P2Y2 Receptor Signaling in Health and Disease
Abstract
1. Introduction
1.1. Nucleotide Receptors (P2X Receptors and P2Y Receptors)
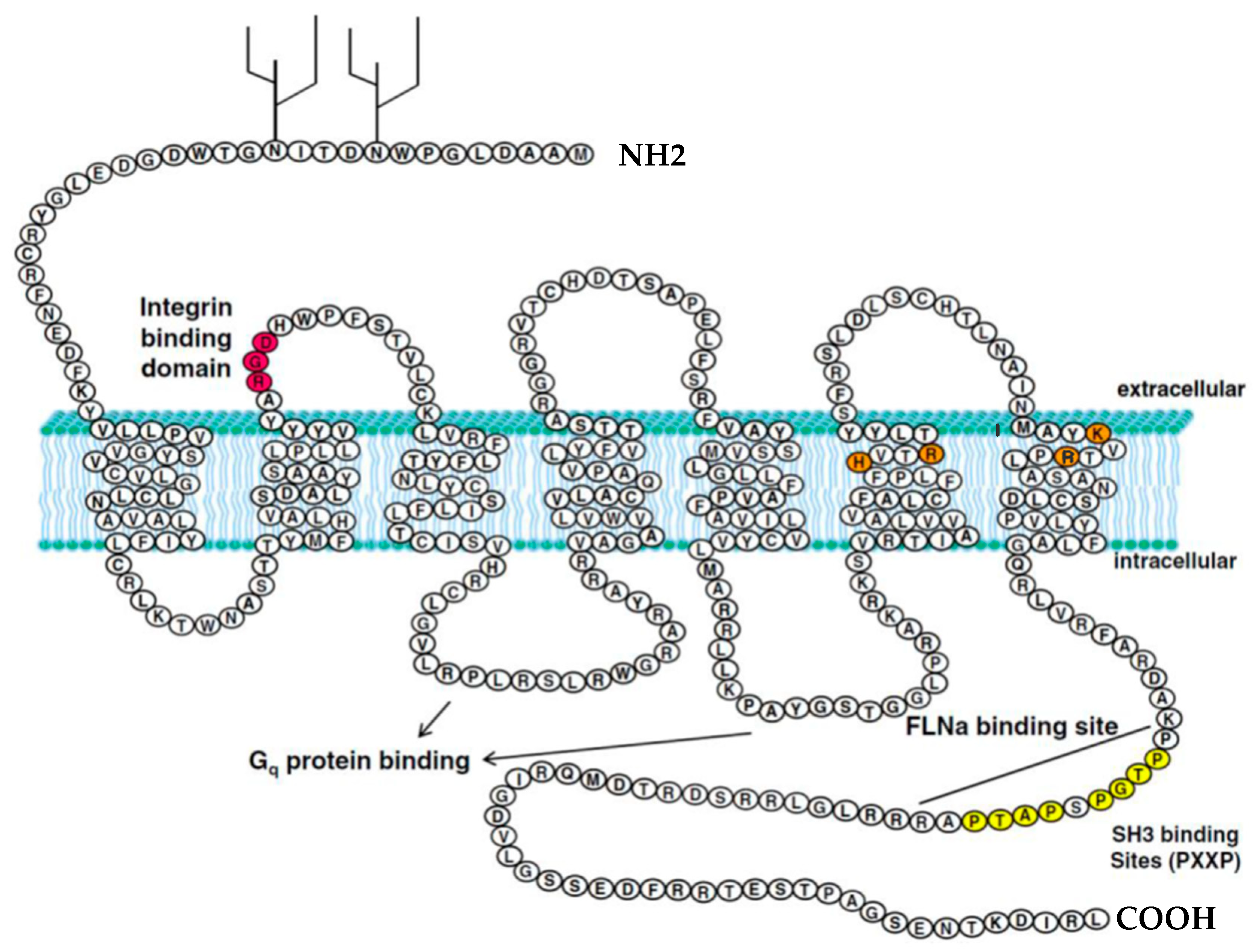
1.2. Structure of the P2Y2 Receptor
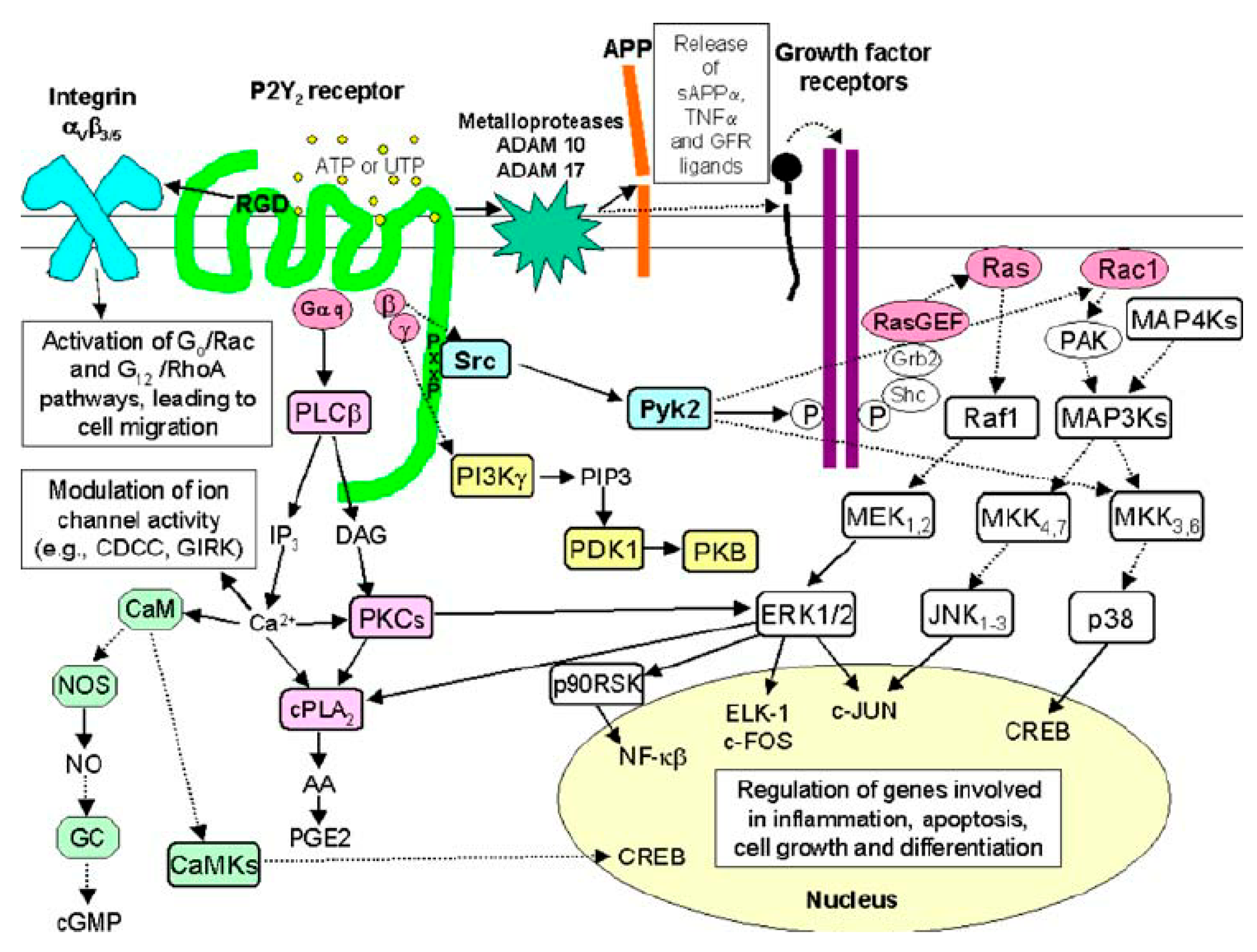
1.3. P2Y2 Receptor Signaling
1.4. Physiological Functions of the P2Y2 Receptor
2. Pathological Roles of the P2Y2 Receptor
2.1. Neurological Disorders: Alzheimer’s Disease
2.2. Ocular Disorders: Dry Eye Disease
2.3. Respiratory Disorders: Cystic Fibrosis
2.4. Inflammatory Disorders: Inflammatory Bowel Disease
2.5. Oncology: Breast Cancer
2.6. Cardiovascular Disorders: Blood Pressure Regulation
2.7. Infectious Diseases: HIV-1
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Idzko, M.; Ferrari, D.; Eltzschig, H.K. Nucleotide signalling during inflammation. Nature 2014, 509, 310–317. [Google Scholar] [CrossRef]
- Alberto, A.V.P.; da Silva Ferreira, N.C.; Bonavita, A.G.C.; Nihei, O.K.; de Farias, F.P.; da Cunha Bisaggio, R.; de Albuquerque, C.; Savino, W.; Coutinho-Silva, R.; Muanis Persechini, P.; et al. Physiologic roles of P2 receptors in leukocytes. J. Leukoc. Biol. 2022, 112, 983–1012. [Google Scholar] [CrossRef]
- Saul, A.; Hausmann, R.; Kless, A.; Nicke, A. Heteromeric assembly of P2X subunits. Front. Cell. Neurosci. 2013, 7, 250. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, K.A.; Delicado, E.G.; Gachet, C.; Kennedy, C.; von Kügelgen, I.; Li, B.; Miras-Portugal, M.T.; Novak, I.; Schöneberg, T.; Perez-Sen, R.; et al. Update of P2Y receptor pharmacology: IUPHAR Review 27. Br. J. Pharmacol. 2020, 177, 2413–2433. [Google Scholar] [CrossRef] [PubMed]
- Wihlborg, A.; Malmsjö, M.; Eyjolfsson, A.; Gustafsson, R.; Jacobson, K.; Erlinge, D. Extracellular nucleotides induce vasodilatation in human arteries via prostaglandins, nitric oxide and endothelium-derived hyperpolarising factor. Br. J. Pharmacol. 2003, 138, 1451–1458. [Google Scholar] [CrossRef]
- Eun, S.Y.; Park, S.W.; Lee, J.H.; Chang, K.C.; Kim, H.J. P2Y2R activation by nucleotides released from oxLDL-treated endothelial cells (ECs) mediates the interaction between ECs and immune cells through RAGE expression and reactive oxygen species production. Free Radic. Biol. Med. 2014, 69, 157–166. [Google Scholar]
- Burnstock, G. Pathophysiology and Therapeutic Potential of Purinergic Signaling. Pharmacol. Rev. 2006, 58, 58–86. [Google Scholar] [CrossRef] [PubMed]
- Marques, R.D.; Praetorius, H.A.; Leipziger, J. P2Y2 receptor knock-out mice display normal NaCl absorption in medullary thick ascending limb. Front. Physiol. 2013, 4, 55874. [Google Scholar] [CrossRef]
- Wu, X.M.; Zhang, N.; Li, J.S.; Yang, Z.H.; Huang, X.L.; Yang, X.F. Purinergic receptors mediate endothelial dysfunction and participate in atherosclerosis. Purinergic Signal 2023, 19, 265–272. [Google Scholar]
- Abbracchio, M.P.; Burnstock, G.; Verkhratsky, A.; Zimmermann, H. Purinergic signalling in the nervous system: An overview. Trends Neurosci. 2009, 32, 19–29. [Google Scholar] [CrossRef]
- Fountain, S.J. Primitive ATP-activated P2X receptors: Discovery, function and pharmacology. Front. Cell. Neurosci. 2013, 7, 247. [Google Scholar]
