Bisphenol AF Induces Hepatic Steatosis via Succinate–SUCNR1-Mediated Macrophage–Hepatocyte Interactions: An Adverse Outcome Pathway Study in Male C57BL/6 Mice
Abstract
1. Introduction
2. Results
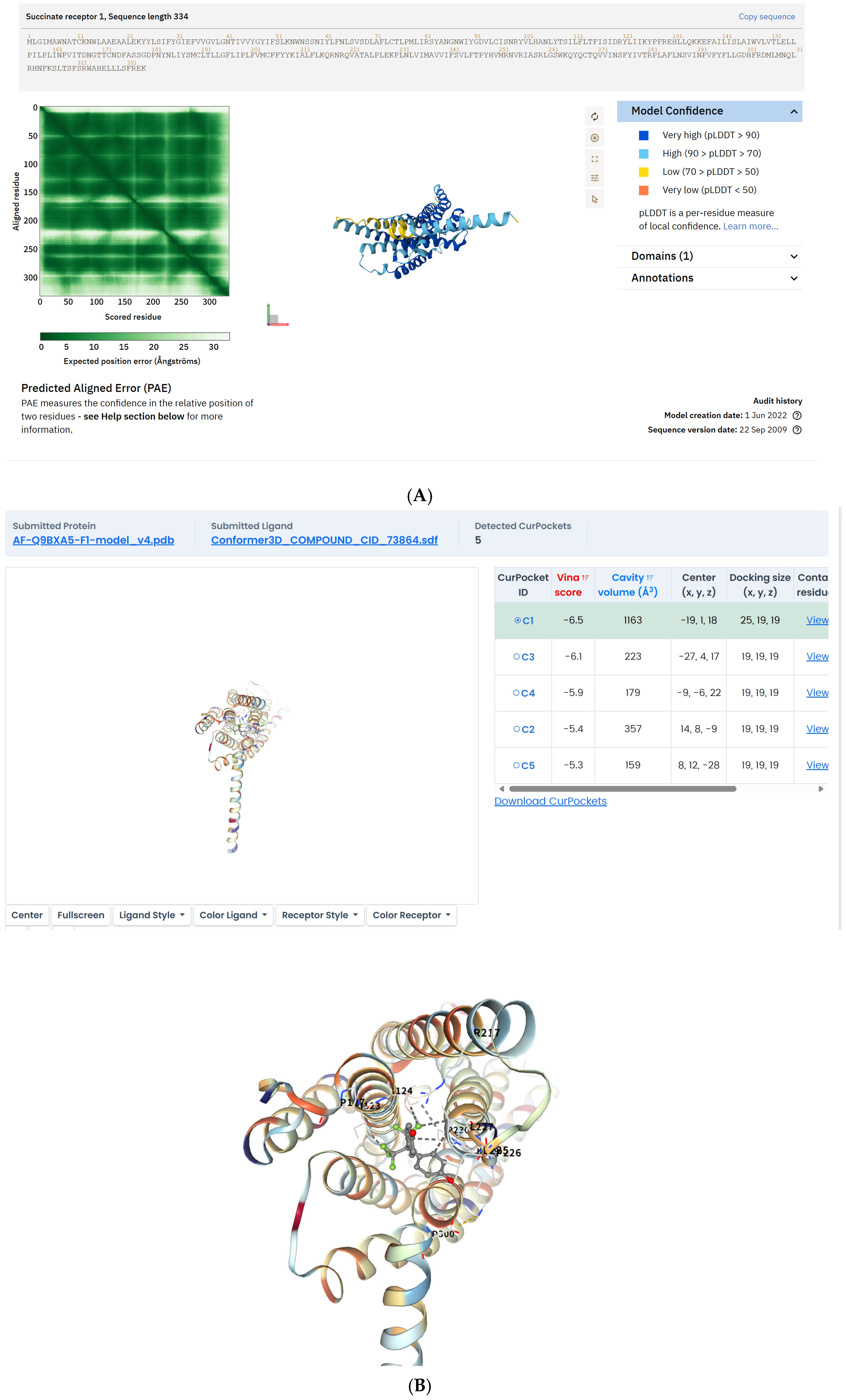
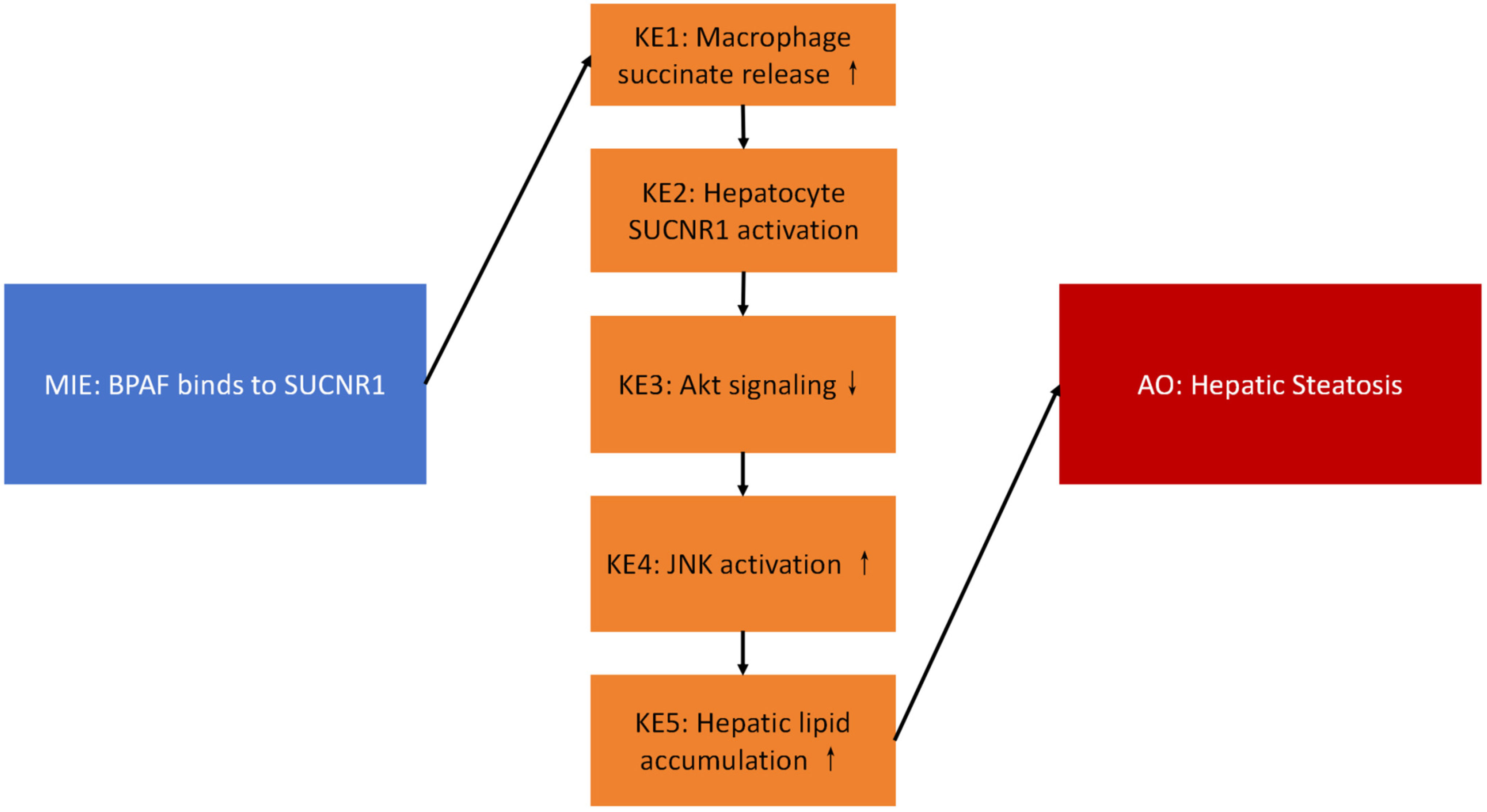
