Neuronal Differentiation and Exosome Profiling of Dental Pulp Stem Cells: Unveiling Their Potential for Nerve Repair
Abstract
1. Introduction
2. Results
2.1. Analysis of hDPSC Conditioned Medium and hDPSC Exosomes
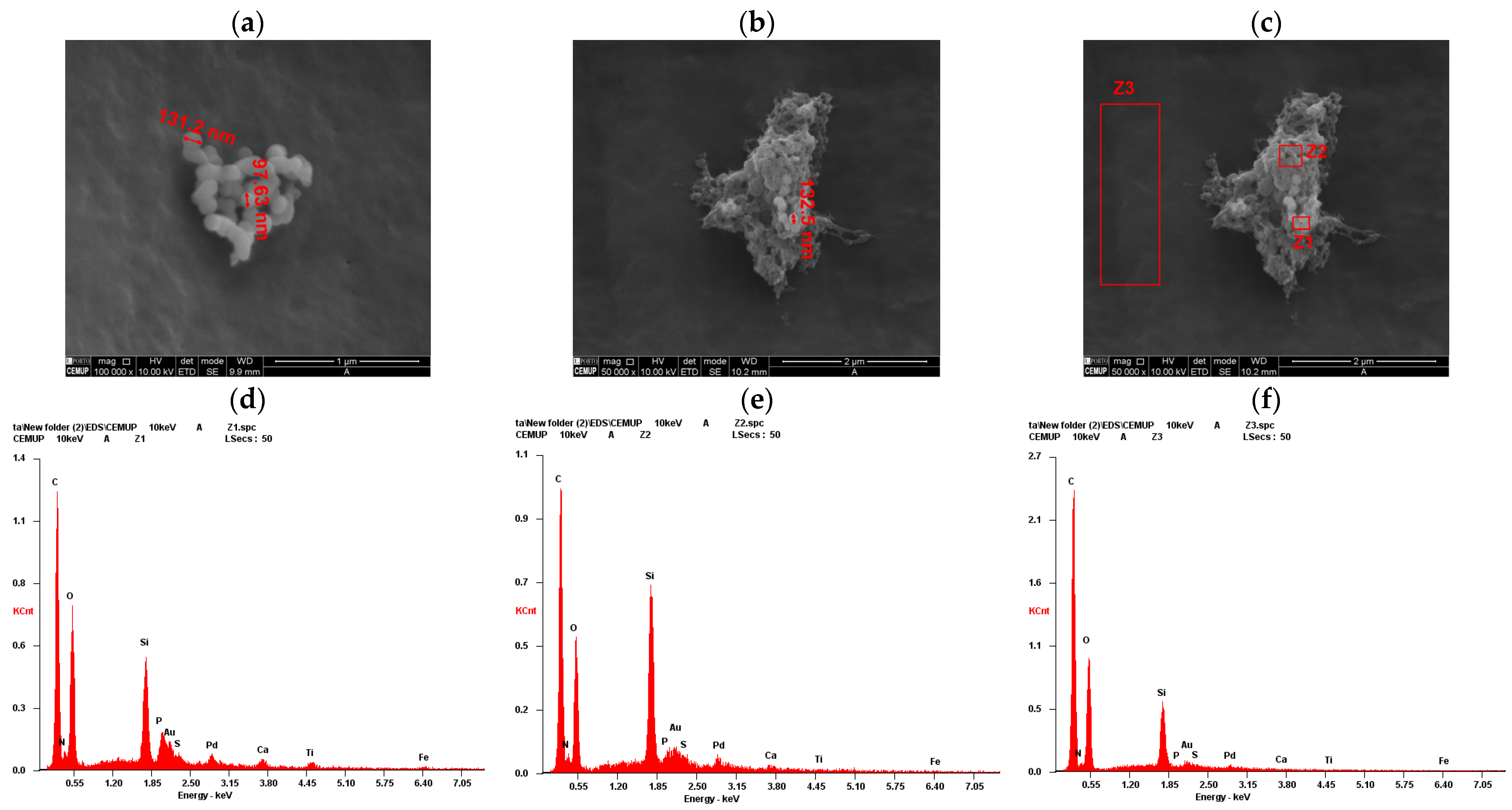
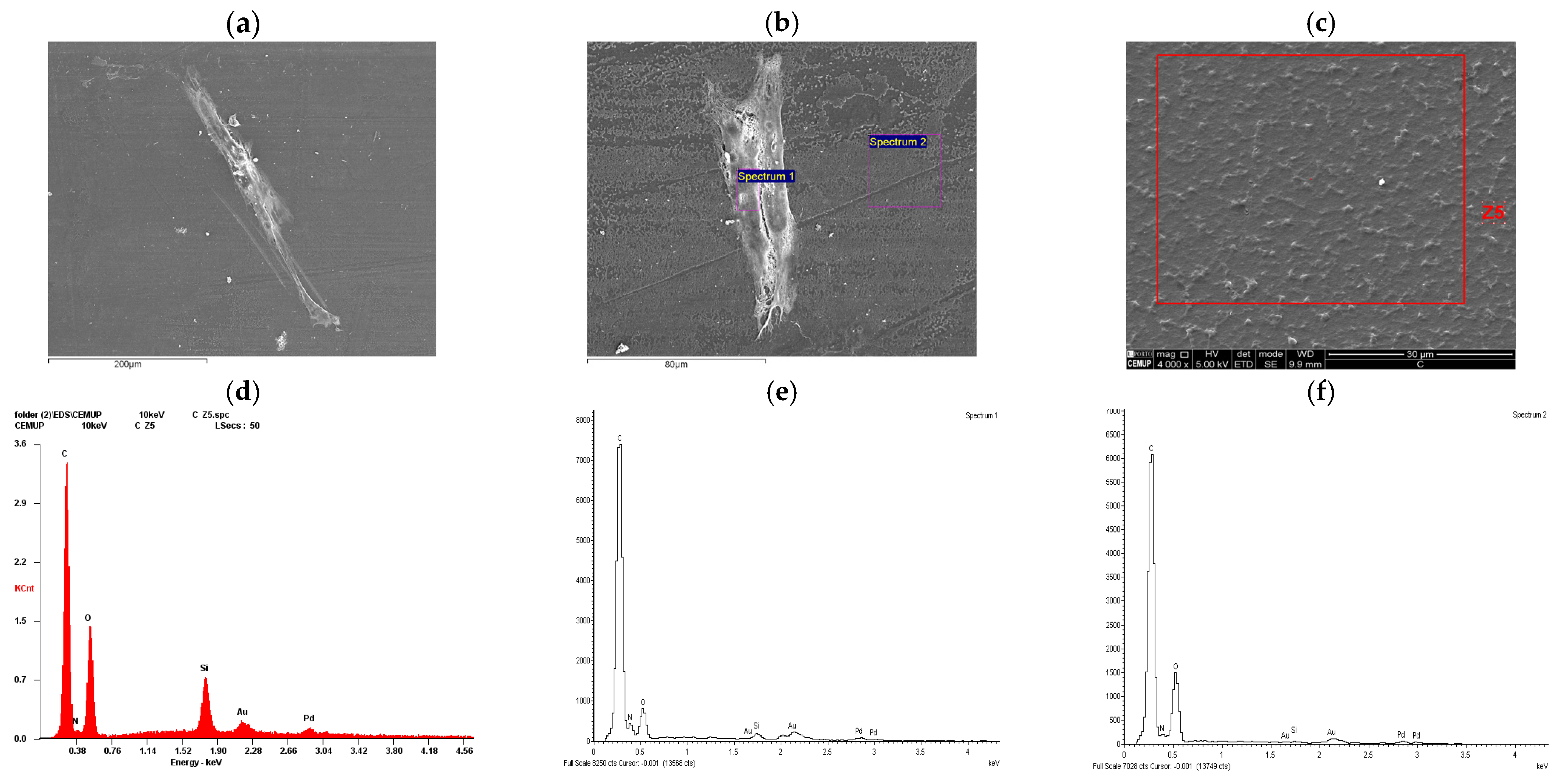
2.2. Scanning Electron Microscopy (SEM) and Energy Dispersive X-Ray Spectroscopy (EDS) Analysis
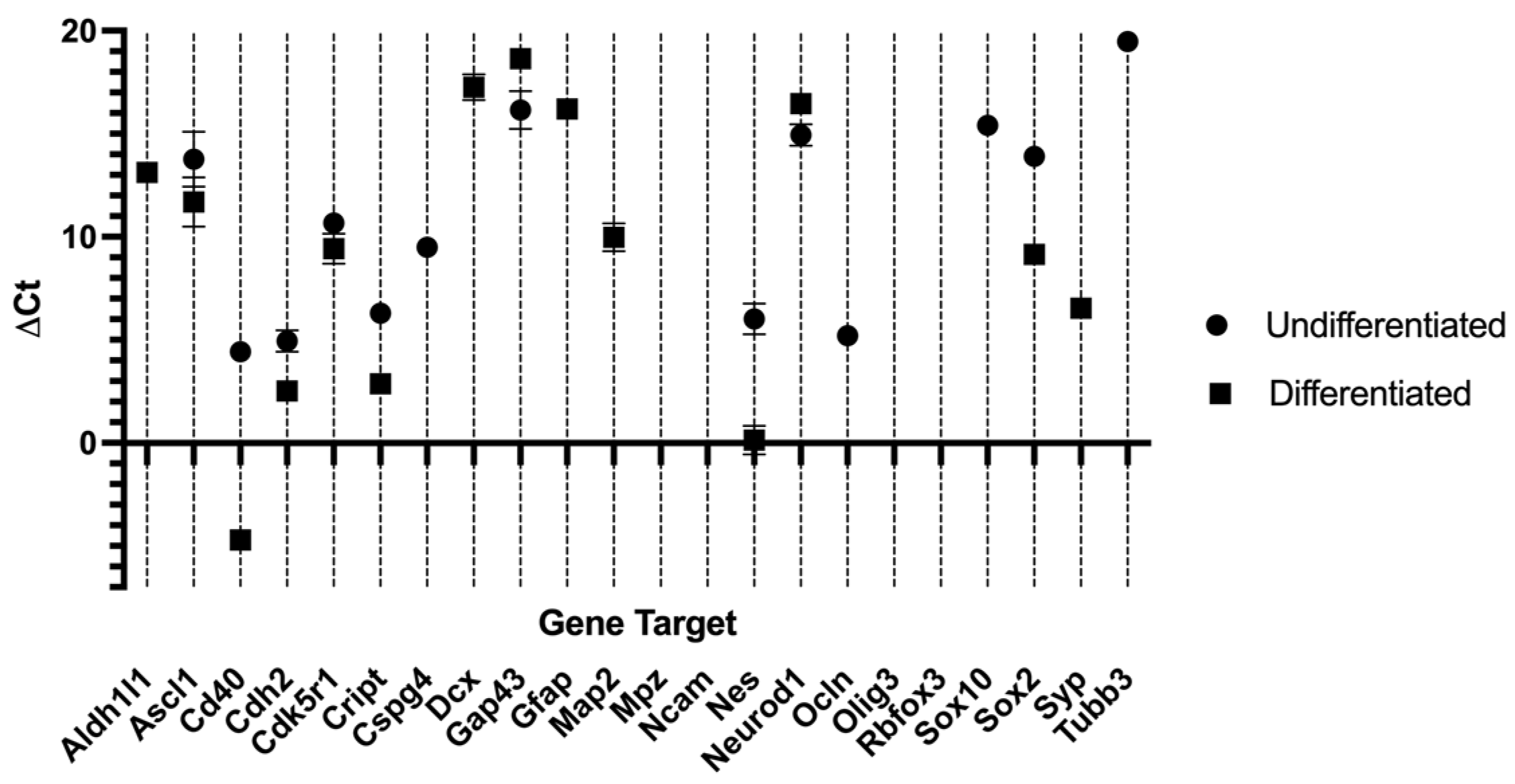
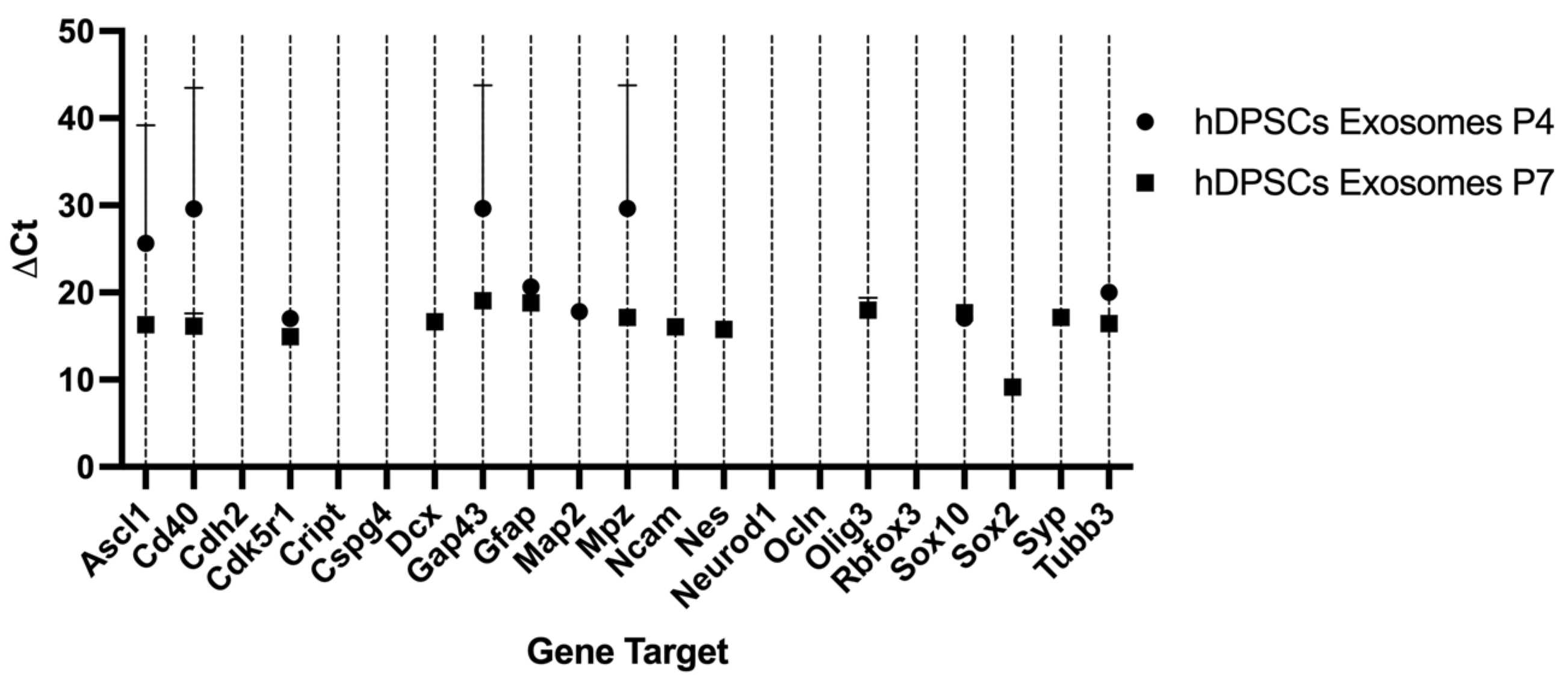
2.3. Reverse Transcriptase Polymerase Chain Reaction (RT-PCR)
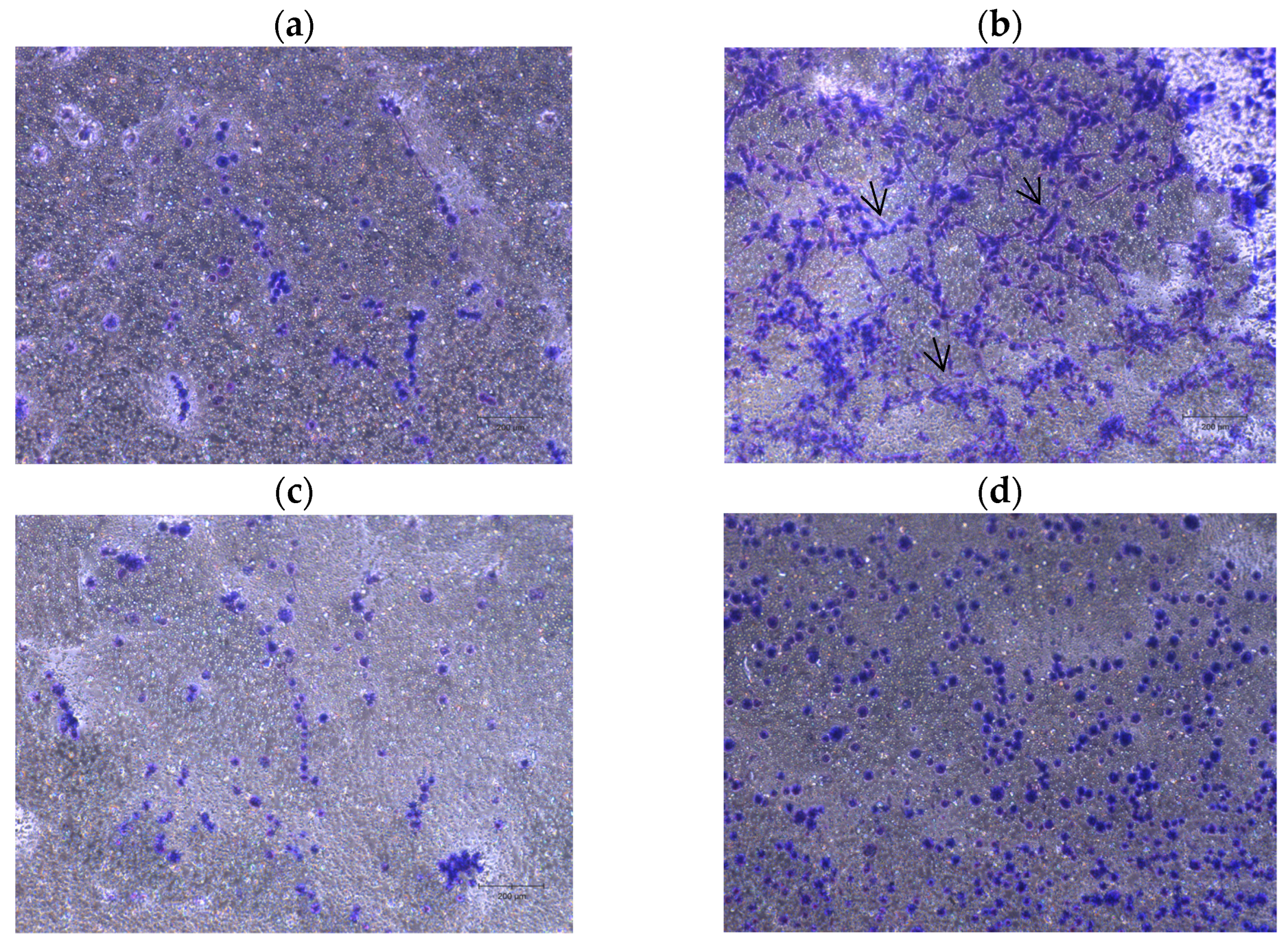
2.4. Neurite Outgrowth Assay
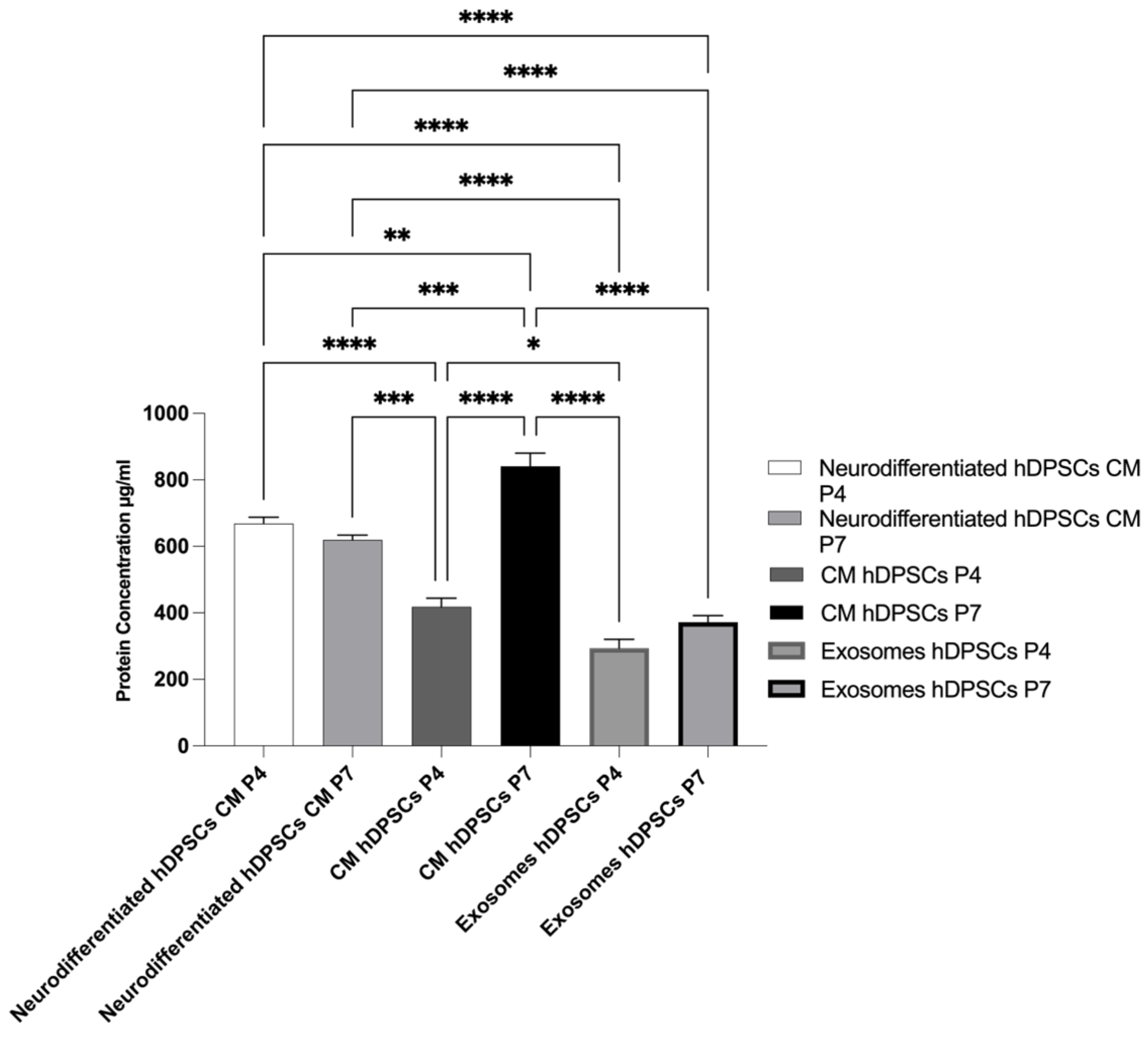
2.5. Total Protein Quantification
3. Discussion
4. Materials and Methods
4.1. Preparation of hDPSCs and Conditioned Medium
- -
- Undifferentiated hDPSCs at P7 and P4, both with unconcentrated CM (1×).
- -
- Neurodifferentiated hDPSCs at P4 and P7 with unconcentrated CM (1×).
- -
- Neurodifferentiated hDPSCs at P4 and P7 with concentrated CM (5×).
- -
- Exosomes isolated from hDPSCs at P4 and P7 with unconcentrated CM (1×).
- -
- Exosomes isolated from hDPSCs at P4 and P7 with concentrated CM (5×).
4.2. Analysis of hDPSC Conditioned Medium
4.3. Exosome Isolation from Cell Culture Media
4.4. Neurogenic Differentiation Assay
4.5. Scanning Electron Microscopy
4.6. RT-PCR
4.6.1. RNA Isolation and cDNA Synthesis from hDPSCs
4.6.2. RNA Isolation and cDNA Synthesis from Exosomes Derived from hDPSCs
4.7. Quantitative RT-PCR Assay
- -
- Ct < 29: Strong positive reaction, indicating a high abundance of the target nucleic acid.
- -
- 30 < Ct < 39: Moderate gene expression, with a detectable but lower quantity of the target nucleic acid.
- -
- Ct > 39: Weak signal, suggesting minimal target nucleic acid presence or possible environmental contamination.