- Illes, P.; Ulrich, H.; Chen, J.F.; Tang, Y. Purinergic Receptors in Cognitive Disturbances. Neurobiol. Dis. 2023, 185, 106229. Available online: https://www.sciencedirect.com/science/article/pii/S0969996123002449 (accessed on 1 October 2025). [CrossRef]
- Khalafalla, F.G.; Greene, S.; Khan, H.; Ilves, K.; Monsanto, M.M.; Alvarez, R.; Chavarria, M.; Nguyen, J.; Norman, B.; Dembitsky, W.P.; et al. P2Y2 Nucleotide Receptor Prompts Human Cardiac Progenitor Cell Activation by Modulating Hippo Signaling. Circ. Res. 2017, 121, 1224–1236. [Google Scholar] [CrossRef]
- Pawlowska, R.; Radzikowska-Cieciura, E.; Jafari, S.; Fastyn, J.; Korkus, E.; Gendaszewska-Darmach, E.; Zhao, G.; Snaar-Jagalska, E.; Chworos, A. Double-modified, thio and methylene ATP analogue facilitates wound healing in vitro and in vivo. Sci. Rep. 2024, 14, 13148. [Google Scholar] [CrossRef]
- Xu, P.; Feng, X.; Luan, H.; Wang, J.; Ge, R.; Li, Z.; Bian, J. Current knowledge on the nucleotide agonists for the P2Y2 receptor. Bioorganic Med. Chem. 2018, 26, 366–375. [Google Scholar] [CrossRef]
- Lan, B.; Zhang, S.; Chen, K.; Dai, S.; Fei, J.; Gao, K.; Sun, X.; Lin, B.; Liu, X. Structural insight into the self-activation and G-protein coupling of P2Y2 receptor. Cell Discov. 2025, 11, 47. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Aranguez, A.; Pintor, J. Focus on Molecules: Purinergic P2Y2 receptor. Exp. Eye Res. 2012, 105, 83–84. [Google Scholar] [CrossRef]
- Peterson, T.S.; Camden, J.M.; Wang, Y.; Seye, C.I.; Wood, W.G.; Sun, G.Y.; Erb, L.; Petris, M.J.; Weisman, G.A. P2Y2 Nucleotide Receptor-Mediated Responses in Brain Cells. Mol. Neurobiol. 2010, 41, 356–366. [Google Scholar] [CrossRef]
- Seye, C.I.; Gadeau, A.-P.; Daret, D.; Dupuch, F.; Alzieu, P.; Capron, L.; Desgranges, C. Overexpression of the P2Y2 Purinoceptor in Intimal Lesions of the Rat Aorta. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 3602–3610. [Google Scholar] [CrossRef] [PubMed]
- Velázquez, B.; Garrad, R.C.; Weisman, G.A.; González, F.A. Differential agonist-induced desensitization of P2Y2 nucleotide receptors by ATP and UTP. Mol. Cell. Biochem. 2000, 206, 75–89. [Google Scholar] [CrossRef]
- Zemskov, E.; Lucas, R.; Verin, A.D.; Umapathy, N.S. P2Y receptors as regulators of lung endothelial barrier integrity. J. Cardiovasc. Dis. Res. 2011, 2, 14–22. [Google Scholar] [CrossRef]
- Liebmann, C. G Protein-Coupled Receptors and their Signaling Pathways: Classical Therapeutical Targets Susceptible to Novel Therapeutic Concepts. Curr. Pharm. Des. 2004, 10, 1937–1958. [Google Scholar] [CrossRef]
- Falzone, M.E.; MacKinnon, R. Gβγ activates PIP2 hydrolysis by recruiting and orienting PLCβ on the membrane surface. Proc. Natl. Acad. Sci. USA 2023, 120, e2301121120. [Google Scholar] [CrossRef]
- Land, M.; Rubin, C.S. A Calcium- and Diacylglycerol-Stimulated Protein Kinase C (PKC), Caenorhabditis elegans PKC-2, Links Thermal Signals to Learned Behavior by Acting in Sensory Neurons and Intestinal Cells. Mol. Cell. Biol. 2017, 37, e00192-17. [Google Scholar] [CrossRef]
