Properties of Heterochannels Kv(1.1-1.2)2 with Mutation T226R in the Kv1.1 Subunit
Abstract
1. Introduction
2. Results
2.1. Design of the Protein Constructs
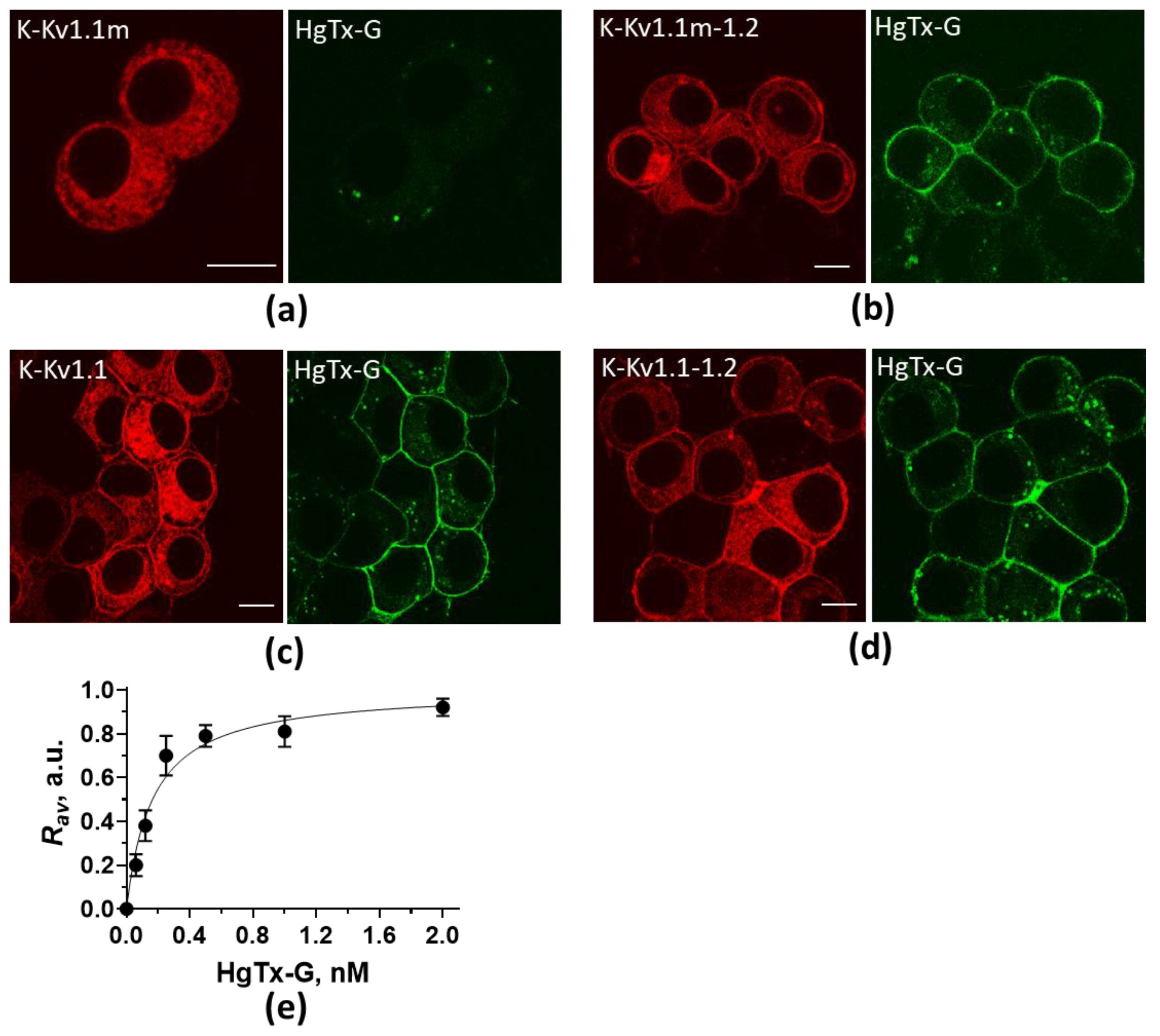
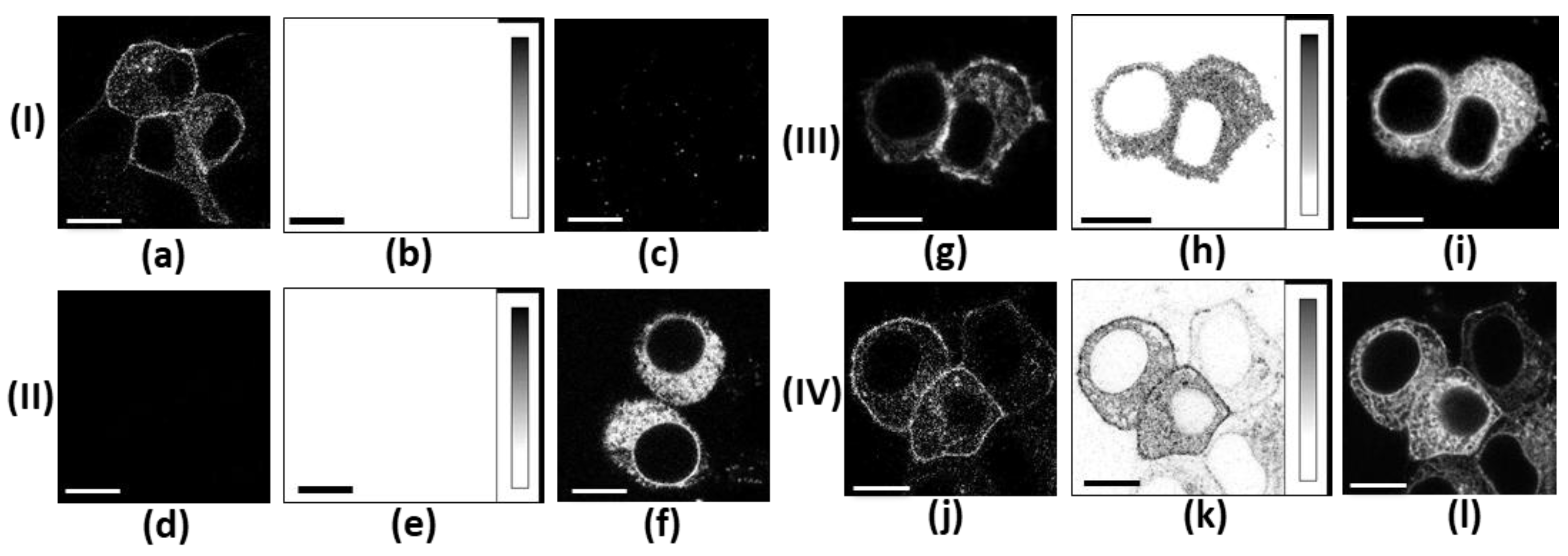
2.2. Distribution of K-Kv1.1m and K-Kv1.1m-1.2 in Cells
2.3. Interactions Between α-Subunits Kv1.2 and Kv1.1m in Cells
2.4. Electrophysiological Properties of Heterochannels Kv(1.1m-1.2)2
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Construction of Expression Plasmids
4.3. Cell Culture and Transfection
4.4. Electrophysiology Measurements
4.5. Confocal Microscopy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EA1 | episodic ataxia type 1 |
| FRET | Förster resonance energy transfer |
References
- Kamaleddin, M.A. Biophysical Properties of the Membrane Influence Spike Initiation Dynamics and Neuronal Excitability: A Focus on Kv1 Channels in Myelinated Axons. Proc. R. Soc. B Biol. Sci. 2025, 292, 20250687. [Google Scholar] [CrossRef]
- Shvetsova, A.A.; Gaynullina, D.K.; Tarasova, O.S.; Schubert, R. Remodeling of Arterial Tone Regulation in Postnatal Development: Focus on Smooth Muscle Cell Potassium Channels. Int. J. Mol. Sci. 2021, 22, 5413. [Google Scholar] [CrossRef]
- Carey, M.; Kehlenbrink, S.; Hawkins, M. Evidence for Central Regulation of Glucose Metabolism. J. Biol. Chem. 2013, 288, 34981–34988. [Google Scholar] [CrossRef]
- Glaudemans, B.; Knoers, N.V.A.M.; Hoenderop, J.G.J.; Bindels, R.J.M. New Molecular Players Facilitating Mg2+ Reabsorption in the Distal Convoluted Tubule. Kidney Int. 2010, 77, 17–22. [Google Scholar] [CrossRef]
- Bradding, P.; Wulff, H. The K+ Channels KCa3.1 and Kv1.3 as Novel Targets for Asthma Therapy. Br. J. Pharmacol. 2009, 157, 1330–1339. [Google Scholar] [CrossRef]