4.8. Neurite Outgrowth Assay
4.9. Total Protein Quantification
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CM | Conditioned Medium |
| EDS | Energy Dispersive X-ray spectroscopy |
| EVs | Extracellular Vesicles |
| hDPSCs | Human Dental Pulp Stem Cells |
| hDPSCs-CM | Human Dental Pulp Stem Cells Conditioned Medium |
| ISEV | International Society for Extracellular Vesicles |
| MSCs | Mesenchymal Stem Cells |
| NGC | Nerve Guide Conduit |
| PNIs | Peripheral Nerve Injuries |
| RT-PCR | Reverse Transcriptase Polymerase Chain Reaction |
| SEM | Scanning Electron Microscopy |
References
- Lopes, B.; Sousa, P.; Alvites, R.; Branquinho, M.; Sousa, A.C.; Mendonça, C.; Atayde, L.M.; Luís, A.L.; Varejão, A.S.P.; Maurício, A.C. Peripheral Nerve Injury Treatments and Advances: One Health Perspective. Int. J. Mol. Sci. 2022, 23, 918. [Google Scholar] [CrossRef]
- Lopes, B.; Sousa, A.C.; Sousa, P.; Moreira, A.d.S.; Coelho, A.F.; Atayde, L.; Salgado, A.J.; Geuna, S.; Alvites, R.; Maurício, A.C.; et al. In vitro evaluation of dental pulp stem cells for sciatic nerve regeneration: Foundations for future in vivo applications. Front. Cell Dev. Biol. 2025, 13, 1528213. [Google Scholar] [CrossRef]
- Alvites, R.; Caseiro, A.R.; Pedrosa, S.S.; Branquinho, M.V.; Ronchi, G.; Geuna, S.; Varejão, A.S.; Maurício, A.C.; Spurkland, A. Peripheral nerve injury and axonotmesis: State of the art and recent advances. Cogent Med. 2018, 5, 1466404. [Google Scholar] [CrossRef]
- Grosu-Bularda, A.; Vancea, C.-V.; Hodea, F.-V.; Cretu, A.; Bordeanu-Diaconescu, E.-M.; Dumitru, C.-S.; Ratoiu, V.-A.; Teodoreanu, R.-N.; Lascar, I.; Hariga, C.-S. Optimizing Peripheral Nerve Regeneration: Surgical Techniques, Biomolecular and Regenerative Strategies—A Narrative Review. Int. J. Mol. Sci. 2025, 26, 3895. [Google Scholar] [CrossRef]
- Zhu, J.; Fu, Q.; Song, L.; Liu, L.; Zheng, Z.; Xu, Y.; Zhang, Z.; Karimi-Maleh, H. Advances in Peripheral Nerve Injury Repair with the Application of Nanomaterials. J. Nanomater. 2022, 2022, 7619884. [Google Scholar] [CrossRef]
- Alvites, R.D.; Branquinho, M.V.; Sousa, A.C.; Lopes, B.; Sousa, P.; Prada, J.; Pires, I.; Ronchi, G.; Raimondo, S.; Luís, A.L.; et al. Effects of Olfactory Mucosa Stem/Stromal Cell and Olfactory Ensheating Cells Secretome on Peripheral Nerve Regeneration. Biomolecules 2022, 12, 818. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Qu, F.; Pei, Y.; Lei, S.; Xia, S.; Liang, J.; Li, S.; Sun, X.; Liu, L. Repairing sciatic nerve injury with self-assembling peptide nanofiber scaffold-containing chitosan conduit. Front. Neurol. 2022, 13, 867711. [Google Scholar] [CrossRef] [PubMed]
- Alvites, R.D.; Branquinho, M.V.; Sousa, A.C.; Amorim, I.; Magalhães, R.; João, F.; Almeida, D.; Amado, S.; Prada, J.; Pires, I.; et al. Combined Use of Chitosan and Olfactory Mucosa Mesenchymal Stem/Stromal Cells to Promote Peripheral Nerve Regeneration In Vivo. Stem Cells Int. 2021, 2021, 6613029. [Google Scholar] [CrossRef]
- Sousa, A.C.; Alvites, R.; Lopes, B.; Sousa, P.; Moreira, A.; Coelho, A.; Rêma, A.; Biscaia, S.; Cordeiro, R.; Faria, F.; et al. Hybrid scaffolds for bone tissue engineering: Integration of composites and bioactive hydrogels loaded with hDPSCs. Biomater. Adv. 2024, 166, 214042. [Google Scholar] [CrossRef] [PubMed]
- Lopes, B.; Coelho, A.; Alvites, R.; Sousa, A.C.; Sousa, P.; Moreira, A.; Atayde, L.; Salgado, A.; Geuna, S.; Maurício, A.C. Animal models in peripheral nerve transection studies: A systematic review on study design and outcomes assessment. Regen. Med. 2023, 19, 189–203. [Google Scholar] [CrossRef]
- Stocco, E.; Barbon, S.; Emmi, A.; Tiengo, C.; Macchi, V.; De Caro, R.; Porzionato, A. Bridging Gaps in Peripheral Nerves: From Current Strategies to Future Perspectives in Conduit Design. Int. J. Mol. Sci. 2023, 24, 9170. [Google Scholar] [CrossRef]
- Riva, E.R.; Özkan, M.; Contreras, E.; Pawar, S.; Zinno, C.; Escarda-Castro, E.; Kim, J.; Wieringa, P.; Stellacci, F.; Micera, S.; et al. Beyond the limiting gap length: Peripheral nerve regeneration through implantable nerve guidance conduits. Biomater. Sci. 2024, 12, 1371–1404. [Google Scholar] [CrossRef]
- Smolinská, V.; Boháč, M.; Danišovič, Ľ. Current Status of the Applications of Conditioned Media Derived from Mesenchymal Stem Cells for Regenerative Medicine. Physiol. Res. 2023, 72, S233–S245. [Google Scholar] [CrossRef]
- Stefańska, K.; Volponi, A.A.; Kulus, M.; Waśko, J.; Farzaneh, M.; Grzelak, J.; Azizidoost, S.; Mozdziak, P.; Bukowska, D.; Antosik, P.; et al. Dental pulp stem cells—A basic research and future application in regenerative medicine. Biomed. Pharmacother. 2024, 178, 116990. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Hatori, A.; Chikazu, D.; Ogasawara, T.; Pan, Z. Application of Dental Pulp Stem Cells for Bone and Neural Tissue Regeneration in Oral and Maxillofacial Region. Stem Cells Int. 2023, 2023, 2026572. [Google Scholar] [CrossRef] [PubMed]
- Mattei, V.; Monache, S.D. Dental Pulp Stem Cells (DPSCs) and Tissue Regeneration: Mechanisms Mediated by Direct, Paracrine, or Autocrine Effects. Biomedicines 2023, 11, 386. [Google Scholar] [CrossRef]
- Tsuruta, T.; Sakai, K.; Watanabe, J.; Katagiri, W.; Hibi, H.; Papaccio, G. Dental pulp-derived stem cell conditioned medium to regenerate peripheral nerves in a novel animal model of dysphagia. PLoS ONE 2018, 13, e0208938. [Google Scholar] [CrossRef]
- Wang, W.; Xu, Z.; Liu, M.; Cai, M.; Liu, X. Prospective applications of extracellular vesicle-based therapies in regenerative medicine: Implications for the use of dental stem cell-derived extracellular vesicles. Front. Bioeng. Biotechnol. 2023, 11, 1278124. [Google Scholar] [CrossRef] [PubMed]
- Alvites, R.; Branquinho, M.; Sousa, A.C.; Lopes, B.; Sousa, P.; Maurício, A.C. Mesenchymal Stem/Stromal Cells and Their Paracrine Activity—Immunomodulation Mechanisms and How to Influence the Therapeutic Potential. Pharmaceutics 2022, 14, 381. [Google Scholar] [CrossRef]
- Sagaradze, G.; Grigorieva, O.; Nimiritsky, P.; Basalova, N.; Kalinina, N.; Akopyan, Z.; Efimenko, A. Conditioned Medium from Human Mesenchymal Stromal Cells: Towards the Clinical Translation. Int. J. Mol. Sci. 2019, 20, 1656. [Google Scholar] [CrossRef]
- Ledesma-Martínez, E.; Mendoza-Núñez, V.M.; Santiago-Osorio, E.; Nurzynska, D. Mesenchymal Stem Cells Derived from Dental Pulp: A Review. Stem Cells Int. 2015, 2016, 4709572. [Google Scholar] [CrossRef]
- Gwam, C.; Mohammed, N.; Ma, X. Stem cell secretome, regeneration, and clinical translation: A narrative review. Ann. Transl. Med. 2021, 9, 70. [Google Scholar] [CrossRef]
- Bar, J.K.; Lis-Nawara, A.; Grelewski, P.G. Dental Pulp Stem Cell-Derived Secretome and Its Regenerative Potential. Int. J. Mol. Sci. 2021, 22, 12018. [Google Scholar] [CrossRef] [PubMed]
- Younes, R.; Issa, Y.; Jdaa, N.; Chouaib, B.; Brugioti, V.; Challuau, D.; Raoul, C.; Scamps, F.; Cuisinier, F.; Hilaire, C. The Secretome of Human Dental Pulp Stem Cells and Its Components GDF15 and HB-EGF Protect Amyotrophic Lateral Sclerosis Motoneurons against Death. Biomedicines 2023, 11, 2152. [Google Scholar] [CrossRef] [PubMed]
- Nor, N.H.M.; Mansor, N.I.; Kashim, M.I.A.M.; Mokhtar, M.H.; Hatta, F.A.M. From Teeth to Therapy: A Review of Therapeutic Potential within the Secretome of Stem Cells from Human Exfoliated Deciduous Teeth. Int. J. Mol. Sci. 2023, 24, 11763. [Google Scholar] [CrossRef]
- Liu, Y.; Xiong, W.; Li, J.; Feng, H.; Jing, S.; Liu, Y.; Zhou, H.; Li, D.; Fu, D.; Xu, C.; et al. Application of dental pulp stem cells for bone regeneration. Front. Med. 2024, 11, 1339573. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Faria, H.V.; Salgado, A.J.; Teixeira, F.G. Mesenchymal Stem Cells-derived Exosomes: A New Possible Therapeutic Strategy for Parkinson’s Disease? Cells 2019, 8, 118. [Google Scholar] [CrossRef]
