Long Non-Coding RNA 1810026B05Rik Mediates Cerebral Ischemia/Reperfusion-Induced Neuronal Injury Through NF-κB Pathway Activation
Abstract
1. Introduction
2. Results
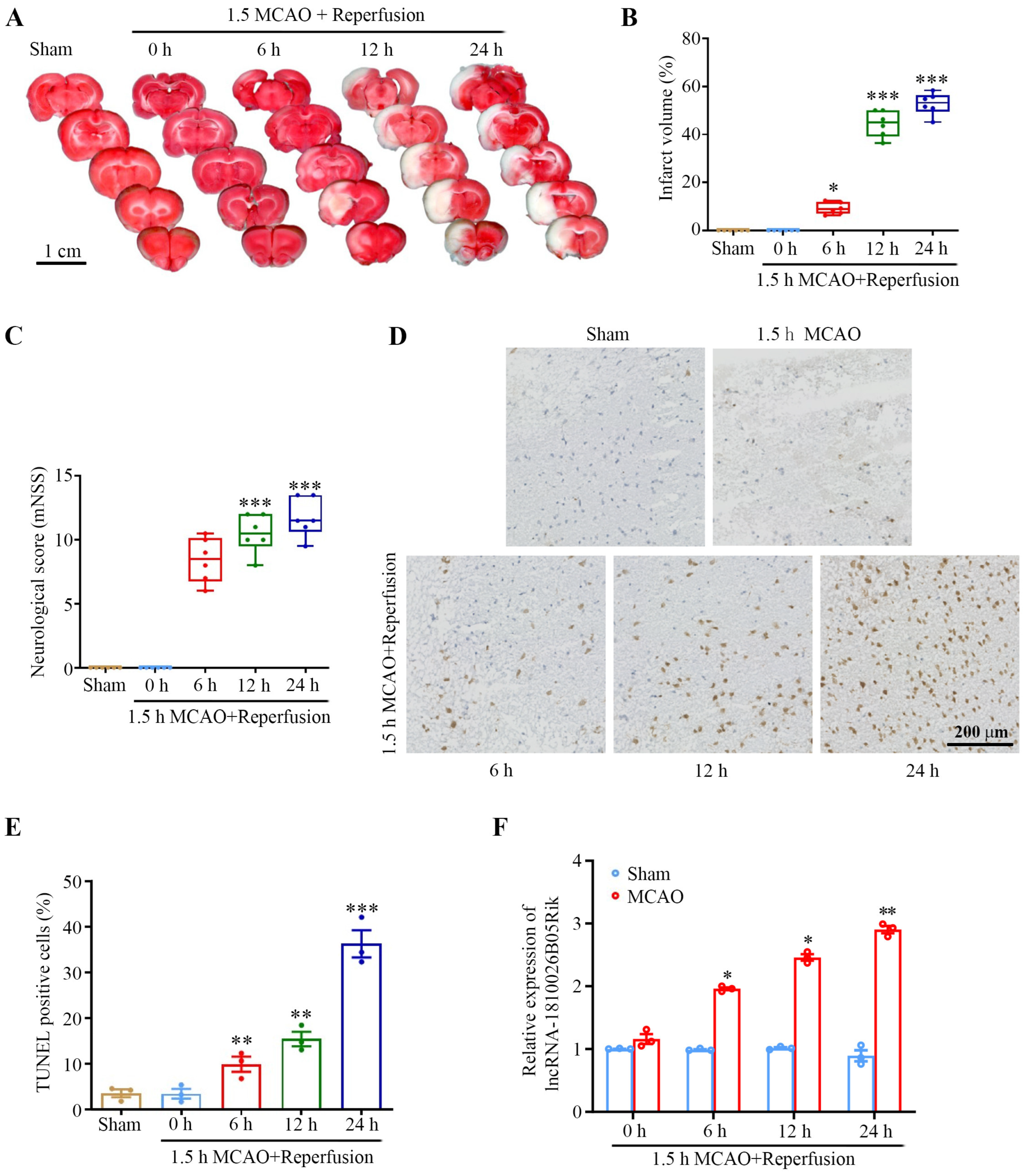
2.1. lncRNA-1810026B05Rik Was Significantly Increased After Ischemic/Reperfusion (I/R) In Vivo and In Vitro
2.2. The Subcellular Localization of lncRNA-1810026B05Rik on OGD/R-Induced Hypoxic Injury in Primary Rat Neurons
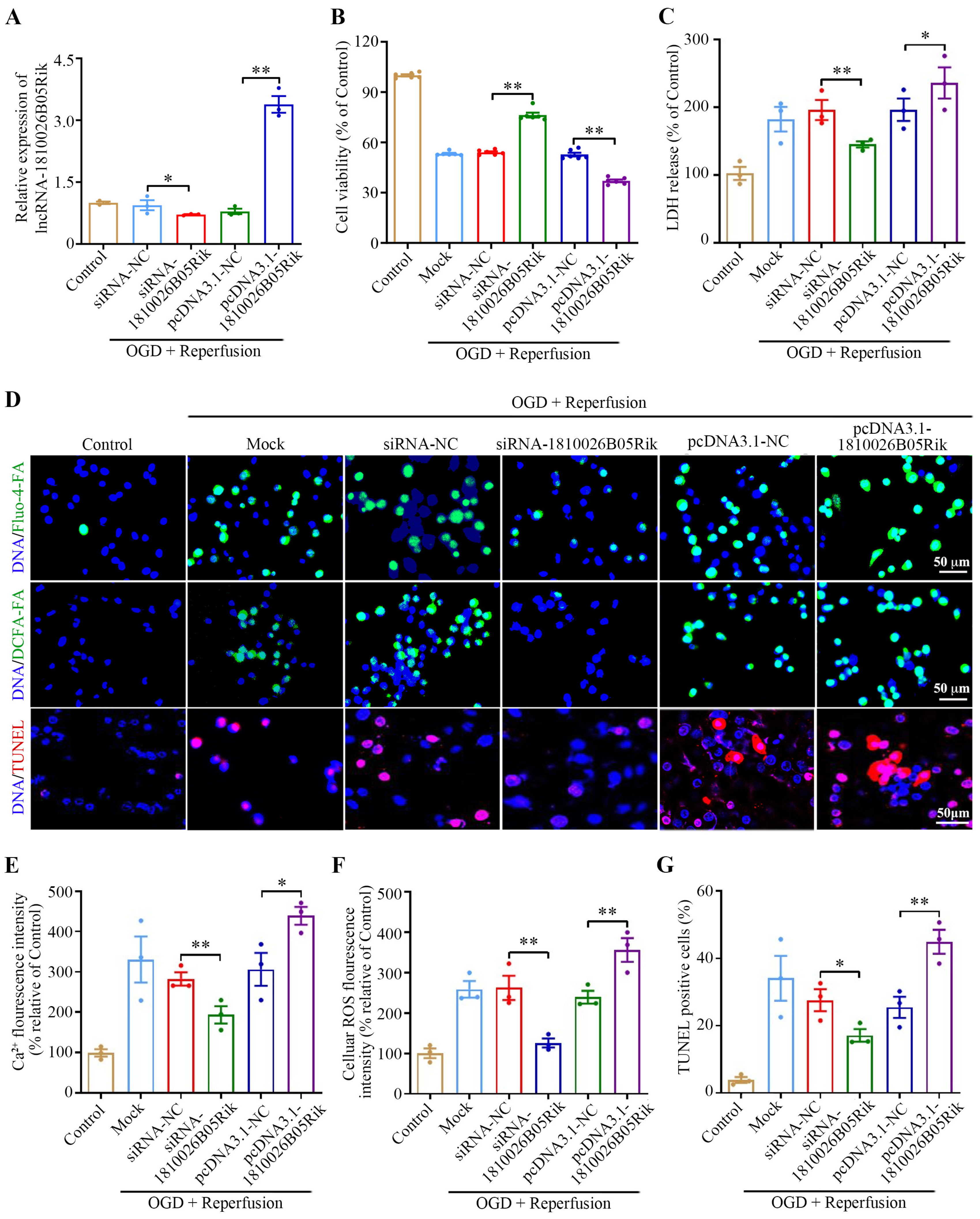
2.3. Effect of lncRNA-1810026B05Rik on OGD/R-Induced Hypoxic Injury in Neurons
2.4. lncRNA-1810026B05Rik Contributed to NF-κB Activation in OGD/R-Treated Neurons
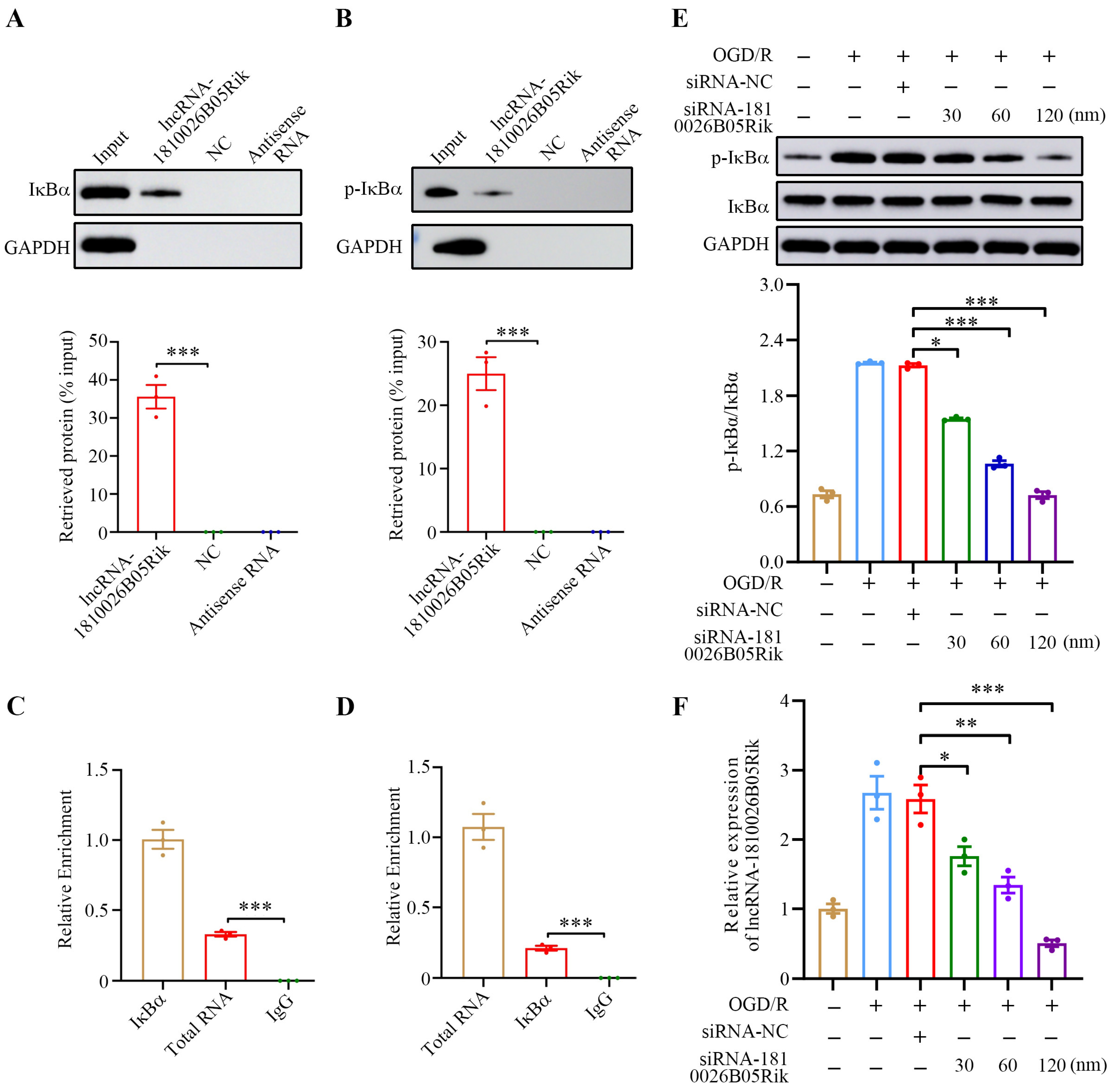
2.5. lncRNA-1810026B05Rik Contributed to IκBα Phosphorylation in OGD/R-Treated Neurons
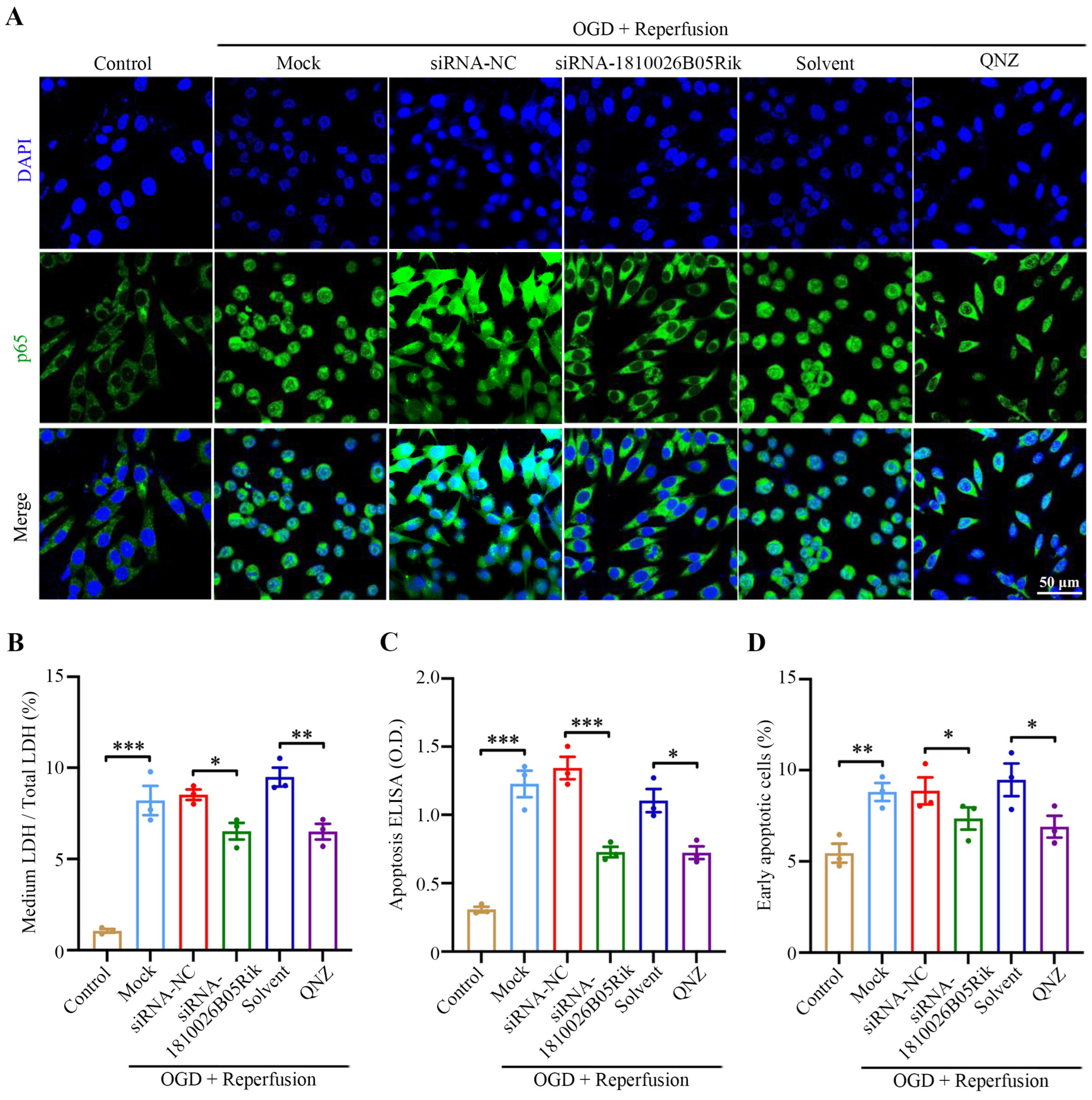
2.6. Knockdown of lncRNA-1810026B05Rik Functioned as NF-κB Inhibitor and Relieved OGD/R-Induced Cell Death and Necrosis
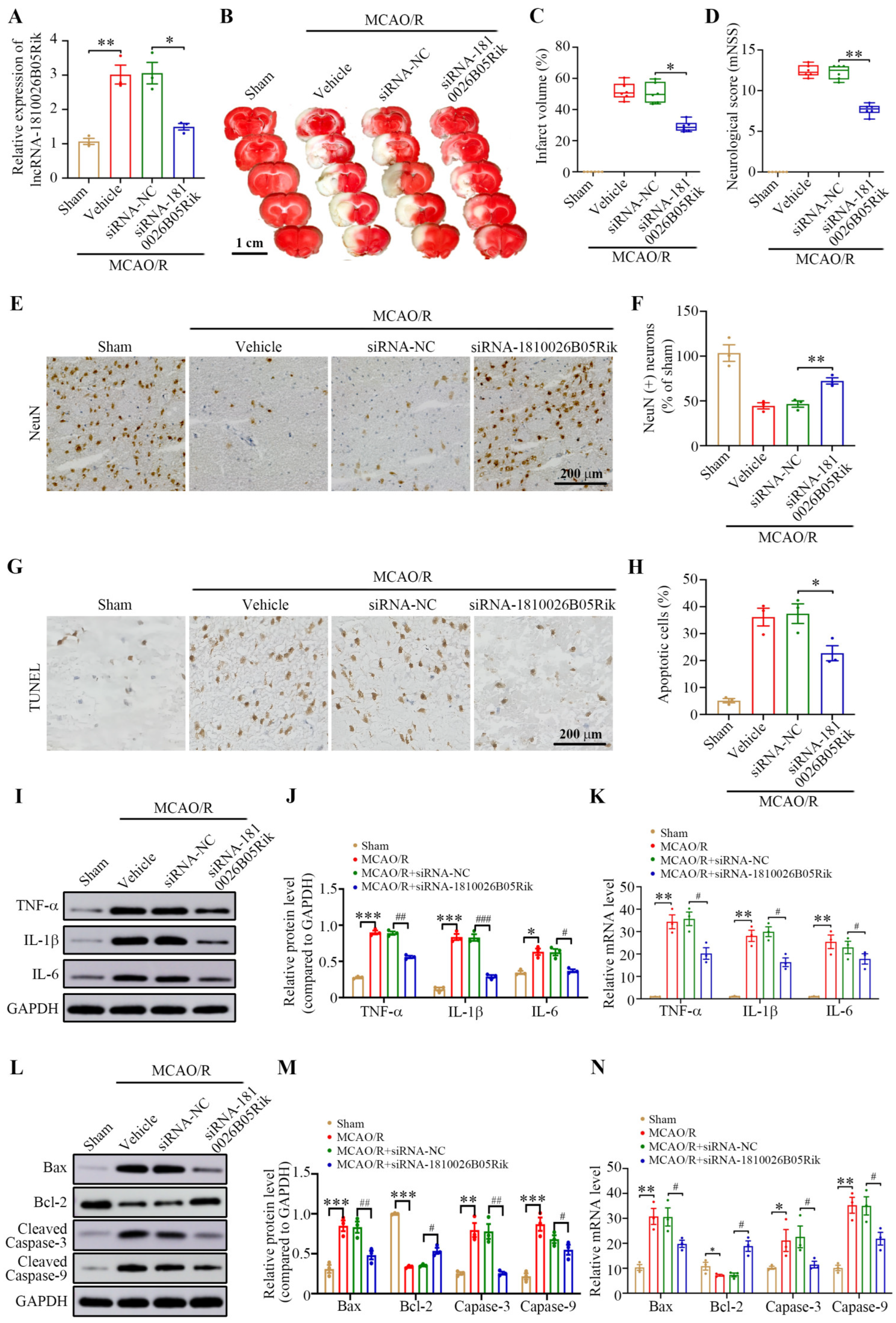
2.7. Knockdown of lncRNA-1810026B05Rik Protects Against Cerebral I/R Injury by Activating the NF-κB Pathway
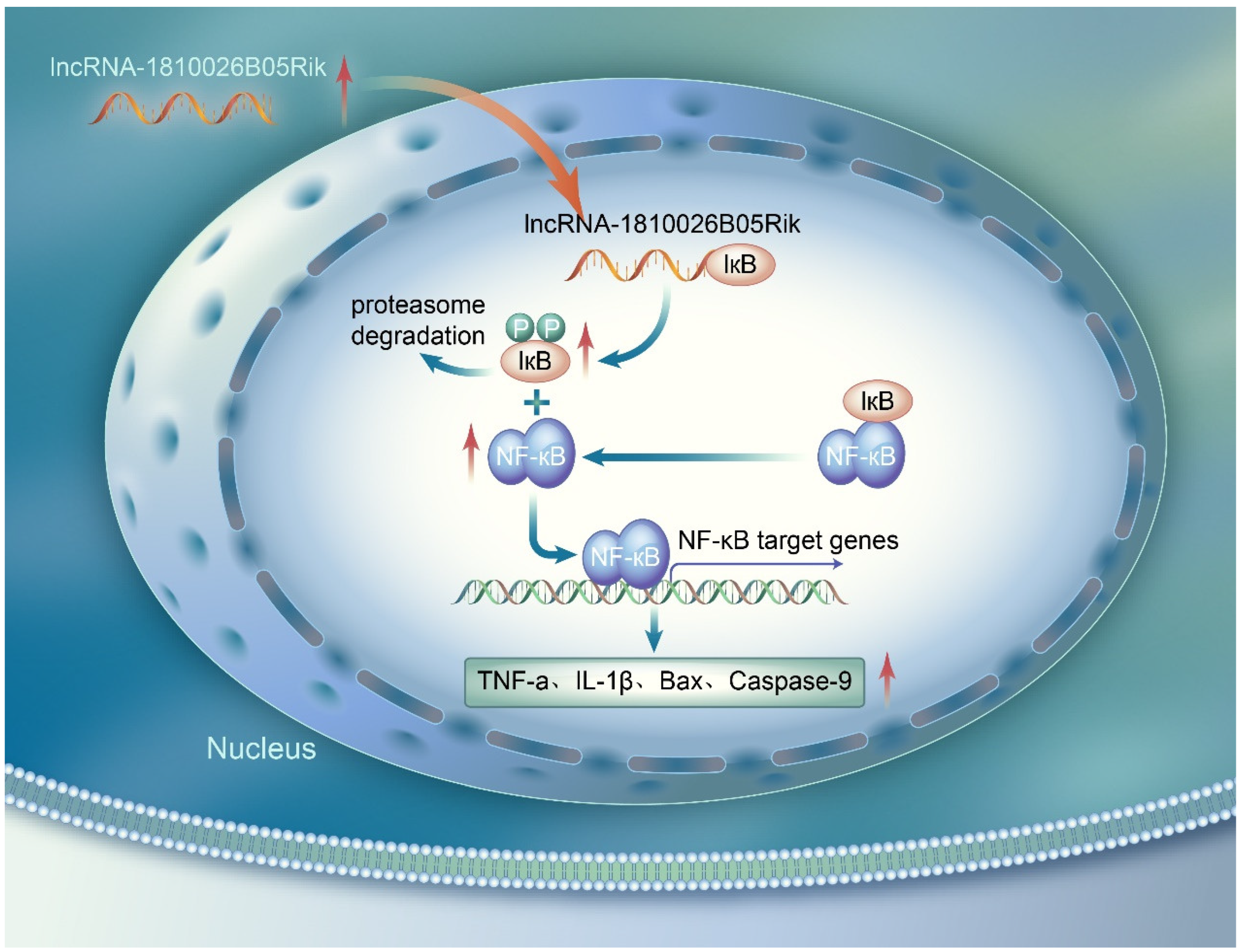
3. Discussion
4. Materials and Methods
4.1. Animal and Focal Cerebral Ischemia
4.2. In Vivo lncRNA-1810026B05Rik Down Expression
4.3. Neurological Function and Infarct Volume Evaluation
4.4. Cell Culture and OGD Treatment
4.5. Cell Transfection
4.6. RNA Fluorescence In Situ Hybridization (RNA FISH)
4.7. Cell Viability Assay
4.8. Cell Apoptosis and Death Assays
4.9. Measurement of Intracellular Reactive Oxygen Species (ROS) Levels
4.10. Determination of Intracellular Ca2+ of Neurons
4.11. TUNEL Staining
4.12. RNA-Binding Protein Immunoprecipitation (RIP) Assay
4.13. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.14. Western Blotting
