Development of Resistance to Damping-Off in Rice, Oryza sativa L., Using CRISPR/Cas9
Abstract
1. Introduction
2. Results
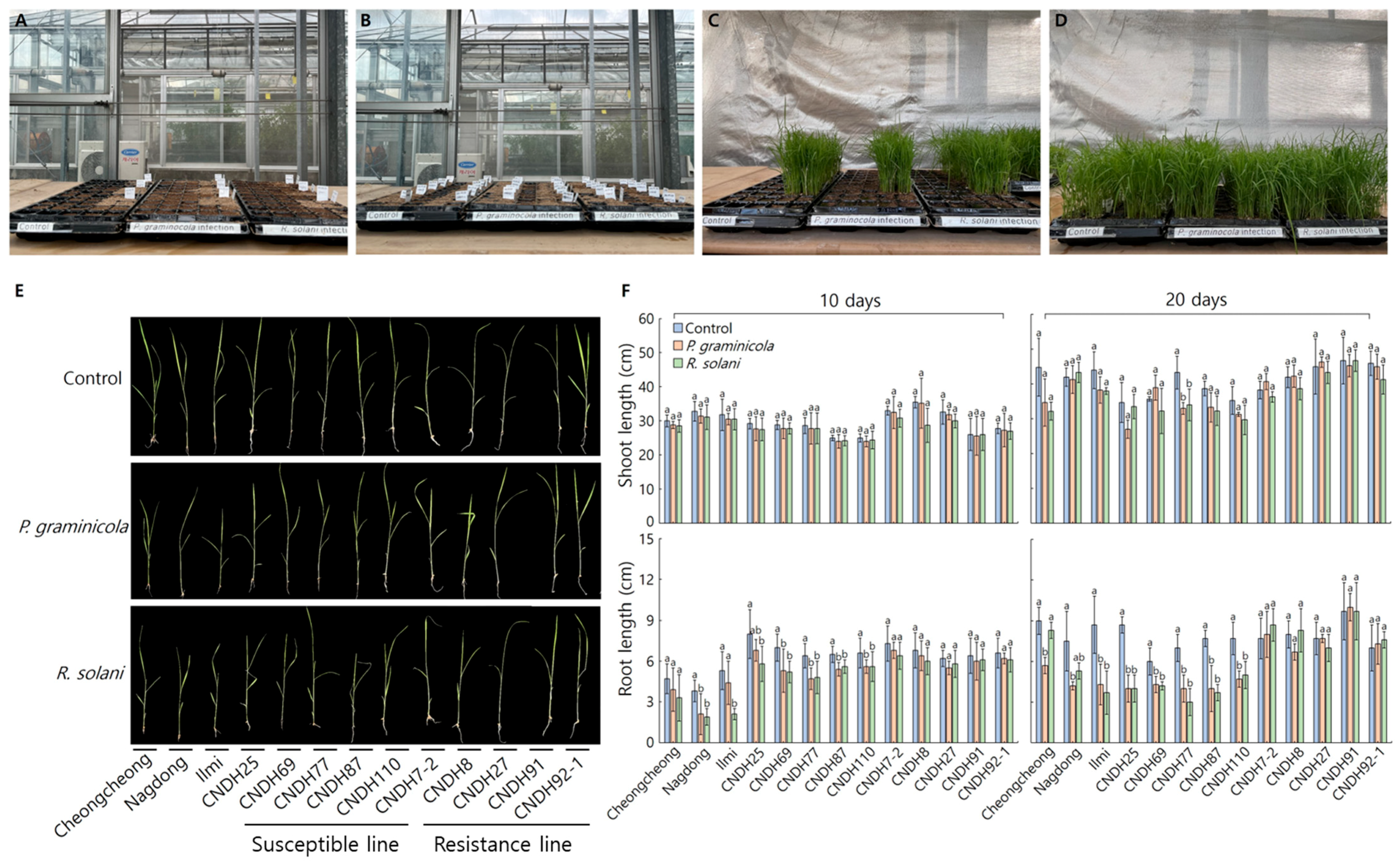
2.1. Significance Analysis of OsDGTq1 Through Field Experiments
Field Investigation of OsDGTq1
2.2. CRISPR/Cas9-Mediated Editing of OsDGTq1
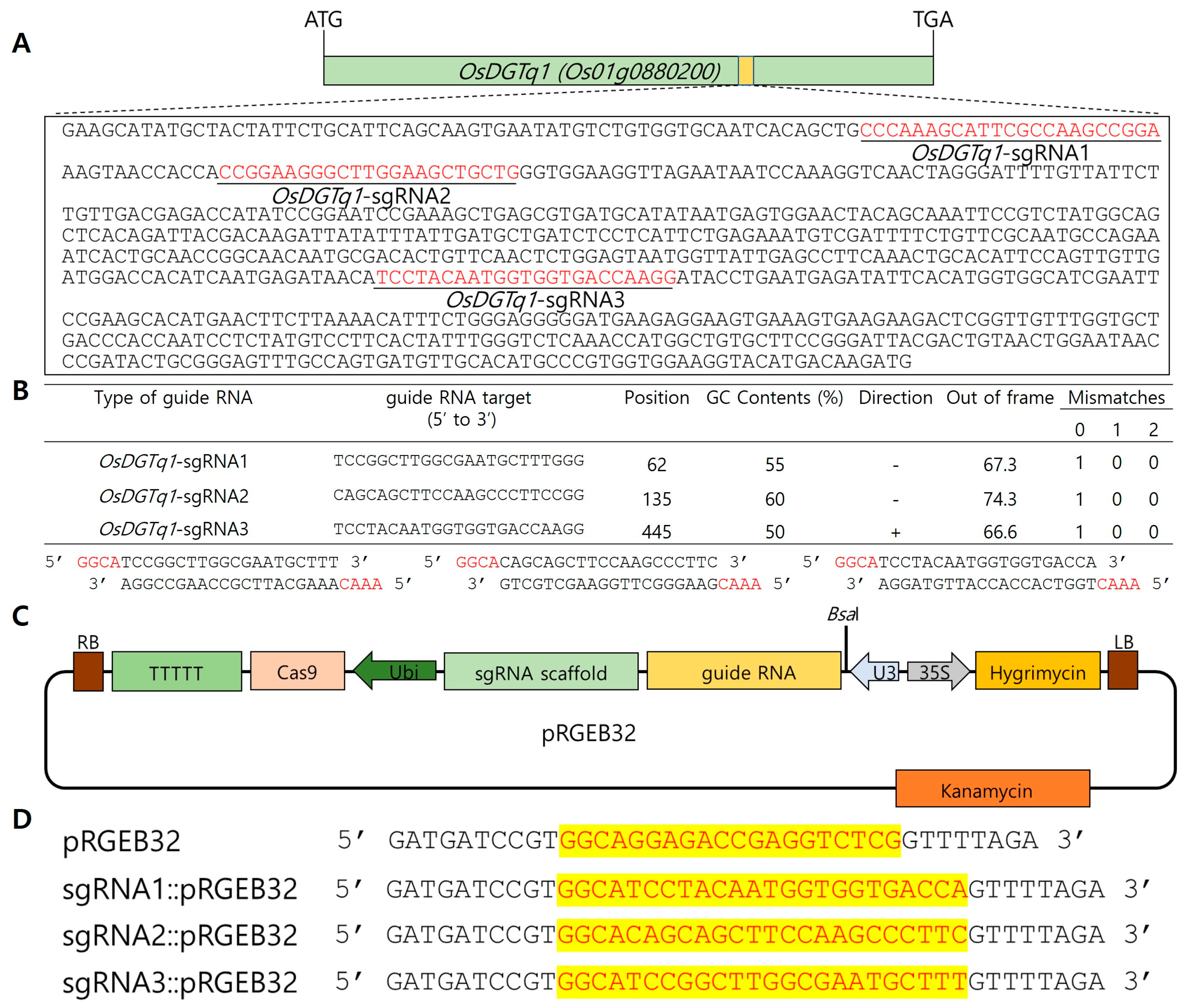
2.2.1. SgRNA Design and Development of OsDGTq1 Genome-Edited Rice Using CRISPR/Cas9
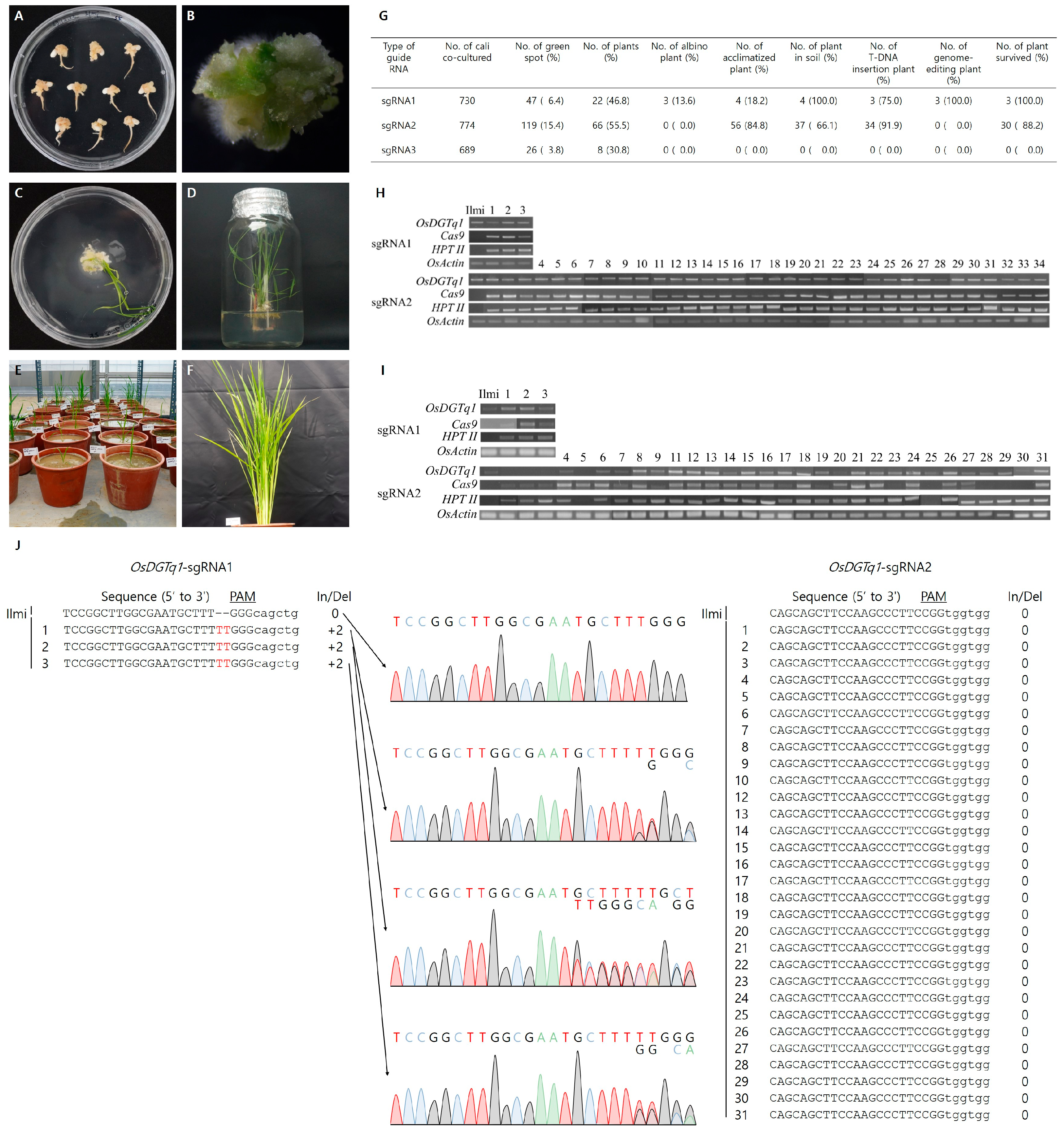
2.2.2. Genotype Analysis and Gene Expression Analysis of OsDGTq1 Regenerated Plants
2.2.3. Nomenclature of G0 Transgenic Lines and Evaluation of Agronomic Traits
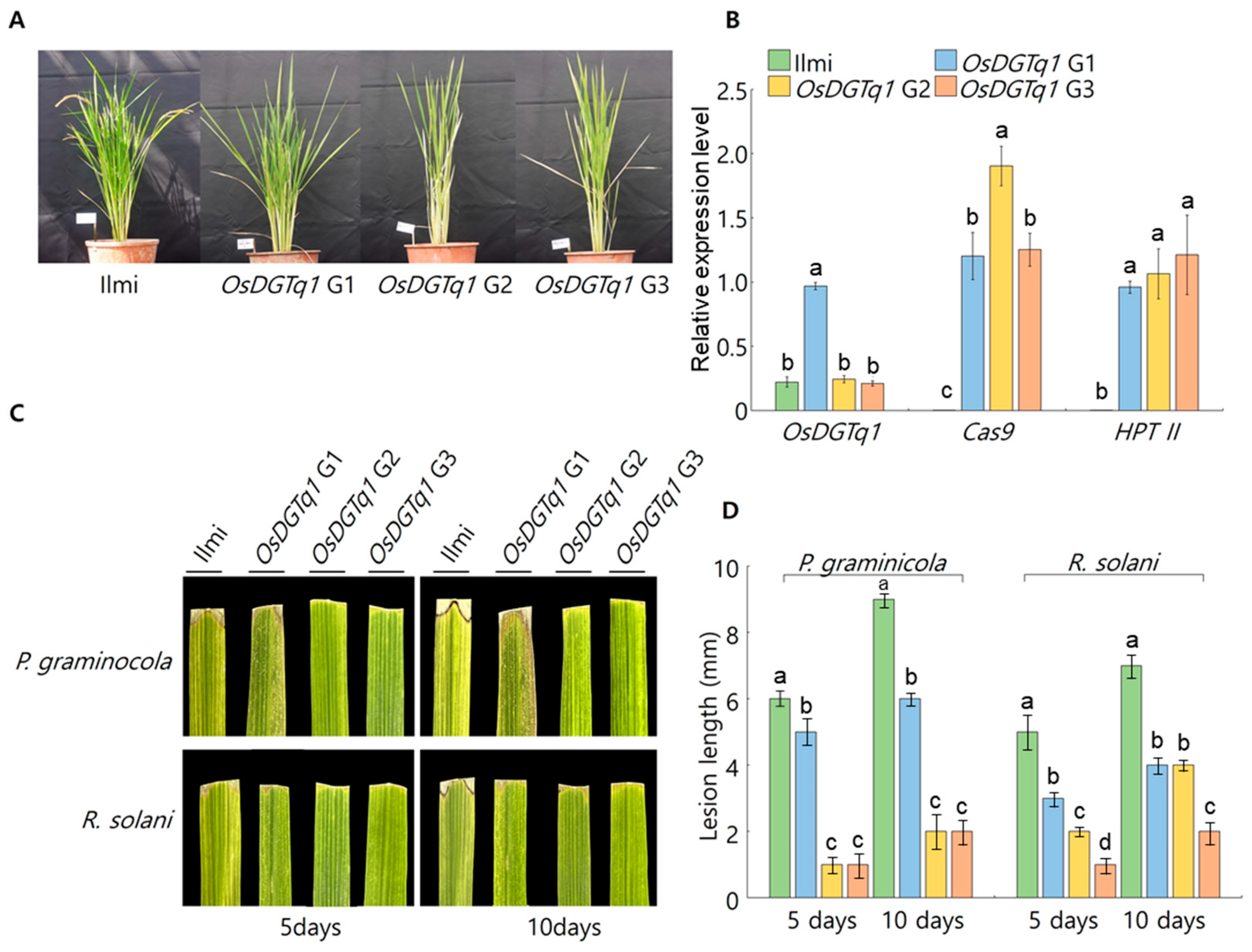
2.2.4. Analysis of Target Gene Expression Levels in Genome-Edited Lines
2.2.5. Predicted Structural Consequences of Frameshift Mutation in OsDGTq1
2.2.6. Investigation of G0 Genome-Edited Lines Leaf Response to Damping-Off Pathogen Strains
3. Discussion
4. Materials and Methods
4.1. In Planta Functional Analysis of Genes
4.1.1. Isolation of P. graminocola and R. solani
4.1.2. Inoculation with Damping-Off Pathogens in the CNDH Population at the Seedling Stage
4.2. CRISPR/Cas9-Mediated Genome Editing
4.2.1. Plant Material
4.2.2. Design of Single Guide RNA for Genome Editing
4.2.3. Genome Editing in Rice Using CRISPR/Cas9 Vector Construction
4.2.4. Agrobacterium-Mediated Rice Transformation and Regeneration
4.3. Molecular Analysis of Transgenic Plants
4.3.1. DNA Extraction
4.3.2. RNA Extraction
4.3.3. cDNA Synthesis
4.3.4. Genotype Analysis of Regenerated Plants
4.3.5. Analysis of Gene Expression in Regenerated Plants
4.3.6. Investigation of Agricultural Traits in Transgenic Lines
