In Search of Molecular Correlates of Fibromyalgia: The Quest for Objective Diagnosis and Effective Treatments
Abstract
1. Introduction
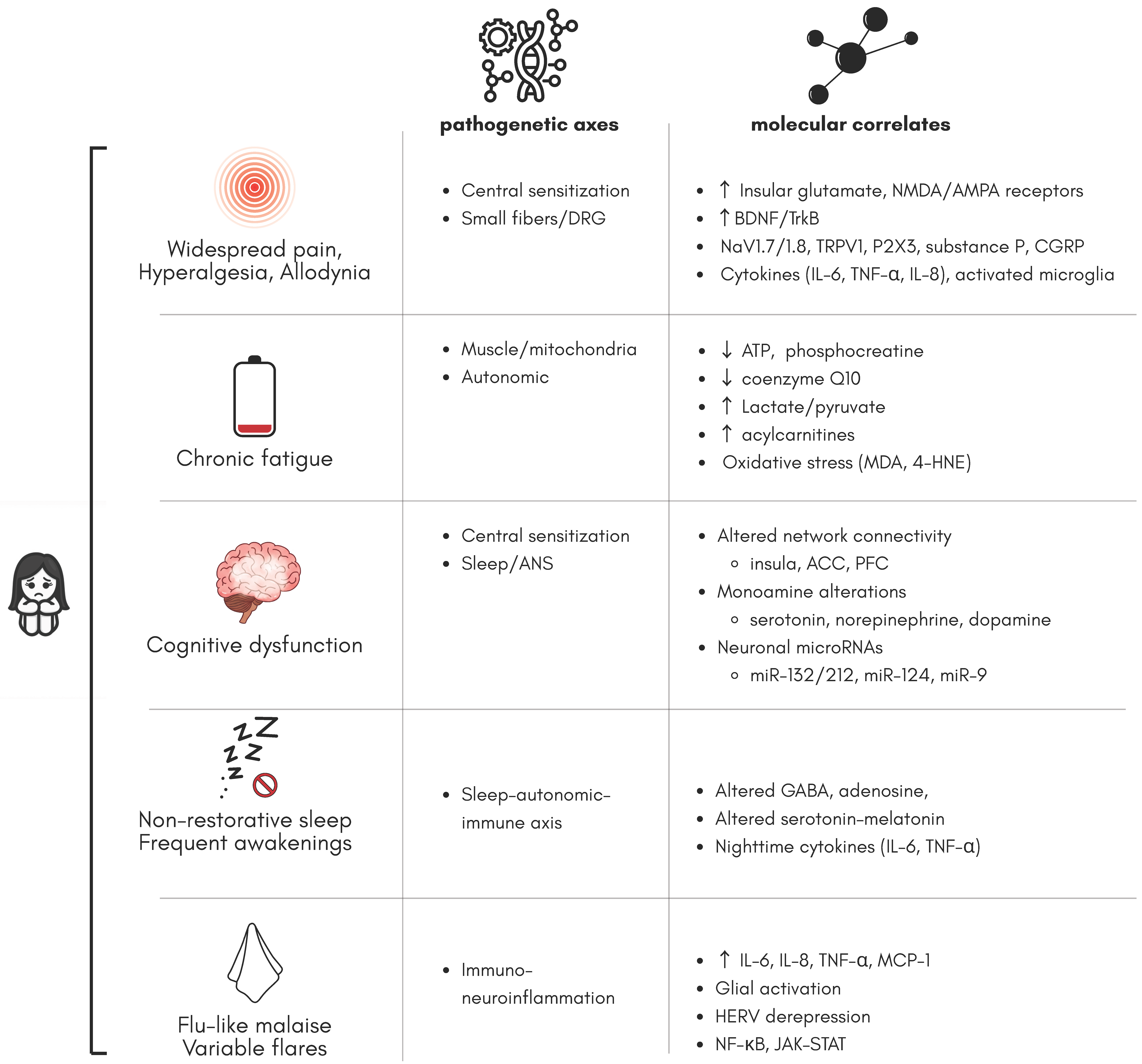
2. Pathogenetic Mechanisms and Molecular Correlates
2.1. Skeletal Muscle and Mitochondrial Bioenergetics
2.2. Dorsal Root Ganglia and Small-Fiber Pathology
2.3. Sensitization and Pain Network Plasticity
2.4. Immune Signaling, Low-Grade Inflammation, and Symptom Modulation
2.5. Autonomic Dysregulation and Interoceptive Burden
2.6. Sleep Architecture and Bidirectional Symptom Escalation
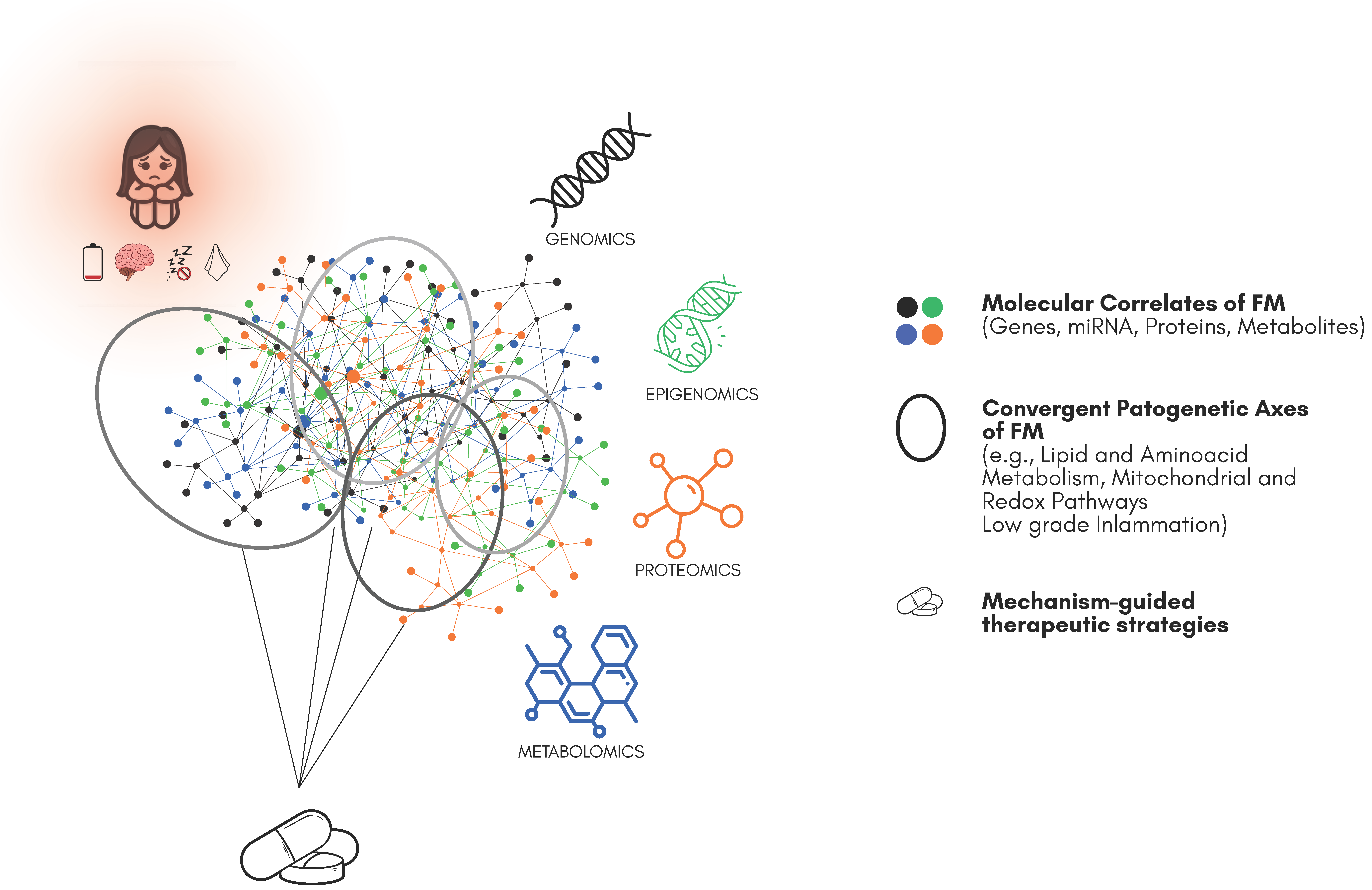
3. Omics Approaches in Fibromyalgia
3.1. Genomics, Epigenetics, and Transcriptomics
3.2. Proteomics and Metabolomics
3.3. Analytical Approaches for Module Discovery and Endotyping
4. Therapeutic Strategies and Drug Repurposing in Fibromyalgia
4.1. Emerging and Adjunctive Approaches: Where They Come From and What They Target
4.2. Mechanism-Anchored Repurposing: Linking Molecules, Pathways, and Drugs
4.3. Future Directions: From Panels to Trials
5. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Siracusa, R.; Paola, R.D.; Cuzzocrea, S.; Impellizzeri, D. Fibromyalgia: Pathogenesis, Mechanisms, Diagnosis and Treatment Options Update. Int. J. Mol. Sci. 2021, 22, 3891. [Google Scholar] [CrossRef]
- Bigatti, S.M.; Hernandez, A.M.; Cronan, T.A.; Rand, K.L. Sleep disturbances in fibromyalgia syndrome: Relationship to pain and depression. Arthritis Care Res. 2008, 59, 961–967. [Google Scholar] [CrossRef]
- Clauw, D.J. Fibromyalgia: A Clinical Review. JAMA 2014, 311, 1547–1555. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Smythe, H.A.; Yunus, M.B.; Bennett, R.M.; Bombardier, C.; Goldenberg, D.L.; Tugwell, P.; Campbell, S.M.; Abeles, M.; Clark, P.; et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990, 33, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, L.P. Worldwide epidemiology of fibromyalgia. Curr. Pain Headache Rep. 2013, 17, 356. [Google Scholar] [CrossRef]
- Marques, A.P.; de Sousa do Espírito Santo, A.; Berssaneti, A.A.; Matsutani, L.A.; Yuan, S.L.K. Prevalence of fibromyalgia: Literature review update. Rev. Bras. Reumatol. (Engl. Ed.) 2017, 57, 356–363. [Google Scholar] [CrossRef]
- Sarzi-Puttini, P.; Giorgi, V.; Marotto, D.; Atzeni, F. Fibromyalgia: An update on clinical characteristics, aetiopathogenesis and treatment. Nat. Rev. Rheumatol. 2020, 16, 645–660. [Google Scholar] [CrossRef]
- Üçeyler, N.; Häuser, W.; Sommer, C. A systematic review on the effectiveness of treatment with antidepressants in fibromyalgia syndrome. Arthritis Care Res. 2008, 59, 1279–1298. [Google Scholar] [CrossRef]
- Becker, S.; Schweinhardt, P. Dysfunctional Neurotransmitter Systems in Fibromyalgia, Their Role in Central Stress Circuitry and Pharmacological Actions on These Systems. Pain Res. Treat. 2012, 2012, 741746. [Google Scholar] [CrossRef]
- Buskila, D. Pediatric fibromyalgia. Rheum. Dis. Clin. N. Am. 2009, 35, 253–261. [Google Scholar] [CrossRef]
- Bennett, R.M.; Jones, J.; Turk, D.C.; Russell, I.J.; Matallana, L. An internet survey of 2,596 people with fibromyalgia. BMC Musculoskelet. Disord. 2007, 8, 27. [Google Scholar] [CrossRef]
- Macfarlane, G.J.; Kronisch, C.; Dean, L.E.; Atzeni, F.; Häuser, W.; Fluß, E.; Choy, E.; Kosek, E.; Amris, K.; Branco, J.; et al. EULAR revised recommendations for the management of fibromyalgia. Ann. Rheum. Dis. 2017, 76, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Cordero, M.D.; Moreno-Fernández, A.M.; de Miguel, M.; Bonal, P.; Campa, F.; Jiménez-Jiménez, L.M.; Ruiz-Losada, A.; Sánchez-Domínguez, B.; Alcázar, J.A.S.; Salviati, L.; et al. Coenzyme Q10 distribution in blood is altered in patients with Fibromyalgia. Clin. Biochem. 2009, 42, 732–735. [Google Scholar] [CrossRef] [PubMed]
- Woolf, C.J. Central sensitization: Implications for the diagnosis and treatment of pain. Pain 2011, 152, S2–S15. [Google Scholar] [CrossRef] [PubMed]
- Branco, J.C.; Bannwarth, B.; Failde, I.; Abello Carbonell, J.; Blotman, F.; Spaeth, M.; Saraiva, F.; Nacci, F.; Thomas, E.; Caubère, J.P.; et al. Prevalence of fibromyalgia: A survey in five European countries. Semin. Arthritis Rheum. 2010, 39, 448–453. [Google Scholar] [CrossRef]
- Fitzcharles, M.A.; Ste-Marie, P.A.; Pereira, J.X. Fibromyalgia: Evolving concepts over the past 2 decades. CMAJ 2013, 185, E645–E651. [Google Scholar] [CrossRef]
- Goldenberg, D.L.; Burckhardt, C.; Crofford, L. Management of Fibromyalgia Syndrome. JAMA 2004, 292, 2388–2395. [Google Scholar] [CrossRef]
- Mohapatra, G.; Dachet, F.; Coleman, L.J.; Gillis, B.; Behm, F.G. Identification of unique genomic signatures in patients with fibromyalgia and chronic pain. Sci. Rep. 2024, 14, 3949. [Google Scholar] [CrossRef]
- Phillips, K.; Clauw, D.J. Central pain mechanisms in chronic pain states—Maybe it is all in their head. Best Pract. Res. Clin. Rheumatol. 2011, 25, 141–154. [Google Scholar] [CrossRef]
