Multiscale Interactome-Guided Discovery Candidate Herbs and Active Ingredients Against Hyperthyroidism by Biased Random Walk Algorithm
Abstract
1. Introduction
2. Results
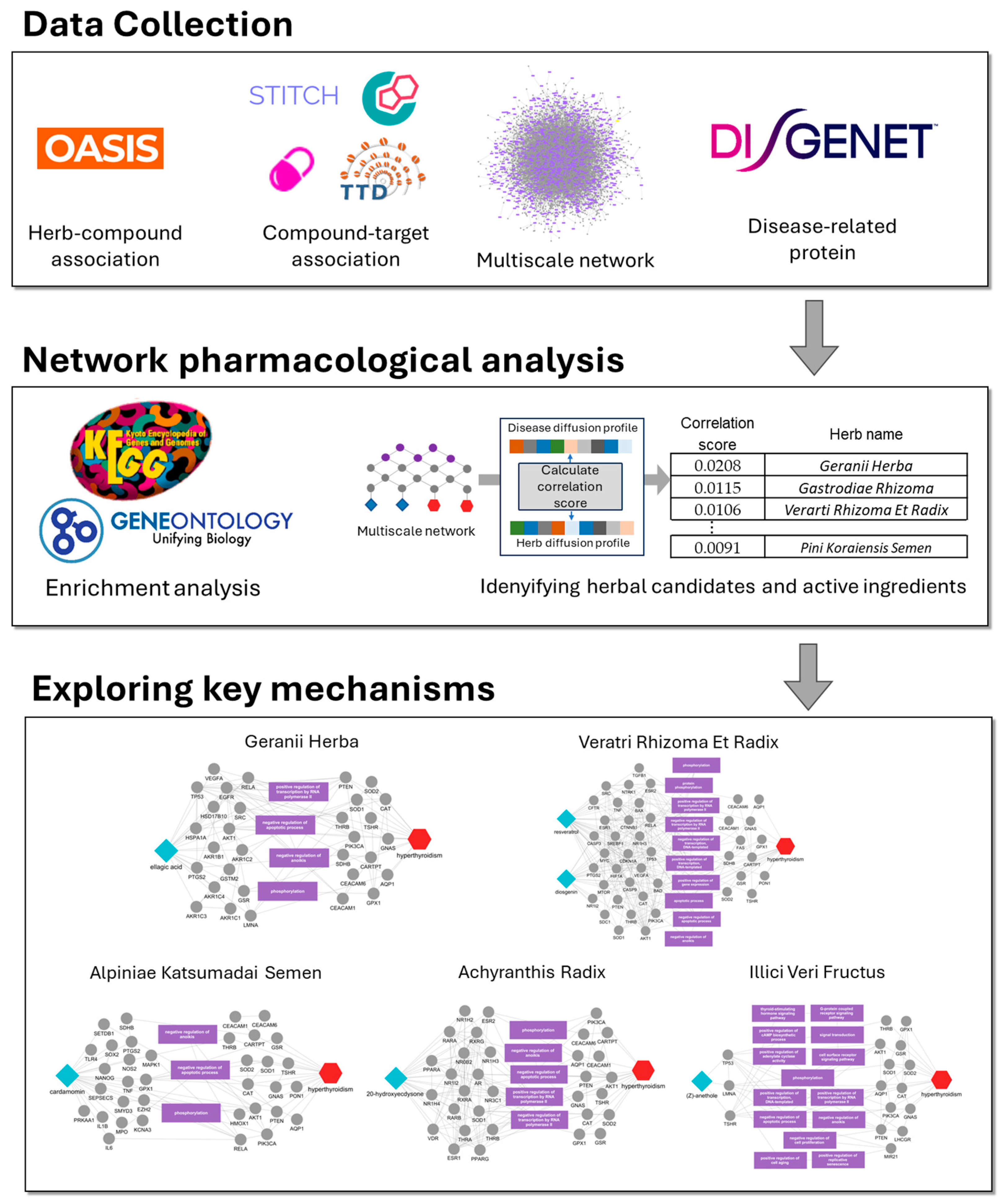
2.1. Identification of Candidate Herbs for Hyperthyroidism
2.2. Herb-Ingredient-Target Network Construction of the Top 10 Herbs
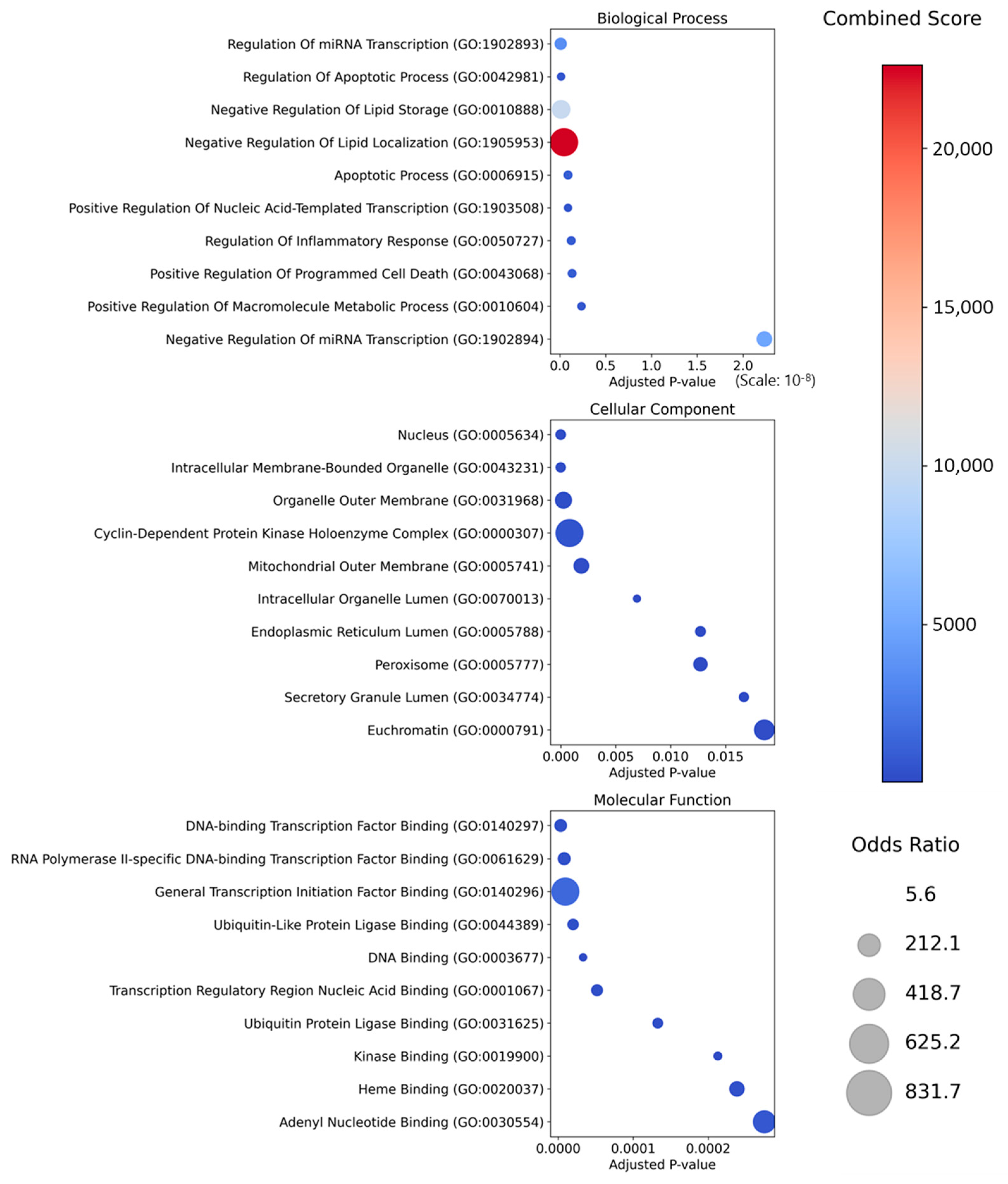
2.3. Enrichment Analysis of the Top 10 Herb Targets
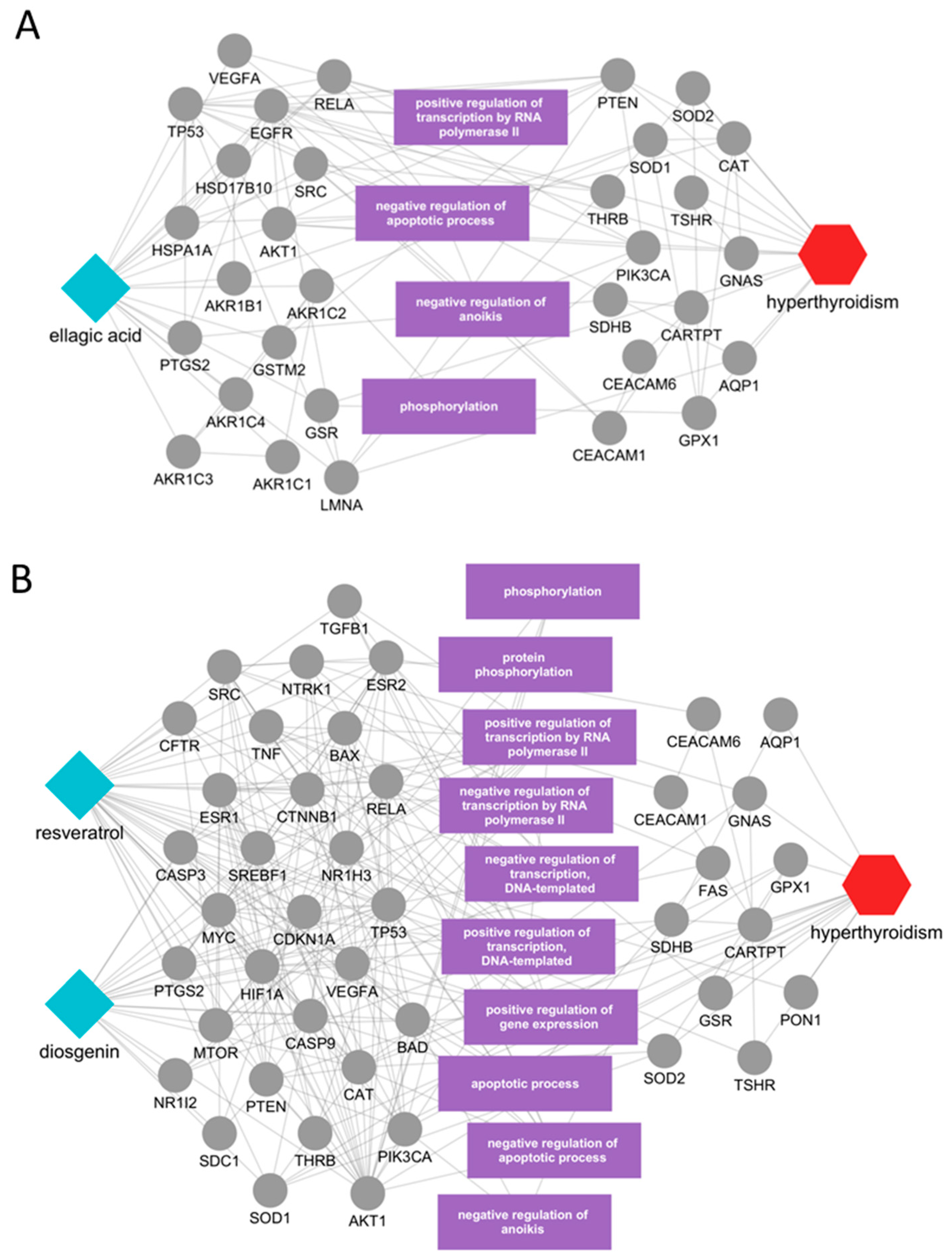
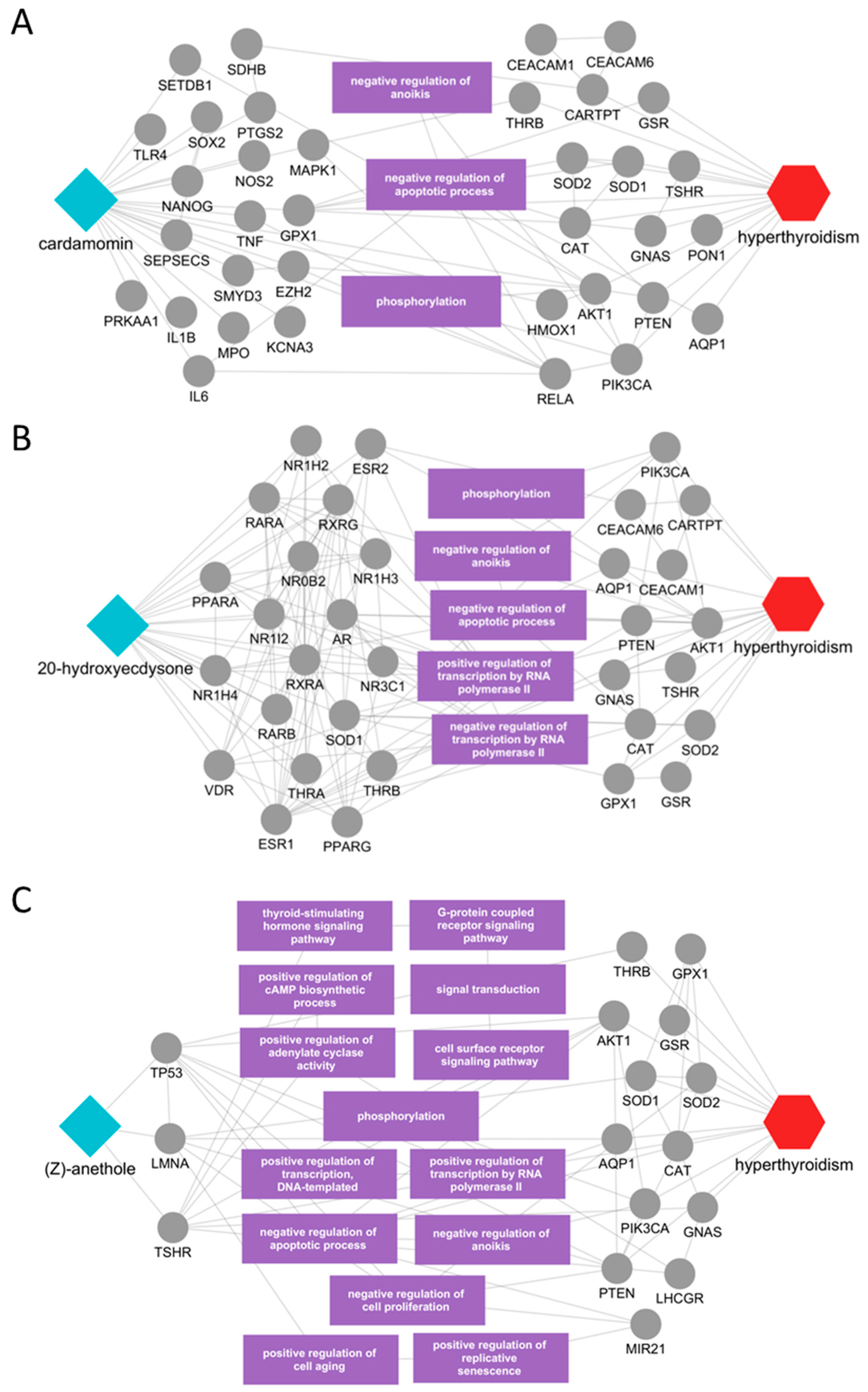
2.4. Identification of Potential Active Ingredients and Multiscale Network Mechanism Analysis of Candidate Herbs
3. Discussion
4. Materials and Methods
4.1. Herb-Ingredient-Target Network Construction
4.2. Enrichment Analysis
4.3. Disease Related Targets
4.4. Multiscale Network Analysis for Predicting Disease Associations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, S.Y.; Pearce, E.N. Hyperthyroidism: A Review. JAMA 2023, 330, 1472–1483. [Google Scholar] [CrossRef]
- Wiersinga, W.M.; Poppe, K.G.; Effraimidis, G. Hyperthyroidism: Aetiology, Pathogenesis, Diagnosis, Management, Complications, and Prognosis. Lancet Diabetes Endocrinol. 2023, 11, 282–298. [Google Scholar] [CrossRef]
- Moon, J.H.; Yi, K.H. The Diagnosis and Management of Hyperthyroidism in Korea: Consensus Report of the Korean Thyroid Association. Endocrinol. Metab. 2013, 28, 275–279. [Google Scholar] [CrossRef]
- Azizi, F.; Malboosbaf, R. Safety of Long-Term Antithyroid Drug Treatment? A Systematic Review. J. Endocrinol. Investig. 2019, 42, 1273–1283. [Google Scholar] [CrossRef]
- Azizi, F. Long-Term Treatment of Hyperthyroidism with Antithyroid Drugs: 35 Years of Personal Clinical Experience. Thyroid 2020, 30, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Lai, Y.; Gan, D.; Liu, Q.; Wang, Y.; He, X.; An, Y.; Gao, T. Possible Molecular Exploration of Herbal Pair Haizao-Kunbu in the Treatment of Graves’ Disease by Network Pharmacology, Molecular Docking, and Molecular Dynamic Analysis. Front. Endocrinol. 2023, 14, 1236549. [Google Scholar] [CrossRef] [PubMed]