4.3.7. Analysis of Gene Expression Levels in the Genome-Edited Rice
4.3.8. Inoculation with Damping-Off Pathogens in Leaves of Genome-Edited Plants
4.3.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- My, N.H.; Demont, M.; Verbeke, W. Inclusiveness of consumer access to food safety: Evidence from certified rice in Vietnam. Glob. Food Secur. 2021, 28, 100491. [Google Scholar] [CrossRef]
- Guo, Y.; Fu, Y.; Hao, F.; Zhang, X.; Wu, W.; Jin, X.; Bryant, C.R.; Senthilnath, J. Integrated phenology and climate in rice yields prediction using machine learning methods. Ecol. Indic. 2021, 120, 106935. [Google Scholar] [CrossRef]
- Bennett, A.J.; Bending, G.D.; Chandler, D.; Hilton, S.; Mills, P. Meeting the demand for crop production: The challenge of yield decline in crops grown in short rotations. Biol. Rev. 2012, 87, 52–71. [Google Scholar] [CrossRef]
- Van Buyten, E.; Banaay, C.; Vera Cruz, C.; Höfte, M. Identity and variability of Pythium species associated with yield decline in aerobic rice cultivation in the Philippines. Plant Pathol. 2013, 62, 139–153. [Google Scholar] [CrossRef]
- Wright, E. Influence of temperature and moisture on damping-off of American and Siberian elm, black locust, and desertwillow. Trans. Br. Mycol. Soc. 1957, 41, 483–490. [Google Scholar]
- Lamichhane, J.R.; Dachbrodt-Saaydeh, S.; Kudsk, P.; Messéan, A. Toward a reduced reliance on conventional pesticides in European agriculture. Plant Dis. 2016, 100, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Menzies, J. Survival of microbial plant pathogens in soil. Bot. Rev. 1963, 29, 79–122. [Google Scholar] [CrossRef]
