CRISPR-Cas12a-Based Isothermal Detection of Mammarenavirus machupoense Virus: Optimization and Evaluation of Multiplex Capability
Abstract
1. Introduction
2. Results
2.1. Target Sequence Selection
2.2. Design and Optimization of the RT-RPA/DETECTR Assay
2.2.1. Guide RNA Selection
2.2.2. Primer Selection
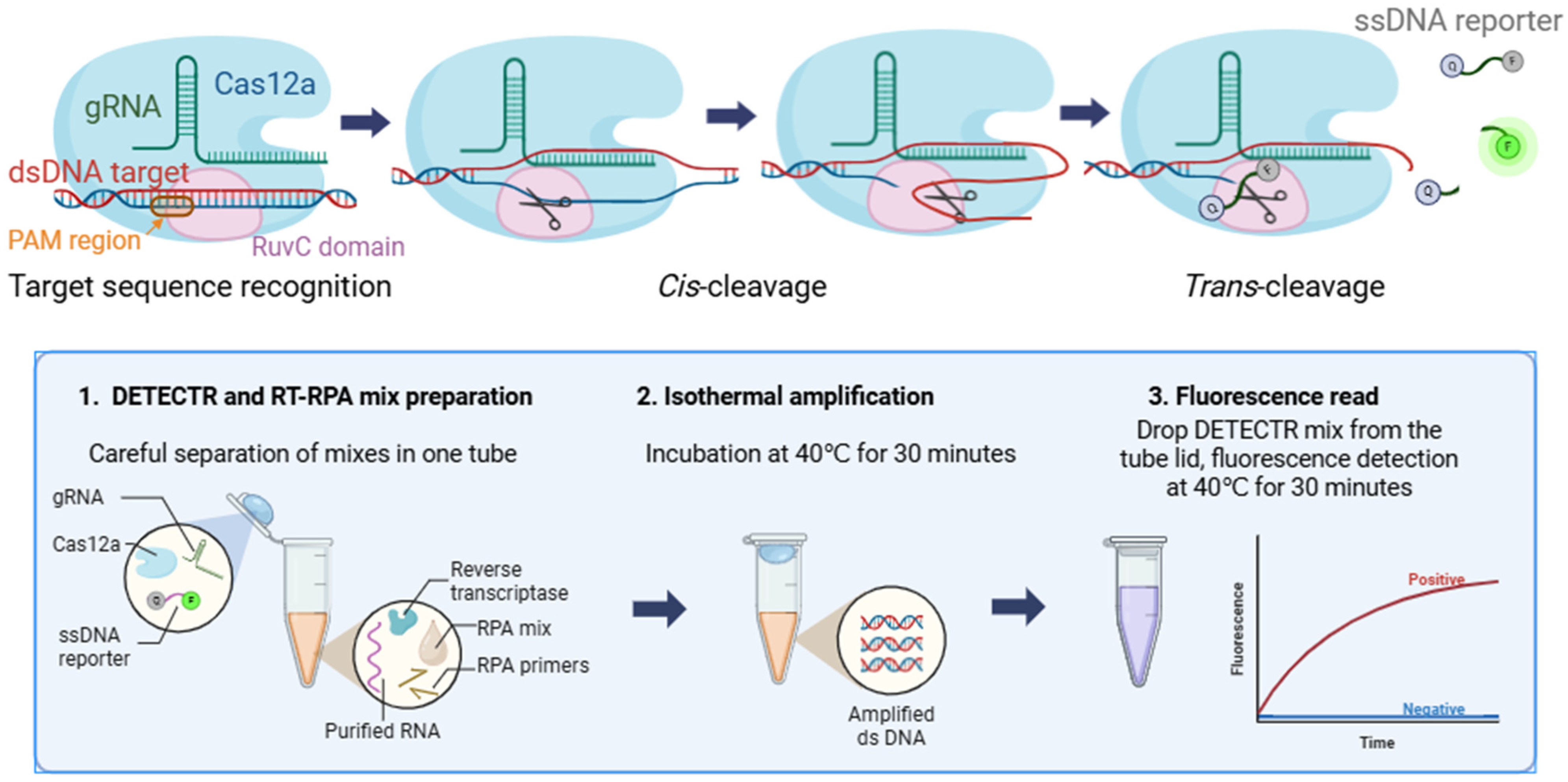
2.2.3. RPA/DETECTR Assay
2.3. Limit of Detection
2.4. Impact of Nucleotide Substitutions
2.5. Multiplex RPA/DETECTR Assay for Detection of MACV Genetic Variants
2.6. Multiplex RT-RPA/DETECTR for Virus-like ARPs
2.7. Clinical Validation of RT-RPA/DETECTR
3. Discussion
4. Materials and Methods
4.1. Positive Control Recombinant Plasmid Construction
4.2. DNA and RNA Positive Controls
4.3. Production and Purification of Armored RNA Particles (ARP)
4.4. RNA Extraction, Purification, and Droplet Digital PCR
4.5. DETECTR Assay
4.6. RPA Assay
4.7. Single-Tube RT-RPA/DETECTR
4.8. Limit of Detection (LOD) Estimation
4.9. Clinical Validation
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BHF | Bolivian hemorrhagic fever |
| MACV | Mammarenavirus machupoense |
| GTOV | Mammarenavirus guanaritoense |
| SABV | Mammarenavirus brazilense |
| JUNV | Mammarenavirus juninense |
| HENV | Henipavirus hendraense |
| NPV | Henipavirus nipahense |
| BSL-4 | Biosafety Level-4 |
| DNA | Deoxyribonucleic acid |
| dsDNA | double-stranded DNA |
| RNA | Ribonucleic acid |
| CRISPR | Clustered regularly interspaced short palindromic repeats |
| PAM | Protospacer-adjacent motif |
| DETECTR | DNA endonuclease-targeted CRISPR trans-reporter |
| SHERLOCK | Specific high-sensitivity enzymatic reporter unlocking |
| PCR | Polymerase chain reaction |
| RT-PCR | Reverse transcription polymerase chain reaction |
| ddPCR | Droplet digital PCR |
| RPA | Recombinase polymerase amplification |
| RT-RPA | Reverse transcription recombinase polymerase amplification |
| LAMP | Loop-mediated isothermal amplification |
| NASBA | Nuclear acid sequence-based amplification |
| ELISA | Enzyme-linked immunosorbent assay |
| POC | Point-of-care |
| LOD | Limit of detection |
| NTC | No-template control |
| ARP | Armored RNA particle |
References
