Challenges in Polyglutamine Diseases: From Dysfunctional Neuronal Circuitries to Neuron-Specific CAG Repeat Instability
Abstract
1. Nine Human-Specific Diseases Need Better-Defined Primary Pathogenicity
1.1. The Polyglutamine Disease Family and the Mutated Genes
1.2. Dysfunction and Degeneration of the Motor Coordination Network Stations
1.3. Expression and Dysfunction of the polyQ-Related Genes and Proteins
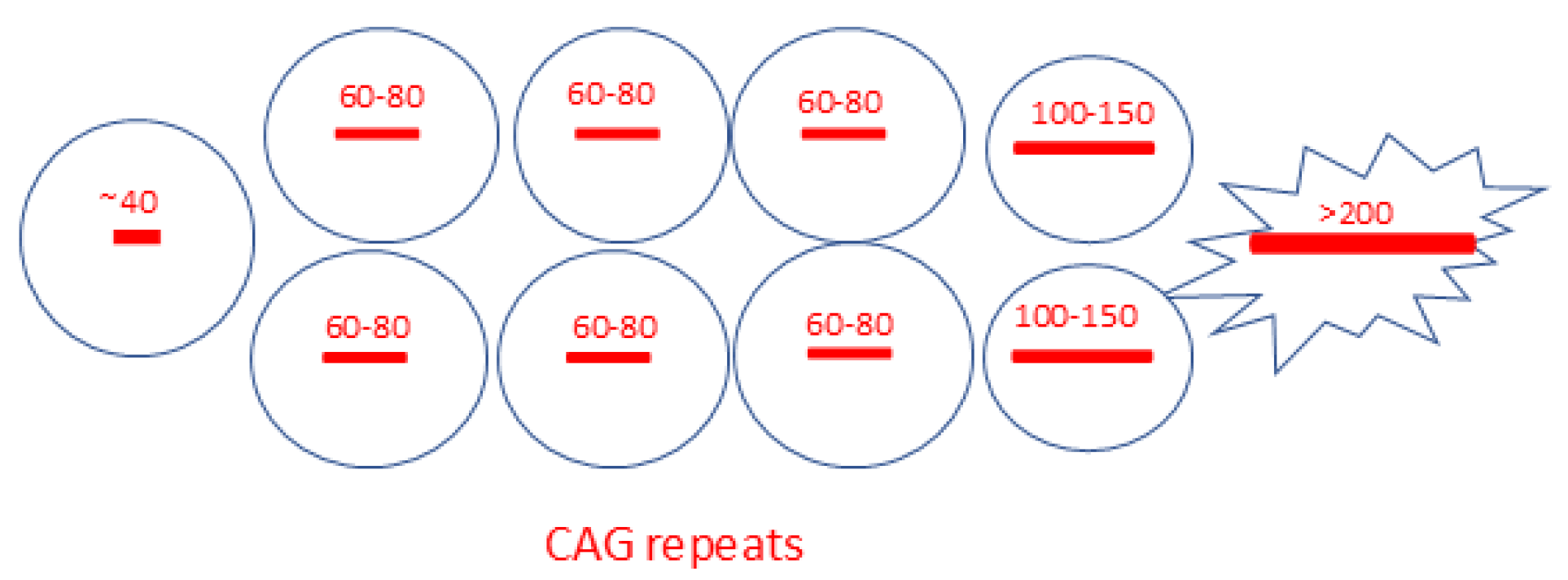
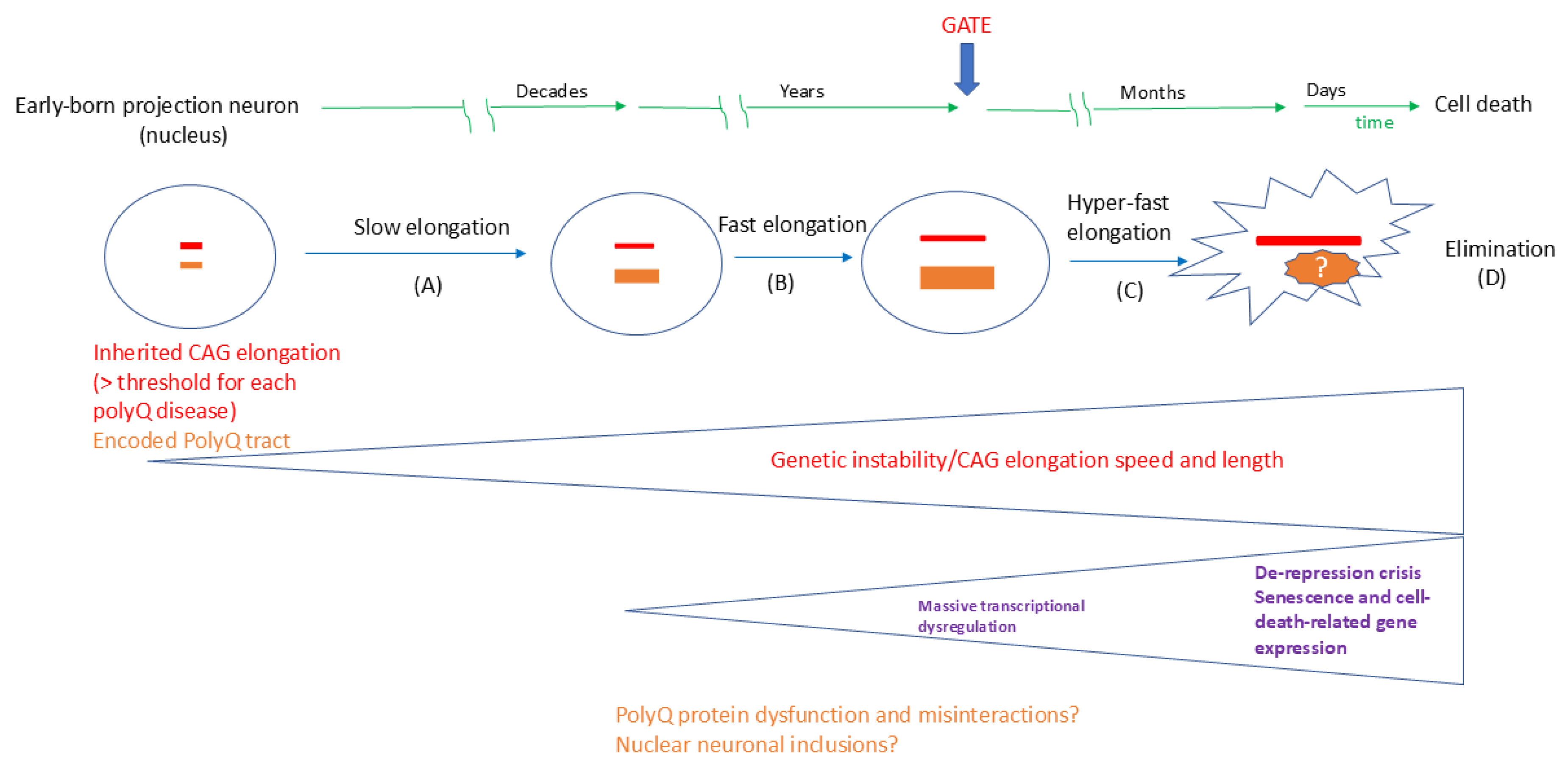
1.4. The Hyper-Expansion of the Repeat Elongation Is the Novel Link for the Neuronal-Specific Pathogenesis
2. Approaches for Linking Neuronal-Type-Specific Vulnerability and Genetic Instability
2.1. The Neuronal Complexity, Excitability, and Vulnerability to Repeat Elongation in the Basal Ganglia Circuits
2.2. The Neuronal Complexity, Excitability, and Vulnerability to Repeat Elongation in the Cerebellar Circuits
2.3. DNA Repair and Somatic Instability in the Neurons of the Motor Coordination Network
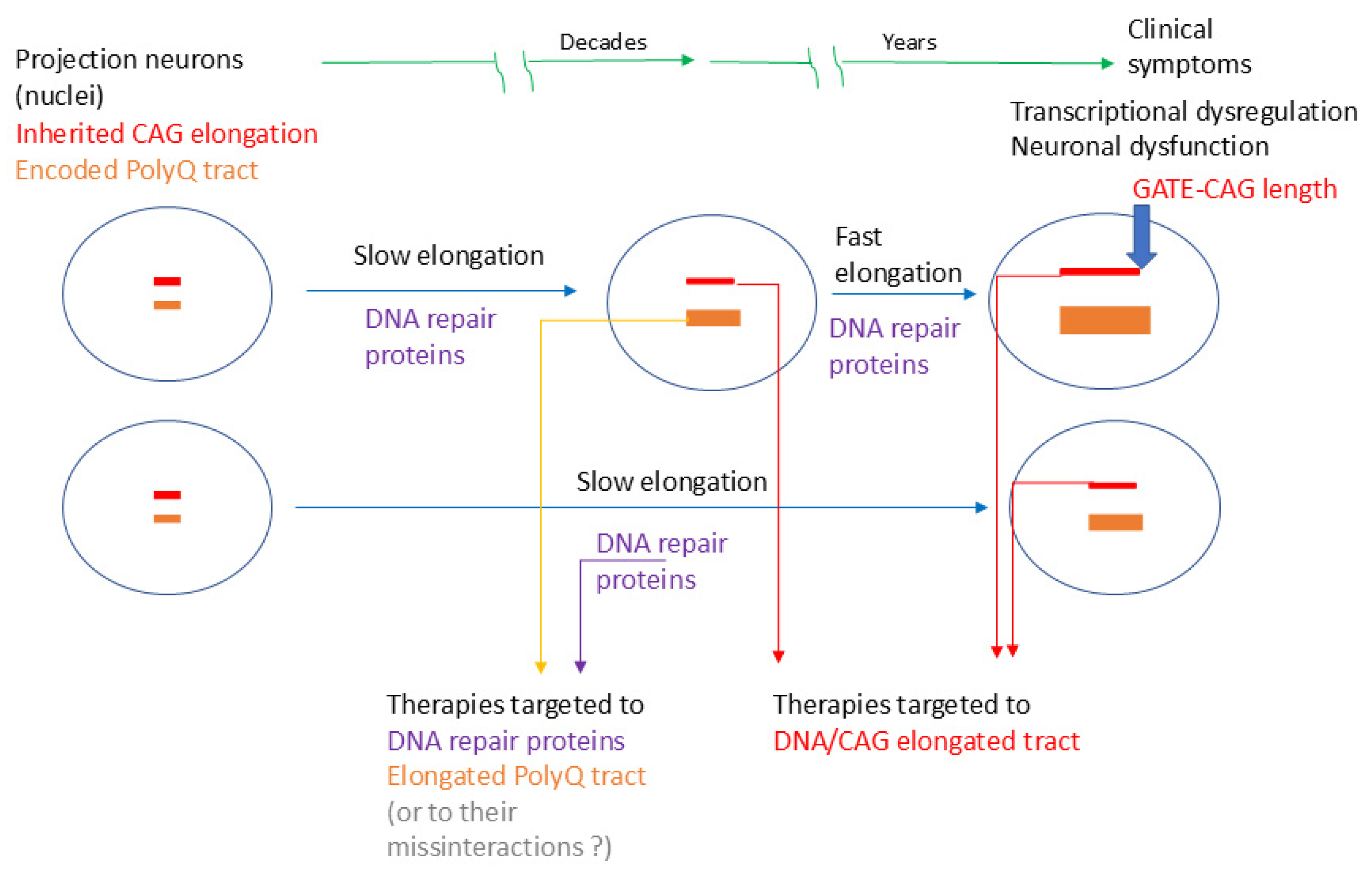
2.4. Approaches to Therapeutic Targets Based on the ELongATE Model
3. Conclusions and Outlook
Funding
Acknowledgments
Conflicts of Interest
References
- La Spada, A.R.; Taylor, J.P. Repeat expansion disease: Progress and puzzles in disease pathogenesis. Nat. Rev. Genet. 2010, 11, 247–258. [Google Scholar] [CrossRef] [PubMed]
- La Spada, A.R.; Taylor, J.P. Polyglutamines placed into context. Neuron 2003, 38, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, A.P.; Shakkottai, V.G.; Albin, R.L. Polyglutamine Repeats in Neurodegenerative Diseases. Annu. Rev. Pathol. 2019, 14, 1–27. [Google Scholar] [CrossRef]
- Stoyas, C.A.; La Spada, A.R. The CAG-polyglutamine repeat diseases: A clinical, molecular, genetic, and pathophysiologic nosology. Handb. Clin. Neurol. 2018, 147, 143–170. [Google Scholar]
- Paulson, H.L.; Shakkottai, V.G.; Clark, H.B.; Orr, H.T. Polyglutamine spinocerebellar ataxias—From genes to potential treatments. Nat. Rev. Neurosci. 2017, 18, 613–626. [Google Scholar] [CrossRef]
- Klockgether, T.; Mariotti, C.; Paulson, H.L. Spinocerebellar ataxia. Nat. Rev. Dis. Primers. 2019, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Rüb, U.; Schöls, L.; Paulson, H.; Auburger, G.; Kermer, P.; Jen, J.C.; Seidel, K.; Korf, H.W.; Deller, T. Clinical features, neurogenetics, and neuropathology of the polyglutamine spinocerebellar ataxias type 1, 2, 3, 6, and 7. Prog. Neurobiol. 2013, 104, 38–66. [Google Scholar] [CrossRef]
- Gardiner, S.L.; Boogaard, M.W.; Trompet, S.; de Mutsert, R.; Rosendaal, F.R.; Gussekloo, J.; Jukema, J.W.; Roos, R.A.C.; Aziz, N.A. Prevalence of Carriers of Intermediate and Pathological Polyglutamine Disease-Associated Alleles Among Large Population-Based Cohorts. JAMA Neurol. 2019, 76, 650–656. [Google Scholar] [CrossRef]
- Johnson, S.L.; Tsou, W.L.; Prifti, M.V.; Harris, A.L.; Todi, S.V. A survey of protein interactions and posttranslational modifications that influence the polyglutamine diseases. Front. Mol. Neurosci. 2022, 15, 974167. [Google Scholar] [CrossRef]
- McLoughlin, H.S.; Moore, L.R.; Paulson, H.L. Pathogenesis of SCA3 and implications for other polyglutamine diseases. Neurobiol. Dis. 2020, 134, 104635. [Google Scholar] [CrossRef]
- Rüb, U.; Hoche, F.; Brunt, E.R.; Heinsen, H.; Seidel, K.; Del Turco, D.; Paulson, H.L.; Bohl, J.; von Gall, C.; Vonsattel, J.P.; et al. Degeneration of the cerebellum in Huntington’s disease (HD): Possible relevance for the clinical picture and potential gateway to pathological mechanisms of the disease process. Brain Pathol. 2013, 23, 165–177. [Google Scholar] [CrossRef]