- Tao, M.; Ye, W.; Wu, Y.; Chang, W.; Liu, F.; Zhu, Y. Identification and Validation of Five Novel Protein Targets for Type 2 Diabetes Mellitus. Sci. Rep. 2025, 15, 12127. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, M.J.; Sala, L.; Denjoy, I.; Wada, Y.; Kozek, K.; Crotti, L.; Dagradi, F.; Kotta, M.C.; Spazzolini, C.; Leenhardt, A.; et al. Continuous Bayesian Variant Interpretation Accounts for Incomplete Penetrance among Mendelian Cardiac Channelopathies. Genet. Med. 2023, 25, 100355. [Google Scholar] [CrossRef] [PubMed]
- Alam, K.A.; Svalastoga, P.; Martinez, A.; Glennon, J.C.; Haavik, J. Potassium Channels in Behavioral Brain Disorders. Molecular Mechanisms and Therapeutic Potential: A Narrative Review. Neurosci. Biobehav. Rev. 2023, 152, 105301. [Google Scholar] [CrossRef] [PubMed]
- Villa, C.; Combi, R. Potassium Channels and Human Epileptic Phenotypes: An Updated Overview. Front. Cell. Neurosci. 2016, 10, 81. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, J. Voltage-Gated Potassium Channels and Genetic Epilepsy. Front. Neurol. 2024, 15, 1466075. [Google Scholar] [CrossRef]
- Paulhus, K.; Ammerman, L.; Glasscock, E. Clinical Spectrum of KCNA1 Mutations: New Insights into Episodic Ataxia and Epilepsy Comorbidity. Int. J. Mol. Sci. 2020, 21, 2802. [Google Scholar] [CrossRef] [PubMed]
- Paulhus, K.; Glasscock, E. Novel Genetic Variants Expand the Functional, Molecular, and Pathological Diversity of KCNA1 Channelopathy. Int. J. Mol. Sci. 2023, 24, 8826. [Google Scholar] [CrossRef] [PubMed]
- D’adamo, M.C.; Liantonio, A.; Rolland, J.F.; Pessia, M.; Imbrici, P. Kv1.1 Channelopathies: Pathophysiological Mechanisms and Therapeutic Approaches. Int. J. Mol. Sci. 2020, 21, 2935. [Google Scholar] [CrossRef]
- Gonzalez, C.; Baez-Nieto, D.; Valencia, I.; Oyarzun, I.; Rojas, P.; Naranjo, D.; Latorre, R. K+ Channels: Function-Structural Overview. Compr. Physiol. 2012, 2, 2087–2149. [Google Scholar] [CrossRef]
- Coleman, S.K.; Newcombe, J.; Pryke, J.; Dolly, J.O. Subunit Composition of Kv1 Channels in Human CNS. J. Neurochem. 1999, 73, 849–858. [Google Scholar] [CrossRef]