4.15. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Behrouzifar, S.; Vakili, A.; Bandegi, A.R.; Kokhaei, P. Neuroprotective nature of adipokine resistin in the early stages of focal cerebral ischemia in a stroke mouse model. Neurochem. Int. 2018, 114, 99–107. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, M.; Zhang, X.; Kuai, Y.; Mei, Y.; Tan, Q.; Zhong, K.; Sun, X.; Tan, W. Isosteviol sodium protects against ischemic stroke by modulating microglia/macrophage polarization via disruption of GAS5/miR-146a-5p sponge. Sci. Rep. 2019, 9, 1–16. [Google Scholar] [CrossRef]
- Singh, N.; Santos, T.; Ali, A.B.; Khan, H.; Kibrik, P.; Storch, J.; Bai, H.; Awad, M.; Patel, R.; Huber, M. Contraindications to tissue plasminogen activator thrombolysis for acute lower extremity ischemia. Vascular 2025, 33, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Khanra, S.; Paul, N.; Mukherjee, S. Early Marked Behavioral Symptoms in Bilateral Posterior Cerebral Artery Stroke: A Disguised Presentation. Indian J. Psychol. Med. 2018, 40, 96–98. [Google Scholar] [CrossRef]
- Pan, Q.; Liu, Y.; Wang, G.; Wen, Z.; Wang, Y. MTMR14 protects against cerebral stroke through suppressing PTEN-regulated autophagy. Biochem. Biophys. Res. Commun. 2020, 529, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Akıl, E.; Akıl, M.A.; Varol, S.; Özdemir, H.H.; Yücel, Y.; Arslan, D.; Akyüz, A.; Alan, S. Echocardiographic Epicardial Fat Thickness and Neutrophil to Lymphocyte Ratio Are Novel Inflammatory Predictors of Cerebral Ischemic Stroke. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2014, 23, 2328–2334. [Google Scholar] [CrossRef]
- Fesenko, I.A.; Kirov, I.V.; Filippova, A.A. Impact of Noncoding Part of the Genome on the Proteome Plasticity of the Eukaryotic Cell. Russ. J. Bioorganic Chem. 2018, 44, 397–402. [Google Scholar] [CrossRef]
