Advances in Mammalian Metallomics: New Insights into Metal Dynamics and Biological Significance
Abstract
1. Introduction
2. The Advancements in Metallomics Technologies
2.1. Application of Spectroscopy in Metallomics
2.2. Application of Mass Spectrometry in Metallomics
2.3. Application of Imaging Techniques in Metallomics
2.4. Challenges in the Standardization of Metal Detection
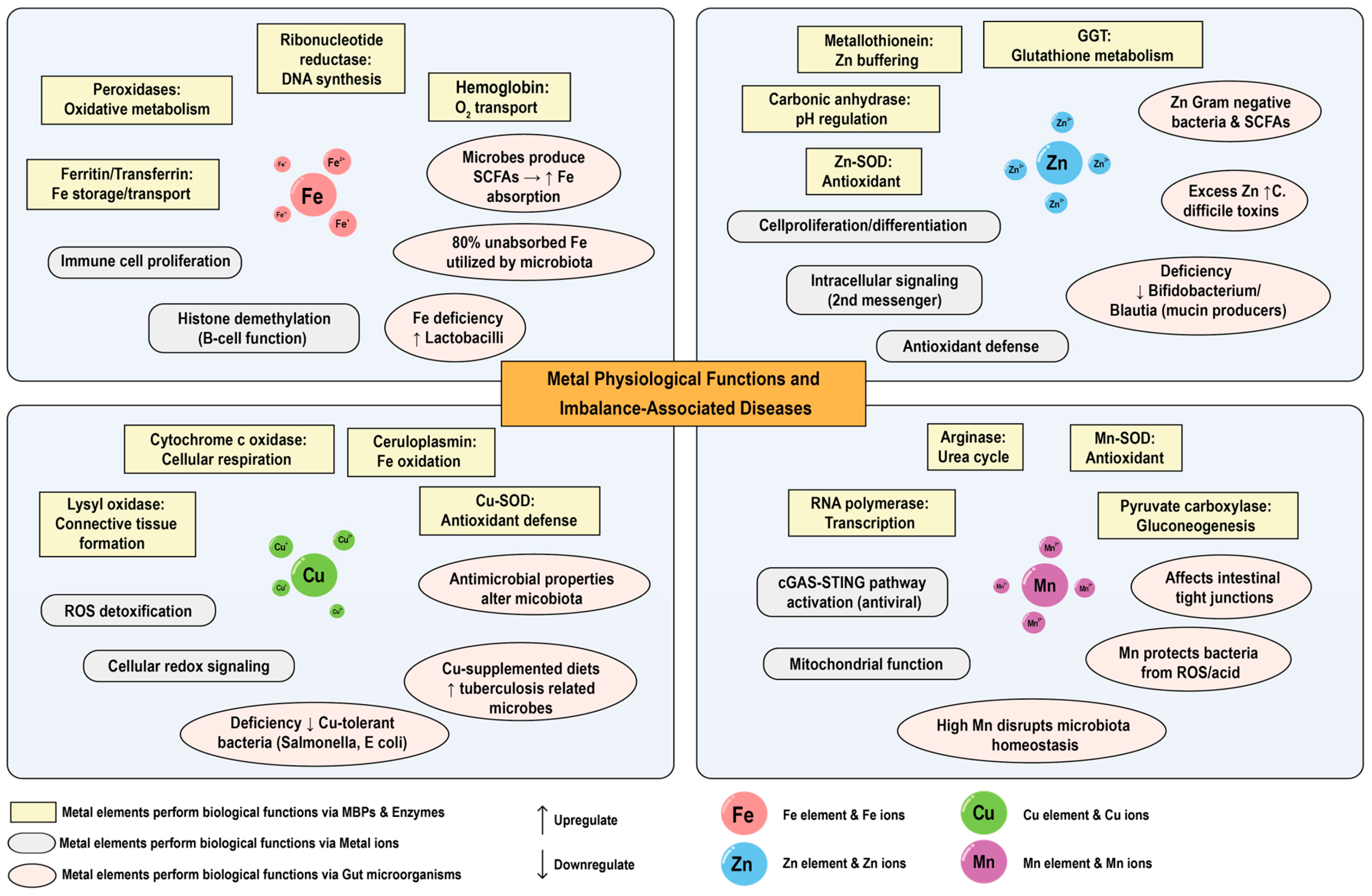
3. Physiological Functions of Metal Elements in Mammals
3.1. Biological Functions of Metal Elements
3.2. The Association Between Metal Imbalance and Diseases
3.3. The Applications of Metal Detection in Clinical Diagnosis
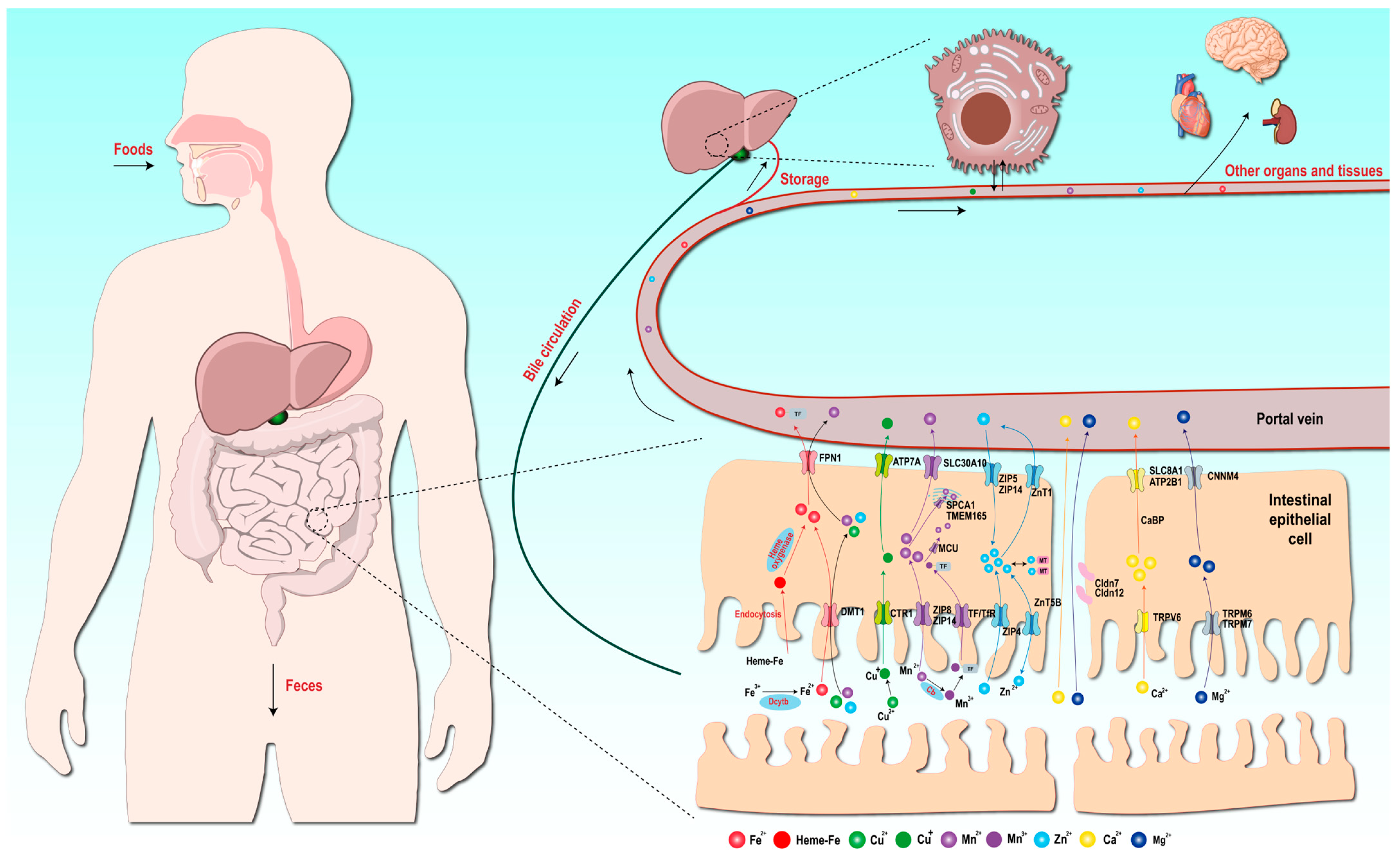
4. Absorption Mechanisms of Metal Elements
4.1. Absorption Mechanism of Iron Elements
4.2. Absorption Mechanism of Copper Elements
4.3. Absorption Mechanism of Manganese Elements
4.4. Absorption Mechanism of Zinc Elements
4.5. Absorption Mechanisms of Other Macroelements
5. Dynamic Landscape of the Metal Elements in Mammals
5.1. Dynamics of Metal Elements in Milk During Lactation
5.2. Dynamics of Metal Elements in Mammals with Age
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cook, T.R.; Vajpayee, V.; Lee, M.H.; Stang, P.J.; Chi, K.-W. Biomedical and Biochemical Applications of Self-Assembled Metallacycles and Metallacages. Acc. Chem. Res. 2013, 46, 2464–2474. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, J. Bioinformatics of Metalloproteins and Metalloproteomes. Molecules 2020, 25, 3366. [Google Scholar] [CrossRef]
- Gujre, N.; Mitra, S.; Soni, A.; Agnihotri, R.; Rangan, L.; Rene, E.R.; Sharma, M.P. Speciation, Contamination, Ecological and Human Health Risks Assessment of Heavy Metals in Soils Dumped with Municipal Solid Wastes. Chemosphere 2021, 262, 128013. [Google Scholar] [CrossRef] [PubMed]
- Mohmand, J.; Eqani, S.A.M.A.S.; Fasola, M.; Alamdar, A.; Mustafa, I.; Ali, N.; Liu, L.; Peng, S.; Shen, H. Human Exposure to Toxic Metals via Contaminated Dust: Bio-Accumulation Trends and Their Potential Risk Estimation. Chemosphere 2015, 132, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.-X.; Wang, Z.-H.; Sun, Y.-D.; Wang, L.-L.; Li, M.; Liu, Y.-T.; Zhang, H.-M.; Jing, P.-W.; Shi, Q.-F.; Yu, Y.-H. Molecular Mechanism of Plant Response to Copper Stress: A Review. Environ. Exp. Bot. 2024, 218, 105590. [Google Scholar] [CrossRef]
- Shahid, M.; Dumat, C.; Khalid, S.; Schreck, E.; Xiong, T.; Niazi, N.K. Foliar Heavy Metal Uptake, Toxicity and Detoxification in Plants: A Comparison of Foliar and Root Metal Uptake. J. Hazard. Mater. 2017, 325, 36–58. [Google Scholar] [CrossRef]
- Busser, B.; Moncayo, S.; Coll, J.-L.; Sancey, L.; Motto-Ros, V. Elemental Imaging Using Laser-Induced Breakdown Spectroscopy: A New and Promising Approach for Biological and Medical Applications. Coord. Chem. Rev. 2018, 358, 70–79. [Google Scholar] [CrossRef]
- Mu, Q.; Chen, L.; Gao, X.; Shen, S.; Sheng, W.; Min, J.; Wang, F. The Role of Iron Homeostasis in Remodeling Immune Function and Regulating Inflammatory Disease. Sci. Bull. 2021, 66, 1806–1816. [Google Scholar] [CrossRef]
- Skalny, A.V.; Aschner, M.; Lei, X.G.; Gritsenko, V.A.; Santamaria, A.; Alekseenko, S.I.; Prakash, N.T.; Chang, J.-S.; Sizova, E.A.; Chao, J.C.J.; et al. Gut Microbiota as a Mediator of Essential and Toxic Effects of Zinc in the Intestines and Other Tissues. Int. J. Mol. Sci. 2021, 22, 13074. [Google Scholar] [CrossRef]
- Chen, B.; Yu, P.; Chan, W.N.; Xie, F.; Zhang, Y.; Liang, L.; Leung, K.T.; Lo, K.W.; Yu, J.; Tse, G.M.K.; et al. Cellular Zinc Metabolism and Zinc Signaling: From Biological Functions to Diseases and Therapeutic Targets. Signal Transduct. Target. Ther. 2024, 9, 6. [Google Scholar] [CrossRef]
- Chen, L.; Min, J.; Wang, F. Copper Homeostasis and Cuproptosis in Health and Disease. Signal Transduct. Target. Ther. 2022, 7, 378. [Google Scholar] [CrossRef]
- Mezzanotte, M.; Stanga, S. Brain Iron Dyshomeostasis and Ferroptosis in Alzheimer’s Disease Pathophysiology: Two Faces of the Same Coin. Aging Dis. 2024, 16, 2615–2640. [Google Scholar] [CrossRef]
- Doroszkiewicz, J.; Farhan, J.A.; Mroczko, J.; Winkel, I.; Perkowski, M.; Mroczko, B. Common and Trace Metals in Alzheimer’s and Parkinson’s Diseases. Int. J. Mol. Sci. 2023, 24, 15721. [Google Scholar] [CrossRef] [PubMed]
- Ilich, J.Z. Osteosarcopenic Adiposity Syndrome Update and the Role of Associated Minerals and Vitamins. Proc. Nutr. Soc. 2021, 80, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Fedosova, N.U.; Habeck, M.; Nissen, P. Structure and Function of Na, K-ATPase—The Sodium-Potassium Pump. Compr. Physiol. 2022, 12, 2659–2679. [Google Scholar] [CrossRef]
- Garza-Lombo, C.; Posadas, Y.; Quintanar, L.; Gonsebatt, M.E.; Franco, R. Neurotoxicity Linked to Dysfunctional Metal Ion Homeostasis and Xenobiotic Metal Exposure: Redox Signaling and Oxidative Stress. Antioxid. Redox Signal. 2018, 28, 1669–1703. [Google Scholar] [CrossRef]
- Wang, X.; An, P.; Gu, Z.; Luo, Y.; Luo, J. Mitochondrial Metal Ion Transport in Cell Metabolism and Disease. Int. J. Mol. Sci. 2021, 22, 7525. [Google Scholar] [CrossRef]
- Jomova, K.; Makova, M.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Rhodes, C.J.; Valko, M. Essential Metals in Health and Disease. Chem.-Biol. Interact. 2022, 367, 110173. [Google Scholar] [CrossRef]
- Zoroddu, M.A.; Aaseth, J.; Crisponi, G.; Medici, S.; Peana, M.; Nurchi, V.M. The Essential Metals for Humans: A Brief Overview. J. Inorg. Biochem. 2019, 195, 120–129. [Google Scholar] [CrossRef]
- Das, N.; Raymick, J.; Sarkar, S. Role of Metals in Alzheimer’s Disease. Metab. Brain Dis. 2021, 36, 1627–1639. [Google Scholar] [CrossRef]
- Wang, S.; Qin, M.; Fan, X.; Jiang, C.; Hou, Q.; Ye, Z.; Zhang, X.; Yang, Y.; Xiao, J.; Wallace, K.; et al. The Role of Metal Ions in Stroke: Current Evidence and Future Perspectives. Ageing Res. Rev. 2024, 101, 102498. [Google Scholar] [CrossRef]
- Morel, J.-D.; Sauzéat, L.; Goeminne, L.J.E.; Jha, P.; Williams, E.; Houtkooper, R.H.; Aebersold, R.; Auwerx, J.; Balter, V. The Mouse Metallomic Landscape of Aging and Metabolism. Nat. Commun. 2022, 13, 607. [Google Scholar] [CrossRef]
- Lönnerdal, B. Trace Element Transport in the Mammary Gland. Annu. Rev. Nutr. 2007, 27, 165–177. [Google Scholar] [CrossRef]
