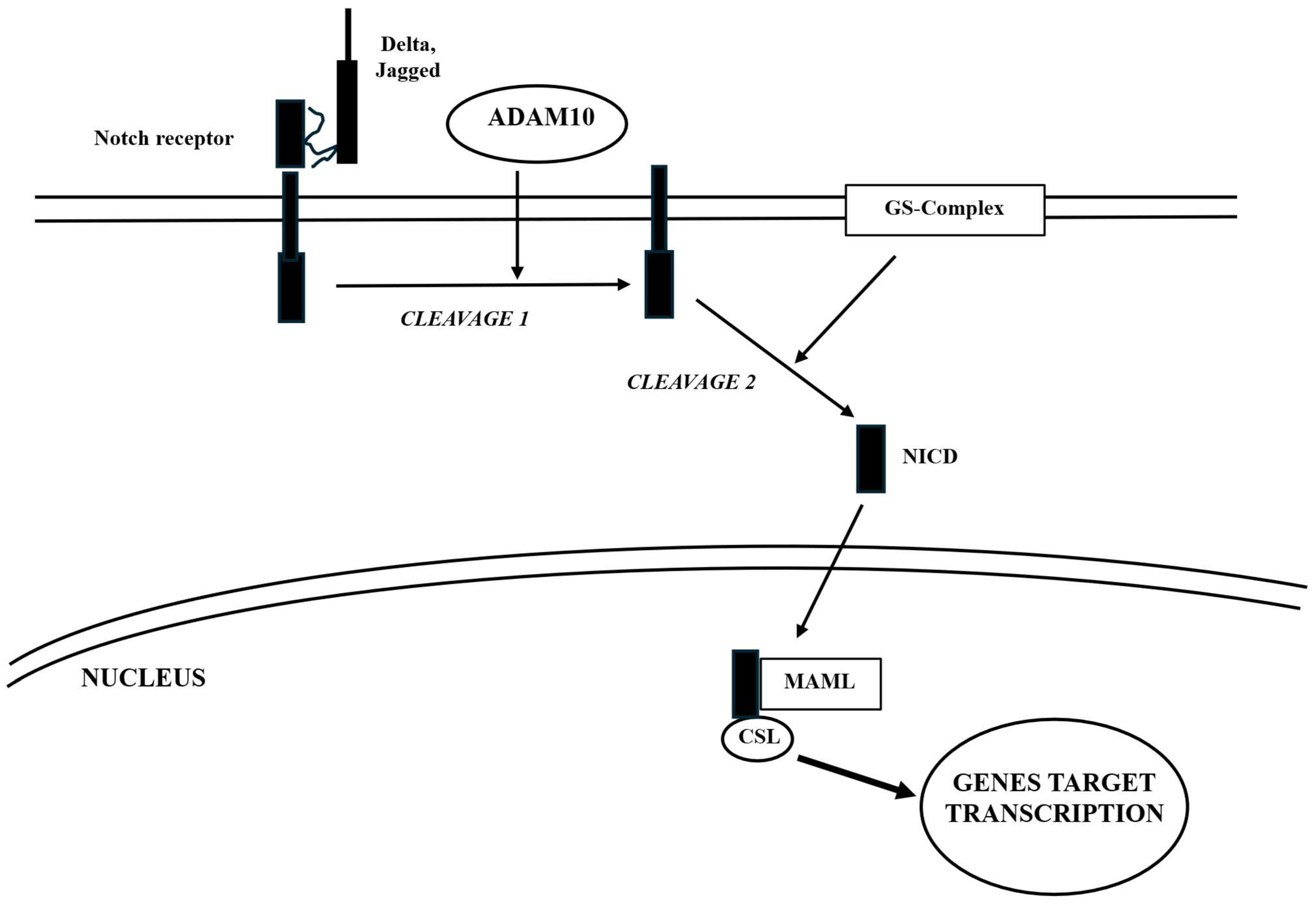
5.2. Notch
5.3. TNF-α
6. Emerging Technologies and Future Directions
7. An Emblematic Condition: Dementia
8. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Quiros-Gonzalez, I.; Yadav, V.K. Central genes, pathways and modules that regulate bone mass. Arch. Biochem. Biophys. 2014, 561, 130–136. [Google Scholar] [CrossRef]
- Dimitri, P.; Rosen, C. The Central Nervous System and Bone Metabolism: An Evolving Story. Calcif. Tissue Int. 2016, 100, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Takeda, S.; Xu, R.; Ochi, H.; Sunamura, S.; Sato, T.; Shibata, S.; Yoshida, Y.; Gu, Z.; Kimure, A.; et al. Sema3A regulates bone-mass accrual through sensory innervations. Nature 2013, 497, 490–493, Correction in Nature 2013, 500, 612. [Google Scholar] [CrossRef] [PubMed]
- Crosstalk Between the Brain and Bone—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/22783328/ (accessed on 8 April 2025).
- Zhou, R.; Guo, Q.; Xiao, Y.; Guo, Q.; Huang, Y.; Li, C.; Luo, X. Endocrine role of bone in the regulation of energy metabolism. Bone Res. 2021, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Oury, F.; Khrimian, L.; Denny, C.A.; Gardin, A.; Chamouni, A.; Goeden, N.; Huang, Y.; Lee, H.; Srnivas, P.; Gao, X.-B.; et al. Maternal and offspring pools of osteocalcin influence brain development and functions. Cell 2013, 155, 228–241. [Google Scholar] [CrossRef]
- Khrimian, L.; Obri, A.; Karsenty, G. Modulation of cognition and anxiety-like behavior by bone remodeling. Mol. Metab. 2017, 6, 1610–1615. [Google Scholar] [CrossRef]
- Sato, S.; Hanada, R.; Kimura, A.; Abe, T.; Matsumoto, T.; Iwasaki, M.; Inose, H.; Ida, T.; Mieda, M.; Takeuci, Y.; et al. Central control of bone remodeling by neuromedin U. Nat. Med. 2007, 13, 1234–1240. [Google Scholar] [CrossRef]
- Cummings, S.R.; Eastell, R. Risk and Prevention of Fracture in Patients with Major Medical Illnesses: A Mini-Review. J. Bone Miner. Res. 2016, 31, 2069–2072. [Google Scholar] [CrossRef]
- Mezuk, B.; Eaton, W.W.; Golden, S.H. Depression and osteoporosis: Epidemiology and potential mediating pathways. Osteoporos. Int. 2007, 19, 1–12. [Google Scholar] [CrossRef]
- Herrán, A.; Amado, J.A.; García-Unzueta, M.T.; Vázquez-Barquero, J.L.; Perera, L.; González-Macías, J. Increased bone remodeling in first-episode major depressive disorder. Psychosom. Med. 2000, 62, 779–782. [Google Scholar] [CrossRef]
- Shi, T.; Shen, S.; Shi, Y.; Wang, Q.; Zhang, G.; Lin, J.; Chen, J.; Bai, F.; Zhang, L.; Wang, Y.; et al. Osteocyte-derived sclerostin impairs cognitive function during ageing and Alzheimer’s disease progression. Nat. Metab. 2024, 6, 531–549. [Google Scholar] [CrossRef]
- Du, J.; Li, A.; Shi, D.; Chen, X.; Wang, Q.; Liu, Z.; Sun, K.; Guo, T. Association of APOE-ε4, Osteoarthritis, β-Amyloid, and Tau Accumulation in Primary Motor and Somatosensory Regions in Alzheimer Disease. Neurology 2023, 101, E40–E49. [Google Scholar] [CrossRef] [PubMed]
- E Llabre, J.; Gil, C.; Amatya, N.; Lagalwar, S.; Possidente, B.; Vashishth, D. Degradation of Bone Quality in a Transgenic Mouse Model of Alzheimer’s Disease. J. Bone Miner. Res. 2020, 37, 2548–2565. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Chen, M. The brain-bone axis: Unraveling the complex interplay between the central nervous system and skeletal metabolism. Eur. J. Med. Res. 2024, 29, 317. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Yoon, S.; Park, J.E.; Lee, J.I.; Kim, H.Y.; Nam, H.J.; Cho, H. MeCP2 dysfunction prevents proper BMP signaling and neural progenitor expansion in brain organoid. Ann. Clin. Transl. Neurol. 2023, 10, 1170–1185. [Google Scholar] [CrossRef]
- Kim, S.U. Human neural stem cells genetically modified for brain repair in neurological disorders. Neuropathology 2004, 24, 159–171. [Google Scholar] [CrossRef]
- Banfi, G.; Lombardi, G.; Colombini, A.; Lippi, G. Bone metabolism markers in sports medicine. Sports Med. 2010, 40, 697–714. [Google Scholar] [CrossRef]
- Barbosa-Nuñez, J.A.; Haro-González, J.N.; García-Márquez, E.; Espinosa-Andrews, H.; Padilla-Camberos, E.; Herrera-Rodríguez, S.E. Proteins and peptides responsible for bone remodeling. Differentiation 2025, 144, 100872. [Google Scholar] [CrossRef]
- Wróbel, E.; Wojdasiewicz, P.; Mikulska, A.; Szukiewicz, D. β-Catenin: A Key Molecule in Osteoblast Differentiation. Biomolecules 2025, 15, 1043. [Google Scholar] [CrossRef]
- Jia, L.; Piña-Crespo, J.; Li, Y. Restoring Wnt/β-catenin signaling is a promising therapeutic strategy for Alzheimer’s disease. Mol. Brain 2019, 12, 104. [Google Scholar] [CrossRef]
- Dengler-Crish, C.M.; Ball, H.C.; Lin, L.; Novak, K.M.; Cooper, L.N. Evidence of Wnt/β-catenin alterations in brain and bone of a tauopathy mouse model of Alzheimer’s disease. Neurobiol. Aging 2018, 67, 148–158. [Google Scholar] [CrossRef]
- Xue, C.; Chu, Q.; Shi, Q.; Zeng, Y.; Lu, J.; Li, L. Wnt signaling pathways in biology and disease: Mechanisms and therapeutic advances. Signal Transduct. Target. Ther. 2025, 10, 106. [Google Scholar] [CrossRef] [PubMed]
- García-Velázquez, L.; Arias, C. The emerging role of Wnt signaling dysregulation in the understanding and modification of age-associated diseases. Ageing Res. Rev. 2017, 37, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Alarcón, M.A.; Medina, M.A.; Hu, Q.; Avila, M.E.; Bustos, B.I.; Pérez-Palma, E.; Peralta, A.; Salazar, P.; Ugarte, G.D.; Reyes, A.E.; et al. A novel functional low-density lipoprotein receptor-related protein 6 gene alternative splice variant is associated with Alzheimer’s disease. Neurobiol. Aging 2013, 34, 1709.e9–1709.e18. [Google Scholar] [CrossRef] [PubMed]
- Pederson, L.; Ruan, M.; Westendorf, J.J.; Khosla, S.; Oursler, M.J. Regulation of bone formation by osteoclasts involves Wnt/BMP signaling and the chemokine sphingosine-1-phosphate. Proc. Natl. Acad. Sci. USA 2008, 105, 20764–20769. [Google Scholar] [CrossRef]
- Florencio-Silva, R.; da Silva Sasso, G.R.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. BioMed Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, J.; Liu, P.; Wang, Q.; Liu, L.; Zhao, H. The RANK/RANKL/OPG system and tumor bone metastasis: Potential mechanisms and therapeutic strategies. Front. Endocrinol. 2022, 13, 1063815. [Google Scholar] [CrossRef]
- Harper, E.; Forde, H.; Davenport, C.; Rochfort, K.D.; Smith, D.; Cummins, P.M. Vascular calcification in type-2 diabetes and cardiovascular disease: Integrative roles for OPG, RANKL and TRAIL. Vasc. Pharmacol. 2016, 82, 30–40. [Google Scholar] [CrossRef]
- Xu, Y.; An, Z.; Hou, K.; Zhou, M.; Liu, Y.; Wang, J.; Hashimoto, M.; Wei, J. ACE2 Alleviates Microglia Neuroinflammation by RANK-RANKL-OPG Axis in Parkinson’s Disease. Inflammation 2025, 1–15. [Google Scholar] [CrossRef]
- Ahern, E.; Smyth, M.J.; Dougall, W.C.; Teng, M.W.L. Roles of the RANKL-RANK axis in antitumour immunity—Implications for therapy. Nat. Rev. Clin. Oncol. 2018, 15, 676–693. [Google Scholar] [CrossRef]
- Nakagawa, N.; Kinosaki, M.; Yamaguchi, K.; Shima, N.; Yasuda, H.; Yano, K.; Morinaga, T.; Higashio, K. RANK is the essential signaling receptor for osteoclast differentiation factor in osteoclastogenesis. Biochem. Biophys. Res. Commun. 1998, 253, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Josien, R.; Li, H.-L.; Ingulli, E.; Sarma, S.; Wong, B.R.; Vologodskaia, M.; Steinman, R.M.; Chio, Y. TRANCE, a tumor necrosis factor family member, enhances the longevity and adjuvant properties of dendritic cells in vivo. J. Exp. Med. 2000, 191, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Green, E.; Choi, Y.; A Flavell, R. Pancreatic lymph node-derived CD4 (+) CD25 (+) Treg cells: Highly potent regulators of diabetes that require TRANCE-RANK signals. Immunity 2002, 16, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Hofbauer, L.C.; Heufelder, A.E. Role of receptor activator of nuclear factor-kappaB ligand and osteoprotegerin in bone cell biology. J. Mol. Med. 2001, 79, 243–253. [Google Scholar] [CrossRef]
- Liu, C.; Chen, X.; Zhi, X.; Weng, W.; Li, Q.; Li, X.; Zou, Y.; Su, J.; Hu, H.-G. Structure-based development of an osteoprotegerin-like glycopeptide that blocks RANKL/RANK interactions and reduces ovariectomy-induced bone loss in mice. Eur. J. Med. Chem. 2018, 145, 661–672. [Google Scholar] [CrossRef]
- Honma, M.; Ikebuchi, Y.; Suzuki, H. RANKL as a key figure in bridging between the bone and immune system: Its physiological functions and potential as a pharmacological target. Pharmacol. Ther. 2021, 218, 107682. [Google Scholar] [CrossRef]
- Pérez-Chacón, G.; Santamaría, P.G.; Redondo-Pedraza, J.; González-Suárez, E. RANK/RANKL Signaling Pathway in Breast Development and Cancer. Adv. Exp. Med. Biol. 2025, 1464, 309–345. [Google Scholar] [CrossRef]
- Kichev, A.; Eede, P.; Gressens, P.; Thornton, C.; Hagberg, H. Implicating Receptor Activator of NF-κB (RANK)/RANK Ligand Signalling in Microglial Responses to Toll-Like Receptor Stimuli. Dev. Neurosci. 2017, 39, 192–206. [Google Scholar] [CrossRef]
- Muzio, L.; Viotti, A.; Martino, G. Microglia in Neuroinflammation and Neurodegeneration: From Understanding to Therapy. Front. Neurosci. 2021, 15, 742065. [Google Scholar] [CrossRef]
- Yu, Z.; Ling, Z.; Lu, L.; Zhao, J.; Chen, X.; Xu, P.; Zou, X. Regulatory Roles of Bone in Neurodegenerative Diseases. Front. Aging Neurosci. 2020, 12, 610581. [Google Scholar] [CrossRef]
- Cummings, S.R.; Martin, J.S.; McClung, M.R.; Siris, E.S.; Eastell, R.; Reid, I.R.; Delmas, P.; Zoog, H.B.; Austin, M.; Wang, A.; et al. Denosumab for Prevention of Fractures in Postmenopausal Women with Osteoporosis. N. Engl. J. Med. 2009, 361, 756–765, Correction in N. Engl. J. Med. 2009, 361, 1914. [Google Scholar] [CrossRef] [PubMed]
- Beaudoin, C.; Jean, S.; Bessette, L.; Ste-Marie, L.-G.; Moore, L.; Brown, J.P. Denosumab compared to other treatments to prevent or treat osteoporosis in individuals at risk of fracture: A systematic review and meta-analysis. Osteoporos. Int. 2016, 27, 2835–2844. [Google Scholar] [CrossRef] [PubMed]
- Denosumab Treatment of Severe Disuse Osteoporosis in a Boy with Spinal Muscular Atrophy—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/29228533/ (accessed on 22 July 2025).