- Gan, D.; Gao, T.-S.; Ma, L.; Lu, H.; Dai, H.; Liu, Q.-Y.; Lai, Y.-W.; Liu, X.-H.; Peng, Z.-D.; Chen, R.-Y. Clinical Efficacy of Chinese Herbal Medicine Formula for Graves’ Hyperthyroidism: A Multicentre, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J. Ethnopharmacol. 2025, 338, 119106. [Google Scholar] [CrossRef]
- Liu, Y.; Shan, Z.; Endocrine Metabolic Diseases Group of the Chinese Geriatrics Society, Chinese Medical Association Thyroid Group of the Chinese Society of Endocrinology; Cheng, M.; Cao, C.X.; Cao, X.P.; Chen, P.; Feng, S.Z.; He, J.R. Expert Consensus on Diagnosis and Treatment for Elderly with Thyroid Diseases in China. Aging Med. 2021, 4, 70–92. [Google Scholar] [CrossRef]
- Qian, W.-W.; Yang, S.-Q.; Hu, S.-M.; Wang, X.-L.; Zhu, Y.; Zhou, T. Enzymatic Degradation, Antioxidant and Immunoregulatory Activities of Polysaccharides from Brown Algae Sargassum Fusiforme. J. Food Meas. Charact. 2021, 15, 1960–1972. [Google Scholar] [CrossRef]
- Zhu, X.; Yao, Q.; Yang, P.; Zhao, D.; Yang, R.; Bai, H.; Ning, K. Multi-Omics Approaches for in-Depth Understanding of Therapeutic Mechanism for Traditional Chinese Medicine. Front. Pharmacol. 2022, 13, 1031051. [Google Scholar] [CrossRef]
- Lee, W.-Y.; Lee, C.-Y.; Kim, Y.-S.; Kim, C.-E. The Methodological Trends of Traditional Herbal Medicine Employing Network Pharmacology. Biomolecules 2019, 9, 362. [Google Scholar] [CrossRef]
- Li, L.; Yang, L.; Yang, L.; He, C.; He, Y.; Chen, L.; Dong, Q.; Zhang, H.; Chen, S.; Li, P. Network Pharmacology: A Bright Guiding Light on the Way to Explore the Personalized Precise Medication of Traditional Chinese Medicine. Chin. Med. 2023, 18, 146. [Google Scholar] [CrossRef]
- Li, X.; Liu, Z.; Liao, J.; Chen, Q.; Lu, X.; Fan, X. Network Pharmacology Approaches for Research of Traditional Chinese Medicines. Chin. J. Nat. Med. 2023, 21, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Lin, J.; Wu, X.; Pu, H.; Guan, Y.; Xiao, P.; He, C.; Jiang, B. Novel Active Compounds and the Anti-Diabetic Mechanism of Mulberry Leaves. Front. Pharmacol. 2022, 13, 986931. [Google Scholar] [CrossRef]