- Takemata, N.; Ohta, K. Role of non-coding RNA transcription around gene regulatory elements in transcription factor recruitment. RNA Biol. 2017, 14, 1–5. [Google Scholar] [CrossRef]
- Mercer, T.R.; Mattick, J.S. Structure and function of long noncoding RNAs in epigenetic regulation. Nat. Struct. Mol. Biol. 2013, 20, 300–307. [Google Scholar] [CrossRef]
- Tao, H.; Yang, J.J.; Shi, K.H. Non-coding RNAs as direct and indirect modulators of epigenetic mechanism regulation of cardiac fibrosis. Expert. Opin. Ther. Targets 2015, 79, 1–10. [Google Scholar] [CrossRef]
- Hauptman, N.; Glava?, D. Long Non-Coding RNA in Cancer. Int. J. Mol. Ences 2013, 14, 4655–4669. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, B.; Shi, X.; Sun, X. Long noncoding RNAs: Potential therapeutic targets in cardiocerebrovascular diseases. Pharmacol. Ther. 2020, 221, 107744. [Google Scholar] [CrossRef] [PubMed]
- Mirzajani, S.; Ghafouri-Fard, S.; Habibabadi, J.M.; Arsang-Jang, S.; Omrani, M.D.; Fesharaki, S.S.H.; Sayad, A.; Taheri, M. Expression Analysis of lncRNAs in Refractory and Non-Refractory Epileptic Patients. J. Mol. Neurosci. 2020, 70, 689–698. [Google Scholar] [CrossRef]
- Zhu, W.; Tian, L.; Yue, X.; Liu, J.; Fu, Y.; Yan, Y. LncRNA Expression Profiling of Ischemic Stroke During the Transition From the Acute to Subacute Stage. Front. Neurol. 2019, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Barangi, S.; Hayes, A.W.; Reiter, R.; Karimi, G. The therapeutic role of long non-coding RNAs in human diseases: A focus on the recent insights into autophagy. Pharmacol. Res. 2019, 142, 22–29. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, L.; Zhang, X.; Hamblin, M.H.; Zhu, T.; Meng, F.; Li, Y.; Chen, Y.E.; Yin, K.J. Altered long non-coding RNA transcriptomic profiles in brain microvascular endothelium after cerebral ischemia. Exp. Neurol. 2016, 277, 162–170. [Google Scholar] [CrossRef]
