Stem-Centered Drought Tolerance in Mikania micrantha During the Dry Season
Abstract
1. Introduction
2. Results
2.1. Change in Phenotypes and Water Content During Dry and Wet Seasons
2.2. Changes in Stem Structural Characteristics During Dry and Wet Seasons
2.3. Changes in Pigment Content During Dry and Wet Seasons
2.4. Changes in Antioxidant Capacity During Dry and Wet Seasons
2.5. Changes in Osmotic Adjustment Substances During Dry and Wet Seasons
2.6. Expression Patterns of bZIP Transcription Factors in Response to Drought and Their Tissue Specificity
3. Discussion
3.1. Stem Structural Optimization Enhances Water Retention
3.2. Balance and Distribution of Photosynthetic Pigments
3.3. Accumulation of Antioxidant Substances
4. Materials and Methods
4.1. Plant Growth and Collection
4.2. Determination of Relative Water Content (RWC)
4.3. Observation of Stem Structure
4.4. Determination of Chlorophyll and Anthocyanin Content
4.5. Determination of Malondialdehyde (MDA) and Soluble Sugar (SS) Content
4.6. Determination of Activities of Enzymatic Antioxidants and Soluble Protein Content
4.7. Determination of Flavonoid and Total Phenol Content
4.8. Screening and Identification of bZIP Gene Family Members in M. micrantha
4.9. Expression Pattern Analysis of bZIP Gene Family Members in M. micrantha
4.10. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| RWC | Relative Water Content |
| Chl | Chlorophyll |
| SS | Soluble Sugar |
| SP | Soluble Protein |
| POD | Peroxidase |
| APX | Ascorbate Peroxidase |
| SOD | Superoxide Dismutase |
| CAT | Catalase |
| GSH | Glutathione |
| FW | Fresh Weight |
| TW | Turgid Weight |
| DW | Dry Weight |
| MDA | Malondialdehyde |
| TBA | Thiobarbituric Acid |
| TCA | Trichloroacetic Acid |
| PBS | Phosphate Buffer |
| EDTA | Ethylenediaminetetraacetic Acid |
| PVP | Polyvinylpyrrolidone |
| NBT | Nitroblue Tetrazolium |
| SD | Mean ± Standard Deviation |
References
- Mikhaylov, A.; Moiseev, N.; Aleshin, K.; Burkhardt, T. Global Climate Change and Greenhouse Effect. Entrep. Sustain. Issues 2020, 7, 2897. [Google Scholar] [CrossRef]