- Kumaki, D.; Nakamura, Y.; Sakai, N.; Kosho, T.; Nakamura, A.; Hirabayashi, S.; Suzuki, T.; Kamimura, M.; Kato, H. Efficacy of Denosumab for Glucocorticoid-Induced Osteoporosis in an Adolescent Patient with Duchenne Muscular Dystrophy A Case Report. J. Bone Jt. Surg. Case Connect 2018, 8, E22. [Google Scholar] [CrossRef] [PubMed]
- Marcadet, L.; Juracic, E.S.; Khan, N.; Bouredji, Z.; Yagita, H.; Ward, L.M.; Russel Tupling, A.; Argaw, A.; Frenette, J. RANKL Inhibition Reduces Cardiac Hypertrophy in mdx Mice and Possibly in Children with Duchenne Muscular Dystrophy. Cells 2023, 12, 1538. [Google Scholar] [CrossRef]
- Bouchon, A.; Dietrich, J.; Colonna, M. Cutting edge: Inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J. Immunol. 2000, 164, 4991–4995. [Google Scholar] [CrossRef]
- Allcock, R.J.N.; Barrow, A.D.; Forbes, S.; Beck, S.; Trowsdale, J. The human TREM gene cluster at 6p21.1 encodes both activating and inhibitory single IgV domain receptors and includes NKp44. Eur. J. Immunol. 2003, 33, 567–577. [Google Scholar] [CrossRef]
- Molloy, E. Triggering Receptor Expressed on Myeloid Cells (TREM) family and the application of its antagonists. Recent Pat. Anti-Infect. Drug Discov. 2009, 4, 51–56. [Google Scholar] [CrossRef]
- Quan, D.N.; Cooper, M.D.; Potter, J.L.; Roberts, M.H.; Cheng, H.; A Jarvis, G. TREM-2 binds to lipooligosaccharides of Neisseria gonorrhoeae and is expressed on reproductive tract epithelial cells. Mucosal Immunol. 2008, 1, 229–238. [Google Scholar] [CrossRef]
- Turnbull, I.R.; Colonna, M. Activating and inhibitory functions of DAP12. Nat. Rev. Immunol. 2007, 7, 155–161. [Google Scholar] [CrossRef]
- Neumann, H.; Takahashi, K. Essential role of the microglial triggering receptor expressed on myeloid cells-2 (TREM2) for central nervous tissue immune homeostasis. J. Neuroimmunol. 2007, 184, 92–99. [Google Scholar] [CrossRef]
- Zheng, H.; Jia, L.; Liu, C.-C.; Rong, Z.; Zhong, L.; Yang, L.; Chen, X.-F.; Fryer, J.D.; Wang, X.; Zhang, Y.-W.; et al. TREM2 Promotes Microglial Survival by Activating Wnt/β-Catenin Pathway. J. Neurosci. 2017, 37, 1772–1784. [Google Scholar] [CrossRef]
- Chen, H.-L.; Chew, L.-J.; Packer, R.J.; Gallo, V. Modulation of the Wnt/beta-catenin pathway in human oligodendroglioma cells by Sox17 regulates proliferation and differentiation. Cancer Lett. 2013, 335, 361–371. [Google Scholar] [CrossRef]
- Ulrich, J.D.; Holtzman, D.M. TREM2 Function in Alzheimer’s Disease and Neurodegeneration. CS Chem. Neurosci. 2016, 7, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wu, X.; Li, X.; Jiang, L.-L.; Gui, X.; Liu, Y.; Sun, Y.; Zhu, B.; Pina-Crespo, J.C.; Zhang, M.; et al. TREM2 Is a Receptor for β-Amyloid that Mediates Microglial Function. Neuron 2018, 97, 1023–1031.e7. [Google Scholar] [CrossRef] [PubMed]
- Paloneva, J.; Kestilä, M.; Wu, J.; Salminen, A.; Böhling, T.; Ruotsalainen, V.; Hakola, P.; Bakker, A.B.; Philips, J.H.; Pekkarinen, P.; et al. Loss-of-function mutations in TYROBP (DAP12) result in a presenile dementia with bone cysts. Nat. Genet. 2000, 25, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Paloneva, J.; Manninen, T.; Christman, G.; Hovanes, K.; Mandelin, J.; Adolfsson, R.; Bianchin, M.; Bird, T.; Miranda, R.; Salmaggi, A.; et al. Mutations in two genes encoding different subunits of a receptor signaling complex result in an identical disease phenotype. Am. J. Hum. Genet. 2002, 71, 656–662. [Google Scholar] [CrossRef]
- Bianchin, M.M.; Capella, H.M.; Chaves, D.L.; Steindel, M.; Grisard, E.C.; Ganev, G.G.; da Silva Junior, J.P.; Evaldo, S.N.; Poffo, M.A.; Walz, R.; et al. Nasu-Hakola disease (polycystic lipomembranous osteodysplasia with sclerosing leukoencephalopathy—PLOSL): A dementia associated with bone cystic lesions. From clinical to genetic and molecular aspects. Cell. Mol. Neurobiol. 2004, 24, 1–24. [Google Scholar] [CrossRef]
- Cignarella, F.; Filipello, F.; Bollman, B.; Cantoni, C.; Locca, A.; Mikesell, R.; Manis, M.; Ibrahim, A.; Deng, L.; Benitez, B.A.; et al. TREM2 activation on microglia promotes myelin debris clearance and remyelination in a model of multiple sclerosis. Acta Neuropathol. 2020, 140, 513–534. [Google Scholar] [CrossRef]
- Price, B.R.; Sudduth, T.L.; Weekman, E.M.; Johnson, S.; Hawthorne, D.; Woolums, A.; Wilcock, D.M. Therapeutic Trem2 activation ameliorates amyloid-beta deposition and improves cognition in the 5XFAD model of amyloid deposition. J. Neuroinflamm. 2020, 17, 238. [Google Scholar] [CrossRef]
- Mirescu, C.; Dejanovic, B.; Larson, K.C.; Kiragasi, B.; Gergits, F.W.; Figley, M.D.; Renoux, A.; Huang, L.; Pandya, B.; Houze, J.; et al. Pharmacological and functional characterization of the first small molecule TREM2 agonist, VG-3927, for the treatment of Alzheimer’s disease. Alzheimer’s Dement. 2024, 20, e089318. [Google Scholar] [CrossRef]
- Battafarano, G.; Rossi, M.; De Martino, V.; Marampon, F.; Borro, L.; Secinaro, A.; Fattore, A. Strategies for Bone Regeneration: From Graft to Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 1128. [Google Scholar] [CrossRef]
- Ohnishi, M.; Nakatani, T.; Lanske, B.; Razzaque, M.S. Reversal of mineral ion homeostasis and soft-tissue calcification of klotho knockout mice by deletion of vitamin D 1alpha-hydroxylase. Kidney Int. 2009, 75, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Brownstein, C.A.; Adler, F.; Nelson-Williams, C.; Iijima, J.; Li, P.; Imura, A.; Nabeshima, Y.; Reyes-Mugica, M.; Carpenter, T.; Lifton, R. A translocation causing increased alpha-klotho level results in hypophosphatemic rickets and hyperparathyroidism. Proc. Natl. Acad. Sci. USA 2008, 105, 3455–3460. [Google Scholar] [CrossRef] [PubMed]
- Kuro, O.M. Aging and FGF23-klotho system. Vitam. Horm. 2021, 115, 317–332. [Google Scholar] [CrossRef]
- Liu, P.; Chen, L.; Bai, X.; Karaplis, A.; Miao, D.; Gu, N. Impairment of spatial learning and memory in transgenic mice overexpressing human fibroblast growth factor-23. Brain Res. 2011, 1412, 9–17. [Google Scholar] [CrossRef]
- Drew, D.A.; Tighiouart, H.; Scott, T.M.; Lou, K.V.; Fan, L.; Shaffi, K.; Weiner, D.; Sarnak, J. FGF-23 and cognitive performance in hemodialysis patients. Hemodial. Int. 2013, 18, 78–86. [Google Scholar] [CrossRef]
- Drew, D.A.; Weiner, D.E. Cognitive impairment in chronic kidney disease: Keep vascular disease in mind. Kidney Int. 2014, 85, 505–507. [Google Scholar] [CrossRef]
- Laszczyk, A.M.; Nettles, D.; Pollock, T.A.; Fox, S.; Garcia, M.L.; Wang, J.; Darryl Quarles, L.; King, G. FGF-23 Deficiency Impairs Hippocampal-Dependent Cognitive Function. Eneuro 2019, 6, 1–10. [Google Scholar] [CrossRef]
- Huang, S.; Li, Z.; Liu, Y.; Gao, D.; Zhang, X.; Hao, J.; Yang, F. Neural regulation of bone remodeling: Identifying novel neural molecules and pathways between brain and bone. J. Cell. Physiol. 2018, 234, 5466–5477. [Google Scholar] [CrossRef]
- Yue, R.; Zhou, B.O.; Shimada, I.S.; Zhao, Z.; Morrison, S.J. Leptin Receptor Promotes Adipogenesis and Reduces Osteogenesis by Regulating Mesenchymal Stromal Cells in Adult Bone Marrow. Cell Stem Cell 2016, 18, 782–796. [Google Scholar] [CrossRef]
- Sharan, K.; Yadav, V.K. Hypothalamic control of bone metabolism. Best Pr. Res. Clin. Endocrinol. Metab. 2014, 28, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Elefteriou, F.; Ahn, J.D.; Takeda, S.; Starbuck, M.; Yang, X.; Liu, X.; Kondo, H.; Richards, W.; Bannon, T.; Noda, M.; et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature 2005, 434, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Ducy, P.; Amling, M.; Takeda, S.; Priemel, M.; Schilling, A.F.; Beil, F.T.; Shen, J.; Vinson, C.; Rueger, J.; Karsenty, G. Leptin inhibits bone formation through a hypothalamic relay: A central control of bone mass. Cell 2000, 100, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Sims, N.A.; Martin, T.J. Coupling the activities of bone formation and resorption: A multitude of signals within the basic multicellular unit. BoneKEy Rep. 2014, 3, 481. [Google Scholar] [CrossRef]
- Tang, P.; Yin, P.; Lv, H.; Zhang, L.; Zhang, L. The role of semaphorin 3a in the skeletal system. Crit. Rev. Eukaryot. Gene Expr. 2015, 25, 47–57. [Google Scholar] [CrossRef]
- Hadjidakis, D.J.; Androulakis, I.I. Bone remodeling. Ann. N. Y. Acad. Sci. 2006, 1092, 385–396. [Google Scholar] [CrossRef]
- Xiao, W.; Wang, Y.; Pacios, S.; Li, S.; Graves, D.T. Cellular and Molecular Aspects of Bone Remodeling. Front. Oral Biol. 2016, 18, 9–16. [Google Scholar] [CrossRef]
- Charles, J.F.; Aliprantis, A.O. Osteoclasts: More than “bone eaters”. Trends Mol. Med. 2014, 20, 449–459. [Google Scholar] [CrossRef]
- Ahima, R.S.; Prabakaran, D.; Mantzoros, C.; Qu, D.; Lowell, B.; Maratos-Flier, E.; Filer, J. Role of leptin in the neuroendocrine response to fasting. Nature 1996, 382, 250–252. [Google Scholar] [CrossRef]
- Baver, S.B.; Hope, K.; Guyot, S.; Bjørbaek, C.; Kaczorowski, C.; O’Connell, K.M.S. Leptin modulates the intrinsic excitability of AgRP/NPY neurons in the arcuate nucleus of the hypothalamus. J. Neurosci. 2014, 34, 5486–5496. [Google Scholar] [CrossRef]
- Holloway, W.R.; Collier, F.M.; Aitken, C.J.; Myers, D.E.; Hodge, J.M.; Malakellis, M.; Gough, T.; Collier, G.; Nicholson, G. Leptin inhibits osteoclast generation. J. Bone Miner. Res. 2002, 17, 200–209. [Google Scholar] [CrossRef]