- Hu, Q.; Wei, S.; Wen, J.; Zhang, W.; Jiang, Y.; Qu, C.; Xiang, J.; Zhao, Y.; Peng, X.; Ma, X. Network Pharmacology Reveals the Multiple Mechanisms of Xiaochaihu Decoction in the Treatment of Non-Alcoholic Fatty Liver Disease. BioData Min. 2020, 13, 11. [Google Scholar] [CrossRef]
- Jang, B.; Kim, Y.; Song, J.; Kim, Y.-W.; Lee, W.-Y. Identifying Herbal Candidates and Active Ingredients against Postmenopausal Osteoporosis Using Biased Random Walk on a Multiscale Network. Int. J. Mol. Sci. 2024, 25, 12322. [Google Scholar] [CrossRef]
- Inui, S.; Tsujimoto, N.; Toda, N.; Itami, S. Suppression of Thyroid Function by Seaweed “Kombu”(Laminaria Japonica) Supplement Seen in a Patient with Alopecia Areata: A Case Report. Open Dermatol. J. 2010. [Google Scholar] [CrossRef]
- Zava, T.T.; Zava, D.T. Assessment of Japanese Iodine Intake Based on Seaweed Consumption in Japan: A Literature-Based Analysis. Thyroid Res. 2011, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, R.; Saha, I.; Maity, A.; Ray, P.P.; Maiti, B. Arecoline Ameliorates Hyperthyroid Condition in Mice under Cold Stress. Arch. Physiol. Biochem. 2018, 124, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Auf’mkolk, M.; Köhrle, J.; Gumbinger, H.; Winterhoff, H.; Hesch, R.-D. Antihormonal Effects of Plant Extracts: Iodothyronine Deiodinase of Rat Liver Is Inhibited by Extracts and Secondary Metabolites of Plants. Horm. Metab. Res. 1984, 16, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Xin, J.; Cheng, W.; Yu, Y.; Chen, J.; Zhang, X.; Shao, S. Diosgenin from Dioscorea Nipponica Rhizoma against Graves’ Disease—On Network Pharmacology and Experimental Evaluation. Front. Pharmacol. 2022, 12, 806829. [Google Scholar] [CrossRef]
- Majumdar, J.; Chakraborty, P.; Mitra, A.; Sarkar, N.K.; Sarkar, S. Fenugreek, a Potent Hypoglycaemic Herb Can Cause Central Hypothyroidism Via Leptin–a Threat to Diabetes Phytotherapy. Exp. Clin. Endocrinol. Diabetes 2017, 125, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, C.; Zitnik, M.; Leskovec, J. Identification of Disease Treatment Mechanisms through the Multiscale Interactome. Nat. Commun. 2021, 12, 1796. [Google Scholar] [CrossRef]
