Protein Corona as a Result of Interaction of Protein Molecules with Inorganic Nanoparticles
Abstract
1. Introduction
2. Parameters Affecting the Interaction of Nanoparticles with Proteins
2.1. Chemical Composition of Nanoparticles
2.2. Size of Nanoparticles
2.3. Surface Properties of Nanoparticles
2.4. Physicochemical Properties of the Colloid
3. Influence of Nanoparticles on Protein Structure
4. Binding Constants of Proteins with Inorganic Nanoparticles
5. Sizes of Complex of Nanoparticles with Proteins and the Thickness of the Protein Corona on the Surface of Nanoparticles
6. Change in the ξ-Potential of the System During the Interaction of Proteins with Nanoparticles
7. Cytotoxicity of Nanoparticle–Protein Complexes
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ab | Antibody |
| α-syn | α-synuclein |
| α-La | α-Lactalbumin |
| AC | Acetate buffer |
| AGP | Human α-1-acidglyco-protein |
| ALA | α-lipoic acid |
| ALD | Aldolase |
| AOT | Sodium bis(2-ethylhexyl) sulfosuccinate |
| APOA1 | Apolipoprotein A1 |
| APOH | Apolipoprotein H |
| Apt | Aptamer |
| BBS | Borate Buffered Saline |
| BGG | Bovine γ-globulin |
| BGL | β-glucosidase |
| BHb | Bovine hemoglobin |
| BLG | β-lactoglobulin |
| BSA | Bovine Serum Albumin |
| BSPP | Bis(2-sulfonatophenyl)phenylphosphine |
| CAT | Catalase |
| CBB | Carbonate-Bicarbonate Buffer |
| CD | Circular dichroism |
| GN | Glutenin |
| CRP | C-reactive protein |
| CTAB | Cetyltrimethylammonium bromide |
| CTAC | Cetrimonium chloride |
| CT | Chymotrypsin |
| Cys | Cysteine |
| Cyt C | Cytochrome C |
| DHLA | Dihydrolipoic Acid |
| DLS | Dynamic light scattering |
| DMEM | Dulbecco’s Modified Eagle Medium |
| EGFP | Green fluorescent protein |
| EG6OH | (11-mercaptoundecyl)hexa(ethylene glycol) |
| EMb | Equine skeletal myoglobin |
| FBG | Fibrinogen |
| FBS | Fetal Bovine Serum |
| FTIR | Fourier Transform Infrared Spectroscopy |
| GA | Glutaric acid |
| GD | Gliadin |
| Gly | Glycine |
| GME | Glutathione monoethyl ester |
| Hb | Hemoglobin |
| HbA1c | Riftia pachyptila coelomic hemoglobin |
| HbAm | Arenicola marina hemoglobin |
| HEPES | 4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid |
| HEWL | Hen egg white lysozyme |
| HSA | Human Serum Albumin |
| IgG | Immunoglobulin G |
| LECT2 | Leukocyte cell-derived chemotaxin-2 |
| LDH | Lactate dehydrogenase |
| 18 LPC | 1-Palmitoyl-2-lysophosphatidylcholine |
| LYZ | Lysozyme |
| Luc | Luciferase |
| Mb | Myoglobin |
| MES | 2-(N-morpholino)ethanesulfonic acid |
| MRI | Magnetic resonance imaging |
| Nar | Naringenin |
| OA | Oleic acid |
| oxyHb | Oxyhemoglobin |
| OVA | Ovalbumin |
| PBS | Phosphate-Buffered Saline |
| PEG | Polyethylene Glycol |
| PEI | Polyethylenimine |
| C-/L-PEOXA | Linear/cyclic poly(2-ethyl-2-oxazoline) |
| Phe | Phenylalanine |
| PLL | Poly-L-lysine |
| PPh | Polyphenols |
| Pro | Proline |
| PVA | Polyvinyl alcohol |
| PVP | Polyvinylpyrrolidone |
| QOR | Quinone oxidoreductase |
| SA | Sodium alginate |
| SOD | Superoxide dismutase |
| SP-B | Surfactant Protein B |
| SPI | Soy Protein Isolate |
| SPR | Surface plasmon resonance |
| SC | Sodium Citrate |
| T80 | Tween 80 |
| TE | Solution of Tris and EDTA |
| TEM | Transmission electron microscope |
| TF | Transferrin |
| TFE | Trifluoroethanol |
| TM | Tropomyosin |
| Tris-HCl | Tris(hydroxymethyl)aminomethane Hydrochloride |
| TPN | Tiopronin |
| TRP | Trypsin |
| Trp214 | Amino acid residue of tryptophan |
| WP | Whey protein |
References
- Gao, J.; Xia, Z.; Gunasekar, S.; Jiang, C.; Karp, J.M.; Joshi, N. Precision drug delivery to the central nervous system using engineered nanoparticles. Nat. Rev. Mater. 2024, 9, 567–588. [Google Scholar] [CrossRef]
- Farooq, M.U.; Lawrie, C.H.; Deng, N.-N. Engineering nanoparticles for cancer immunotherapy: Current achievements, key considerations and future perspectives. Chem. Eng. J. 2024, 486, 150356. [Google Scholar] [CrossRef]
- Truong, T.T.; Mondal, S.; Doan, V.H.M.; Tak, S.; Choi, J.; Oh, H.; Nguyen, T.D.; Misra, M.; Lee, B.; Oh, J. Precision-engineered metal and metal-oxide nanoparticles for biomedical imaging and healthcare applications. Adv. Colloid Interface Sci. 2024, 332, 103263. [Google Scholar] [CrossRef] [PubMed]
- Sengar, A. Precision in practice: Nanotechnology and targeted therapies for personalized care. Int. J. Adv. Nano Comput. Anal. 2024, 3, 56–67. [Google Scholar] [CrossRef]
- Umair, M.; Zafar, S.H.; Cheema, M.; Usman, M. New insights into the environmental application of hybrid nanoparticles in metal contaminated agroecosystem: A review. J. Environ. Manag. 2024, 349, 119553. [Google Scholar] [CrossRef] [PubMed]
- Selim, M.M.; El-Safty, S.; Tounsi, A.; Shenashen, M. A review of magnetic nanoparticles used in nanomedicine. APL Mater. 2024, 12, 010601. [Google Scholar] [CrossRef]
- Kumarasamy, R.V.; Natarajan, P.M.; Umapathy, V.R.; Roy, J.R.; Mironescu, M.; Palanisamy, C.P. Clinical applications and therapeutic potentials of advanced nanoparticles: A comprehensive review on completed human clinical trials. Front. Nanotechnol. 2024, 6, 1479993. [Google Scholar] [CrossRef]
- Lapusan, R.; Borlan, R.; Focsan, M. Advancing MRI with magnetic nanoparticles: A comprehensive review of translational research and clinical trials. Nanoscale Adv. 2024, 6, 2234–2259. [Google Scholar] [CrossRef]
- Lim, S.H.; Wong, T.W.; Tay, W.X. Overcoming colloidal nanoparticle aggregation in biological milieu for cancer therapeutic delivery: Perspectives of materials and particle design. Adv. Colloid Interface Sci. 2024, 325, 103094. [Google Scholar] [CrossRef]
- Nanda, S.S.; Yi, D.K. Recent advances in synergistic effect of nanoparticles and its biomedical application. Int. J. Mol. Sci. 2024, 25, 3266. [Google Scholar] [CrossRef]
- Ji, Y.; Wang, Y.; Wang, X.; Lv, C.; Zhou, Q.; Jiang, G.; Yan, B.; Chen, L. Beyond the promise: Exploring the complex interactions of nanoparticles within biological systems. J. Hazard. Mater. 2024, 468, 133800. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Cortez-Jugo, C.; Ju, Y.; Caruso, F. Approaching Two Decades: Biomolecular Coronas and Bio–Nano Interactions. ACS Nano 2024, 18, 33257–33263. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Marets, C.; Boudon, J.; Millot, N.; Saviot, L.; Maurizi, L. In vivo protein corona on nanoparticles: Does the control of all material parameters orient the biological behavior? Nanoscale Adv. 2021, 3, 1209–1229. [Google Scholar] [CrossRef] [PubMed]
- Shemetov, A.A.; Nabiev, I.; Sukhanova, A. Molecular Interaction of Proteins and Peptides with Nanoparticles. ACS Nano 2012, 6, 4585–4602. [Google Scholar] [CrossRef]
- Meesaragandla, B.; García, I.; Biedenweg, D.; Toro-Mendoza, J.; Coluzza, I.; Liz-Marzán, L.M.; Delcea, M. H-Bonding-mediated binding and charge reorganization of proteins on gold nanoparticles. Phys. Chem. Chem. Phys. 2020, 22, 4490–4500. [Google Scholar] [CrossRef]
- Kopp, M.; Kollenda, S.; Epple, M. Nanoparticle–Protein Interactions: Therapeutic Approaches and Supramolecular Chemistry. Acc. Chem. Res. 2017, 50, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zarić, S.D.; Zrilić, S.S.; Gofman, I.; Heck, B.; Reiter, G. London dispersion forces and steric effects within nanocomposites tune interaction energies and chain conformation. Commun. Chem. 2025, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Greco, A.; Corbo, C. Could selection of biomolecular corona constituents through nanoparticle design produce more effective localized therapies? Nanomedicine 2025, 20, 1219–1222. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.F.; Picco, A.S.; Galdino, F.E.; Albuquerque, L.J.C.; Berret, J.-F.; Cardoso, M.B. Nanoparticle–protein interaction: Demystifying the correlation between protein corona and aggregation phenomena. ACS Appl. Mater. Interfaces 2022, 14, 28559–28569. [Google Scholar] [CrossRef] [PubMed]
- Salvati, A. The biomolecular corona of nanomedicines: Effects on nanomedicine outcomes and emerging opportunities. Curr. Opin. Biotechnol. 2024, 87, 103101. [Google Scholar] [CrossRef]
- Piella, J.; Bastús, N.G.; Puntes, V. Size-Dependent Protein–Nanoparticle Interactions in Citrate-Stabilized Gold Nanoparticles: The Emergence of the Protein Corona. Bioconjugate Chem. 2016, 28, 88–97. [Google Scholar] [CrossRef]
- Kwon, M.J.; Lee, J.; Wark, A.W.; Lee, H.J. Nanoparticle-Enhanced Surface Plasmon Resonance Detection of Proteins at Attomolar Concentrations: Comparing Different Nanoparticle Shapes and Sizes. Anal. Chem. 2012, 84, 1702–1707. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, C.C.; Payne, C.K. Nanoparticle–Cell Interactions: Molecular Structure of the Protein Corona and Cellular Outcomes. Acc. Chem. Res. 2014, 47, 2651–2659. [Google Scholar] [CrossRef] [PubMed]
- Saei, A.A.; Mahmoudi, M. Multi-omics exploration of biomolecular corona in nanomedicine therapeutics and diagnostics. Nanomedicine 2024, 19, 1223–1226. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, Y.; Tang, Q.; Yin, D.; Tang, C.; He, E.; Zou, L.; Peng, Q. The protein corona and its effects on nanoparticle-based drug delivery systems. Acta Biomater. 2021, 129, 57–72. [Google Scholar] [CrossRef]
- Simon, J.; Kuhn, G.; Fichter, M.; Gehring, S.; Landfester, K.; Mailänder, V. Unraveling the in vivo protein corona. Cells 2021, 10, 132. [Google Scholar] [CrossRef]
- Natarajan, P.; Tomich, J.M. Understanding the influence of experimental factors on bio-interactions of nanoparticles: Towards improving correlation between in vitro and in vivo studies. Arch. Biochem. Biophys. 2020, 694, 108592. [Google Scholar] [CrossRef]
- Shan, H.; Guo, Y.; Li, J.; Liu, Z.; Chen, S.; Dashnyam, B.; McClements, D.J.; Cao, C.; Xu, X.; Yuan, B. Impact of whey protein corona formation around TiO2 nanoparticles on their physiochemical properties and gastrointestinal fate. J. Agric. Food Chem. 2024, 72, 4958–4976. [Google Scholar] [CrossRef]
- Corbo, C.; Molinaro, R.; Parodi, A.; Toledano Furman, N.E.; Salvatore, F.; Tasciotti, E. The Impact of Nanoparticle Protein Corona on Cytotoxicity, Immunotoxicity and Target Drug Delivery. Nanomedicine 2015, 11, 81–100. [Google Scholar] [CrossRef]
- Okyem, S.; Awotunde, O.; Ogunlusi, T.; Riley, M.B.; Driskell, J.D. Probing the mechanism of antibody-triggered aggregation of gold nanoparticles. Langmuir 2021, 37, 2993–3000. [Google Scholar] [CrossRef] [PubMed]
- Carreón-Álvarez, C.; Sánchez-García, J.L.; Sanabria-Ayala, V.; Ortiz-Frade, L.A.; García-Rodríguez, M.E.; Rodríguez-López, J.L.; López-Revilla, R. Multibranched gold nanoparticles coated with serum proteins fit for photothermal tumor ablation. AIP Adv. 2020, 10, 125030. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, J.L.; Lazarovits, J.; Chan, W.C. An analysis of the binding function and structural organization of the protein corona. J. Am. Chem. Soc. 2020, 142, 8827–8836. [Google Scholar] [CrossRef] [PubMed]
- Flint, Z.; Grannemann, H.; Baffour, K.; Koti, N.; Taylor, E.; Grier, E.; Sutton, C.; Johnson, D.; Dandawate, P.; Patel, R. Mechanistic insights behind the self-assembly of human insulin under the influence of surface-engineered gold nanoparticles. ACS Chem. Neurosci. 2024, 15, 2359–2371. [Google Scholar] [CrossRef] [PubMed]
- Ghouri, M.D.; Tariq, A.; Saleem, J.; Muhaymin, A.; Cai, R.; Chen, C. Protein corona potentiates the recovery of nanoparticle-induced disrupted tight junctions in endothelial cells. Nanoscale Horiz. 2025, 10, 179–189. [Google Scholar] [CrossRef]
- Sun, Y.; Zhen, T.; Li, Y.; Wang, Y.; Wang, M.; Li, X.; Sun, Q. Interaction of food-grade titanium dioxide nanoparticles with pepsin in simulated gastric fluid. LWT 2020, 134, 110208. [Google Scholar] [CrossRef]