- Buijsen, R.A.M.; Toonen, L.J.A.; Gardiner, S.L.; van Roon-Mom, W.M.C. Genetics, Mechanisms, and Therapeutic Progress in Polyglutamine Spinocerebellar Ataxias. Neurotherapeutics 2019, 16, 263–286. [Google Scholar] [CrossRef]
- Cubo, E.; Martinez-Horta, S.I.; Santalo, F.S.; Descalls, A.M.; Calvo, S.; Gil-Polo, C.; Muñoz, I.; Llano, K.; Mariscal, N.; Diaz, D.; et al. Clinical manifestations of homozygote allele carriers in Huntington’s disease. Neurology 2019, 92, e2101–e2108. [Google Scholar] [CrossRef]
- Fusilli, C.; Migliore, S.; Mazza, T.; Consoli, F.; De Luca, A.; Barbagallo, G.; Ciammola, A.; Gatto, E.M.; Cesarini, M.; Etcheverry, J.L.; et al. Biological and clinical manifestations of juvenile Huntington’s disease: A retrospective analysis. Lancet Neurol. 2018, 17, 986–993. [Google Scholar] [CrossRef]
- Babovic-Vuksanovic, D.; Snow, K.; Patterson, M.C.; Michels, V.V. Spinocerebellar ataxia type 2 (SCA 2) in an infant with extreme CAG repeat expansion. Am. J. Med. Genet. 1998, 79, 383–387. [Google Scholar] [CrossRef]
- Paciorkowski, A.R.; Shafrir, Y.; Hrivnak, J.; Patterson, M.C.; Tennison, M.B.; Clark, H.B.; Gomez, C.M. Massive expansion of SCA2 with autonomic dysfunction, retinitis pigmentosa, and infantile spasms. Neurology 2011, 77, 1055–1060. [Google Scholar] [CrossRef]
- Donis, K.C.; Saute, J.A.; Krum-Santos, A.C.; Furtado, G.V.; Mattos, E.P.; Saraiva-Pereira, M.L.; Torman, V.L.; Jardim, L.B. Spinocerebellar ataxia type 3/Machado-Joseph disease starting before adolescence. Neurogenetics 2016, 17, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, H.; Xia, Y.; Jiang, H.; Shen, L.; Wang, S.; Shen, R.; Huang, L.; Wang, J.; Xu, Q.; et al. A neuropathological study at autopsy of early onset spinocerebellar ataxia 6. J. Clin. Neurosci. 2010, 17, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Donis, K.C.; Mattos, E.P.; Silva, A.A.; Furtado, G.V.; Saraiva-Pereira, M.L.; Jardim, L.B.; Saute, J.A. Infantile spinocerebellar ataxia type 7: Case report and a review of the literature. J. Neurol. Sci. 2015, 354, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Grunseich, C.; Kats, I.R.; Bott, L.C.; Rinaldi, C.; Kokkinis, A.; Fox, D.; Chen, K.L.; Schindler, A.B.; Mankodi, A.K.; Shrader, J.A.; et al. Early onset and novel features in a spinal and bulbar muscular atrophy patient with a 68 CAG repeat. Neuromuscul. Disord. 2014, 24, 978–981. [Google Scholar] [CrossRef]
- Bostan, A.C.; Strick, P.L. The basal ganglia and the cerebellum: Nodes in an integrated network. Nat. Rev. Neurosci. 2018, 19, 338–350. [Google Scholar] [CrossRef]
- Groenewegen, H.J. The basal ganglia and motor control. Neural Plast. 2003, 10, 107–120. [Google Scholar] [CrossRef]
- Gerfen, C.R.; Surmeier, D.J. Modulation of striatal projection systems by dopamine. Annu. Rev. Neurosci. 2011, 34, 441–466. [Google Scholar] [CrossRef]
- Stacho, M.; Häusler, A.N.; Brandstetter, A.; Iannilli, F.; Mohlberg, H.; Schiffer, C.; Smaers, J.B.; Amunts, K. Phylogenetic reduction of the magnocellular red nucleus in primates and intersubject variability in humans. Front. Neuroanat. 2024, 18, 1331305. [Google Scholar] [CrossRef]
- Plotkin, J.L.; Goldberg, J.A. Thinking Outside the Box (and Arrow): Current Themes in Striatal Dysfunction in Movement Disorders. Neuroscientist 2019, 25, 359–379. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.A.; Wallén-Mackenzie, Å. Architecture of the subthalamic nucleus. Commun. Biol. 2024, 7, 78. [Google Scholar] [CrossRef] [PubMed]
- De Zeeuw, C.I.; Simpson, J.I.; Hoogenraad, C.C.; Galjart, N.; Koekkoek, S.K.; Ruigrok, T.J. Microcircuitry and function of the inferior olive. Trends Neurosci. 1998, 21, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Landwehrmeyer, G.B.; McNeil, S.M.; Dure, L.S.; Ge, P.; Aizawa, H.; Huang, Q.; Ambrose, C.M.; Duyao, M.P.; Bird, E.D.; Bonilla, E.; et al. Huntington’s disease gene: Regional and cellular expression in the brain of normal and affected individuals. Ann. Neurol. 1995, 37, 218–230. [Google Scholar] [CrossRef]