- Mouttet, R.; Escobar-Gutiérrez, A.; Esquibet, M.; Gentzbittel, L.; Mugniéry, D.; Reignault, P.; Sarniguet, C.; Castagnone-Sereno, P. Banning of methyl bromide for seed treatment: Could Ditylenchus dipsaci again become a major threat to alfalfa production in Europe? Pest Manag. Sci. 2014, 70, 1017–1022. [Google Scholar] [CrossRef]
- Miller, S.A.; Ferreira, J.P.; LeJeune, J.T. Antimicrobial use and resistance in plant agriculture: A one health perspective. Agriculture 2022, 12, 289. [Google Scholar] [CrossRef]
- Mores, A.; Borrelli, G.M.; Laidò, G.; Petruzzino, G.; Pecchioni, N.; Amoroso, L.G.M.; Desiderio, F.; Mazzucotelli, E.; Mastrangelo, A.M.; Marone, D. Genomic approaches to identify molecular bases of crop resistance to diseases and to develop future breeding strategies. Int. J. Mol. Sci. 2021, 22, 5423. [Google Scholar] [CrossRef]
- Rajput, M.; Choudhary, K.; Kumar, M.; Vivekanand, V.; Chawade, A.; Ortiz, R.; Pareek, N. RNA interference and CRISPR/Cas gene editing for crop improvement: Paradigm shift towards sustainable agriculture. Plants 2021, 10, 1914. [Google Scholar] [CrossRef] [PubMed]
- Newsom, S.; Parameshwaran, H.P.; Martin, L.; Rajan, R. The CRISPR-Cas mechanism for adaptive immunity and alternate bacterial functions fuels diverse biotechnologies. Front. Cell. Infect. Microbiol. 2021, 10, 619763. [Google Scholar] [CrossRef] [PubMed]
- Morshedzadeh, F.; Ghanei, M.; Lotfi, M.; Ghasemi, M.; Ahmadi, M.; Najari-Hanjani, P.; Sharif, S.; Mozaffari-Jovin, S.; Peymani, M.; Abbaszadegan, M.R. An update on the application of CRISPR technology in clinical practice. Mol. Biotechnol. 2024, 66, 179–197. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-R.; Kim, E.-G.; Jang, Y.-H.; Yun, B.-J.; Kim, K.-M. Selection strategy for damping-off resistance gene by biotechnology in rice plant. Plant Soil 2022, 474, 277–296, Correction in Plant Soil 2022, 474, 297–298. [Google Scholar] [CrossRef]
- Bandumula, N. Rice production in Asia: Key to global food security. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2018, 88, 1323–1328. [Google Scholar] [CrossRef]
- Kaushik, B.; Singh, K.; Tiwari, D.K.; Singh, U.K. Impact of climate change on crop yield due to pests and crop diseases: Future projections. Microsc. Microanal. 2023, 29, 56–58. [Google Scholar] [CrossRef]
- Al-Abdalall, A.H.A. Assessment of yield loss caused by root rots in wheat and barley. J. Food Agric. Environ. 2010, 8, 638–641. [Google Scholar]
- Bourne, R.R.; Flaxman, S.R.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.H.; Leasher, J.; Limburg, H. Magnitude, temporal trends, and projections of the global prevalence of blindness and distance and near vision impairment: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e888–e897. [Google Scholar] [CrossRef]
- Schneider, P.; Asch, F. Rice production and food security in Asian Mega deltas—A review on characteristics, vulnerabilities and agricultural adaptation options to cope with climate change. J. Agron. Crop Sci. 2020, 206, 491–503. [Google Scholar] [CrossRef]
- Mc Carthy, U.; Uysal, I.; Badia-Melis, R.; Mercier, S.; O’Donnell, C.; Ktenioudaki, A. Global food security–Issues, challenges and technological solutions. Trends Food Sci. Technol. 2018, 77, 11–20, Correction in Trends Food Sci. Technol. 2022, 123, 404. [Google Scholar] [CrossRef]