- Chinn, S.; Caldwell, W.; Gritsenko, K. Fibromyalgia Pathogenesis and Treatment Options Update. Curr. Pain Headache Rep. 2016, 20, 25. [Google Scholar] [CrossRef]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Häuser, W.; Katz, R.L.; Mease, P.J.; Russell, A.S.; Russell, I.J.; Walitt, B. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin. Arthritis Rheum. 2016, 46, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Arnold, L.M.; Clauw, D.J.; Dunegan, L.J.; Turk, D.C. A Framework for Fibromyalgia Management for Primary Care Providers. Mayo Clin. Proc. 2012, 87, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Drewes, A.M.; Andreasen, A.; Schrøder, H.D.; Høgsaa, B.; Jennum, P. Pathology of Skeletal Muscle in Fibromyalgia: A Histo-Immuno-Chemical and Ultrastructural Study. Br. J. Rheumatol. 1993, 32, 479–483. [Google Scholar] [CrossRef]
- Schmidt-Wilcke, T.; Ichesco, E.; Hampson, J.P.; Kairys, A.; Peltier, S.; Harte, S.; Clauw, D.J.; Harris, R.E. Resting state connectivity correlates with drug and placebo response in fibromyalgia patients. NeuroImage Clin. 2014, 6, 252–261. [Google Scholar] [CrossRef]
- Yunus, M.B.; Masi, A.T.; Aldag, J.C. Preliminary Criteria for Primary Fibromyalgia Syndrome (PFS): Multivariate Analysis of a Consecutive Series of PFS, Other Pain Patients, and Normal Subjects. Clin. Exp. Rheumatol. 1989, 7, 63–69. [Google Scholar]
- Sánchez-Domínguez, B.; Bullón, P.; Román-Malo, L.; Marín-Aguilar, F.; Alcocer-Gómez, E.; Carrión, Á.M.; Sánchez-Alcázar, J.A.; Cordero, M.D. Oxidative stress, mitochondrial dysfunction and, inflammation common events in skin of patients with Fibromyalgia. Mitochondrion 2015, 21, 69–75. [Google Scholar] [CrossRef]
- Häuser, W.; Walitt, B.; Fitzcharles, M.A.; Sommer, C. Review of pharmacological therapies in fibromyalgia syndrome. Arthritis Res. Ther. 2014, 16, 201. [Google Scholar] [CrossRef]
- Carville, S.F.; Arendt-Nielsen, L.; Bliddal, H.; Blotman, F.; Branco, J.C.; Buskila, D.; Da Silva, J.A.P.; Danneskiold-Samsøe, B.; Dincer, F.; Henriksson, K.G.; et al. EULAR evidence-based recommendations for the management of fibromyalgia syndrome. Ann. Rheum. Dis. 2008, 67, 536–541, Erratum in Ann. Rheum. Dis. 2015, 74, 476. [Google Scholar] [CrossRef]
- Buskila, D.; Neumann, L.; Hazanov, I.; Carmi, R. Familial aggregation in the fibromyalgia syndrome. Semin. Arthritis Rheum. 1996, 26, 605–611. [Google Scholar] [CrossRef]
- Cassisi, G.; Sarzi-Puttini, P.; Cazzola, M. Chronic widespread pain and fibromyalgia: Could there be some relationship with infections and vaccinations? Clin. Exp. Rheumatol. 2011, 29, S118–S126. [Google Scholar]
- Lantos, P.M. Chronic Lyme Disease. Infect. Dis. Clin. N. Am. 2015, 29, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Winslow, B.T.; Vandal, C.; Dang, L. Fibromyalgia: Diagnosis and Management. Am. Fam. Physician 2023, 107, 137–144. [Google Scholar] [PubMed]
- Epstein, L.J.; Kristo, D.; Strollo, P.J.; Friedman, N.; Malhotra, A.; Patil, S.P.; Ramar, K.; Rogers, R.; Schwab, R.J.; Weaver, T.E.; et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J. Clin. Sleep Med. 2009, 5, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Garber, J.R.; Cobin, R.H.; Gharib, H.; Hennessey, J.V.; Klein, I.; Mechanick, J.I.; Pessah-Pollack, R.; Singer, P.A.; Woeber, K.A. Clinical practice guidelines for hypothyroidism in adults: Cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr. Pract. 2012, 18, 988–1028. [Google Scholar] [CrossRef]
- Camaschella, C. Iron-deficiency anemia. N. Engl. J. Med. 2015, 372, 1832–1843. [Google Scholar] [CrossRef]
- Raj, S.R. Postural tachycardia syndrome (POTS). Circulation 2013, 127, 2336–2342. [Google Scholar] [CrossRef]
- Drossman, D.A.; Hasler, W.L. Rome IV-Functional GI Disorders: Disorders of Gut-Brain Interaction. Gastroenterology 2016, 150, 1257–1261. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5); American Psychiatric Publishing: Arlington, VA, USA, 2013. [Google Scholar]
- Dolcino, M.; Tinazzi, E.; Puccetti, A.; Lunardi, C. Gene Expression Profiling in Fibromyalgia Indicates an Autoimmune Origin of the Disease and Opens New Avenues for Targeted Therapy. J. Clin. Med. 2020, 9, 1814. [Google Scholar] [CrossRef]
- D’Agnelli, S.; Arendt-Nielsen, L.; Gerra, M.C.; Zatorri, K.; Boggiani, L.; Baciarello, M.; Bignami, E. Fibromyalgia: Genetics and epigenetics insights may provide the basis for the development of diagnostic biomarkers. Mol. Pain 2019, 15, 1–12. [Google Scholar] [CrossRef]
- Gkouvi, A.; Tsiogkas, S.G.; Bogdanos, D.P.; Gika, H. Proteomics in Patients with Fibromyalgia Syndrome: A Systematic Review of Observational Studies. Curr. Pain Headache Rep. 2024, 28, 565. [Google Scholar] [CrossRef]
- Cordero, M.D.; Alcocer-Gómez, E.; Culic, O.; Carrión, A.M.; de Miguel, M.; Díaz-Parrado, E.; Pérez-Villegas, E.M.; Bullón, P.; Battino, M.; Sánchez-Alcazar, J.A. NLRP3 Inflammasome Is Activated in Fibromyalgia: The Effect of Coenzyme Q10. Antioxidants Redox Signal. 2014, 20, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Fulle, S.; Mecocci, P.; Fanó, G.; Vecchiet, I.; Vecchini, A.; Racciotti, D.; Cherubini, A.; Pizzigallo, E.; Vecchiet, L.; Senin, U.; et al. Specific oxidative alterations in vastus lateralis muscle of patients with the diagnosis of chronic fatigue syndrome. Free Radic. Biol. Med. 2000, 29, 1252–1259. [Google Scholar] [CrossRef] [PubMed]