- Li, C.; Xu, W.; Chu, S.; Zheng, Z.; Xiao, Y.; Li, L.; Bi, H.; Wei, L. The Chemical Speciation, Spatial Distribution and Toxicity of Mercury from Tibetan Medicine Zuotai, β-HgS and HgCl2 in Mouse Kidney. J. Trace Elem. Med. Biol. 2018, 45, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Jim, V.; LaViolette, C.; Briehl, M.M.; Ingram, J.C. Spatial Distribution of Uranium in Mice Kidneys Detected by Laser Ablation Inductively Coupled Plasma Mass Spectrometry. J. Appl. Bioanal. 2017, 3, 43–48. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sun, S.; Xie, E.; Xu, S.; Ji, S.; Wang, S.; Shen, J.; Wang, R.; Shen, X.; Su, Y.; Song, Z.; et al. The Intestinal Transporter SLC30A1 Plays a Critical Role in Regulating Systemic Zinc Homeostasis. Adv. Sci. 2024, 11, e2406421. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Wei, T.; Wang, X.; Liu, Y.; Tan, Z.; Zhang, Y.; Feng, T.; Cheng, Y.; Wang, F.; Ma, B.; et al. Discovery of Metal-Binding Proteins by Thermal Proteome Profiling. Nat. Chem. Biol. 2024, 20, 770–778. [Google Scholar] [CrossRef]
- Evans, E.H.; Pisonero, J.; Smith, C.M.M.; Taylor, R.N. Atomic Spectrometry Update: Review of Advances in Atomic Spectrometry and Related Techniques. J. Anal. At. Spectrom. 2024, 39, 1188–1211. [Google Scholar] [CrossRef]
- Xu, M.-L.; Gao, Y.; Han, X.X.; Zhao, B. Detection of Pesticide Residues in Food Using Surface-Enhanced Raman Spectroscopy: A Review. J. Agric. Food Chem. 2017, 65, 6719–6726. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; Bezerra, M.A.; Santos, A.S.; dos Santos, W.N.L.; Novaes, C.G.; de Oliveira, O.M.C.; Oliveira, M.L.; Garcia, R.L. Atomic Absorption Spectrometry—A Multi Element Technique. TrAC Trends Anal. Chem. 2018, 100, 1–6. [Google Scholar] [CrossRef]
- Çoker, C.; Ersöz, B.; Habif, S.; Çetiner, N.; Gültekin, Y. Elemental Analysis of Serum by Inductively Coupled Plasma Atomic Emission Spectroscopy in Comparison to Atomic Absorption Spectroscopy. Turk. J. Med. Sci. 1996, 26, 553–557. [Google Scholar] [CrossRef]
- Offenbacher, E.; Spencer, H.; Dowling, H.; Pi-Sunyer, F. Metabolic Chromium Balances in Men. Am. J. Clin. Nutr. 1986, 44, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Griffin, J.L.; Walker, L.A.; Shore, R.F.; Nicholson, J.K. Metabolic Profiling of Chronic Cadmium Exposure in the Rat. Chem. Res. Toxicol. 2001, 14, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Rossipal, E.; Krachler, M. Pattern of Trace Elements in Human Milk during the Course of Lactation. Nutr. Res. 1998, 18, 11–24. [Google Scholar] [CrossRef]
- Keen, C.L.; Lönnerdal, B.; Clegg, M.; Hurley, L.S. Developmental Changes in Composition of Rat Milk: Trace Elements, Minerals, Protein, Carbohydrate and Fat. J. Nutr. 1981, 111, 226–236. [Google Scholar] [CrossRef]
- Bulska, E.; Wagner, B. Quantitative Aspects of Inductively Coupled Plasma Mass Spectrometry. Philos. Trans. A Math. Phys. Eng. Sci. 2016, 374, 20150369. [Google Scholar] [CrossRef]
- Douvris, C.; Vaughan, T.; Bussan, D.; Bartzas, G.; Thomas, R. How ICP-OES Changed the Face of Trace Element Analysis: Review of the Global Application Landscape. Sci. Total Environ. 2023, 905, 167242. [Google Scholar] [CrossRef]
- Jagodić, J.; Rovčanin, B.; Krstić, Đ.; Paunović, I.; Živaljević, V.; Manojlović, D.; Stojsavljević, A. Elemental Profiling of Adrenal Adenomas in Solid Tissue and Blood Samples by ICP-MS and ICP-OES. Microchem. J. 2021, 165, 106194. [Google Scholar] [CrossRef]
- Parra-Arroyo, L.; González-González, R.B.; Castillo-Zacarías, C.; Melchor Martínez, E.M.; Sosa-Hernández, J.E.; Bilal, M.; Iqbal, H.M.N.; Barceló, D.; Parra-Saldívar, R. Highly Hazardous Pesticides and Related Pollutants: Toxicological, Regulatory, and Analytical Aspects. Sci. Total Environ. 2022, 807, 151879. [Google Scholar] [CrossRef]
- Li, F.; Ge, L.; Tang, Z.; Chen, Y.; Wang, J. Recent Developments on XRF Spectra Evaluation. Appl. Spectrosc. Rev. 2020, 55, 263–287. [Google Scholar] [CrossRef]
- Fernández Ruiz, R. TXRF Spectrometry in the Bioanalytical Sciences: A Brief Review. X-Ray Spectrom. 2022, 51, 279–293. [Google Scholar] [CrossRef]
- da Costa, M.V.; Lima, G.J.d.O.; Guilherme, L.R.G.; Carneiro, M.A.C.; Ribeiro, B.T. Towards Direct and Eco-Friendly Analysis of Plants Using Portable X-Ray Fluorescence Spectrometry: A Methodological Approach. Chemosphere 2023, 339, 139613. [Google Scholar] [CrossRef]
- Maltsev, A.S.; Pashkova, G.V.; Fernández-Ruiz, R.; Demonterova, E.I.; Shuliumova, A.N.; Umarova, N.N.; Shergin, D.L.; Mukhamedova, M.M.; Chubarov, V.M.; Mikheeva, E.A. Characterization of Archaeological Ceramics from Eastern Siberia by Total-Reflection X-Ray Fluorescence Spectrometry and Principal Component Analysis. Spectrochim. Acta Part. B At. Spectrosc. 2021, 175, 106012. [Google Scholar] [CrossRef]
- Pashkova, G.V.; Smagunova, A.N.; Finkelshtein, A.L. X-Ray Fluorescence Analysis of Milk and Dairy Products: A Review. TrAC Trends Anal. Chem. 2018, 106, 183–189. [Google Scholar] [CrossRef]
- Lossow, K.; Schlörmann, W.; Tuchtenhagen, M.; Schwarz, M.; Schwerdtle, T.; Kipp, A.P. Measurement of Trace Elements in Murine Liver Tissue Samples: Comparison between ICP-MS/MS and TXRF. J. Trace Elem. Med. Biol. 2023, 78, 127167. [Google Scholar] [CrossRef] [PubMed]
- Vogiatzis, C.G.; Zachariadis, G.A. Tandem Mass Spectrometry in Metallomics and the Involving Role of ICP-MS Detection: A Review. Anal. Chim. Acta 2014, 819, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Shi, S. A Novel Strategy to Determine the Compositions of Inorganic Elements in Fruit Wines Using ICP-MS/MS. Food Chem. 2019, 299, 125172. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Shi, S.-Y.; Chen, X.-Q. Accurate Quantification of Toxic Elements in Medicine Food Homologous Plants Using ICP-MS/MS. Food Chem. 2018, 245, 692–697. [Google Scholar] [CrossRef]
- Baudry, J.; Kopp, J.F.; Boeing, H.; Kipp, A.P.; Schwerdtle, T.; Schulze, M.B. Changes of Trace Element Status during Aging: Results of the EPIC-Potsdam Cohort Study. Eur. J. Nutr. 2020, 59, 3045–3058. [Google Scholar] [CrossRef]
- Ling, W.; Zhao, G.; Wang, W.; Wang, C.; Zhang, L.; Zhang, H.; Lu, D.; Ruan, S.; Zhang, A.; Liu, Q.; et al. Metallomic Profiling and Natural Copper Isotopic Signatures of Childhood Autism in Serum and Red Blood Cells. Chemosphere 2023, 330, 138700. [Google Scholar] [CrossRef]
- Theiner, S.; Schoeberl, A.; Schweikert, A.; Keppler, B.K.; Koellensperger, G. Mass Spectrometry Techniques for Imaging and Detection of Metallodrugs. Curr. Opin. Chem. Biol. 2021, 61, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Sedmera, D. Mass Cytometry Imaging in Physiology. Acta Physiol. 2022, 235, e13822. [Google Scholar] [CrossRef] [PubMed]
- Schaier, M.; Theiner, S.; Baier, D.; Braun, G.; Berger, W.; Koellensperger, G. Multiparametric Tissue Characterization Utilizing the Cellular Metallome and Immuno-Mass Spectrometry Imaging. JACS Au 2023, 3, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Funke, S.K.I.; Sperling, M.; Karst, U. Weighted Linear Regression Improves Accuracy of Quantitative Elemental Bioimaging by Means of LA-ICP-MS. Anal. Chem. 2021, 93, 15720–15727. [Google Scholar] [CrossRef]