- Latimer, C.S.; Flanagan, M.E.; Cimino, P.J.; Jayadev, S.; Davis, M.; Hoffer, Z.S.; Montine, T.J.; Gonzalez-Cuyar, L.F.; Bird, T.D.; Keene, C.D. Neuropathological Comparison of Adult Onset and Juvenile Huntington’s Disease with Cerebellar Atrophy: A Report of a Father and Son. J. Huntingtons Dis. 2017, 6, 337–348. [Google Scholar] [CrossRef]
- Hedjoudje, A.; Nicolas, G.; Goldenberg, A.; Vanhulle, C.; Dumant-Forrest, C.; Deverrière, G.; Treguier, P.; Michelet, I.; Guyant-Maréchal, L.; Devys, D.; et al. Morphological features in juvenile Huntington disease associated with cerebellar atrophy—Magnetic resonance imaging morphometric analysis. Pediatr. Radiol. 2018, 48, 1463–1471. [Google Scholar] [CrossRef]
- Pressl, C.; Mätlik, K.; Kus, L.; Darnell, P.; Luo, J.D.; Paul, M.R.; Weiss, A.R.; Liguore, W.; Carroll, T.S.; Davis, D.A.; et al. Selective vulnerability of layer 5a corticostriatal neurons in Huntington’s disease. Neuron 2024, 112, 924–941.e10. [Google Scholar] [CrossRef]
- Singh-Bains, M.K.; Mehrabi, N.F.; Sehji, T.; Austria, M.D.R.; Tan, A.Y.S.; Tippett, L.J.; Dragunow, M.; Waldvogel, H.J.; Faull, R.L.M. Cerebellar degeneration correlates with motor symptoms in Huntington disease. Ann. Neurol. 2019, 85, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Schöls, L.; Reimold, M.; Seidel, K.; Globas, C.; Brockmann, K.; Hauser, T.K.; Auburger, G.; Bürk, K.; den Dunnen, W.; Reischl, G.; et al. No parkinsonism in SCA2 and SCA3 despite severe neurodegeneration of the dopaminergic substantia nigra. Brain 2015, 138, 3316–3326. [Google Scholar] [CrossRef]
- Ishikawa, K.; Tanaka, H.; Saito, M.; Ohkoshi, N.; Fujita, T.; Yoshizawa, K.; Ikeuchi, T.; Watanabe, M.; Hayashi, A.; Takiyama, Y.; et al. Japanese families with autosomal dominant pure cerebellar ataxia map to chromosome 19p13.1-p13.2 and are strongly associated with mild CAG expansions in the spinocerebellar ataxia type 6 gene in chromosome 19p13.1. Am. J. Hum. Genet. 1997, 61, 336–346. [Google Scholar] [CrossRef]
- Ansorge, O.; Giunti, P.; Michalik, A.; Van Broeckhoven, C.; Harding, B.; Wood, N.; Scaravilli, F. Ataxin-7 aggregation and ubiquitination in infantile SCA7 with 180 CAG repeats. Ann. Neurol. 2004, 56, 448–452. [Google Scholar] [CrossRef]
- Horton, L.C.; Frosch, M.P.; Vangel, M.G.; Weigel-DiFranco, C.; Berson, E.L.; Schmahmann, J.D. Spinocerebellar ataxia type 7: Clinical course, phenotype-genotype correlations, and neuropathology. Cerebellum 2013, 12, 176–193. [Google Scholar] [CrossRef]
- Johansson, J.; Forsgren, L.; Sandgren, O.; Brice, A.; Holmgren, G.; Holmberg, M. Expanded CAG repeats in Swedish spinocerebellar ataxia type 7 (SCA7) patients: Effect of CAG repeat length on the clinical manifestation. Hum. Mol. Genet. 1998, 7, 171–176. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Toyoshima, Y.; Takahashi, H. Spinocerebellar Ataxia Type 17 (SCA17). Adv. Exp. Med. Biol. 2018, 1049, 219–231. [Google Scholar] [PubMed][Green Version]
- Nowak, B.; Kozlowska, E.; Pawlik, W.; Fiszer, A. Atrophin-1 Function and Dysfunction in Dentatorubral-Pallidoluysian Atrophy. Mov. Disord. 2023, 38, 526–536. [Google Scholar] [CrossRef]
- Breza, M.; Koutsis, G. Kennedy’s disease (spinal and bulbar muscular atrophy): A clinically oriented review of a rare disease. J. Neurol. 2019, 266, 565–573. [Google Scholar] [CrossRef]
- Echaniz-Laguna, A.; Rousso, E.; Anheim, M.; Cossée, M.; Tranchant, C. A family with early-onset and rapidly progressive X-linked spinal and bulbar muscular atrophy. Neurology 2005, 64, 1458–1460. [Google Scholar] [CrossRef]