- Clos-Garcia, M.; Andrés-Marin, N.; Fernández-Eulate, G.; Abecia, L.; Lavín, J.L.; van Liempd, S.; Cabrera, D.; Royo, F.; Valero, A.; Errazquin, N.; et al. Gut microbiome and serum metabolome analyses identify molecular biomarkers and altered glutamate metabolism in fibromyalgia. eBioMedicine 2019, 46, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Andrés-Rodríguez, L.; Borràs, X.; Feliu-Soler, A.; Pérez-Aranda, A.; Angarita-Osorio, N.; Moreno-Peral, P.; Montero-Marin, J.; García-Campayo, J.; Carvalho, A.F.; Maes, M.; et al. Peripheral immune aberrations in fibromyalgia: A systematic review, meta-analysis and meta-regression. Brain Behav. Immun. 2020, 87, 881–889. [Google Scholar] [CrossRef]
- Piras, C.; Pibiri, M.; Conte, S.; Ferranti, G.; Leoni, V.P.; Liggi, S.; Spada, M.; Muntoni, S.; Caboni, P.; Atzori, L. Metabolomics analysis of plasma samples of patients with fibromyalgia and electromagnetic sensitivity using GC–MS technique. Sci. Rep. 2022, 12, 21923. [Google Scholar] [CrossRef]
- Sluka, K.A.; Clauw, D.J. Neurobiology of fibromyalgia and chronic widespread pain. Neuroscience 2016, 338, 114–129. [Google Scholar] [CrossRef]
- Bazzichi, L.; Rossi, A.; Massimetti, G.; Giannaccini, G.; Giuliano, T.; Feo, F.D.; Ciapparelli, A.; Dell’Osso, L.; Bombardieri, S. Cytokine patterns in fibromyalgia and their correlation with clinical manifestations. Clin. Exp. Rheumatol. 2007, 25, 225–230. [Google Scholar]
- Cohen, H.; Neumann, L.; Shore, M.; Amir, M.; Cassuto, Y.; Buskila, D. Autonomic dysfunction in patients with fibromyalgia: Application of power spectral analysis of heart rate variability. Semin. Arthritis Rheum. 2000, 29, 217–227. [Google Scholar] [CrossRef]
- Choy, E.H.S. The role of sleep in pain and fibromyalgia. Nat. Rev. Rheumatol. 2015, 11, 513–520. [Google Scholar] [CrossRef]
- Bengtsson, A.; Henriksson, K.G.; Jorfeldt, L.; Kågedal, B.; Lennmarken, C.; Lindström, F. Primary Fibromyalgia: A Clinical and Laboratory Study of 55 Patients. Scand. J. Rheumatol. 1986, 15, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.; Maes, M. Mitochondrial dysfunctions in Myalgic Encephalomyelitis/chronic fatigue syndrome explained by activated immuno-inflammatory, oxidative and nitrosative stress pathways. Metab. Brain Dis. 2014, 29, 19–36. [Google Scholar] [CrossRef]
- Mease, P. Fibromyalgia syndrome: Review of clinical presentation, pathogenesis, outcome measures, and treatment. J. Rheumatol. Suppl. 2005, 75, 6–21. [Google Scholar]
- Häuser, W.; Ablin, J.; Fitzcharles, M.A.; Littlejohn, G.; Luciano, M.; Usui, C.; Walitt, B. Fibromyalgia. Nat. Rev. Dis. Primers 2015, 1, 15022. [Google Scholar] [CrossRef]
- Meeus, M.; Nijs, J. Central sensitization: A biopsychosocial explanation for chronic widespread pain in patients with fibromyalgia and chronic fatigue syndrome. Clin. Rheumatol. 2007, 26, 465–473. [Google Scholar] [CrossRef]
- Park, J.H.; Niermann, K.J.; Olsen, N.J. Evidence for Metabolic Abnormalities in the Muscles of Patients with Fibromyalgia. Curr. Rheumatol. Rep. 2000, 2, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Gracely, R.H.; Petzke, F.; Wolf, J.M.; Clauw, D.J. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 2002, 46, 1333–1343. [Google Scholar] [CrossRef] [PubMed]
- Napadow, V.; LaCount, L.; Park, K.; As-Sanie, S.; Clauw, D.J.; Harris, R.E. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum. 2010, 62, 2545–2555. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.B.; Kosek, E.; Petzke, F.; Carville, S.; Fransson, P.; Marcus, H.; Williams, S.C.; Choy, E.; Giesecke, T.; Mainguy, Y.; et al. Evidence of dysfunctional pain inhibition in Fibromyalgia reflected in rACC during provoked pain. Pain 2009, 144, 95–100. [Google Scholar] [CrossRef]
- Albrecht, D.S.; Forsberg, A.; Sandström, A.; Bergan, C.; Kadetoff, D.; Protsenko, E.; Lampa, J.; Lee, Y.C.; Olgart Höglund, C.; Catana, C.; et al. Brain glial activation in fibromyalgia – A multi-site positron emission tomography investigation. Brain Behav. Immun. 2019, 75, 72–83. [Google Scholar] [CrossRef]
- Lund, E.; Kendall, S.A.; Janerot-Sjöberg, B.; Bengtsson, A. Muscle Metabolism in Fibromyalgia Studied by P-31 Magnetic Resonance Spectroscopy During Aerobic and Anaerobic Exercise. Scand. J. Rheumatol. 2003, 32, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Staud, R.; Vierck, C.J.; Cannon, R.L.; Mauderli, A.P.; Price, D.D. Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain 2001, 91, 165–175. [Google Scholar] [CrossRef]
- Giannoccaro, M.P.; Donadio, V.; Incensi, A.; Avoni, P.; Liguori, R. Small nerve fiber involvement in patients referred for fibromyalgia. Muscle Nerve 2014, 49, 757–759. [Google Scholar] [CrossRef]
- Serra, J.; Collado, A.; Solà, R.; Antonelli, F.; Torres, X.; Salgueiro, M.; Quiles, C.; Bostock, H. Hyperexcitable C nociceptors in fibromyalgia. Ann. Neurol. 2014, 75, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Sprott, H.; Salemi, S.; Gay, R.E.; Bradley, L.A.; Alarcón, G.S.; Oh, S.J.; Michel, B.A.; Gay, S. Increased DNA fragmentation and ultrastructural changes in fibromyalgic muscle fibres. Ann. Rheum. Dis. 2004, 63, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Bazzichi, L.; Ciregia, F.; Giusti, L.; Baldini, C.; Giannaccini, G.; Giacomelli, C.; Sernissi, F.; Bombardieri, S.; Lucacchini, A. Detection of potential markers of primary fibromyalgia syndrome in human saliva. Proteom.—Clin. Appl. 2009, 3, 1296–1304. [Google Scholar] [CrossRef] [PubMed]