- Molkova, E.A.; Pustovoy, V.I.; Stepanova, E.V.; Gorudko, I.V.; Astashev, M.E.; Simakin, A.V.; Sarimov, R.M.; Gudkov, S.V. pH-dependent HEWL-AuNPs interactions: Optical study. Molecules 2023, 29, 82. [Google Scholar] [CrossRef]
- Sanchez-Guzman, D.; Giraudon--Colas, G.l.; Marichal, L.; Boulard, Y.; Wien, F.; Degrouard, J.; Baeza-Squiban, A.; Pin, S.; Renault, J.P.; Devineau, S. In situ analysis of weakly bound proteins reveals molecular basis of soft corona formation. ACS Nano 2020, 14, 9073–9088. [Google Scholar] [CrossRef]
- Chubarov, A.S. Serum Albumin for Magnetic Nanoparticles Coating. Magnetochemistry 2022, 8, 13. [Google Scholar] [CrossRef]
- Dhar, S.; Sood, V.; Lohiya, G.; Deivendran, H.; Katti, D.S. Role of physicochemical properties of protein in modulating the nanoparticle-bio interface. J. Biomed. Nanotechnol. 2020, 16, 1276–1295. [Google Scholar] [CrossRef]
- Sabourian, P.; Yazdani, G.; Ashraf, S.S.; Frounchi, M.; Mashayekhan, S.; Kiani, S.; Kakkar, A. Effect of physico-chemical properties of nanoparticles on their intracellular uptake. Int. J. Mol. Sci. 2020, 21, 8019. [Google Scholar] [CrossRef] [PubMed]
- Burmistrov, D.E.; Serov, D.A.; Baimler, I.V.; Gritsaeva, A.V.; Chapala, P.; Simakin, A.V.; Astashev, M.E.; Karmanova, E.E.; Dubinin, M.V.; Nizameeva, G.R.; et al. Polymethyl Methacrylate-like Photopolymer Resin with Titanium Metal Nanoparticles Is a Promising Material for Biomedical Applications. Polymers 2025, 17, 1830. [Google Scholar] [CrossRef] [PubMed]
- Dikovskaya, A.O.; Simakin, A.V.; Baimler, I.V.; Gudkov, S.V. The concentration limit of stability for individual gold nanoparticles in aqueous colloid during water evaporation. Chem. Phys. 2024, 586, 112399. [Google Scholar] [CrossRef]
- Molkova, E.A.; Pustovoi, V.I.; Baimler, I.V.; Simakin, A.V.; Burmistrov, D.E.; Gorudko, I.V.; Gudkov, S.V. Optical Study of the Influence of the Medium Acidity on the Interaction between Gold Nanoparticles and Bovine Serum Albumin in Aqueous Solution. Phys. Wave Phenom. 2024, 32, 232–240. [Google Scholar] [CrossRef]
- Antonova, A.; Kolesnikova, O.; Ivantcova, P.; Gubaidullina, M.; Shipunova, V. Comparative Analysis of Stabilization Methods for Silver Nanoprisms As Promising Nanobiomedicine Agents. Nanobiotechnology Rep. 2024, 19, 921–928. [Google Scholar] [CrossRef]
- Griaznova, O.Y.; Belyaev, I.B.; Sogomonyan, A.S.; Zelepukin, I.V.; Tikhonowski, G.V.; Popov, A.A.; Komlev, A.S.; Nikitin, P.I.; Gorin, D.A.; Kabashin, A.V.; et al. Laser Synthesized Core-Satellite Fe-Au Nanoparticles for Multimodal In Vivo Imaging and In Vitro Photothermal Therapy. Pharmaceutics 2022, 14, 994. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, Z.; Safiabadi Tali, S.H.; Hajimiri, H.; Al-Kassawneh, M.; Jahanshahi-Anbuhi, S. Gold nanoparticles-based colorimetric assays for environmental monitoring and food safety evaluation. Crit. Rev. Anal. Chem. 2024, 54, 2209–2244. [Google Scholar] [CrossRef] [PubMed]
- Orlov, A.V.; Burenin, A.G.; Skirda, A.M.; Nikitin, P.I. Kinetic Analysis of Prostate-Specific Antigen Interaction with Monoclonal Antibodies for Development of a Magnetic Immunoassay Based on Nontransparent Fiber Structures. Molecules 2022, 27, 8077. [Google Scholar] [CrossRef] [PubMed]
- Pushkarev, A.V.; Orlov, A.V.; Znoyko, S.L.; Bragina, V.A.; Nikitin, P.I. Rapid and Easy-to-Use Method for Accurate Characterization of Target Binding and Kinetics of Magnetic Particle Bioconjugates for Biosensing. Sensors 2021, 21, 2802. [Google Scholar] [CrossRef]
- Siddique, S.; Chow, J.C. Gold nanoparticles for drug delivery and cancer therapy. Appl. Sci. 2020, 10, 3824. [Google Scholar] [CrossRef]
- Sarma, P.P.; Rai, A.; Baruah, P.K. Recent advances in the development of antibiotics-coated gold nanoparticles to combat antimicrobial resistance. Antibiotics 2024, 13, 124. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Serov, D.A.; Astashev, M.E.; Semenova, A.A.; Lisitsyn, A.B. Ag2O Nanoparticles as a Candidate for Antimicrobial Compounds of the New Generation. Pharmaceuticals 2022, 15, 968. [Google Scholar] [CrossRef]
- Tukova, A.; Kuschnerus, I.C.; Garcia-Bennett, A.; Wang, Y.; Rodger, A. Gold Nanostars with Reduced Fouling Facilitate Small Molecule Detection in the Presence of Protein. Nanomaterials 2021, 11, 2565. [Google Scholar] [CrossRef] [PubMed]
- Tukova, A.; Nguyen, N.T.T.; Garcia-Bennett, A.; Rodger, A.; Wang, Y. Plasmonic Nanostars: Unique Properties That Distinguish Them from Spherical Nanoparticles from a Biosensing Perspective. Adv. Opt. Mater. 2024, 12, 2401183. [Google Scholar] [CrossRef]
- Tao, X.; Chang, X.; Wan, X.; Guo, Y.; Zhang, Y.; Liao, Z.; Song, Y.; Song, E. Impact of protein corona on noncovalent molecule–gold nanoparticle-based sensing. Anal. Chem. 2020, 92, 14990–14998. [Google Scholar] [CrossRef] [PubMed]
- Bolaños, K.; Celis, F.; Garrido, C.; Campos, M.; Guzmán, F.; Kogan, M.J.; Araya, E. Adsorption of bovine serum albumin on gold nanoprisms: Interaction and effect of NIR irradiation on protein corona. J. Mater. Chem. B 2020, 8, 8644–8657. [Google Scholar] [CrossRef]
- Bae, Y.; Liu, X. Unveiling the effects of protein corona formation on the aggregation kinetics of gold nanoparticles in monovalent and divalent electrolytes. Environ. Pollut. 2024, 346, 123552. [Google Scholar] [CrossRef]
- Barbir, R.; Capjak, I.; Crnković, T.; Debeljak, Ž.; Jurašin, D.D.; Ćurlin, M.; Šinko, G.; Weitner, T.; Vrček, I.V. Interaction of silver nanoparticles with plasma transport proteins: A systematic study on impacts of particle size, shape and surface functionalization. Chem. Biol. Interact. 2021, 335, 109364. [Google Scholar] [CrossRef]
- Sahraei, A.; Mohammadi, F.; Boukherroub, R.; Szunerits, S. Formation of a highly stable and nontoxic protein corona upon interaction of human α-1-acid glycoprotein (AGP) with citrate-stabilized silver nanoparticles. Langmuir 2020, 36, 10321–10330. [Google Scholar] [CrossRef]
- Batista, C.C.; Albuquerque, L.J.; Jäger, A.; Stepanek, P.; Giacomelli, F.C. Probing protein adsorption onto polymer-stabilized silver nanocolloids towards a better understanding on the evolution and consequences of biomolecular coronas. Mater. Sci. Eng. C 2020, 111, 110850. [Google Scholar] [CrossRef] [PubMed]
- Boehmler, D.J.; O’Dell, Z.J.; Chung, C.; Riley, K.R. Bovine serum albumin enhances silver nanoparticle dissolution kinetics in a size-and concentration-dependent manner. Langmuir 2020, 36, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Guglielmelli, A.; D’Aquila, P.; Palermo, G.; Dell’Aglio, M.; Passarino, G.; Strangi, G.; Bellizzi, D. Role of the human serum albumin protein corona in the antimicrobial and photothermal activity of metallic nanoparticles against Escherichia coli bacteria. Acs Omega 2023, 8, 31333–31343. [Google Scholar] [CrossRef] [PubMed]
- Russo Krauss, I.; Picariello, A.; Vitiello, G.; De Santis, A.; Koutsioubas, A.; Houston, J.E.; Fragneto, G.; Paduano, L. Interaction with human serum proteins reveals biocompatibility of phosphocholine-functionalized SPIONs and formation of albumin-decorated nanoparticles. Langmuir 2020, 36, 8777–8791. [Google Scholar] [CrossRef] [PubMed]
- Włodarczyk, A.; Gorgoń, S.; Radoń, A.; Bajdak-Rusinek, K. Magnetite nanoparticles in magnetic hyperthermia and cancer therapies: Challenges and perspectives. Nanomaterials 2022, 12, 1807. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, J.; Soares, S.F.; Amorim, C.O.; Amaral, J.S.; Silva, C.; Martel, F.; Trindade, T.; Daniel-da-Silva, A.L. Magnetic driven nanocarriers for pH-responsive doxorubicin release in cancer therapy. Molecules 2020, 25, 333. [Google Scholar] [CrossRef] [PubMed]
- Kianfar, E. Magnetic nanoparticles in targeted drug delivery: A review. J. Supercond. Nov. Magn. 2021, 34, 1709–1735. [Google Scholar] [CrossRef]
- Kurlandskaya, G.V.; Safronov, A.P.; Shcherbinin, S.V.; Beketov, I.V.; Blyakhman, F.A.; Makarova, E.B.; Korch, M.A.; Svalov, A.V. Magnetic nanoparticles obtained by electrophysical methods: Focus on biomedical applications. Solid State Physics 2021, 63, 1447–1461. [Google Scholar] [CrossRef]
- Nithya, R.; Thirunavukkarasu, A.; Sathya, A.B.; Sivashankar, R. Magnetic materials and magnetic separation of dyes from aqueous solutions: A review. Environ. Chem. Lett. 2021, 19, 1275–1294. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Bahreinizad, H.; Amiri, Z.; Aliabadi, H.A.M.; Salimi-Bani, M.; Nakisa, A.; Davoodi, F.; Tahmasebi, B.; Ahmadpour, F.; Radinekiyan, F. Functionalized magnetic nanoparticles for the separation and purification of proteins and peptides. TrAC Trends Anal. Chem. 2021, 141, 116291. [Google Scholar] [CrossRef]
- Gawali, S.L.; Shelar, S.B.; Gupta, J.; Barick, K.; Hassan, P. Immobilization of protein on Fe3O4 nanoparticles for magnetic hyperthermia application. Int. J. Biol. Macromol. 2021, 166, 851–860. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, G.; Wang, S. Magnetic nanoparticle tracking for one-step protein separation and binding kinetics analysis. J. Electrochem. Soc. 2022, 169, 057509. [Google Scholar] [CrossRef]
- Alnadari, F.; Xue, Y.; Alsubhi, N.H.; Alamoudi, S.A.; Alwabli, A.S.; Al-Quwaie, D.A.; Hamed, Y.S.; Nasiru, M.M.; Ebrahim, A.A.; El-Saadony, M.T. Reusability of immobilized β-glucosidase on sodium alginate-coated magnetic nanoparticles and high productivity applications. J. Saudi Chem. Soc. 2022, 26, 101517. [Google Scholar] [CrossRef]
- Yu, Z.; Li, Q.; Wang, J.; Yu, Y.; Wang, Y.; Zhou, Q.; Li, P. Reactive Oxygen Species-Related Nanoparticle Toxicity in the Biomedical Field. Nanoscale Res. Lett. 2020, 15, 115. [Google Scholar] [CrossRef] [PubMed]
- Timoshnikov, V.A.; Kobzeva, T.V.; Polyakov, N.E.; Kontoghiorghes, G.J. Redox Interactions of Vitamin C and Iron: Inhibition of the Pro-Oxidant Activity by Deferiprone. Int. J. Mol. Sci. 2020, 21, 3967. [Google Scholar] [CrossRef] [PubMed]
- Bychkova, A.V.; Lopukhova, M.V.; Wasserman, L.A.; Pronkin, P.G.; Degtyarev, Y.N.; Shalupov, A.I.; Vasilyeva, A.D.; Lyubov’V, Y.; Kovarski, A.L.; Kononikhin, A.S. Interaction between immunoglobulin G and peroxidase-like iron oxide nanoparticles: Physicochemical and structural features of the protein. Biochim. et Biophys. Acta (BBA) Proteins Proteom. 2020, 1868, 140300. [Google Scholar] [CrossRef] [PubMed]
- Burmistrov, D.E.; Shumeyko, S.A.; Semenova, N.A.; Dorokhov, A.S.; Gudkov, S.V. Selenium Nanoparticles (Se NPs) as Agents for Agriculture Crops with Multiple Activity: A Review. Agronomy 2025, 15, 1591. [Google Scholar] [CrossRef]
- Turovsky, E.A.; Plotnikov, E.Y.; Simakin, A.V.; Gudkov, S.V.; Varlamova, E.G. New magnetic iron nanoparticle doped with selenium nanoparticles and the mechanisms of their cytoprotective effect on cortical cells under ischemia-like conditions. Arch. Biochem. Biophys. 2025, 764, 110241. [Google Scholar] [CrossRef] [PubMed]
- Shabalina, A.V.; Kozlov, V.A.; Popov, I.A.; Gudkov, S.V. A Review on Recently Developed Antibacterial Composites of Inorganic Nanoparticles and Non-Hydrogel Polymers for Biomedical Applications. Nanomaterials 2024, 14, 1753. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Burmistrov, D.E.; Fomina, P.A.; Validov, S.Z.; Kozlov, V.A. Antibacterial Properties of Copper Oxide Nanoparticles (Review). Int. J. Mol. Sci. 2024, 25, 11563. [Google Scholar] [CrossRef]
- Simakin, A.V.; Burmistrov, D.E.; Baimler, I.V.; Gritsaeva, A.V.; Serov, D.A.; Astashev, M.E.; Chapala, P.; Validov, S.Z.; Yanbaev, F.M.; Gudkov, S.V. TiO2 Nanoparticles Obtained by Laser Sintering When Added to Methacrylate Photopolymer Resin Improve Its Physicochemical Characteristics and Impart Antibacterial Properties. Inorganics 2025, 13, 233. [Google Scholar] [CrossRef]