- Odehnalová, N.; Šandriková, V.; Hromadka, R.; Skaličková, M.; Dytrych, P.; Hoskovec, D.; Kejík, Z.; Hajduch, J.; Vellieux, F.; Vašáková, M.K.; et al. The potential of exosomes in regenerative medicine and in the diagnosis and therapies of neurodegenerative diseases and cancer. Front. Med. 2025, 12, 1539714. [Google Scholar] [CrossRef]
- Chansaenroj, J.; Kornsuthisopon, C.; Chansaenroj, A.; Samaranayake, L.P.; Fan, Y.; Osathanon, T. Potential of Dental Pulp Stem Cell Exosomes: Unveiling miRNA-Driven Regenerative Mechanisms. Int. Dent. J. 2024, 75, 415–425. [Google Scholar] [CrossRef]
- Shafiei, M.; Ansari, M.N.M.; Razak, S.I.A.; Khan, M.U.A. A Comprehensive Review on the Applications of Exosomes and Liposomes in Regenerative Medicine and Tissue Engineering. Polymers 2021, 13, 2529. [Google Scholar] [CrossRef]
- Gugliandolo, A.; Mazzon, E. Dental Mesenchymal Stem Cell Secretome: An Intriguing Approach for Neuroprotection and Neuroregeneration. Int. J. Mol. Sci. 2021, 23, 456. [Google Scholar] [CrossRef]
- Lötvall, J.; Hill, A.F.; Hochberg, F.; Buzás, E.I.; Di Vizio, D.; Gardiner, C.; Gho, Y.S.; Kurochkin, I.V.; Mathivanan, S.; Quesenberry, P.; et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles 2014, 3, 26913. [Google Scholar] [CrossRef]
- Dilsiz, N. A comprehensive review on recent advances in exosome isolation and characterization: Toward clinical applications. Transl. Oncol. 2024, 50, 102121. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, F.; Fu, X.; Han, N. Therapeutic Potential of Mesenchymal Stem Cell-Derived Exosomes as Nanomedicine for Peripheral Nerve Injury. Int. J. Mol. Sci. 2024, 25, 7882. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.J.; Chau, Z.L.; Chen, S.; Hill, J.J.; Korpany, K.V.; Liang, N.; Lin, L.; Lin, Y.; Liu, J.K.; Liu, Y.; et al. Exosome Processing and Characterization Approaches for Research and Technology Development. Adv. Sci. 2022, 9, e2103222. [Google Scholar] [CrossRef]
- Zhang, S.; Teo, K.Y.W.; Chuah, S.J.; Lai, R.C.; Lim, S.K.; Toh, W.S. MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterials 2019, 200, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, X.; Liu, Y.; Wang, Z.; Chen, L.; Du, J.; Li, Q.; Zhou, H.; Zhao, R.; Liu, B. Mesenchymal stem cell–derived exosomal miRNAs in neuroprotection and neuronal apoptosis: Mechanistic insights and therapeutic potential. J. Cell. Mol. Med. 2023, 27, 11234–11250. [Google Scholar] [CrossRef]
- Chai, Y.; Liu, Y.; Liu, Z.; Wei, W.; Dong, Y.; Yang, C.; Chen, M. Study on the Role and Mechanism of Exosomes Derived from Dental Pulp Stem Cells in Promoting Regeneration of Myelin Sheath in Rats with Sciatic Nerve Injury. Mol. Neurobiol. 2024, 61, 6175–6188. [Google Scholar] [CrossRef]
- Yavuz, B.; Mutlu, E.C.; Ahmed, Z.; Ben-Nissan, B.; Stamboulis, A. Applications of Stem Cell-Derived Extracellular Vesicles in Nerve Regeneration. Int. J. Mol. Sci. 2024, 25, 5863. [Google Scholar] [CrossRef]
- Namini, M.S.; Daneshimehr, F.; Beheshtizadeh, N.; Mansouri, V.; Ai, J.; Jahromi, H.K.; Ebrahimi-Barough, S. Cell-free therapy based on extracellular vesicles: A promising therapeutic strategy for peripheral nerve injury. Stem Cell Res. Ther. 2023, 14, 254. [Google Scholar] [CrossRef]
- Ezdakova, M.I.; Andreeva, E.R. Impaired Communication through Gap Junctions Reduces the Angiogenic Potential of the Secretome in Mesenchymal Stromal Cell—Endothelial Cell Interactions In Vitro. Bull. Exp. Biol. Med. 2024, 178, 139–144. [Google Scholar] [CrossRef]
- Liu, J.; Ding, Y.; Liu, Z.; Liang, X. Senescence in Mesenchymal Stem Cells: Functional Alterations, Molecular Mechanisms, and Rejuvenation Strategies. Front. Cell Dev. Biol. 2020, 8, 258. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ye, Z.; Wang, X.; Li, J.; Mei, P.L.; Duan, L.; Wang, B.; Xu, C.; Xiong, W.; He, Y. Application of dental pulp stem cell-conditioned medium combined with deep cryopreservation of autologous cranial flaps. Stem Cell Res. Ther. 2025, 16, 272. [Google Scholar] [CrossRef] [PubMed]
- Harrell, C.R.; Jovicic, N.; Djonov, V.; Volarevic, V. Therapeutic Use of Mesenchymal Stem Cell-Derived Exosomes: From Basic Science to Clinics. Pharmaceutics 2020, 12, 474. [Google Scholar] [CrossRef]
- Rastogi, S.; Sharma, V.; Bharti, P.S.; Rani, K.; Modi, G.P.; Nikolajeff, F.; Kumar, S. The Evolving Landscape of Exosomes in Neurodegenerative Diseases: Exosomes Characteristics and a Promising Role in Early Diagnosis. Int. J. Mol. Sci. 2021, 22, 440. [Google Scholar] [CrossRef]
- Chen, G.-Y.; Fu, L.-L.; Ye, H.-P.; Cheng, P.; Feng, H.-C.; Yan, M. Effects of exosomes from human dental pulp stem cells on the biological behavior of human fibroblasts. Sci. Rep. 2025, 15, 1134. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-H.; Liao, Y.-T.; Hsueh, K.-K.; Huang, H.-K.; Chen, T.-M.; Chiang, E.-R.; Hsu, S.-H.; Tseng, T.-C.; Wang, J.-P. Adipose-Derived Mesenchymal Stem Cells From a Hypoxic Culture Improve Neuronal Differentiation and Nerve Repair. Front. Cell Dev. Biol. 2021, 9, 658099. [Google Scholar] [CrossRef]
- Caseiro, A.R.; Pedrosa, S.S.; Ivanova, G.; Branquinho, M.V.; Almeida, A.; Faria, F.; Amorim, I.; Pereira, T.; Maurício, A.C.; Papaccio, G. Mesenchymal Stem/Stromal Cells metabolomic and bioactive factors profiles: A comparative analysis on the umbilical cord and dental pulp derived Stem/Stromal Cells secretome. PLoS ONE 2019, 14, e0221378. [Google Scholar] [CrossRef] [PubMed]
- Sultan, N.; Amin, L.E.; Zaher, A.R.; Grawish, M.E.; Scheven, B.A. Dental pulp stem cells stimulate neuronal differentiation of PC12 cells. Neural Regen Res. 2021, 16, 1821–1828. [Google Scholar] [CrossRef]
- Sultan, N.; Amin, L.E.; Zaher, A.R.; Grawish, M.E.; Scheven, B.A. Neurotrophic effects of dental pulp stem cells on trigeminal neuronal cells. Sci Rep. 2020, 10, 19694. [Google Scholar] [CrossRef]
- Zou, J.; Dai, Y.; Chen, Y.; Wang, B.; Zhang, Z.; Li, X.; Wu, J.; Xu, X.; Li, W.; Zhou, J.; et al. Exosomes derived from odontogenic stem cells: Its role in the dentin–pulp complex. Regen Ther. 2023, 24, 135–146. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.Y.; Ren, J.L.; Xu, F.; Chen, F.M. Dental stem cell-derived extracellular vesicles as promising therapeutic agents in the treatment of diseases. Int. J. Oral Sci. 2022, 14, 2. [Google Scholar] [CrossRef] [PubMed]
- Pisciotta, A.; Bertoni, L.; Vallarola, A.; Bertani, G.; Mecugni, D.; Carnevale, G. Neural crest-derived stem cells from dental pulp and tooth-associated stem cells for peripheral nerve regeneration. Neural Regen. Res. 2020, 15, 373–381. [Google Scholar] [CrossRef]
- Man, R.C.; Fung, C.H.; Typek, J.P.; Liu, R.; Zhu, D.; Liu, J.; Chen, F.M.; Zhao, Y. Insights into the effects of the dental stem cell secretome on nerve regeneration: Towards cell-free treatment. Stem Cells Int. 2019, 2019, 4596150. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.; Valente, S.G.; Sabongi, R.G.; Dos Santos, J.B.G.; Leite, V.M.; Ulrich, H.; Nery, A.A.; da Silva Fernandes, M.J. Bone marrow-derived mesenchymal stem cells versus adipose-derived mesenchymal stem cells for peripheral nerve regeneration. Neural Regen. Res. 2018, 13, 100–104. [Google Scholar] [CrossRef]
- Pardo-Rodríguez, B.; Baraibar, A.M.; Manero-Roig, I.; Luzuriaga, J.; Salvador-Moya, J.; Polo, Y.; Basanta-Torres, R.; Unda, F.; Mato, S.; Ibarretxe, G.; et al. Functional differentiation of human dental pulp stem cells into neuron-like cells exhibiting electrophysiological activity. Stem Cell Res. Ther. 2025, 16, 10. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, Y.; Zhang, T.; Qu, J.; Liu, Q.; Chen, Y.; Xu, B.; Shen, J. Dental pulp stem cells conditioned medium-functionalized microspheres for endodontic regeneration. Front. Cell Dev. Biol. 2025, 13, 1627220. [Google Scholar] [CrossRef]