- Menero-Valdés, P.; Álvarez, L.; González-Iglesias, H.; Fernández, B.; Pereiro, R. Unveiling Compositional Images of Specific Proteins in Individual Cells by LA-ICP-MS: Labelling with Ruthenium Red and Metal Nanoclusters. Anal. Chim. Acta 2024, 1317, 342906. [Google Scholar] [CrossRef]
- Liu, J.; Cui, J.; Wei, X.; Li, W.; Liu, C.; Li, X.; Chen, M.; Fan, Y.; Wang, J. Investigation on Selenium and Mercury Interactions and the Distribution Patterns in Mice Organs with LA-ICP-MS Imaging. Anal. Chim. Acta 2021, 1182, 338941. [Google Scholar] [CrossRef]
- López-Fernández, H.; de Pessôa, G.S.; Arruda, M.A.Z.; Capelo-Martínez, J.L.; Fdez-Riverola, F.; Glez-Peña, D.; Reboiro-Jato, M. LA-iMageS: A Software for Elemental Distribution Bioimaging Using LA-ICP-MS Data. J. Cheminform 2016, 8, 65. [Google Scholar] [CrossRef]
- Westerhausen, M.T.; Bishop, D.P.; Dowd, A.; Wanagat, J.; Cole, N.; Doble, P.A. Super-Resolution Reconstruction for Two- and Three-Dimensional LA-ICP-MS Bioimaging. Anal. Chem. 2019, 91, 14879–14886. [Google Scholar] [CrossRef]
- Togao, M.; Nakayama, S.M.M.; Ikenaka, Y.; Mizukawa, H.; Makino, Y.; Kubota, A.; Matsukawa, T.; Yokoyama, K.; Hirata, T.; Ishizuka, M. Bioimaging of Pb and STIM1 in Mice Liver, Kidney and Brain Using Laser Ablation Inductively Coupled Plasma Mass Spectrometry (LA-ICP-MS) and Immunohistochemistry. Chemosphere 2020, 238, 124581. [Google Scholar] [CrossRef]
- Katsarou, A.; Pantopoulos, K. Basics and Principles of Cellular and Systemic Iron Homeostasis. Mol. Asp. Med. 2020, 75, 100866. [Google Scholar] [CrossRef]
- Ma, Y.; Fei, Y.; Ding, S.; Jiang, H.; Fang, J.; Liu, G. Trace Metal Elements: A Bridge between Host and Intestinal Microorganisms. Sci. China Life Sci. 2023, 66, 1976–1993. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Wang, H.; Xu, H.; Liu, Y.; Ma, B.; Chen, X.; Zeng, X.; Wang, X.; Wang, B.; Shiau, C.; et al. Co-Evolution-Based Prediction of Metal-Binding Sites in Proteomes by Machine Learning. Nat. Chem. Biol. 2023, 19, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Bielik, V.; Kolisek, M. Bioaccessibility and Bioavailability of Minerals in Relation to a Healthy Gut Microbiome. Int. J. Mol. Sci. 2021, 22, 6803. [Google Scholar] [CrossRef] [PubMed]
- Narasaki, Y.; You, A.S.; Malik, S.; Moore, L.W.; Bross, R.; Cervantes, M.K.; Daza, A.; Kovesdy, C.P.; Nguyen, D.; Kalantar-Zadeh, K.; et al. Dietary Potassium Intake, Kidney Function, and Survival in a Nationally Representative Cohort. Am. J. Clin. Nutr. 2022, 116, 1123–1134. [Google Scholar] [CrossRef]
- Jaques, D.A.; Wuerzner, G.; Ponte, B. Sodium Intake as a Cardiovascular Risk Factor: A Narrative Review. Nutrients 2021, 13, 3177. [Google Scholar] [CrossRef]
- Bernal, A.; Zafra, M.A.; Simon, M.J.; Mahia, J. Sodium Homeostasis, a Balance Necessary for Life. Nutrients 2023, 15, 395. [Google Scholar] [CrossRef]
- Crichton, R. Chapter 15—Nickel and Cobalt: Evolutionary Relics. In Biological Inorganic Chemistry, 3rd ed.; Crichton, R., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 435–457. ISBN 978-0-12-811741-5. [Google Scholar]
- Kieninger, C.; Baker, J.A.; Podewitz, M.; Wurst, K.; Jockusch, S.; Lawrence, A.D.; Deery, E.; Gruber, K.; Liedl, K.R.; Warren, M.J.; et al. Zinc Substitution of Cobalt in Vitamin B12: Zincobyric Acid and Zincobalamin as Luminescent Structural B12-Mimics. Angew. Chem. Int. Ed. Engl. 2019, 58, 14568–14572. [Google Scholar] [CrossRef]
- Adamus, J.P.; Ruszczyńska, A.; Wyczałkowska-Tomasik, A. Molybdenum’s Role as an Essential Element in Enzymes Catabolizing Redox Reactions: A Review. Biomolecules 2024, 14, 869. [Google Scholar] [CrossRef]
- Kumar, S.; Vinella, D.; De Reuse, H. Nickel, an Essential Virulence Determinant of Helicobacter Pylori: Transport and Trafficking Pathways and Their Targeting by Bismuth. Adv. Microb. Physiol. 2022, 80, 1–33. [Google Scholar] [CrossRef]
- Wolfram, L.; Bauerfeind, P. Activities of Urease and Nickel Uptake of Helicobacter Pylori Proteins Are Media- and Host-Dependent. Helicobacter 2009, 14, 264–270. [Google Scholar] [CrossRef]
- Shannon, M.C.; Hill, G.M. Trace Mineral Supplementation for the Intestinal Health of Young Monogastric Animals. Front. Vet. Sci. 2019, 6, 73. [Google Scholar] [CrossRef]
- Lee, Y.; Layman, D.; Bell, R.; Norton, H. Response of Glutathione-Peroxidase and Catalase to Excess Dietary Iron in Rats. J. Nutr. 1981, 111, 2195–2202. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Guan, Y.; Lv, M.; Zhang, R.; Guo, Z.; Wei, X.; Du, X.; Yang, J.; Li, T.; Wan, Y.; et al. Manganese Increases the Sensitivity of the cGAS-STING Pathway for Double-Stranded DNA and Is Required for the Host Defense against DNA Viruses. Immunity 2018, 48, 675–687.e7. [Google Scholar] [CrossRef] [PubMed]
- Horning, K.J.; Caito, S.W.; Tipps, K.G.; Bowman, A.B.; Aschner, M. Manganese Is Essential for Neuronal Health. In Annual Review of Nutrition; Bowman, B.A., Stover, P.J., Eds.; Annual Reviews: Palo Alto, CA, USA, 2015; Volume 35, pp. 71–108. ISBN 978-0-8243-2835-1. [Google Scholar]
- Crichton, R. Chapter 4—Biological Ligands for Metal Ions. In Biological Inorganic Chemistry, 3rd ed.; Crichton, R., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 81–118. ISBN 978-0-12-811741-5. [Google Scholar]
- Crichton, R. Chapter 1—An Overview of the Role of Metals in Biology. In Biological Inorganic Chemistry, 3rd ed.; Crichton, R., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 1–18. ISBN 978-0-12-811741-5. [Google Scholar]
- Hosmane, N.S. Chapter 12—Bioinorganic Chemistry and Applications. In Advanced Inorganic Chemistry; Hosmane, N.S., Ed.; Academic Press: Boston, MA, USA, 2017; pp. 225–249. ISBN 978-0-12-801982-5. [Google Scholar]
- Billesbolle, C.B.; Azumaya, C.M.; Kretsch, R.C.; Powers, A.S.; Gonen, S.; Schneider, S.; Arvedson, T.; Dror, R.O.; Cheng, Y.; Manglik, A. Structure of Hepcidin-Bound Ferroportin Reveals Iron Homeostatic Mechanisms. Nature 2020, 586, 807–811. [Google Scholar] [CrossRef] [PubMed]
- Scholz, G.A.; Xie, S.; Arsiwala, T.; Guggisberg, D.; Vogel, M.; Bachmann, M.; Moller, B. Low Iron Diet Improves Clinical Arthritis in the Mouse Model of Collagen-Induced Arthritis. Cells 2024, 13, 1792. [Google Scholar] [CrossRef]
- Liu, S.; Wei, Y.; Liang, Y.; Du, P.; Lei, P.; Yu, D.; Zhang, H. Engineering Nanozymes for Tumor Therapy via Ferroptosis Self-Amplification. Adv. Healthc. Mater. 2024, 13, 2400307. [Google Scholar] [CrossRef]