- Raqeeb, A.; Sheng, J.; Ao, N.; Braun, A.P. Purinergic P2Y2 receptors mediate rapid Ca2+ mobilization, membrane hyperpolarization and nitric oxide production in human vascular endothelial cells. Cell Calcium 2011, 49, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Flores, R.V.; Hernández-Pérez, M.G.; Aquino, E.; Garrad, R.C.; Weisman, G.A.; Gonzalez, F.A. Agonist-induced phosphorylation and desensitization of the P2Y2 nucleotide receptor. Mol. Cell. Biochem. 2005, 280, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Erb, L.; Liao, Z.; Seye, C.I.; Weisman, G.A. P2 receptors: Intracellular signaling. Pflug. Arch. 2006, 452, 552–562. [Google Scholar] [CrossRef]
- Woods, L.T.; Forti, K.M.; Shanbhag, V.C.; Camden, J.M.; Weisman, G.A. P2Y receptors for extracellular nucleotides: Contributions to cancer progression and therapeutic implications. Biochem. Pharmacol. 2021, 187, 114406. [Google Scholar] [CrossRef] [PubMed]
- Elliott, M.R.; Chekeni, F.B.; Trampont, P.C.; Lazarowski, E.R.; Kadl, A.; Walk, S.F.; Park, D.; Woodson, R.I.; Ostankovich, M.; Sharma, P.; et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 2009, 461, 282–286. [Google Scholar] [CrossRef]
- Seye, C.I.; Yu, N.; González, F.A.; Erb, L.; Weisman, G.A. The P2Y2 Nucleotide Receptor Mediates Vascular Cell Adhesion Molecule-1 Expression through Interaction with VEGF Receptor-2 (KDR/Flk-1). J. Biol. Chem. 2004, 279, 35679–35686. [Google Scholar] [CrossRef]
- Müller, T.; Robaye, B.; Vieira, R.P.; Ferrari, D.; Grimm, M.; Jakob, T.; Martin, S.F.; di Virgilio, F.; Boeynaems, J.-.M.; Virchow, J.C.; et al. The purinergic receptor P2Y2 receptor mediates chemotaxis of dendritic cells and eosinophils in allergic lung inflammation. Allergy 2010, 65, 1545–1553. [Google Scholar] [CrossRef]
- Jasmer, K.J.; Woods, L.T.; Forti, K.M.; Martin, A.L.; Camden, J.M.; Colonna, M.; Weisman, G.A. P2Y2 receptor antagonism resolves sialadenitis and improves salivary flow in a Sjögren’s syndrome mouse model. Arch. Oral Biol. 2021, 124, 105067. [Google Scholar] [CrossRef]
- Ferrari, D.; Idzko, M.; Dichmann, S.; Purlis, D.; Virchow, C.; Norgauer, J.; Chiozzi, P.; di Virgilio, F.; Luttmann, W. P2 purinergic receptors of human eosinophils: Characterization and coupling to oxygen radical production. FEBS Lett. 2000, 486, 217–224. [Google Scholar] [CrossRef]
- Jin, J.; Rao Dasari, V.; Sistare, F.D.; Kunapuli, S.P. Distribution of P2Y receptor subtypes on haematopoietic cells. Br. J. Pharmacol. 1998, 123, 789–794. [Google Scholar] [CrossRef]
- Wang, L.; Jacobsen, S.E.W.; Bengtsson, A.; Erlinge, D. P2 receptor mRNA expression profiles in human lymphocytes, monocytes and CD34+ stem and progenitor cells. BMC Immunol. 2004, 5, 16. [Google Scholar] [CrossRef]
- Gorini, S.; Callegari, G.; Romagnoli, G.; Mammi, C.; Mavilio, D.; Rosano, G.; Fini, M.; di Virgilio, F.; Gulinelli, S.; Falzoni, S.; et al. ATP secreted by endothelial cells blocks CX3CL1-elicited natural killer cell chemotaxis and cytotoxicity via P2Y11 receptor activation. Blood 2010, 116, 4492–4500. [Google Scholar] [CrossRef] [PubMed]
- Woods, L.; Camden, J.; Khalafalla, M.; Petris, M.; Erb, L.; Ambrus, J.; Weisman, G. P2Y 2 R deletion ameliorates sialadenitis in IL-14α-transgenic mice. Oral Dis. 2018, 24, 761–771. [Google Scholar] [CrossRef]
- Neumann, A.; Attah, I.; Al-Hroub, H.; Namasivayam, V.; Müller, C.E. Discovery of P2Y2 Receptor Antagonist Scaffolds through Virtual High-Throughput Screening. J. Chem. Inf. Model. 2022, 62, 1538–1549. [Google Scholar] [CrossRef] [PubMed]