- Cordero, M.D.; Miguel, M.D.; Fernández, A.M.M.; López, I.M.C.; Maraver, J.G.; Cotán, D.; Izquierdo, L.G.; Bonal, P.; Campa, F.; Bullon, P.; et al. Mitochondrial dysfunction and mitophagy activation in blood mononuclear cells of fibromyalgia patients: Implications in the pathogenesis of the disease. Arthritis Res. Ther. 2010, 12, R17. [Google Scholar] [CrossRef]
- Gerdle, B.; Ghafouri, B.; Lund, E.; Bengtsson, A.; Lundberg, P.; van Ettinger-Veenstra, H.; Leinhard, O.D.; Forsgren, M.F. Evidence of Mitochondrial Dysfunction in Fibromyalgia: Deviating Muscle Energy Metabolism Detected Using Microdialysis and Magnetic Resonance. J. Clin. Med. 2020, 9, 3527. [Google Scholar] [CrossRef]
- Inferrera, F.; Marino, Y.; D’Amico, R.; Impellizzeri, D.; Cordaro, M.; Siracusa, R.; Gugliandolo, E.; Fusco, R.; Cuzzocrea, S.; Paola, R.D. Impaired mitochondrial quality control in fibromyalgia: Mechanisms involved in skeletal muscle alteration. Arch. Biochem. Biophys. 2024, 758, 110083. [Google Scholar] [CrossRef]
- Macchi, C.; Giachi, A.; Fichtner, I.; Pedretti, S.; Sarzi-Puttini, P.; Mitro, N.; Corsini, A.; Ruscica, M.; Gualtierotti, R. Mitochondrial function in patients affected with fibromyalgia syndrome is impaired and correlates with disease severity. Sci. Rep. 2024, 14, 30247. [Google Scholar] [CrossRef]
- Martínez-Lavín, M. Dorsal Root Ganglia: Fibromyalgia Pain Factory? Clin. Rheumatol. 2021, 40, 783–787. [Google Scholar] [CrossRef]
- Oaklander, A.L.; Herzog, Z.D.; Downs, H.M.; Klein, M.M. Objective evidence that small-fiber polyneuropathy underlies some illnesses currently labeled as fibromyalgia. Pain 2013, 154, 2310–2316. [Google Scholar] [CrossRef] [PubMed]
- Üçeyler, N.; Zeller, D.; Kahn, A.K.; Kewenig, S.; Kittel-Schneider, S.; Schmid, A.; Casanova-Molla, J.; Reiners, K.; Sommer, C. Small fibre pathology in patients with fibromyalgia syndrome. Brain 2013, 136, 1857–1867. [Google Scholar] [CrossRef] [PubMed]
- Solano, C.; Martinez, A.; Becerril, L.; Vargas, A.; Figueroa, J.; Navarro, C.; Ramos-Remus, C.; Martinez-Lavin, M. Autonomic Dysfunction in Fibromyalgia Assessed by the Composite Autonomic Symptoms Scale (COMPASS). J. Clin. Rheumatol. 2009, 15, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Park, D.J.; Kim, S.H.; Nah, S.S.; Lee, J.H.; Kim, S.K.; Lee, Y.A.; Hong, S.J.; Kim, H.S.; Lee, H.S.; Kim, H.A.; et al. Association between catechol-O-methyl transferase gene polymorphisms and fibromyalgia in a Korean population: A case-control study. Eur. J. Pain 2016, 20, 1131–1139. [Google Scholar] [CrossRef]
- Hackshaw, K.V. The Search for Biomarkers in Fibromyalgia. Diagnostics 2021, 11, 156. [Google Scholar] [CrossRef]
- Vargas-Alarcón, G.; Alvarez-Leon, E.; Fragoso, J.M.; Vargas, A.; Martinez, A.; Vallejo, M.; Martinez-Lavin, M. A SCN9A gene-encoded dorsal root ganglia sodium channel polymorphism associated with severe fibromyalgia. BMC Musculoskelet. Disord. 2012, 13, 23. [Google Scholar] [CrossRef]
- Bäckryd, E.; Tanum, L.; Lind, A.L.; Larsson, A.; Gordh, T. Evidence of both systemic inflammation and neuroinflammation in fibromyalgia patients, as assessed by a multiplex protein panel applied to the cerebrospinal fluid and to plasma. J. Pain Res. 2017, 10, 515–525. [Google Scholar] [CrossRef]
- Wolfe, F.; Walitt, B. Culture, science and the changing nature of fibromyalgia. Nat. Rev. Rheumatol. 2013, 9, 751–755. [Google Scholar] [CrossRef]
- Roizenblatt, S.; Moldofsky, H.; Benedito-Silva, A.A.; Tufik, S. Alpha sleep characteristics in fibromyalgia. Arthritis Rheum. 2001, 44, 222–230. [Google Scholar] [CrossRef]
- Moldofsky, H.; Inhaber, N.H.; Guinta, D.R.; Alvarez-Horine, S.B. Effects of Sodium Oxybate on Sleep Physiology and Sleep/Wake-related Symptoms in Patients with Fibromyalgia Syndrome: A Double-blind, Randomized, Placebo-controlled Study. J. Rheumatol. 2010, 37, 2156–2166. [Google Scholar] [CrossRef]
- Dib-Hajj, S.D.; Yang, Y.; Black, J.A.; Waxman, S.G. The NaV1.7 sodium channel: From molecule to man. Nat. Rev. Neurosci. 2013, 14, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Mork, P.J.; Nilsen, T.I.L. Sleep problems and risk of fibromyalgia: Longitudinal data on an adult female population in Norway. Arthritis Rheum. 2012, 64, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Häuser, W.; Petzke, F.; Sommer, C. Comparative Efficacy and Harms of Duloxetine, Milnacipran, and Pregabalin in Fibromyalgia Syndrome. J. Pain 2010, 11, 505–521. [Google Scholar] [CrossRef] [PubMed]
- Busch, A.J.; Barber, K.A.; Overend, T.J.; Peloso, P.M.J.; Schachter, C.L. Exercise for treating fibromyalgia syndrome. Cochrane Database Syst. Rev. 2007, 4, CD003786. [Google Scholar] [CrossRef]
- Bernardy, K.; Füber, N.; Köllner, V.; Häuser, W. Efficacy of Cognitive-Behavioral Therapies in Fibromyalgia Syndrome—A Systematic Review and Metaanalysis of Randomized Controlled Trials. J. Rheumatol. 2010, 37, 1991–2005. [Google Scholar] [CrossRef]
- Lee, Y.H.; Song, G.G. Comparative efficacy and tolerability of duloxetine, pregabalin, and milnacipran for the treatment of fibromyalgia: A Bayesian network meta-analysis of randomized controlled trials. Rheumatol. Int. 2016, 36, 663–672. [Google Scholar] [CrossRef]
- Younger, J.; Noor, N.; McCue, R.; Mackey, S. Low-dose naltrexone for the treatment of fibromyalgia: Findings of a small, randomized, double-blind, placebo-controlled, counterbalanced, crossover trial assessing daily pain levels. Arthritis Rheum. 2013, 65, 529–538. [Google Scholar] [CrossRef]