- Lee, J.; Kim, M.; Itoh, T.Q.; Lim, C. Ataxin-2: A versatile posttranscriptional regulator and its implication in neural function. Wiley Interdiscip. Rev. RNA 2018, 9, e1488. [Google Scholar] [CrossRef]
- Saudou, F.; Humbert, S. The Biology of Huntingtin. Neuron 2016, 89, 910–926. [Google Scholar] [CrossRef]
- Handsaker, R.E.; Kashin, S.; Reed, N.M.; Tan, S.; Lee, W.S.; McDonald, T.M.; Morris, K.; Kamitaki, N.; Mullally, C.D.; Morakabati, N.R.; et al. Long somatic DNA-repeat expansion drives neurodegeneration in Huntington’s disease. Cell 2025, 188, 623–639.e19. [Google Scholar] [CrossRef] [PubMed]
- Bäuerlein, F.J.B.; Saha, I.; Mishra, A.; Kalemanov, M.; Martínez-Sánchez, A.; Klein, R.; Dudanova, I.; Hipp, M.S.; Hartl, F.U.; Baumeister, W.; et al. In Situ Architecture and Cellular Interactions of PolyQ Inclusions. Cell 2017, 171, 179–187.e10. [Google Scholar] [CrossRef]
- Paulson, H.L.; Perez, M.K.; Trottier, Y.; Trojanowski, J.Q.; Subramony, S.H.; Das, S.S.; Vig, P.; Mandel, J.L.; Fischbeck, K.H.; Pittman, R.N. Intranuclear inclusions of expanded polyglutamine protein in spinocerebellar ataxia type 3. Neuron 1997, 19, 333–344. [Google Scholar] [CrossRef]
- Figiel, M.; Szlachcic, W.J.; Switonski, P.M.; Gabka, A.; Krzyzosiak, W.J. Mouse models of polyglutamine diseases: Review and data table. Part I. Mol. Neurobiol. 2012, 46, 393–429. [Google Scholar] [CrossRef]
- Switonski, P.M.; Szlachcic, W.J.; Gabka, A.; Krzyzosiak, W.J.; Figiel, M. Mouse models of polyglutamine diseases in therapeutic approaches: Review and data table. Part II. Mol. Neurobiol. 2012, 46, 430–466. [Google Scholar] [CrossRef]
- Hasegawa, A.; Ikeuchi, T.; Koike, R.; Matsubara, N.; Tsuchiya, M.; Nozaki, H.; Homma, A.; Idezuka, J.; Nishizawa, M.; Onodera, O. Long-term disability and prognosis in dentatorubral-pallidoluysian atrophy: A correlation with CAG repeat length. Mov. Disord. 2010, 25, 1694–1700. [Google Scholar] [CrossRef] [PubMed]
- Lippi, A.; Krisko, A. Protein aggregation: A detrimental symptom or an adaptation mechanism? J. Neurochem. 2024, 168, 1426–1441. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, T.K.; Paul, S. Protein-misfolding diseases and chaperone-based therapeutic approaches. FEBS J. 2006, 273, 1331–1349. [Google Scholar] [CrossRef] [PubMed]
- Valastyan, J.S.; Lindquist, S. Mechanisms of protein-folding diseases at a glance. Dis. Model. Mech. 2014, 7, 9–14. [Google Scholar] [CrossRef]
- Berchowitz, L.E.; Kabachinski, G.; Walker, M.R.; Carlile, T.M.; Gilbert, W.V.; Schwartz, T.U.; Amon, A. Regulated Formation of an Amyloid-like Translational Repressor Governs Gametogenesis. Cell 2015, 163, 406–418. [Google Scholar] [CrossRef]
- Tabrizi, S.J.; Ghosh, R.; Leavitt, B.R. Huntingtin Lowering Strategies for Disease Modification in Huntington’s Disease. Neuron 2019, 101, 801–819. [Google Scholar] [CrossRef]
- Reilmann, R. Concern about Tominersen in Patients with Huntington’s Disease. N. Engl. J. Med. 2024, 390, 1058–1059. [Google Scholar]
- Azevedo, F.A.; Carvalho, L.R.; Grinberg, L.T.; Farfel, J.M.; Ferretti, R.E.; Leite, R.E.; Jacob Filho, W.; Lent, R.; Herculano-Houzel, S. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J. Comp. Neurol. 2009, 513, 532–541. [Google Scholar] [CrossRef]
- Lent, R.; Azevedo, F.A.; Andrade-Moraes, C.H.; Pinto, A.V. How many neurons do you have? Some dogmas of quantitative neuroscience under revision. Eur. J. Neurosci. 2012, 35, 1–9. [Google Scholar] [CrossRef]
- Tezenas du Montcel, S.; Durr, A.; Bauer, P.; Figueroa, K.P.; Ichikawa, Y.; Brussino, A.; Forlani, S.; Rakowicz, M.; Schöls, L.; Mariotti, C.; et al. Modulation of the age at onset in spinocerebellar ataxia by CAG tracts in various genes. Brain 2014, 137 Pt 9, 2444–2455. [Google Scholar] [CrossRef] [PubMed]