- He, Z.; Zhang, Z.; Valè, G.; San Segundo, B.; Chen, X.; Pasupuleti, J. Disease and pest resistance in rice. Front. Plant Sci. 2023, 14, 1333904. [Google Scholar] [CrossRef]
- Pino-Otín, M.R.; Ballestero, D.; Navarro, E.; Mainar, A.M.; Val, J. Effects of the insecticide fipronil in freshwater model organisms and microbial and periphyton communities. Sci. Total Environ. 2021, 764, 142820. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Poopal, R.-K.; Ramesh, M. Synthetic organic chemicals (flame retardants and pesticides) with neurotoxic potential induced behavioral impairment on zebrafish (Danio rerio): A non-invasive approach for neurotoxicology. Environ. Sci. Pollut. Res. 2021, 28, 37534–37546. [Google Scholar] [CrossRef] [PubMed]
- Podevin, N.; Davies, H.V.; Hartung, F.; Nogué, F.; Casacuberta, J.M. Site-directed nucleases: A paradigm shift in predictable, knowledge-based plant breeding. Trends Biotechnol. 2013, 31, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Vacheron, J.; Desbrosses, G.; Bouffaud, M.-L.; Touraine, B.; Moënne-Loccoz, Y.; Muller, D.; Legendre, L.; Wisniewski-Dyé, F.; Prigent-Combaret, C. Plant growth-promoting rhizobacteria and root system functioning. Front. Plant Sci. 2013, 4, 356. [Google Scholar] [CrossRef]
- Park, J.-R.; Kim, E.-G.; Jang, Y.-H.; Jan, R.; Farooq, M.; Ubaidillah, M.; Kim, K.-M. Applications of CRISPR/Cas9 as new strategies for short breeding to drought gene in rice. Front. Plant Sci. 2022, 13, 850441. [Google Scholar] [CrossRef]
- Wada, N.; Ueta, R.; Osakabe, Y.; Osakabe, K. Precision genome editing in plants: State-of-the-art in CRISPR/Cas9-based genome engineering. BMC Plant Biol. 2020, 20, 234. [Google Scholar] [CrossRef]