- Eyring, V.; Waugh, D.W.; Bodeker, G.E.; Cordero, E.; Akiyoshi, H.; Austin, J.; Beagley, S.R.; Boville, B.A.; Braesicke, P.; Brühl, C.; et al. Multimodel Projections of Stratospheric Ozone in the 21st Century. J. Geophys. Res. Atmos. 2007, 112, D16303. [Google Scholar] [CrossRef]
- Arnell, N.W. Climate Change and Global Water Resources. Glob. Environ. Change 1999, 9, S31–S49. [Google Scholar] [CrossRef]
- Wang, L.; Chen, W. A CMIP5 Multimodel Projection of Future Temperature, Precipitation, and Climatological Drought in China. Int. J. Climatol. 2014, 34, 2059–2078. [Google Scholar] [CrossRef]
- Yi, C.; Wei, S.; Hendrey, G. Warming Climate Extends Dryness-Controlled Areas of Terrestrial Carbon Sequestration. Sci. Rep. 2014, 4, 5472. [Google Scholar] [CrossRef]
- Ding, J.; Mack, R.N.; Lu, P.; Ren, M.; Huang, H. China’s Booming Economy Is Sparking and Accelerating Biological Invasions. BioScience 2008, 58, 317–324. [Google Scholar] [CrossRef]
- Paini, D.R.; Sheppard, A.W.; Cook, D.C.; De Barro, P.J.; Worner, S.P.; Thomas, M.B. Global Threat to Agriculture from Invasive Species. Proc. Natl. Acad. Sci. USA 2016, 113, 7575–7579. [Google Scholar] [CrossRef]
- Horvitz, N.; Wang, R.; Wan, F.; Nathan, R. Pervasive Human-Mediated Large-Scale Invasion: Analysis of Spread Patterns and Their Underlying Mechanisms in 17 of China’s Worst Invasive Plants. J. Ecol. 2017, 105, 85–94. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Ye, W.H.; Cao, H.L.; Feng, H.L. Mikania micrantha HBK in China—An Overview. Weed Res. 2004, 44, 42–49. [Google Scholar] [CrossRef]
- Shao, H.; Peng, S.; Wei, X.; Zhang, D.; Zhang, C. Potential Allelochemicals from an Invasive Weed Mikania micrantha HBK. J. Chem. Ecol. 2005, 31, 1657–1668. [Google Scholar] [CrossRef] [PubMed]
- Cock, M.J.W. Potential Biological Control Agents for Mikania micrantha HBK from the Neotropical Region. Int. J. Pest. Manag. 1982, 28, 242–254. [Google Scholar]
- Wang, R.; Xia, W.N.; Liu, S.; Qin, Z.; Liang, K.M.; Su, Y.J.; Zhang, J.E. Effects of Water Stress on the Growth and Allelopathic Potential of Invasive Plant Mikania micrantha HBK. Allelopath. J. 2016, 39, 143–152. [Google Scholar]
- Zhang, L.L.; Wen, D.Z.; Fu, S.L. Responses of Photosynthetic Parameters of Mikania micrantha and Chromolaena odorata to Contrasting Irradiance and Soil Moisture. Biol. Plant. 2009, 53, 517–522. [Google Scholar] [CrossRef]