- Gharaei, M.A.; Xue, Y.; Mustafa, K.; Lie, S.A.; Fristad, I. Human dental pulp stromal cell conditioned medium alters endothelial cell behavior. Stem Cell Res. Ther. 2018, 9, 69. [Google Scholar] [CrossRef]
- El Moshy, S.; Radwan, I.A.; Rady, D.; Abbass, M.M.S.; El-Rashidy, A.A.; Sadek, K.M.; Dörfer, C.E.; El-Sayed, K.M.F. Dental Stem Cell-Derived Secretome/Conditioned Medium: The Future for Regenerative Therapeutic Applications. Stem Cells Int. 2020, 2020, 7593402. [Google Scholar] [CrossRef]
- Maccaferri, M.; Pisciotta, A.; Carnevale, G.; Salvarani, C.; Pignatti, E. Human dental pulp stem cells modulate pro-inflammatory macrophages both through cell-to-cell contact and paracrine signaling. Front. Immunol. 2024, 15, 1440974. [Google Scholar] [CrossRef]
- Barone, L.; Cucchiara, M.; Palano, M.T.; Bassani, B.; Gallazzi, M.; Rossi, F.; Raspanti, M.; Zecca, P.A.; De Antoni, G.; Pagiatakis, C.; et al. Dental pulp mesenchymal stem cell (DPSCs)-derived soluble factors, produced under hypoxic conditions, support angiogenesis via endothelial cell activation and generation of M2-like macrophages. J. Biomed. Sci. 2024, 31, 99. [Google Scholar] [CrossRef]
- Li, P.; Ou, Q.; Shi, S.; Shao, C. Immunomodulatory properties of mesenchymal stem cells/dental stem cells and their therapeutic applications. Cell. Mol. Immunol. 2023, 20, 558–569. [Google Scholar] [CrossRef]
- Alraies, A.; Alaidaroos, N.Y.A.; Waddington, R.J.; Moseley, R.; Sloan, A.J. Variation in human dental pulp stem cell ageing profiles reflect contrasting proliferative and regenerative capabilities. BMC Cell Biol. 2017, 18, 12. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Yamamoto, S.; Chikenji, T.S. Role of cellular senescence in inflammation and regeneration. Inflamm. Regen. 2024, 44, 28. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Naruphontjirakul, P.; Huang, T.-Y.; Wu, Y.-C.; Cheng, W.-H.; Su, W.-T. The Exosomes of Stem Cells from Human Exfoliated Deciduous Teeth Suppress Inflammation in Osteoarthritis. Int. J. Mol. Sci. 2024, 25, 8560. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; Bartold, P.M.; Gulati, K.; Moran, C.S.; Ivanovski, S.; Han, P. Periodontal and Dental Pulp Cell-Derived Small Extracellular Vesicles: A Review of the Current Status. Nanomaterials 2021, 11, 1858. [Google Scholar] [CrossRef] [PubMed]
- Mai, Z.; Chen, H.; Ye, Y.; Hu, Z.; Sun, W.; Cui, L.; Zhao, X. Translational and Clinical Applications of Dental Stem Cell-Derived Exosomes. Front. Genet. 2021, 12, 750990. [Google Scholar] [CrossRef]
- Arimura, Y.; Shindo, Y.; Yamanaka, R.; Mochizuki, M.; Hotta, K.; Nakahara, T.; Ito, E.; Yoshioka, T.; Oka, K.; Haque, N. Peripheral-neuron-like properties of differentiated human dental pulp stem cells (hDPSCs). PLoS ONE 2021, 16, e0251356. [Google Scholar] [CrossRef]
- Sousa, A.C.; Biscaia, S.; Alvites, R.; Branquinho, M.; Lopes, B.; Sousa, P.; Valente, J.; Franco, M.; Santos, J.D.; Mendonça, C.; et al. Assessment of 3D-Printed Polycaprolactone, Hydroxyapatite Nanoparticles and Diacrylate Poly(ethylene glycol) Scaffolds for Bone Regeneration. Pharmaceutics 2022, 14, 2643. [Google Scholar] [CrossRef]
- Brunello, G.; Zanotti, F.; Trentini, M.; Zanolla, I.; Pishavar, E.; Favero, V.; Favero, R.; Favero, L.; Bressan, E.; Bonora, M.; et al. Exosomes Derived from Dental Pulp Stem Cells Show Different Angiogenic and Osteogenic Properties in Relation to the Age of the Donor. Pharmaceutics 2022, 14, 908. [Google Scholar] [CrossRef]
- Harley-Troxell, M.E.; Steiner, R.; Advincula, R.C.; Anderson, D.E.; Dhar, M. Interactions of Cells and Biomaterials for Nerve Tissue Engineering: Polymers and Fabrication. Polymers 2023, 15, 3685. [Google Scholar] [CrossRef]
- Liu, Y.; Zou, Y.; Liu, X.; Wang, Y.; Sun, J.; Yuan, C.; Wang, P. The effect of differentiation of human dental pulp stem cells to glial cells on the sensory nerves of the dental pulp. Int. J. Dent. 2024, 2024, 3746794. [Google Scholar] [CrossRef]
- Gao, Y.; Tian, Z.; Liu, Q.; Wang, T.; Ban, L.-K.; Lee, H.H.-C.; Umezawa, A.; Almansour, A.I.; Arumugam, N.; Kumar, R.S.; et al. Neuronal Cell Differentiation of Human Dental Pulp Stem Cells on Synthetic Polymeric Surfaces Coated With ECM Proteins. Front. Cell Dev. Biol. 2022, 10, 893241. [Google Scholar] [CrossRef] [PubMed]
- Seonwoo, H.; Jang, K.-J.; Lee, D.; Park, S.; Lee, M.; Park, S.; Lim, K.-T.; Kim, J.; Chung, J.H. Neurogenic Differentiation of Human Dental Pulp Stem Cells on Graphene-Polycaprolactone Hybrid Nanofibers. Nanomaterials 2018, 8, 554. [Google Scholar] [CrossRef]
- Ellis, P.; Fagan, B.M.; Magness, S.T.; Hutton, S.; Taranova, O.; Hayashi, S.; McMahon, A.; Rao, M.; Pevny, L. SOX2, a Persistent Marker for Multipotential Neural Stem Cells Derived from Embryonic Stem Cells, the Embryo or the Adult. Dev. Neurosci. 2004, 26, 148–165. [Google Scholar] [CrossRef]
- Suzuki, S.; Namiki, J.; Shibata, S.; Mastuzaki, Y.; Okano, H. The Neural Stem/Progenitor Cell Marker Nestin Is Expressed in Proliferative Endothelial Cells, but Not in Mature Vasculature. J. Histochem. Cytochem. 2010, 58, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Vainorius, G.; Novatchkova, M.; Michlits, G.; Baar, J.C.; Raupach, C.; Lee, J.; Yelagandula, R.; Wernig, M.; Elling, U. Ascl1 and Ngn2 convert mouse embryonic stem cells to neurons via functionally distinct paths. Nat. Commun. 2023, 14, 5341. [Google Scholar] [CrossRef] [PubMed]
- Alimperti, S.; Andreadis, S.T. CDH2 and CDH11 act as regulators of stem cell fate decisions. Stem Cell Res. 2015, 14, 270–282. [Google Scholar] [CrossRef]
- Al-Maswary, A.A.; O’rEilly, M.; Holmes, A.P.; Walmsley, A.D.; Cooper, P.R.; Scheven, B.A.; Hetman, M. Exploring the neurogenic differentiation of human dental pulp stem cells. PLoS ONE 2022, 17, e0277134. [Google Scholar] [CrossRef]
- Luzuriaga, J.; Polo, Y.; Pastor-Alonso, O.; Pardo-Rodríguez, B.; Larrañaga, A.; Unda, F.; Sarasua, J.-R.; Pineda, J.R.; Ibarretxe, G. Advances and Perspectives in Dental Pulp Stem Cell Based Neuroregeneration Therapies. Int. J. Mol. Sci. 2021, 22, 3546. [Google Scholar] [CrossRef] [PubMed]
- Geuna, S.; Raimondo, S.; Fregnan, F.; Haastert-Talini, K.; Grothe, C.; Kirik, D. In vitro models for peripheral nerve regeneration. Eur. J. Neurosci. 2015, 43, 287–296. [Google Scholar] [CrossRef]
- Li, D.; Zou, X.-Y.; El-Ayachi, I.; Romero, L.O.; Yu, Z.; Iglesias-Linares, A.; Cordero-Morales, J.F.; Huang, G.T.-J. Human Dental Pulp Stem Cells and Gingival Mesenchymal Stem Cells Display Action Potential Capacity In Vitro after Neuronogenic Differentiation. Stem Cell Rev. Rep. 2018, 15, 67–81. [Google Scholar] [CrossRef]
- Kucharova, K.; Stallcup, W. The NG2 proteoglycan promotes oligodendrocyte progenitor proliferation and developmental myelination. Neuroscience 2009, 166, 185–194. [Google Scholar] [CrossRef]
- Fujiwara, S.; Hoshikawa, S.; Ueno, T.; Hirata, M.; Saito, T.; Ikeda, T.; Kawaguchi, H.; Nakamura, K.; Tanaka, S.; Ogata, T.; et al. SOX10 Transactivates S100B to Suppress Schwann Cell Proliferation and to Promote Myelination. PLoS ONE 2014, 9, e115400. [Google Scholar] [CrossRef]
- Pummi, K.P.; Heape, A.M.; Grénman, R.A.; Peltonen, J.T.; Peltonen, S.A. Tight Junction Proteins ZO-1, Occludin, and Claudins in Developing and Adult Human Perineurium. J. Histochem. Cytochem. 2004, 52, 1037–1046. [Google Scholar] [CrossRef]