- Scott, V.E.S.; Muniz, Z.M.; Sewing, S.; Lichtinghagen, R.; Parcej, D.N.; Pongs, O.; Dolly, J.O. Antibodies Specific for Distinct Kv Subunits Unveil a HeteroOligomeric Basis for Subtypes of α-Dendrotoxin-Sensitive K+ Channels in Bovine Brain. Biochemistry 1994, 33, 1617–1623. [Google Scholar] [CrossRef]
- Wang, H.; Kunkel, D.D.; Martin, T.M.; Schwartzkroin, P.A.; Tempel, B.L. Heteromultimeric K+ Channels in Terminal and Juxtaparanodal Regions of Neurons. Nature 1993, 365, 75–79. [Google Scholar] [CrossRef]
- Lorincz, A.; Nusser, Z. Cell-Type-Dependent Molecular Composition of the Axon Initial Segment. J. Neurosci. 2008, 28, 14329–14340. [Google Scholar] [CrossRef]
- Pinatel, D.; Faivre-Sarrailh, C. Assembly and Function of the Juxtaparanodal Kv1 Complex in Health and Disease. Life 2020, 11, 8. [Google Scholar] [CrossRef]
- Ovsepian, S.V.; Leberre, M.; Steuber, V.; O’Leary, V.B.; Leibold, C.; Oliver Dolly, J. Distinctive Role of KV1.1 Subunit in the Biology and Functions of Low Threshold K(+) Channels with Implications for Neurological Disease. Pharmacol. Ther. 2016, 159, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Simeone, T.A.; Simeone, K.A.; Samson, K.K.; Kim, D.Y.; Rho, J.M. Loss of the Kv1.1 Potassium Channel Promotes Pathologic Sharp Waves and High Frequency Oscillations in in Vitro Hippocampal Slices. Neurobiol. Dis. 2013, 54, 68–81. [Google Scholar] [CrossRef]
- Smart, S.L.; Lopantsev, V.; Zhang, C.L.; Robbins, C.A.; Wang, H.; Chiu, S.Y.; Schwartzkroin, P.A.; Messing, A.; Tempel, B.L. Deletion of the K(v)1.1 Potassium Channel Causes Epilepsy in Mice. Neuron 1998, 20, 809–819. [Google Scholar] [CrossRef]
- Bagchi, B.; Al-Sabi, A.; Kaza, S.; Scholz, D.; O’Leary, V.B.; Dolly, J.O.; Ovsepian, S.V. Disruption of Myelin Leads to Ectopic Expression of K(V)1.1 Channels with Abnormal Conductivity of Optic Nerve Axons in a Cuprizone-Induced Model of Demyelination. PLoS ONE 2014, 9, e87736. [Google Scholar] [CrossRef]