- Upadhyay, J.; Farr, O.M.; Mantzoros, C.S. The role of leptin in regulating bone metabolism. Metabolism 2015, 64, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.T.; Kalra, S.P.; Wong, C.P.; A Philbrick, K.; Lindenmaier, L.B.; Boghossian, S.; Iwaniec, U. Peripheral leptin regulates bone formation. J. Bone Miner. Res. 2012, 28, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Patel, M.S.; Bradley, A.; Wagner, E.F.; Karsenty, G. The molecular clock mediates leptin-regulated bone formation. Cell 2005, 122, 803–815. [Google Scholar] [CrossRef] [PubMed]
- Gordeladze, J.O.; Drevon, C.A.; Syversen, U.; Reseland, J.E. Leptin stimulates human osteoblastic cell proliferation, de novo collagen synthesis, and mineralization: Impact on differentiation markers, apoptosis, and osteoclastic signaling. J. Cell. Biochem. 2002, 85, 825–836. [Google Scholar] [CrossRef]
- Chan, J.L.; Mantzoros, C.S. Leptin and the hypothalamic-pituitary regulation of the gonadotropin-gonadal axis. Pituitary 2001, 4, 87–92. [Google Scholar] [CrossRef]
- Isozaki, O.; Tsushima, T.; Miyakawa, M.; Demura, H.; Seki, H. Interaction between leptin and growth hormone (GH)/IGF-I axis. Endocr. J. 1999, 46, S17–S24. [Google Scholar] [CrossRef]
- Roubos, E.W.; Dahmen, M.; Kozicz, T.; Xu, L. Leptin and the hypothalamo-pituitary-adrenal stress axis. Gen. Comp. Endocrinol. 2012, 177, 28–36. [Google Scholar] [CrossRef]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef]
- Lempesis, I.G.; Georgakopoulou, V.E. Physiopathological mechanisms related to inflammation in obesity and type 2 diabetes mellitus. World J. Exp. Med. 2023, 13, 7–16. [Google Scholar] [CrossRef]
- Picó, C.; Palou, M.; Pomar, C.A.; Rodríguez, A.M.; Palou, A. Leptin as a key regulator of the adipose organ. Rev. Endocr. Metab. Disord. 2021, 23, 13–30. [Google Scholar] [CrossRef]
- Mraz, M.; Haluzik, M. The role of adipose tissue immune cells in obesity and low-grade inflammation. J. Endocrinol. 2014, 222, 113–127. [Google Scholar] [CrossRef]
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E. Leptin and Obesity: Role and Clinical Implication. Front. Endocrinol. 2021, 12, 585887. [Google Scholar] [CrossRef]
- Fà, M.; Puzzo, D.; Piacentini, R.; Staniszewski, A.; Zhang, H.; Baltrons, M.A.; Li Puma, D.; Chatterjee, I.; Li, J.; Saeed, F.; et al. Extracellular Tau Oligomers Produce an Immediate Impairment of LTP and Memory. Sci. Rep. 2016, 6, 19393. [Google Scholar] [CrossRef]
- Li, S.; Hong, S.; Shepardson, N.E.; Walsh, D.M.; Shankar, G.M.; Selkoe, D. Soluble Oligomers of Amyloid β Protein Facilitate Hippocampal Long-Term Depression by Disrupting Neuronal Glutamate Uptake. Neuron 2009, 62, 788–801. [Google Scholar] [CrossRef]
- Moult, P.R.; Harvey, J. NMDA receptor subunit composition determines the polarity of leptin-induced synaptic plasticity. Neuropharmacology 2011, 61, 924–936. [Google Scholar] [CrossRef]
- Mejido, D.C.; Peny, J.A.; Vieira, M.N.; Ferreira, S.T.; De Felice, F.G. Insulin and leptin as potential cognitive enhancers in metabolic disorders and Alzheimer’s disease. Neuropharmacology 2020, 171, 108115. [Google Scholar] [CrossRef] [PubMed]
- Forny-Germano, L.; De Felice, F.G.; Vieira, M.N.D.N. The role of leptin and adiponectin in obesity-associated cognitive decline and Alzheimer’s disease. Front. Neurosci. 2019, 13, 1027. [Google Scholar] [CrossRef] [PubMed]
- Paz-Filho, G.J.; Babikian, T.; Asarnow, R.; Esposito, K.; Erol, H.K.; Wong, M.L.; Delibasi, T.; Licinio, J. Leptin Replacement Improves Cognitive Development. PLoS ONE 2008, 3, e3098, Correction in PLoS ONE 2008, 3, e3098. [Google Scholar] [CrossRef]
- Doherty, G.; Holiday, A.; Malekizadeh, Y.; Manolescu, C.; Duncan, S.; Flewitt, I.; Hamilton, K.; MacLeod, B.; Ainge, J.; Harvey, J. Leptin-based hexamers facilitate memory and prevent amyloid-driven AMPA receptor internalisation and neuronal degeneration. J. Neurochem. 2022, 165, 809–826. [Google Scholar] [CrossRef]
- Malekizadeh, Y.; Holiday, A.; Redfearn, D.; Ainge, J.A.; Doherty, G.; Harvey, J. A Leptin Fragment Mirrors the Cognitive Enhancing and Neuroprotective Actions of Leptin. Cereb. Cortex 2016, 27, 4769–4782. [Google Scholar] [CrossRef] [PubMed]
- Grinspoon, S.; Gulick, T.; Askari, H.; Landt, M.; Lee, K.; Anderson, E.; Ma, Z.; Vignati, L.; Bowsher, R.; Herzog, D.; et al. Serum leptin levels in women with anorexia nervosa. J. Clin. Endocrinol. Metab. 1996, 81, 3861–3863. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.H.; Mantzoros, C. Role of leptin in human reproductive disorders. J. Endocrinol. 2014, 223, T49–T62. [Google Scholar] [CrossRef] [PubMed]
- Welt, C.K.; Chan, J.L.; Bullen, J.; Murphy, R.; Smith, P.; DePaoli, A.M.; Karalis, A.; Mantzoros, C. Recombinant Human Leptin in Women with Hypothalamic Amenorrhea. N. Engl. J. Med. 2004, 351, 987–997. [Google Scholar] [CrossRef]
- Baldock, P.A.; Sainsbury, A.; Couzens, M.; Enriquez, R.F.; Thomas, G.P.; Gardiner, E.M.; Herzog, H. Hypothalamic Y2 receptors regulate bone formation. J. Clin. Investig. 2002, 109, 915–921. [Google Scholar] [CrossRef]
- Allison, S.J.; A Baldock, P.; Enriquez, R.F.; Lin, E.; During, M.; Gardiner, E.M.; Eisman, J.; Sainsbury, A.; Herzog, H. Critical interplay between neuropeptide Y and sex steroid pathways in bone and adipose tissue homeostasis. J. Bone Miner. Res. 2009, 24, 294–304. [Google Scholar] [CrossRef]
- Baldock, P.A.; Sainsbury, A.; Allison, S.; Lin, E.J.D.; Couzens, M.; Boey, D.; Enriquez, R.; During, M.; Herzog, H.; Gardiner, E. Hypothalamic control of bone formation: Distinct actions of leptin and Y2 receptor pathways. J. Bone Miner. Res. 2005, 20, 1851–1857. [Google Scholar] [CrossRef]
- Baldock, P.A.; Lee, N.J.; Driessler, F.; Lin, S.; Allison, S.; Stehrer, B.; Lin, E.; Zhang, L.; Enriquez, R.; Wong, I.; et al. Neuropeptide Y knockout mice reveal a central role of NPY in the coordination of bone mass to body weight. PLoS ONE 2009, 4, e8415. [Google Scholar] [CrossRef]
- Horsnell, H.; Baldock, P.A. Osteoblastic Actions of the Neuropeptide Y System to Regulate Bone and Energy Homeostasis. Curr. Osteoporos. Rep. 2016, 14, 26–31. [Google Scholar] [CrossRef]
- Xie, W.; Li, F.; Han, Y.; Qin, Y.; Wang, Y.; Chi, X.; Xiao, J.; Li, Z. Neuropeptide Y1 receptor antagonist promotes osteoporosis and microdamage repair and enhances osteogenic differentiation of bone marrow stem cells via cAMP/PKA/CREB pathway. Aging 2020, 12, 8120–8136. [Google Scholar] [CrossRef]
- Xie, W.; Li, F.; Han, Y.; Li, Z.; Xiao, J. Neuropeptides are associated with pain threshold and bone microstructure in ovariectomized rats. Neuropeptides 2020, 81, 101995. [Google Scholar] [CrossRef]
- Baldock, P.A.; Allison, S.J.; Lundberg, P.; Lee, N.J.; Slack, K.; Lin, E.-J.D.; Enriquez, R.; McDonald, M.; Zhang, L.; During, M.; et al. Novel role of Y1 receptors in the coordinated regulation of bone and energy homeostasis. J. Biol. Chem. 2007, 282, 19092–19102. [Google Scholar] [CrossRef]
- Mahar, I.; Albuquerque, M.S.; Mondragon-Rodriguez, S.; Cavanagh, C.; Davoli, M.A.; Chabot, J.G.; William, S.; Mechawar, N.; Quirion, R.; Kranric, S. Phenotypic alterations in hippocampal NPY- and PV-expressing interneurons in a presymptomatic transgenic mouse model of Alzheimer’s disease. Front. Aging Neurosci. 2017, 8, 327. [Google Scholar] [CrossRef] [PubMed]
- Ramos, B.; Baglietto-Vargas, D.; del Rio, J.C.; Moreno-Gonzalez, I.; Santa-Maria, C.; Jimenez, S.; Caballero, C.; Lopez-Tellez, J.; Khan, Z.; Ruano, D.; et al. Early neuropathology of somatostatin/NPY GABAergic cells in the hippocampus of a PS1 × APP transgenic model of Alzheimer’s disease. Neurobiol. Aging 2006, 27, 1658–1672. [Google Scholar] [CrossRef] [PubMed]
- Kowall, N.W.; Beal, M.F. Cortical somatostatin, neuropeptide Y, and NADPH diaphorase neurons: Normal anatomy and alterations in alzheimer’s disease. Ann. Neurol. 1988, 23, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Martel, J.-C.; Alagar, R.; Robitaille, Y.; Quirion, R. Neuropeptide Y receptor binding sites in human brain. Possible alteration in Alzheimer’s disease. Brain Res. 1990, 519, 228–235. [Google Scholar] [CrossRef]
- Alom, J.; Galard, R.; Catalan, R.; Castellanos, J.; Schwartz, S.; Tolosa, E. Cerebrospinal fluid neuropeptide Y in alzheimer’s disease. Eur. Neurol. 1990, 30, 211–213. [Google Scholar] [CrossRef]
- Koide, S.; Onishi, H.; Hashimoto, H.; Kai, T.; Yamagami, S. Plasma neuropeptide Y is reduced in patients with Alzheimer’s disease. Neurosci. Lett. 1995, 198, 149–151. [Google Scholar] [CrossRef]
- Rose, J.B.; Crews, L.; Rockenstein, E.; Adame, A.; Mante, M.; Hersh, L.B.; Gage, F.H.; Spencer, B.; Poktar, R.; Marr, R.A.; et al. Neuropeptide Y fragments derived from neprilysin processing are neuroprotective in a transgenic model of Alzheimer’s disease. J. Neurosci. 2009, 29, 1115. [Google Scholar] [CrossRef]
- dos Santos, V.V.; Santos, D.B.; Lach, G.; Rodrigues, A.L.S.; Farina, M.; De Lima, T.C.; Prediger, R.D. Neuropeptide Y (NPY) prevents depressive-like behavior, Spatial memory deficits and oxidative stress following amyloid-β (Aβ1-40) administration in mice. Behav. Brain Res. 2013, 244, 107–115. [Google Scholar] [CrossRef]
- Yulyaningsih, E.; Zhang, L.; Herzog, H.; Sainsbury, A. NPY receptors as potential targets for anti-obesity drug development. Br. J. Pharmacol. 2011, 163, 1170–1202. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Neves, J.; Cavadas, C.; de Almeida, L. Neuropeptide Y (NPY) intranasal delivery alleviates Machado–Joseph disease. Sci. Rep. 2021, 11, 3345. [Google Scholar] [CrossRef] [PubMed]