- Sances, S.; Ho, R.; Vatine, G.; West, D.; Laperle, A.; Meyer, A.; Godoy, M.; Kay, P.S.; Mandefro, B.; Hatata, S. Human iPSC-Derived Endothelial Cells and Microengineered Organ-Chip Enhance Neuronal Development. Stem Cell Rep. 2018, 10, 1222–1236. [Google Scholar] [CrossRef]
- Turner, M.; Galloway, A.; Vigorito, E. Noncoding RNA and its associated proteins as regulatory elements of the immune system. Nat. Immunol. 2014, 15, 484–491. [Google Scholar] [CrossRef]
- Meller, V.H.; Joshi, S.S.; Deshpande, N. Modulation of Chromatin by Noncoding RNA. Annu. Rev. Genet. 2015, 49, 673. [Google Scholar] [CrossRef]
- Rutenbergschoenberg, M.; Sexton, A.N.; Simon, M. The Properties of Long Noncoding RNAs That Regulate Chromatin. Annu. Rev. Genom. Hum. Genet. 2016, 17, 69–94. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, E.; Tamamaki, N.; Nakamura, T.; Kataoka, K.; Tokutomi, Y.; Dong, Y.F.; Fukuda, M.; Matsuba, S.; Ogawa, H.; Kim-Mitsuyama, S. Excess salt causes cerebral neuronal apoptosis and inflammation in stroke-prone hypertensive rats through angiotensin II-induced NADPH oxidase activation. Stroke J. Cereb. Circ. 2008, 39, 3049–3056. [Google Scholar] [CrossRef]
- Tian, G.H.; Tao, S.S.; Chen, M.T.; Li, Y.S.; Li, Y.P.; Shang, H.C.; Tang, X.Y.; Chen, J.X.; Tang, H.B. Electroacupuncture Treatment Alleviates Central Poststroke Pain by Inhibiting Brain Neuronal Apoptosis and Aberrant Astrocyte Activation. Neural Plast. 2016, 2016, 1437148. [Google Scholar] [CrossRef]