- Harjunpää, H.; Llort Asens, M.; Guenther, C.; Fagerholm, S.C. Cell Adhesion Molecules and Their Roles and Regulation in the Immune and Tumor Microenvironment. Front. Immunol. 2019, 10, 1078. [Google Scholar] [CrossRef] [PubMed]
- Baker, O.J.; Camden, J.M.; Rome, D.E.; Seye, C.I.; Weisman, G.A. P2Y2 nucleotide receptor activation up-regulates vascular cell adhesion molecular-1 expression and enhances lymphocyte adherence to a human submandibular gland cell line. Mol. Immunol. 2008, 45, 65–75, Erratum in Mol. Immunol. 2008, 45, 3319. [Google Scholar] [CrossRef]
- Apolloni, S.; Montilli, C.; Finocchi, P.; Amadio, S. Membrane compartments and purinergic signalling: P2X receptors in neurodegenerative and neuroinflammatory events. FEBS J. 2009, 276, 354–364. [Google Scholar] [CrossRef]
- Puchałowicz, K.; Tarnowski, M.; Baranowska-Bosiacka, I.; Chlubek, D.; Dziedziejko, V. P2X and P2Y Receptors—Role in the Pathophysiology of the Nervous System. Int. J. Mol. Sci. 2014, 15, 23672–23704. [Google Scholar] [CrossRef]
- Kong, Q.; Peterson, T.S.; Baker, O.; Stanley, E.; Camden, J.; Seye, C.I.; Erb, L.; Simonyi, A.; Wood, W.G.; Sun, G.Y.; et al. Interleukin-1β enhances nucleotide-induced and α-secretase-dependent amyloid precursor protein processing in rat primary cortical neurons via up-regulation of the P2Y2 receptor. J. Neurochem. 2009, 109, 1300–1310. [Google Scholar] [CrossRef] [PubMed]
- Franke, H. Role of G protein-coupled receptors (GPCRs) for purines and pyrimidines in mediating degeneration and regeneration under neuroinflammatory processes. Purinergic Signal 2011, 7, 1–5. [Google Scholar] [CrossRef]
- Weisman, G.A.; Yu, N.; Liao, Z.; Gonzaléz, F.; Erb, L.; Seye, C.I. P2 Receptors in Health and Disease. Biotechnol. Genet. Eng. Rev. 2006, 22, 171–196. [Google Scholar] [CrossRef]
- Brambilla, R.; Abbracchio, M.P. Modulation of Cyclooxygenase-2 and Brain Reactive Astrogliosis by Purinergic P2 Receptors. Ann. N. Y. Acad. Sci. 2001, 939, 54–62. [Google Scholar] [CrossRef]
- Brambilla, R.; Burnstock, G.; Bonazzi, A.; Ceruti, S.; Cattabeni, F.; Abbracchio, M.P. Cyclo-oxygenase-2 mediates P2Y receptor-induced reactive astrogliosis. Br. J. Pharmacol. 1999, 126, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.N.; Fan, H.; Song, J.T. Targeting purinergic receptors to attenuate inflammation of dry eye. Purinergic Signal. 2023, 19, 199–206. [Google Scholar] [CrossRef]
- Baldini, C.; Rossi, C.; Ferro, F.; Santini, E.; Seccia, V.; Donati, V.; Solini, A. The P2X7 receptor–inflammasome complex has a role in modulating the inflammatory response in primary S jögren’s syndrome. J. Intern. Med. 2013, 274, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Pintor, J.; Sánchez-Nogueiro, J.; Irazu, M.; Mediero, A.; Peláez, T.; Peral, A. Immunolocalisation of P2Y receptors in the rat eye. Purinergic Signal. 2004, 1, 83–90. [Google Scholar] [CrossRef]
- Choi, J.Y.; Shin, J.-H.; Kim, J.L.; Jung, S.H.; Son, E.J.; Song, M.H.; Kim, S.H.; Yoon, J.-H. P2Y2 agonist induces mucin secretion via Ca2+- and inositol 1,4,5-triphosphate-dependent pathway in human middle ear epithelial cells. Hear. Res. 2005, 209, 24–31. [Google Scholar] [CrossRef]