- Lee, W.-Y.; Park, K.-I.; Bak, S.-B.; Lee, S.; Bae, S.-J.; Kim, M.-J.; Park, S.-D.; Kim, C.O.; Kim, J.-H.; Kim, Y.W. Evaluating Current Status of Network Pharmacology for Herbal Medicine Focusing on Identifying Mechanisms and Therapeutic Effects. J. Adv. Res. 2025, 76, 799–815. [Google Scholar] [CrossRef] [PubMed]
- Ross, D.S. Radioiodine Therapy for Hyperthyroidism. N. Engl. J. Med. 2011, 364, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, P.; Mosekilde, L. Hyperthyroidism, Bone Mineral, and Fracture Risk—A Meta-Analysis. Thyroid 2003, 13, 585–593. [Google Scholar] [CrossRef]
- Williams, G.R.; Bassett, J.D. Thyroid Diseases and Bone Health. J. Endocrinol. Investig. 2018, 41, 99–109. [Google Scholar] [CrossRef]
- Biondi, B.; Cooper, D.S. The Clinical Significance of Subclinical Thyroid Dysfunction. Endocr. Rev. 2008, 29, 76–131. [Google Scholar] [CrossRef]
- Mendoza, A.; Hollenberg, A.N. New Insights into Thyroid Hormone Action. Pharmacol. Ther. 2017, 173, 135–145. [Google Scholar] [CrossRef]
- El-Haddad, A.E.; Farag, M.A. Alpinia Katsumadai Seed from a Condiment to Ethnomedicine to Nutraceutical, a Comprehensive Review of Its Chemistry and Health Benefits. J. Tradit. Complement. Med. 2024. [Google Scholar] [CrossRef]
- Hong, M.; Li, S.; Tan, H.Y.; Wang, N.; Tsao, S.-W.; Feng, Y. Current Status of Herbal Medicines in Chronic Liver Disease Therapy: The Biological Effects, Molecular Targets and Future Prospects. Int. J. Mol. Sci. 2015, 16, 28705–28745. [Google Scholar] [CrossRef]
- Fang, Y.; Lei, C.; Wang, Z.; Liu, L.; Chen, Y.; Chen, F.; Li, J.; Tu, Z.; Tao, Q.; Xu, Y. Regulation of Transient Receptor Potential Ankyrin 1 by Traditional Chinese Medicine Drugs and Their Active Ingredients. Front. Pharmacol. 2025, 16, 1604765. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-R.; Niu, Y.-S.; Zhou, H.-L. Achyranthes Bidentata Blume (Amaranthaceae): A Review of Its Botany, Traditional Uses, Phytochemistry, Pharmacology, and Toxicology. J. Pharm. Pharmacol. 2024, 76, 930–966. [Google Scholar] [CrossRef]