- Liu, B.; Yan, J.; Li, W.; Yin, L.; Li, P.; Yu, H.; Xing, L.; Cai, M.; Wang, H.; Zhao, M.; et al. Mikania micrantha Genome Provides Insights into the Molecular Mechanism of Rapid Growth. Nat. Commun. 2020, 11, 340. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, Y.; Zheng, Y.; Cai, M.; Peng, C.; Li, W. Physiological and Transcriptomic Responses of Mikania micrantha Stem to Shading Yield Novel Insights into Its Invasiveness. Biol. Invasions 2021, 23, 2927–2943. [Google Scholar] [CrossRef]
- Yu, H.; Le Roux, J.J.; Jiang, Z.; Sun, F.; Peng, C.; Li, W. Soil Nitrogen Dynamics and Competition During Plant Invasion: Insights from Mikania micrantha Invasions in China. New Phytol. 2021, 229, 3440–3452. [Google Scholar] [CrossRef]
- Khan, A.A.; Wang, Y.-F.; Akbar, R.; Alhoqail, W.A. Mechanistic insights and future perspectives of drought stress management in staple crops. Front. Plant Sci. 2025, 27, 1547452. [Google Scholar] [CrossRef]
- Huang, J.-G.; Guo, X.; Rossi, S.; Zhai, L.; Yu, B.; Zhang, S.; Zhang, M. Intra-Annual Wood Formation of Subtropical Chinese Red Pine Shows Better Growth in Dry Season Than Wet Season. Tree Physiol. 2018, 38, 1225–1236. [Google Scholar] [CrossRef]
- Cai, M.; Huang, J.; Chen, M.; Chen, L.; Zhang, X.; Chen, M.; Wu, J.; Pan, Y.; Peng, C. The role and synthesis mechanism of anthocyanins in Sphagneticola trilobata stems under low temperature. Biol. Invasions 2024, 26, 2851–2867. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, J.; Ke, W.; Cai, M.; Chen, G.; Peng, C. Responses of Sphagneticola trilobata, Sphagneticola calendulacea and Their Hybrid to Drought Stress. Int. J. Mol. Sci. 2021, 22, 11288. [Google Scholar] [CrossRef] [PubMed]
- Davidson, A.M.; Jennions, M.; Nicotra, A.B. Do invasive species show higher phenotypic plasticity than native species and if so, is it adaptive? A meta-analysis. Ecol. Lett. 2011, 14, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Chapin, F.S., III; Oechel, W.C.; Van Cleve, K.; Lawrence, W. The Role of Mosses in the Phosphorus Cycling of an Alaskan Black Spruce Forest. Oecologia 1987, 74, 310–315. [Google Scholar] [CrossRef]