- Oliver, D.; Yuan, S.; McSwiggin, H.; Yan, W. Pervasive genotypic mosaicism in founder mice derived from genome editing through pronuclear injection. PLoS ONE 2015, 10, e0129457. [Google Scholar] [CrossRef]
- Fernandez, A.; O’Leary, C.; O’Byrne, K.J.; Burgess, J.; Richard, D.J.; Suraweera, A. Epigenetic mechanisms in DNA double strand break repair: A clinical review. Front. Mol. Biosci. 2021, 8, 685440. [Google Scholar] [CrossRef]
- Alok, A.; Chauhan, H.; Upadhyay, S.K.; Pandey, A.; Kumar, J.; Singh, K. Compendium of plant-specific CRISPR vectors and their technical advantages. Life 2021, 11, 1021. [Google Scholar] [CrossRef]
- Lee, K.; Eggenberger, A.L.; Banakar, R.; McCaw, M.E.; Zhu, H.; Main, M.; Kang, M.; Gelvin, S.B.; Wang, K. CRISPR/Cas9-mediated targeted T-DNA integration in rice. Plant Mol. Biol. 2019, 99, 317–328. [Google Scholar] [CrossRef]
- Penagos-Puig, A.; Furlan-Magaril, M. Heterochromatin as an important driver of genome organization. Front. Cell Dev. Biol. 2020, 8, 579137. [Google Scholar] [CrossRef] [PubMed]
- Jupe, F.; Rivkin, A.C.; Michael, T.P.; Zander, M.; Motley, S.T.; Sandoval, J.P.; Slotkin, R.K.; Chen, H.; Castanon, R.; Nery, J.R. The complex architecture and epigenomic impact of plant T-DNA insertions. PLoS Genet. 2019, 15, e1007819. [Google Scholar] [CrossRef]
- Phillips, R.L.; Kaeppler, S.M.; Olhoft, P. Genetic instability of plant tissue cultures: Breakdown of normal controls. Proc. Natl. Acad. Sci. USA 1994, 91, 5222–5226. [Google Scholar] [CrossRef]