- Weng, X.; Lin, P.; Liu, F.; Chen, J.; Li, H.; Huang, L.; Zhen, C.; Xu, H.; Liu, X.; Ye, H. Achyranthes Bidentata Polysaccharides Activate the Wnt/Β-Catenin Signaling Pathway to Promote Chondrocyte Proliferation. Int. J. Mol. Med. 2014, 34, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Pan, R.-L.; Hu, W.-Q.; Pan, J.; Huang, L.; Luan, C.-C.; Shen, H.-M. Achyranthes Bidentata Polypeptides Prevent Apoptosis by Inhibiting the Glutamate Current in Cultured Hippocampal Neurons. Neural Regen. Res. 2020, 15, 1086–1093. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhu, Y.; Peng, W.; Wang, H.; Yuan, Y.; Gu, X. Achyranthes Bidentata Polypeptide Protects Schwann Cells from Apoptosis in Hydrogen Peroxide-Induced Oxidative Stress. Front. Neurosci. 2018, 12, 868. [Google Scholar] [CrossRef]
- Zou, Q.; Huang, Y.; Zhang, W.; Lu, C.; Yuan, J. A Comprehensive Review of the Pharmacology, Chemistry, Traditional Uses and Quality Control of Star Anise (Illicium Verum Hook. F.): An Aromatic Medicinal Plant. Molecules 2023, 28, 7378. [Google Scholar] [CrossRef] [PubMed]
- Chouksey, D.; Upmanyu, N.; Pawar, R. Central Nervous System Activity of Illicium Verum Fruit Extracts. Asian Pac. J. Trop. Med. 2013, 6, 869–875. [Google Scholar] [CrossRef]
- Kowshik, J.; Giri, H.; Kishore, T.K.K.; Kesavan, R.; Vankudavath, R.N.; Reddy, G.B.; Dixit, M.; Nagini, S. Ellagic Acid Inhibits Vegf/Vegfr2, Pi3k/Akt and Mapk Signaling Cascades in the Hamster Cheek Pouch Carcinogenesis Model. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Cancer Agents) 2014, 14, 1249–1260. [Google Scholar] [CrossRef]
- Nallasamy, P.; Kang, Z.Y.; Sun, X.; Anandh Babu, P.V.; Liu, D.; Jia, Z. Natural Compound Resveratrol Attenuates Tnf-Alpha-Induced Vascular Dysfunction in Mice and Human Endothelial Cells: The Involvement of the Nf-Κb Signaling Pathway. Int. J. Mol. Sci. 2021, 22, 12486. [Google Scholar] [CrossRef]