- Lochhead, J.J.; Thorne, R.G. Intranasal delivery of biologics to the central nervous system. Adv. Drug Deliv. Rev. 2012, 64, 614–628. [Google Scholar] [CrossRef] [PubMed]
- Perälä, N.; Sariola, H.; Immonen, T. More than nervous: The emerging roles of plexins. Differentiation 2012, 83, 77–91. [Google Scholar] [CrossRef]
- Hayashi, M.; Nakashima, T.; Taniguchi, M.; Kodama, T.; Kumanogoh, A.; Takayanagi, H. Osteoprotection by semaphorin 3A. Nature 2012, 485, 69–74. [Google Scholar] [CrossRef]
- Behar, O.; Golden, J.A.; Mashimo, H.; Schoen, F.J.; Fishman, M.C. Semaphorin III is needed for normal patterning and growth of nerves, bones and heart. Nature 1996, 383, 525–528. [Google Scholar] [CrossRef]
- Li, Z.; Hao, J.; Duan, X.; Wu, N.; Zhou, Z.; Yang, F.; Li, J.; Zhao, Z.; Huang, S. The role of semaphorin 3A in bone remodeling. Front. Cell. Neurosci. 2017, 11, 40. [Google Scholar] [CrossRef]
- Li, Y.; Yang, L.; He, S.; Hu, J. The effect of semaphorin 3A on fracture healing in osteoporotic rats. J. Orthop. Sci. 2015, 20, 1114–1121. [Google Scholar] [CrossRef]
- Janssen, B.J.C.; Robinson, R.A.; Pérez-Brangulí, F.; Bell, C.H.; Mitchell, K.J.; Siebold, C.; Jones, E.Y. Structural basis of semaphoring-plexin signalling. Nature 2010, 467, 1118–1122. [Google Scholar] [CrossRef]
- Dacquin, R.; Domenget, C.; Kumanogoh, A.; Kikutani, H.; Jurdic, P.; Machuca-Gayet, I. Control of bone resorption by semaphorin 4D is dependent on ovarian function. PLoS ONE 2011, 6, e26627. [Google Scholar] [CrossRef]
- Negishi-Koga, T.; Shinohara, M.; Komatsu, N.; Bito, H.; Kodama, T.; Friedel, R.H.; Takayanagi, H. Suppression of bone formation by osteoclastic expression of semaphorin 4D. Nat. Med. 2011, 17, 1473–1480. [Google Scholar] [CrossRef]
- Delorme, G.; Saltel, F.; Bonnelye, E.; Jurdic, P.; Machuca-Gayet, I. Expression and function of semaphorin 7A in bone cells. Biol. Cell 2005, 97, 589–597. [Google Scholar] [CrossRef]
- Koh, J.M.; Oh, B.; Lee, J.Y.; Lee, J.K.; Kimm, K.; Kim, G.S.; Park, B.L.; Cheong, H.S.; Shin, H.D.; Hong, J.M.; et al. Association study of semaphorin 7a (sema7a) polymorphisms with bone mineral density and fracture risk in postmenopausal Korean women. J. Hum. Genet. 2006, 51, 112–117. [Google Scholar] [CrossRef]
- Beaulieu, J.; Espinoza, S.; Gainetdinov, R.R. Dopamine receptors—IUPHAR Review 13. Br. J. Pharmacol. 2014, 172, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Paus, T. Primate anterior cingulate cortex: Where motor control, drive and cognition interface. Nat. Rev. Neurosci. 2001, 2, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Luciana, M.; Collins, P.F.; Depue, R.A. Opposing roles for dopamine and serotonin in the modulation of human spatial working memory functions. Cereb. Cortex 1998, 8, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Z.; Chen, Z.; Tu, S.; Mai, Z.; Chen, L.; Kang, X.; Chen, X.; Wei, J.; Wang, Y.; Peng, Y.; et al. Dopamine Suppresses Osteogenic Differentiation of Rat Bone Marrow-Derived Mesenchymal Stem Cells via AKT/GSK-3 β/β-Catenin Signaling Pathway. Stem Cells Int. 2022, 2022, 4154440. [Google Scholar] [CrossRef]
- Wang, L.; Han, L.; Xue, P.; Hu, X.; Wong, S.W.; Deng, M.; Tseng, H.C.; Huang, B.-W.; Ko, C. Dopamine suppresses osteoclast differentiation via cAMP/PKA/CREB pathway. Cell. Signal. 2020, 78, 109847. [Google Scholar] [CrossRef]
- Cederberg, K.L.J.; Silvestri, R.; Walters, A.S. Vitamin D and Restless Legs Syndrome: A Review of Current Literature. Tremor Other Hyperkinetic Mov. 2023, 13, 12. [Google Scholar] [CrossRef]
- Antonelli, F.; Strafella, A.P. Behavioral disorders in Parkinson’s disease: The role of dopamine. Park. Relat. Disord. 2014, 20 (Suppl. S1), S10–S12. [Google Scholar] [CrossRef]
- Taggart, H.; Crawford, V. Reduced bone density of the hip in elderly patients with Parkinson’s disease. Age Ageing 1995, 24, 326–328. [Google Scholar] [CrossRef] [PubMed]
- Handa, K.; Kiyohara, S.; Yamakawa, T.; Ishikawa, K.; Hosonuma, M.; Sakai, N.; Karakawa, A.; Chatani, M.; Tsuji, M.; Inagaki, K.; et al. Bone loss caused by dopaminergic degeneration and levodopa treatment in Parkinson’s disease model mice. Sci. Rep. 2019, 9, 13768. [Google Scholar] [CrossRef] [PubMed]
- Brenner, B.; Harney, J.T.; Ahmed, B.A.; Jeffus, B.C.; Unal, R.; Mehta, J.L.; Kilic, F. Plasma serotonin levels and the platelet serotonin transporter. J. Neurochem. 2007, 102, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Kanova, M.; Kohout, P. Serotonin-Its Synthesis and Roles in the Healthy and the Critically Ill. Int. J. Mol. Sci. 2021, 22, 4837. [Google Scholar] [CrossRef]
- Ducy, P.; Karsenty, G. The two faces of serotonin in bone biology. J. Cell Biol. 2010, 191, 7–13. [Google Scholar] [CrossRef]
- El-Merahbi, R.; Löffler, M.; Mayer, A.; Sumara, G. The roles of peripheral serotonin in metabolic homeostasis. FEBS Lett. 2015, 589, 1728–1734. [Google Scholar] [CrossRef]
- Berger, M.; Gray, J.A.; Roth, B.L. The expanded biology of serotonin. Annu. Rev. Med. 2009, 60, 355–366. [Google Scholar] [CrossRef]
- Sun, W.; Ye, B.; Chen, S.; Zeng, L.; Lu, H.; Wan, Y.; Gao, Q.; Chen, K.; Qu, Y.; Wu, B.; et al. Neuro–bone tissue engineering: Emerging mechanisms, potential strategies, and current challenges. Bone Res. 2023, 11, 65. [Google Scholar] [CrossRef]
- Bliziotes, M. Update in serotonin and bone. J. Clin. Endocrinol. Metab. 2010, 95, 4124–4132. [Google Scholar] [CrossRef]
- Durham, E.; Zhang, Y.; LaRue, A.; Bradshaw, A.; Cray, J. Selective serotonin reuptake inhibitors (SSRI) affect murine bone lineage cells. Life Sci. 2020, 255, 117827. [Google Scholar] [CrossRef]
- Wang, C.-X.; Ge, X.-Y.; Wang, M.-Y.; Ma, T.; Zhang, Y.; Lin, Y. Dopamine D1 receptor-mediated activation of the ERK signaling pathway is involved in the osteogenic differentiation of bone mesenchymal stem cells. Stem Cell Res. Ther. 2020, 11, 12. [Google Scholar] [CrossRef]
- Huang, H.H.; Brennan, T.C.; Muir, M.M.; Mason, R.S. Functional alpha1- and beta2-adrenergic receptors in human osteoblasts. J. Cell. Physiol. 2009, 220, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.-P.; Xia, W.F. Regulation of bone metabolism mediated by β-adrenergic receptor and its clinical application. World J. Clin. Cases 2021, 9, 8967–8973. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Xie, K.; Wu, S.; Chen, Y. Osteoporosis and Fracture Risk Associated with Novel Antidepressants: A Systematic Review and Meta-Analysis. Actas Urol. Espanolas 2024, 52, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Rajakumaraswamy, N.; Maze, M. alpha2-Adrenoceptor agonists: Shedding light on neuroprotection? Br. Med. Bull. 2005, 71, 77–92. [Google Scholar] [CrossRef]
- Madrigal, J.L.M.; Kalinin, S.; Richardson, J.C.; Feinstein, D.L. Neuroprotective actions of noradrenaline: Effects on glutathione synthesis and activation of peroxisome proliferator activated receptor delta. J. Neurochem. 2007, 103, 2092–2101. [Google Scholar] [CrossRef]
- Wang, X.; Xu, J.; Kang, Q. Neuromodulation of bone: Role of different peptides and their interactions (Review). Mol. Med. Rep. 2021, 23, 32. [Google Scholar] [CrossRef]
- Sohn, R.; Rösch, G.; Junker, M.; Meurer, A.; Zaucke, F.; Jenei-Lanzl, Z. Adrenergic signalling in osteoarthritis. Cell. Signal. 2021, 82, 109948. [Google Scholar] [CrossRef]
- Nishiura, T.; Abe, K. α1-Adrenergic receptor stimulation induces the expression of receptor activator of nuclear factor κB ligand gene via protein kinase C and extracellular signal-regulated kinase pathways in MC3T3-E1 osteoblast-like cells. Arch. Oral Biol. 2007, 52, 778–785. [Google Scholar] [CrossRef]
- Hamrick, M.W.; Ferrari, S.L. Leptin and the sympathetic connection of fat to bone. Osteoporos. Int. 2007, 19, 905–912. [Google Scholar] [CrossRef]
- Pierroz, D.D.; Bonnet, N.; Bianchi, E.N.; Bouxsein, M.L.; A Baldock, P.; Rizzoli, R.; Ferrari, S.L. Deletion of β-adrenergic receptor 1, 2, or both leads to different bone phenotypes and response to mechanical stimulation. J. Bone Miner. Res. 2012, 27, 1252–1262. [Google Scholar] [CrossRef]
- Ma, Y.; Krueger, J.J.; Redmon, S.N.; Uppuganti, S.; Nyman, J.S.; Hahn, M.K.; Elefteriou, F. Extracellular norepinephrine clearance by the norepinephrine transporter is required for skeletal homeostasis. J. Biol. Chem. 2013, 288, 30105–30113. [Google Scholar] [CrossRef]
- Jing, X.-Z.; Yuan, X.-Z.; Luo, X.; Zhang, S.-Y.; Wang, X.-P. An Update on Nondopaminergic Treatments for Motor and Non-motor Symptoms of Parkinson’s Disease. Curr. Neuropharmacol. 2023, 21, 1806–1826. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Shankar, R.; Gamelli, R.; Jones, S. Dynamic norepinephrine alterations in bone marrow: Evidence of functional innervation. J. Neuroimmunol. 1999, 96, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Bulut, S.D.; İspir, G.Z.; Bulut, S.; Aygün, E. Comparison of the Effect of Selective Serotonin and Norepinephrine Reuptake Inhibitors on Bone Mineral Density with Selective Serotonin Reuptake Inhibitors and Healthy Controls. J. Clin. Densitom. 2024, 28, 101538. [Google Scholar] [CrossRef] [PubMed]
- Tsigos, C.; Chrousos, G.P. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J. Psychosom. Res. 2002, 53, 865–871. [Google Scholar] [CrossRef]