- Yarmishyn, A.A.; Kremenskoy, M.; Batagov, A.O.; Preuss, A.; Wong, J.H.; Kurochkin, I.V. Genome-Wide Analysis of mRNAs Associated with Mouse Peroxisomes. BMC Genom. 2016, 17, 1028. [Google Scholar] [CrossRef]
- Aboelnga, M.M. Exploring the Structure Function Relationship of Heme Peroxidases: Molecular Dynamics Study on Cytochrome c Peroxidase Variants. Comput. Biol. Med. 2022, 146, 105544. [Google Scholar] [CrossRef]
- Klaessens, S.; Stroobant, V.; Hoffmann, D.; Gyrd-Hansen, M.; Pilotte, L.; Vigneron, N.; De Plaen, E.; Van den Eynde, B.J. Tryptophanemia Is Controlled by a Tryptophan-Sensing Mechanism Ubiquitinating Tryptophan 2,3-Dioxygenase. Proc. Natl. Acad. Sci. USA 2021, 118, e2022447118. [Google Scholar] [CrossRef]
- Ward, D.M.; Cloonan, S.M. Mitochondrial Iron in Human Health and Disease. In Annual Review of Physiology; Nelson, M.T., Walsh, K., Eds.; Annual Reviews: Palo Alto, CA, USA, 2019; Volume 81, pp. 453–482. ISBN 978-0-8243-0381-5. [Google Scholar]
- Puig, S.; Ramos-Alonso, L.; Maria Romero, A.; Martinez-Pastor, M.T. The Elemental Role of Iron in DNA Synthesis and Repair. Metallomics 2017, 9, 1483–1500. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, W.; Yan, F.; Dai, W.; Fang, F.; Gao, Y.; Cui, W. Amelioration Effects of the Soybean Lecithin-Gallic Acid Complex on Iron-Overload-Induced Oxidative Stress and Liver Damage in C57BL/6J Mice. Pharm. Biol. 2023, 61, 37–49. [Google Scholar] [CrossRef]
- Solomon, E.I.; Gipson, R.R. Chapter Two—Spectroscopic Definition of Ferrous Active Sites in Non-Heme Iron Enzymes. In Methods in Enzymology; Bridwell-Rabb, J., Ed.; Mononuclear Non-heme Iron Dependent Enzymes Part A; Academic Press: Cambridge, MA, USA, 2024; Volume 703, pp. 29–49. [Google Scholar]
- Crichton, R. Chapter 13—Iron: Essential for Almost All Life. In Biological Inorganic Chemistry, 3rd ed.; Crichton, R., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 363–404. ISBN 978-0-12-811741-5. [Google Scholar]
- Boyd, S.D.; Ullrich, M.S.; Skopp, A.; Winkler, D.D. Copper Sources for Sod1 Activation. Antioxidants 2020, 9, 500. [Google Scholar] [CrossRef]
- Ramos, D.; Mar, D.; Ishida, M.; Vargas, R.; Gaite, M.; Montgomery, A.; Linder, M.C. Mechanism of Copper Uptake from Blood Plasma Ceruloplasmin by Mammalian Cells. PLoS ONE 2016, 11, e0149516. [Google Scholar] [CrossRef]
- Kalpage, H.A.; Wan, J.; Morse, P.T.; Zurek, M.P.; Turner, A.A.; Khobeir, A.; Yazdi, N.; Hakim, L.; Liu, J.; Vaishnav, A.; et al. Cytochrome c Phosphorylation: Control of Mitochondrial Electron Transport Chain Flux and Apoptosis. Int. J. Biochem. Cell Biol. 2020, 121, 105704. [Google Scholar] [CrossRef] [PubMed]
- Richter, O.-M.H.; Ludwig, B. Cytochrome c Oxidase--Structure, Function, and Physiology of a Redox-Driven Molecular Machine. Rev. Physiol. Biochem. Pharmacol. 2003, 147, 47–74. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, L.M.; Libedinsky, A.; Elorza, A.A. Role of Copper on Mitochondrial Function and Metabolism. Front. Mol. Biosci. 2021, 8, 711227. [Google Scholar] [CrossRef] [PubMed]
- Smith-Mungo, L.I.; Kagan, H.M. Lysyl Oxidase: Properties, Regulation and Multiple Functions in Biology. Matrix Biol. 1998, 16, 387–398. [Google Scholar] [CrossRef]
- Schmidt, K.; Ralle, M.; Schaffer, T.; Jayakanthan, S.; Bari, B.; Muchenditsi, A.; Lutsenko, S. ATP7A and ATP7B Copper Transporters Have Distinct Functions in the Regulation of Neuronal Dopamine—Hydroxylase. J. Biol. Chem. 2018, 293, 20085–20098. [Google Scholar] [CrossRef]
- Arredondo, M.; Nunez, M.T. Iron and Copper Metabolism. Mol. Asp. Med. 2005, 26, 313–327. [Google Scholar] [CrossRef]
- Vest, K.E.; Zhu, X.; Cobine, P.A. Chapter 12—Copper Disposition in Yeast. In Clinical and Translational Perspectives on WILSON DISEASE; Kerkar, N., Roberts, E.A., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 115–126. ISBN 978-0-12-810532-0. [Google Scholar]
- Crichton, R. Chapter 14—Copper—Coping with Dioxygen. In Biological Inorganic Chemistry, 3rd ed.; Crichton, R., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 405–433. ISBN 978-0-12-811741-5. [Google Scholar]
- Carpenè, E.; Andreani, G.; Isani, G. Metallothionein Functions and Structural Characteristics. J. Trace Elem. Med. Biol. 2007, 21, 35–39. [Google Scholar] [CrossRef]
- He, X.; Ge, C.; Xia, J.; Xia, Z.; Zhao, L.; Huang, S.; Wang, R.; Pan, J.; Cheng, T.; Xu, P.-F.; et al. The Zinc Transporter SLC39A10 Plays an Essential Role in Embryonic Hematopoiesis. Adv. Sci. 2023, 10, e2205345. [Google Scholar] [CrossRef]
- Pajarillo, E.A.B.; Lee, E.; Kang, D.-K. Trace Metals and Animal Health: Interplay of the Gut Microbiota with Iron, Manganese, Zinc, and Copper. Anim. Nutr. 2021, 7, 750–761. [Google Scholar] [CrossRef]
- Wessels, I.; Maywald, M.; Rink, L. Zinc as a Gatekeeper of Immune Function. Nutrients 2017, 9, 1286. [Google Scholar] [CrossRef]
- McCall, K.A.; Huang, C.C.; Fierke, C.A. Function and Mechanism of Zinc Metalloenzymes. J. Nutr. 2000, 130, 1437S–1446S. [Google Scholar] [CrossRef] [PubMed]
- Crichton, R. Chapter 12—Zinc—Lewis Acid and Gene Regulator. In Biological Inorganic Chemistry, 3rd ed.; Crichton, R., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 339–362. ISBN 978-0-12-811741-5. [Google Scholar]
- Huang, X.; Li, L.; Wang, J.; Min, J.; Wang, F. Functional discoveries and mechanistic studies of manganese transporters. Chinese Bulletin of Life Sciences. 2018, 30, 603–614. [Google Scholar] [CrossRef]
- Shi, J.-H.; Chen, Y.-X.; Feng, Y.; Yang, X.; Lin, J.; Wang, T.; Wei, C.-C.; Ma, X.-H.; Yang, R.; Cao, D.; et al. Fructose Overconsumption Impairs Hepatic Manganese Homeostasis and Ammonia Disposal. Nat. Commun. 2023, 14, 7934. [Google Scholar] [CrossRef]
- Crichton, R. Chapter 16—Manganese—Oxygen Generation and Detoxification. In Biological Inorganic Chemistry, 3rd ed.; Crichton, R., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 459–473. ISBN 978-0-12-811741-5. [Google Scholar]
- Tainer, J.A.; Getzoff, E.D.; Richardson, J.S.; Richardson, D.C. Structure and Mechanism of Copper, Zinc Superoxide Dismutase. Nature 1983, 306, 284–287. [Google Scholar] [CrossRef]
- Jabara, H.H.; Boyden, S.E.; Chou, J.; Ramesh, N.; Massaad, M.J.; Benson, H.; Bainter, W.; Fraulino, D.; Rahimov, F.; Sieff, C.; et al. A Missense Mutation in TFRC, Encoding Transferrin Receptor 1, Causes Combined Immunodeficiency. Nat. Genet. 2016, 48, 74–78. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, C.; Wu, Q.; An, P.; Huang, L.; Wang, J.; Chen, C.; Chen, X.; Zhang, F.; Ma, L.; et al. Iron-Dependent Histone 3 Lysine 9 Demethylation Controls B Cell Proliferation and Humoral Immune Responses. Nat. Commun. 2019, 10, 2935. [Google Scholar] [CrossRef]