- Patterson, M.; Grant, A.; Paessler, S. Epidemiology and Pathogenesis of Bolivian Hemorrhagic Fever. Curr. Opin. Virol. 2014, 5, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Silva-Ramos, C.R.; Faccini-Martínez, Á.A.; Calixto, O.-J.; Hidalgo, M. Bolivian Hemorrhagic Fever: A Narrative Review. Travel Med. Infect. Dis. 2021, 40, 102001. [Google Scholar] [CrossRef]
- Aguilar, P.V.; Camargo, W.; Vargas, J.; Guevara, C.; Roca, Y.; Felices, V.; Laguna-Torres, A.; Tesh, R.; Ksiazek, T.G.; Kochel, T.J. Reemergence of Bolivian Hemorrhagic Fever, 2007–2008. Emerg. Infect. Dis. 2009, 15, 1526–1528. [Google Scholar] [CrossRef]
- Ortiz-Prado, E.; Vasconez-Gonzalez, J.; Becerra-Cardona, D.A.; Farfán-Bajaña, M.J.; García-Cañarte, S.; López-Cortés, A.; Izquierdo-Condoy, J.S. Hemorrhagic Fevers Caused by South American Mammarenaviruses: A Comprehensive Review of Epidemiological and Environmental Factors Related to Potential Emergence. Travel Med. Infect. Dis. 2025, 64, 102827. [Google Scholar] [CrossRef]
- Yuan, Z. From Origin to the Present: Establishment, Mechanism, Evolutions and Biomedical Applications of the CRISPR/Cas-Based Macromolecular System in Brief. Molecules 2025, 30, 947. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.S.; Ma, E.; Harrington, L.B.; Da Costa, M.; Tian, X.; Palefsky, J.M.; Doudna, J.A. CRISPR-Cas12a Target Binding Unleashes Indiscriminate Single-Stranded DNase Activity. Science 2018, 360, 436–439. [Google Scholar] [CrossRef]
- Kellner, M.J.; Koob, J.G.; Gootenberg, J.S.; Abudayyeh, O.O.; Zhang, F. SHERLOCK: Nucleic Acid Detection with CRISPR Nucleases. Nat. Protoc. 2019, 14, 2986–3012. [Google Scholar] [CrossRef]
- Misiurina, M.A.; Chirinskaite, A.V.; Fotina, A.S.; Zelinsky, A.A.; Sopova, J.V.; Leonova, E.I. New PAM Improves the Single-Base Specificity of CrRNA-Guided LbCas12a Nuclease. Life 2022, 12, 1927. [Google Scholar] [CrossRef] [PubMed]
- Huyke, D.A.; Ramachandran, A.; Bashkirov, V.I.; Kotseroglou, E.K.; Kotseroglou, T.; Santiago, J.G. Enzyme Kinetics and Detector Sensitivity Determine Limits of Detection of Amplification-Free CRISPR-Cas12 and CRISPR-Cas13 Diagnostics. Anal. Chem. 2022, 94, 9826–9834. [Google Scholar] [CrossRef]
- Misra, C.S.; Rangu, S.S.; Phulsundar, R.D.; Bindal, G.; Singh, M.; Shashidhar, R.; Saha, T.K.; Rao, A.V.S.S.N.; Rath, D. An Improved, Simple and Field-Deployable CRISPR-Cas12a Assay for the Detection of SARS-CoV-2. J. Appl. Microbiol. 2022, 133, 2668–2677. [Google Scholar] [CrossRef]