- Li, Y.-Y.; Huang, S.-S.; Lee, M.-M.; Deng, J.-S.; Huang, G.-J. Anti-Inflammatory Activities of Cardamonin from Alpinia Katsumadai through Heme Oxygenase-1 Induction and Inhibition of Nf-Κb and Mapk Signaling Pathway in the Carrageenan-Induced Paw Edema. Int. Immunopharmacol. 2015, 25, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Orie, N.N.; Raees, A.; Aljaber, M.Y.; Mohamed-Ali, N.; Bensmail, H.; Hamza, M.M.; Al-Ansari, N.; Beotra, A.; Mohamed-Ali, V.; Almaadheed, M. 20-Hydroxyecdysone Dilates Muscle Arterioles in a Nitric Oxide-Dependent, Estrogen Er-Β Receptor-Independent Manner. Phytomed. Plus 2021, 1, 100078. [Google Scholar] [CrossRef]
- Yancu, D.; Vaillancourt, C.; Sanderson, J.T. Evaluating the Effects on Steroidogenesis of Estragole and Trans-Anethole in a Feto-Placental Co-Culture Model. Mol. Cell. Endocrinol. 2019, 498, 110583. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z. Drugbank 5.0: A Major Update to the Drugbank Database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.; Li, F.; Zhou, Y.; Zhang, Y.; Wang, Z.; Zhang, R.; Zhu, J.; Ren, Y.; Tan, Y. Therapeutic Target Database 2020: Enriched Resource for Facilitating Research and Early Development of Targeted Therapeutics. Nucleic Acids Res. 2020, 48, D1031–D1041. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Santos, A.; Von Mering, C.; Jensen, L.J.; Bork, P.; Kuhn, M. Stitch 5: Augmenting Protein–Chemical Interaction Networks with Tissue and Affinity Data. Nucleic Acids Res. 2016, 44, D380–D384. [Google Scholar] [CrossRef]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A. Enrichr: A Comprehensive Gene Set Enrichment Analysis Web Server 2016 Update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef]
- Fang, Z.; Liu, X.; Peltz, G. Gseapy: A Comprehensive Package for Performing Gene Set Enrichment Analysis in Python. Bioinformatics 2023, 39, btac757. [Google Scholar] [CrossRef]
- Gene Ontology (GO) Consortium. The Gene Ontology Resource: 20 Years and Still Going Strong. Nucleic Acids Res. 2019, 47, D330–D338. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. Kegg: New Perspectives on Genomes, Pathways, Diseases and Drugs. Nucleic Acids Res. 2017, 45, D353–D361. [Google Scholar] [CrossRef] [PubMed]