- Chen, T.; White, J.F.; Li, C. Fungal Endophyte Epichloë bromicola Infection Regulates Anatomical Changes to Account for Salt Stress Tolerance in Wild Barley (Hordeum brevisubulatum). Plant Soil. 2021, 461, 533–546. [Google Scholar] [CrossRef]
- Li, X.Y.; Wang, X.Y.; Li, L.G.; Bai, Z.H. A Study of Morphological Anatomy of Root and Stem on Cerasus humilis. J. Inn. Mong. Agric. Univ. (Nat. Sci. Ed.) 2014, 35, 26–30. [Google Scholar]
- Zulfiqar, F.; Younis, A.; Riaz, A.; Mansoor, F.; Hameed, M.; Akram, N.A.; Abideen, Z. Morpho-Anatomical Adaptations of Two Tagetes erecta L. Cultivars with Contrasting Response to Drought Stress. Pak. J. Bot. 2020, 52, 801–810. [Google Scholar] [CrossRef]
- Yaqoob, U.; Jan, N.; Raman, P.V.; Siddique, K.H.M.; John, R. Crosstalk between Brassinosteroid Signaling, ROS Signaling and Phenylpropanoid Pathway During Abiotic Stress in Plants: Does It Exist? Plant Stress. 2022, 4, 100075. [Google Scholar] [CrossRef]
- Liu, F.; Liu, Q.; Liang, X.; Huang, H.; Zhang, S. Morphological, Anatomical, and Physiological Assessment of Ramie [Boehmeria nivea (L.) Gaud.] Tolerance to Soil Drought. Genet. Resour. Crop Evol. 2005, 52, 497–506. [Google Scholar] [CrossRef]
- Wang, Z.C.; Wang, L.; Zhou, M.Y.; He, D.M.; Bi, H.H.; Ge, X.; Shen, J.C.; Fu, S.L. Comparison on Leaf Structure Characteristics and Branch Hydraulic Function of Three Carya illinoinensis Cultivars. J. Plant Res. Environ. 2021, 30, 38–45. [Google Scholar]
- Maherali, H.; Pockman, W.T.; Jackson, R.B. Adaptive Variation in the Vulnerability of Woody Plants to Xylem Cavitation. Ecology 2004, 85, 2184–2199. [Google Scholar] [CrossRef]
- Boughalleb, F.; Abdellaoui, R.; Ben-Brahim, N.; Neffati, M. Anatomical Adaptations of Astragalus gombiformis Pomel. Under Drought Stress. Open Life Sci. 2014, 9, 1215–1225. [Google Scholar]
- Guo, Y.Y.; Yu, H.Y.; Kong, D.S.; Yan, F.; Zhang, Y.J. Effects of Drought Stress on Growth and Chlorophyll Fluorescence of Lycium ruthenicum Murr. Seedlings. Photosynth. 2016, 54, 524–531. [Google Scholar] [CrossRef]
- Zaefyzadeh, M.; Quliyev, R.A.; Babayeva, S.M.; Abbasov, M.A. The Effect of the Interaction Between Genotypes and Drought Stress on the Superoxide Dismutase and Chlorophyll Content in Durum Wheat Landraces. Turk. J. Biol. 2009, 33, 1–7. [Google Scholar] [CrossRef]