- Yang, H.; Hao, C.; Nan, Z.; Sun, R. Bovine hemoglobin adsorption onto modified silica nanoparticles: Multi-spectroscopic measurements based on kinetics and protein conformation. Int. J. Biol. Macromol. 2020, 155, 208–215. [Google Scholar] [CrossRef]
- Dembélé, J.; Liao, J.-H.; Liu, T.-P.; Chen, Y.-P. Overcoming cytosolic delivery barriers of proteins using denatured protein-conjugated mesoporous silica nanoparticles. ACS Appl. Mater. Interfaces 2022, 15, 432–451. [Google Scholar] [CrossRef] [PubMed]
- Precupas, A.; Gheorghe, D.; Botea-Petcu, A.; Leonties, A.R.; Sandu, R.; Popa, V.T.; Mariussen, E.; Naouale, E.Y.; Rundén-Pran, E.; Dumit, V. Thermodynamic parameters at bio–nano interface and nanomaterial toxicity: A case study on BSA interaction with ZnO, SiO2, and TiO2. Chem. Res. Toxicol. 2020, 33, 2054–2071. [Google Scholar] [CrossRef] [PubMed]
- Mitjans, M.; Marics, L.; Bilbao, M.; Maddaleno, A.S.; Piñero, J.J.; Vinardell, M.P. Size matters? A comprehensive in vitro study of the impact of particle size on the toxicity of ZnO. Nanomaterials 2023, 13, 1800. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Alshehri, M.A.; Alamery, S.F.; Khan, J.M. Impact of metal nanoparticles on the structure and function of metabolic enzymes. Int. J. Biol. Macromol. 2021, 188, 576–585. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, D.; Yu, H. Fabrication of protein-coated titanium dioxide nanoparticles for cellular uptake fluorescence imaging and treatment of colorectal cancer. Mater. Res. Express 2021, 8, 125008. [Google Scholar] [CrossRef]
- Vian, R.; Salehi, H.; Lapierre, M.; Cuisinier, F.; Cavaillès, V.; Balme, S. Adsorption of proteins on TiO2 particles influences their aggregation and cell penetration. Food Chem. 2021, 360, 130003. [Google Scholar] [CrossRef] [PubMed]
- Díez-Pascual, A.M. Carbon-Based Nanomaterials. Int. J. Mol. Sci. 2021, 22, 7726. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.; Flahaut, E.; Golzio, M. Overview of Carbon Nanotubes for Biomedical Applications. Materials 2019, 12, 624. [Google Scholar] [CrossRef] [PubMed]
- Negri, V.; Pacheco-Torres, J.; Calle, D.; López-Larrubia, P. Carbon Nanotubes in Biomedicine. Top. Curr. Chem. 2020, 378, 15. [Google Scholar] [CrossRef]
- Chen, H.; Huang, J.; Fam, D.; Tok, A. Horizontally Aligned Carbon Nanotube Based Biosensors for Protein Detection. Bioengineering 2016, 3, 23. [Google Scholar] [CrossRef]
- Wayu, M.B.; Pannell, M.J.; Labban, N.; Case, W.S.; Pollock, J.A.; Leopold, M.C. Functionalized carbon nanotube adsorption interfaces for electron transfer studies of galactose oxidase. Bioelectrochemistry 2019, 125, 116–126. [Google Scholar] [CrossRef]
- Serda, M.; Korzuch, J.; Dreszer, D.; Krzykawska-Serda, M.; Musioł, R. Interactions between modified fullerenes and proteins in cancer nanotechnology. Drug Discov. Today 2023, 28, 103704. [Google Scholar] [CrossRef] [PubMed]
- Di Giosia, M.; Bomans, P.H.H.; Bottoni, A.; Cantelli, A.; Falini, G.; Franchi, P.; Guarracino, G.; Friedrich, H.; Lucarini, M.; Paolucci, F.; et al. Proteins as supramolecular hosts for C60: A true solution of C60 in water. Nanoscale 2018, 10, 9908–9916. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zhu, Y.; Yao, X.; Liu, H. Carbon Nanoparticles Inhibit the Aggregation of Prion Protein as Revealed by Experiments and Atomistic Simulations. J. Chem. Inf. Model. 2018, 59, 1909–1918. [Google Scholar] [CrossRef] [PubMed]
- Grassian, V.H. When Size Really Matters: Size-Dependent Properties and Surface Chemistry of Metal and Metal Oxide Nanoparticles in Gas and Liquid Phase Environments. J. Phys. Chem. C 2008, 112, 18303–18313. [Google Scholar] [CrossRef]
- Xu, M.; Qi, Y.; Liu, G.; Song, Y.; Jiang, X.; Du, B. Size-Dependent In Vivo Transport of Nanoparticles: Implications for Delivery, Targeting, and Clearance. ACS Nano 2023, 17, 20825–20849. [Google Scholar] [CrossRef]
- Eun, C. Effect of surface curvature on diffusion-limited reactions on a curved surface. J. Chem. Phys. 2017, 147, 184112. [Google Scholar] [CrossRef]
- Zhang, N.; Xiong, G.; Liu, Z. Toxicity of metal-based nanoparticles: Challenges in the nano era. Front. Bioeng. Biotechnol. 2022, 10, 1001572. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, G.; Liu, Q.S.; Liu, W.; Qu, G.; Hu, L.; Long, Y.; Cai, Z.; Zhao, X.; Jiang, G. Identification and interaction mechanism of protein corona on silver nanoparticles with different sizes and the cellular responses. J. Hazard. Mater. 2021, 414, 125582. [Google Scholar] [CrossRef]
- Buckley, A.; Warren, J.; Hussain, R.; Smith, R. Synchrotron radiation circular dichroism spectroscopy reveals that gold and silver nanoparticles modify the secondary structure of a lung surfactant protein B analogue. Nanoscale 2023, 15, 4591–4603. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.-M.; Chen, W.-Q.; Lu, Y.-Q.; Han, J.-Y.; Liu, Y.; Jiang, F.-L. A model beyond protein corona: Thermodynamics and binding stoichiometries of the interactions between ultrasmall gold nanoclusters and proteins. Nanoscale 2020, 12, 4573–4585. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, V.; Velasco, B.; Arellano, L.G.; Domínguez-Arca, V.; Cambón, A.; Pardo, A.; Topete, A.; Rosales-Rivera, L.C.; Soltero, J.A.; Barbosa, S. Role of surface functionalization and biomolecule structure on protein corona adsorption and conformation onto anisotropic metallic nanoparticles. J. Mol. Liq. 2024, 398, 124240. [Google Scholar] [CrossRef]
- Zhuo, Y.; Zhao, Y.-G.; Zhang, Y. Enhancing Drug Solubility, Bioavailability, and Targeted Therapeutic Applications through Magnetic Nanoparticles. Molecules 2024, 29, 4854. [Google Scholar] [CrossRef] [PubMed]
- Solak, K.; Mavi, A.; Yılmaz, B. Disulfiram-loaded functionalized magnetic nanoparticles combined with copper and sodium nitroprusside in breast cancer cells. Mater. Sci. Eng. C 2021, 119, 111452. [Google Scholar] [CrossRef] [PubMed]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Zhu, H.; Lu, Y.; Xia, J.; Liu, Y.; Chen, J.; Lee, J.; Koh, K.; Chen, H. Aptamer-assisted protein orientation on silver magnetic nanoparticles: Application to sensitive leukocyte cell-derived chemotaxin 2 surface plasmon resonance sensors. Anal. Chem. 2022, 94, 2109–2118. [Google Scholar] [CrossRef]
- Lee, K.; Oh, E.; Stewart, M.H.; Susumu, K.; Díaz, S.A.; Green, C.M.; Nag, O.K.; Delehanty, J.B. Adsorption of hemoglobin onto gold nanoparticles: Role of ligand coating on assembly formation and protein structure. J. Nanoparticle Res. 2025, 27, 2. [Google Scholar] [CrossRef]
- González-García, L.E.; MacGregor, M.N.; Visalakshan, R.M.; Lazarian, A.; Cavallaro, A.A.; Morsbach, S.; Mierczynska-Vasilev, A.; Mailänder, V.; Landfester, K.; Vasilev, K. Nanoparticles surface chemistry influence on protein corona composition and inflammatory responses. Nanomaterials 2022, 12, 682. [Google Scholar] [CrossRef]
- Ghosh, G.; Panicker, L. Protein–nanoparticle interactions and a new insight. Soft Matter 2021, 17, 3855–3875. [Google Scholar] [CrossRef]
- Gao, Y.; He, Y.; Zhang, H.; Zhang, Y.; Gao, T.; Wang, J.-H.; Wang, S. Zwitterion-functionalized mesoporous silica nanoparticles for enhancing oral delivery of protein drugs by overcoming multiple gastrointestinal barriers. J. Colloid Interface Sci. 2021, 582, 364–375. [Google Scholar] [CrossRef]
- Yu, Q.; Zhao, L.; Guo, C.; Yan, B.; Su, G. Regulating Protein Corona Formation and Dynamic Protein Exchange by Controlling Nanoparticle Hydrophobicity. Front. Bioeng. Biotechnol. 2020, 8, 210. [Google Scholar] [CrossRef] [PubMed]
- Lunardi, C.N.; Gomes, A.J.; Rocha, F.S.; De Tommaso, J.; Patience, G.S. Experimental methods in chemical engineering: Zeta potential. Can. J. Chem. Eng. 2021, 99, 627–639. [Google Scholar] [CrossRef]
- Najahi-Missaoui, W.; Arnold, R.D.; Cummings, B.S. Safe nanoparticles: Are we there yet? Int. J. Mol. Sci. 2020, 22, 385. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Ito, S.; Yoshino, F.; Suzuki, Y.; Zhao, L.; Komatsu, N. Polyglycerol grafting shields nanoparticles from protein corona formation to avoid macrophage uptake. ACS Nano 2020, 14, 7216–7226. [Google Scholar] [CrossRef]
- Rampado, R.; Crotti, S.; Caliceti, P.; Pucciarelli, S.; Agostini, M. Recent Advances in Understanding the Protein Corona of Nanoparticles and in the Formulation of “Stealthy” Nanomaterials. Front. Bioeng. Biotechnol. 2020, 8, 166. [Google Scholar] [CrossRef]
- Zalba, S.; Ten Hagen, T.L.; Burgui, C.; Garrido, M.J. Stealth nanoparticles in oncology: Facing the PEG dilemma. J. Control. Release 2022, 351, 22–36. [Google Scholar] [CrossRef]
- Barbalinardo, M.; Bertacchini, J.; Bergamini, L.; Magarò, M.S.; Ortolani, L.; Sanson, A.; Palumbo, C.; Cavallini, M.; Gentili, D. Surface properties modulate protein corona formation and determine cellular uptake and cytotoxicity of silver nanoparticles. Nanoscale 2021, 13, 14119–14129. [Google Scholar] [CrossRef]
- Schroffenegger, M.; Leitner, N.S.; Morgese, G.; Ramakrishna, S.N.; Willinger, M.; Benetti, E.M.; Reimhult, E. Polymer topology determines the formation of protein corona on core–shell nanoparticles. ACS Nano 2020, 14, 12708–12718. [Google Scholar] [CrossRef]
- Guido, V.S.; Olivieri, P.H., Jr.; Brito, M.L.; Prezoto, B.C.; Martinez, D.S.; Oliva, M.L.V.; Sousa, A.A. Stealth and Biocompatible Gold Nanoparticles through Surface Coating with a Zwitterionic Derivative of Glutathione. Langmuir 2024, 40, 12167–12178. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, H.; Maity, A.; Singh, K.; Bagchi, D.; Prasad, A.; Chakraborty, A. Effect of Lipid Corona on Phenylalanine-Functionalized Gold Nanoparticles to Develop Stable and Corona-Free Systems. Langmuir 2024, 40, 4531–4543. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, Q.; Yu, H.; Guo, Y.; Cheng, Y.; Qian, H.; Xie, Y.; Yao, W. Protein corona formed on the TiO2 nanoparticles promotes the hydrolysis of collagen in simulated gastrointestinal fluids. Food Biosci. 2023, 53, 102786. [Google Scholar] [CrossRef]
- Hussain, Z.; Khan, S.; Imran, M.; Sohail, M.; Shah, S.W.A.; de Matas, M. PEGylation: A promising strategy to overcome challenges to cancer-targeted nanomedicines: A review of challenges to clinical transition and promising resolution. Drug Deliv. Transl. Res. 2019, 9, 721–734. [Google Scholar] [CrossRef]
- Wen, P.; Ke, W.; Dirisala, A.; Toh, K.; Tanaka, M.; Li, J. Stealth and pseudo-stealth nanocarriers. Adv. Drug Deliv. Rev. 2023, 198, 114895. [Google Scholar] [CrossRef]
- Marques, C.; Hajipour, M.J.; Marets, C.; Oudot, A.; Safavi-sohi, R.; Guillemin, M.; Borchard, G.; Jordan, O.; Saviot, L.; Maurizi, L. Identification of the Proteins Determining the Blood Circulation Time of Nanoparticles. ACS Nano 2023, 17, 12458–12470. [Google Scholar] [CrossRef]
- Alam, D.; Naaz, F.; Islam, A.; Sardar, M.; Ahmad, T. Role of sugar osmolytes and their nano-counterparts as inhibitors in protein fibrillation. J. Mol. Liq. 2023, 386, 122479. [Google Scholar] [CrossRef]
- Prajapati, K.P.; Panigrahi, A.; Purohit, S.; Ansari, M.; Dubey, K.; Behera, R.K.; Anand, B.G.; Kar, K. Osmoprotectant coated thermostable gold nanoparticles efficiently restrict temperature-induced amyloid aggregation of insulin. J. Phys. Chem. Lett. 2021, 12, 1803–1813. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Dar, T.A.; Wijayasinghe, Y.S.; Sahoo, D.; Poddar, N.K. Nano-Osmolyte conjugation: Tailoring the osmolyte-protein interactions at the Nanoscale. ACS Omega 2023, 8, 47367–47379. [Google Scholar] [CrossRef] [PubMed]