- Terakado, K.; Yogo, T.; Kohara, Y.; Soeta, S.; Nezu, Y.; Harada, Y.; Hara, Y.; Amasaki, H.; Tagawa, M. Conjunctival expression of the P2Y2 receptor and the effects of 3% diquafosol ophthalmic solution in dogs. Vet. J. 2014, 202, 48–52. [Google Scholar] [CrossRef]
- Mantelli, F.; Argüeso, P. Functions of ocular surface mucins in health and disease. Curr. Opin. Allergy Clin. Immunol. 2008, 8, 477–483. [Google Scholar] [CrossRef]
- Montés-Micó, R.; Cervino, A.; Ferrer-Blasco, T.; García-Lázaro, S.; Madrid-Costa, D. The Tear Film and the optical Quality of the Eye. Ocul. Surf. 2010, 8, 185–192. [Google Scholar] [CrossRef]
- Park, J.H.; Moon, S.-H.; Kang, D.H.; Um, H.J.; Kang, S.-S.; Kim, J.Y.; Tchah, H. Diquafosol Sodium Inhibits Apoptosis and Inflammation of Corneal Epithelial Cells Via Activation of Erk1/2 and RSK: In Vitro and In Vivo Dry Eye Model. Investig. Opthalmology Vis. Sci. 2018, 59, 5108. [Google Scholar] [CrossRef]
- Skalicky, S.; Lau, O.; Samarawickrama, C. P2Y2 receptor agonists for the treatment of dry eye disease: A review. Clin. Ophthalmol. 2014, 8, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Jumblatt, J.E.; Jumblatt, M.M. Regulation of Ocular Mucin Secretion by P2Y2Nucleotide Receptors in Rabbit and Human Conjunctiva. Exp. Eye Res. 1998, 67, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, N.; Sonomura, Y.; Kato, H.; Komuro, A.; Kinoshita, S. Three percent diquafosol ophthalmic solution as an additional therapy to existing artificial tears with steroids for dry-eye patients with Sjögren’s syndrome. Eye 2015, 29, 1204–1212. [Google Scholar] [CrossRef]
- Yang, C.; Su, L.; Wang, Y.; Liu, L. UTP regulation of ion transport in alveolar epithelial cells involves distinct mechanisms. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2009, 297, L439–L454. [Google Scholar] [CrossRef] [PubMed]
- Wirsching, E.; Fauler, M.; Fois, G.; Frick, M. P2 Purinergic Signaling in the Distal Lung in Health and Disease. Int. J. Mol. Sci. 2020, 21, 4973. [Google Scholar] [CrossRef]
- Faria, D.; Schreiber, R.; Kunzelmann, K. CFTR is activated through stimulation of purinergic P2Y2 receptors. Pflug. Arch. 2009, 457, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Cressman, V.L.; Lazarowski, E.; Homolya, L.; Boucher, R.C.; Koller, B.H.; Grubb, B.R. Effect of loss of P2Y2 receptor gene expression on nucleotide regulation of murine epithelial Cl− transport. J. Biol. Chem. 1999, 274, 26461–26468. [Google Scholar] [CrossRef]
- Kreda, S.M.; Davis, C.W.; Rose, M.C. CFTR, mucins, and mucus obstruction in cystic fibrosis. Cold Spring Harb. Perspect. Med. 2012, 2, a009589. [Google Scholar] [CrossRef]
- Hanssens, L.S.; Duchateau, J.; Casimir, G.J. CFTR Protein: Not Just a Chloride Channel? Cells 2021, 10, 2844. [Google Scholar] [CrossRef] [PubMed]
- Ratjen, F.; Durham, T.; Navratil, T.; Schaberg, A.; Accurso, F.J.; Wainwright, C.; Barnes, M.; Moss, R.B. Long term effects of denufosol tetrasodium in patients with cystic fibrosis. J. Cyst. Fibros. 2012, 11, 539–549. [Google Scholar] [CrossRef]
- Chang, J.T. Pathophysiology of Inflammatory Bowel Diseases. N. Engl. J. Med. 2020, 383, 2652–2664. [Google Scholar]