- Zhu, Y.-F.; Wu, Y.-X.; Hu, Y.; Jia, X.-M.; Zhao, T.; Cheng, L.; Wang, Y.-X. Tolerance of Two Apple Rootstocks to Short-Term Salt Stress: Focus on Chlorophyll Degradation, Photosynthesis, Hormone and Leaf Ultrastructures. Acta Physiol. Plant. 2019, 41, 87. [Google Scholar] [CrossRef]
- Cirillo, V.; D’Amelia, V.; Esposito, M.; Amitrano, C.; Carillo, P.; Carputo, D.; Maggio, A. Anthocyanins Are Key Regulators of Drought Stress Tolerance in Tobacco. Biology 2021, 10, 139. [Google Scholar] [CrossRef]
- Steyn, W.J.; Wand, S.J.E.; Holcroft, D.M.; Jacobs, G. Anthocyanins in Vegetative Tissues: A Proposed Unified Function in Photoprotection. New Phytol. 2002, 155, 349–361. [Google Scholar] [CrossRef]
- Cai, M.-L.; Ding, W.-Q.; Zhai, J.-J.; Zheng, X.-T.; Yu, Z.-C.; Zhang, Q.-L.; Lin, X.-H.; Chow, W.S.; Peng, C.-L. Photosynthetic Compensation of Non-Leaf Organ Stems of the Invasive Species Sphagneticola trilobata (L.) Pruski at Low Temperature. Photosynth. Res. 2021, 149, 121–134. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Riadh, K.; Gopi, R.; Manivannan, P.; Inès, J.; Al-Juburi, H.J.; Chang-Xing, Z.; Hong-Bo, S.; Panneerselvam, R. Antioxidant Defense Responses: Physiological Plasticity in Higher Plants Under Abiotic Constraints. Acta Physiol. Plant. 2009, 31, 427–436. [Google Scholar] [CrossRef]
- Zhao, P.; Chen, X.; Xue, X.; Wang, Y.; Wang, Y.; Li, H.; Xue, R.; Li, Y. Improvement of polyamine synthesis maintains photosynthetic function in wheat during drought stress and rewatering at the grain filling stage. Plant Growth Regul. 2024, 102, 497–513. [Google Scholar] [CrossRef]
- Hong, W.; Jin, J.Y. Effects of Zinc Deficiency and Drought on Plant Growth and Metabolism of Reactive Oxygen Species in Maize (Zea mays L.). Agric. Sci. China 2007, 6, 988–995. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, J.; Zhao, X.; Liu, G.; Yang, C.; Zhan, L. A Novel LEA Gene from Tamarix androssowii Confers Drought Tolerance in Transgenic Tobacco. Plant Sci. 2006, 171, 655–662. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat Tolerance in Plants: An Overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Drought Stress Effects on Growth, ROS Markers, Compatible Solutes, Phenolics, Flavonoids, and Antioxidant Activity in Amaranthus tricolor. Appl. Biochem. Biotechnol. 2018, 186, 999–1016. [Google Scholar] [CrossRef]