- Zhe, X.; Zhang, S.; Ren, H. Effect of intravenous thrombolysis with butylphthalide, edaravone and recombinant tissue plasminogen activator (rt-PA) on serum inflammatory factors in patients with ischemic stroke. Trop. J. Pharm. Res. 2024, 23, 1569–1575. [Google Scholar] [CrossRef]
- Meng, Q.T.; Chen, R.; Chen, C.; Su, K.; Li, W.; Tang, L.H.; Liu, H.M.; Xue, R.; Sun, Q.; Leng, Y.; et al. Transcription factors Nrf2 and NF-κB contribute to inflammation and apoptosis induced by intestinal ischemia-reperfusion in mice. Int. J. Mol. Med. 2017, 40, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Zhang, R.; Chen, Y.; Zhu, Z.; Ma, C. Raf Kinase Inhibitor Protein Attenuates Ischemic-Induced Microglia Cell Apoptosis and Activation Through NF-κB Pathway. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2017, 41, 1125–1134. [Google Scholar] [CrossRef]
- Venna, V.R.; Weston, G.; Benashski, S.E.; Tarabishy, S.; Liu, F.; Li, J.; Conti, L.H.; Mccullough, L.D. NF-κB contributes to the detrimental effects of social isolation after experimental stroke. Acta Neuropathol. 2012, 124, 425–438. [Google Scholar] [CrossRef]
- Cui, L.; Duchamp, N.S.; Boston, D.J.; Ren, X.; Zhang, X.; Hu, H.; Zhao, L.R. NF-κB is involved in brain repair by stem cell factor and granulocyte-colony stimulating factor in chronic stroke. Exp. Neurol. 2015, 263, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Li, T.; Ji, K.; Zhou, X.; Yao, W.; Zhou, L.; Huang, P.; Zhong, K. Safranal alleviates pentetrazole-induced epileptic seizures in mice by inhibiting the NF-κB signaling pathway and mitochondrial-dependent apoptosis through GSK-3β inactivation. J. Ethnopharmacol. 2024, 333, 118408. [Google Scholar] [CrossRef]