- Zhao, Y.; Peng, X.; Wang, Q.; Zhang, Z.; Wang, L.; Xu, Y.; Yang, H.; Bai, J.; Geng, D. Crosstalk Between the Neuroendocrine System and Bone Homeostasis. Endocr. Rev. 2023, 45, 95–124. [Google Scholar] [CrossRef]
- Donat, A.; Jiang, S.; Xie, W.; Knapstein, P.R.; Albertsen, L.-C.; Kokot, J.L.; Sevecke, J.; Augustin, R.; Jahn, D.; Yorgan, T.A.; et al. The selective norepinephrine reuptake inhibitor reboxetine promotes late-stage fracture healing in mice. IScience 2023, 26, 107761. [Google Scholar] [CrossRef]
- Hakam, A.E.; Duarte, P.M.; Mbadu, M.P.; Aukhil, I.; da Silva, H.D.P.; Chang, J. Association of different antidepressant classes with clinical attachment level and alveolar bone loss in patients with periodontitis: A retrospective study. J. Periodontal Res. 2021, 57, 75–84. [Google Scholar] [CrossRef]
- Van Hooft, J.A.; Dougherty, J.J.; Endeman, D.; A Nichols, R.; Wadman, W.J. Gabapentin inhibits presynaptic Ca2+ influx and synaptic transmission in rat hippocampus and neocortex. Eur. J. Pharmacol. 2002, 449, 221–228. [Google Scholar] [CrossRef]
- Fernandez, P.C.R.; Wright, C.S.; Warden, S.J.; Hum, J.; Farach-Carson, M.C.; Thompson, W.R. Effects of Gabapentin and Pregabalin on Calcium Homeostasis: Implications for Physical Rehabilitation of Musculoskeletal Tissues. Curr. Osteoporos. Rep. 2022, 20, 365–378. [Google Scholar] [CrossRef]
- Rocha, S.; Ferraz, R.; Prudêncio, C.; Fernandes, M.H.; Costa-Rodrigues, J. Differential effects of antiepileptic drugs on human bone cells. J. Cell. Physiol. 2019, 234, 19691–19701. [Google Scholar] [CrossRef] [PubMed]
- Delmas, P.D. Treatment of postmenopausal osteoporosis. Lancet 2002, 359, 2018–2026. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, M.; Erlandsson, M.; Alatalo, S.; Windahl, S.; Andersson, G.; Halleen, J.M.; Carlsten, H.; Gustafsson, J.A.; Ohlsson, C. Estrogen receptor alpha, but not estrogen receptor beta, is involved in the regulation of the OPG/RANKL (osteoprotegerin/receptor activator of NF-kappa B ligand) ratio and serum interleukin-6 in male mice. J. Endocrinol. 2001, 171, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Blake, J.; Cosman, F.A.; Lewiecki, E.M.; McClung, M.R.; Pinkerton, J.; Shapiro, M. Management of osteoporosis in postmenopausal women: The 2021 position statement of The North American Menopause Society. Menopause 2021, 28, 973–997. [Google Scholar] [CrossRef]
- Morale, M.; Serra, P.; L’ePiscopo, F.; Tirolo, C.; Caniglia, S.; Testa, N.; Gennuso, F.; Giaquinta, G.; Rocchitta, G.; Desole, M.S.; et al. Estrogen, neuroinflammation and neuroprotection in Parkinson’s disease: Glia dictates resistance versus vulnerability to neurodegeneration. Neuroscience 2006, 138, 869–878. [Google Scholar] [CrossRef]
- Alexander, M.W.; Wu, C.-Y.; Coughlan, G.T.; Puri, T.; Buckley, R.F.; Palta, P.; Swardfager, W.; Masellis, M.; Gaela, L.A.M.; Einstein, G.; et al. Associations Between Age at Menopause, Vascular Risk, and 3-Year Cognitive Change in the Canadian Longitudinal Study on Aging. Neurology 2024, 102, e209298. [Google Scholar] [CrossRef]
- Song, Y.J.; Li, S.R.; Li, X.W.; Chen, X.; Wei, Z.X.; Liu, Q.; Cheng, Y. The Effect of Estrogen Replacement Therapy on Alzheimer’s Disease and Parkinson’s Disease in Postmenopausal Women: A Meta-Analysis. Front. Neurosci. 2020, 14, 157. [Google Scholar] [CrossRef]
- Chen, P.; Li, B.; Ou-Yang, L. Role of estrogen receptors in health and disease. Front. Endocrinol. 2022, 13, 839005. [Google Scholar] [CrossRef]
- Souberbielle, J.-C.; Cavalier, E.; Cormier, C. How to manage an isolated elevated PTH? Ann. Endocrinol. 2015, 76, 134–141. [Google Scholar] [CrossRef]
- Goltzman, D. Physiology of Parathyroid Hormone. Endocrinol. Metab. Clin. N. Am. 2018, 47, 743–758. [Google Scholar] [CrossRef]
- Sell, J.; Ramirez, S.; Partin, M. Parathyroid Disorders. Am. Fam. Physician 2022, 105, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; O’Brien, C.A. Osteocyte RANKL: New insights into the control of bone remodeling. J. Bone Miner. Res. 2012, 27, 499–505. [Google Scholar] [CrossRef]
- O’BRien, C.A.; Nakashima, T.; Takayanagi, H. Osteocyte control of osteoclastogenesis. Bone 2013, 54, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.C.; Sakata, T.; Pfleger, L.L.; Bencsik, M.; Halloran, B.P.; Bikle, D.D.; Nisselson, R.A. PTH Differentially Regulates Expression of RANKL and OPG. J. Bone Miner. Res. 2004, 19, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Szymczak, J.; Bohdanowicz-Pawlak, A. Osteoprotegerin, RANKL, and bone turnover in primary hyperparathyroidism: The effect of parathyroidectomy and treatment with alendronate. Horm. Metab. Res. 2013, 45, 759–764. [Google Scholar] [CrossRef]
- Nakchbandi, I.A.; Lang, R.; Kinder, B.; Insogna, K.L. The role of the receptor activator of nuclear factor-κB ligand/osteoprotegerin cytokine system in primary hyperparathyroidism. J. Clin. Endocrinol. Metab. 2008, 93, 967–973. [Google Scholar] [CrossRef]
- Silva, B.C.; Bilezikian, J.P. Skeletal abnormalities in Hypoparathyroidism and in Primary Hyperparathyroidism. Rev. Endocr. Metab. Disord. 2020, 22, 789–802. [Google Scholar] [CrossRef]
- Kaleem, I.; Alexander, J.; Hisbulla, M.; Kannichamy, V.; Mishra, V.; Banerjee, A.; Gandhi, A.B.; Khan, S. A Review of the Relationship of the Cerebrospinal Fluid Changes During the Dysregulation of Parathyroid Hormone with Psychiatric or Neurological Manifestations. Cureus 2021, 13, e12679. [Google Scholar] [CrossRef]
- Bühler, G.; Balabanova, S.; Milowski, S.; Rosenthal, J.; Antoniadis, G.; Mohr, K.; Richter, K.M. Detection of immunoreactive parathyroid hormone-related protein in human cerebrospinal fluid. Exp. Clin. Endocrinol. Diabetes 1997, 105, 336–340. [Google Scholar] [CrossRef]
- Chatterjee, O.; Nakchbandi, I.A.; Philbrick, W.M.; Dreyer, B.E.; Zhang, J.P.; Kaczmarek, L.K.; Brines, M.L.; Broadus, A.E. Endogenous parathyroid hormone-related protein functions as a neuroprotective agent. Brain Res. 2002, 930, 58–66. [Google Scholar] [CrossRef]
- Funk, J.L.; Trout, C.R.; Wei, H.; Stafford, G.; Reichlin, S. Parathyroid hormone-related protein (PTHrP) induction in reactive astrocytes following brain injury: A possible mediator of CNS inflammation. Brain Res. 2001, 915, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Macica, C.M.; Liang, G.; Lankford, K.L.; Broadus, A.E. Induction of parathyroid hormone-related peptide following peripheral nerve injury: Role as a modulator of Schwann cell phenotype. Glia 2006, 53, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Funk, J.L.; Migliati, E.; Chen, G.; Wei, H.; Wilson, J.; Downey, K.J.; Mullarky, P.J.; Coull, B.M.; McDonagh, P.F.; Ritter, L.S. Parathyroid hormone-related protein induction in focal stroke: A neuroprotective vascular peptide. Am. J. Physiol. Integr. Comp. Physiol. 2003, 284, R1021–R1030. [Google Scholar] [CrossRef] [PubMed]
- Dettori, C.; Ronca, F.; Scalese, M.; Saponaro, F. Parathyroid Hormone (PTH)-Related Peptides Family: An Intriguing Role in the Central Nervous System. J. Pers. Med. 2023, 13, 714. [Google Scholar] [CrossRef]
- Fisher, A.; Srikusalanukul, W.; Davis, M.; Smith, P. Hip fracture type: Important role of parathyroid hormone (PTH) response to hypovitaminosis D. Bone 2010, 47, 400–407. [Google Scholar] [CrossRef]
- Bailey, S.; Poundarik, A.A.; Sroga, G.E.; Vashishth, D. Structural role of osteocalcin and its modification in bone fracture. Appl. Phys. Rev. 2023, 10, 011410. [Google Scholar] [CrossRef]
- Ivaska, K.K.; Hentunen, T.A.; Vääräniemi, J.; Ylipahkala, H.; Pettersson, K.; Väänänen, H.K. Release of intact and fragmented osteocalcin molecules from bone matrix during bone resorption in vitro. J. Biol. Chem. 2004, 279, 18361–18369. [Google Scholar] [CrossRef]
- Vergatti, A.; Abate, V.; Iannuzzo, G.; Barbato, A.; De Filippo, G.; Rendina, D. The bone-heart axis in the pathogenesis of cardiovascular diseases: A narrative review. Nutr. Metab. Cardiovasc. Dis. 2025, 35, 103872. [Google Scholar] [CrossRef]
- Karsenty, G. Osteocalcin: A Multifaceted Bone-Derived Hormone. Annu. Rev. Nutr. 2023, 43, 55–71. [Google Scholar] [CrossRef]
- Qian, Z.; Li, H.; Yang, H.; Yang, Q.; Lu, Z.; Wang, L.; Chen, Y.; Li, X. Osteocalcin attenuates oligodendrocyte differentiation and myelination via GPR37 signaling in the mouse brain. Sci. Adv. 2021, 7, eabi5811. [Google Scholar] [CrossRef]
- Khrimian, L.; Obri, A.; Ramos-Brossier, M.; Rousseaud, A.; Moriceau, S.; Nicot, A.S.; Mera, P.; Kosmidis, S.; Karnavas, T.; Saudou, F.; et al. Gpr158 mediates osteocalcin’s regulation of cognition. J. Exp. Med. 2017, 214, 2859–2873. [Google Scholar] [CrossRef]
- Kosmidis, S.; Polyzos, A.; Harvey, L.; Youssef, M.; Denny, C.A.; Dranovsky, A.; Kandel, E.R. RbAp48 Protein Is a Critical Component of GPR158/OCN Signaling and Ameliorates Age-Related Memory Loss. Cell Rep. 2018, 25, 959–973.e6. [Google Scholar] [CrossRef]