- Piñero, J.; Ramírez-Anguita, J.M.; Saüch-Pitarch, J.; Ronzano, F.; Centeno, E.; Sanz, F.; Furlong, L.I. The Disgenet Knowledge Platform for Disease Genomics: 2019 Update. Nucleic Acids Res. 2020, 48, D845–D855. [Google Scholar] [CrossRef] [PubMed]
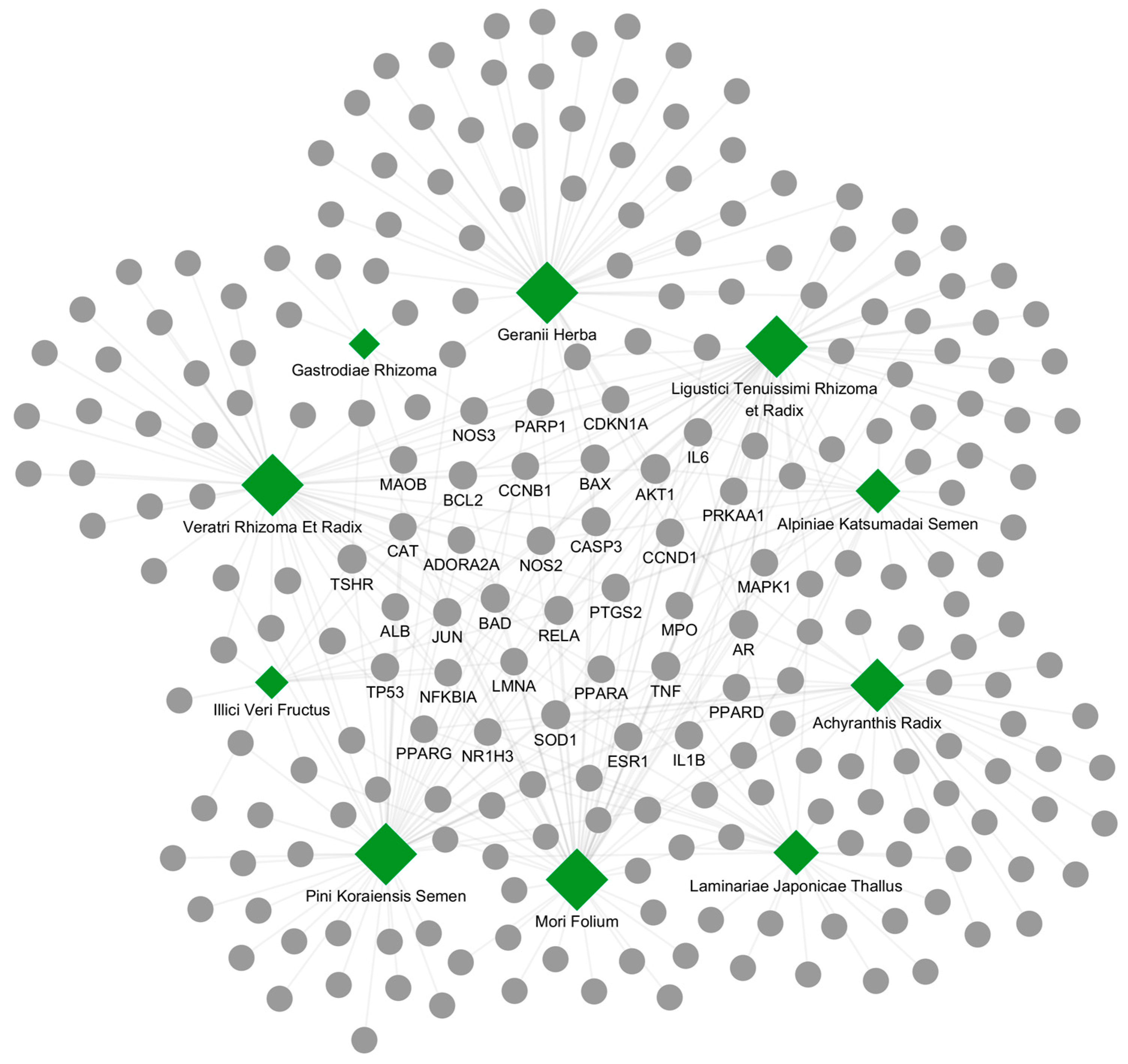
| Herb Name (Latin) | Correlation Score † | Overlap (p-Value #) | Enrichment | References (PMID) |
|---|---|---|---|---|
| Geranii Herba * | 0.021 | 2/50 (6.0 × 10−3) | 16.91 | - |
| Veratri Rhizoma Et Radix * | 0.011 | 5/50 (6.6 × 10−8) | 42.27 | |
| Laminariae Japonicae Thallus | 0.010 | 2/27 (1.7 × 10−3) | 31.31 | 18689954 [17] 21975053 [18] |
| Alpiniae Katsumadai Semen * | 0.010 | 2/26 (1.6 × 10−3) | 32.52 | - |
| Achyranthis Radix * | 0.010 | 2/38 (3.5 × 10−3) | 22.25 | - |
| Illici Veri Fructus * | 0.010 | 1/11 (2.6 × 10−2) | 38.43 | - |
| Ligustici Tenuissimi Rhizoma et Radix | 0.010 | 4/50 (4.2 × 10−6) | 33.82 | - |
| Mori Folium | 0.010 | 4/50 (4.2 × 10−6) | 33.82 | - |
| Pini Koraiensis Semen | 0.010 | 4/50 (4.2 × 10−6) | 33.82 | - |
| Arecae Semen | 0.009 | 3/50 (0.2 × 10−3) | 25.36 | 29278926 [19] |
| Term | Overlap | Adjusted p-Value | Combined Score | Genes |
|---|---|---|---|---|
| MAPK signaling pathway | 8/294 | 3.3 × 10−8 | 351.17 | JUN; IL1B; CASP3; AKT1; MAPK1; TP53; TNF; RELA |
| Thyroid hormone signaling pathway | 6/121 | 6.1 × 10−8 | 593.35 | CCND1; BAD; AKT1; MAPK1; ESR1; TP53 |
| Calcium signaling pathway | 3/240 | 8.4 × 10−3 | 37.28 | ADORA2A; NOS2; NOS3 |
| HIF-1 signaling pathway | 8/109 | 1.2 × 10−11 | 1463.04 | IL6; CDKN1A; NOS2; NOS3; BCL2; AKT1; MAPK1; RELA |
| p53 signaling pathway | 7/73 | 4.0 × 10−11 | 1804.99 | CDKN1A; CCNB1; CCND1; CASP3; BCL2; BAX; TP53 |
| mTOR signaling pathway | 4/154 | 1.5 × 10−4 | 150.43 | PRKAA1; AKT1; MAPK1; TNF |
| PI3K-Akt signaling pathway | 11/354 | 1.4 × 10−11 | 657.11 | IL6; CDKN1A; PRKAA1; CCND1; NOS3; BAD; BCL2; AKT1; MAPK1; TP53; RELA |