- Wu, Z.; Song, Y.; Wang, Y.; Zhou, H.; Chen, L.; Zhan, Y.; Li, T.; Xie, G.; Wu, H. Biological role of mitochondrial TLR4-mediated NF-κB signaling pathway in central nervous system injury. Cell Biochem. Funct. 2024, 42, e4056. [Google Scholar] [CrossRef]
- Liu, H.; Wu, X.; Luo, J.; Wang, X.; Guo, H.; Feng, D.; Zhao, L.; Bai, H.; Song, M.; Liu, X.; et al. Pterostilbene Attenuates Astrocytic Inflammation and Neuronal Oxidative Injury After Ischemia-Reperfusion by Inhibiting NF-κB Phosphorylation. Front. Immunol. 2019, 10, 2408. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Lin, C.; Yuan, L.; Chen, L.; Guo, P.; Li, P.; Wang, W.; Zhang, X. Preactivation of Notch1 in remote ischemic preconditioning reduces cerebral ischemia-reperfusion injury through crosstalk with the NF-κB pathway. J. Neuroinflamm. 2019, 16, 181. [Google Scholar] [CrossRef] [PubMed]
- Rom, A.; Melamed, L.; Gil, N.; Goldrich, M.J.; Kadir, R.; Golan, M.; Biton, I.; Perry, R.B.; Ulitsky, I. Regulation of CHD2 expression by the Chaserr long noncoding RNA gene is essential for viability. Nat. Commun. 2019, 10, 5092. [Google Scholar] [CrossRef]
- Anilkumar, S.; Wright-Jin, E. NF-κB as an inducible regulator of inflammation in the central nervous system. Cells 2024, 13, 485. [Google Scholar] [CrossRef]
- Yi, H.; Peng, R.; Zhang, L.-y.; Sun, Y.; Peng, H.-m.; Liu, H.-d.; Yu, L.-j.; Li, A.-l.; Zhang, Y.-j.; Jiang, W.-h. LincRNA-Gm4419 knockdown ameliorates NF-κB/NLRP3 inflammasome-mediated inflammation in diabetic nephropathy. Cell Death Dis. 2017, 8, e2583. [Google Scholar] [CrossRef]
- Foulds, C.E.; Panigrahi, A.K.; Coarfa, C.; Lanz, R.B.; O’Malley, B.W. Long Noncoding RNAs as Targets and Regulators of Nuclear Receptors. Curr. Top. Microbiol. Immunol. 2016, 394, 143–176. [Google Scholar] [CrossRef]
- Howell, J.A.; Bidwell, G.L., 3rd. Targeting the NF-κB pathway for therapy of ischemic stroke. Ther. Deliv. 2020, 11, 113–123. [Google Scholar] [CrossRef]