- Zuberi, S.; Eunson, L.; Spauschus, A.; De Silva, R.; Tolmie, J.; Wood, N.; McWilliam, R.; Stephenson, J.; Kullmann, D.; Hanna, M.G. A Novel Mutation in the Human Voltage-Gated Potassium Channel Gene (Kv1.1) Associates with Episodic Ataxia Type 1 and Sometimes with Partial Epilepsy. Brain 1999, 122, 817–825. [Google Scholar] [CrossRef]
- Scheffer, H.; Brunt, E.R.P.; Mol, G.J.J.; Van der Vlies, P.; Stulp, R.P.; Verlind, E.; Mantel, G.; Averyanov, Y.N.; Hofstra, R.M.W.; Buys, C.H.C.M. Three Novel KCNA1 Mutations in Episodic Ataxia Type I Families. Hum. Genet. 1998, 102, 464–466. [Google Scholar] [CrossRef]
- Çomu, S.; Giuliani, M.; Narayanan, V. Episodic Ataxia and Myokymia Syndrome: A New Mutation of Potassium Channel Gene Kv1.1. Ann. Neurol. 1996, 40, 684–687. [Google Scholar] [CrossRef]
- Chen, H.; Von Hehn, C.; Kaczmarek, L.K.; Ment, L.R.; Pober, B.R.; Hisama, F.M. Functional Analysis of a Novel Potassium Channel (KCNA1) Mutation in Hereditary Myokymia. Neurogenetics 2007, 8, 131–135. [Google Scholar] [CrossRef]
- Kinali, M.; Jungbluth, H.; Eunson, L.H.; Sewry, C.A.; Manzur, A.Y.; Mercuri, E.; Hanna, M.G.; Muntoni, F. Expanding the Phenotype of Potassium Channelopathy: Severe Neuromyotonia and Skeletal Deformities without Prominent Episodic Ataxia. Neuromuscul. Disord. 2004, 14, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Brownstein, C.A.; Beggs, A.H.; Rodan, L.; Shi, J.; Towne, M.C.; Pelletier, R.; Cao, S.; Rosenberg, P.A.; Urion, D.K.; Picker, J.; et al. Clinical Heterogeneity Associated with KCNA1 Mutations Include Cataplexy and Nonataxic Presentations. Neurogenetics 2016, 17, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Rea, R.; Spauschus, A.; Eunson, L.H.; Hanna, M.G.; Kullmann, D.M. Variable K(+) Channel Subunit Dysfunction in Inherited Mutations of KCNA1. J. Physiol. 2002, 538, 5–23. [Google Scholar] [CrossRef] [PubMed]
- Spauschus, A.; Eunson, L.; Hanna, M.G.; Kullmann, D.M. Functional Characterization of a Novel Mutation in KCNA1 in Episodic Ataxia Type 1 Associated with Epilepsy. Ann. N. Y. Acad. Sci. 1999, 868, 442–446. [Google Scholar] [CrossRef]