- Lajmorak, A.; Seyyed Ebrahimi, S.A.; Yazdian, F.; Lalegani, Z.; Hamawandi, B. The Effect of Trehalose Coating for Magnetite Nanoparticles on Stability of Egg White Lysozyme. Int. J. Mol. Sci. 2022, 23, 9657. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Bhuyan, M.; Natu, K.; Sahoo, D.; Bose, K.; Poddar, N.K. Proline-Functionalized ZnO (Zinc Oxide) Nano-Osmolytes: Potent Catalysts for Protein Aggregation Versus Pristine ZnO. BioNanoScience 2025, 15, 54. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Gao, T.; Lou, C.; Wang, H.; Liu, Y.; Cao, A. Folding of flexible protein fragments and design of nanoparticle-based artificial antibody targeting lysozyme. J. Phys. Chem. B 2022, 126, 5045–5054. [Google Scholar] [CrossRef]
- Shanwar, S.; Liang, L.; Nechaev, A.V.; Bausheva, D.K.; Balalaeva, I.V.; Vodeneev, V.A.; Roy, I.; Zvyagin, A.V.; Guryev, E.L. Controlled formation of a protein corona composed of denatured BSA on upconversion nanoparticles improves their colloidal stability. Materials 2021, 14, 1657. [Google Scholar] [CrossRef]
- Rónavári, A.; Bélteky, P.; Boka, E.; Zakupszky, D.; Igaz, N.; Szerencsés, B.; Pfeiffer, I.; Kónya, Z.; Kiricsi, M. Polyvinyl-Pyrrolidone-Coated Silver Nanoparticles—The Colloidal, Chemical, and Biological Consequences of Steric Stabilization under Biorelevant Conditions. Int. J. Mol. Sci. 2021, 22, 8673. [Google Scholar] [CrossRef]
- Bashiri, G.; Padilla, M.S.; Swingle, K.L.; Shepherd, S.J.; Mitchell, M.J.; Wang, K. Nanoparticle protein corona: From structure and function to therapeutic targeting. Lab Chip 2023, 23, 1432–1466. [Google Scholar] [CrossRef]
- Abarca-Cabrera, L.; Xu, L.; Berensmeier, S.; Fraga-García, P. Competition at the Bio-nano Interface: A Protein, a Polysaccharide, and a Fatty Acid Adsorb onto Magnetic Nanoparticles. ACS Appl. Bio Mater. 2022, 6, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Shan, H.; Zhao, Q.; Guo, Y.; Gao, M.; Xu, X.; McClements, D.J.; Cao, C.; Yuan, B. Impact of pH on the formation and properties of whey protein coronas around TiO2 nanoparticles. J. Agric. Food Chem. 2023, 71, 5756–5769. [Google Scholar] [CrossRef] [PubMed]
- Pandit, S.; Kundu, S. Fluorescence quenching and related interactions among globular proteins (BSA and lysozyme) in presence of titanium dioxide nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127253. [Google Scholar] [CrossRef]
- Peng, Z.G.; Hidajat, K.; Uddin, M.S. Adsorption of bovine serum albumin on nanosized magnetic particles. J. Colloid Interface Sci. 2004, 271, 277–283. [Google Scholar] [CrossRef]
- Bychkova, A.V.; Lopukhova, M.V.; Wasserman, L.A.; Degtyarev, Y.N.; Kovarski, A.L.; Chakraborti, S.; Mitkevich, V.A. The influence of pH and ionic strength on the interactions between human serum albumin and magnetic iron oxide nanoparticles. Int. J. Biol. Macromol. 2022, 194, 654–665. [Google Scholar] [CrossRef]
- Bemelmans, B.J.N.; Verschoor, J.C.; Erné, B.H.; Tromp, R.H. Adsorption of whey protein and sodium caseinate onto colloidal chromium oxide as a model for the pre-fouling of steel. Colloids Surf. B Biointerfaces 2025, 255, 114949. [Google Scholar] [CrossRef]
- Lee, J.G.; Lannigan, K.; Shelton, W.A.; Meissner, J.; Bharti, B. Adsorption of Myoglobin and Corona Formation on Silica Nanoparticles. Langmuir 2020, 36, 14157–14165. [Google Scholar] [CrossRef] [PubMed]
- Schwaminger, S.P.; Fraga-García, P.; Blank-Shim, S.A.; Straub, T.; Haslbeck, M.; Muraca, F.; Dawson, K.A.; Berensmeier, S. Magnetic One-Step Purification of His-Tagged Protein by Bare Iron Oxide Nanoparticles. ACS Omega 2019, 4, 3790–3799. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Zhang, J.; Wu, Z. Investigation of silver nanoparticles interaction with human proteins (IgG & Fib). Colloid Interface Sci. Commun. 2023, 53, 100701. [Google Scholar] [CrossRef]
- Li, M.; Zhang, X.; Li, S.; Shao, X.; Chen, H.; Lv, L.; Huang, X. Probing protein dissociation from gold nanoparticles and the influence of temperature from the protein corona formation mechanism. RSC Adv. 2021, 11, 18198–18204. [Google Scholar] [CrossRef] [PubMed]
- Khabatova, V.V.; Serov, D.A.; Tikhonova, I.V.; Astashev, M.E.; Nagaev, E.I.; Sarimov, R.M.; Matveyeva, T.A.; Simakin, A.V.; Gudkov, S.V. Selenium Nanoparticles Can Influence the Immune Response Due to Interactions with Antibodies and Modulation of the Physiological State of Granulocytes. Pharmaceutics 2022, 14, 2772. [Google Scholar] [CrossRef] [PubMed]
- Nagaev, E.I.; Baimler, I.V.; Baryshev, A.S.; Astashev, M.E.; Gudkov, S.V. Effect of Laser-Induced Optical Breakdown on the Structure of Bsa Molecules in Aqueous Solutions: An Optical Study. Molecules 2022, 27, 6752. [Google Scholar] [CrossRef] [PubMed]
- Serov, D.A.; Nagaev, E.I.; Kuleshova, A.I.; Reut, V.E.; Astashev, M.E. The Effect of Surgical Laser Radiation on the Structure of Bovine Serum Albumin in vitro. Biophysics 2023, 68, 376–382. [Google Scholar] [CrossRef]
- Shamim, N.; Hong, L.; Hidajat, K.; Uddin, M.S. Thermosensitive-polymer-coated magnetic nanoparticles: Adsorption and desorption of Bovine Serum Albumin. J. Colloid Interface Sci. 2006, 304, 1–8. [Google Scholar] [CrossRef]
- Liang, Y.-Y.; Zhang, L.-M. Bioconjugation of Papain on Superparamagnetic Nanoparticles Decorated with Carboxymethylated Chitosan. Biomacromolecules 2007, 8, 1480–1486. [Google Scholar] [CrossRef]
- Wang, S.-N.; Zhang, C.-R.; Qi, B.-K.; Sui, X.-N.; Jiang, L.-Z.; Li, Y.; Wang, Z.-J.; Feng, H.-X.; Wang, R.; Zhang, Q.-Z. Immobilized alcalase alkaline protease on the magnetic chitosan nanoparticles used for soy protein isolate hydrolysis. Eur. Food Res. Technol. 2014, 239, 1051–1059. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Jiang, X.-P.; Li, Y.; Zeng, S.; Zhang, Y.-W. Preparation Fe3O4@chitosan magnetic particles for covalent immobilization of lipase from Thermomyces lanuginosus. Int. J. Biol. Macromol. 2015, 75, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Anirudhan, T.S.; Rejeena, S.R.; Binusree, J. Adsorptive Separation of Myoglobin from Aqueous Solutions Using Iron Oxide Magnetic Nanoparticles Modified with Functionalized Nanocrystalline Cellulose. J. Chem. Eng. Data 2013, 58, 1329–1339. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Abdelmonem, A.M.; Behzadi, S.; Clement, J.H.; Dutz, S.; Ejtehadi, M.R.; Hartmann, R.; Kantner, K.; Linne, U.; Maffre, P.; et al. Temperature: The “Ignored” Factor at the NanoBio Interface. ACS Nano 2013, 7, 6555–6562. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Lohse, S.E.; Murphy, C.J.; Fathizadeh, A.; Montazeri, A.; Suslick, K.S. Variation of Protein Corona Composition of Gold Nanoparticles Following Plasmonic Heating. Nano Lett. 2013, 14, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Molkova, E.A.; Matveeva, T.A.; Baimler, I.V.; Sarimov, R.M.; Gudkov, S.V.; Dorokhov, A.S.; Izmailov, A.Y. Investigation of the Influence of pH and Temperature of an Aqueous Solution on the Stability of the Gold Nanoparticles–Lysozyme Protein System by Optical Methods. Phys. Wave Phenom. 2025, 33, 192–206. [Google Scholar] [CrossRef]
- Molkova, E.A.; Sarimov, R.M.; Matveeva, T.A.; Simakin, A.V.; Akopdzhanov, A.G.; Serov, D.A.; Rebezov, M.B.; Astashev, M.E.; Sergienko, K.V.; Sevostyanov, M.A.; et al. Optical Investigation of the Combined Effect of pH and Temperature on the Interactions of BSA Protein with Iron Oxide Nanoparticles. Colloids Interfaces 2025, 9, 45. [Google Scholar] [CrossRef]
- Gorshkov, V.; Bubis, J.A.; Solovyeva, E.M.; Gorshkov, M.V.; Kjeldsen, F. Protein corona formed on silver nanoparticles in blood plasma is highly selective and resistant to physicochemical changes of the solution. Environ. Sci. Nano 2019, 6, 1089–1098. [Google Scholar] [CrossRef]
- Liu, W.; Worms, I.; Slaveykova, V.I. Interaction of silver nanoparticles with antioxidant enzymes. Environ. Sci. Nano 2020, 7, 1507–1517. [Google Scholar] [CrossRef]
- Mohammad-Beigi, H.; Hayashi, Y.; Zeuthen, C.M.; Eskandari, H.; Scavenius, C.; Juul-Madsen, K.; Vorup-Jensen, T.; Enghild, J.J.; Sutherland, D.S. Mapping and identification of soft corona proteins at nanoparticles and their impact on cellular association. Nat. Commun. 2020, 11, 4535. [Google Scholar] [CrossRef]
- Wang, C.; Chen, B.; He, M.; Hu, B. Composition of Intracellular Protein Corona around Nanoparticles during Internalization. ACS Nano 2021, 15, 3108–3122. [Google Scholar] [CrossRef]
- Marycleopha, M.; Yaou Balarabe, B.; Adjama, I.; Moussa, H.; Anandaram, H.; Abdoul Razak, M.W. Anhydrous sol-gel synthesis of TiO2 nanoparticles: Evaluating their impact on protein interactions in biological systems. J. Trace Elem. Miner. 2024, 7, 100114. [Google Scholar] [CrossRef]
- Sarimov, R.M.; Nagaev, E.I.; Matveyeva, T.A.; Binhi, V.N.; Burmistrov, D.E.; Serov, D.A.; Astashev, M.E.; Simakin, A.V.; Uvarov, O.V.; Khabatova, V.V. Investigation of aggregation and disaggregation of self-assembling nano-sized clusters consisting of individual iron oxide nanoparticles upon interaction with HEWL protein molecules. Nanomaterials 2022, 12, 3960. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, K.M.; Payne, C.K. Concentration and composition of the protein corona as a function of incubation time and serum concentration: An automated approach to the protein corona. Anal. Bioanal. Chem. 2022, 414, 7265–7275. [Google Scholar] [CrossRef] [PubMed]
- Logashina, Y.; Lubova, K.; Maleeva, E.; Palikov, V.; Palikova, Y.; Dyachenko, I.; Andreev, Y. Analysis of Structural Determinants of Peptide MS 9a-1 Essential for Potentiating of TRPA1 Channel. Mar. Drugs 2022, 20, 465. [Google Scholar] [CrossRef] [PubMed]
- Ajmal, M.R. Protein misfolding and aggregation in proteinopathies: Causes, mechanism and cellular response. Diseases 2023, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Poothong, J.; Jang, I.; Kaufman, R.J. Defects in protein folding and/or quality control cause protein aggregation in the endoplasmic reticulum. In Cellular Biology of the Endoplasmic Reticulum; Springer: Berlin/Heidelberg, Germany, 2021; pp. 115–143. [Google Scholar] [CrossRef]
- Spencer, B.G.; Finnie, J.W. The role of endoplasmic reticulum stress in cell survival and death. J. Comp. Pathol. 2020, 181, 86–91. [Google Scholar] [CrossRef]
- Rinauro, D.J.; Chiti, F.; Vendruscolo, M.; Limbocker, R. Misfolded protein oligomers: Mechanisms of formation, cytotoxic effects, and pharmacological approaches against protein misfolding diseases. Mol. Neurodegener. 2024, 19, 20. [Google Scholar] [CrossRef] [PubMed]
- Mitroshina, E.; Kalinina, E.; Vedunova, M. Optogenetics in Alzheimer’s Disease: Focus on Astrocytes. Antioxidants 2023, 12, 1856. [Google Scholar] [CrossRef] [PubMed]
- Mitroshina, E.V.; Kalinina, E.P.; Kalyakulina, A.I.; Teplyakova, A.V.; Vedunova, M.V. The Effect of the Optogenetic Stimulation of Astrocytes on Neural Network Activity in an In Vitro Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2024, 25, 12237. [Google Scholar] [CrossRef]
- Miles, A.; Janes, R.W.; Wallace, B.A. Tools and methods for circular dichroism spectroscopy of proteins: A tutorial review. Chem. Soc. Rev. 2021, 50, 8400–8413. [Google Scholar] [CrossRef]
- Haque, M.A.; Kaur, P.; Islam, A.; Hassan, M.I. Application of circular dichroism spectroscopy in studying protein folding, stability, and interaction. In Advances in Protein Molecular and Structural Biology Methods; Elsevier: Amsterdam, The Netherlands, 2022; pp. 213–224. [Google Scholar]
- Pignataro, M.F.; Herrera, M.G.; Dodero, V.I. Evaluation of peptide/protein self-assembly and aggregation by spectroscopic methods. Molecules 2020, 25, 4854. [Google Scholar] [CrossRef]
- De Meutter, J.; Goormaghtigh, E. Evaluation of protein secondary structure from FTIR spectra improved after partial deuteration. Eur. Biophys. J. 2021, 50, 613–628. [Google Scholar] [CrossRef]
- Usoltsev, D.; Sitnikova, V.; Kajava, A.; Uspenskaya, M. FTIR spectroscopy study of the secondary structure changes in human serum albumin and trypsin under neutral salts. Biomolecules 2020, 10, 606. [Google Scholar] [CrossRef]