- Gusella, J.F.; Lee, J.M.; MacDonald, M.E. Huntington’s disease: Nearly four decades of human molecular genetics. Hum. Mol. Genet. 2021, 30, R254–R263. [Google Scholar] [CrossRef] [PubMed]
- Gusella, J.F. CAG Repeat Not Polyglutamine Length Determines Timing of Huntington’s Disease Onset. Cell 2019, 178, 887–900.e14. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.E.B.; Black, H.F.; Collins, J.A.; Gall-Duncan, T.; Caron, N.S.; Pearson, C.E.; Hayden, M.R. Interrupting sequence variants and age of onset in Huntington’s disease: Clinical implications and emerging therapies. Lancet Neurol. 2020, 19, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Siletti, K.; Hodge, R.; Mossi Albiach, A.; Lee, K.W.; Ding, S.L.; Hu, L.; Lönnerberg, P.; Bakken, T.; Casper, T.; Clark, M.; et al. Transcriptomic diversity of cell types across the adult human brain. Science 2023, 382, eadd7046. [Google Scholar] [CrossRef] [PubMed]
- Apsley, E.J.; Becker, E.B.E. Purkinje Cell Patterning-Insights from Single-Cell Sequencing. Cells 2022, 11, 2918. [Google Scholar] [CrossRef] [PubMed]
- Elam, J.S.; Glasser, M.F.; Harms, M.P.; Sotiropoulos, S.N.; Andersson, J.L.R.; Burgess, G.C.; Curtiss, S.W.; Oostenveld, R.; Larson-Prior, L.J.; Schoffelen, J.M.; et al. The Human Connectome Project: A retrospective. Neuroimage 2021, 244, 118543. [Google Scholar] [CrossRef]
- Gritton, H.J.; Howe, W.M.; Romano, M.F.; DiFeliceantonio, A.G.; Kramer, M.A.; Saligrama, V.; Bucklin, M.E.; Zemel, D.; Han, X. Unique contributions of parvalbumin and cholinergic interneurons in organizing striatal networks during movement. Nat. Neurosci. 2019, 22, 586–597. [Google Scholar] [CrossRef]
- Vogt Weisenhorn, D.M.; Giesert, F.; Wurst, W. Diversity matters—Heterogeneity of dopaminergic neurons in the ventral mesencephalon and its relation to Parkinson’s Disease. J. Neurochem. 2016, 139, 8–26. [Google Scholar] [CrossRef]
- Blumenstock, S.; Dudanova, I. Cortical and Striatal Circuits in Huntington’s Disease. Front. Neurosci. 2020, 14, 82. [Google Scholar] [CrossRef]
- Pedroarena, C.M.; Schwarz, C. Efficacy and short-term plasticity at GABAergic synapses between Purkinje and cerebellar nuclei neurons. J. Neurophysiol. 2003, 89, 704–715. [Google Scholar] [CrossRef]
- Person, A.L.; Raman, I.M. Synchrony and neural coding in cerebellar circuits. Front. Neural Circuits 2012, 11, 97. [Google Scholar] [CrossRef]
- Callahan, J.W.; Abercrombie, E.D. Relationship between subthalamic nucleus neuronal activity and electrocorticogram is altered in the R6/2 mouse model of Huntington’s disease. J. Physiol. 2015, 593, 3727–3738. [Google Scholar] [CrossRef]
- Callahan, J.W.; Abercrombie, E.D. Age-dependent alterations in the cortical entrainment of subthalamic nucleus neurons in the YAC128 mouse model of Huntington’s disease. Neurobiol. Dis. 2015, 78, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Du, Y.; Broussard, G.J.; Kislin, M.; Yuede, C.M.; Zhang, S.; Dietmann, S.; Gabel, H.; Zhao, G.; Wang, S.S.; et al. Transcriptomic mapping uncovers Purkinje neuron plasticity driving learning. Nature 2022, 605, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Kozareva, V.; Martin, C.; Osorno, T.; Rudolph, S.; Guo, C.; Vanderburg, C.; Nadaf, N.; Regev, A.; Regehr, W.G.; Macosko, E. A transcriptomic atlas of mouse cerebellar cortex comprehensively defines cell types. Nature 2021, 598, 214–219, Correction in Nature 2022, 602, E21. [Google Scholar] [CrossRef] [PubMed]
- Serra, H.G.; Byam, C.E.; Lande, J.D.; Tousey, S.K.; Zoghbi, H.Y.; Orr, H.T. Gene profiling links SCA1 pathophysiology to glutamate signaling in Purkinje cells of transgenic mice. Hum. Mol. Genet. 2004, 13, 2535–2543. [Google Scholar] [CrossRef]