- Fasching, C.L.; Servellita, V.; McKay, B.; Nagesh, V.; Broughton, J.P.; Sotomayor-Gonzalez, A.; Wang, B.; Brazer, N.; Reyes, K.; Streithorst, J.; et al. COVID-19 Variant Detection with a High-Fidelity CRISPR-Cas12 Enzyme. J. Clin. Microbiol. 2022, 60, e00261-22. [Google Scholar] [CrossRef]
- Dai, B.; Xiang, A.; Qu, D.; Chen, G.; Wang, L.; Wang, W.; Zhai, D.; Wang, L.; Lu, Z. Rapid and Sensitive Assay of Helicobacter pylori with One-Tube RPA-CRISPR/Cas12 by Portable Array Detector for Visible Analysis of Thermostatic Nucleic Acid Amplification. Front. Microbiol. 2022, 13, 858247. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, J.; Gao, E.; Lu, Y.; Qiu, H. A Rapid Visualization Method for Detecting Rotavirus A by Combining Nuclear Acid Sequence-Based Amplification with the CRISPR-Cas12a Assay. J. Med. Microbiol. 2024, 73, 001892. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Yin, K.; Li, Z.; Lalla, R.V.; Ballesteros, E.; Sfeir, M.M.; Liu, C. Ultrasensitive and Visual Detection of SARS-CoV-2 Using All-in-One Dual CRISPR-Cas12a Assay. Nat. Commun. 2020, 11, 4711. [Google Scholar] [CrossRef] [PubMed]
- Broughton, J.P.; Deng, X.; Yu, G.; Fasching, C.L.; Servellita, V.; Singh, J.; Miao, X.; Streithorst, J.A.; Granados, A.; Sotomayor-Gonzalez, A.; et al. CRISPR–Cas12-Based Detection of SARS-CoV-2. Nat. Biotechnol. 2020, 38, 870–874. [Google Scholar] [CrossRef]
- Ding, R.; Long, J.; Yuan, M.; Zheng, X.; Shen, Y.; Jin, Y.; Yang, H.; Li, H.; Chen, S.; Duan, G. CRISPR/Cas12-Based Ultra-Sensitive and Specific Point-of-Care Detection of HBV. Int. J. Mol. Sci. 2021, 22, 4842. [Google Scholar] [CrossRef]
- Yu, F.; Zhang, K.; Wang, Y.; Li, D.; Cui, Z.; Huang, J.; Zhang, S.; Li, X.; Zhang, L. CRISPR/Cas12a-Based on-Site Diagnostics of Cryptosporidium parvum IId-Subtype-Family from Human and Cattle Fecal Samples. Parasites Vectors 2021, 14, 208. [Google Scholar] [CrossRef]
- Zhang, A.; Sun, B.; Zhang, J.; Cheng, C.; Zhou, J.; Niu, F.; Luo, Z.; Yu, L.; Yu, C.; Dai, Y.; et al. CRISPR/Cas12a Coupled with Recombinase Polymerase Amplification for Sensitive and Specific Detection of Aphelenchoides besseyi. Front. Bioeng. Biotechnol. 2022, 10, 912959. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cheng, M.; Yang, S.; Xing, C.; Li, Q.; Zhu, Y.; Ji, Y.; Du, Y. CRISPR/Cas12a Combined with RPA for Detection of T. Gondii in Mouse Whole Blood. Parasites Vectors 2023, 16, 256. [Google Scholar] [CrossRef]