- Grbic, D.M.; Degagné, E.; Langlois, C.; Dupuis, A.A.; Gendron, F.P. Intestinal Inflammation Increases the Expression of the P2Y6 Receptor on Epithelial Cells and the Release of CXC Chemokine Ligand 8 by UDP. J. Immunol. 2008, 180, 2659–2668. [Google Scholar] [CrossRef] [PubMed]
- Degagné, E.; Degrandmaison, J.; Grbic, D.M.; Vinette, V.; Arguin, G.; Gendron, F.P. P2Y2 receptor promotes intestinal microtubule stabilization and mucosal re-epithelization in experimental colitis. J. Cell. Physiol. 2013, 228, 99–109. [Google Scholar] [PubMed]
- Erb, L.; Liu, J.; Ockerhausen, J.; Kong, Q.; Garrad, R.C.; Griffin, K.; Neal, C.; Krugh, B.; Santiago-Pérez, L.I.; González, F.A.; et al. An Rgd Sequence in the P2Y2 Receptor Interacts with αVβ3 Integrins and Is Required for Go-Mediated Signal Transduction. J. Cell Biol. 2001, 153, 491–502. [Google Scholar] [CrossRef]
- de Araújo, J.B.; Kerkhoff, V.V.; de Oliveira Maciel, S.F.V.; de Resende ESilva, D.T. Targeting the purinergic pathway in breast cancer and its therapeutic applications. Purinergic Signal 2021, 17, 179–200. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.L.; Gong, Y.; Ji, P.; Xie, Y.F.; Jiang, Y.Z.; Liu, G.Y. Targeting nucleotide metabolism: A promising approach to enhance cancer immunotherapy. J. Hematol. Oncol. 2022, 15, 45. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhong, M.; Xiong, Y.; Gao, Z.; Wu, Z.; Liu, Y.; Hong, X. Emerging roles of nucleotide metabolism in cancer development: Progress and prospect. Aging 2021, 13, 13349–13358. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.H.; Kim, Y.; Kim, M.; Jang, J.; Lee, S. Emerging Roles of Vascular Cell Adhesion Molecule-1 (VCAM-1) in Immunological Disorders and Cancer. Int. J. Mol. Sci. 2018, 19, 1057. [Google Scholar] [CrossRef]
- Rosette, C.; Roth, R.B.; Oeth, P.; Braun, A.; Kammerer, S.; Ekblom, J.; Denissenko, M.F. Role of ICAM1 in invasion of human breast cancer cells. Carcinogenesis 2005, 26, 943–950. [Google Scholar] [CrossRef]
- Jin, H.; Eun, S.Y.; Lee, J.S.; Park, S.W.; Lee, J.H.; Chang, K.C.; Kim, H.J. P2Y2 receptor activation by nucleotides released from highly metastatic breast cancer cells increases tumor growth and invasion via crosstalk with endothelial cells. Breast Cancer Res. BCR 2014, 16, R77. [Google Scholar] [CrossRef]
- Zhang, J.L.; Liu, Y.; Yang, H.; Zhang, H.Q.; Tian, X.X.; Fang, W.G. ATP-P2Y2-β-catenin axis promotes cell invasion in breast cancer cells. Cancer Sci. 2017, 108, 1318–1327. [Google Scholar] [CrossRef]
- Joo, Y.N.; Jin, H.; Eun, S.Y.; Park, S.W.; Chang, K.C.; Kim, H.J. P2Y2R activation by nucleotides released from the highly metastatic breast cancer cell contributes to pre-metastatic niche formation by mediating lysyl oxidase secretion, collagen crosslinking, and monocyte recruitment. Oncotarget 2014, 5, 9322–9334. [Google Scholar] [CrossRef]
- Mendis, S.; Davis, S.; Norrving, B. Organizational Update: The World Health Organization Global Status Report on Noncommunicable Diseases 2014; One More Landmark Step in the Combat Against Stroke and Vascular Disease. Stroke 2015, 46, e121–e122. [Google Scholar] [CrossRef]
- Ostchega, Y.; Fryar, C.D.; Nwankwo, T.; Nguyen, D.T. Hypertension Prevalence Among Adults Aged 18 and Over: United States, 2017–2018; NCHS Data Brief; National Center for Health Statistics: Hyattsville, MD, USA, 2020; pp. 1–8.