- Knowles, R.; Dehorter, N.; Ellender, T. From Progenitors to Progeny: Shaping Striatal Circuit Development and Function. J. Neurosci. 2021, 41, 9483–9502. [Google Scholar] [CrossRef]
- Sepp, M.; Leiss, K.; Murat, F.; Okonechnikov, K.; Joshi, P.; Leushkin, E.; Spänig, L.; Mbengue, N.; Schneider, C.; Schmidt, J.; et al. Cellular development and evolution of the mammalian cerebellum. Nature 2024, 625, 788–796. [Google Scholar] [CrossRef]
- Flower, M.D.; Tabrizi, S.J. The breaking point where repeat expansion triggers neuronal collapse in Huntington’s disease. Cell Genom. 2025, 5, 100816. [Google Scholar] [CrossRef]
- Iyer, R.R.; Pluciennik, A. DNA Mismatch Repair and its Role in Huntington’s Disease. J. Huntingtons Dis. 2021, 10, 75–94. [Google Scholar] [CrossRef]
- Telenius, H.; Kremer, B.; Goldberg, Y.P.; Theilmann, J.; Andrew, S.E.; Zeisler, J.; Adam, S.; Greenberg, C.; Ives, E.J.; Clarke, L.A.; et al. Somatic and gonadal mosaicism of the Huntington disease gene CAG repeat in brain and sperm. Nat. Genet. 1994, 6, 409–414, Erratum in Nat. Genet. 1994, 7, 113. [Google Scholar] [CrossRef]
- Malik, I.; Kelley, C.P.; Wang, E.T.; Todd, P.K. Molecular mechanisms underlying nucleotide repeat expansion disorders. Nat. Rev. Mol. Cell Biol. 2021, 22, 589–607, Correction in Nat. Rev. Mol. Cell Biol. 2021, 22, 644. [Google Scholar] [CrossRef]
- Bunting, E.L.; Hamilton, J.; Tabrizi, S.J. Polyglutamine diseases. Curr. Opin. Neurobiol. 2022, 72, 39–47. [Google Scholar] [CrossRef]
- Kennedy, L.; Evans, E.; Chen, C.M.; Craven, L.; Detloff, P.J.; Ennis, M.; Shelbourne, P.F. Dramatic tissue-specific mutation length increases are an early molecular event in Huntington disease pathogenesis. Hum. Mol. Genet. 2003, 12, 3359–3367. [Google Scholar] [CrossRef]
- Mätlik, K.; Baffuto, M.; Kus, L.; Deshmukh, A.L.; Davis, D.A.; Paul, M.R.; Carroll, T.S.; Caron, M.C.; Masson, J.Y.; Pearson, C.E.; et al. Cell-type-specific CAG repeat expansions and toxicity of mutant Huntingtin in human striatum and cerebellum. Nat Genet. 2024, 56, 383–394. [Google Scholar] [CrossRef]
- Kim, G.D.; Lim, C.; Park, J. A practical handbook on single-cell RNA sequencing data quality control and downstream analysis. Mol. Cells 2024, 47, 100103. [Google Scholar] [CrossRef]
- Gao, R.; Matsuura, T.; Coolbaugh, M.; Zühlke, C.; Nakamura, K.; Rasmussen, A.; Siciliano, M.J.; Ashizawa, T.; Lin, X. Instability of expanded CAG/CAA repeats in spinocerebellar ataxia type 17. Eur. J. Hum. Genet. 2008, 16, 215–222. [Google Scholar] [CrossRef]
- Genetic Modifiers of Huntington’s Disease (GeM-HD) Consortium. Identification of Genetic Factors that Modify Clinical Onset of Huntington’s Disease. Cell 2015, 162, 516–526. [Google Scholar] [CrossRef]
- Malaiya, S.; Cortes-Gutierrez, M.; Herb, B.R.; Coffey, S.R.; Legg, S.R.W.; Cantle, J.P.; Colantuoni, C.; Carroll, J.B.; Ament, S.A. Single-Nucleus RNA-Seq Reveals Dysregulation of Striatal Cell Identity Due to Huntington’s Disease Mutations. J. Neurosci. 2021, 41, 5534–5552. [Google Scholar] [CrossRef] [PubMed]
- Herranz, N.; Gil, J. Mechanisms and functions of cellular senescence. J. Clin. Investig. 2018, 128, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- von Schimmelmann, M.; Feinberg, P.A.; Sullivan, J.M.; Ku, S.M.; Badimon, A.; Duff, M.K.; Wang, Z.; Lachmann, A.; Dewell, S.; Ma’ayan, A.; et al. Polycomb repressive complex 2 (PRC2) silences genes responsible for neurodegeneration. Nat. Neurosci. 2016, 19, 1321–1330. [Google Scholar] [CrossRef]
- Finneran, D.; Desjarlais, T.; Morgan, D.; Gordon, M.N. Differential effects of neuronal Cdkn2a overexpression in mouse brain. Alzheimer’s Dement. 2023, 19, e078485. [Google Scholar] [CrossRef]