- Zhang, K.; Sun, Z.; Shi, K.; Yang, D.; Bian, Z.; Li, Y.; Gou, H.; Jiang, Z.; Yang, N.; Chu, P.; et al. RPA-CRISPR/Cas12a-Based Detection of Haemophilus parasuis. Animals 2023, 13, 3317. [Google Scholar] [CrossRef]
- Li, Y.; Shi, Z.; Hu, A.; Cui, J.; Yang, K.; Liu, Y.; Deng, G.; Zhu, C.; Zhu, L. Rapid One-Tube RPA-CRISPR/Cas12 Detection Platform for Methicillin-Resistant Staphylococcus Aureus. Diagnostics 2022, 12, 829. [Google Scholar] [CrossRef]
- Miao, J.; Zuo, L.; He, D.; Fang, Z.; Berthet, N.; Yu, C.; Wong, G. Rapid Detection of Nipah Virus Using the One-Pot RPA-CRISPR/Cas13a Assay. Virus Res. 2023, 332, 199130. [Google Scholar] [CrossRef]
- Rasool, H.M.H.; Chen, Q.; Gong, X.; Zhou, J. CRISPR/Cas System and Its Application in the Diagnosis of Animal Infectious Diseases. FASEB J. 2024, 38, e70252. [Google Scholar] [CrossRef]
- Zhang, X. Development of CRISPR-Mediated Nucleic Acid Detection Technologies and Their Applications in the Livestock Industry. Genes 2022, 13, 2007. [Google Scholar] [CrossRef]
- Gootenberg, J.S.; Abudayyeh, O.O.; Lee, J.W.; Essletzbichler, P.; Dy, A.J.; Joung, J.; Verdine, V.; Donghia, N.; Daringer, N.M.; Freije, C.A.; et al. Nucleic Acid Detection with CRISPR-Cas13a/C2c2. Science 2017, 356, 438–442. [Google Scholar] [CrossRef]
- Chen, S.-J.; Rai, C.-I.; Wang, S.-C.; Chen, Y.-C. Point-of-Care Testing for Infectious Diseases Based on Class 2 CRISPR/Cas Technology. Diagnostics 2023, 13, 2255. [Google Scholar] [CrossRef] [PubMed]
- Dolgova, A.S.; Stukolova, O.A. High-Fidelity PCR Enzyme with DNA-Binding Domain Facilitates de Novo Gene Synthesis. 3 Biotech 2017, 7, 128. [Google Scholar] [CrossRef] [PubMed]
- Pasloske, B.L.; Walkerpeach, C.R.; Obermoeller, R.D.; Winkler, M.; DuBois, D.B. Armored RNA Technology for Production of Ribonuclease-Resistant Viral RNA Controls and Standards. J. Clin. Microbiol. 1998, 36, 3590–3594. [Google Scholar] [CrossRef] [PubMed]

| Detection System | Target Name, Fragment Length | Test Material | Published LOD | Recalculated LOD | Reference |
|---|---|---|---|---|---|
| Cas12a-DETECTR system after pre-amplification by LAMP | Hepatitis B virus polymerase coding region, 188 nt | standard plasmids | 1 copy/µl | 103 copies/ml | [16] |