- Yang, X.; Lu, M.; Wang, Y.; Wang, Y.; Liu, Z.; Chen, S. Response Mechanism of Plants to Drought Stress. Horticulturae 2021, 7, 50. [Google Scholar] [CrossRef]
- Mohagheghian, B.; Saeidi, G.; Arzani, A. Phenolic compounds, antioxidant enzymes, and oxidative stress in barley (Hordeum vulgare L.) genotypes under field drought-stress conditions. BMC Plant Biol. 2025, 25, 709. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Ilyas, N. Role of Nanosilicab to Boost the Activities of Metabolites in Triticum aestivum Facing Drought Stress. Plant Soil. 2022, 477, 99–115. [Google Scholar] [CrossRef]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive Oxygen Species Homeostasis and Signalling During Drought and Salinity Stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef]
- Ababaf, M.; Omidi, H.; Bakhshandeh, A. Changes in Antioxidant Enzymes Activities and Alkaloid Amount of Catharanthus roseus in Response to Plant Growth Regulators Under Drought Condition. Ind. Crops Prod. 2021, 167, 113505. [Google Scholar] [CrossRef]
- Haddad, R.; Kamangar, A. The Ameliorative Effect of Silicon and Potassium on Drought Stressed Grape (Vitis vinifera L.) Leaves. Iran. J. Genet. Plant Breed. 2015, 4, 48–58. [Google Scholar]
- Yang, S.; Xu, K.; Chen, S.; Li, T.; Xia, H.; Chen, L.; Liu, H.; Luo, L. A stress-responsive bZIP transcription factor OsbZIP62 improves drought and oxidative tolerance in rice. BMC Plant Biol. 2019, 19, 260. [Google Scholar] [CrossRef]
- Guo, Y.Y.; Yu, H.Y.; Yang, M.M.; Kong, D.S.; Zhang, Y.J. Effect of Drought Stress on Lipid Peroxidation, Osmotic Adjustment and Antioxidant Enzyme Activity of Leaves and Roots of Lycium ruthenicum Murr. Seedling. Russ. J. Plant Physiol. 2018, 65, 244–250. [Google Scholar] [CrossRef]
- Wach, D.; Skowron, P. An overview of plant responses to the drought stress at morphological, physiological and biochemical levels. Pol. J. Agron. 2022, 4, 25–34. [Google Scholar]