- Latremoliere, A.; Cheng, L.; DeLisle, M.; Wu, C.; Chew, S.; Hutchinson, E.B.; Sheridan, A.; Alexandre, C.; Latremoliere, F.; Sheu, S.-H.; et al. Neuronal-Specific TUBB3 Is Not Required for Normal Neuronal Function but Is Essential for Timely Axon Regeneration. Cell Rep. 2018, 24, 1865–1879.e9. [Google Scholar] [CrossRef]
- Gusel’nIkova, V.V.; Korzhevskiy, D.E. NeuN As a Neuronal Nuclear Antigen and Neuron Differentiation Marker. Acta Nat. 2015, 7, 42–47. [Google Scholar] [CrossRef]
- LeBlanc, S.E.; Jang, S.-W.; Ward, R.M.; Wrabetz, L.; Svaren, J. Direct Regulation of Myelin Protein Zero Expression by the Egr2 Transactivator. J. Biol. Chem. 2006, 281, 5453–5460. [Google Scholar] [CrossRef]
- Monache, S.D.; Pulcini, F.; Santilli, F.; Martellucci, S.; Santacroce, C.; Fabrizi, J.; Angelucci, A.; Sorice, M.; Mattei, V. Hypoxia Induces DPSC Differentiation versus a Neurogenic Phenotype by the Paracrine Mechanism. Biomedicines 2022, 10, 1056. [Google Scholar] [CrossRef] [PubMed]
- Holahan, M.R. A Shift from a Pivotal to Supporting Role for the Growth-Associated Protein (GAP-43) in the Coordination of Axonal Structural and Functional Plasticity. Front. Cell. Neurosci. 2017, 11, 266. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Strata, P. Structural plasticity of climbing fibers and the growth-associated protein GAP-43. Front. Neural Circuits 2013, 7, 25. [Google Scholar] [CrossRef]
- Libberecht, K.; Dirkx, N.; Vangansewinkel, T.; Vandendries, W.; Lambrichts, I.; Wolfs, E. The Influence of Lysosomal Stress on Dental Pulp Stem Cell-Derived Schwann Cells. Biomolecules 2024, 14, 405. [Google Scholar] [CrossRef] [PubMed]
- Candelise, N.; Santilli, F.; Fabrizi, J.; Caissutti, D.; Spinello, Z.; Moliterni, C.; Lancia, L.; Monache, S.D.; Mattei, V.; Misasi, R. The Importance of Stem Cells Isolated from Human Dental Pulp and Exfoliated Deciduous Teeth as Therapeutic Approach in Nervous System Pathologies. Cells 2023, 12, 1686. [Google Scholar] [CrossRef]
- Bassett, C.; Triplett, H.; Lott, K.; Howard, K.M.; Kingsley, K. Differential Expression of MicroRNA (MiR-27, MiR-145) among Dental Pulp Stem Cells (DPSCs) Following Neurogenic Differentiation Stimuli. Biomedicines 2023, 11, 3003. [Google Scholar] [CrossRef]
- Radwitz, J.; Hausrat, T.J.; Heisler, F.F.; Janiesch, P.C.; Pechmann, Y.; Rübhausen, M.; Kneussel, M. Tubb3 expression levels are sensitive to neuronal activity changes and determine microtubule growth and kinesin-mediated transport. Cell. Mol. Life Sci. 2022, 79, 575. [Google Scholar] [CrossRef]
- Jurga, A.M.; Paleczna, M.; Kadluczka, J.; Kuter, K.Z. Beyond the GFAP-Astrocyte Protein Markers in the Brain. Biomolecules 2021, 11, 1361. [Google Scholar] [CrossRef] [PubMed]
- Konnova, E.A.; Deftu, A.-F.; Chung, P.C.S.; Pertin, M.; Kirschmann, G.; Decosterd, I.; Suter, M.R. Characterisation of GFAP-Expressing Glial Cells in the Dorsal Root Ganglion after Spared Nerve Injury. Int. J. Mol. Sci. 2023, 24, 15559. [Google Scholar] [CrossRef]
- Mester, T.; Raychaudhuri, N.; Gillespie, E.F.; Chen, H.; Smith, T.J.; Douglas, R.S.; Pietropaolo, M. CD40 Expression in Fibrocytes Is Induced by TSH: Potential Synergistic Immune Activation. PLoS ONE 2016, 11, e0162994. [Google Scholar] [CrossRef]
- Xia, L.; Li, P.; Bi, W.; Yang, R.; Zhang, Y. CDK5R1 promotes Schwann cell proliferation, migration, and production of neurotrophic factors via CDK5/BDNF/TrkB after sciatic nerve injury. Neurosci. Lett. 2023, 817, 137514. [Google Scholar] [CrossRef]
- Ao, C.; Li, C.; Chen, J.; Tan, J.; Zeng, L. The role of Cdk5 in neurological disorders. Front. Cell. Neurosci. 2022, 16, 951202. [Google Scholar] [CrossRef] [PubMed]
- Sugimori, M.; Nagao, M.; Parras, C.M.; Nakatani, H.; Lebel, M.; Guillemot, F.; Nakafuku, M. Ascl1 is required for oligodendrocyte development in the spinal cord. Development 2008, 135, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Carney, T.J.; Dutton, K.A.; Greenhill, E.; Delfino-Machín, M.; Dufourcq, P.; Blader, P.; Kelsh, R.N. A direct role for Sox10 in specification of neural crest-derived sensory neurons. Development 2006, 133, 4619–4630. [Google Scholar] [CrossRef]
- Tordoff, E.; Allen, J.; Elgart, K.; Elsherbini, A.; Kalia, V.; Wu, H.; Eren, E.; Kapogiannis, D.; Gololobova, O.; Witwer, K.; et al. A novel multiplexed immunoassay for surface-exposed proteins in plasma extracellular vesicles. J. Extracell. Vesicles 2024, 13, e70007. [Google Scholar] [CrossRef]
- Ahmad, P.; Estrin, N.; Farshidfar, N.; Zhang, Y.; Miron, R.J. Isolation methods of exosomes derived from dental stem cells. Int. J. Oral Sci. 2025, 17, 50. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhou, Y.; Li, H.J. Advances in mesenchymal stem cell exosomes: A review. Stem Cell Res. Ther. 2021, 12, 71. [Google Scholar] [CrossRef]
- O’COnnor, C.; Mullally, R.E.; McComish, S.F.; O’SUllivan, J.; Woods, I.; Schoen, I.; Garre, M.; Caldwell, M.A.; Dervan, A.; O’BRien, F.J. Neurotrophic extracellular matrix proteins promote neuronal and iPSC astrocyte progenitor cell- and nano-scale process extension for neural repair applications. Am. J. Anat. 2024, 246, 585–601. [Google Scholar] [CrossRef]
- Xu, X.-M.; Deng, L.-X.; Liu, N.-K.; Wen, R.N.; Yang, S.-N.; Wen, X. Laminin-coated multifilament entubulation, combined with Schwann cells and glial cell line-derived neurotrophic factor, promotes unidirectional axonal regeneration in a rat model of thoracic spinal cord hemisection. Neural Regen. Res. 2021, 16, 186–191. [Google Scholar] [CrossRef]
- Speer, J.; Barcellona, M.; Jing, L.; Liu, B.; Lu, M.; Kelly, M.; Buchowski, J.; Zebala, L.; Luhmann, S.; Gupta, M.; et al. Integrin-mediated interactions with a laminin-presenting substrate modulate biosynthesis and phenotypic expression for cells of the human nucleus pulposus. Eur. Cells Mater. 2021, 41, 793–810. [Google Scholar] [CrossRef]
- Arimori, T.; Miyazaki, N.; Mihara, E.; Takizawa, M.; Taniguchi, Y.; Cabañas, C.; Sekiguchi, K.; Takagi, J. Structural mechanism of laminin recognition by integrin. Nat. Commun. 2021, 12, 4012. [Google Scholar] [CrossRef]
- Nolan, A.M.; Collins, L.M.; Wyatt, S.L.; Gutierrez, H.; O’Keeffe, G.W. The neurite growth inhibitory effects of soluble TNFα on developing sympathetic neurons are dependent on developmental age. Differentiation 2014, 88, 124–130. [Google Scholar] [CrossRef]
- Popova, D.; Jacobsson, S.O.P. A fluorescence microplate screen assay for the detection of neurite outgrowth and neurotoxicity using an antibody against βIII-tubulin. Toxicol. Vitr. 2014, 28, 411–418. [Google Scholar] [CrossRef] [PubMed]
- A Sierra-Fonseca, J.; Najera, O.; Martinez-Jurado, J.; Walker, E.M.; Varela-Ramirez, A.; Khan, A.M.; Miranda, M.; Lamango, N.S.; Roychowdhury, S. Nerve growth factor induces neurite outgrowth of PC12 cells by promoting Gβγ-microtubule interaction. BMC Neurosci. 2014, 15, 132. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, J.; Caron, V.; Tremblay, A. SUMOylation of nuclear receptor Nor1/NR4A3 coordinates microtubule cytoskeletal dynamics and stability in neuronal cells. Cell Biosci. 2024, 14, 91. [Google Scholar] [CrossRef]
- DeVault, L.; Mateusiak, C.; Palucki, J.; Brent, M.; Milbrandt, J.; DiAntonio, A.; Wiche, G. The response of Dual-leucine zipper kinase (DLK) to nocodazole: Evidence for a homeostatic cytoskeletal repair mechanism. PLoS ONE 2024, 19, e0300539. [Google Scholar] [CrossRef]
- Plessis-Belair, J.; Russo, T.; Riessland, M.; Sher, R.B. Nuclear Import Defects Drive Cell Cycle Dysregulation in Neurodegeneration. Aging Cell 2025, 24, e70091. [Google Scholar] [CrossRef]