- Olsson, A.-M.; Salmén, L. The association of water to cellulose and hemicellulose in paper examined by FTIR spectroscopy. Carbohydr. Res. 2004, 339, 813–818. [Google Scholar] [CrossRef]
- Kafle, B.; Böcker, U.; Wubshet, S.G.; Dankel, K.; Måge, I.; OFarrell, M.; Afseth, N.K. Fourier-transform infrared spectroscopy for characterization of liquid protein solutions: A comparison of two sampling techniques. Vib. Spectrosc. 2023, 124, 103490. [Google Scholar] [CrossRef]
- dos Santos Rodrigues, F.H.; Delgado, G.G.; da Costa, T.S.; Tasic, L. Applications of fluorescence spectroscopy in protein conformational changes and intermolecular contacts. BBA Adv. 2023, 3, 100091. [Google Scholar] [CrossRef] [PubMed]
- Gudkov, S.V.; Sarimov, R.M.; Astashev, M.E.; Pishchalnikov, R.Y.; Yanykin, D.V.; Simakin, A.V.; Shkirin, A.V.; Serov, D.A.; Konchekov, E.M.; Gusein-zade Namik Guseynaga, O.; et al. Modern physical methods and technologies in agriculture. Phys. Uspekhi 2024, 67, 194–210. [Google Scholar] [CrossRef]
- Siddiquee, M.A.; ud din Parray, M.; Mehdi, S.H.; Alzahrani, K.A.; Alshehri, A.A.; Malik, M.A.; Patel, R. Green synthesis of silver nanoparticles from Delonix regia leaf extracts: In-vitro cytotoxicity and interaction studies with bovine serum albumin. Mater. Chem. Phys. 2020, 242, 122493. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, X.; Yan, C.; Wang, G. The morphology dependent interaction between silver nanoparticles and bovine serum albumin. Materials 2023, 16, 5821. [Google Scholar] [CrossRef]
- Bhunia, A.K.; Kamilya, T.; Saha, S. Study of the adsorption of human hemoglobin to silver (Ag) nanoparticle surface for the detection of the unfolding of hemoglobin. Plasmonics 2022, 17, 1139–1156. [Google Scholar] [CrossRef]
- Dell’Aglio, M.; Salajková, Z.; Mallardi, A.; Sportelli, M.C.; Kaiser, J.; Cioffi, N.; De Giacomo, A. Sensing nanoparticle-protein corona using nanoparticle enhanced Laser Induced Breakdown Spectroscopy signal enhancement. Talanta 2021, 235, 122741. [Google Scholar] [CrossRef]
- Wang, G.; Wang, W.; Shangguan, E.; Gao, S.; Liu, Y. Effects of gold nanoparticle morphologies on interactions with proteins. Mater. Sci. Eng. C 2020, 111, 110830. [Google Scholar] [CrossRef]
- Halder, K.; Sengupta, P.; Chaki, S.; Saha, R.; Dasgupta, S. Understanding conformational changes in human serum albumin and its interactions with gold nanorods: Do flexible regions play a role in corona formation? Langmuir 2023, 39, 1651–1664. [Google Scholar] [CrossRef]
- Halder, K.; Dasgupta, S. Temperature dependent human serum albumin Corona formation: A case study on gold nanorods and nanospheres. Int. J. Biol. Macromol. 2025, 290, 138581. [Google Scholar] [CrossRef] [PubMed]
- Rajan, D.; Rajamanikandan, R.; Ilanchelian, M. Investigating the biophysical interaction of serum albumins-gold nanorods using hybrid spectroscopic and computational approaches with the intent of enhancing cytotoxicity efficiency of targeted drug delivery. J. Mol. Liq. 2023, 377, 121541. [Google Scholar] [CrossRef]
- Dai, J.; Chen, C.; Yin, M.; Li, H.; Li, W.; Zhang, Z.; Wang, Q.; Du, Z.; Xu, X.; Wang, Y. Interactions between gold nanoparticles with different morphologies and human serum albumin. Front. Chem. 2023, 11, 1273388. [Google Scholar] [CrossRef] [PubMed]
- Rajan, D.; Rajamanikandan, R.; Ilanchelian, M. Morphological and biophysical insights into the gold nanorods binding interaction of haemoglobin/myoglobin by hybrid spectroscopic approaches with bacterial cytotoxicity evaluation. J. Mol. Liq. 2022, 353, 118777. [Google Scholar] [CrossRef]
- Shanmugaraj, K.; Sharma, A.S.; Sasikumar, T.; Mangalaraja, R.V.; Ilanchelian, M. Insight into the binding and conformational changes of hemoglobin/lysozyme with bimetallic alloy nanoparticles using various spectroscopic approaches. J. Mol. Liq. 2020, 300, 111747. [Google Scholar] [CrossRef]
- Meesaragandla, B.; Karanth, S.; Janke, U.; Delcea, M. Biopolymer-coated gold nanoparticles inhibit human insulin amyloid fibrillation. Sci. Rep. 2020, 10, 7862. [Google Scholar] [CrossRef]
- Jouya Talaei, A.; Zarei, N.; Hasan, A.; Haj Bloukh, S.; Edis, Z.; Abbasi Gamasaee, N.; Heidarzadeh, M.; Mahdi Nejadi Babadaei, M.; Shahpasand, K.; Sharifi, M.; et al. Fabrication of inorganic alumina particles at nanoscale by a pulsed laser ablation technique in liquid and exploring their protein binding, anticancer and antipathogenic activities. Arab. J. Chem. 2021, 14, 102923. [Google Scholar] [CrossRef]
- Richter-Bisson, Z.W.; Nie, H.-Y.; Hedberg, Y.S. Serum protein albumin and chromium: Mechanistic insights into the interaction between ions, nanoparticles, and protein. Int. J. Biol. Macromol. 2024, 278, 134845. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, S.; Verma, R.; Mehrotra, S.; Patnaik, S.; Pandey, A.K.; Priya, S.; Sharma, S.K. Promiscuous interaction of titanium dioxide nanoparticles leads to alterations in structural stability and interferes with luciferase folding. J. Nanoparticle Res. 2023, 25, 163. [Google Scholar] [CrossRef]
- Pang, C.; Zhang, N.; Falahati, M. Acceleration of α-synuclein fibril formation and associated cytotoxicity stimulated by silica nanoparticles as a model of neurodegenerative diseases. Int. J. Biol. Macromol. 2021, 169, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Ortelli, S.; Costa, A.L.; Zanoni, I.; Blosi, M.; Geiss, O.; Bianchi, I.; Mehn, D.; Fumagalli, F.; Ceccone, G.; Guerrini, G. TiO2@ BSA nano-composites investigated through orthogonal multi-techniques characterization platform. Colloids Surf. B Biointerfaces 2021, 207, 112037. [Google Scholar] [CrossRef] [PubMed]
- Barkhade, T.; Mahapatra, S.K.; Banerjee, I. A Protein and Membrane Integrity Study of TiO2 Nanoparticles-Induced Mitochondrial Dysfunction and Prevention by Iron Incorporation. J. Membr. Biol. 2021, 254, 217–237. [Google Scholar] [CrossRef] [PubMed]
- Maity, A.; Mondal, A.; Kundu, S.; Shome, G.; Misra, R.; Singh, A.; Pal, U.; Mandal, A.K.; Bera, K.; Maiti, N.C. Naringenin-functionalized gold nanoparticles and their role in α-synuclein stabilization. Langmuir 2023, 39, 7231–7248. [Google Scholar] [CrossRef]
- Sarimov, R.; Matveeva, T.; Serov, D.; Nikitin, P.; Bashkin, S.; Loboyko, A.; Binhi, V. Effect of Magnetic Fields on Lysozyme Renaturation. Opera Medica et Physiol. 2024, 11, 149–165. [Google Scholar] [CrossRef]
- Mozhaeva, V.; Sarimov, R. Study of the Enhanced Green Fluorescent Protein Fluorescence Changes During its Deand Renaturation, and Upon the Addition of Nanoparticles. Opera Medica et Physiol. 2023, 10, 49–57. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, W.; Liu, Z.; Liu, X.; Song, E.; Song, Y. Dual effects of fibrinogen decoration on the tuning of silica nanoparticles-induced autophagic response: The implication of sedimentation and internalization. J. Hazard. Mater. 2021, 408, 124467. [Google Scholar] [CrossRef]
- Lynch, I.; Dawson, K.A. Protein–Nanoparticle Interactions. In Nano-Enabled Medical Applications; Taylor & Francis Group: Oxfordshire, UK, 2020; pp. 231–250. [Google Scholar] [CrossRef]
- Sousa, A.A.; Schuck, P.; Hassan, S.A. Biomolecular interactions of ultrasmall metallic nanoparticles and nanoclusters. Nanoscale Adv. 2021, 3, 2995–3027. [Google Scholar] [CrossRef]
- Nastyshyn, S.; Pop-Georgievski, O.; Stetsyshyn, Y.; Budkowski, A.; Raczkowska, J.; Hruby, M.; Lobaz, V. Protein corona of SiO2 nanoparticles with grafted thermoresponsive copolymers: Calorimetric insights on factors affecting entropy vs. enthalpy-driven associations. Appl. Surf. Sci. 2022, 601, 154201. [Google Scholar] [CrossRef]
- Sapp, A.D.; Díaz-Cano, C.E.; Lengyel, J.; Abarca-Cabrera, L.; Fraga-García, P. Amino Acid Adsorption Onto Magnetic Nanoparticles Reveals Correlations With Physicochemical Parameters. ChemNanoMat 2024, 10, e202400280. [Google Scholar] [CrossRef]
- Song, L.; Wang, J.; Duan, W. Denatured BSA-mediated co-assembly with iron oxide nanoparticles and their effect on protein fibrillation. Chem. Phys. Lett. 2023, 830, 140790. [Google Scholar] [CrossRef]
- Marichal, L.; Degrouard, J.; Gatin, A.; Raffray, N.; Aude, J.-C.; Boulard, Y.; Combet, S.; Cousin, F.; Hourdez, S.; Mary, J. From protein corona to colloidal self-assembly: The importance of protein size in protein–nanoparticle interactions. Langmuir 2020, 36, 8218–8230. [Google Scholar] [CrossRef] [PubMed]
- Avellán-Llaguno, R.D.; Zhang, X.; Zhao, P.; Velez, A.; Cruz, M.; Kikuchi, J.; Dong, S.; Huang, Q. Differential aggregation of polystyrene and titanium dioxide nanoparticles under various salinity conditions and against multiple proteins types. Environ. Sci. Pollut. Res. 2022, 29, 74173–74184. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.K.; Mansoori, A.; Jana, S.K.; Kumar, A.; Ghorai, T.K. Biosynthesis of silver nanoparticles using green tea aqueous leaf extract and their biological and chemotherapeutic activity. J. Mol. Struct. 2025, 1320, 139690. [Google Scholar] [CrossRef]
- Baruah, K.; Konthoujam, I.; Lyndem, S.; Aguan, K.; Roy, A.S. Complexation of turmeric and curcumin mediated silver nanoparticles with human serum albumin: Further investigation into the protein-corona formation, anti-bacterial effects and cell cytotoxicity studies. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 294, 122540. [Google Scholar] [CrossRef]
- Jung, E.-B.; Yu, J.; Choi, S.-J. Interaction between ZnO nanoparticles and albumin and its effect on cytotoxicity, cellular uptake, intestinal transport, toxicokinetics, and acute oral toxicity. Nanomaterials 2021, 11, 2922. [Google Scholar] [CrossRef]
- Bing, J.; Xiao, X.; McClements, D.J.; Biao, Y.; Chongjiang, C. Protein corona formation around inorganic nanoparticles: Food plant proteins-TiO2 nanoparticle interactions. Food Hydrocoll. 2021, 115, 106594. [Google Scholar] [CrossRef]
- Sekimukai, H.; Iwata-Yoshikawa, N.; Fukushi, S.; Tani, H.; Kataoka, M.; Suzuki, T.; Hasegawa, H.; Niikura, K.; Arai, K.; Nagata, N. Gold nanoparticle-adjuvanted S protein induces a strong antigen-specific IgG response against severe acute respiratory syndrome-related coronavirus infection, but fails to induce protective antibodies and limit eosinophilic infiltration in lungs. Microbiol. Immunol. 2020, 64, 33–51. [Google Scholar] [CrossRef]
- Tizro, P.; Choi, C.; Khanlou, N. Sample Preparation for Transmission Electron Microscopy. In Biobanking; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2019; pp. 417–424. [Google Scholar] [CrossRef]
- Larichev, Y.V. Application of DLS for metal nanoparticle size determination in supported catalysts. Chem. Pap. 2021, 75, 2059–2066. [Google Scholar] [CrossRef]
- Bell, N.C.; Minelli, C.; Tompkins, J.; Stevens, M.M.; Shard, A.G. Emerging Techniques for Submicrometer Particle Sizing Applied to Stöber Silica. Langmuir 2012, 28, 10860–10872. [Google Scholar] [CrossRef]
- Babick, F. Dynamic light scattering (DLS). In Characterization of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2020; pp. 137–172. [Google Scholar] [CrossRef]
- Souza, T.G.; Ciminelli, V.S.; Mohallem, N.D.S. A comparison of TEM and DLS methods to characterize size distribution of ceramic nanoparticles. J. Phys. Conf. Ser. 2016, 733, 012039. [Google Scholar] [CrossRef]
- Pavani, P.; Kumar, K.; Rani, A.; Venkatesu, P.; Lee, M.-J. The influence of sodium phosphate buffer on the stability of various proteins: Insights into protein-buffer interactions. J. Mol. Liq. 2021, 331, 115753. [Google Scholar] [CrossRef]
- Artymiuk, P.J.; Blake, C.C.F.; Grace, D.E.P.; Oatley, S.J.; Phillips, D.C.; Sternberg, M.J.E. Crystallographic studies of the dynamic properties of lysozyme. Nature 1979, 280, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Sarimov, R.M.; Binhi, V.N.; Matveeva, T.A.; Penkov, N.V.; Gudkov, S.V. Unfolding and Aggregation of Lysozyme under the Combined Action of Dithiothreitol and Guanidine Hydrochloride: Optical Studies. Int. J. Mol. Sci. 2021, 22, 2710. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef]