| Wnt signaling pathway | 4/166 | 2.0 × 10−4 | 134.66 | JUN; CCND1; TP53; PPARD |
| Parathyroid hormone synthesis, secretion and action | 3/106 | 8.4 × 10−4 | 128.09 | CDKN1A; BCL2; MAPK1 |
| Name | PubChem Compound ID | Correlation Score | Overlap (p-Value #) | References (PMID) |
|---|---|---|---|---|
| Geranii Herba | ||||
| ellagic acid | 5281855 | 0.007 | 2/107 (1.4 × 10−2) | 6724503 [20] |
| Veratri Rhizoma Et Radix | ||||
| resveratrol * | 445154 | 0.013 | 8/326 (1.2 × 10−8) | - |
| diosgenin | 99474 | 0.011 | 3/25 (8.6 × 10−6) | 35140607 [21] 28407664 [22] |
| Alpiniae Katsumadai Semen | ||||
| cardamomin * | 641785 | 0.012 | 2/22 (6.0 × 10−4) | - |
| Achyranthis Radix | ||||
| 20-hydroxyecdysone * | 5459840 | 0.009 | 2/37 (1.7 × 10−3) | - |
| Illici Veri Fructus | ||||
| (Z)-anethole * | 1549040 | 0.025 | 1/3 (5.0 × 10−3) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.-H.; Kim, J.-H.; Han, Y.; Kim, S.; Jin, H.; Lee, W.-Y. Multiscale Interactome-Guided Discovery Candidate Herbs and Active Ingredients Against Hyperthyroidism by Biased Random Walk Algorithm. Int. J. Mol. Sci. 2025, 26, 9789. https://doi.org/10.3390/ijms26199789
Han S-H, Kim J-H, Han Y, Kim S, Jin H, Lee W-Y. Multiscale Interactome-Guided Discovery Candidate Herbs and Active Ingredients Against Hyperthyroidism by Biased Random Walk Algorithm. International Journal of Molecular Sciences. 2025; 26(19):9789. https://doi.org/10.3390/ijms26199789
Chicago/Turabian StyleHan, Seok-Hoon, Ji-Hwan Kim, Yewon Han, Sangjin Kim, Hyowon Jin, and Won-Yung Lee. 2025. "Multiscale Interactome-Guided Discovery Candidate Herbs and Active Ingredients Against Hyperthyroidism by Biased Random Walk Algorithm" International Journal of Molecular Sciences 26, no. 19: 9789. https://doi.org/10.3390/ijms26199789
APA StyleHan, S.-H., Kim, J.-H., Han, Y., Kim, S., Jin, H., & Lee, W.-Y. (2025). Multiscale Interactome-Guided Discovery Candidate Herbs and Active Ingredients Against Hyperthyroidism by Biased Random Walk Algorithm. International Journal of Molecular Sciences, 26(19), 9789. https://doi.org/10.3390/ijms26199789