- Glatigny, M.; Moriceau, S.; Rivagorda, M.; Ramos-Brossier, M.; Nascimbeni, A.C.; Lante, F.; Shanley, M.R.; Boudarene, N.; Rousseaud, A.; Friedman, A.K.; et al. Autophagy Is Required for Memory Formation and Reverses Age-Related Memory Decline. Curr. Biol. 2019, 29, 435–448.e8. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.M.; Singh, P.; Khrimian, L.; Morgan, D.A.; Chowdhury, S.; Arteaga-Solis, E.; Horvath, T.L.; Domingos, A.I.; Marlsand, A.L.; Yadav, V.K.; et al. Mediation of the Acute Stress Response by the Skeleton. Cell Metab. 2019, 30, 890–902.e8. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Xu, X.-Y.; Xu, R.-Z.; Zhen, Y.-F.; Xu, G.; Li, Y.K. Decreased serum undercarboxylated osteocalcin is associated with cognitive impairment in male patients with type 2 diabetes. J. Diabetes Complicat. 2018, 32, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.; Zhang, D.; Ma, D.-N.; Hou, Y.-F.; Zhuang, Q.-Q.; Gong, Y.-L.; Sun, K.-H.; Zhao, H.-Y.; Tao, B.; Yang, Y.-Y.; et al. Osteocalcin ameliorates cognitive dysfunctions in a mouse model of Alzheimer’s Disease by reducing amyloid β burden and upregulating glycolysis in neuroglia. Cell Death Discov. 2023, 9, 46. [Google Scholar] [CrossRef]
- Wu, Y.; Ma, Y.; Liu, Z.; Geng, Q.; Chen, Z.; Zhang, Y. Alterations of myelin morphology and oligodendrocyte development in early stage of Alzheimer’s disease mouse model. Neurosci. Lett. 2017, 642, 102–106. [Google Scholar] [CrossRef]
- Chen, J.-F.; Liu, K.; Hu, B.; Li, R.-R.; Xin, W.; Chen, H.; Wang, F.; Chen, L.; Li, R.-X.; Ren, S.-H.; et al. Enhancing myelin renewal reverses cognitive dysfunction in a murine model of Alzheimer’s disease. Neuron 2021, 109, 2292–2307.e5. [Google Scholar] [CrossRef]
- Tkach, V.; Bock, E.; Berezin, V. The role of RhoA in the regulation of cell morphology and motility. Cell Motil. Cytoskelet. 2005, 61, 21–33. [Google Scholar] [CrossRef]
- Shen, D.-W.; Pouliot, L.M.; Gillet, J.-P.; Ma, W.; Johnson, A.C.; Hall, M.D.; Gottesman, M.M. The transcription factor GCF2 is an upstream repressor of the small GTPAse RhoA, regulating membrane protein trafficking, sensitivity to doxorubicin, and resistance to cisplatin. Mol. Pharm. 2012, 9, 1822–1833. [Google Scholar] [CrossRef]
- Chi, X.; Wang, S.; Huang, Y.; Stamnes, M.; Chen, J.L. Roles of rho GTPases in intracellular transport and cellular transformation. Int. J. Mol. Sci. 2013, 14, 7089–7108. [Google Scholar] [CrossRef]
- Loirand, G.; GuérIn, P.; Pacaud, P. Rho kinases in cardiovascular physiology and pathophysiology. Circ. Res. 2006, 98, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhang, W.; Chen, J.; Li, S.; Guo, G. Rho-associated protein kinase modulates neurite extension by regulating microtubule remodeling and vinculin distribution. Neural Regen. Res. 2013, 8, 3027–3035. [Google Scholar] [CrossRef] [PubMed]
- McBeath, R.; Pirone, D.M.; Nelson, C.M.; Bhadriraju, K.; Chen, C.S. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell 2004, 6, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, A.B.; Hall, A. Rho GTPases: Biochemistry and biology. Annu. Rev. Cell Dev. Biol. 2005, 21, 247–269. [Google Scholar] [CrossRef]
- Zhou, X.; Zheng, Y. Cell type-specific signaling function of RhoA GTPase: Lessons from mouse gene targeting. J. Biol. Chem. 2013, 288, 36179–36188. [Google Scholar] [CrossRef]
- Stankiewicz, T.R.; Linseman, D.A. Rho family GTPases: Key players in neuronal development, neuronal survival, and neurodegeneration. Front. Cell. Neurosci. 2014, 8, 314. [Google Scholar] [CrossRef]
- Nakayama, A.Y.; Harms, M.B.; Luo, L. Small GTPases Rac and Rho in the maintenance of dendritic spines and branches in hippocampal pyramidal neurons. J. Neurosci. 2000, 20, 5329–5338. [Google Scholar] [CrossRef]
- Govek, E.; Hatten, M.E.; Van Aelst, L. The role of Rho GTPase proteins in CNS neuronal migration. Dev. Neurobiol. 2010, 71, 528–553. [Google Scholar] [CrossRef]
- Duman, J.G.; Mulherkar, S.; Tu, Y.K.; Cheng, J.X.; Tolias, K.F. Mechanisms for spatiotemporal regulation of Rho-GTPase signaling at synapses. Neurosci. Lett. 2015, 601, 4–10. [Google Scholar] [CrossRef]
- Fujita, Y.; Yamashita, T. Axon growth inhibition by RhoA/ROCK in the central nervous system. Front. Neurosci. 2014, 8, 338. [Google Scholar] [CrossRef] [PubMed]
- Loirand, G. Rho Kinases in Health and Disease: From Basic Science to Translational Research. Pharmacol. Rev. 2015, 67, 1074–1095. [Google Scholar] [CrossRef] [PubMed]
- Chellaiah, M.A.; Soga, N.; Swanson, S.; McAllister, S.; Alvarez, U.; Wang, D.; Dowdy, S.F.; Hruska, K.A. Rho-A is critical for osteoclast podosome organization, motility, and bone resorption. J. Biol. Chem. 2000, 275, 11993–12002. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Wang, J.; Zhao, J.; Xia, M.; Wang, H.; Sun, L.; Zhang, W.-B. RhoA/ROCK-TAZ Axis regulates bone formation within calvarial trans-sutural distraction osteogenesis. Cell. Signal. 2024, 121, 111300. [Google Scholar] [CrossRef]
- D’Angelo, R.C.; Wicha, M.S. Stem cells in normal development and cancer. Prog. Mol. Biol. Transl. Sci. 2010, 95, 113–158. [Google Scholar] [CrossRef]
- Artavanis-Tsakonas, S.; Rand, M.D.; Lake, R.J. Notch signaling: Cell fate control and signal integration in development. Science 1999, 284, 770–776. [Google Scholar] [CrossRef]
- Lampada, A.; Taylor, V. Notch signaling as a master regulator of adult neurogenesis. Front. Neurosci. 2023, 17, 1179011. [Google Scholar] [CrossRef]
- Zanotti, S.; Canalis, E. Notch Signaling and the Skeleton. Endocr. Rev. 2016, 37, 223–253. [Google Scholar] [CrossRef]
- Yoon, K.; Gaiano, N. Notch signaling in the mammalian central nervous system: Insights from mouse mutants. Nat. Neurosci. 2005, 8, 709–715, Erratum in Nat. Neurosci. 2005, 8, 1411. [Google Scholar] [CrossRef]
- Ho, D.M.; Artavanis-Tsakonas, S.; Louvi, A. The Notch pathway in CNS homeostasis and neurodegeneration. Wiley Interdiscip. Rev. Dev. Biol. 2019, 9, e358. [Google Scholar] [CrossRef]
- Canalis, E.; Adams, D.J.; Boskey, A.; Parker, K.; Kranz, L.; Zanotti, S. Notch signaling in osteocytes differentially regulates cancellous and cortical bone remodeling. J. Biol. Chem. 2013, 288, 25614–25625. [Google Scholar] [CrossRef]
- Luo, Z.; Shang, X.; Zhang, H.; Wang, G.; Massey, P.A.; Barton, S.R.; Kevil, C.G.; Dong, Y. Notch Signaling in Osteogenesis, Osteoclastogenesis, and Angiogenesis. Am. J. Pathol. 2019, 189, 1495–1500. [Google Scholar] [CrossRef] [PubMed]
- Tezuka, K.-I.; Yasuda, M.; Watanabe, N.; Morimura, N.; Kuroda, K.; Miyatani, S.; Hozumi, N. Stimulation of osteoblastic cell differentiation by Notch. J. Bone Miner. Res. 2002, 17, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Shu, B.; Tian, Y.; Chelly, M.; Morandi, M.M.; Barton, S.; Shang, X.; Dong, Y. Notch activation promotes osteoblast mineralization by inhibition of apoptosis. J. Cell. Physiol. 2018, 233, 6921–6928. [Google Scholar] [CrossRef] [PubMed]
- Deregowski, V.; Gazzerro, E.; Priest, L.; Rydziel, S.; Canalis, E. Notch 1 overexpression inhibits osteoblastogenesis by suppressing Wnt/β-catenin but not bone morphogenetic protein signaling. J. Biol. Chem. 2006, 281, 6203–6210. [Google Scholar] [CrossRef]
- Cao, J.; Wei, Y.; Lian, J.; Yang, L.; Zhang, X.; Xie, J.; Liu, Q.; Luo, J.; He, B.; Tang, M. Notch signaling pathway promotes osteogenic differentiation of mesenchymal stem cells by enhancing BMP9/Smad signaling. Int. J. Mol. Med. 2017, 40, 378–388. [Google Scholar] [CrossRef]
- Lin, G.L.; Hankenson, K.D. Integration of BMP, Wnt, and notch signaling pathways in osteoblast differentiation. J. Cell. Biochem. 2011, 112, 3491–3501. [Google Scholar] [CrossRef]
- Kode, A.; Mosialou, I.; Manavalan, S.J.; Rathinam, C.V.; Friedman, R.A.; Teruya-Feldstein, J.; Bhagat, G.; Berman, E.; Kousteni, S. FoxO1-dependent induction of acute myeloid leukemia by osteoblasts in mice. Leukemia 2015, 30, 1–13. [Google Scholar] [CrossRef]
- Carey, K.A.; Farnfield, M.M.; Tarquinio, S.D.; Cameron-Smith, D. Impaired expression of Notch signaling genes in aged human skeletal muscle. J. Gerontol. Ser. A 2007, 62, 9–17. [Google Scholar] [CrossRef]
- Engin, F.; Yao, Z.; Yang, T.; Zhou, G.; Bertin, T.; Jiang, M.M.; Chen, Y.; Wang, L.; Zheng, H.; Sutton, R.E.; et al. Dimorphic effects of Notch signaling in bone homeostasis. Nat. Med. 2008, 14, 299–305. [Google Scholar] [CrossRef]
- Canalis, E.; Parker, K.; Feng, J.Q.; Zanotti, S. Osteoblast lineage-specific effects of notch activation in the skeleton. Endocrinology 2013, 154, 623–634. [Google Scholar] [CrossRef]
- Yorgan, T.; Vollersen, N.; Riedel, C.; Jeschke, A.; Peters, S.; Busse, B.; Amling, M.; Schinke, T. Osteoblast-specific Notch2 inactivation causes increased trabecular bone mass at specific sites of the appendicular skeleton. Bone 2016, 87, 136–146. [Google Scholar] [CrossRef]
- Fukushima, H.; Nakao, A.; Okamoto, F.; Shin, M.; Kajiya, H.; Sakano, S.; Bigas, A.; Jimi, E.; Okabe, K. The Association of Notch2 and NF-κB Accelerates RANKL-Induced Osteoclastogenesis. Mol. Cell. Biol. 2008, 28, 6402–6412. [Google Scholar] [CrossRef]
- Engin, F.; Bertin, T.; Ma, O.; Jiang, M.M.; Wang, L.; Sutton, R.E.; Donehower, L.A.; Lee, B. Notch signaling contributes to the pathogenesis of human osteosarcomas. Hum. Mol. Genet. 2009, 18, 1464–1470. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.P.M. How the NOTCH pathway contributes to the ability of osteosarcoma cells to metastasize. Cancer Treat. Res. 2009, 152, 479–496. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Setoguchi, T.; Hirotsu, M.; Gao, H.; Sasaki, H.; Matsunoshita, Y.; Komiya, S. Inhibition of Notch pathway prevents osteosarcoma growth by cell cycle regulation. Br. J. Cancer 2009, 100, 1957–1965. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wei, J.; Xiao, W.; Xie, W.; Ru, Q.; Chen, L.; Wu, Y.; Mobasheri, A.; Li, Y. Insights into the Notch signaling pathway in degenerative musculoskeletal disorders: Mechanisms and perspectives. Biomed. Pharmacother. 2023, 169, 115884. [Google Scholar] [CrossRef]