- Vivas, C.V.; Duarte, E.L.; Barreto, Y.B.; deOliveira, C.L.P.; Toma, S.H.; Santos, J.J.; Araki, K.; Alencar, A.M.; Bloise, A.C. Interactions Between Silver Nanoparticles and Culture Medium Biomolecules with Dose and Time Dependencies. J. Fluoresc. 2024, 35, 835–854. [Google Scholar] [CrossRef]
- Kumar, M.; Jaiswal, V.D.; Pangam, D.S.; Bhatia, P.; Kulkarni, A.; Dongre, P. Biophysical study of DC electric field induced stable formation of albumin-gold nanoparticles corona and curcumin binding. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 305, 123469. [Google Scholar] [CrossRef]
- Terracciano, R.; Zhang, A.; Butler, E.B.; Demarchi, D.; Hafner, J.H.; Grattoni, A.; Filgueira, C.S. Effects of surface protein adsorption on the distribution and retention of intratumorally administered gold nanoparticles. Pharmaceutics 2021, 13, 216. [Google Scholar] [CrossRef]
- Pavase, T.R.; Lin, H.; Soomro, M.A.; Zheng, H.; Li, X.; Wang, K.; Li, Z. Visual detection of tropomyosin, a major shrimp allergenic protein using gold nanoparticles (AuNPs)-assisted colorimetric aptasensor. Mar. Life Sci. Technol. 2021, 3, 382–394. [Google Scholar] [CrossRef] [PubMed]
- Baruah, K.; Singh, A.K.; Kumari, K.; Nongbri, D.L.; Jha, A.N.; Singha Roy, A. Interactions of Turmeric-and Curcumin-Functionalized Gold Nanoparticles with Human Serum Albumin: Exploration of Protein Corona Formation, Binding, Thermodynamics, and Antifibrillation Studies. Langmuir 2023, 40, 1381–1398. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Fu, F.; Wang, W.; Wang, W.; Huang, Z.; Huang, Y.; Pan, X.; Wu, C. Plasma protein corona forming upon fullerene nanocomplex: Impact on both counterparts. Particuology 2023, 73, 26–36. [Google Scholar] [CrossRef]
- Bai, Y.; Wu, X.; Ouyang, P.; Shi, M.; Li, Q.; Maimaiti, T.; Lan, S.; Yang, S.-T.; Chang, X.-L. Surface modification mediates the interaction between fullerene and lysozyme: Protein structure and antibacterial activity. Environ. Sci. Nano 2021, 8, 76–85. [Google Scholar] [CrossRef]
- Wu, H.; Chen, M.; Shang, M.; Li, X.; Mu, K.; Fan, S.; Jiang, S.; Li, W. Insights into the binding behavior of bovine serum albumin to black carbon nanoparticles and induced cytotoxicity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 200, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, F.; Moeeni, M.; Li, C.; Boukherroub, R.; Szunerits, S. Interaction of cellulose and nitrodopamine coated superparamagnetic iron oxide nanoparticles with alpha-lactalbumin. RSC Adv. 2020, 10, 9704–9716. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, F.F.; Almeida, M.R.; Soares, S.F.; Trindade, T.; Freire, M.G.; Daniel-da-Silva, A.L.; Tavares, A.P. Recovery of immunoglobulin G from rabbit serum using κ-carrageenan-modified hybrid magnetic nanoparticles. Int. J. Biol. Macromol. 2020, 150, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, K.M.; Pho, T.; Champion, J.A.; Payne, C.K. Automation and low-cost proteomics for characterization of the protein corona: Experimental methods for big data. Anal. Bioanal. Chem. 2020, 412, 6543–6551. [Google Scholar] [CrossRef]
- Sahin, S.; Ozmen, I. Covalent immobilization of trypsin on polyvinyl alcohol-coated magnetic nanoparticles activated with glutaraldehyde. J. Pharm. Biomed. Anal. 2020, 184, 113195. [Google Scholar] [CrossRef]
- António, M.; Lima, T.; Vitorino, R.; Daniel-da-Silva, A.L. Label-free dynamic light scattering assay for C-reactive protein detection using magnetic nanoparticles. Anal. Chim. Acta 2022, 1222, 340169. [Google Scholar] [CrossRef]
- Nakhjiri, M.Z.; Asadi, S.; Hasan, A.; Babadaei, M.M.N.; Vahdani, Y.; Rasti, B.; Ale-Ebrahim, M.; Arsalan, N.; Goorabjavari, S.V.M.; Haghighat, S. Exploring the interaction of synthesized nickel oxide nanoparticles through hydrothermal method with hemoglobin and lymphocytes: Bio-thermodynamic and cellular studies. J. Mol. Liq. 2020, 317, 113893. [Google Scholar] [CrossRef]
- Vahdati, M.; Tohidi Moghadam, T. Synthesis and characterization of selenium nanoparticles-lysozyme nanohybrid system with synergistic antibacterial properties. Sci. Rep. 2020, 10, 510. [Google Scholar] [CrossRef]
- Shourni, S.; Javadi, A.; Hosseinpour, N.; Bahramian, A.; Raoufi, M. Characterization of protein corona formation on nanoparticles via the analysis of dynamic interfacial properties: Bovine serum albumin-silica particle interaction. Colloids Surf. A Physicochem. Eng. Asp. 2022, 638, 128273. [Google Scholar] [CrossRef]
- Bukackova, M.; Marsalek, R. Interaction of BSA with ZnO, TiO2, and CeO2 nanoparticles. Biophys. Chem. 2020, 267, 106475. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ding, L.; Yao, C.; Li, C.; Zhang, J.; Yin, X.; Wu, M.; Wang, Y. Effect of Transferrin on Cellular Uptake or Expulsion of Titanium Dioxide Nanoparticles. Nano 2020, 15, 2050121. [Google Scholar] [CrossRef]
- Yan, C.; Sharma, P.; Chen, Q.; Li, B.; Shang, J. Coupled impact of proteins with different molecular weights and surface charges on TiO 2 mobility. Environ. Sci. Nano 2022, 9, 2773–2787. [Google Scholar] [CrossRef]
- Yu, L.; Andriola, A. Quantitative gold nanoparticle analysis methods: A review. Talanta 2010, 82, 869–875. [Google Scholar] [CrossRef]
- Sengottiyan, S.; Mikolajczyk, A.; Jagiełło, K.; Swirog, M.; Puzyn, T. Core, coating, or corona? The importance of considering protein coronas in nano-QSPR modeling of zeta potential. ACS Nano 2023, 17, 1989–1997. [Google Scholar] [CrossRef]
- Pochapski, D.J.; Carvalho dos Santos, C.; Leite, G.W.; Pulcinelli, S.H.; Santilli, C.V. Zeta potential and colloidal stability predictions for inorganic nanoparticle dispersions: Effects of experimental conditions and electrokinetic models on the interpretation of results. Langmuir 2021, 37, 13379–13389. [Google Scholar] [CrossRef]
- Salis, A.; Boström, M.; Medda, L.; Cugia, F.; Barse, B.; Parsons, D.F.; Ninham, B.W.; Monduzzi, M. Measurements and theoretical interpretation of points of zero charge/potential of BSA protein. Langmuir 2011, 27, 11597–11604. [Google Scholar] [CrossRef]
- Oliva, F.Y.; Avalle, L.B.; Cámara, O.R.; De Pauli, C.P. Adsorption of human serum albumin (HSA) onto colloidal TiO2 particles, Part I. J. Colloid Interface Sci. 2003, 261, 299–311. [Google Scholar] [CrossRef]
- Xiong, P.; Huang, X.; Ye, N.; Lu, Q.; Zhang, G.; Peng, S.; Wang, H.; Liu, Y. Cytotoxicity of Metal-Based Nanoparticles: From Mechanisms and Methods of Evaluation to Pathological Manifestations. Adv. Sci. 2022, 9, e2106049. [Google Scholar] [CrossRef]
- Khang, D.; Lee, Y.K.; Choi, E.-J.; Webster, T.J.; Kim, S.-H. Effect of the protein corona on nanoparticles for modulating cytotoxicity and immunotoxicity. Int. J. Nanomed. 2014, 10, 97–113. [Google Scholar] [CrossRef]
- Cai, R.; Chen, C. The Crown and the Scepter: Roles of the Protein Corona in Nanomedicine. Adv. Mater. 2018, 31, e1805740. [Google Scholar] [CrossRef] [PubMed]
- Kus-Liśkiewicz, M.; Fickers, P.; Ben Tahar, I. Biocompatibility and Cytotoxicity of Gold Nanoparticles: Recent Advances in Methodologies and Regulations. Int. J. Mol. Sci. 2021, 22, 10952. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.M.; Thorat, N.D.; Shete, P.B.; Bedge, P.A.; Gavde, S.; Joshi, M.G.; Tofail, S.A.M.; Bohara, R.A. Comprehensive cytotoxicity studies of superparamagnetic iron oxide nanoparticles. Biochem. Biophys. Rep. 2018, 13, 63–72. [Google Scholar] [CrossRef]
- Ruess, D.; Grau, S.; Hoevels, M.; Treuer, H.; Goldbrunner, R.; Ruge, M.I. Surg-24application of Nanotherm®by Stereotactic Guidance: A Technical Note. Neuro-Oncol. 2015, 17, v219. [Google Scholar] [CrossRef]
- Bullivant, J.; Zhao, S.; Willenberg, B.; Kozissnik, B.; Batich, C.; Dobson, J. Materials Characterization of Feraheme/Ferumoxytol and Preliminary Evaluation of Its Potential for Magnetic Fluid Hyperthermia. Int. J. Mol. Sci. 2013, 14, 17501–17510. [Google Scholar] [CrossRef] [PubMed]
- Lockwood, Z.A.; Jirousek, M.R.; Basilion, J.P.; Burda, C. Redefining cancer photodynamic therapy with gold nanoparticles. Photochem. Photobiol. 2025, 101, 1120–1138. [Google Scholar] [CrossRef] [PubMed]
- Kadria-Vili, Y.; Schwartz, J.A.; Polascik, T.J.; Goodrich, G.P.; Jorden, D.; Pinder, D.; Halas, N.J.; Rastinehad, A.R. A Detailed Clinical Case of Localized Prostate Tumors Treated with Nanoparticle-Assisted Sub-Ablative Laser Ablation. Nanomaterials 2024, 14, 1261. [Google Scholar] [CrossRef]
- Tamarkin, L.; Myer, L.; Haynes, R.; Paciotti, G. CYT-6091 (Aurimune): A colloidal gold-based tumor-targeted nanomedicine. Nanomedicine: Nanotechnology. Biol. Med. 2006, 2, 273–274. [Google Scholar] [CrossRef]
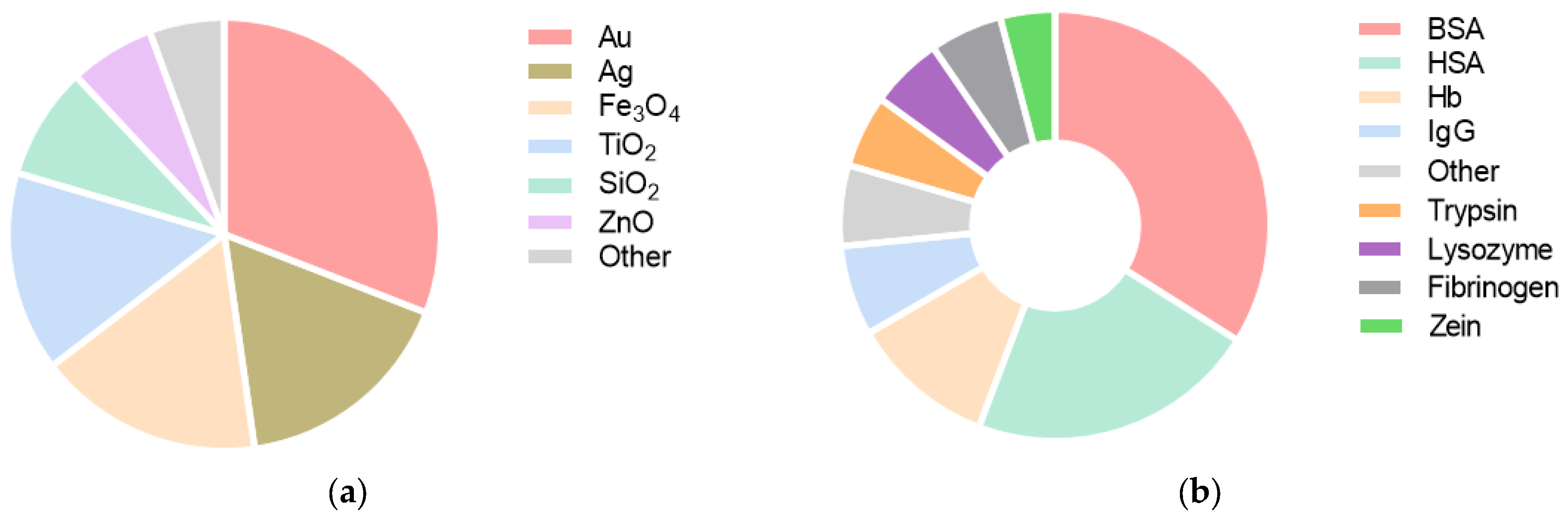

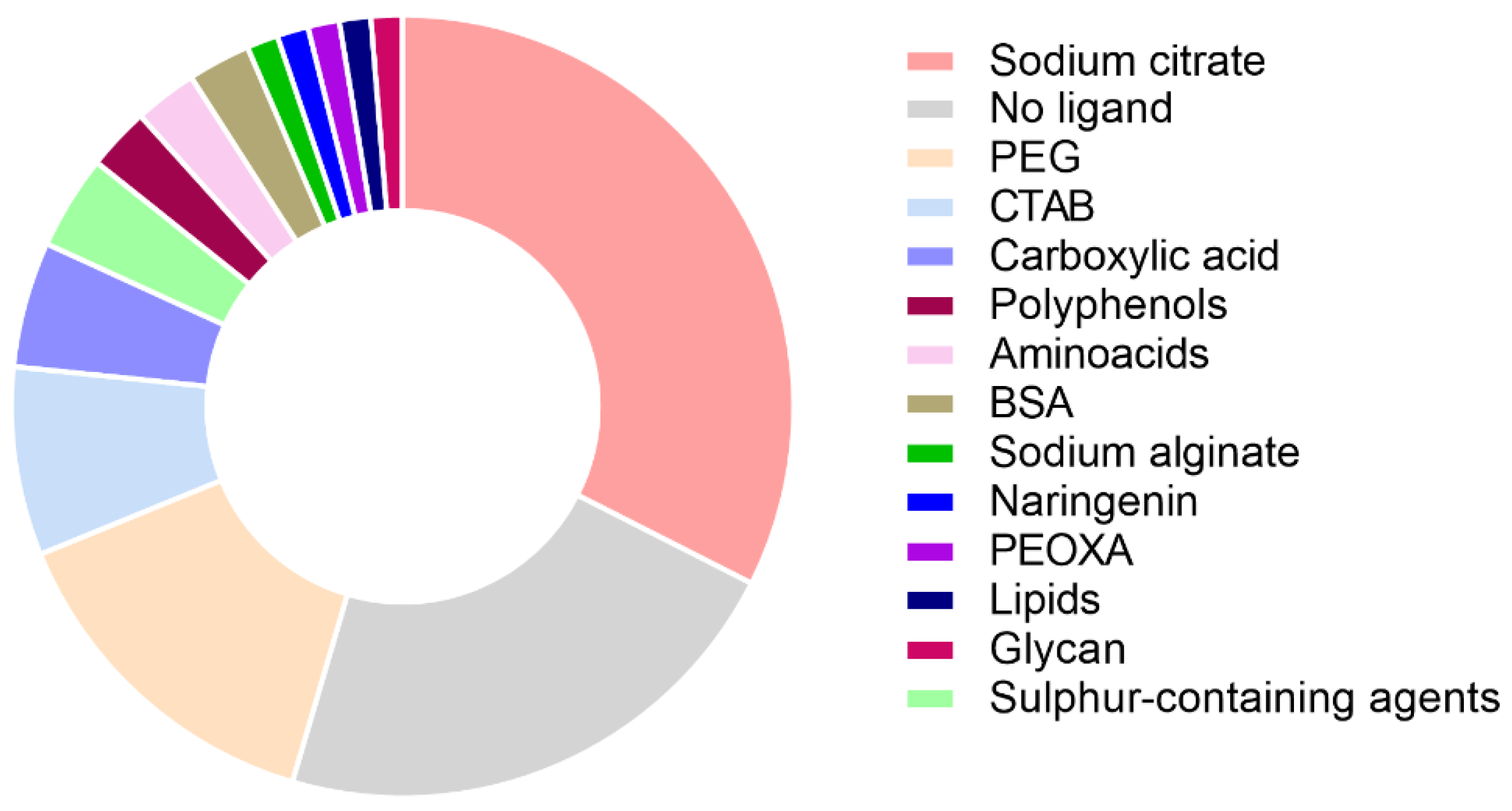
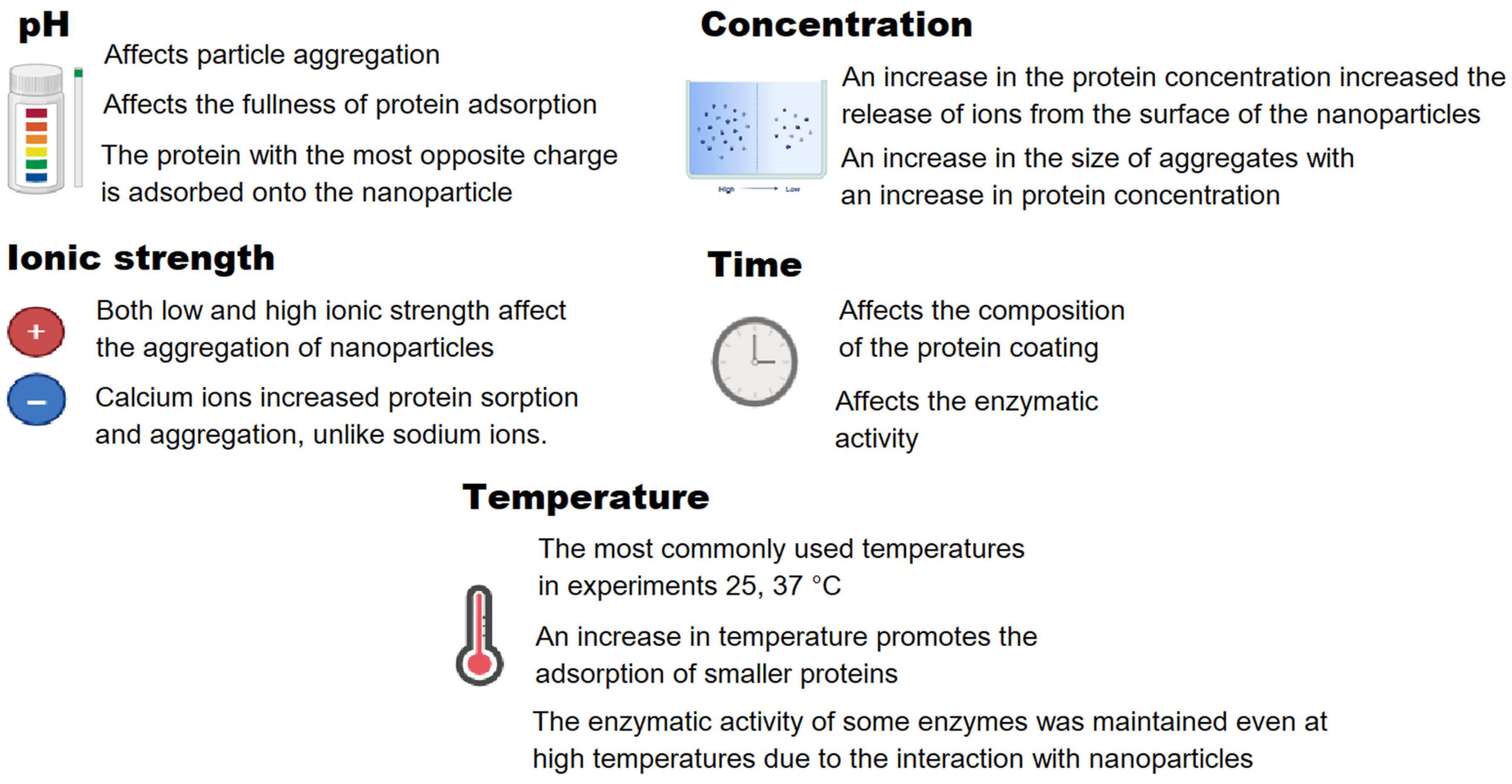


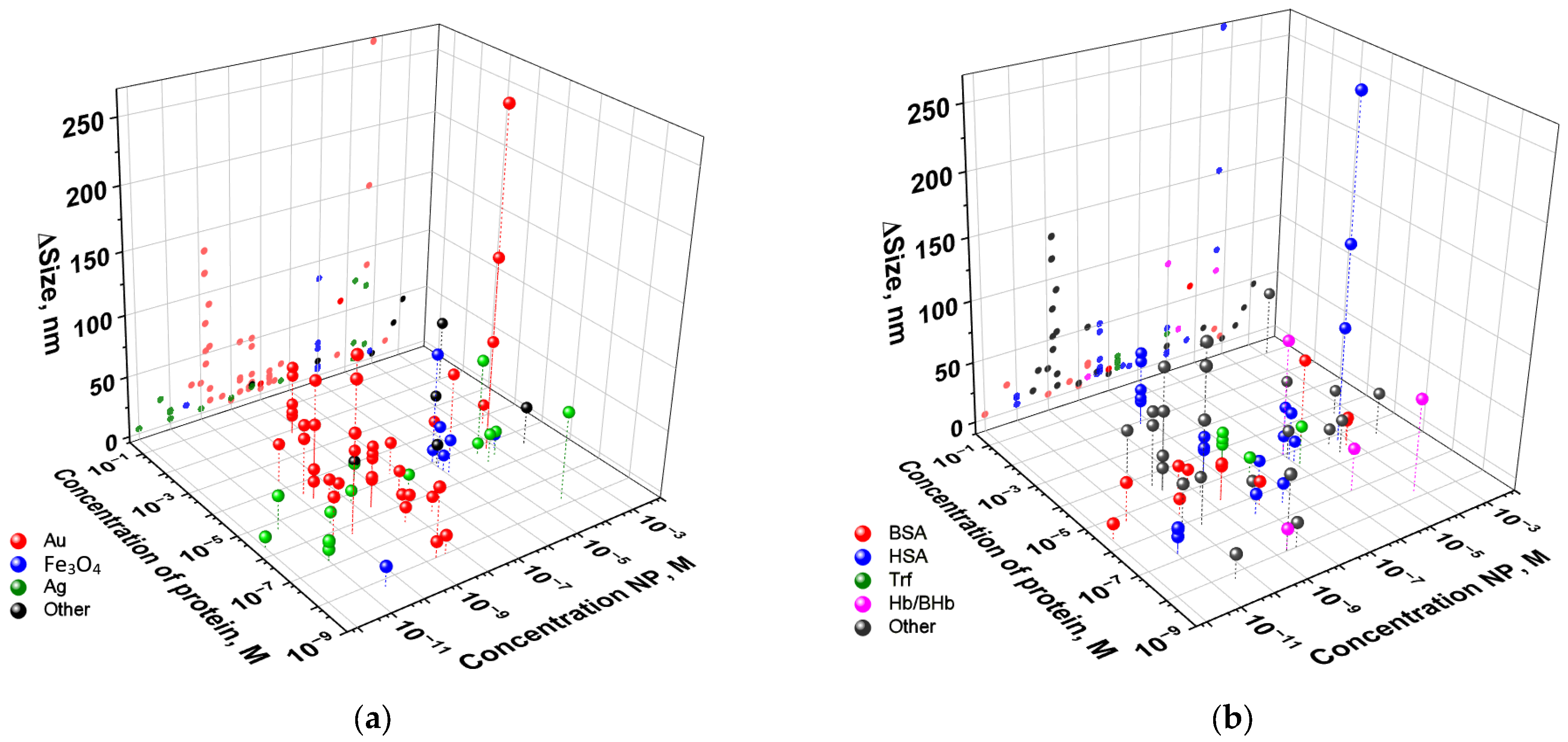
| Protein | Medium | Nanoparticle | Nanoparticle + Protein | Conclusions | Ref. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Cprotein | pH | Buffer | Material | Ligands NP | Shape | Size, nm | ζ, mV | Size, nm | ζ, mV | K | ||
| BSA | 8 nM | 6.5 | SC | Ag | SC | Spherical | 15 25 42 (DLS) | −28 −38 −39 | 16 25 44 (DLS) | −26 −38 −34 | Ka = 1.7 × 107 M−1 Ka = 2.1 × 107 M−1 Ka = 2.2 × 107 M−1 | A slight increase in the binding constant was observed with an increase in the size of the nanoparticles. | [60] |
| BSA | 2.0 μM | 7.4 | PBS | Ag | - | Spherical | 141 (DLS) | −19 | - | - | Ka = 6.77 × 109 M−1 | Stable complexes are formed when BSA interacts with Ag nanoparticles | [208] |
| BSA | 0.06–5 μM | 7.4 | PBS | Ag | - | Spherical | 73 (DLS) | - | - | - | Ka = 25.5 × 104 M−1 | After interaction, a decrease in the content of α-helices was observed; the process was spontaneous (ΔG < 0) | [179] |
| BSA | 2 µM–10 mM | 7.4 | PBS | Ag | SC CTAC SC | Spherical Rods Triangles | 85 87 71 (DLS) | - | 89 91 64 (DLS) | - | Kd = 5.5 × 10−11 M−1 Kd = 1.55 × 10−10 M−1 Kd = 9.57 × 10−5 M−1 | The size and shape of nanoparticles significantly affect the interaction with BSA. In the presence of BSA, nanotriangles gradually evolved into nanodisks. | [180] |
| BSA | 10 μM | 7.4 | PBS | Ag | PVP Tween 20 CTAB PLL AOT SC | Spherical Triangles Cubic | 7–70 (TEM) | - | - | - | Ka = 0.07–6 × 104 M−1 | The observed conformational changes in BSA did not correlate with the Ka value. The interaction of the protein with nanoparticles depends on the interaction of the protein with the functional groups of nanoparticles. | [57] |
| BSA IgG LYZ | 5 μM | 7.4 | NaCl | Ag | PEI | Spherical | 32 (DLS) | +13 | 55 - - | - | Ka = 3.4 × 106 M−1 - - | BSA adsorption onto silver nanoparticles functionalized with PEI was observed. The adsorption of IgG and lysozyme did not occur. | [59] |
| CAT SOD | 2 µM 5 µM | 7.4 | HEPES | Ag | SC | Spherical | 20 (TEM) 24 (DLS) | - | 33–43 29 (DLS) | - | - | The interaction of CAT with nanoparticles led to changes in the secondary structure of the protein with a loss of enzymatic activity, unlike SOD. | [157] |
| FBS | - | 8.3 | DMEM | Ag | SC | Spherical | 42 (DLS) | −34 | 50 (DLS) | −15 | Ka = 105 M−1 | The composition and thickness of the protein corona depend on the concentration and incubation time. Hydrophobic interactions are the determining factors of the interaction | [223] |
| FBS | - | 7.4 | DMEM | Ag | SC EG6OH | Spherical | 22 (DLS) | −40 | 85 (DLS) | −29 | - | The composition of serum proteins adsorbed on nanoparticles strongly depends on the density of the EG6OH ligand. Functional groups affected toxicity and cellular uptake. | [117] |