- Lee, S.-H.; Shon, Y.G.; Kim, C.Y.; Chun, H.J.; Cheong, Y.H.; Kim, Z.H.; Choe, Z.R.; Choi, Y.J.; Cho, M.J. Variations in the morphology of rice plants regenerated from protoplasts using different culture procedures. Plant Cell Tissue Organ Cult. 1999, 57, 179–187. [Google Scholar] [CrossRef]
- Rongbai, L.; Pandey, M.; Pandey, S.; Dwivedi, D. Agro-morphological characterization of ovary culture-derived plants of rice (Oryza sativa L.). Euphytica 1999, 106, 197–203. [Google Scholar] [CrossRef]
- Carsono, N.; Yoshida, T. Variation in spikelet-related traits of rice plants regenerated from mature seed-derived callus culture. Plant Prod. Sci. 2007, 10, 86–90. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, S.-K.; Park, J.-R.; Kim, E.-G.; Kim, K.-M. Development of Resistance to Damping-Off in Rice, Oryza sativa L., Using CRISPR/Cas9. Int. J. Mol. Sci. 2025, 26, 9761. https://doi.org/10.3390/ijms26199761
Jeong S-K, Park J-R, Kim E-G, Kim K-M. Development of Resistance to Damping-Off in Rice, Oryza sativa L., Using CRISPR/Cas9. International Journal of Molecular Sciences. 2025; 26(19):9761. https://doi.org/10.3390/ijms26199761
Chicago/Turabian StyleJeong, Seung-Kyo, Jae-Ryoung Park, Eun-Gyeong Kim, and Kyung-Min Kim. 2025. "Development of Resistance to Damping-Off in Rice, Oryza sativa L., Using CRISPR/Cas9" International Journal of Molecular Sciences 26, no. 19: 9761. https://doi.org/10.3390/ijms26199761
APA StyleJeong, S.-K., Park, J.-R., Kim, E.-G., & Kim, K.-M. (2025). Development of Resistance to Damping-Off in Rice, Oryza sativa L., Using CRISPR/Cas9. International Journal of Molecular Sciences, 26(19), 9761. https://doi.org/10.3390/ijms26199761