- Verkest, C.; Schaefer, I.; Nees, T.A.; Wang, N.; Jegelka, J.M.; Taberner, F.J.; Lechner, S.G. Intrinsically disordered intracellular domains control key features of the mechanically-gated ion channel PIEZO2. Nat. Commun. 2022, 13, 1365. [Google Scholar] [CrossRef]
- Borghi, R.; Petrini, S.; Apollonio, V.; Trivisano, M.; Specchio, N.; Moreno, S.; Bertini, E.; Tartaglia, M.; Compagnucci, C. Altered cytoskeleton dynamics in patient-derived iPSC-based model of PCDH19 clustering epilepsy. Front. Cell Dev. Biol. 2025, 12, 1518533. [Google Scholar] [CrossRef]
- Lasser, M.; Tiber, J.; Lowery, L.A. The Role of the Microtubule Cytoskeleton in Neurodevelopmental Disorders. Front. Cell. Neurosci. 2018, 12, 165. [Google Scholar] [CrossRef]
- Sánchez-Huertas, C.; Herrera, E. With the Permission of Microtubules: An Updated Overview on Microtubule Function During Axon Pathfinding. Front. Mol. Neurosci. 2021, 14, 759404. [Google Scholar] [CrossRef] [PubMed]
- Verstraelen, P.; Detrez, J.R.; Verschuuren, M.; Kuijlaars, J.; Nuydens, R.; Timmermans, J.-P.; De Vos, W.H. Dysregulation of Microtubule Stability Impairs Morphofunctional Connectivity in Primary Neuronal Networks. Front. Cell. Neurosci. 2017, 11, 173. [Google Scholar] [CrossRef] [PubMed]
- Sferra, A.; Nicita, F.; Bertini, E. Microtubule Dysfunction: A Common Feature of Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 7354. [Google Scholar] [CrossRef]
- Harvey, A.; Yen, T.-Y.; Aizman, I.; Tate, C.; Case, C.; Rojas, M. Proteomic Analysis of the Extracellular Matrix Produced by Mesenchymal Stromal Cells: Implications for Cell Therapy Mechanism. PLoS ONE 2013, 8, e79283. [Google Scholar] [CrossRef] [PubMed]
- Abd-elwahab, S.A.-e.; Khamis, N.H.; Rifaai, R.A.; El-Tahawy, N.F.G.; Ibrahim, R.A. Mesenchymal-Stem Cell-Derived Conditioned Media Versus Exosomes in the Treatment of Rat Model of Polycystic Ovary: An Attempt to Understand the Underlying Mechanisms (Biochemical and Histological Study). Microsc. Microanal. 2023, 29, 1244–1257. [Google Scholar] [CrossRef]
- Novais, A.; Lesieur, J.; Sadoine, J.; Slimani, L.; Baroukh, B.; Saubaméa, B.; Schmitt, A.; Vital, S.; Poliard, A.; Hélary, C.; et al. Priming Dental Pulp Stem Cells from Human Exfoliated Deciduous Teeth with Fibroblast Growth Factor-2 Enhances Mineralization Within Tissue-Engineered Constructs Implanted in Craniofacial Bone Defects. Stem Cells Transl. Med. 2019, 8, 844–857. [Google Scholar] [CrossRef]
- Alvites, R.; Lopes, B.; Coelho, A.; Sousa, A.C.; Sousa, P.; Moreira, A.; Rêma, A.; Simões, M.; Mendonça, C.; Atayde, L.; et al. Combined use of olfactory mucosal mesenchymal stem cells conditioned medium and neural guide conduits promotes nerve regeneration in an ovine model. Front. Cell Dev. Biol. 2025, 13, 1598736. [Google Scholar] [CrossRef]
- Kunimatsu, R.; Nakajima, K.; Awada, T.; Tsuka, Y.; Abe, T.; Ando, K.; Hiraki, T.; Kimura, A.; Tanimoto, K. Comparative characterization of stem cells from human exfoliated deciduous teeth, dental pulp, and bone marrow-derived mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2018, 501, 193–198. [Google Scholar] [CrossRef]
- Dogny, C.; André-Lévigne, D.; Kalbermatten, D.F.; Madduri, S. Therapeutic potential and challenges of mesenchymal stem cell-derived exosomes for peripheral nerve regeneration: A systematic review. Int. J. Mol. Sci. 2024, 25, 6489. [Google Scholar] [CrossRef]
- Zhou, T.; Rong, M.; Wang, Z.; Chu, H.; Chen, C.; Zhang, J.; Tian, Z. Conditioned medium derived from 3D tooth germs: A novel cocktail for stem cell priming and early in vivo pulp regeneration. Cell Prolif. 2021, 54, e13129. [Google Scholar] [CrossRef]
- Sousa, A.C.; Biscaia, S.; Alvites, R.; Branquinho, M.; Lopes, B.; Sousa, P.; Valente, J.; Franco, M.; Santos, J.D.; Atayde, L.; et al. Production, Characterisation, and In Vitro Evaluation of 3D Printed PCL/HANp/PEGDA Scaffold for Bone Regeneration. Mater. Proc. 2022, 8, 79. [Google Scholar]
- Caseiro, A.R.; Ivanova, G.; Pedrosa, S.S.; Branquinho, M.V.; Georgieva, P.; Barbosa, P.P.; Santos, J.D.; Magalhães, R.; Teixeira, P.; Pereira, T.; et al. Human umbilical cord blood plasma as an alternative to animal sera for mesenchymal stromal cells in vitro expansion—A multicomponent metabolomic analysis. PLoS ONE 2018, 13, e0203936. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.; Pereira, I.; Pereira, J.E.; Maltez, L.; Brandão, A.; Alvites, R.; Sousa, A.C.; Branquinho, M.; Caseiro, A.R.; Pedrosa, S.S.; et al. Dextrin hydrogel loaded with a macroporous Bonelike® scaffold and dental pulp stem cells for critical-sized defect repair. Materialia 2023, 30, 101859. [Google Scholar] [CrossRef]
- Sousa, P.; Lopes, B.; Sousa, A.C.; Moreira, A.d.S.; Rêma, A.; Alvites, R.; Geuna, S.; Alves, N.; Maurício, A.C. Rat Hair Follicle Stem Cell-Derived Exosomes: Isolation, Characterization and Comparative Analysis of Their In Vitro Wound Healing Potential. Int. J. Mol. Sci. 2025, 26, 5081. [Google Scholar] [CrossRef] [PubMed]
- Alvites, R.D.; Branquinho, M.V.; Caseiro, A.R.; Amorim, I.; Pedrosa, S.S.; Rêma, A.; Faria, F.; Porto, B.; Oliveira, C.; Teixeira, P.; et al. Rat Olfactory Mucosa Mesenchymal Stem/Stromal Cells (OM-MSCs): A Characterization Study. Int. J. Cell Biol. 2020, 2020, 2938258. [Google Scholar] [CrossRef]
- Clerici, M.; Nelemans, L.C.; Buzgo, M.; Simaite, A. Pitfalls of Accurate Protein Determination inside PLGA Nanoparticles Using the Micro BCA Assay. In Proceedings of the International Electronic Conference on Pharmaceutics, Online, 1–15 December 2020; p. 28. [Google Scholar]
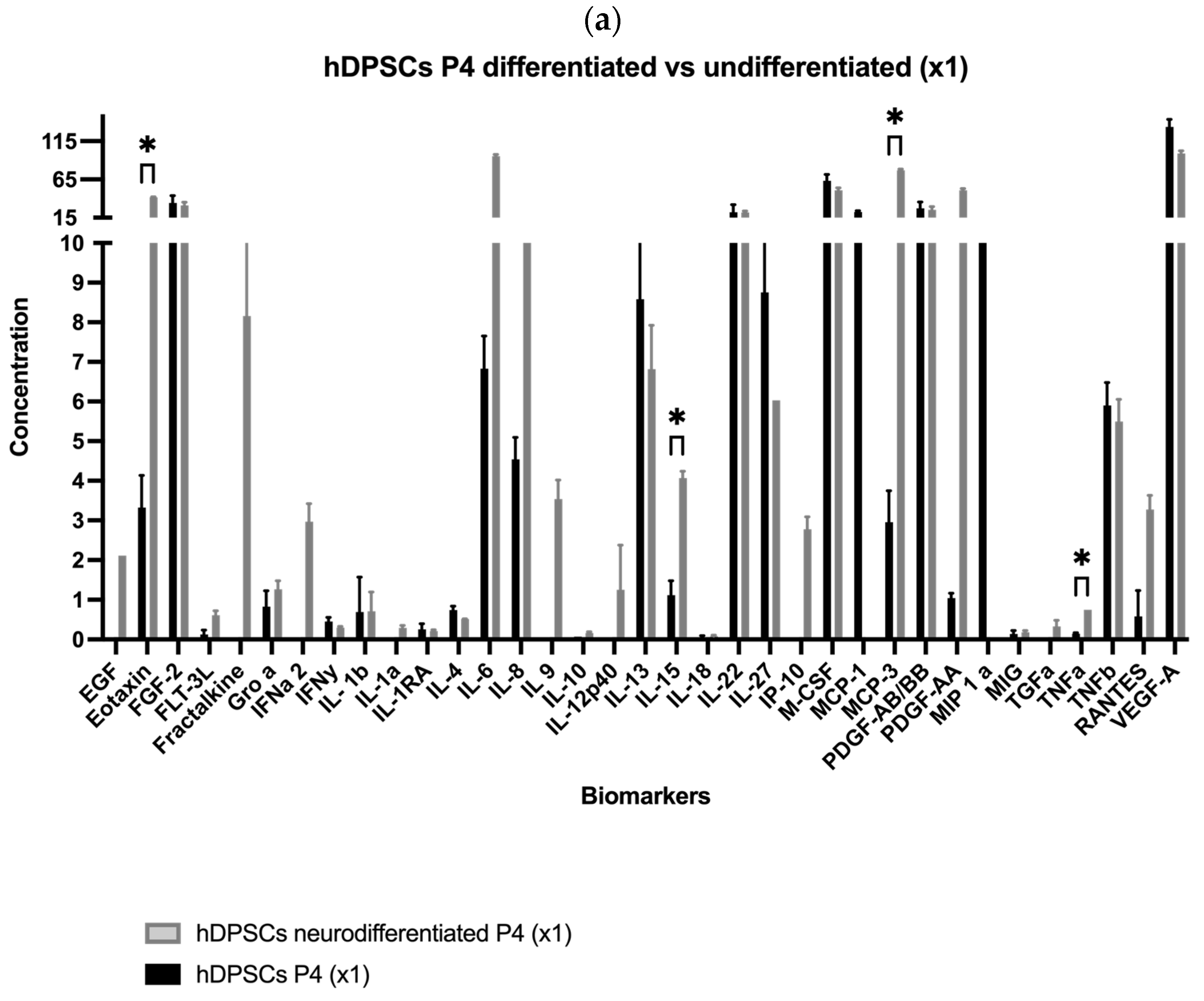
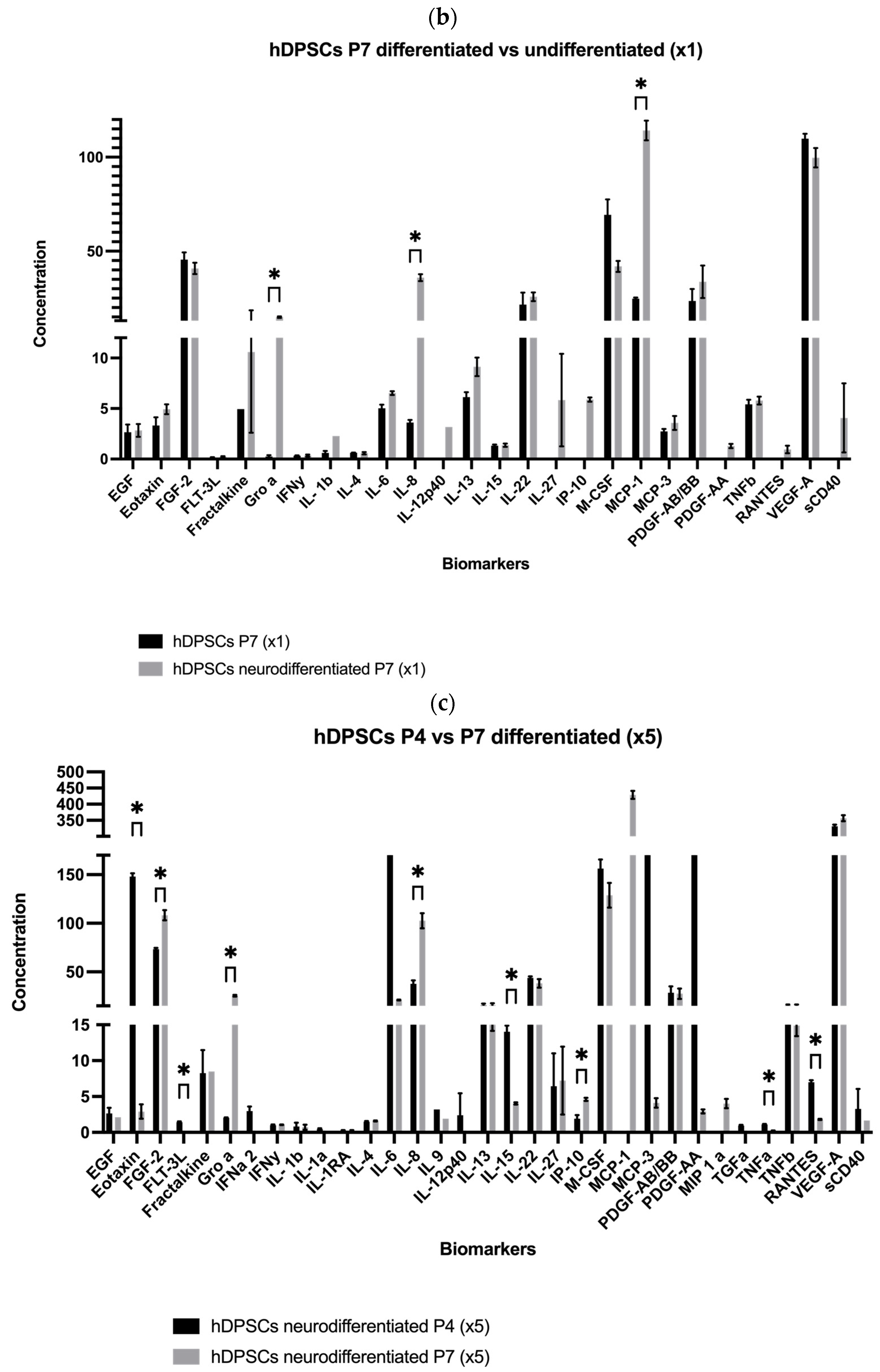

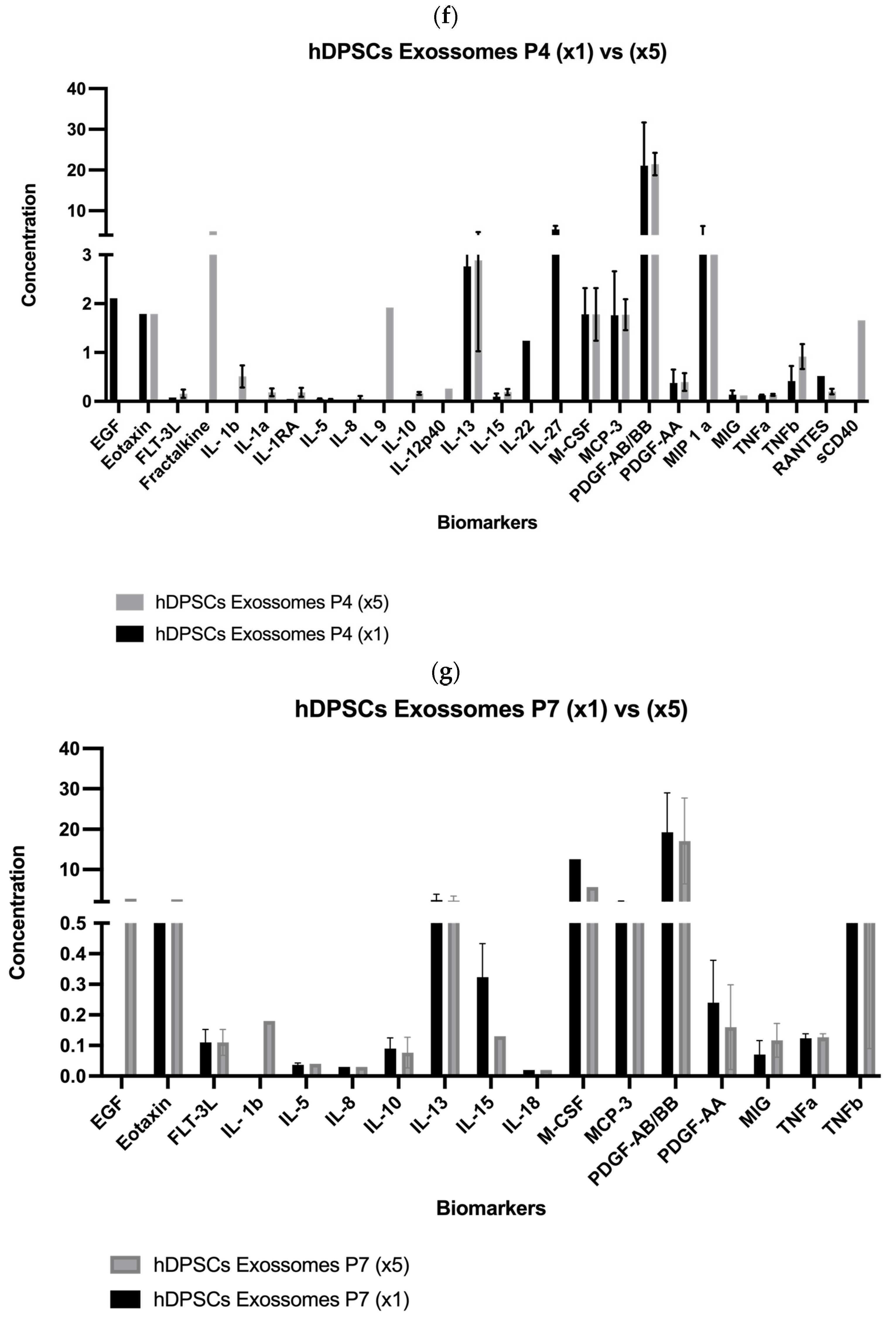

| Biomolecule | Mean ± SEM (P4) (×1) | Mean ± SEM (P4) (×5) | Mean ± SEM (P7) (×1) | Mean ± SEM (P7) (×5) |
|---|---|---|---|---|
| EGF | 1.40 ± 0.00 | 1.77 ± 0.77 | 2.84 ± 0.63 | 0.70 ± 0.00 |
| Eotaxin | 41.66 ± 0.65 | 148.06 ± 3.26 | 4.93 ± 0.49 | 2.90 ± 1.00 |
| FGF-2 | 31.24 ± 4.03 | 73.33 ± 1.42 | 40.83 ± 3.00 | 108.31 ± 5.20 |
| FLT-3L | 0.61 ± 0.11 | 1.42 ± 0.13 | 0.23 ± 0.06 | 0.25 ± 0.00 |
| Fractalkine | 5.44 ± 4.54 | 8.27 ± 3.21 | 7.06 ± 7.98 | 2.83 ± 0.00 |
| Groα | 1.26 ± 0.21 | 2.03 ± 0.06 | 14.93 ± 0.41 | 25.46 ± 0.91 |
| IFN α 2 | 2.96 ± 0.45 | 1.98 ± 0.64 | nd | nd |
| IFNγ | 0.3 ± 0.03 | 1.01 ± 0.09 | 0.35 ± 0.10 | 1.06 ± 0.03 |
| IL-1α | 0.29 ± 0.06 | 0.46 ± 0.12 | nd | nd |
| IL-1b | 0.71 ± 0.49 | 0.81 ± 0.55 | 0.76 ± 0.00 | 0.72 ± 0.36 |
| IL-1RA | 0.21 ± 0.03 | 0.25 ± 0.06 | nd | 0.19 ± 0.13 |
| IL-4 | 0.51 ± 0.01 | 1.48 ± 0.09 | 0.58 ± 0.11 | 1.58 ± 0.06 |
| IL-6 | 95.44 ± 2.04 | 254.07 ± 5.16 | 6.53 ± 0.19 | 21.07 ± 0.44 |
| IL-8 | 11.7 ± 0.51 | 37.67 ± 3.48 | 35.89 ± 1.83 | 102.60 ± 7.78 |
| IL-9 | 2.36 ± 0.48 | 2.13 ± 0.00 | nd | 0.64 ± 0.00 |
| IL-10 | 0.16 ± 0.03 | nd | nd | nd |
| IL-12p40 | 1.25 ± 1.21 | 2.39 ± 3.06 | 2.11 ± 0.00 | nd |
| IL-13 | 6.82 ± 1.10 | 15.56 ± 1.74 | 9.13 ± 0.92 | 15.93 ± 1.77 |
| IL-15 | 4.07 ± 0.17 | 14.02 ± 0.87 | 1.37 ± 0.17 | 15.93 ± 1.77 |
| IL-18 | 0.08 ± 0.02 | nd | nd | nd |
| IL-22 | 21.07 ± 2.09 | 43.86 ± 1.52 | 17.17 ± 2.32 | 38.18 ± 4.45 |
| IL-27 | 2.01 ± 0.00 | 4.29 ± 4.59 | 5.83 ± 4.58 | 7.21 ± 4.73 |
| IP-10 | 2.77 ± 0.31 | 1.91 ± 0.50 | 5.89 ± 0.21 | 4.60 ± 0.21 |
| M-CSF | 50.78 ± 3.15 | 156.36 ± 9.17 | 41.92 ± 2.91 | 128.81 ± 12.65 |
| MCP-1 | nd | nd | 114.2 ± 5.22 | 428.88 ± 12.34 |
| MCP-3 | 76.98 ± 1.59 | 217.32 ± 10.82 | 3.57 ± 0.69 | 4.11 ± 0.66 |
| MIG | 0.18 ± 0.05 | nd | nd | nd |
| MIP | nd | nd | nd | 4.02 ± 0.65 |
| PDGF-AA | 50.84 ± 2.28 | 192.09 ± 5.22 | 1.29 ± 0.21 | 2.92 ± 0.28 |
| PDGF-AB/BB | 25.45 ± 3.93 | 28.45 ± 6.69 | 33.69 ± 8.64 | 27.52 ± 5.32 |
| TGFα | 0.22 ± 0.15 | nd | nd | 0.23 ± 0.04 |
| TNFα | 0.75 ± 0.00 | 1.11 ± 0.60 | nd | 14.90 ± 1.49 |