- Das, I.; Saha, K.; Mukhopadhyay, D.; Roy, S.; Raychaudhuri, G.; Chatterjee, M.; Mitra, P.K. Impact of Iron Deficiency Anemia on Cell-Mediated and Humoral Immunity in Children: A Case Control Study. J. Nat. Sci. Biol. Med. 2014, 5, 158–163. [Google Scholar] [CrossRef]
- Mohideen, K.; Chandrasekaran, K.; Kareema, M.; Jeyanthikumari, T.; Dhungel, S.; Ghosh, S. Assessment of Antioxidant Enzyme Superoxide Dismutase (SOD) in Oral Cancer: Systematic Review and Meta-Analysis. Dis. Markers 2024, 2024, 2264251. [Google Scholar] [CrossRef] [PubMed]
- Robinett, N.G.; Peterson, R.L.; Culotta, V.C. Eukaryotic Copper-Only Superoxide Dismutases (SODs): A New Class of SOD Enzymes and SOD-like Protein Domains. J. Biol. Chem. 2018, 293, 4636–4643. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.I.; Sarmento-Ribeiro, A.B.; Gonçalves, A.C. Zinc: From Biological Functions to Therapeutic Potential. Int. J. Mol. Sci. 2023, 24, 4822. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Chen, M.; Yan, S.; Han, Y.; Yuan, H.; Liu, Q.; Lu, D.; Li, L.; Wang, K.; Liu, F.; et al. Zinc Ions Activate AKT and Promote Prostate Cancer Cell Proliferation via Disrupting AKT Intramolecular Interaction. Oncogene 2024, 44, 8–18. [Google Scholar] [CrossRef]
- Rolles, B.; Maywald, M.; Rink, L. Intracellular Zinc during Cell Activation and Zinc Deficiency. J. Trace Elem. Med. Biol. 2021, 68, 126864. [Google Scholar] [CrossRef]
- Liu, Q.; Barker, S.; Knutson, M.D. Iron and Manganese Transport in Mammalian Systems. Biochim. Biophys. Acta-Mol. Cell Res. 2021, 1868, 118890. [Google Scholar] [CrossRef]
- Ma, J.; Piao, X.; Mahfuz, S.; Long, S.; Wang, J. The Interaction among Gut Microbes, the Intestinal Barrier and Short Chain Fatty Acids. Anim. Nutr. 2022, 9, 159–174. [Google Scholar] [CrossRef]
- Peng, Z.; Liao, Y.; Yang, W.; Liu, L. Metal(Loid)-Gut Microbiota Interactions and Microbiota-Related Protective Strategies: A Review. Environ. Int. 2024, 192, 109017. [Google Scholar] [CrossRef]
- Xiao, L.; Tang, R.; Wang, J.; Wan, D.; Yin, Y.; Xie, L. Gut Microbiota Bridges the Iron Homeostasis and Host Health. Sci. China-Life Sci. 2023, 66, 1952–1975. [Google Scholar] [CrossRef]
- Zhu, Q.; Chen, B.; Zhang, F.; Zhang, B.; Guo, Y.; Pang, M.; Huang, L.; Wang, T. Toxic and Essential Metals: Metabolic Interactions with the Gut Microbiota and Health Implications. Front. Nutr. 2024, 11, 1448388. [Google Scholar] [CrossRef]
- Paganini, D.; Zimmermann, M.B. The Effects of Iron Fortification and Supplementation on the Gut Microbiome and Diarrhea in Infants and Children: A Review. Am. J. Clin. Nutr. 2017, 106, 1688S–1693S. [Google Scholar] [CrossRef]
- Chang, P. Microbial Metabolite-Receptor Interactions in the Gut Microbiome. Curr. Opin. Chem. Biol. 2024, 83, 102539. [Google Scholar] [CrossRef]
- Linder, M.C. Copper Homeostasis in Mammals, with Emphasis on Secretion and Excretion. A Review. Int. J. Mol. Sci. 2020, 21, 4932. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, Y.; Zhao, A.; Cai, X.; Yu, A.; Xu, Q.; Liu, W.; Zhang, N.; Wu, S.; Chen, Y.; et al. High Dietary Copper Intake Induces Perturbations in the Gut Microbiota and Affects Host Ovarian Follicle Development. Ecotox. Environ. Safe 2023, 255, 114810. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, J.; Dong, Z.; Li, G.; Wang, J.; Li, Y.; Wan, D.; Yang, H.; Yin, Y. Effect of Dietary Copper on Intestinal Microbiota and Antimicrobial Resistance Profiles of Escherichia Coli in Weaned Piglets. Front. Microbiol. 2019, 10, 2808. [Google Scholar] [CrossRef] [PubMed]
- Djoko, K.Y.; Phan, M.-D.; Peters, K.M.; Walker, M.J.; Schembri, M.A.; McEwan, A.G. Interplay between Tolerance Mechanisms to Copper and Acid Stress in Escherichia coli. Proc. Natl. Acad. Sci. USA 2017, 114, 6818–6823. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, L. The Interplay between Copper Metabolism and Microbes: In Perspective of Host Copper-Dependent ATPases ATP7A/B. Front. Cell. Infect. Microbiol. 2023, 13, 1267931. [Google Scholar] [CrossRef]
- Maares, M.; Haase, H. A Guide to Human Zinc Absorption: General Overview and Recent Advances of In Vitro Intestinal Models. Nutrients 2020, 12, 762. [Google Scholar] [CrossRef]
- Reed, S.; Neuman, H.; Moscovich, S.; Glahn, R.P.; Koren, O.; Tako, E. Chronic Zinc Deficiency Alters Chick Gut Microbiota Composition and Function. Nutrients 2015, 7, 9768–9784. [Google Scholar] [CrossRef]
- Zackular, J.P.; Moore, J.L.; Jordan, A.T.; Juttukonda, L.J.; Noto, M.J.; Nicholson, M.R.; Crews, J.D.; Semler, M.W.; Zhang, Y.; Ware, L.B.; et al. Dietary Zinc Alters the Microbiota and Decreases Resistance to Clostridium Difficile Infection. Nat. Med. 2016, 22, 1502. [Google Scholar] [CrossRef]
- Zackular, J.P.; Skaar, E.P. The Role of Zinc and Nutritional Immunity in Clostridium difficile Infection. Gut Microbes 2018, 9, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, Z.; Wang, P.; Yu, X.; Ding, H.; Wang, Z.; Feng, J. Effect of Long-Term and Short-Term Imbalanced Zn Manipulation on Gut Microbiota and Screening for Microbial Markers Sensitive to Zinc Status. Microbiol. Spectr. 2021, 9, e00483-21. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Kolba, N.; Tako, E. The Effect of Dietary Zinc and Zinc Physiological Status on the Composition of the Gut Microbiome in Vivo. Crit. Rev. Food Sci. Nutr. 2024, 64, 6432–6451. [Google Scholar] [CrossRef] [PubMed]
- Bosma, E.F.; Rau, M.H.; van Gijtenbeek, L.A.; Siedler, S. Regulation and Distinct Physiological Roles of Manganese in Bacteria. Fems Microbiol. Rev. 2021, 45, fuab028. [Google Scholar] [CrossRef]
- Seymour, M.; Wright, Z.; Waters, L. Manganese Homeostasis in Bacteria: Interaction of the Small Protein MntS and Manganese Exporter MntP in E. coli. FASEB J. 2022, 36. [Google Scholar] [CrossRef]
- Tinkov, A.A.; Martins, A.C.; Avila, D.S.; Gritsenko, V.A.; Skalny, A.; Santamaria, A.; Lee, E.; Bowman, A.B.; Aschner, M. Gut Microbiota as a Potential Player in Mn-Induced Neurotoxicity. Biomolecules 2021, 11, 1292. [Google Scholar] [CrossRef]
- Li, C.-Y.; Li, X.-Y.; Shen, L.; Ji, H.-F. Regulatory Effects of Transition Metals Supplementation/Deficiency on the Gut Microbiota. Appl. Microbiol. Biotechnol. 2021, 105, 1007–1015. [Google Scholar] [CrossRef]
- Atwood, C.S.; Perry, G.; Zeng, H.; Kato, Y.; Jones, W.D.; Ling, K.-Q.; Huang, X.; Moir, R.D.; Wang, D.; Sayre, L.M.; et al. Copper Mediates Dityrosine Cross-Linking of Alzheimer’s Amyloid-Beta. Biochemistry 2004, 43, 560–568. [Google Scholar] [CrossRef]
- Mezzaroba, L.; Alfieri, D.F.; Colado Simão, A.N.; Vissoci Reiche, E.M. The Role of Zinc, Copper, Manganese and Iron in Neurodegenerative Diseases. Neurotoxicology 2019, 74, 230–241. [Google Scholar] [CrossRef]