- Southwell, A.L.; Smith-Dijak, A.; Kay, C.; Sepers, M.; Villanueva, E.B.; Parsons, M.P.; Xie, Y.; Anderson, L.; Felczak, B.; Waltl, S.; et al. An enhanced Q175 knock-in mouse model of Huntington disease with higher mutant huntingtin levels and accelerated disease phenotypes. Hum. Mol. Genet. 2016, 25, 3654–3675. [Google Scholar] [CrossRef] [PubMed]
- Phan, B.N.; Ray, M.H.; Xue, X.; Fu, C.; Fenster, R.J.; Kohut, S.J.; Bergman, J.; Haber, S.N.; McCullough, K.M.; Fish, M.K.; et al. Single nuclei transcriptomics in human and non-human primate striatum in opioid use disorder. Nat. Commun. 2024, 15, 878. [Google Scholar] [CrossRef] [PubMed]
- Kacher, R.; Lejeune, F.X.; David, I.; Boluda, S.; Coarelli, G.; Leclere-Turbant, S.; Heinzmann, A.; Marelli, C.; Charles, P.; Goizet, C.; et al. CAG repeat mosaicism is gene specific in spinocerebellar ataxias. Am. J. Hum. Genet. 2024, 111, 913–926. [Google Scholar] [CrossRef]
- Chong, S.S.; McCall, A.E.; Cota, J.; Subramony, S.H.; Orr, H.T.; Hughes, M.R.; Zoghbi, H.Y. Gametic and somatic tissue-specific heterogeneity of the expanded SCA1 CAG repeat in spinocerebellar ataxia type 1. Nat. Genet. 1995, 10, 344–350. [Google Scholar] [CrossRef]
- Tanaka, F.; Sobue, G.; Doyu, M.; Ito, Y.; Yamamoto, M.; Shimada, N.; Yamamoto, K.; Riku, S.; Hshizume, Y.; Mitsuma, T. Differential pattern in tissue-specific somatic mosaicism of expanded CAG trinucleotide repeats in dentatorubral-pallidoluysian atrophy, Machado-Joseph disease, and X-linked recessive spinal and bulbar muscular atrophy. J. Neurol. Sci. 1996, 135, 43–50. [Google Scholar] [CrossRef]
- Hashida, H.; Goto, J.; Suzuki, T.; Jeong, S.; Masuda, N.; Ooie, T.; Tachiiri, Y.; Tsuchiya, H.; Kanazawa, I. Single cell analysis of CAG repeat in brains of dentatorubral-pallidoluysian atrophy (DRPLA). J. Neurol. Sci. 2001, 190, 87–93. [Google Scholar] [CrossRef]
- Oh, Y.M.; Lee, S.W. Patient-derived neuron model: Capturing age-dependent adult-onset degenerative pathology in Huntington’s disease. Mol. Cells 2024, 47, 100046. [Google Scholar] [CrossRef]
- Manley, K.; Shirley, T.L.; Flaherty, L.; Messer, A. Msh2 deficiency prevents in vivo somatic instability of the CAG repeat in Huntington disease transgenic mice. Nat. Genet. 1999, 23, 471–473. [Google Scholar] [CrossRef] [PubMed]
- Phadte, A.S.; Bhatia, M.; Ebert, H.; Abdullah, H.; Elrazaq, E.A.; Komolov, K.E.; Pluciennik, A. FAN1 removes triplet repeat extrusions via a PCNA- and RFC-dependent mechanism. Proc. Natl. Acad. Sci. USA 2023, 120, e2302103120. [Google Scholar] [CrossRef]
- Pinto, R.M.; Dragileva, E.; Kirby, A.; Lloret, A.; Lopez, E.; St Claire, J.; Panigrahi, G.B.; Hou, C.; Holloway, K.; Gillis, T.; et al. Mismatch repair genes Mlh1 and Mlh3 modify CAG instability in Huntington’s disease mice: Genome-wide and candidate approaches. PLoS Genet. 2013, 9, e1003930. [Google Scholar] [CrossRef]
- Nakamori, M.; Panigrahi, G.B.; Lanni, S.; Gall-Duncan, T.; Hayakawa, H.; Tanaka, H.; Luo, J.; Otabe, T.; Li, J.; Sakata, A.; et al. A slipped-CAG DNA-binding small molecule induces trinucleotide-repeat contractions in vivo. Nat. Genet. 2020, 52, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Gall-Duncan, T.; Ko, S.Y.; Quick, I.K.; Khan, M.; Feng, K.; Kelley, C.P.; Coleman, A.; Touze, A.; Tang, S.; Mehkary, M.; et al. Interventionally targeting somatic CAG expansions can be a rapid disease-modifying therapeutic avenue: Preclinical evidence. bioRxiv 2025. [Google Scholar] [CrossRef] [PubMed]
- Hasuike, Y.; Tanaka, H.; Gall-Duncan, T.; Mehkary, M.; Nakatani, K.; Pearson, C.E.; Tsuji, S.; Mochizuki, H.; Nakamori, M. CAG repeat-binding small molecule improves motor coordination impairment in a mouse model of Dentatorubral-pallidoluysian atrophy. Neurobiol. Dis. 2022, 163, 105604. [Google Scholar] [CrossRef]