| Hb | 1 µg/mL | 6.8 | Water | Ag | - | Spherical | 33 (DLS) | - | 100 (DLS) | - | - | Interaction between a protein and nanoparticles leads to an increase in the content of β-layers and a decrease in the number of α-helices | [181] |
| HSA | 0.15 µM | 7.3 | PBS | Ag | SC | Spherical | 20 40 80 (TEM) | −38.8 −32.9 −37.2 | 24 51 90 (TEM) | −13 −12 −12 | Kcat = 0.188 s−1 Kcat = 0.207 s−1 Kcat = 0.186 s−1 | The most significant changes in the secondary structure of the protein were observed when HSA interacted with smaller particles. | [99] |
| HSA | 3 μM | 7.4 | Tris-HCl | Ag | PPh | Spherical | 10–20 (DLS) | −36 | 23–30 | −27– (−20) | Kq = 2 × 1010 M−1 × s−1 | HSA binds to nanoparticles primarily through hydrophobic association; electrostatic interaction is not the main driving force | [209] |
| AGP | 48 μM | 7.4 | PBS | Ag | SC | Spherical | 10 (TEM) 21 (DLS) | −69 | 25 | −76 | Ka = 109 M−1 | The formation of a protein corona was accompanied by the preservation of the native protein structure and led to a significant decrease in the cytotoxicity of nanoparticles | [58] |
| IgG | 5 µM–0.5 M | 7.0 | PBS | Ag | - | Spherical | 100–262 (DLS) | - | 174–253 (DLS) | −35–(−7) | - | The aggregation and interaction of nanoparticles with protein is influenced by the ionic strength and protein concentration | [142] |
| LECT2 | 0–100 ng/mL | 7.4 | PBS | Ag@ MNP | Apt | Spherical | 216 (DLS) | −17 | - | −21 | - | Compared with the traditional method of random protein immobilization, orientation using an aptamer on Ag@MNP nanoparticles increases the efficiency of binding to the LECT2 protein. | [106] |
| HSA | 25 μM | - | Water | Ag Au Pt | - | Spherical | 9 10 10 (DLS) | - | 18 21 30 (DLS) | - | - | HSA is considered a biocompatible coating for metal nanoparticles in antibacterial therapy | [61] |
| HSA | 0.1 mg/mL | 7.5 | PBS | Al2O3 | - | Spherical | 118 (DLS) | −31 | - | - | KSV = 1.73–5.92 M−1 | Al2O3 nanoparticles induce partial unfolding of HSA molecules near aromatic residues, but do not cause significant changes in the secondary structure of HSA even at high concentrations. | [191] |
| anti-HRP ab | 0.03 g/L | 6–8.5 | PBS, NaOH | Au | SC | Spherical | 60 (DLS) | −22 | 87–200 (DLS) | - | - | The surface charge of the adsorbed protein can be used to control the aggregation of nanoparticles. | [30] |
| APOA1 | 5.0 μg/mL | 7.4 | TE | Au | SC | Spherical Stars | 44 58 (DLS) | −39 −35 | 108 146 (DLS) | −15 −12 | - | Precoating of gold nanoparticles with APOA1 protein reduces the expression of ZO-1 in endothelial cells and increases the ability to overcome biological barriers. | [34] |
| α-Syn | 200 μM | 7.3 | PBS | Au | Nar | Spherical | 24 (DLS) | −22 | 50–100 (DLS) | - | Ka = 5.02 × 106 M−1 | Nanoparticles functionalized with naringenin effectively slow down the aggregation and formation of α-synuclein fibrils. | [197] |
| BHb EMb | 10−6 M | 7.4 | PBS | Au | - | Rods | 60 (DLS) | +35 | - | 18 13 | Ka = 5.93 × 108 M−1 | The interaction of proteins with gold nanoparticles causes changes in the secondary structure. | [188] |
| BLG | 50 μM | 7.2 | PBS | Au | - | Spherical | 40 (DLS) | - | 52 (DLS) | - | Ka= 29 × 105 M−1 | Temperature plays a key role in protein adsorption on the surface of nanoparticles. As the temperature increases, the binding ability decreases, and the amount of adsorbed protein decreases too. | [143] |
| BSA | 15 μM | 7.4 | PBS | Au | - | Spherical | 24 (DLS) | −34 | 33 (DLS) | −16 | - | Exposure to a constant electric field leads to an increase in the thickness of the protein corona in the solution. | [224] |
| BSA | 1 mg/mL | 7.4 | PBS | Au | PEG | Triangles | 90 (DLS) | −27 | 93 (DLS) | −18 | Ksv = 3 × 108 M−1 | The gold nanotriangles retained photothermal properties after interaction with BSA. A decrease in the content of α-helices in the secondary structure was observed | [55] |
| BSA | 0.5–15 μM | 6.0 | PBS | Au | - | Spherical | 30 (DLS) | - | 39 (DLS) | −11 | - | The protein corona on gold nanoparticles affects the intracellular distribution and retention of particles. | [225] |
| BSA | 1 mg/mL | 7.4 | PBS | Au | - | Multibranched | 125 (DLS) | −24 | 146 (DLS) | −72 | - | The interaction of nanoparticles with the BSA reduces the tendency of nanoparticles to aggregate. | [31] |
| BSA | 0–2 μM | 7.4 | PBS | Au | CTAB PEG-COOH | Rods | 39 × 9.5 (TEM) | 56 −17 | 57 43 (DLS) | 22 −22 | - | PEG functionalization reduces the adsorption of proteins on the surface of nanoparticles in comparison with CTAB-coated nanoparticles. However, a protein corona still forms on PEG AuNPs, which persists even at high protein concentrations. | [102] |
| FBG | 0–0.4 μM | CTAB PEG-COOH | 56 −17 | 59 43 (DLS) | 21 −22 | ||||||||
| TM | 10–200 nM | - | NaCl | Au | Apt | Spherical | 13 (TEM) | - | - | - | - | A spectrophotometric technique has been developed to detect tropomyosin protein at nanomolar concentrations using aptamer-functionalized AuNPs | [226] |
| Hb | 0–100 nM | 7.4 | PBS | Au | ALA SC | Spherical | 25 (DLS) | −28 −21 | - 50–80 (DLS) | −24 −18 | - | There is a significant loss of the α-helix structure after interaction with citrate nanoparticles. | [107] |
| HSA | 5 μg/L–0.50 g/L | 7.4 | CBB | Au | - | Spherical | 69 (DLS) | - | 70 (DLS) | - | - | The colloidal stability of nanoparticles in the presence of a protein is influenced by the concentration of HSA, the ionic strength, and the valence of the cation in the salt solution. The presence of Ca2+ promotes additional adsorption of HSA on nanoparticles, which leads to particle aggregation. | [56] |
| HSA | 10/100/200 μM | 7.0 | Water | Au | CTAB | Rods | 35 × 12 (TEM) | - | - | - | - | Changes in the tryptophan microenvironment of the protein in comparison with the native protein are higher at a lower protein concentration. | [184] |
| HSA | 7.5 mM | 7.4 | PBS | Au | SC PEG-OMe PEG-COOH PEG-NH2 Glycan | Spherical | 19 45 47 47 35 (DLS) | −14 −2 −9 4 −3 | 70 55 60 67 79 (DLS) | −15 −5 −12 6 −6 | - | Electrostatic interactions and hydrogen bonds play an important role in binding nanoparticles to proteins. Neutral PEG-OMe gold nanoparticles do not cause structural changes, but positively charged PEG-NH2 nanoparticles cause conformational changes in HSA at any pH. | [15] |
| HSA | 0–50 μM | 7.4 | Tris-HCl | Au | PPh | Spherical | 17 (TEM) | −20 | 30 (TEM) | −10 | Kb = 104 M−1 | Nanoparticles can have an inhibitory effect on the formation of amyloid fibrils | [227] |
| HSA | 6 μM | 7.3 | Tris-HCl | Au | CTAB | Stars Rods Flowers | 20 (TEM) 28 (DLS) 40 (TEM) 68 (DLS) 42 (TEM) 79 (DLS) | +60 +64 +11 | 295 (DLS) 220 (DLS) 164 (DLS) | +26 +26 −9 | Ka = 1.7 × 105 M−1 Ka = 5.24 × 105 M−1 Ka = 1.73 × 106 M−1 | Nanoflowers have a higher ability to interact with HSA compared to other nanoparticles. | [187] |
| HSA cyt C | 0.25 μM 0.9 μM | 10.5 | CBB | Au | - | Spherical | 13 (DLS) | −55 | 21 18 | −21 −60 | - | Both proteins have a high affinity for the surface of gold nanoparticles due to free external thiols. | [182] |
| HSA Histone | 0.25–10 μM 0.01–10 μM | 7.4 | PBS | Au | Phe Lipids | Spherical | 30 (DLS) | −42 | 38 40 (DLS) | −31 50 | Ka = 4.22 × 106 M−1 Ka = 1.18 × 104 M−1 | The functionalization of the nanoparticle surface by fats provides increased resistance to protein adsorption. | [120] |
| HSA TF | 1 mg/mL | 7.4 | PBS | Au | DHLA | Spherical | 1.7 (TEM) | −20 | 34 (DLS) - | −40– (−15) −35 (−10) | - | Nanoparticles have little effect on the secondary structure of HSA and no effect on the secondary structure of transferrin. | [101] |
| FBG TRP | 10−11–10−4 M | 7.0 | PBS | Au | SC | Spherical | 35 (TEM) | −31.7 | - | −25 −10.6 | Kd = 1.58 × 108 M−1 Kd = 6.45 × 107 M−1 | Nanorods and nanostars can induce large changes in the secondary structures of proteins, unlike spherical nanoparticles. | [183] |
| FBG TRP | CTAC | Rods | 48 × 14 (TEM) | +44.8 | +17.3 +36.5 | - | |||||||
| IgG | 1 μM | 7.4 | PBS | Au | SC | Spherical | 60 (TEM) 69 (DLS) | −21 | 94 (DLS) | −11 | - | The biological effect of the protein corona can be manipulated by changing the structure of proteins. | [32] |
| Insulin | 10 μM | 7.4 | PBS | Au | Pro HydroxyPro | Spherical | 10 (DLS) | −72 −45 | - | - | Ka = 1.303 × 104 M−1 Ka = 0.9904 × 103 M−1 | Osmolite-functionalized nanoparticles contribute to the preservation of the native protein structure under aggregation conditions. | [126] |
| Insulin | 20 μM | 3.0, 7.4 | Gly-HCl PBS | Au | PEG200 PEG1500 PEG6000 PEG10000 | Spherical | 56 59 51 50 (DLS) | −25 −20 −10 −5 | 65 78 141 105 | - | - | Longer fibrils were formed when the protein interacted with nanoparticles that were functionalized by PEG with a higher molecular weight. | [33] |
| S-protein SARS-CoV-2 | 5 ng/µL | 7.5 | PBS | Au | BSPP | Spherical | 52 (TEM) 121 (DLS) | - | 93 (TEM) 173 (DLS) | - | - | The conjugate of protein and AuNPs causes a strong reaction of antigen-specific IgG. | [212] |
| TF | 2 mg/mL | 7.4 | PBS | Au | SC PEG TPN Cys GME | Spherical | 5 23 6 21 7 (DLS) | −34 −24 −24 −9 0.4 | 17 24 14 39 6 | −26 −24 −32 −18 −2 | - | Functionalization of nanoparticles with glutathione monoethyl ether resulted in resistance to protein adsorption and aggregation, as well as low cytotoxicity compared to other functional groups. | [119] |
| BHb Lys | 0.08–2.40 × 10−8 M | - | Water | Au/Ag | SC | Spherical | 24 (DLS) | −31 | 32 75 (DLS) | −10 −5 | Ksv = 5.7 × 109 M−1 Ksv = 5.35 × 109 M−1 | Nanoparticles influenced the structure of the BHb and Lys proteins | [189] |
| SP-B analog | 4 µg/mL | 5.2–6.4 | TFE/ PBS | Au | - | Spherical | 5 10 20 (DLS) | −17 −13 −15 | 139 85 131 | −11 5 10 | - | The content of the α-helical structure in the protein decreased when interacting with nanoparticles, and the efficiency of the process depends on the size. | [100] |
| Ag | 10 (DLS) | −9 | 126 | +2 | |||||||||
| BSA | 4 µM | - | Water | C60 | T80 | Spherical | 243 (TEM) | −13 | 357 | −14 | - | The C60 nanocomplex affects the secondary structure of the protein. The adsorption of BSA proceeded continuously, while FBG was partially desorbed after 4 h | [228] |
| FBG | 0.6 µM | 412 | −22 | ||||||||||
| Hb | 4 µM | 540 | +9 | ||||||||||
| LYZ | 50 µg/mL | 6.2 | PBS | C60 | NaOH | Spherical | 0.67 nm (DLS) | - | - | - | - | Fullerene functionalized by a hydrophilic group suppresses the enzymatic activity of lysozyme | [229] |
| BSA | 2 µM | 7.0 | PBS | C | - | Spherical | 360 (DLS) | - | 343 | - | Kq = 0.98 × 107 L mol−1 s−1 | BSA coating decreases carbon nanoparticle cytotoxicity against BEAS-2B cells and is accompanied by a slight reduction in the protein’s α-helical content | [230] |
| HSA | 0.15 mM | 7.2–7.4 | MES TRIS | Cr2O3 | - | Spherical | 100 (TEM) | −18.29 | - | −10.66 | - | BSA binds to nanoparticles in a preferred orientation due to electrostatic interaction | [192] |
| α-La | 2.5 μM | - | Water | Fe3O4 | Cellulose | Spherical | 14 (DLS) | −23 | - | −32 | Ka = 9.68 × 1011 M−1 | There are no conformational changes in the protein during interaction with nanoparticles. There is a strong interaction with protein during the functionalization of magnetic particles by cellulose in comparison with magnetic particles. | [231] |
| Anti-BSA | 6 nM | 7.4 | PBS | Fe3O4 | BSA | Spherical | 89 (DLS) | - | 100 (DLS) | - | Ka = 1.3 × 105 M−1 × s−1 | Iron oxide nanoparticles can be used to extract the target protein. The kinetics of binding were measured in solution without elution or reimmobilization. | [70] |
| BGL | 5 mg/mL | 4–9 | PBS | Fe3O4 | SA | Spherical | 10–40 (DLS) | - | 40–50 (DLS) | - | Kcat = 20.86 s−1 | The enzyme was more stable when interacting with iron oxide nanoparticles at different pH and temperature than the enzyme in the absence of nanoparticles under the same conditions. | [71] |