- Bae, Y.; Yang, T.; Zeng, H.-C.; Campeau, P.M.; Chen, Y.; Bertin, T.; Dawson, B.C.; Munivez, E.; Tao, J.; Lee, B.H. miRNA-34c regulates Notch signaling during bone development. Hum. Mol. Genet. 2012, 21, 2991–3000. [Google Scholar] [CrossRef]
- Shiozawa, Y.; Pienta, K.J.; Taichman, R.S. Hematopoietic stem cell niche is a potential therapeutic target for bone metastatic tumors. Clin. Cancer Res. 2011, 17, 5553–5558. [Google Scholar] [CrossRef]
- Lian, J.B.; Stein, G.S.; van Wijnen, A.J.; Stein, J.L.; Hassan, M.Q.; Gaur, T.; Zhang, Y. MicroRNA control of bone formation and homeostasis. Nat. Rev. Endocrinol. 2012, 8, 212–227. [Google Scholar] [CrossRef]
- Inose, H.; Ochi, H.; Kimura, A.; Fujita, K.; Xu, R.; Sato, S.; Iwasaki, M.; Sunamura, S.; Takeuchi, Y.; Fukumoto, S.; et al. A microRNA regulatory mechanism of osteoblast differentiation. Proc. Natl. Acad. Sci. USA 2009, 106, 20794–20799. [Google Scholar] [CrossRef]
- Dilawar, M.; Yu, X.; Jin, Y.; Yang, J.; Lin, S.; Liao, J.; Dai, Q.; Zhang, X.; Nisar, M.F.; Chen, G. Notch signaling pathway in osteogenesis, bone development, metabolism, and diseases. FASEB J. 2025, 39, e70417. [Google Scholar] [CrossRef]
- Baek, K.; Hwang, H.R.; Park, H.; Kwon, A.; Qadir, A.S.; Ko, S.H.; Woo, K.M.; Ryoo, H.-M.; Kim, G.-S.; Baek, J.-H. TNF-α upregulates sclerostin expression in obese mice fed a high-fat diet. J. Cell. Physiol. 2013, 229, 640–650. [Google Scholar] [CrossRef]
- Marini, F.; Giusti, F.; Palmini, G.; Brandi, M.L. Role of Wnt signaling and sclerostin in bone and as therapeutic targets in skeletal disorders. Osteoporos. Int. 2022, 34, 213–238. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, T.; Shah, M.; Doyle, C.; Greenslade, K.; Penney, M.; Creeke, P.; Kotian, A.; Ke, H.Z.; Naidoo, V.; Holdsworth, G. Recombinant sclerostin inhibits bone formation in vitro and in a mouse model of sclerosteosis. J. Orthop. Transl. 2021, 29, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Ashifa, N.; Viswanathan, K.; Sundaram, R.; Srinivasan, S. Sclerostin and its role as a bone modifying agent in periodontal disease. J. Oral Biosci. 2021, 63, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, R.; Koide, M.; Udagawa, N.; Kobayashi, Y. Positive and Negative Regulators of Sclerostin Expression. Int. J. Mol. Sci. 2022, 23, 4895. [Google Scholar] [CrossRef]
- Roggia, C.; Tamone, C.; Cenci, S.; Pacifici, R.; Isaia, G.C. Role of TNF-Alpha Producing T-Cells in Bone Loss Induced by Estrogen Deficiency. Minerva Med. 2004, 95, 125–132. Available online: https://pubmed.ncbi.nlm.nih.gov/15272247/ (accessed on 25 July 2025).
- Cao, J.J. Effects of obesity on bone metabolism. J. Orthop. Surg. Res. 2011, 6, 30. [Google Scholar] [CrossRef]
- Pedersen, M.; Bruunsgaard, H.; Weis, N.; Hendel, H.W.; Andreassen, B.U.; Eldrup, E.; Dela, F.; Pedersen, B.K. Circulating levels of TNF-alpha and IL-6-relation to truncal fat mass and muscle mass in healthy elderly individuals and in patients with type-2 diabetes. Mech. Ageing Dev. 2003, 124, 495–502. [Google Scholar] [CrossRef]
- Bastard, J.P.; Maachi, M.; Lagathu, C.; Kim, M.J.; Caron, M.; Vidal, H.; Capeau, J.; Feve, B. Recent Advances in the Relationship Between Obesity, Inflammation, and Insulin Resistance—PubMed 2006:4–12. Available online: https://pubmed.ncbi.nlm.nih.gov/16613757/ (accessed on 25 July 2025).
- Lin, P.; Gan, Y.B.; He, J.; Lin, S.E.; Xu, J.K.; Chang, L.; Zhao, L.-M.; Zhu, J.; Zhang, L.; Huang, S.; et al. Advancing skeletal health and disease research with single-cell RNA sequencing. Mil. Med. Res. 2024, 11, 33. [Google Scholar] [CrossRef]
- Cohen, Y.C.; Zada, M.; Wang, S.-Y.; Bornstein, C.; David, E.; Moshe, A.; Baoguo, L.; Shlomi-Loubaton, S.; Gatt, M.E.; Gur, C.; et al. Identification of resistance pathways and therapeutic targets in relapsed multiple myeloma patients through single-cell sequencing. Nat. Med. 2021, 27, 491–503. [Google Scholar] [CrossRef]
- Park, Y.; Kc, N.; Paneque, A.; Cole, P.D. Tau, Glial Fibrillary Acidic Protein, and Neurofilament Light Chain as Brain Protein Biomarkers in Cerebrospinal Fluid and Blood for Diagnosis of Neurobiological Diseases. Int. J. Mol. Sci. 2024, 25, 6295. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, A.; Chen, M.; Cao, J. Advances in Circulating Biomarkers for Neurodegenerative Diseases, Traumatic Brain Injuries, and Central Nervous System Tumors. Ann. Lab. Med. 2025, 45, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Sosa-Ortiz, A.L.; Acosta-Castillo, I.; Prince, M.J. Epidemiology of dementias and Alzheimer’s disease. Arch. Med. Res. 2012, 43, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.M.; Mendelson, D.A. Epidemiology of fragility fractures. Arch. Med. Res. 2012, 43, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Hebert, L.E.; Weuve, J.; Scherr, P.A.; Evans, D.A. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 2013, 80, 1778–1783. [Google Scholar] [CrossRef]
- Sharma, S.; Mueller, C.; Stewart, R.; Veronese, N.; Vancampfort, D.; Koyanagi, A.; Lamb, S.E.; Perera, G.; Stubbs, B. Predictors of Falls and Fractures Leading to Hospitalization in People with Dementia: A Representative Cohort Study. J. Am. Med. Dir. Assoc. 2018, 19, 607–612. [Google Scholar] [CrossRef]
- Jeon, J.H.; Park, J.H.; Oh, C.; Chung, J.K.; Song, J.Y.; Kim, S.; Lee, S.H.; Jang, J.W.; Kim, Y.J. Dementia is Associated with an Increased Risk of Hip Fractures: A Nationwide Analysis in Korea. J. Clin. Neurol. 2019, 15, 243–249. [Google Scholar] [CrossRef]
- Zhou, R.; Zhou, H.; Rui, L.; Xu, J. Bone loss and osteoporosis are associated with conversion from mild cognitive impairment to Alzheimer’s disease. Curr. Alzheimer Res. 2014, 11, 706–713. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, H.; Qiu, F.; He, J.; Chen, J. Cognitive impairment and risks of osteoporosis: A systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2022, 106, 104879. [Google Scholar] [CrossRef]
- Zhao, Y.; Shen, L.; Ji, H.F. Alzheimer’s disease and risk of hip fracture: A meta-analysis study. Sci. World J. 2012, 2012, 872173. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Wang, L. Alzheimer’s Disease is an Important Risk Factor of Fractures: A Meta-analysis of Cohort Studies. Mol. Neurobiol. 2016, 54, 3230–3235. [Google Scholar] [CrossRef] [PubMed]
- Hsu, B.; Bleicher, K.; Waite, L.M.; Naganathan, V.; Blyth, F.M.; Handelsman, D.J.; Le Couter, D.G.; Seibel, M.J.; Cumming, R.G. Community-dwelling older men with dementia are at high risk of hip fracture, but not any other fracture: The Concord Health and Aging in Men Project. Geriatr. Gerontol. Int. 2018, 18, 1479–1484. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.H.; Chuang, C.S.; Hung, C.H.; Lin, C.L.; Sung, F.C.; Tang, C.H.; Hsu, H.-C.; Chung, C.-J. Fracture as an independent risk factor of dementia: A nationwide population-based cohort study. Medicine 2014, 93, e188, Erratum in Medicine 2016, 95, e158e. [Google Scholar] [CrossRef]
- Kuo, L.Y.; Hsu, P.T.; Wu, W.T.; Lee, R.P.; Wang, J.H.; Chen, H.W.; Chen, I.H.; Yu, T.C.; Peng, C.H.; Liu, K.L.; et al. The incidence of mental disorder increases after hip fracture in older people: A nationwide cohort study. BMC Geriatr. 2021, 21, 249. [Google Scholar] [CrossRef]
- Takahashi, S.; Hoshino, M.; Tsujio, T.; Terai, H.; Suzuki, A.; Namikawa, T.; Kato, M.; Matsumura, A.; Takayama, K.; Nakamura, H. Risk factors for cognitive decline following osteoporotic vertebral fractures: A multicenter cohort study. J. Orthop. Sci. 2017, 22, 834–839. [Google Scholar] [CrossRef]
- Yaffe, K.; Browner, W.; Cauley, J.; Launer, L.; Harris, T. Association between bone mineral density and cognitive decline in older women. J. Am. Geriatr. Soc. 1999, 47, 1176–1182. [Google Scholar] [CrossRef]
- Zhou, R.; Deng, J.; Zhang, M.; Zhou, H.D.; Wang, Y.J. Association between bone mineral density and the risk of Alzheimer’s disease. J. Alzheimer’s Dis. 2011, 24, 101–108. [Google Scholar] [CrossRef]
- Kostev, K.; Hadji, P.; Jacob, L. Impact of Osteoporosis on the Risk of Dementia in Almost 60,000 Patients Followed in General Practices in Germany. J. Alzheimer’s Dis. 2018, 65, 401–407. [Google Scholar] [CrossRef]
- Kang, H.G.; Park, H.Y.; Ryu, H.U.; Suk, S.H. Bone mineral loss and cognitive impairment: The PRESENT project. Medicine 2018, 97, e12755. [Google Scholar] [CrossRef]
- Stefanidou, M.M.; O’donnell, A.B.; Himali, J.J.; DeCarli, C.M.; Satizabal, C.; Beiser, A.S.; Seshadri, S.; Zadly, T. Bone Mineral Density Measurements and Association with Brain Structure and Cognitive Function: The Framingham Offspring Cohort. Alzheimer Dis. Assoc. Disord. 2021, 35, 291–297, Erratum in Alzheimer Dis. Assoc. Disord. 2022, 36, 95. [Google Scholar] [CrossRef] [PubMed]
- Schurman, C.A.; Burton, J.B.; Rose, J.; Ellerby, L.M.; Alliston, T.; Schilling, B. Molecular and Cellular Crosstalk between Bone and Brain: Accessing Bidirectional Neural and Musculoskeletal Signaling during Aging and Disease. J. Bone Metab. 2023, 30, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Haberland, M.; Schilling, A.F.; Rueger, J.M.; Amling, M. Brain and bone: Central regulation of bone mass. A new paradigm in skeletal biology. J. Bone Jt. Surg. 2001, 83, 1871–1876. [Google Scholar] [CrossRef] [PubMed]