| One-pot RPA-CRISPR/Cas13a assay | Nipah virus (AY988601.1) N gene, 231 nt | cDNA from healthy human plasma mixed with synthetic Nipah virus N gene RNA | 103 copies/µl | 106 copies/ml | [22] |
| AIOD-CRISPR assay | SARS-CoV-2 N gene, 316 nt | a positive control plasmid, clinical swab samples | 3 copies per reaction with two gRNAs, 3 × 103 copies per reaction with one gRNA | 3 × 103 copies/,mL 3 × 106 copies/ml | [14] |
| DETECTR assay (Cas12 detection with lateral-flow analysis after pre-amplification by RT-LAMP) | SARS-CoV-2 E gene (WHO E amplicon) and N gene (CDC N2 amplicon) | synthetic gene fragments, nasopharyngeal swab | 10 copies/ µL | 2 × 104 copies/ml | [15] |
| CRISPR-Cas13a/C2c2 (RT-RPA with T7 RNA polymerase and subsequent SHERLOCK detection assay by Cas13a) | Zika virus, 343 nt | lentiviruses harboring genomic fragments, clinical samples (serum or urine) | 2 aM | 1.2 × 103 copies/ml | [25] |
| Multiplex, single-tube RT-RPA/DETECTR assay | M. machupoense virus, 136 nt | virus-like ARPs | 80 aM | 5 × 104 copies/ml | this work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapitonova, M.A.; Shabalina, A.V.; Dedkov, V.G.; Dolgova, A.S. CRISPR-Cas12a-Based Isothermal Detection of Mammarenavirus machupoense Virus: Optimization and Evaluation of Multiplex Capability. Int. J. Mol. Sci. 2025, 26, 9754. https://doi.org/10.3390/ijms26199754
Kapitonova MA, Shabalina AV, Dedkov VG, Dolgova AS. CRISPR-Cas12a-Based Isothermal Detection of Mammarenavirus machupoense Virus: Optimization and Evaluation of Multiplex Capability. International Journal of Molecular Sciences. 2025; 26(19):9754. https://doi.org/10.3390/ijms26199754
Chicago/Turabian StyleKapitonova, Marina A., Anna V. Shabalina, Vladimir G. Dedkov, and Anna S. Dolgova. 2025. "CRISPR-Cas12a-Based Isothermal Detection of Mammarenavirus machupoense Virus: Optimization and Evaluation of Multiplex Capability" International Journal of Molecular Sciences 26, no. 19: 9754. https://doi.org/10.3390/ijms26199754
APA StyleKapitonova, M. A., Shabalina, A. V., Dedkov, V. G., & Dolgova, A. S. (2025). CRISPR-Cas12a-Based Isothermal Detection of Mammarenavirus machupoense Virus: Optimization and Evaluation of Multiplex Capability. International Journal of Molecular Sciences, 26(19), 9754. https://doi.org/10.3390/ijms26199754