| BSA | 1 mg/mL | 6.0 | PBS | Fe3O4 | GA | Spherical | 38 (DLS) | −24.5 | 98 (DLS) | −16.7 | - | Nanoparticles in the presence of BSA were heated more efficiently than nanoparticles without BSA and showed significant internalization in cancer cells. | [69] |
| BSA | 0–50 µM | 7.4 | PBS | Fe3O4 | SC | Spherical | 18 (TEM) 30 (DLS) | −28.2 | 37 (DLS) | −18.4 | Ka = 4.6 × 105 M−1 | Denaturation of BSA in interaction with nanoparticles leads to a transition from the formation of a monolayer on nanoparticles to the formation of stable complexes. | [205] |
| FBS | - | 7.4 | PBS | Fe3O4 | PVA | Spherical | 8 (TEM) | +2 | 150–276 (DLS) | −4 | - | Proteins responsible for the long-term circulation of nanoparticles were found: osteopontin, lipoprotein lipase, coagulation factor VII, matrix protein GLA, secreted phosphoprotein 24, alpha-2H glycoprotein, and apolipoprotein C-I. | [124] |
| HEWL | 0.01–5 mg/mL | 4–11.8 | Water NaOH | Fe3O4 | SC | Spherical | 3 (DLS) | −32 | 246–462 (DLS) | −25–(+10) | - | Nanoparticles are involved in the formation of large aggregates, the size of which depends on the concentration of protein and nanoparticles. The addition of HEWL to clusters of nanoparticles leads to the disintegration of clusters into individual nanoparticles. | [161] |
| HSA | 2 mg/mL 8 mg/mL | 6.0–7.5 | PBS | Fe3O4 | - | Spherical | 50 (DLS) | - | 90 (DLS) | - | Ka = (1.3–5.8) × 104 M−1 | The interaction of HSA with iron oxide nanoparticles depends on the pH and ionic strength in the solution. | [138] |
| HSA | 0.29–0.91 g/L | 7.4 | HEPES | Fe3O4 | L-PEOXA C-PEOXA | Spherical | 52 21 (DLS) | - | 60 10–50 (DLS) | - | - | Cyclic PEOXA quantitatively prevents protein adsorption on nanoparticles. However, the dense shell of linear PEOXA cannot prevent a weak but significant interaction with HSA. | [118] |
| HSA TF | 0.2 mg/mL | 7.4 | PBS | Fe3O4 | Bilayer of OA and 18 LPC | Spherical | 16 (DLS) | −8 | 22 20–40 (DLS) | −17 −17 | Ka = 6.2 × 108 M−1 - | The protein structure did not change significantly after interaction with nanoparticles. The conjugate of HSA with nanoparticles did not affect the properties of the lipid bilayer. | [62] |
| IgG | 0.6 mg/mL | 3.0–9.0 | AC CBB PBS | Fe3O4 | κ-carrageenan | Spherical | 70 (TEM) | −25 | - | - | - | Magnetic nanoparticles may be a promising material for IgG isolation and purification. | [232] |
| IgG | 0.12–4.8 mg/mL | 6.6 | Tris-HCl | Fe3O4 | SC | Spherical | 50 (TEM) | - | - | - | - | Structural and conformational changes in the protein were observed when IgG interacted with nanoparticles. | [74] |
| OVA | 2.93 mg/mL | 7.4 | PBS | Fe3O4 | Methyl-modified | Spherical | 10 (DLS) | −38 | 251 (DLS) | −26 | - | Iron oxide nanoparticles were used for magnetic separation of ovalbumin from the medium. | [233] |
| TRP | 0.75 mg/mL | 4–11 | SC PBS CBB | Fe3O4 | PVA | Spherical | 244 (DLS) | −22 | - | - | - | Immobilized trypsin showed efficient proteolytic activity within a shorter period (15 min) compared to free trypsin (24 h) | [234] |
| CRP | 0.1–40 mg/L | 7.4 | Tris-HCl | Fe3O4@ SiO2/ COOH | EDTA/ TMS | Spherical | 53 (TEM) 750 (DLS) | −65 | 920 (DLS) | −65 | - | The effect of protein concentration on changes in the hydrodynamic characteristics of nanoparticles is shown. Such nanoparticles can be used for magnetic separation of proteins from solution. | [235] |
| Hb | 3 μM | 7.4 | Tris-HCl | NiO | - | Spherical | 20–50 (TEM) 175 (DLS) | −29 | - | - | Ksv = 3.24 × 104 M−1 | It has been shown that nanoparticles interact with hydrophilic amino acid residues in hemoglobin. | [236] |
| BSA HEWL | 1 mg/mL | 4.0–9.0 | AC BBS | TiO2 | SC | Spherical, cubical | 21 (TEM) | −11.6 | - | - | Kd = 130–500 M−1 Kd = 750–2000 M−1 | The binding affinity of TiO2 nanoparticles with lysozyme is higher than with BSA. | [136] |
| LYZ | 0.1–0.2 mg/mL | 6.2 | PBS | Se | SC | Spherical | 35 (SEM) 71 (DLS) | −30.2 | 84 (DLS) | 3 | - | The synergistic antibacterial activity of SeNPs and lysozyme against E. coli and S. aureus has been shown. | [237] |
| α-Syn | 100 µM | 7.4 | HEPES, NaCl | SiO2 | - | Spherical | 20 (TEM) | - | - | - | ka = 0.083 ± 0.011 h−1 | The presence of nanoparticles accelerates the amyloid fibrillation of the protein. Amyloid fibrils formed after interaction with nanoparticles were more toxic in comparison with those obtained without nanoparticles. | [194] |
| BHb | 2–10 μM | 7.4 | PBS | SiO2 | - | Spherical | 95 84 (DLS) | 18 40 | 165–190 320–370 (DLS) | 6 24 | - | The nanoparticles induced conformational changes in the protein around Tyr residues and heme degradation. | [80] |
| BSA | 10−7–10−3 M | 7.4 | PBS | SiO2 | - | Spherical | 9 (TEM) | - | - | - | - | Significant adsorption of BSA on nanoparticles was observed. | [238] |
| BSA LYZ BGG | 27.2 g/L 19.1 g/L 0.119 g/L | 7.4 | PBS | SiO2 | - | Spherical | 23 (TEM) 31 (DLS) | −39 | 250 5000 3000 | - | - | The authors proposed an approach to differentiate between different types of aggregates. | [19] |
| Mb | 0.1–5 mg/mL | 4.0–10.0 | Water | SiO2 | - | Spherical | 30 (DLS) | −45 | - | - | - | Changing the ionic strength of the solution allows you to control the adsorption–desorption of protein on the surface of the nanoparticle. | [140] |
| Mb Hb HbA1c HbAm | 0.01–2 g/L | 7.0 | PBS | SiO2 | - | Spherical | 33.6 | −17 | - | - | Ka = 1.7 × 105 Ka = 1.9 × 106 Ka = 1.1 × 106 Ka = 5.7 × 106 | Large proteins interacted with nanoparticles with greater affinity than small proteins. | [206] |
| oxyHb | 1 mM | 7.0 | PBS | SiO2 | - | Spherical | 26 (DLS) | −42 | 30 (DLS) | - | Ka = 2.1 × 105 M−1 | Nanoparticles affect the secondary structure of weakly bound proteins | [29] |
| BSA | 0.51 mM | 2–8 | Water | SiO2 TiO2 ZnO | - | Spherical | 155 493 520 | −32 −28 −31 | 162 475 147 | −38 −31 −31 | Ka = 1.1 × 105 M−1 Ka = 1.3 × 106 M−1 Ka = 1.8 × 105 M−1 | The affinity of the interaction of BSA with nanoparticles decreases in the line: ZnO > SiO2 > TiO2. The interaction between the particles and the protein is spontaneous (ΔG < 0). Minor changes in the secondary structure of the protein were observed during adsorption on nanoparticles. | [82] |
| WP | 100 μg/mL | 2–11 | Water | TiO2 | - | Spherical | 20–40 (TEM) | −12 | - | −18 | - | The formation of a protein corona on the surface of nanoparticles increases their antioxidant activity, which can reduce the negative biological effects of nanoparticles. | [135] |
| ALD CAT LDH QOR | 1 mg/mL | 7.5 | PBS | Al2O3 ZnO Fe3O4 CuO NiO | - | Spherical | 40 35–45 35–45 70 40 (DLS) | - | - | - | - | The effect of copper nanoparticles had a significant effect on the conformation, stability, and activity of metabolic enzymes in comparison with other nanoparticles. | [84] |
| BSA | 1 mg/mL | 2–12 | Water | CeO2 TiO2 ZnO | - | Spherical | 58 68 78 (DLS) | −30–(+43) −26–(+33) −69–(+13) | - | - | - | The content of the α-helical structure decreases, and the content of the disordered structure of BSA increases with the addition of nanoparticles. | [239] |
| BSA | 100–1000 μM | - | Water | TiO2 | - | Cubic | 130–260 (DLS) | −38 | 500 (DLS) | −30 | Ka = 8.76 × 102 M−1 | The values of hydrodynamic diameters and charges in colloids are important variables for analyzing the effect of the ionic force on the colloidal properties of nanoparticles. | [207] |
| Pepsin | 3.2 mg/mL | 1.2 | HCl NaCl | TiO2 | - | Spherical | 163 (DLS) | +27 | 208 (DLS) | +2 | - | Nanoparticles have no noticeable effect on the secondary structure of pepsin. However, the interaction of pepsin with nanoparticles led to a decrease in enzyme activity. | [35] |
| Luc | 3 µM | 7.4 | Tris–AC | TiO2 | - | Spherical | 24 (DLS) | −11 | 50 (DLS) | −4 | - | The interaction of luciferase with nanoparticles leads to a change in the secondary structure of the protein and promotes invalid folding of luciferase. | [193] |
| TF | 2.5 mg/mL | 7.4 | PBS | TiO2 | - | Spherical | 30–50 (TEM) | - | 50–70 (TEM) | - | - | Modification of the surface of nanoparticles with protein increased the stability of nanoparticles and their penetration into cells. | [240] |
| BSA Collagen Zein LYZ | 0.02 mg/mL | 3.5–10.4 | Water NaOH | TiO2 | - | Spherical | - | - | 80, 1500 10–60 10–60 5–30 (DLS) | - | - | The greatest absorption by SW1417 cells was observed for titanium nanoparticles functionalized with BSA. | [85] |
| BLG | - | 7.4 | PBS | TiO2 | - | Spherical | 3200 | - | 400 | - | - | The interaction of nanoparticles with β-lactoglobulin affects the secondary structure of the protein. There are no changes in the protein structure when nanoparticles interact with gelatin. It has been established that β-lactoglobulin promotes more efficient penetration of nanoparticles into cells than gelatin. | [86] |
| BSA | 2–16 mg/mL | 7.0 | PBS | TiO2 | - | Spherical | 300 (DLS) | 200–250 (DLS) | - | - | The concentration of BSA affected the thickness of the adsorbed layer. Spatial repulsion caused by an increase in the thickness of the adsorption layer of the protein increases the stability of the nanoparticles. | [241] | |
| BSA | 100 μg/mL | 4–5 | Water HCl NaOH | TiO2 | - | Spherical | 600 (DLS) | +41 | 2500 (DLS) | −18 | - | There is an increase in the percentage of β-structures and a decrease in α-helices in the BSA structure when interacting with nanoparticles. | [195] |
| GN GD SPI Zein | 100 mg/mL | 7.0 7.0 9.0 7.0 | water | TiO2 | - | Spherical | 32 (TEM) 760 (DLS) | −18.7 | 180 2733 300 200 (DLS) | +22.6 +61.4 −15.6 +18.9 | - | There is an increase in the percentage of β-structures in proteins when proteins bind to TiO2. | [211] |
| TRP | 0.01 g/mL | 7.4 | PBS | TiO2 | - | Spherical | 60 (TEM) 100 (DLS) | 0.7 | - | - | Ksv = 1.56 × 106 M−1 | The content of β-sheets in trypsin decreased when interacting with nanoparticles, while the content of random structures increased when the protein binds to TiO2. | [121] |
| BSA FBG | 2 mg/mL | 7.4 | PBS | ZnO | - | Spherical | 50 (DLS) | - | 806 30 (DLS) | - | - | The interaction of nanoparticles with fibrinogen avoids the effect of nanoparticle agglomeration. | [83] |
| BSA Casein Zein | 1 mg/mL | 7.4 | Water | ZnO | - | Spherical | 78 (SEM) | +16 | 380 600 1600 (DLS) | −22 −21 +8 | - Ka = 2.1 × 107 M−1 Ka = 2.2 × 107 M−1 | Cellular uptake increases with the functionalization of ZnO nanoparticles by BSA protein relative to nanoparticles without protein. | [210] |
| BSA | 10 mM | 7.4 | PBS | ZnO | Pro | Spherical | 702 (DLS) | −12 | 10 (DLS) | - | Kb = 0.3875 M−1 | Zinc oxide nanoparticles interact to a greater level with hydrophobic protein groups. A mixture of ZnO and proline at high temperature promotes protein fibrillation. | [129] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarimov, R.M.; Molkova, E.A.; Simakin, A.V.; Dorokhov, A.S.; Gudkov, S.V. Protein Corona as a Result of Interaction of Protein Molecules with Inorganic Nanoparticles. Int. J. Mol. Sci. 2025, 26, 9771. https://doi.org/10.3390/ijms26199771
Sarimov RM, Molkova EA, Simakin AV, Dorokhov AS, Gudkov SV. Protein Corona as a Result of Interaction of Protein Molecules with Inorganic Nanoparticles. International Journal of Molecular Sciences. 2025; 26(19):9771. https://doi.org/10.3390/ijms26199771
Chicago/Turabian StyleSarimov, Ruslan M., Elena A. Molkova, Alexander V. Simakin, Alexey S. Dorokhov, and Sergey V. Gudkov. 2025. "Protein Corona as a Result of Interaction of Protein Molecules with Inorganic Nanoparticles" International Journal of Molecular Sciences 26, no. 19: 9771. https://doi.org/10.3390/ijms26199771
APA StyleSarimov, R. M., Molkova, E. A., Simakin, A. V., Dorokhov, A. S., & Gudkov, S. V. (2025). Protein Corona as a Result of Interaction of Protein Molecules with Inorganic Nanoparticles. International Journal of Molecular Sciences, 26(19), 9771. https://doi.org/10.3390/ijms26199771