| TNFβ | 5.49 ± 0.56 | 15.2 ± 1.30 | 5.79 ± 0.39 | 1.80 ± 0.07 |
| RANTES | 3.27 ± 0.35 | 7.00 ± 0.27 | 0.95 ± 0.37 | 356.41 ± 9.34 |
| VEGF-A | 98.89 ± 3.39 | 331.81 ± 4.57 | 99.72 ± 5.17 | 0.55 ± 0.00 |
| sCD40 | nd | 3.27 ± 2.79 | 2.72 ± 3.41 | nd |
| Biomolecule | Mean ± SEM (Exosomes P4) (×1) | Mean ± SEM (Exosomes P4) (×5) | Mean ± SEM (Exosomes P7) (×1) | Mean ± SEM (Exosomes P7) (×5) |
|---|---|---|---|---|
| EGF | 0.70 ± 0.00 | nd | nd | 0.91 ± 0.00 |
| Eotaxin | 0.60 ± 0.65 | 0.60 ± 0.00 | 0.41 ± 0.00 | 0.86 ± 0.00 |
| FGF-2 | 0.08 ± 0.00 | nd | nd | nd |
| FLT-3L | nd | 0.16 ± 0.09 | 0.07 ± 0.04 | 0.07 ± 0.04 |
| Fractalkine | nd | 3.3 ± 0.00 | nd | nd |
| IL-1α | nd | 0.12 ± 0.07 | nd | nd |
| IL-1b | nd | 0.34 ± 0.23 | nd | 0.06 ± 0.00 |
| IL-1RA | 0.03 ± 0.00 | 0.12 ± 0.09 | nd | 0.19 ± 0.13 |
| IL-5 | 0.05 ± 0.01 | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.04 ± 0.00 |
| IL-8 | 0.01 ± 0.00 | 0.06 ± 0.05 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| IL-9 | nd | 0.64 ± 0.00 | nd | nd |
| IL-10 | 0.03 ± 0.00 | 0.16 ± 0.03 | 0.09 ± 0.03 | 0.08 ± 0.05 |
| IL-12p40 | nd | 0.09 ± 0.00 | nd | nd |
| IL-13 | 2.76 ± 1.17 | 2.89 ± 1.86 | 2.44 ± 1.42 | 2.29 ± 1.12 |
| IL-15 | 0.09 ± 0.07 | 0.19 ± 0.06 | 0.32 ± 0.11 | 0.04 ± 0.00 |
| IL-18 | nd | nd | nd | nd |
| IL-22 | 0.41 ± 0.00 | nd | nd | nd |
| IL-27 | 3.60 ± 0.88 | nd | nd | nd |
| M-CSF | 1.78 ± 0.54 | 1.78 ± 0.54 | 12.58 ± 0.00 | 5.66 ± 0.00 |
| MCP-3 | 1.76 ± 0.90 | 1.77 ± 0.32 | 1.34 ± 0.18 | 0.27 ± 0.00 |
| MIG | 0.14 ± 0.09 | 0.08 ± 0.00 | 0.07 ± 0.05 | 0.12 ± 0.05 |
| MIP | 2.10 ± 3.07 | 1.31 ± 0.00 | nd | nd |
| PDGF-AA | 0.38 ± 0.27 | 0.40 ± 0.18 | 0.24 ± 0.14 | 0.16 ± 0.14 |
| PDGF-AB/BB | 21.07 ± 10.62 | 21.45 ± 2.77 | 12.84 ± 9.70 | 17.06 ± 10.61 |
| TNFα | 0.13 ± 0.01 | 0.13 ± 0.02 | 0.12 ± 0.01 | 0.13 ± 0.01 |
| TNFβ | 0.42 ± 0.31 | 0.92 ± 0.25 | 0.56 ± 0.38 | 0.63 ± 0.54 |
| RANTES | 0.17 ± 0.00 | 0.13 ± 0.06 | nd | nd |
| sCD40 | nd | 0.55 ± 0.00 | nd | nd |
| Cytokine/Factor | P4 | P7 | Function in Nerve Regeneration |
|---|---|---|---|
| FGF-2 | ✓ | ✓ | Angiogenesis, Schwann cell proliferation, axon outgrowth |
| VEGF-A | ✓ | — | Angiogenesis, neuroprotection |
| PDGF-AA | ✓ | — | Schwann cell support, tissue remodeling |
| IL-6 | ✓ | — | Inflammation resolution, glial modulation |
| IL-8 | ✓ | ✓ | Angiogenesis, chemotaxis |
| IL-22 | ✓ | ✓ | Tissue protection, neurotrophic signaling |
| M-CSF | ✓ | ✓ | Macrophage activation and polarization |
| MCP-3 | ✓ | — | Monocyte/macrophage recruitment |
| MCP-1 | — | ✓ | Monocyte chemotaxis, immune modulation |
| Eotaxin | ✓ | — | Chemokine signaling, potential glial effects |
| RANTES | — | ✓ | T-cell chemotaxis, axon growth, remyelination |
| Undifferentiated | Differentiated | ||||||
|---|---|---|---|---|---|---|---|
| Target Gene | Ct Average DPSCs | ΔCt DPSCs | Ct Average DPSCs | ΔCt DPSCs | ΔΔCt | RQ | Regulation |
| Aldh1l1 | nd | nd | 33.24 ± 0.02 | 13.14 | nd | nd | nd |
| Ascl1 | 33.66 ± 1.33 | 14.36 | 31.78 ± 1.19 | 11.68 | −2.68 | 6.41 | ↑ |
| Cd40 | 24.32 ± 0.28 | 5.02 | 15.4 ± 0.00 | −4.70 | −9.70 | 843.36 | ↑ |
| Cdh2 | 24.85 ± 0.52 | 5.55 | 22.63 ± 0.07 | 2.53 | −3.02 | 8.11 | ↑ |
| Cdk5r1 | 30.47 ± 0.05 | 11.17 | 29.52 ± 0.73 | 9.42 | −1.75 | 3.36 | ↑ |
| Cript | 25.59 ± 0.01 | 6.29 | 23.08 ± 0.28 | 2.98 | −3.31 | 9.92 | ↑ |
| Cspg4 | 28.4 ± 0.00 | 9.10 | nd | nd | nd | nd | nd |
| Dcx | nd | nd | 37.35 ± 0.64 | 17.25 | nd | nd | nd |
| Gap43 | 36.05 ± 0.91 | 16.75 | 38.75 ± 0.28 | 18.65 | 1.90 | 0.27 | ↓ |
| Gfap | nd | nd | 36.3 ± 0.13 | 16.20 | nd | nd | nd |
| Map2 | nd | nd | 30.08 ± 0.67 | 9.98 | nd | nd | nd |
| Mpz | nd | nd | nd | nd | nd | nd | nd |
| Ncam | nd | nd | nd | nd | nd | nd | nd |
| Nes | 25.91 ± 0.75 | 6.61 | 20.24 ± 0.70 | 0.14 | −6.47 | 88.65 | ↑ |
| Neurod1 | 34.84 ± 0.52 | 15.54 | 36.56 ± 0.34 | 16.46 | 0.92 | 0.53 | N |
| Ocln | 24.51 ± 0.00 | 5.21 | nd | nd | nd | nd | nd |
| Olig3 | nd | nd | nd | nd | nd | nd | nd |
| Rbfox3 | nd | nd | nd | nd | nd | nd | nd |
| Sox10 | 35.3 ± 0.13 | 16.00 | nd | nd | nd | nd | nd |
| Sox2 | 33.21 ± 0.00 | 13.91 | 29.25 ± 0.20 | 9.15 | −4.76 | 37.10 | ↑ |
| Syp | nd | nd | 26.68 ± 0.11 | 6.58 | nd | nd | nd |
| Tubb3 | 38.78 ± 0.00 | 19.48 | nd | nd | nd | nd | nd |
| hDPSCs Exosomes P4 | hDPSCs Exosomes P7 | ||||||
|---|---|---|---|---|---|---|---|
| Target Gene | Ct Average Exo P4 | ΔCt Exo P4 | Ct Average Exo P7 | ΔCt Exo P7 | ΔΔCt | RQ | Regulation |
| Aldh1l1 | nd | nd | nd | nd | nd | nd | nd |
| Ascl1 | 35.31 ± 0.00 | 16.01 | 36.41 ± 0.37 | 16.31 | 0.30 | 0.81 | N |
| Cd40 | 39.27 ± 0.23 | 19.97 | 36.23 ± 1.46 | 16.13 | −3.84 | 14.32 | ↑ |
| Cdh2 | nd | nd | nd | nd | nd | nd | nd |
| Cdk5r1 | 36.33 ± 0.00 | 17.03 | 35.05 ± 0.06 | 14.95 | −2.08 | 4.23 | ↑ |
| Cript | nd | nd | nd | nd | nd | nd | nd |
| Cspg4 | nd | nd | nd | nd | nd | nd | nd |
| Dcx | nd | nd | 36.74 ± 0.00 | 16.64 | nd | nd | nd |
| Gap43 | 39.28 ± 0.50 | 19.98 | 39.15 ± 0.36 | 19.05 | −0.93 | 1.91 | N |
| Gfap | 39.93 ± 0.00 | 20.63 | 38.92 ± 0.00 | 18.82 | 1.81 | 0.29 | ↓ |
| Map2 | 37.11 ± 0.00 | 17.81 | nd | nd | nd | nd | nd |
| Mpz | 39.28 ± 0.50 | 19.98 | 37.25 ± 0.81 | 17.15 | 2.83 | 0.14 | ↓ |
| Ncam | nd | nd | 36.18 ± 0.00 | 16.08 | nd | nd | nd |
| Nes | nd | nd | 35.85 ± 0.64 | 15.75 | nd | nd | nd |
| Neurod1 | nd | nd | nd | nd | nd | nd | nd |
| Ocln | nd | nd | nd | nd | nd | nd | nd |
| Olig3 | nd | nd | 38.09 ± 1.40 | 17.99 | nd | nd | nd |
| Rbfox3 | nd | nd | nd | nd | nd | nd | nd |
| Sox10 | 36.38 ± 0.00 | 17.08 | 37.79 ± 0.00 | 17.69 | −0.61 | 1.53 | N |
| Sox2 | nd | nd | 36.79 ± 0.00 | 16.69 | nd | nd | nd |
| Syp | nd | nd | 37.24 ± 0.00 | 17.14 | nd | nd | nd |
| Tubb3 | 39.30 ± 0.00 | 20.00 | 36.54 ± 0.00 | 16.44 | 3.56 | 0.08 | ↓ |
| Neurodifferentiated hDPSCs CM P4 | Neurodifferentiated hDPSCs CM P7 | CM hDPSCs P4 | CM hDPSCs P7 | Exosomes hDPSCs P4 | Exosomes hDPSCs P7 | |
|---|---|---|---|---|---|---|
| Neurodifferentiated hDPSCs CM P4 | ns | **** | ** | **** | **** | |
| Neurodifferentiated hDPSCs CM P7 | *** | *** | **** | **** | ||
| CM hDPSCs P4 | **** | * | ns | |||
| CM hDPSCs P7 | **** | **** | ||||
| Exosomes hDPSCs P4 | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, B.; Sousa, P.; de Sousa Moreira, A.; Sousa, A.C.; Rêma, A.; Atayde, L.; Salgado, A.J.; Geuna, S.; Alvites, R.; Maurício, A.C. Neuronal Differentiation and Exosome Profiling of Dental Pulp Stem Cells: Unveiling Their Potential for Nerve Repair. Int. J. Mol. Sci. 2025, 26, 9723. https://doi.org/10.3390/ijms26199723
Lopes B, Sousa P, de Sousa Moreira A, Sousa AC, Rêma A, Atayde L, Salgado AJ, Geuna S, Alvites R, Maurício AC. Neuronal Differentiation and Exosome Profiling of Dental Pulp Stem Cells: Unveiling Their Potential for Nerve Repair. International Journal of Molecular Sciences. 2025; 26(19):9723. https://doi.org/10.3390/ijms26199723
Chicago/Turabian StyleLopes, Bruna, Patrícia Sousa, Alícia de Sousa Moreira, Ana Catarina Sousa, Alexandra Rêma, Luís Atayde, António J. Salgado, Stefano Geuna, Rui Alvites, and Ana Colette Maurício. 2025. "Neuronal Differentiation and Exosome Profiling of Dental Pulp Stem Cells: Unveiling Their Potential for Nerve Repair" International Journal of Molecular Sciences 26, no. 19: 9723. https://doi.org/10.3390/ijms26199723
APA StyleLopes, B., Sousa, P., de Sousa Moreira, A., Sousa, A. C., Rêma, A., Atayde, L., Salgado, A. J., Geuna, S., Alvites, R., & Maurício, A. C. (2025). Neuronal Differentiation and Exosome Profiling of Dental Pulp Stem Cells: Unveiling Their Potential for Nerve Repair. International Journal of Molecular Sciences, 26(19), 9723. https://doi.org/10.3390/ijms26199723