- Zhou, P.; Li, J.; Jiang, H.; Jin, Q.; Wang, Y.; Xu, Y. Analysis of bZIP gene family in lotus (Nelumbo) and functional study of NnbZIP36 in regulating anthocyanin synthesis. BMC Plant Biol. 2023, 23, 429. [Google Scholar] [CrossRef]
- Sun, F.; Ou, Q.; Yu, H.; Li, N.; Peng, C. The invasive plant Mikania micrantha affects the soil foodweb and plant-soil nutrient contents in orchards. Soil. Biol. Biochem. 2019, 139, 107630. [Google Scholar] [CrossRef]
- Ogbaga, C.C.; Stepien, P.; Johnson, G.N. Sorghum (Sorghum bicolor) Varieties Adopt Strongly Contrasting Strategies in Response to Drought. Physiol. Plant. 2014, 152, 389–401. [Google Scholar] [CrossRef]
- Hamann, T.; Smets, E.; Lens, F. A Comparison of Paraffin and Resin-Based Techniques Used in Bark Anatomy. Taxon 2011, 60, 841–851. [Google Scholar] [CrossRef]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as Well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Yemets, O.; Gauslaa, Y.; Solhaug, K.A. Monitoring with Lichens—Conductivity Methods Assess Salt and Heavy Metal Damage More Efficiently Than Chlorophyll Fluorescence. Ecol. Indic. 2015, 55, 59–64. [Google Scholar] [CrossRef]
- Tan, W.; Liu, J.; Dai, T.; Jing, Q.; Cao, W.; Jiang, D. Alterations in Photosynthesis and Antioxidant Enzyme Activity in Winter Wheat Subjected to Post-Anthesis Water-Logging. Photosynthetica 2008, 46, 21–27. [Google Scholar] [CrossRef]
- Du, H.; Wang, Z.; Huang, B. Differential Responses of Warm-Season and Cool-Season Turfgrass Species to Heat Stress Associated with Antioxidant Enzyme Activity. J. Am. Soc. Hortic. Sci. 2009, 134, 417–422. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen Peroxide Is Scavenged by Ascorbate-Specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Park, Y.-S.; Jung, S.-T.; Kang, S.-G.; Heo, B.G.; Arancibia-Avila, P.; Toledo, F.; Drzewiecki, J.; Namiesnik, J.; Gorinstein, S. Antioxidants and Proteins in Ethylene-Treated Kiwifruits. Food Chem. 2008, 107, 640–648. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of Total Phenolic Content and Other Oxidation Substrates in Plant Tissues Using Folin–Ciocalteu Reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cai, M.; Chen, M.; Ke, W.; Pan, Y.; Huang, J.; Zhang, J.; Peng, C. Genome-Wide Characterization of PIN Auxin Efflux Carrier Gene Family in Mikania micrantha. Int. J. Mol. Sci. 2022, 23, 10183. [Google Scholar] [CrossRef]