- Lederman, S.; Arnold, L.M.; Vaughn, B.; Kelley, M.; Sullivan, G.M. Efficacy and Safety of Sublingual Cyclobenzaprine for the Treatment of Fibromyalgia: Results From a Randomized, Double-Blind, Placebo-Controlled Trial. Arthritis Care Res. 2023, 75, 2359–2368. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef]
- Sirota, M.; Dudley, J.T.; Kim, J.; Chiang, A.P.; Morgan, A.A.; Sweet-Cordero, A.; Sage, J.; Butte, A.J. Discovery and Preclinical Validation of Drug Indications Using Compendia of Public Gene Expression Data. Sci. Transl. Med. 2011, 3, 96ra77. [Google Scholar] [CrossRef]
- Cook, D.B.; Lange, G.; Ciccone, D.S.; Liu, W.C.; Steffener, J.; Natelson, B.H. Functional imaging of pain in patients with primary fibromyalgia. J. Rheumatol. 2004, 31, 364–378. [Google Scholar] [PubMed]
- Barabási, A.L.; Gulbahce, N.; Loscalzo, J. Network medicine: A network-based approach to human disease. Nat. Rev. Genet. 2011, 12, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.B.; Maixner, D.W.; Fillingim, R.B.; Slade, G.; Gracely, R.H.; Ambrose, K.; Zaykin, D.V.; Hyde, C.; John, S.; Tan, K.; et al. Large candidate gene association study reveals genetic risk factors and therapeutic targets for fibromyalgia. Arthritis Rheum. 2012, 64, 584–593. [Google Scholar] [CrossRef]
- Legangneux, E.; Mora, J.J.; Spreux-Varoquaux, O.; Thorin, I.; Herrou, M.; Alvado, G.; Gomeni, C. Cerebrospinal fluid biogenic amine metabolites, plasma-rich platelet serotonin and [3H]imipramine reuptake in the primary fibromyalgia syndrome. Rheumatology 2001, 40, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.T.; Atzeni, F.; Beasley, M.; Flüß, E.; Sarzi-Puttini, P.; Macfarlane, G.J. The prevalence of fibromyalgia in the general population: A comparison of the American College of Rheumatology 1990, 2010, and modified 2010 classification criteria. Arthritis Rheumatol. 2015, 67, 568–575. [Google Scholar] [CrossRef]
- Castro-Marrero, J.; Cordero, M.D.; Sáez-Francas, N.; Jimenez-Gutierrez, C.; Aguilar-Montilla, F.J.; Aliste, L.; Alegre-Martin, J. Could Mitochondrial Dysfunction Be a Differentiating Marker Between Chronic Fatigue Syndrome and Fibromyalgia? Antioxid. Redox Signal. 2013, 19, 1855–1860. [Google Scholar] [CrossRef]
- Larson, A.A.; Giovengo, S.L.; Russell, J.I.; Michalek, J.E. Changes in the concentrations of amino acids in the cerebrospinal fluid that correlate with pain in patients with fibromyalgia: Implications for nitric oxide pathways. Pain 2000, 87, 201–211. [Google Scholar] [CrossRef]
- Russell, J.I.; Holman, A.J.; Swick, T.J.; Alvarez-Horine, S.; Wang, G.Y.; Guinta, D.; for the Sodium Oxybate 06-008 FM Study Group. Sodium oxybate reduces pain, fatigue, and sleep disturbance and improves functionality in fibromyalgia: Results from a 14-week, randomized, double-blind, placebo-controlled study. Pain 2011, 152, 1007–1017. [Google Scholar] [CrossRef]
- Üçeyler, N.; Häuser, W.; Sommer, C. Systematic review with meta-analysis: Cytokines in fibromyalgia syndrome. BMC Musculoskelet. Disord. 2011, 12, 245. [Google Scholar] [CrossRef]
- Leinders, M.; Doppler, K.; Klein, T.; Deckart, M.; Rittner, H.; Sommer, C.; Üçeyler, N. Increased Cutaneous miR-let-7d Expression Correlates with Small Nerve Fiber Pathology in Patients with Fibromyalgia Syndrome. Pain 2016, 157, 2493–2503. [Google Scholar] [CrossRef]
- Albrecht, D.S.; Granziera, C.; Hooker, J.M.; Loggia, M.L. In Vivo Imaging of Human Neuroinflammation. ACS Chem. Neurosci. 2016, 7, 470–483, Erratum in ACS Chem. Neurosci. 2018, 9, 1515. [Google Scholar] [CrossRef] [PubMed]
- Kadetoff, D.; Lampa, J.; Westman, M.; Andersson, M.; Kosek, E. Evidence of central inflammation in fibromyalgia-increased cerebrospinal fluid interleukin-8 levels. J. Neuroimmunol. 2012, 242, 33–38. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, D.C.; Maschietto, M.; Galhardoni, R.; Gouveia, G.; Chile, T.; Krepischi, A.C.V.; Dale, C.S.; Brunoni, A.R.; Parravano, D.C.; Moscoso, A.S.C.; et al. Epigenetics insights into chronic pain: DNA hypomethylation in fibromyalgia—A controlled pilot-study. Pain 2017, 158, 1473–1480. [Google Scholar] [CrossRef]
- Gerra, M.C.; Carnevali, D.; Ossola, P.; González-Villar, A.; Pedersen, I.S.; Triñanes, Y.; Donnini, C.; Manfredini, M.; Arendt-Nielsen, L.; de-la Peña, M.T.C. DNA Methylation Changes in Fibromyalgia Suggest the Role of the Immune-Inflammatory Response and Central Sensitization. J. Clin. Med. 2021, 10, 4992. [Google Scholar] [CrossRef]
- Malafoglia, V.; Ilari, S.; Gioia, C.; Vitiello, L.; Tenti, M.; Iannuccelli, C.; Cristiani, C.M.; Garofalo, C.; Passacatini, L.C.; Viglietto, G.; et al. An Observational Study on Chronic Pain Biomarkers in Fibromyalgia and Osteoarthritis Patients: Which Role for Mu Opioid Receptor’s Expression on NK Cells? Biomedicines 2023, 11, 931. [Google Scholar] [CrossRef]
- Malafoglia, V.; Raffaeli, W.; Ilari, S.; Gioia, C.; Iannuccelli, C.; Tenti, M.; Vitiello, L.; Proietti, S.; Lupacchini, L.; Passacatini, L.C.; et al. Mu-opioid receptor expression on B cells as a potential biomarker for chronic pain: A follow-up study with patients with fibromyalgia. Pain Rep. 2025, 10, e1283. [Google Scholar] [CrossRef]