- Li, X.; Ding, H.; Jing, J.; Qian, S.; Ma, Y.; Lv, M.; Gao, Y.; Zhang, Y.; Li, T. Sulfasalazine improves neuronal function in mice with ischemic stroke by inhibiting the STING/NF-κB pathway. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025, 398, 5797–5810. [Google Scholar] [CrossRef]
- He, L.; Lei, R.; Li, S.; Zhao, X.; He, X.; Yang, X.; Liu, P.; Zhang, D.; Jiang, Y. Hirudin promotes cerebral angiogenesis and exerts neuroprotective effects in MCAO/R rats by activating the Wnt/β-catenin pathway. J. Stroke Cerebrovasc. Dis. 2025, 34, 108218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Han, S.; Zheng, Y.; Li, J.; Ma, L.; Tan, J.; Kang, X.; Gong, R.; Chen, S.; Shi, S. The mechanism of promoting angiogenesis after cerebral infarction by scalp acupuncture based on MATN2/WNT3a/β-catenin. J. Mol. Histol. 2025, 56, 186. [Google Scholar] [CrossRef] [PubMed]
- Gu, D.M.; Lu, P.H.; Zhang, K.; Xiang, W.; Wang, Q. EGFR mediates astragaloside IV-induced Nrf2 activation to protect cortical neurons against in vitro ischemia/reperfusion damages. Biochem. Biophys. Res. Commun. 2015, 457, 391–397. [Google Scholar] [CrossRef]
- Wen, Y.; Yu, Y.; Fu, X. LncRNA Gm4419 contributes to OGD/R injury of cerebral microglial cells via IκB phosphorylation and NF-κB activation. Biochem. Biophys. Res. Commun. 2017, 487, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Liu, J.; Du, X.; Qi, M.; She, T.; Xue, K.; Wu, X.; Xu, L.; Peng, B.; Zhang, Y. ROS exhaustion reverses the effects of hyperbaric oxygen on hemorrhagic transformation through reactivating microglia in post-stroke hyperglycemic mice. Sci. Rep. 2024, 14, 21410. [Google Scholar] [CrossRef]