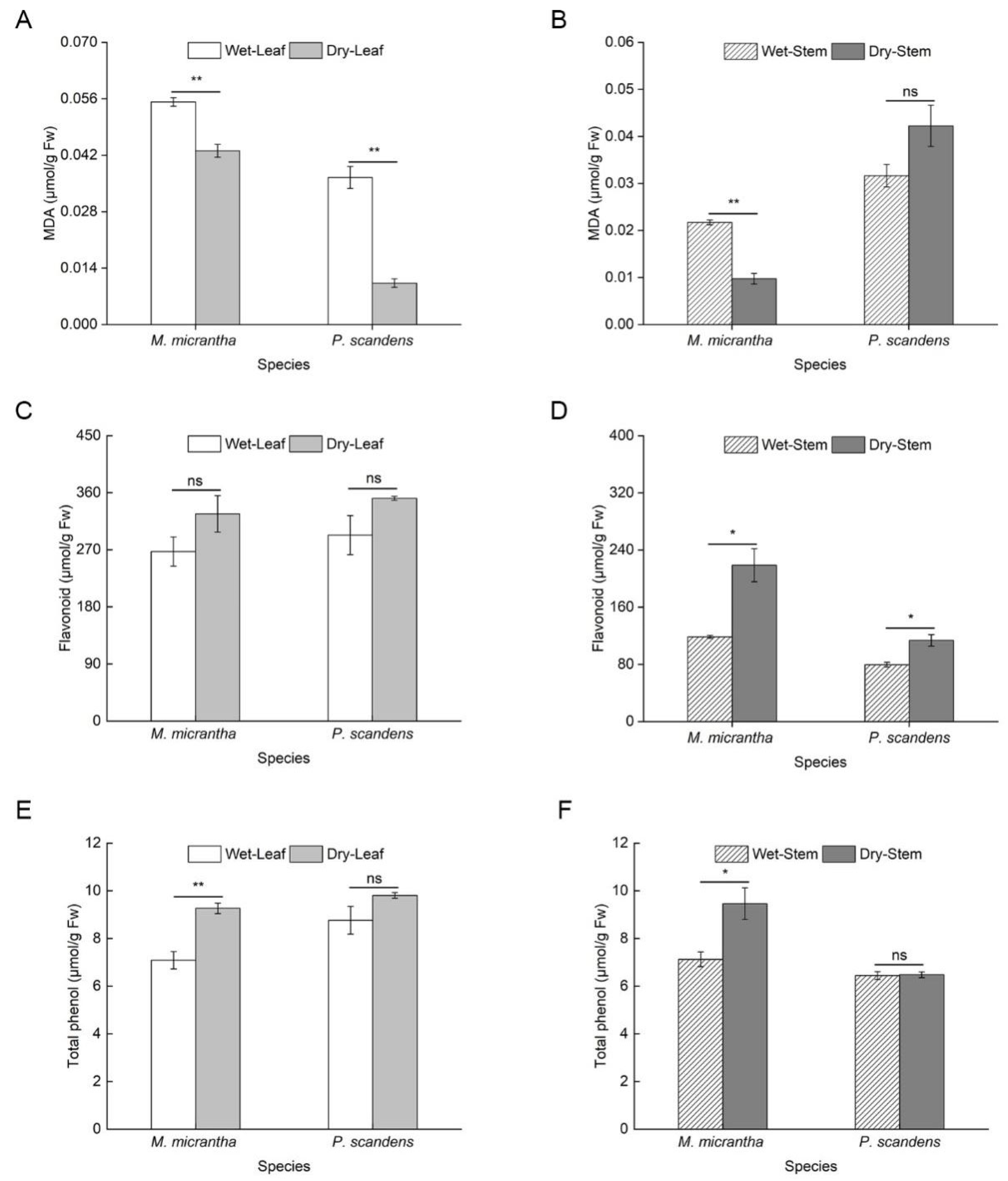
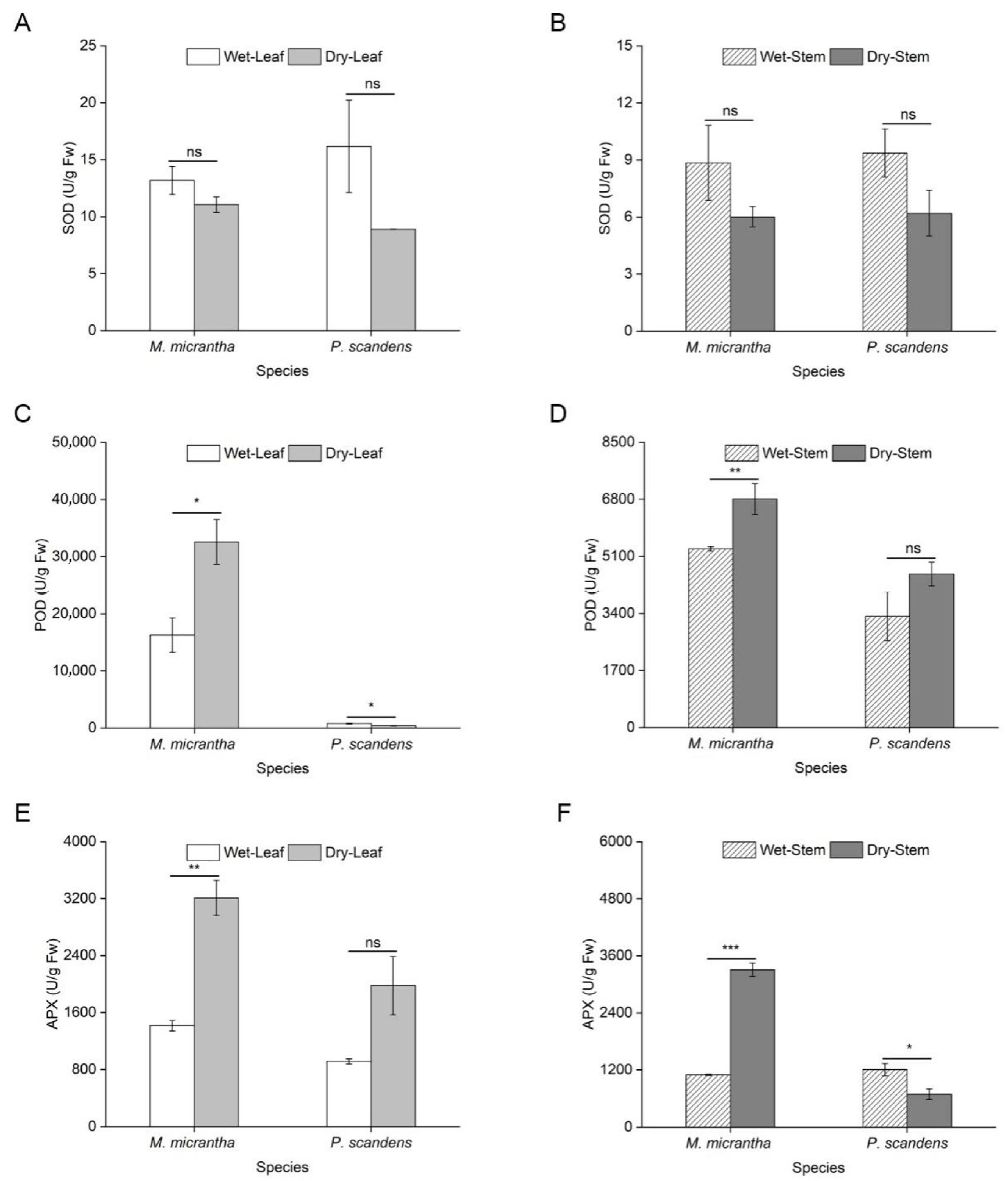
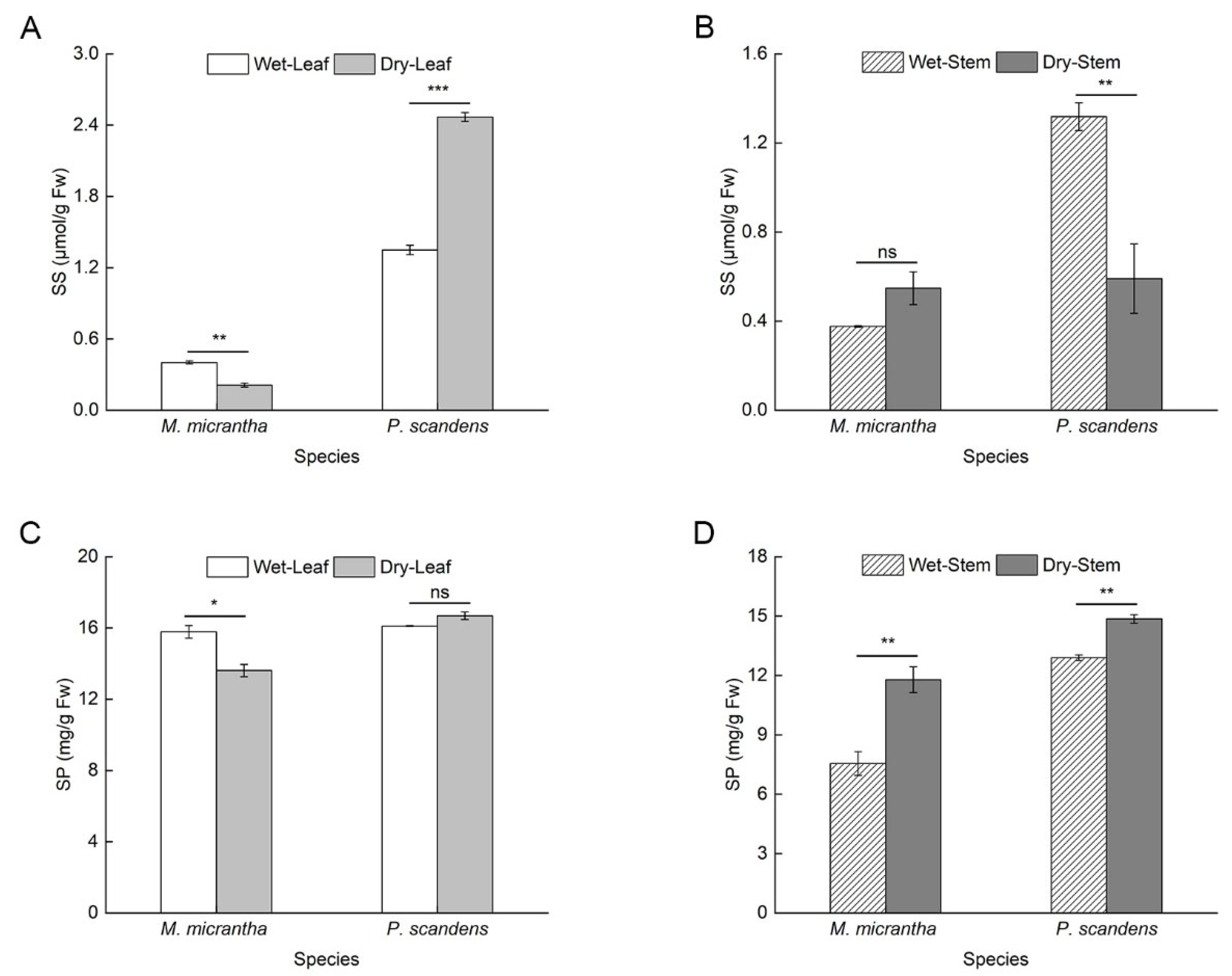
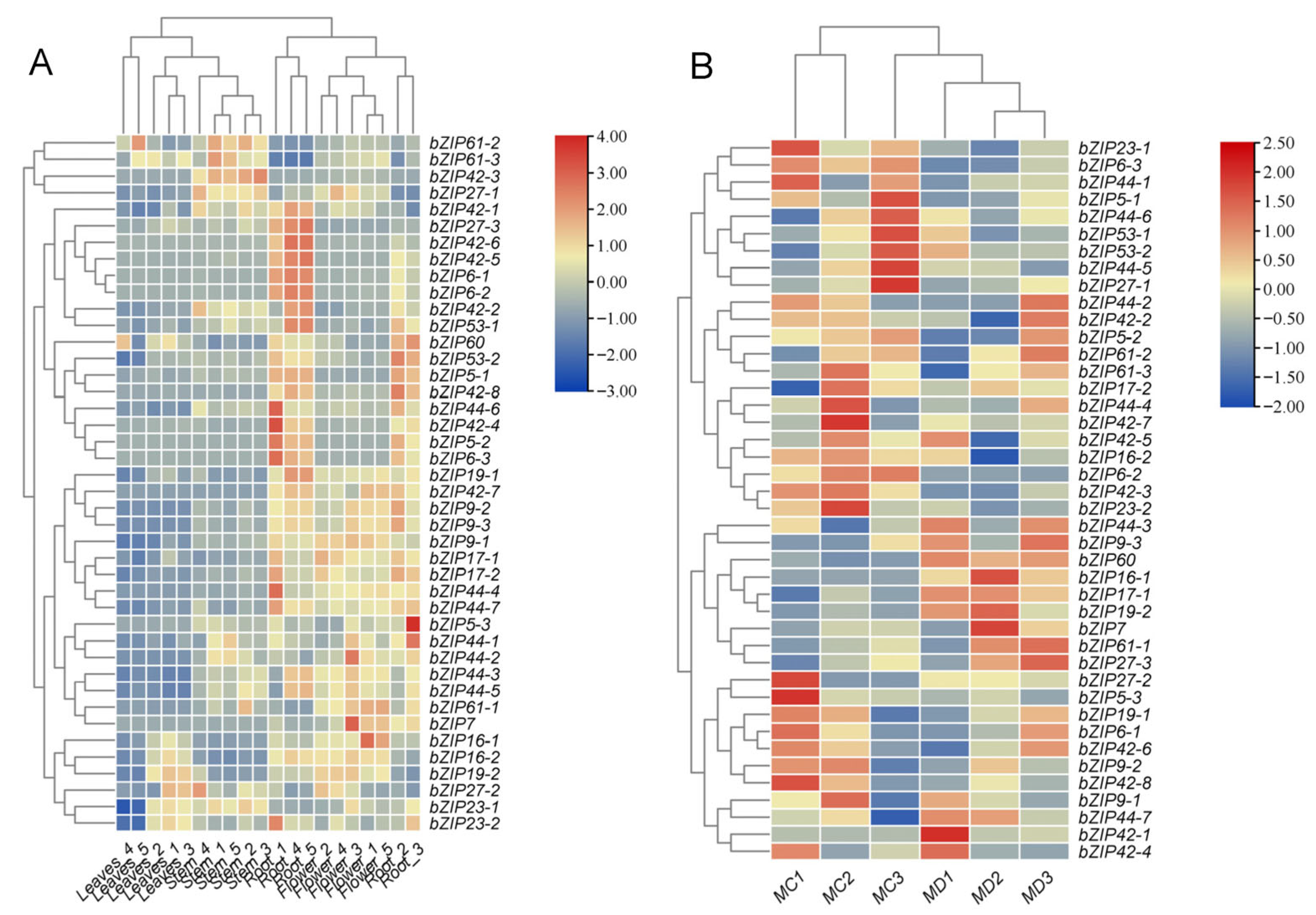
| Plant Organ | M. micrantha | P. scandens | Soil Moisture Content (%) | |||
|---|---|---|---|---|---|---|
| Wet | Dry | Wet | Dry | Wet | Dry | |
| Leaf-RWC (%) | 88.68 ± 1.15 | 79.69 ± 0.19 ** | 92.14 ± 0.25 | 87.71 ± 1.63 ns | 26.60 ± 0.96 | 2.52 ± 0.04 *** |
| Stem-RWC (%) | 83.99 ± 0.45 | 81.31 ± 1.67 ns | 94.75 ± 1.00 | 83.84 ± 4.17 ns | ||
| Indicators | M. micrantha | P. scandens | ||
|---|---|---|---|---|
| Wet | Dry | Wet | Dry | |
| Cortex proportion (%) | 23.97 ± 1.01 | 29.97 ± 0.72 ** | 28.38 ± 1.27 | 35.56 ± 0.42 ** |
| Medulla proportion (%) | 47.8 ± 1.03 | 41.79 ± 1.34 * | 37.35 ± 2.53 | 31.07 ± 0.52 ns |
| Catheter diameter (μm) | 126.07 ± 13.69 | 91.79 ± 14.76 ns | 109.97 ± 13.78 | 88.59 ± 3.77 ns |
| Catheter/vascular bundles (Individual/Individual) | 3.05 ± 0.07 | 3.24 ± 0.23 ns | 2.62 ± 0.06 | 2.77 ± 0.03 ns |
| vessel wall thickness (μm) | 1.91 ± 0.13 | 2.4 ± 0.10 * | 1.67 ± 0.04 | 1.67 ± 0.02 ns |
| Catheter density (Individual/mm2) | 28.4 ± 2.36 | 53.89 ± 7.35 * | 29.88 ± 6.19 | 30.97 ± 2.17 ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, M.; Chen, M.; Zhang, J.; Peng, C. Stem-Centered Drought Tolerance in Mikania micrantha During the Dry Season. Int. J. Mol. Sci. 2025, 26, 9722. https://doi.org/10.3390/ijms26199722
Cai M, Chen M, Zhang J, Peng C. Stem-Centered Drought Tolerance in Mikania micrantha During the Dry Season. International Journal of Molecular Sciences. 2025; 26(19):9722. https://doi.org/10.3390/ijms26199722
Chicago/Turabian StyleCai, Minling, Minghao Chen, Junjie Zhang, and Changlian Peng. 2025. "Stem-Centered Drought Tolerance in Mikania micrantha During the Dry Season" International Journal of Molecular Sciences 26, no. 19: 9722. https://doi.org/10.3390/ijms26199722
APA StyleCai, M., Chen, M., Zhang, J., & Peng, C. (2025). Stem-Centered Drought Tolerance in Mikania micrantha During the Dry Season. International Journal of Molecular Sciences, 26(19), 9722. https://doi.org/10.3390/ijms26199722