- Irwin, M.R. Why Sleep Is Important for Health: A Psychoneuroimmunology Perspective. Annu. Rev. Psychol. 2015, 66, 143–172. [Google Scholar] [CrossRef]
- Giménez-Orenga, K.; Bayarri, S.; Andrés-Rodríguez, L.; Salvador, A.; Martín-Serrano, G.; Varela-Rodríguez, C.; Feliu-Soler, A.; Ribes, R.; Garcia-Campayo, J.; Luciano, J.V.; et al. HERV activation segregates ME/CFS from fibromyalgia while defining a novel nosologic entity. Elife 2025, 14, RP104441. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lukkahatai, N.; Walitt, B.; Espina, A.; Wang, D.; Saligan, L.N. Comparing Genomic Profiles of Women With and Without Fibromyalgia. Biol. Res. Nurs. 2015, 17, 373–383. [Google Scholar] [CrossRef]
- Subramanian, A.; Narayan, R.; Corsello, S.M.; Peck, D.D.; Natoli, T.E.; Lu, X.; Gould, J.; Davis, J.F.; Tubelli, A.A.; Asiedu, J.K.; et al. A Next Generation Connectivity Map: L1000 Platform and the First 1,000,000 Profiles. Cell 2017, 171, 1437–1452.e17. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Walitt, B.; Klose, P.; Fitzcharles, M.A.; Phillips, T.; Häuser, W. Cannabinoids for fibromyalgia. Cochrane Database Syst. Rev. 2016, 7, CD011694. [Google Scholar] [CrossRef] [PubMed]
- Iannuccelli, C.; Favretti, M.; Dolcini, G.; Di Carlo, M.; Pellegrino, G.; Bazzichi, L.; Atzeni, F.; Lucini, D.; Varrassi, G.; Leoni, M.L.G.; et al. Fibromyalgia: One year in review 2025. Clin. Exp. Rheumatol. 2025, 43, 957–969. [Google Scholar] [CrossRef] [PubMed]
- Noppers, I.; Niesters, M.; Swartjes, M.; Bauer, M.; Aarts, L.; Geleijnse, N.; Mooren, R.; Dahan, A.; Sarton, E. Absence of long-term analgesic effect from a short-term S-ketamine infusion on fibromyalgia pain: A randomized, prospective, double blind, active placebo-controlled trial. Eur. J. Pain 2011, 15, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Skrabek, R.Q.; Galimova, L.; Ethans, K.; Perry, D. Nabilone for the Treatment of Pain in Fibromyalgia. J. Pain 2008, 9, 164–173. [Google Scholar] [CrossRef]
- Ware, M.A.; Fitzcharles, M.A.; Joseph, L.; Shir, Y. The Effects of Nabilone on Sleep in Fibromyalgia: Results of a Randomized Controlled Trial. Anesth. Analg. 2010, 110, 604–610. [Google Scholar] [CrossRef]
- García-Domínguez, M. Fibromyalgia and Inflammation: Unrevealing the Connection. Cells 2025, 14, 271. [Google Scholar] [CrossRef]
- Ablin, J.N. Fibromyalgia: Are you a genetic/environmental disease? Pain Rep. 2025, 10, e1256. [Google Scholar] [CrossRef]
- Filipovic, T.; Filipović, A.; Nikolic, D.; Gimigliano, F.; Stevanov, J.; Hrkovic, M.; Bosanac, I. Fibromyalgia: Understanding, Diagnosis and Modern Approaches to Treatment. J. Clin. Med. 2025, 14, 955. [Google Scholar] [CrossRef]
- Sedda, S.; Cadoni, M.P.L.; Medici, S.; Aiello, E.; Erre, G.L.; Nivoli, A.M.; Carru, C.; Coradduzza, D. Fibromyalgia, Depression, and Autoimmune Disorders: An Interconnected Web of Inflammation. Biomedicines 2025, 13, 503. [Google Scholar] [CrossRef]
- White, K.P.; Speechley, M.; Harth, M.; Ostbye, T. The London Fibromyalgia Epidemiology Study: The prevalence of fibromyalgia syndrome in London, Ontario. J. Rheumatol. 1999, 26, 1570–1576. [Google Scholar] [PubMed]
- Mas, A.; Carmona, L.; Valverde, M.; Ribas, B.; Group, E.S. Prevalence and impact of fibromyalgia on function and quality of life in individuals from the general population: Results from a nationwide study in Spain. Clin. Exp. Rheumatol. 2008, 26, 885–892. [Google Scholar]
- Clauw, D.J. Fibromyalgia and related conditions. Mayo Clin. Proc. 2015, 90, 680–692. [Google Scholar] [CrossRef] [PubMed]
- Arnold, L.M.; Bennett, R.M.; Crofford, L.J.; Dean, L.E.; Clauw, D.J.; Goldenberg, D.L.; Fitzcharles, M.A.; Paiva, E.S.; Staud, R.; Sarzi-Puttini, P.; et al. AAPT Diagnostic Criteria for Fibromyalgia. J. Pain 2019, 20, 611–628. [Google Scholar] [CrossRef]
- Walitt, B.; Urrútia, G.; Nishishinya, M.B.; Cantrell, S.E.; Häuser, W. Selective serotonin reuptake inhibitors for fibromyalgia syndrome. Cochrane Database Syst. Rev. 2015, 2015, CD011735. [Google Scholar] [CrossRef]
- Cordero, M.D.; Alcocer-Gómez, E.; de Miguel, M.; Culic, O.; Carrión, A.M.; Alvarez-Suarez, J.M.; Bullón, P.; Battino, M.; Fernández-Rodríguez, A.; Sánchez-Alcazar, J.A. Can Coenzyme Q10 Improve Clinical and Molecular Parameters in Fibromyalgia? Antioxid. Redox Signal. 2013, 19, 1356–1361. [Google Scholar] [CrossRef]
- Moldofsky, H. Sleep and pain. Sleep Med. Rev. 2001, 5, 385–396. [Google Scholar] [CrossRef]
- Fitzcharles, M.A.; Ste-Marie, P.A.; Goldenberg, D.L.; Pereira, J.X.; Abbey, S.; Choinière, M.; Ko, G.; Moulin, D.E.; Panopalis, P.; Proulx, J.; et al. 2012 Canadian Guidelines for the diagnosis and management of fibromyalgia syndrome: Executive summary. Pain Res. Manag. 2013, 18, 119–126. [Google Scholar] [CrossRef]
- Docampo, E.; Escaramís, G.; Gratacòs, M.; Villatoro, S.; Puig, A.; Kogevinas, M.; Collado, A.; Carbonell, J.; Rivera, J.; Vidal, J.; et al. Genome-wide analysis of single nucleotide polymorphisms and copy number variants in fibromyalgia suggest a role for the central nervous system. Pain 2014, 155, 1102–1109. [Google Scholar] [CrossRef]
- Vogel, C.; Marcotte, E.M. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 2012, 13, 227–232. [Google Scholar] [CrossRef]