- Messerli, F.H.; Williams, B.; Ritz, E. Essential hypertension. Lancet 2007, 370, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Rossier, B.C.; Pradervand, S.; Schild, L.; Hummler, E. Epithelial sodium channel and the control of sodium balance: Interaction between genetic and environmental factors. Annu. Rev. Physiol. 2002, 64, 877–897. [Google Scholar] [CrossRef]
- Kortenoeven, M.L.A.; Pedersen, N.B.; Rosenbaek, L.L.; Fenton, R.A. Vasopressin regulation of sodium transport in the distal nephron and collecting duct. Am. J. Physiol. Ren. Physiol. 2015, 309, F280–F299. [Google Scholar]
- Mironova, E.; Suliman, F.; Stockand, J.D. Renal Na+ excretion consequent to pharmacogenetic activation of Gq-DREADD in principal cells. Am. J. Physiol. Ren. Physiol. 2019, 316, F758–F767. [Google Scholar] [CrossRef]
- Stockand, J.D.; Mironova, E.; Bugaj, V.; Rieg, T.; Insel, P.A.; Vallon, V.; Peti-Peterdi, J.; Pochynyuk, O. Purinergic inhibition of ENaC produces aldosterone escape. J. Am. Soc. Nephrol. JASN 2010, 21, 1903–1911. [Google Scholar] [CrossRef]
- Pochynyuk, O.; Rieg, T.; Bugaj, V.; Schroth, J.; Fridman, A.; Boss, G.R.; Insel, P.A.; Stockand, J.D.; Vallon, V. Dietary Na+ inhibits the open probability of the epithelial sodium channel in the kidney by enhancing apical P2Y2-receptor tone. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2010, 24, 2056–2065. [Google Scholar] [CrossRef]
- Pochynyuk, O.; Bugaj, V.; Rieg, T.; Insel, P.A.; Mironova, E.; Vallon, V.; Stockand, J.D. Paracrine regulation of the epithelial Na+ channel in the mammalian collecting duct by purinergic P2Y2 receptor tone. J. Biol. Chem. 2008, 283, 36599–36607. [Google Scholar] [CrossRef] [PubMed]
- Mironova, E.; Peti-Peterdi, J.; Bugaj, V.; Stockand, J.D. Diminished paracrine regulation of the epithelial Na+ channel by purinergic signaling in mice lacking connexin 30. J. Biol. Chem. 2011, 286, 1054–1060. [Google Scholar] [CrossRef]
- Bugaj, V.; Sansom, S.C.; Wen, D.; Hatcher, L.I.; Stockand, J.D.; Mironova, E. Flow-sensitive K+-coupled ATP secretion modulates activity of the epithelial Na+ channel in the distal nephron. J. Biol. Chem. 2012, 287, 38552–38558. [Google Scholar] [CrossRef] [PubMed]
- Pochynyuk, O.; Bugaj, V.; Vandewalle, A.; Stockand, J.D. Purinergic control of apical plasma membrane PI(4,5)P2 levels sets ENaC activity in principal cells. Am. J. Physiol. Ren. Physiol. 2008, 294, F38–F46. [Google Scholar] [CrossRef] [PubMed]
- Pochynyuk, O.; Tong, Q.; Staruschenko, A.; Stockand, J.D. Binding and direct activation of the epithelial Na+ channel (ENaC) by phosphatidylinositides. J. Physiol. 2007, 580 Pt 2, 365–372. [Google Scholar] [CrossRef]
- Pochynyuk, O.; Tong, Q.; Medina, J.; Vandewalle, A.; Staruschenko, A.; Bugaj, V.; Stockand, J.D. Molecular determinants of PI(4,5)P2 and PI(3,4,5)P3 regulation of the epithelial Na+ channel. J. Gen. Physiol. 2007, 130, 399–413. [Google Scholar] [CrossRef]
- Pochynyuk, O.; Tong, Q.; Staruschenko, A.; Ma, H.P.; Stockand, J.D. Regulation of the epithelial Na+ channel (ENaC) by phosphatidylinositides. Am. J. Physiol. Ren. Physiol. 2006, 290, F949–F957. [Google Scholar] [CrossRef]
- Archer, C.R.; Enslow, B.T.; Carver, C.M.; Stockand, J.D. Phosphatidylinositol 4,5-bisphosphate directly interacts with the β and γ subunits of the sodium channel ENaC. J. Biol. Chem. 2020, 295, 7958–7969. [Google Scholar] [CrossRef]
- Rieg, T.; Bundey, R.A.; Chen, Y.; Deschenes, G.; Junger, W.; Insel, P.A.; Vallon, V. Mice lacking P2Y2 receptors have salt-resistant hypertension and facilitated renal Na+ and water reabsorption. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2007, 21, 3717–3726. [Google Scholar] [CrossRef]
- Rieg, T.; Gerasimova, M.; Boyer, J.L.; Insel, P.A.; Vallon, V. P2Y2 receptor activation decreases blood pressure and increases renal Na+ excretion. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R510–R518. [Google Scholar]