- Tabrizi, S.J.; Estevez-Fraga, C.; van Roon-Mom, W.M.C.; Flower, M.D.; Scahill, R.I.; Wild, E.J.; Muñoz-Sanjuan, I.; Sampaio, C.; Rosser, A.E.; Leavitt, B.R. Potential disease-modifying therapies for Huntington’s disease: Lessons learned and future opportunities. Lancet Neurol. 2022, 21, 645–658. [Google Scholar] [CrossRef]
- Gall-Duncan, T.; Sato, N.; Yuen, R.K.C.; Pearson, C.E. Advancing genomic technologies and clinical awareness accelerate the discovery of disease-associated tandem repeat sequences. Genome Res. 2022, 32, 1–27. [Google Scholar] [CrossRef] [PubMed]
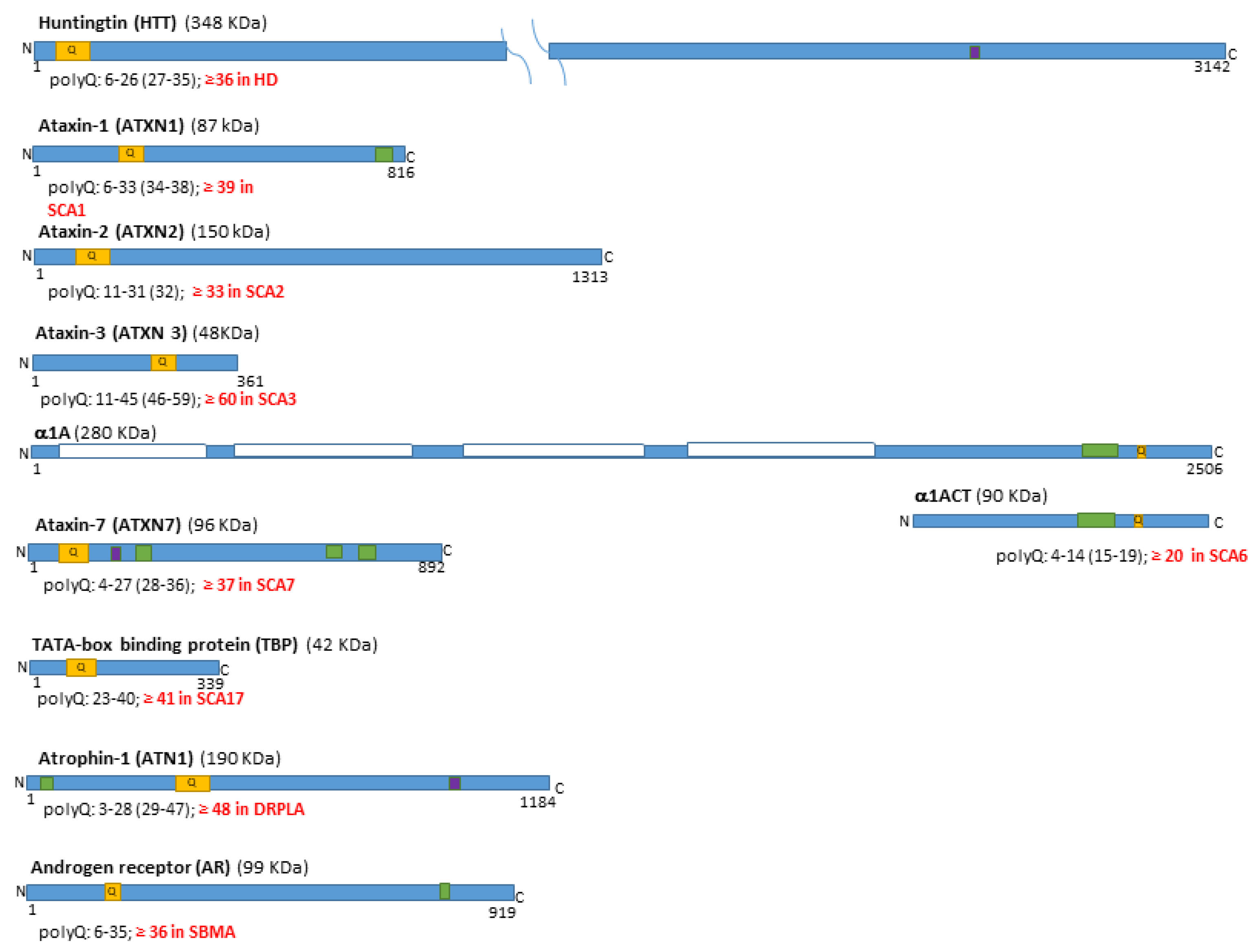
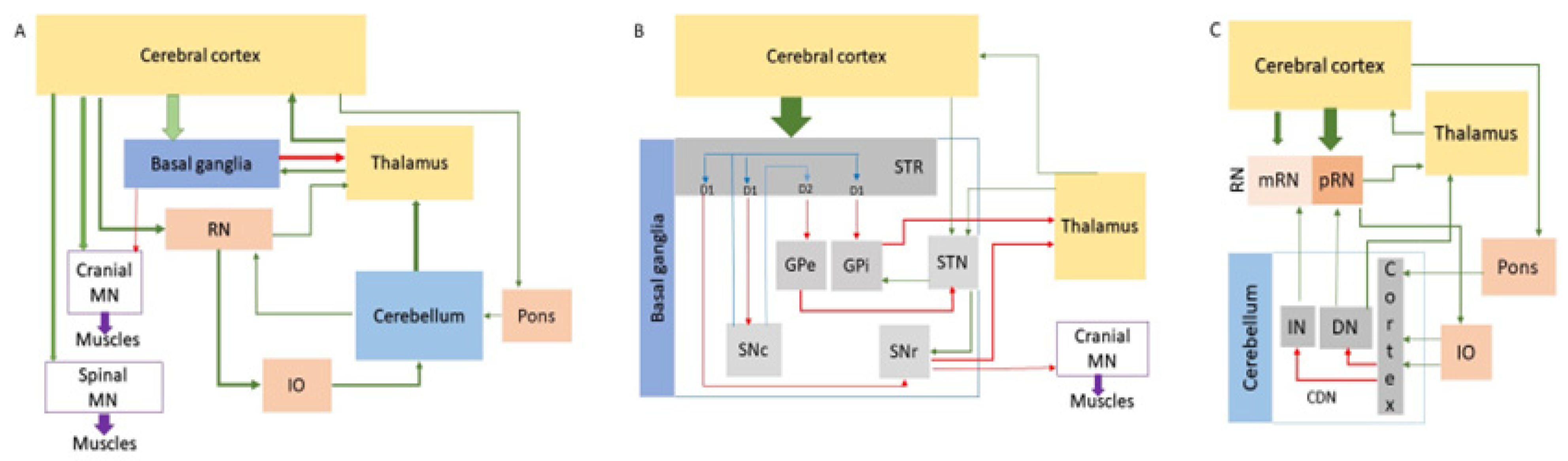
| Disease | Forebrain | Midbrain | Hindbrain | SC | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CC | TH | R | Basal Ganglia | RN | CN | Pons | Cerebellum | MO | MN | WM | |||||||
| STR | GP | STN | SN | PN | CN | CBC | CDN | IO | CN | ||||||||
| HD | |||||||||||||||||
| SCA1 | |||||||||||||||||
| SCA2 | |||||||||||||||||
| SCA3 | |||||||||||||||||
| SCA6 | |||||||||||||||||
| SCA7 | |||||||||||||||||
| SCA17 | |||||||||||||||||
| DRPLA | |||||||||||||||||
| SBMA | |||||||||||||||||
| Genes | WB | CNS Regions | Neural Cell Types | H | K | B | M | S | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NC | BG | R | TH | MB | CB | P | MO | SC | N | A | O | MG | ||||||||
| HTT | ||||||||||||||||||||
| ATXN1 | ||||||||||||||||||||
| ATXN2 | ||||||||||||||||||||
| ATXN3 | ||||||||||||||||||||
| CACNA1A | 0–9 | |||||||||||||||||||
| ATXN7 | 10–19 | |||||||||||||||||||
| TBP | 20–99 | |||||||||||||||||||
| ATN1 | 100–199 | |||||||||||||||||||
| AR | >200 | |||||||||||||||||||
| Disease | Most Affected Projection Neurons of the Motor Coordination Network (Patients and Animal Models) | PolyQ Proteins | CAG-Repeat Elongations Patient/Animal Models * | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SPNs | GPPNs/ SNrPNs | STNPNs | PNs | IOPNs | DCPNs | RNPNs | DY | AG/ NI | GI | CAG Repeat Size in Neurons | |
| HD | +++ | ++ | + | ++ | + | ++ | + | + | + | ++/+ * | >150/144 *, 150*, 175 * |
| SCA1 | + | + | + | +++ | ++ | ++ | ++ | + | + | ++/+ | ?/154 * |
| SCA2 | - | ++ | + | ++ | ++ | ++ | ++ | + | + | +/+ * | ?/127 * |
| SCA3 | ++ | ++ | ++ | ++ | ++ | +++ | ++ | + | + | +/+ * | ?/148 * |
| SCA6 | - | - | - | +++ | ++ | ++ | ++ | + | + | +/+ * | ?/84 * |
| SCA7 | - | + | + | ++ | ++ | ++ | ++ | + | + | +/+ * | ?/266 * |
| SCA17 | ++ | ++ | ++ | +++ | ++ | + | + | + | + | +/+ * | ?/105 * |
| DRPLA | - | +++ | +++ | ++ | + | +++ | +++ | + | + | ++/++ * | ?/129 * |
| SBMA | - | - | - | + | + | ++ | + | + | + | +/+ * | ?/112 *, 113 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deleanu, R. Challenges in Polyglutamine Diseases: From Dysfunctional Neuronal Circuitries to Neuron-Specific CAG Repeat Instability. Int. J. Mol. Sci. 2025, 26, 9755. https://doi.org/10.3390/ijms26199755
Deleanu R. Challenges in Polyglutamine Diseases: From Dysfunctional Neuronal Circuitries to Neuron-Specific CAG Repeat Instability. International Journal of Molecular Sciences. 2025; 26(19):9755. https://doi.org/10.3390/ijms26199755
Chicago/Turabian StyleDeleanu, Roxana. 2025. "Challenges in Polyglutamine Diseases: From Dysfunctional Neuronal Circuitries to Neuron-Specific CAG Repeat Instability" International Journal of Molecular Sciences 26, no. 19: 9755. https://doi.org/10.3390/ijms26199755
APA StyleDeleanu, R. (2025). Challenges in Polyglutamine Diseases: From Dysfunctional Neuronal Circuitries to Neuron-Specific CAG Repeat Instability. International Journal of Molecular Sciences, 26(19), 9755. https://doi.org/10.3390/ijms26199755