- Offenbaecher, M.; Bondy, B.; de Jonge, S.; Glatzeder, K.; Krüger, M.; Schoeps, P.; Ackenheil, M. Possible association of fibromyalgia with a polymorphism in the serotonin transporter gene regulatory region. Arthritis Rheum. 1999, 42, 2482–2488. [Google Scholar] [CrossRef]
- Cohen, H.; Buskila, D.; Neumann, L.; Ebstein, R.P. Confirmation of an association between fibromyalgia and serotonin transporter promoter region (5-HTTLPR) polymorphism, and relationship to anxiety-related personality traits. Arthritis Rheum. 2002, 46, 845–847. [Google Scholar] [CrossRef] [PubMed]
- Gürsoy, S.; Erdal, E.; Herken, H.; Madenci, E.; Alasehirli, B. Association of T102C polymorphism of the 5-HT2A receptor gene with psychiatric status in fibromyalgia syndrome. Rheumatol. Int. 2001, 21, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Gürsoy, S.; Erdal, E.; Herken, H.; Madenci, E.; Alasehirli, B. Significance of catechol-O-methyltransferase gene polymorphism in fibromyalgia syndrome. Rheumatol. Int. 2003, 23, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H.; Neumann, L.; Glazer, Y.; Ebstein, R.P.; Buskila, D. The relationship between a common catechol-O-methyltransferase (COMT) polymorphism val(158)met and fibromyalgia. Clin. Exp. Rheumatol. 2009, 27, S51–S56. [Google Scholar]
- Inanir, A.; Karakuş, N.; Ateş, O.; Sezer, S. Clinical symptoms in fibromyalgia are associated to catechol-O-methyltransferase (COMT) gene Val158Met polymorphism. Xenobiotica 2014, 44, 434–439. [Google Scholar] [CrossRef]
- Buskila, D.; Cohen, H.; Neumann, L.; Ebstein, R.P. An association between fibromyalgia and the dopamine D4 receptor exon III repeat polymorphism and relationship to novelty seeking personality traits. Mol. Psychiatry 2004, 9, 730–731, Erratum in Mol. Psychiatry 2004, 9, 973; Erratum in Mol. Psychiatry 2004, 9, 1052. [Google Scholar] [CrossRef]
- Park, D.J.; Kim, S.H.; Nah, S.S.; Lee, J.H.; Kim, S.K.; Lee, Y.A.; Hong, S.J.; Kim, H.S.; Lee, H.S.; Kim, H.A.; et al. Association between brain-derived neurotrophic factor gene polymorphisms and fibromyalgia in a Korean population: A multicenter study. Arthritis Res. Ther. 2018, 20, 220. [Google Scholar] [CrossRef]
- da Silveira Alves, C.F.; Caumo, W.; Silvestri, J.M.; Zortea, M.; Dos Santos, V.S.; Cardoso, D.F. Pain catastrophizing is associated with the Val66Met polymorphism of the brain-derived neurotrophic factor in fibromyalgia. Adv. Rheumatol. 2020, 60, 39. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, N.; Wei, M.; Pan, Q.; Cheng, C.; Lu, K.E.; Mo, J.; Chen, Y. Methylation factors as biomarkers of fibromyalgia. Ann. Transl. Med. 2023, 11, 169. [Google Scholar] [CrossRef]
- Jones, E.A.; Asaad, F.; Patel, N.; Jain, E.; Abd-Elsayed, A. Management of Fibromyalgia: An Update. Biomedicines 2024, 12, 1266. [Google Scholar] [CrossRef]
- Jurado-Priego, L.N.; Cueto-Ureña, C.; Ramírez-Expósito, M.J.; Martínez-Martos, J.M. Fibromyalgia: A Review of the Pathophysiological Mechanisms and Multidisciplinary Treatment Strategies. Biomedicines 2024, 12, 1543. [Google Scholar] [CrossRef] [PubMed]
- Bjersing, J.L.; Lundborg, C.; Bokarewa, M.I.; Mannerkorpi, K. Profile of Cerebrospinal microRNAs in Fibromyalgia. PLoS ONE 2013, 8, e78762. [Google Scholar] [CrossRef] [PubMed]
- Cerdá-Olmedo, G.; Mena-Durán, A.V.; Monsalve, V.; Oltra, E. Identification of a MicroRNA Signature for the Diagnosis of Fibromyalgia. PLoS ONE 2015, 10, e0121903. [Google Scholar] [CrossRef] [PubMed]
- Bjersing, J.L.; Bokarewa, M.I.; Mannerkorpi, K. Profile of circulating microRNAs in fibromyalgia and their relation to symptom severity: An exploratory study. Rheumatol. Int. 2015, 35, 635–642. [Google Scholar] [CrossRef]
- Ovrom, E.A.; Mostert, K.A.; Khakhkhar, S.; McKee, D.P.; Yang, P.; Her, Y.F. A Comprehensive Review of the Genetic and Epigenetic Contributions to the Development of Fibromyalgia. Biomedicines 2023, 11, 1119. [Google Scholar] [CrossRef]
- Lamb, J.; Crawford, E.D.; Peck, D.; Modell, J.W.; Blat, I.C.; Wrobel, M.J.; Lerner, J.; Brunet, J.P.; Subramanian, A.; Ross, K.N.; et al. The Connectivity Map: Using Gene-Expression Signatures to Connect Small Molecules, Genes, and Disease. Science 2006, 313, 1929–1935. [Google Scholar] [CrossRef]
- Bruun, K.D.; Christensen, R.; Amris, K.; Vaegter, H.B.; Blichfeldt-Eckhardt, M.R.; Bye-Møller, L.; Holsgaard-Larsen, A.; Toft, P. Naltrexone 6 mg once daily versus placebo in women with fibromyalgia: A randomised, double-blind, placebo-controlled trial. Lancet Rheumatol. 2024, 6, e31–e39. [Google Scholar] [CrossRef]
- Malatji, B.G.; Meyer, H.; Mason, S.; Engelke, U.F.H.; Wevers, R.A.; van Reenen, M.; Reinecke, C.J. A diagnostic biomarker profile for fibromyalgia syndrome based on an NMR metabolomics study of selected patients and controls. BMC Neurol. 2017, 17, 88. [Google Scholar] [CrossRef]
- Ciregia, F.; Giacomelli, C.; Giusti, L.; Boldrini, C.; Piga, I.; Pepe, P.; Consensi, A.; Gori, S.; Lucacchini, A.; Mazzoni, M.R.; et al. Putative salivary biomarkers useful to differentiate patients with fibromyalgia. J. Proteom. 2019, 190, 44–54. [Google Scholar] [CrossRef]
- Khoonsari, P.E.; Musunri, S.; Herman, S.; Svensson, C.I.; Tanum, L.; Gordh, T.; Kultima, K. Systematic analysis of the cerebrospinal fluid proteome of fibromyalgia patients. J. Proteom. 2019, 190, 35–43. [Google Scholar] [CrossRef]