- Stockand, J.D. Vasopressin regulation of renal sodium excretion. Kidney Int. 2010, 78, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Haas, C.B.; Lovászi, M.; Braganhol, E.; Pacher, P.; Haskó, G. Ectonucleotidases in Inflammation, Immunity, and Cancer. J. Immunol. 2021, 206, 1983–1990. [Google Scholar] [CrossRef]
- Séror, C.; Melki, M.-T.; Subra, F.; Raza, S.Q.; Bras, M.; Saïdi, H.; Nardacci, R.; Voisin, L.; Paoletti, A.; Law, F.; et al. Extracellular ATP acts on P2Y2 purinergic receptors to facilitate HIV-1 infection. J. Exp. Med. 2011, 208, 1823–1834. [Google Scholar] [CrossRef] [PubMed]
- Vanderstocken, G.; van de Paar, E.; Robaye, B.; di Pietrantonio, L.; Bondue, B.; Boeynaems, J.-M.; Desmecht, D.; Communi, D. Protective role of P2Y2 receptor against lung infection induced by pneumonia virus of mice. PLoS ONE 2012, 7, e50385. [Google Scholar] [CrossRef] [PubMed]
| Disease/Condition | Mechanisms Involving P2Y2 | Physiological/Pathological Effects |
|---|---|---|
| Neurological: Alzheimer’s disease | ↑ P2Y2 expression in neurons (IL-1β induced); ADAM10/17 activation → ↑ sAPP-α release; ERK1/2 and PI3K signaling; regulation of cofilin phosphorylation; arachidonic acid release in astrocytes | Neuroprotection via sAPP-α and neurite stability; inflammation via COX-2 and AA/PG release |
| Ocular: Dry Eye Disease | Ca2+ mobilization in corneal epithelium; cytokine release (IL-8); mucin secretion from goblet cells; ERK-p90RSK and NF-κB suppression | Dual role: proinflammatory vs. protective; clinical use of diquafosol to stimulate tears and mucins |
| Respiratory: Cystic Fibrosis | P2Y2 on epithelium → Cl−/HCO3− secretion and ENaC inhibition; ATP/UTP-triggered mucin release; immune cell signaling | Promotes airway hydration and mucus clearance; denufosol (agonist) failed in clinical trials |
| Inflammatory: Inflammatory Bowel Disease | ↑ P2Y2 in colon of IBD patients and DSS-colitis mice; COX-2–PGE2 pathway; integrin binding (RGD motif) → cytoskeletal reorganization | Amplifies inflammation (acute); promotes epithelial repair and barrier regeneration (resolution phase) |
| Oncology: Breast Cancer | MAPK and AP-1 signaling; EGFR transactivation via ADAMs; Rac/Rho activation; regulation of ICAM-1 and VCAM-1; β-catenin signaling; lysyl oxidase induction under hypoxia | Promotes tumor cell migration, invasion, adhesion, metastasis, and chemoresistance |
| Cardiovascular: Hypertension & Atherosclerosis | Renal ENaC inhibition via PLC/PIP2 signaling → natriuresis & ↓ BP; αVβ3-integrin interaction → actin remodeling and leukocyte migration | Blood pressure regulation: P2Y2 dysfunction linked to hypertension; possible target for vascular inflammation |
| Infectious: HIV-1 | ATP release via pannexin-1 → P2Y2 activation → proline-rich tyrosine kinase 2 activation → membrane depolarization → viral fusion (Env–CD4/CXCR4 mediated) | Facilitates viral entry, syncytium formation, replication, and spread; receptor upregulated in HIV patients |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salarpour, F.; Sévigny, J. P2Y2 Receptor Signaling in Health and Disease. Int. J. Mol. Sci. 2025, 26, 9815. https://doi.org/10.3390/ijms26199815
Salarpour F, Sévigny J. P2Y2 Receptor Signaling in Health and Disease. International Journal of Molecular Sciences. 2025; 26(19):9815. https://doi.org/10.3390/ijms26199815
Chicago/Turabian StyleSalarpour, Fatemeh, and Jean Sévigny. 2025. "P2Y2 Receptor Signaling in Health and Disease" International Journal of Molecular Sciences 26, no. 19: 9815. https://doi.org/10.3390/ijms26199815
APA StyleSalarpour, F., & Sévigny, J. (2025). P2Y2 Receptor Signaling in Health and Disease. International Journal of Molecular Sciences, 26(19), 9815. https://doi.org/10.3390/ijms26199815