- Luo, M.; Li, Z.; Wang, W.; Zeng, Y.; Liu, Z.; Qiu, J. Long non-coding RNA H19 increases bladder cancer metastasis by associating with EZH2 and inhibiting E-cadherin expression. Cancer Lett. 2013, 333, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Wang, K.; Yeh, S.; Sun, Y.; Liang, L.; Xiao, Y.; Xu, W.; Niu, Y.; Cheng, L.; Maity, S.N.; et al. LncRNA-p21 alters the antiandrogen enzalutamide-induced prostate cancer neuroendocrine differentiation via modulating the EZH2/STAT3 signaling. Nat. Commun. 2019, 10, 2571. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Li, M.; Yao, J.; Jiang, X.; Feng, J.; Shi, X.; Sun, X. Long Non-Coding RNA 1810026B05Rik Mediates Cerebral Ischemia/Reperfusion-Induced Neuronal Injury Through NF-κB Pathway Activation. Int. J. Mol. Sci. 2025, 26, 9756. https://doi.org/10.3390/ijms26199756
Zhang H, Li M, Yao J, Jiang X, Feng J, Shi X, Sun X. Long Non-Coding RNA 1810026B05Rik Mediates Cerebral Ischemia/Reperfusion-Induced Neuronal Injury Through NF-κB Pathway Activation. International Journal of Molecular Sciences. 2025; 26(19):9756. https://doi.org/10.3390/ijms26199756
Chicago/Turabian StyleZhang, Hao, Meng Li, Jiayu Yao, Xuan Jiang, Junxiao Feng, Xingjuan Shi, and Xiaoou Sun. 2025. "Long Non-Coding RNA 1810026B05Rik Mediates Cerebral Ischemia/Reperfusion-Induced Neuronal Injury Through NF-κB Pathway Activation" International Journal of Molecular Sciences 26, no. 19: 9756. https://doi.org/10.3390/ijms26199756
APA StyleZhang, H., Li, M., Yao, J., Jiang, X., Feng, J., Shi, X., & Sun, X. (2025). Long Non-Coding RNA 1810026B05Rik Mediates Cerebral Ischemia/Reperfusion-Induced Neuronal Injury Through NF-κB Pathway Activation. International Journal of Molecular Sciences, 26(19), 9756. https://doi.org/10.3390/ijms26199756