- Pawlitzki, M.; Uebelhör, J.; Sweeney-Reed, C.M.; Stephanik, H.; Hoffmann, J.; Lux, A.; Reinhold, D. Lower Serum Zinc Levels in Patients with Multiple Sclerosis Compared to Healthy Controls. Nutrients 2018, 10, 967. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Jiang, X.; Gu, W. Emerging Mechanisms and Disease Relevance of Ferroptosis. Trends Cell Biol. 2020, 30, 478–490. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Kang, R.; Kroemer, G.; Tang, D. Broadening Horizons: The Role of Ferroptosis in Cancer. Nat. Rev. Clin. Oncol. 2021, 18, 280–296. [Google Scholar] [CrossRef] [PubMed]
- Li, Y. Copper Homeostasis: Emerging Target for Cancer Treatment. IUBMB Life 2020, 72, 1900–1908. [Google Scholar] [CrossRef] [PubMed]
- Blockhuys, S.; Wittung-Stafshede, P. Roles of Copper-Binding Proteins in Breast Cancer. Int. J. Mol. Sci. 2017, 18, 871. [Google Scholar] [CrossRef]
- Skrajnowska, D.; Bobrowska-Korczak, B. Role of Zinc in Immune System and Anti-Cancer Defense Mechanisms. Nutrients 2019, 11, 2273. [Google Scholar] [CrossRef]
- Ehrenpreis, E.D.; Jarrouj, G.; Meader, R.; Wagner, C.; Ellis, M. A Comprehensive Review of Hypomagnesemia. Dis. Mon. 2022, 68, 101285. [Google Scholar] [CrossRef]
- Nakhaee, S.; Rezayee, M.; Mansouri, B.; Hadianfar, A.; Zadeh, A.A.; Zardast, M.; Sefat, M.P.; Mehrpour, O. Comparison of Thyroid Function in Lead-Poisoned Patients and Healthy Individuals in Eastern Iran. Biol. Trace Elem. Res. 2022, 200, 3097–3102. [Google Scholar] [CrossRef]
- Mohsenipour, R.; Aflatoonian, M.; Alimadadi, H.; Rahmani, P.; Esmaeili, N.; Yazdi, M.; Abbasi, F.; Solgi, F.; Sharifi, F.; Vafaii, N.; et al. Lead Poisoning as a Differential Diagnosis in Pediatric Patients with Chronic Abdominal Pain: A Case-Control Study in Tehran-Iran. BMC Gastroenterol. 2024, 24, 344. [Google Scholar] [CrossRef]
- Zabihi, A.; Mehrpour, O.; Nakhaee, S.; Atabati, E. Lead Poisoning and Its Effects on Bone Density. Sci. Rep. 2025, 15, 15619. [Google Scholar] [CrossRef]
- Mao, S.; Huang, S. Zinc and Copper Levels in Bladder Cancer: A Systematic Review and Meta-Analysis. Biol. Trace Elem. Res. 2013, 153, 5–10. [Google Scholar] [CrossRef]
- Wang, W.; Liu, X.; Zhang, C.; Sheng, F.; Song, S.; Li, P.; Dai, S.; Wang, B.; Lu, D.; Zhang, L.; et al. Identification of Two-Dimensional Copper Signatures in Human Blood for Bladder Cancer with Machine Learning. Chem. Sci. 2022, 13, 1648–1656. [Google Scholar] [CrossRef]
- Rolles, B.; Chatain, N.; Görg, R.; Vieri, M.; Tillmann-Tröster, N.; Bourgeois, M.G.; Christen, D.; Walter, J.; Hillerbrand, A.C.; Romine, K.A.; et al. ZIP10 as a Potential Therapeutic Target in Acute Myeloid Leukaemia. Br. J. Haematol. 2025, 207, 767–779. [Google Scholar] [CrossRef]
- Nakanishi, K.; Toyoshima, M.; Ichikawa, G.; Suzuki, S. Zinc Deficiency Is Associated with Gynecologic Cancer Recurrence. Front. Oncol. 2022, 12, 1025060. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tian, Y.; Zhang, H.; Xu, B.; Chen, H. Potential Pathways of Zinc Deficiency-Promoted Tumorigenesis. Biomed. Pharmacother. 2021, 133, 110983. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.J.; Frazer, D.M. Current Understanding of Iron Homeostasis. Am. J. Clin. Nutr. 2017, 106, 1559S–1566S. [Google Scholar] [CrossRef] [PubMed]
- Fuqua, B.K.; Vulpe, C.D.; Anderson, G.J. Intestinal Iron Absorption. J. Trace Elem. Med. Biol. 2012, 26, 115–119. [Google Scholar] [CrossRef]
- Zeidan, R.S.; Han, S.M.; Leeuwenburgh, C.; Xiao, R. Iron Homeostasis and Organismal Aging. Ageing Res Rev 2021, 72, 101510. [Google Scholar] [CrossRef]
- Gunshin, H.; Fujiwara, Y.; Custodio, A.O.; Direnzo, C.; Robine, S.; Andrews, N.C. Slc11a2 Is Required for Intestinal Iron Absorption and Erythropoiesis but Dispensable in Placenta and Liver. J. Clin. Investig. 2005, 115, 1258–1266. [Google Scholar] [CrossRef]
- Go, L.-D.; Rj, S.; At, M. Duodenal Cytochrome B Expression Stimulates Iron Uptake by Human Intestinal Epithelial Cells. J. Nutr. 2008, 138, 991–995. [Google Scholar] [CrossRef]
- Jormakka, M. Structural Insights into Ferroportin Mediated Iron Transport. Biochem. Soc. Trans. 2023, 51, 2143–2152. [Google Scholar] [CrossRef]
- Han, J.; Luo, J.; Wang, C.; Kapilevich, L.; Zhang, X.-A. Roles and Mechanisms of Copper Homeostasis and Cuproptosis in Osteoarticular Diseases. Biomed. Pharmacother. 2024, 174, 116570. [Google Scholar] [CrossRef]
- Nose, Y.; Kim, B.-E.; Thiele, D.J. Ctr1 Drives Intestinal Copper Absorption and Is Essential for Growth, Iron Metabolism, and Neonatal Cardiac Function. Cell Metab. 2006, 4, 235–244. [Google Scholar] [CrossRef]
- Shawki, A.; Anthony, S.R.; Nose, Y.; Engevik, M.A.; Niespodzany, E.J.; Barrientos, T.; Öhrvik, H.; Worrell, R.T.; Thiele, D.J.; Mackenzie, B. Intestinal DMT1 Is Critical for Iron Absorption in the Mouse but Is Not Required for the Absorption of Copper or Manganese. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G635–G647. [Google Scholar] [CrossRef]
- Turnlund, J.R. Human Whole-Body Copper Metabolism. Am. J. Clin. Nutr. 1998, 67, 960S–964S. [Google Scholar] [CrossRef] [PubMed]
- Scheiber, I.; Dringen, R.; Mercer, J.F.B. Copper: Effects of Deficiency and Overload. Met. Ions Life Sci. 2013, 13, 359–387. [Google Scholar] [CrossRef] [PubMed]
- Roth, J.; Ponzoni, S.; Aschner, M. Manganese Homeostasis and Transport. Met. Ions Life Sci. 2013, 12, 169–201. [Google Scholar] [CrossRef] [PubMed]
- Anagianni, S.; Tuschl, K. Genetic Disorders of Manganese Metabolism. Curr. Neurol. Neurosci. Rep. 2019, 19, 33. [Google Scholar] [CrossRef]
- Boycott, K.M.; Beaulieu, C.L.; Kernohan, K.D.; Gebril, O.H.; Mhanni, A.; Chudley, A.E.; Redl, D.; Qin, W.; Hampson, S.; Küry, S.; et al. Autosomal-Recessive Intellectual Disability with Cerebellar Atrophy Syndrome Caused by Mutation of the Manganese and Zinc Transporter Gene SLC39A8. Am. J. Hum. Genet. 2015, 97, 886–893. [Google Scholar] [CrossRef]
- Tuschl, K.; Meyer, E.; Valdivia, L.E.; Zhao, N.; Dadswell, C.; Abdul-Sada, A.; Hung, C.Y.; Simpson, M.A.; Chong, W.K.; Jacques, T.S.; et al. Mutations in SLC39A14 Disrupt Manganese Homeostasis and Cause Childhood-Onset Parkinsonism-Dystonia. Nat. Commun. 2016, 7, 11601. [Google Scholar] [CrossRef]
- Fujishiro, H.; Yano, Y.; Takada, Y.; Tanihara, M.; Himeno, S. Roles of ZIP8, ZIP14, and DMT1 in Transport of Cadmium and Manganese in Mouse Kidney Proximal Tubule Cells. Metallomics 2012, 4, 700–708. [Google Scholar] [CrossRef]