- Wåhlén, K.; Yan, H.; Welinder, C.; Ernberg, M.; Kosek, E.; Mannerkorpi, K.; Gerdle, B.; Ghafouri, B. Proteomic Investigation in Plasma from Women with Fibromyalgia in Response to a 15-Week Resistance Exercise Intervention. Med. Sci. Sports Exerc. 2022, 54, 232–246. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Tejero, J.A.; Martínez-Lara, E.; Peinado, M.A.; del Moral, M.L.; Siles, E. Hydroxytyrosol as a Promising Ally in the Treatment of Fibromyalgia. Nutrients 2020, 12, 2386. [Google Scholar] [CrossRef] [PubMed]
- Atzeni, F.; Talotta, R.; Masala, I.F.; Giacomelli, C.; Conversano, C.; Nucera, V.; Lucchino, B.; Iannuccelli, C.; Di Franco, M.; Bazzichi, L. One year in review 2019: Fibromyalgia. Clin. Exp. Rheumatol. 2019, 37 (Suppl. 116), 3–10. [Google Scholar] [PubMed]
- Arnold, L.M.; Russell, I.J.; Diri, E.W.; Duan, W.R.; Young, J.P.J.; Sharma, U.; Martin, S.A.; Barrett, J.A.; Haig, G. A 14-week, Randomized, Double-Blinded, Placebo-Controlled Monotherapy Trial of Pregabalin in Patients With Fibromyalgia. J. Pain 2008, 9, 792–805. [Google Scholar] [CrossRef]
- Mease, P.J.; Russell, I.J.; Arnold, L.M.; Florian, H.; Young, J.P.J.; Martin, S.A.; Sharma, U. A randomized, double-blind, placebo-controlled, phase III trial of pregabalin in the treatment of patients with fibromyalgia. J. Rheumatol. 2008, 35, 502–514. [Google Scholar]
- Nüesch, E.; Häuser, W.; Bernardy, K.; Barth, J.; Jüni, P. Comparative efficacy of pharmacological and non-pharmacological interventions in fibromyalgia syndrome: Network meta-analysis. Ann. Rheum. Dis. 2013, 72, 955–962. [Google Scholar] [CrossRef]
- Wang, C.; Schmid, C.H.; Rones, R.; Kalish, R.; Yinh, J.; Goldenberg, D.L.; Lee, Y.; McAlindon, T. A Randomized Trial of Tai Chi for Fibromyalgia. N. Engl. J. Med. 2010, 363, 743–754. [Google Scholar] [CrossRef]
- Glombiewski, J.A.; Sawyer, A.T.; Gutermann, J.; Koenig, K.; Rief, W.; Hofmann, S.G. Psychological treatments for fibromyalgia: A meta-analysis. Pain 2010, 151, 280–295. [Google Scholar] [CrossRef]
- Deare, J.C.; Zheng, Z.; Xue, C.C.L.; Liu, J.P.; Shang, J.; Scott, S.W.; Littlejohn, G. Acupuncture for treating fibromyalgia. Cochrane Database Syst. Rev. 2013, 2013, CD007070. [Google Scholar] [CrossRef]
- Pastrak, M.; Abd-Elsayed, A.; Ma, F.; Vrooman, B.; Visnjevac, O. Systematic Review of the Use of Intravenous Ketamine for Fibromyalgia. Ochsner J. 2021, 21, 387–394. [Google Scholar] [CrossRef]
- Minerbi, A.; Gonzalez, E.; Brereton, N.J.B.; Anjarkouchian, A.; Dewar, K.; Fitzcharles, M.A.; Chevalier, S.; Shir, Y. Altered microbiome composition in individuals with fibromyalgia. Pain 2019, 160, 2589–2602. [Google Scholar] [CrossRef]
| Condition | Overlap with FM | Distinguishing Features |
|---|---|---|
| ME/CFS |
|
|
| Hypothyroidism |
|
|
| Iron-deficiency anemia |
|
|
| Inflammatory arthritis (RA, SLE) |
|
|
| Small-fiber neuropathy |
|
|
| Obstructive sleep apnea (OSA) |
|
|
| POTS Autonomic disorders |
|
|
| IBS Functional GI disorders |
|
|
| Major depression Anxiety |
|
|
| Aspect | Description and Challenges |
|---|---|
| Clinical features |
|
| Pathophysiology Complex multi-axis interplay with limited large-scale validation |
|
| Diagnosis |
|
| Treatment Partial efficacy, adherence challenges, and side effects |
|
| Future directions |
|
| Axis | Representative Findings | Clinical Correlates |
|---|---|---|
| Muscle and mitochondria |
|
|
| DRG and small fibers |
|
|
| Sensitization |
|
|
| Immune and neuroinflammation |
|
|
| Autonomic and sleep axes |
|
|
| Molecule/Element | Pathway/Evidence in FM | Clinical/Pathophysiological Implications |
|---|---|---|
| COMT polymorphisms |
|
|
| SLC6A4 (5-HTT) variants |
|
|
| SLC6A4, GRIA4 |
|
|
| DNA methylation (immune/oxidative loci) |
|
|
| GRM2 methylation |
|
|
| HERV expression profiles |
|
|
| let-7 family (let-7a/d) |
|
|
| miR-21-5p |
|
|
| miR-146a-5p, miR-155-5p |
|
|
| miR-27a/b, miR-223-3p |
|
|
| miR-103/107, miR-320a |
|
|
| miR-132/212, miR-124-3p, miR-9-5p |
|
|
| miR-34a-5p |
|
|
| Category | Description and Examples |
|---|---|
| Pharmacological (approved) |
|
| Non-pharmacological (core care) |
|
| Emerging/repurposed |
|
| Future directions |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonomi, S.; Oltra, E.; Alberio, T. In Search of Molecular Correlates of Fibromyalgia: The Quest for Objective Diagnosis and Effective Treatments. Int. J. Mol. Sci. 2025, 26, 9762. https://doi.org/10.3390/ijms26199762
Bonomi S, Oltra E, Alberio T. In Search of Molecular Correlates of Fibromyalgia: The Quest for Objective Diagnosis and Effective Treatments. International Journal of Molecular Sciences. 2025; 26(19):9762. https://doi.org/10.3390/ijms26199762
Chicago/Turabian StyleBonomi, Sveva, Elisa Oltra, and Tiziana Alberio. 2025. "In Search of Molecular Correlates of Fibromyalgia: The Quest for Objective Diagnosis and Effective Treatments" International Journal of Molecular Sciences 26, no. 19: 9762. https://doi.org/10.3390/ijms26199762
APA StyleBonomi, S., Oltra, E., & Alberio, T. (2025). In Search of Molecular Correlates of Fibromyalgia: The Quest for Objective Diagnosis and Effective Treatments. International Journal of Molecular Sciences, 26(19), 9762. https://doi.org/10.3390/ijms26199762