- Karsenty, G.; Oury, F. The central regulation of bone mass, the first link between bone remodeling and energy metabolism. J. Clin. Endocrinol. Metab. 2010, 95, 4795–4801. [Google Scholar] [CrossRef]
- Idelevich, A.; Baron, R. Brain to bone: What is the contribution of the brain to skeletal homeostasis? Bone 2018, 115, 31–42. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, S.; Sun, X. Alzheimer’s Disease and Impaired Bone Microarchitecture, Regeneration and Potential Genetic Links. Life 2023, 13, 373. [Google Scholar] [CrossRef]
- Pan, J.X.; Tang, F.; Xiong, F.; Xiong, L.; Zeng, P.; Wang, B.; Zhao, K.; Guo, H.; Shun, C.; Xia, W.F.; et al. APP promotes osteoblast survival and bone formation by regulating mitochondrial function and preventing oxidative stress. Cell Death Dis. 2018, 9, 1077. [Google Scholar] [CrossRef]
- Fehsel, K.; Christl, J. Comorbidity of osteoporosis and Alzheimer’s disease: Is ‘AKT’ -ing on cellular glucose uptake the missing link? Ageing Res. Rev. 2022, 76, 101592. [Google Scholar] [CrossRef]
- Clark, D.; Nakamura, M.; Miclau, T.; Marcucio, R. Effects of Aging on Fracture Healing. Curr. Osteoporos. Rep. 2017, 15, 601–608. [Google Scholar] [CrossRef]

| Brain–Bone Axis Main Players | Origin | Action | Pathology/ Condition |
|---|---|---|---|
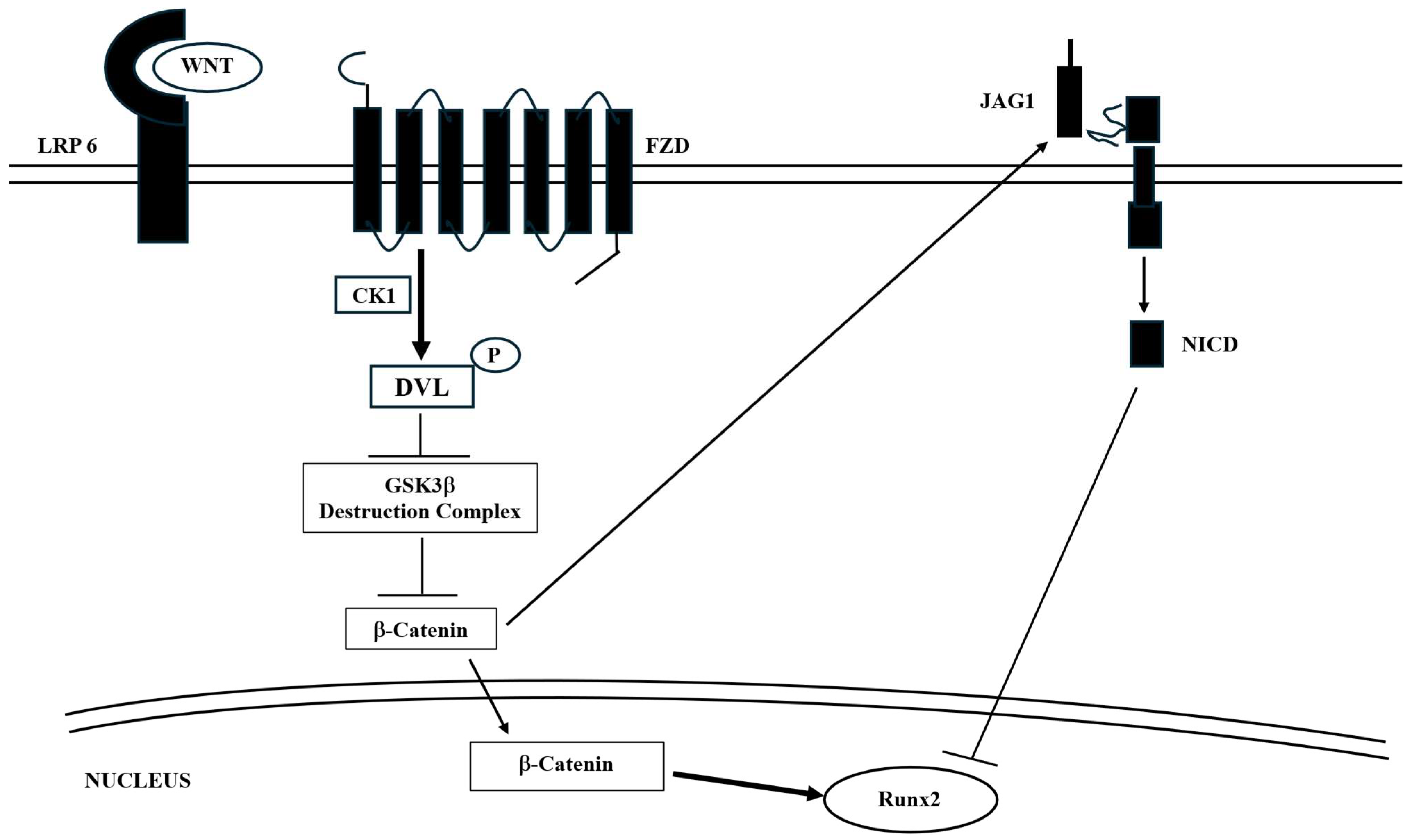
| Wnt-β-catenin pathway | Ubiquitous | - Promotes osteoblast differentiation, proliferation, and mineralization. - Induces OPG expression to prevent bone resorption. | Osteoporosis; AD |
| RANKL/RANK/OPG system | T cells/bone cells/osteoblasts, bone marrow stromal cells, B cells, and dendritic cells | - Pivotal role in regulating bone remodeling by balancing bone formation and resorption. - Crucial role in immune and related processes regulation. - Critical role in modulating neuroinflammatory processes. | Rheumatoid arthritis; osteoporosis; AD |
| TREM2 | Single immunoglobulin variable (IgV) domain receptor family | - Pivotal role in maintaining CNS tissue homeostasis. - Crucial role, in conjunction with the Wnt pathway, in regulating β-catenin signaling to promote osteoblast differentiation and proliferation. | AD; PLOSL |
| FGF-23 | Bone | - Regulation of bone remodeling by influencing the differentiation of osteoblasts and osteocytes. - Plays a pivotal role in promoting neurogenesis, which is essential for preserving cognitive functions. | Cognitive and memory decline; bone remodeling alterations |
| Leptin | Adipocytes | - Pivotal role in bone metabolism by direct and indirect action. Direct action: stimulates the proliferation of osteoblasts while simultaneously inhibiting the activity of osteoclasts. Indirect action: regulation of the hypothalamic–pituitary–adrenal (HPA) and growth hormone/insulin-like growth factor-1 (GH/IGF-1) axes, both of which are essential for maintaining bone homeostasis. - Neuroprotective action on the CNS. | Obesity; AD; anorexia nervosa; hypothalamic amenorrhea |
| Neuropeptide Y (NPY) | Hypothalamus (arcuate nucleus, ARC) | -Neural mediator in bone remodeling processes and the maintenance of bone homeostasis. | AD; PD |
| Semaphorins (Sem) | Ubiquitous | - Regulators of various biological processes such as angiogenesis, immune responses, and bone metabolism. - In particular, in bone: #Sem3A: Regulator of both osteoblast and osteoclast activity, acting as a positive regulator in osteoblastogenesis (enhancing Wnt-induced signaling) and as a negative regulator in osteoclast differentiation. #Sema4D: Exerts an inhibitory effect on osteoblasts while simultaneously stimulating osteoclast formation and activity. #Sema7A: Stimulator of osteoclast and osteoblast migration during bone remodeling. | Cancer; autoimmune diseases (like rheumatoid arthritis and multiple sclerosis); metabolic disorders |
| Dopamine | Hypothalamus and adrenal glands (in smaller percentage) | - Crucial roles in neuromodulation, including movement and motor control, spatial memory function, and cognitive function. - Pivotal role in maintaining bone homeostasis, directly by enhancing the activity of osteoblasts and inhibiting osteoclast activity; indirectly by regulating the secretion of hormones such as PTH and vitamin D. | Osteoporosis; cognitive decline; PD |
| Serotonin (5-HT) | CNS (by serotonergic neurons); gastrointestinal tract and platelets (peripheric production) | - 5-HT produced as a neurotransmitter acts centrally to regulate various functions, including mood, reward, anger, perception, aggression, attention, and memory. At the peripheral level, 5-HT plays a crucial role in regulating major organ functions, such as glucose homeostasis and lipid metabolism. - In bone, 5-HT produced as a neurotransmitter acts centrally to inhibit bone resorption and promote bone formation. Conversely, at the peripheral level, 5-HT directly inhibits bone formation. | Depression; osteoporosis; skeletal integrity loss associated with long-term antidepressive treatment |
| Norepinephrine (NE) | Locus ceruleus | - Crucial role in cognitive processes, particularly cognitive flexibility and active memory. - Significant action on bone metabolism and function by binding to α- and β-ARs on the surface of bone cells. Binding to α1-AR leads to the expression and release of RANKL triggering osteoclastogenesis. Binding to β1-AR has anabolic effects, stimulating osteoblast activity and inhibiting osteoclast activity. Binding to the β2-AR has catabolic effects, leading to inhibition of bone formation. | Cognitive impairments; sleep disturbances; PD; bone fragility and risk fracture; skeletal integrity loss associated with long-term antidepressive treatment |
| Estrogens | Ovaries, corpus luteum, placenta and adipose tissue | - Crucial role in neural development in the adult brain, participating in the processes of differentiation, proliferation, and protection against inflammatory processes in neurons, particularly dopaminergic neurons. -Significant role in the processes of skeletal growth and maintenance of bone mass, amplifying osteoblastic activity and inhibiting osteoclast activity. | Bone loss and an increased risk of osteoporosis; PD |
| Parathormone (PTH) and PTH-related peptides | Parathyroid glands | - Pivotal role in regulating calcium-phosphorus and bone metabolism. PTH stimulates bone resorption by acting on the RRO pathway. - PTHrP plays a protective role in neurons against excitotoxicity and seems to be involved in modulating nerve regeneration and cerebral vasculature. | Primary hypoparathyroidism and hyperparathyroidism with related neurological and bone manifestations, such as reduction in BMD and an increased risk of osteoporotic fractures |
| Osteocalcin (OC) | Mature osteoblasts | - Plays a pivotal role in the mineralization process, stabilizing and facilitating the formation of mineralized bone tissue. - Is involved in the regulation of phosphate-calcium metabolism, which is essential for maintaining skeletal health. - Promotes neurite outgrowth. - Regulate a wide range of neuronal activities associated with cognitive function. | Depression; cognitive disfunction; AD |
| RhoA/ROCK pathway | Various tissues | - Crucial signal transduction system that plays a pivotal role in cell growth, differentiation, migration, and development. - Plays a pivotal role in neural development and survival and in regulating osteoclastic and osteoblastic activity. | Several neurological disorders; osteoporosis |
| Notch | Ubiquitous | Intercellular Notch signaling is crucial for diverse developmental pathways and for maintaining homeostasis in various cell types. - Plays a key role in maintaining the delicate balance between differentiation and the preservation of neural stem cells to assure the proper functioning of the CNS. - Plays a crucial role, in conjunction with the Wnt/β-catenin pathway, in regulating osteoblast differentiation. | Osteoporosis; osteosarcoma |
| TNF-α | Various cell types, particularly mononuclear phagocytes | - TNF-α activates cytocidal functions, playing a crucial role in the host’s defense. - TNF-α increases the expression of RANKL in bone cells, which promotes osteoclast formation and bone resorption. Additionally, it upregulates the expression of sclerostin, which contributes to bone loss by inhibiting the Wnt signaling pathway. | Neuroinflammation; PD; bone loss; Van Buchem’s disease |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massaccesi, L.; Corsi Romanelli, M.M.; Galliera, E. Brain–Bone Axis in Physiological and Pathological Conditions. Int. J. Mol. Sci. 2025, 26, 9822. https://doi.org/10.3390/ijms26199822
Massaccesi L, Corsi Romanelli MM, Galliera E. Brain–Bone Axis in Physiological and Pathological Conditions. International Journal of Molecular Sciences. 2025; 26(19):9822. https://doi.org/10.3390/ijms26199822
Chicago/Turabian StyleMassaccesi, Luca, Massimiliano Marco Corsi Romanelli, and Emanuela Galliera. 2025. "Brain–Bone Axis in Physiological and Pathological Conditions" International Journal of Molecular Sciences 26, no. 19: 9822. https://doi.org/10.3390/ijms26199822
APA StyleMassaccesi, L., Corsi Romanelli, M. M., & Galliera, E. (2025). Brain–Bone Axis in Physiological and Pathological Conditions. International Journal of Molecular Sciences, 26(19), 9822. https://doi.org/10.3390/ijms26199822