- Jursa, T.; Smith, D.R. Ceruloplasmin Alters the Tissue Disposition and Neurotoxicity of Manganese, but Not Its Loading onto Transferrin. Toxicol. Sci. 2009, 107, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Miller, D.S.; Zheng, W. Intracellular Localization and Subsequent Redistribution of Metal Transporters in a Rat Choroid Plexus Model Following Exposure to Manganese or Iron. Toxicol. Appl. Pharmacol. 2008, 230, 167–174. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stiles, L.I.; Ferrao, K.; Mehta, K.J. Role of Zinc in Health and Disease. Clin. Exp. Med. 2024, 24, 38. [Google Scholar] [CrossRef] [PubMed]
- Kondaiah, P.; Yaduvanshi, P.S.; Sharp, P.A.; Pullakhandam, R. Iron and Zinc Homeostasis and Interactions: Does Enteric Zinc Excretion Cross-Talk with Intestinal Iron Absorption? Nutrients 2019, 11, 1885. [Google Scholar] [CrossRef]
- Guo, H.; Wang, S.; Zhang, H.; Li, J.; Wang, C.; Liu, Z.; Chen, J.; Wang, K.; Wei, X.; Wei, Q.; et al. Research Progress on the Molecular Structure, Function, and Application in Tumor Therapy of Zinc Transporter ZIP4. Int. J. Biol. Sci. 2024, 20, 5910–5924. [Google Scholar] [CrossRef]
- Dufner-Beattie, J.; Wang, F.; Kuo, Y.-M.; Gitschier, J.; Eide, D.; Andrews, G.K. The Acrodermatitis Enteropathica Gene ZIP4 Encodes a Tissue-Specific, Zinc-Regulated Zinc Transporter in Mice. J. Biol. Chem. 2003, 278, 33474–33481. [Google Scholar] [CrossRef]
- Guthrie, G.J.; Aydemir, T.B.; Troche, C.; Martin, A.B.; Chang, S.-M.; Cousins, R.J. Influence of ZIP14 (slc39A14) on Intestinal Zinc Processing and Barrier Function. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G171–G178. [Google Scholar] [CrossRef]
- Wang, F.; Kim, B.-E.; Petris, M.J.; Eide, D.J. The Mammalian Zip5 Protein Is a Zinc Transporter That Localizes to the Basolateral Surface of Polarized Cells. J. Biol. Chem. 2004, 279, 51433–51441. [Google Scholar] [CrossRef]
- Kambe, T.; Tsuji, T.; Hashimoto, A.; Itsumura, N. The Physiological, Biochemical, and Molecular Roles of Zinc Transporters in Zinc Homeostasis and Metabolism. Physiol. Rev. 2015, 95, 749–784. [Google Scholar] [CrossRef]
- Isani, G.; Carpenè, E. Metallothioneins, Unconventional Proteins from Unconventional Animals: A Long Journey from Nematodes to Mammals. Biomolecules 2014, 4, 435–457. [Google Scholar] [CrossRef]
- Liu, S.; Wang, N.; Long, Y.; Wu, Z.; Zhou, S. Zinc Homeostasis: An Emerging Therapeutic Target for Neuroinflammation Related Diseases. Biomolecules 2023, 13, 416. [Google Scholar] [CrossRef]
- Beto, J.A. The Role of Calcium in Human Aging. Clin. Nutr. Res. 2015, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Marcus, C.S.; Lengemann, F.W. Absorption of Ca45 and Sr85 from Solid and Liquid Food at Various Levels of the Alimentary Tract of the Rat. J. Nutr. 1962, 77, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Wongdee, K.; Chanpaisaeng, K.; Teerapornpuntakit, J.; Charoenphandhu, N. Intestinal Calcium Absorption. Compr. Physiol. 2021, 11, 2047–2073. [Google Scholar] [CrossRef] [PubMed]
- Alexander, R.T.; Rievaj, J.; Dimke, H. Paracellular Calcium Transport across Renal and Intestinal Epithelia. Biochem. Cell Biol. 2014, 92, 467–480. [Google Scholar] [CrossRef]
- de Baaij, J.H.F.; Hoenderop, J.G.J.; Bindels, R.J.M. Magnesium in Man: Implications for Health and Disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, H.; Huang, J.; Faouzi, M.; Schmitz, C.; Penner, R.; Fleig, A. The TRPM6 Kinase Domain Determines the Mg·ATP Sensitivity of TRPM7/M6 Heteromeric Ion Channels. J. Biol. Chem. 2014, 289, 5217–5227. [Google Scholar] [CrossRef]
- Chamniansawat, S.; Suksridechacin, N.; Thongon, N. Current Opinion on the Regulation of Small Intestinal Magnesium Absorption. World J. Gastroenterol. 2023, 29, 332–342. [Google Scholar] [CrossRef]
- Maret, W. The Quintessence of Metallomics: A Harbinger of a Different Life Science Based on the Periodic Table of the Bioelements. Metallomics 2022, 14, mfac051. [Google Scholar] [CrossRef]
- Sanchez, C.; Fente, C.; Barreiro, R.; Lopez-Racamonde, O.; Cepeda, A.; Regal, P. Association between Breast Milk Mineral Content and Maternal Adherence to Healthy Dietary Patterns in Spain: A Transversal Study. Foods 2020, 9, 659. [Google Scholar] [CrossRef]
- Domellof, M. Iron Requirements in Infancy. Ann. Nutr. Metab. 2011, 59, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Domellöf, M.; Lönnerdal, B.; Abrams, S.A.; Hernell, O. Iron Absorption in Breast-Fed Infants:: Effects of Age, Iron Status, Iron Supplements, and Complementary Foods. Am. J. Clin. Nutr. 2002, 76, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.E.; Deeth, H.C. Magnesium in Milk. Int. Dairy J. 2017, 71, 89–97. [Google Scholar] [CrossRef]
- Montagne, P.; Cuillière, M.L.; Molé, C.; Béné, M.C.; Faure, G. Changes in Lactoferrin and Lysozyme Levels in Human Milk during the First Twelve Weeks of Lactation. In Bioactive Components of Human Milk; Newburg, D.S., Ed.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2001; Volume 501, pp. 241–247. ISBN 978-0-306-46653-3. [Google Scholar]
- Reyes, S.M.; Brockway, M.; McDermid, J.M.; Chan, D.; Granger, M.; Refvik, R.; Sidhu, K.K.; Musse, S.; Monnin, C.; Lotoski, L.; et al. Human Milk Micronutrients and Child Growth and Body Composition in the First 2 Years: A Systematic Review. Adv. Nutr. 2024, 15, 100082. [Google Scholar] [CrossRef]
- Çebi, A.; Şengül, Ü. Toxic Metal and Trace Element Status in the Breast Milk of Turkish New-Born Mothers. J. Trace Elem. Med. Biol. 2022, 74, 127066. [Google Scholar] [CrossRef]
- Long, J.; Guo, S.; Cai, L.; Zhang, T.; Chen, W.; Xie, C. Variation in Milk Minerals and Chemical Components Corresponding to Milking Time and Lactation Day in Sows. Biol. Rhythm. Res. 2020, 51, 1231–1242. [Google Scholar] [CrossRef]
- Liu, L.; Yang, Z.; Li, Y.; Fan, Y.; Chu, C.; Wang, H.; Amantuer, A.; Cao, L.; Hu, B.; Abula, Z.; et al. Mineral Profiles Characteristics in Milk from Dairy Cows in Xinjiang, China, and Production Plan for Season-Dependent High-Calcium Milk Sources. Foods 2025, 14, 1841. [Google Scholar] [CrossRef]
- Robinson, O.; Lau, C.E. How Do Metabolic Processes Age: Evidence from Human Metabolomic Studies. Curr. Opin. Chem. Biol. 2023, 76, 102360. [Google Scholar] [CrossRef]
- Meplan, C. Trace Elements and Ageing, a Genomic Perspective Using Selenium as an Example. J. Trace Elem. Med. Biol. 2011, 25, S11–S16. [Google Scholar] [CrossRef]
- Ma, J.; Geng, S.; Sun, Q.; Zhang, X.; Han, L.; Yao, X.; Zhang, B.; Zhu, L.; Wen, J. Exposure to Metal Mixtures and Young Children’s Growth and Development: A Biomonitoring-Based Study in Eastern China. Ecotox. Environ. Safe 2023, 268, 115726. [Google Scholar] [CrossRef]