- Efremenko, A.V.; Kryukova, E.V.; Kazakov, O.V.; Ignatova, A.A.; Shmatin, I.I.; Korabeynikova, V.N.; Toporova, V.A.; Yakimov, S.A.; Kirpichnikov, M.P.; Nekrasova, O.V.; et al. Heterochannels Kv(1.1-1.2)2 and Their Interactions with Pore Blockers. Cells 2025, 14, 1364. [Google Scholar] [CrossRef]
- Orlov, N.A.; Kryukova, E.V.; Efremenko, A.V.; Yakimov, S.A.; Toporova, V.A.; Kirpichnikov, M.P.; Nekrasova, O.V.; Feofanov, A.V. Interactions of the Kv1.1 Channel with Peptide Pore Blockers: A Fluorescent Analysis on Mammalian Cells. Membranes 2023, 13, 645. [Google Scholar] [CrossRef]
- Efremenko, A.V.; Nekrasova, O.V.; Feofanov, A.V. Formation of Heterotetrameric Potassium Channels Kv1.1–Kv1.2 in Neuro-2A Cells: Analysis by the Förster Resonance Energy Transfer Technique. Biophysics 2025, 70, 69–75. [Google Scholar] [CrossRef]
- Ignatova, A.A.; Kryukova, E.V.; Novoseletsky, V.N.; Kazakov, O.V.; Orlov, N.A.; Korabeynikova, V.N.; Larina, M.V.; Fradkov, A.F.; Yakimov, S.A.; Kirpichnikov, M.P.; et al. New High-Affinity Peptide Ligands for Kv1.2 Channel: Selective Blockers and Fluorescent Probes. Cells 2024, 13, 2096. [Google Scholar] [CrossRef] [PubMed]
- Capera, J.; Serrano-Novillo, C.; Navarro-Pérez, M.; Cassinelli, S.; Felipe, A. The Potassium Channel Odyssey: Mechanisms of Traffic and Membrane Arrangement. Int. J. Mol. Sci. 2019, 20, 734. [Google Scholar] [CrossRef] [PubMed]
- Manganas, L.N.; Wang, Q.; Scannevin, R.H.; Antonucci, D.E.; Rhodes, K.J.; Trimmer, J.S. Identification of a Trafficking Determinant Localized to the Kv1 Potassium Channel Pore. Proc. Natl. Acad. Sci. USA 2001, 98, 14055–14059. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Watanabe, I.; Gomez, B.; Thornhill, W.B. Determinants Involved in Kv1 Potassium Channel Folding in the Endoplasmic Reticulum, Glycosylation in the Golgi, and Cell Surface Expression. J. Biol. Chem. 2001, 276, 39419–39427. [Google Scholar] [CrossRef]