- Pastor, A.J.S.; Desai, G.; Garcia-Villarino, M.; Karagas, M.R.; Kordas, K. Exposure to a Mixture of Metals and Growth Indicators in 6-11-Year-Old Children from the 2013-2016 NHANES. Expo. Health 2021, 13, 173–184. [Google Scholar] [CrossRef]
- Zhang, B.; Podolskiy, D.I.; Mariotti, M.; Seravalli, J.; Gladyshev, V.N. Systematic Age-, Organ-, and Diet-Associated Ionome Remodeling and the Development of Ionomic Aging Clocks. Aging Cell 2020, 19, e13119. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, H.; Guan, Q.; Yang, X.; Yu, Q.; Zhang, M.; Xia, Y. Maternal Exposure to Trace Elements, Toxic Metals, and Longitudinal Changes in Infancy Anthropometry and Growth Trajectories: A Prospective Cohort Study. Environ. Sci. Technol. 2023, 57, 11779–11791. [Google Scholar] [CrossRef]
- Nairz, M.; Weiss, G. Iron in Infection and Immunity. Mol. Asp. Med. 2020, 75, 100864. [Google Scholar] [CrossRef]
- Qiu, W.; Ye, J.; Su, Y.; Zhang, X.; Pang, X.; Liao, J.; Wang, R.; Zhao, C.; Zhang, H.; Hu, L.; et al. Co-Exposure to Environmentally Relevant Concentrations of Cadmium and Polystyrene Nanoplastics Induced Oxidative Stress, Ferroptosis and Excessive Mitophagy in Mice Kidney. Environ. Pollut. 2023, 333, 121947. [Google Scholar] [CrossRef]
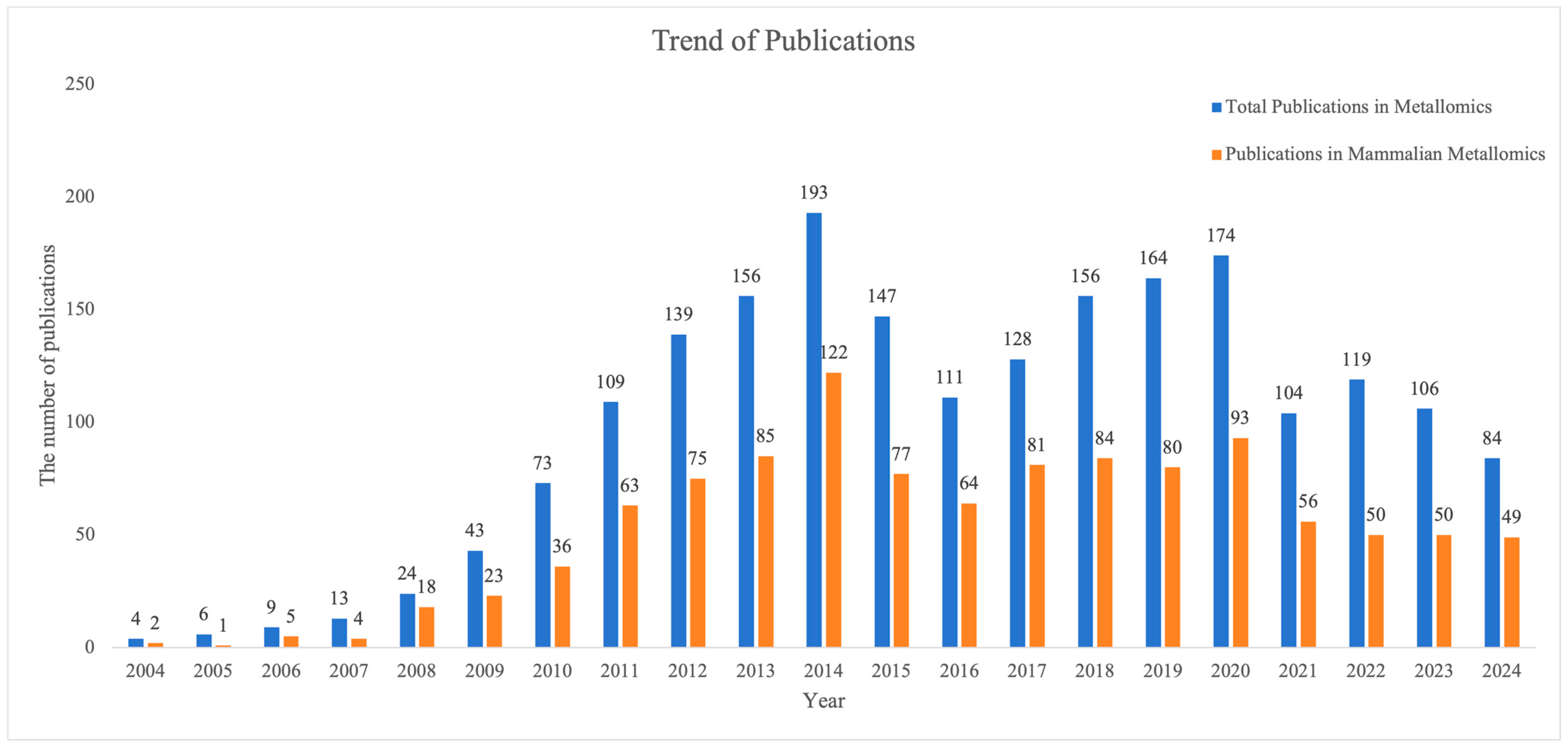
| Category | Technique | Acronym | Detection Limit | Primary Applications | Advantages | Limitations |
|---|---|---|---|---|---|---|
| Spectroscopy | Flame Atomic Absorption Spectroscopy | FAAS | High ppb to ppm range | Milk, serum, feces, tissues | • Low cost • Simple operation • Wide application scope | • Single element only • Moderate sensitivity • Complex sample preparation |
| Graphite Furnace AAS | GFAAS | Sub-ppb level (<1 ppb) | Trace metals in tissues/biofluids | • High sensitivity • Small sample volume | • Single element only • High cost • Slow analysis speed | |
| Inductively Coupled Plasma Optical Emission Spectrometry | ICP-OES | 1–10 ppb (most elements) | Multi-element analysis (e.g., adrenal tissue, nutrition studies) | •Multi-element detection • Wide linear range • Robust performance | • Lower sensitivity vs. ICP-MS • Spectral interferences | |
| X-Ray Fluorescence | XRF | Percentage to sub-ppm levels | Non-destructive analysis (milk, liver, archaeological samples) | • Non-destructive • Minimal sample prep • Multi-element | • Poor trace sensitivity • Matrix effects • Bulk analysis only | |
| Mass Spectrometry | Inductively Coupled Plasma Mass Spectrometry | ICP-MS | ppt level (parts-per-trillion) | Trace metals/isotopes in biomedicine (ASD biomarkers, cancer drug distribution) | • Highest sensitivity • Isotopic analysis • Ultra-wide linear range | • High cost • Complex sample digestion • Polyatomic interferences |
| Imaging | Laser Ablation ICP-MS | LA-ICP-MS | Sub-ppm to ppt (element-dependent) | Spatial metal distribution (e.g., Pb in brain, U/Th accumulation) | • 2D/3D elemental mapping • µm-scale spatial resolution • Semi-quantitative | • Semi-destructive • Requires matrix-matched standards • Limited depth profiling |
| Metal Ion | Primary Absorption Site | Key Transporters | Absorption Mechanism | Regulation and Homeostasis | Associated Diseases |
|---|---|---|---|---|---|
| Iron | Duodenum, proximal jejunum | - DMT1 (apical uptake) - Dcytb (Fe3+ reductase) - FPN1 (basolateral export) - Transferrin (TF) (plasma transport) | - Non-heme Fe: Dietary Fe3+ reduced to Fe2+ by Dcytb→ DMT1-mediated uptake → intracellular storage → FPN1 export → binds TF in plasma. - Heme Fe: Endocytosed → degraded by heme oxygenase → Fe2+ exported via FPN1. | - Hepcidin regulates FPN1 degradation to control systemic Fe. - Excess Fe stored as ferritin. | Anemia (deficiency), neurodegeneration (excess), organ damage |
| Copper | Small intestine | - CTR1 (apical uptake) - ATP7A (basolateral export) - Ceruloplasmin (CP) (plasma transport) | Dietary Cu2+ reduced to Cu+ → CTR1 uptake → ATP7A export → binds CP or albumin in plasma. | - Liver redistributes Cu via ATP7B: • Bound to CP for circulation • Biliary excretion for excess Cu elimination. | Menkes disease (ATP7A defect), Wilson’s disease (ATP7B defect) |
| Manganese | Small intestine | - DMT1, ZIP8, ZIP14 (apical uptake) - SLC30A10, FPN1 (basolateral export) - Transferrin (TF) (Mn3+ transport) | Mn2+ uptake via DMT1/ZIP8/ZIP14 → oxidized to Mn3+ by ceruloplasmin → binds TF for systemic transport. | SLC30A10/FPN1 export excess Mn. - Accumulates in brain via TfR-mediated endocytosis. | Hypermanganemia, Parkinsonism-like syndromes |
| Zinc | Small intestine | - ZIP4 (apical uptake) - ZnT1(basolateral export) - ZIP5/ZIP14 (basolateral uptake) - Metallothionein (MT) (intracellular buffer) | ZIP4 mediates dietary Zn2+ uptake → Zn2+ bound to MT → ZnT1 exports Zn2+ to plasma. ZIP5/14 import Zn2+ from blood. | - MT sequesters excess Zn. - ZIP4 endocytosis downregulates absorption during Zn sufficiency. | Slowed growth, immune dysfunction, dermatitis |
| Calcium | Small intestine | - TRPV6 (apical uptake) - Calbindin (intracellular shuttle) - ATP2B1/SLC8A1 (basolateral export) | Transcellular: TRPV6 uptake → calbindin transport → ATP2B1/SLC8A1 export. Paracellular: Passive diffusion via tight junctions. | Vitamin D upregulates TRPV6/calbindin. PTH regulates renal/bone Ca2+ recycling. | Rickets, osteoporosis, hypercalcemia |
| Magnesium | Small intestine | - TRPM6/TRPM7 (apical uptake) - CNNM4 (basolateral export) - Claudin-7/12 (paracellular transport) | Transcellular: TRPM6/7 uptake → CNNM4 export. Paracellular: Claudin channels facilitate diffusion. | Kidney reabsorption via TRPM6/CNNMs maintains balance. | Hypomagnesemia, cardiac arrhythmias |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, X.; Teng, Y.; Deng, Y.; Zhang, Q.; Hu, C.; Feng, J. Advances in Mammalian Metallomics: New Insights into Metal Dynamics and Biological Significance. Int. J. Mol. Sci. 2025, 26, 9729. https://doi.org/10.3390/ijms26199729
Tian X, Teng Y, Deng Y, Zhang Q, Hu C, Feng J. Advances in Mammalian Metallomics: New Insights into Metal Dynamics and Biological Significance. International Journal of Molecular Sciences. 2025; 26(19):9729. https://doi.org/10.3390/ijms26199729
Chicago/Turabian StyleTian, Xin, Yifan Teng, Yuhang Deng, Qian Zhang, Caihong Hu, and Jie Feng. 2025. "Advances in Mammalian Metallomics: New Insights into Metal Dynamics and Biological Significance" International Journal of Molecular Sciences 26, no. 19: 9729. https://doi.org/10.3390/ijms26199729
APA StyleTian, X., Teng, Y., Deng, Y., Zhang, Q., Hu, C., & Feng, J. (2025). Advances in Mammalian Metallomics: New Insights into Metal Dynamics and Biological Significance. International Journal of Molecular Sciences, 26(19), 9729. https://doi.org/10.3390/ijms26199729