- Orlov, N.A.; Ignatova, A.A.; Kryukova, E.V.; Yakimov, S.A.; Kirpichnikov, M.P.; Nekrasova, O.V.; Feofanov, A.V. Combining MKate2-Kv1.3 Channel and Atto488-Hongotoxin for the Studies of Peptide Pore Blockers on Living Eukaryotic Cells. Toxins 2022, 14, 858. [Google Scholar] [CrossRef]
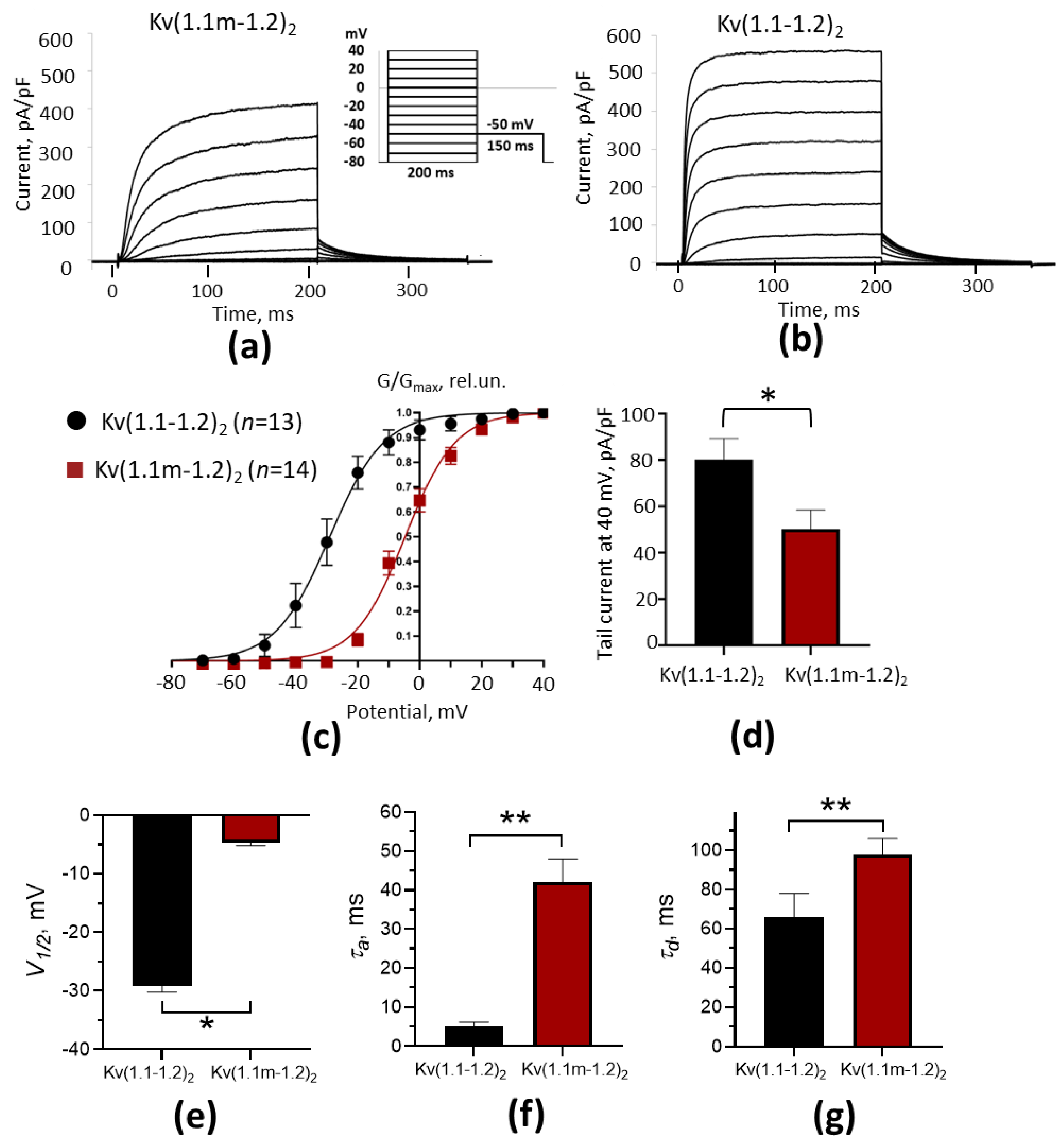
| Channels | V1/2 ¶, mV | τa, ms | τd, ms |
|---|---|---|---|
| Kv(1.1-1.2)2 | −29.0 ± 1.1 (n = 13) | 5.1 ± 1.1 (n = 11) | 66 ± 12 (n = 11) |
| Kv(1.1m-1.2)2 | −4.7 ± 0.5 (n = 14), p * < 0.0001 | 42 ± 6 (n = 11), p * < 0.0001 | 98 ± 8 (n = 11), p * = 0.038 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ignatova, A.A.; Efremenko, A.V.; Abramochkin, D.V.; Dzhumaniiazova, I.; Shmatin, I.I.; Kirpichnikov, M.P.; Feofanov, A.V.; Nekrasova, O.V. Properties of Heterochannels Kv(1.1-1.2)2 with Mutation T226R in the Kv1.1 Subunit. Int. J. Mol. Sci. 2025, 26, 9730. https://doi.org/10.3390/ijms26199730
Ignatova AA, Efremenko AV, Abramochkin DV, Dzhumaniiazova I, Shmatin II, Kirpichnikov MP, Feofanov AV, Nekrasova OV. Properties of Heterochannels Kv(1.1-1.2)2 with Mutation T226R in the Kv1.1 Subunit. International Journal of Molecular Sciences. 2025; 26(19):9730. https://doi.org/10.3390/ijms26199730
Chicago/Turabian StyleIgnatova, Anastasia A., Anastasia V. Efremenko, Denis V. Abramochkin, Irina Dzhumaniiazova, Ivan I. Shmatin, Mikhail P. Kirpichnikov, Alexey V. Feofanov, and Oksana V. Nekrasova. 2025. "Properties of Heterochannels Kv(1.1-1.2)2 with Mutation T226R in the Kv1.1 Subunit" International Journal of Molecular Sciences 26, no. 19: 9730. https://doi.org/10.3390/ijms26199730
APA StyleIgnatova, A. A., Efremenko, A. V., Abramochkin, D. V., Dzhumaniiazova, I., Shmatin, I. I., Kirpichnikov, M. P., Feofanov, A. V., & Nekrasova, O. V. (2025). Properties of Heterochannels Kv(1.1-1.2)2 with Mutation T226R in the Kv1.1 Subunit. International Journal of Molecular Sciences, 26(19), 9730. https://doi.org/10.3390/ijms26199730