2.1. BPAF Binds to SUCNR1 and Stabilizes the Active-State Conformation
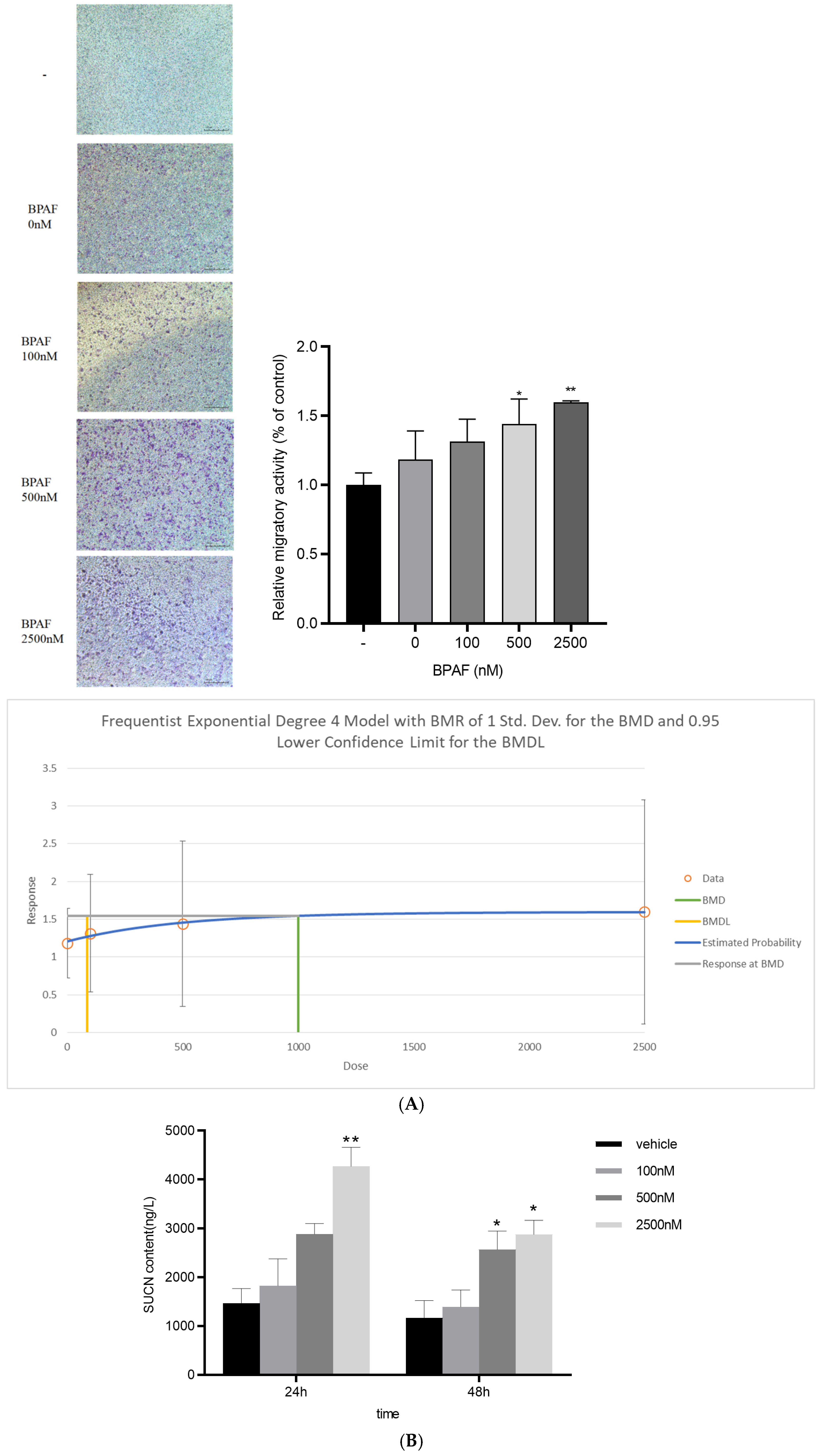
2.2. BPAF Elevates Succinate Release from Macrophages
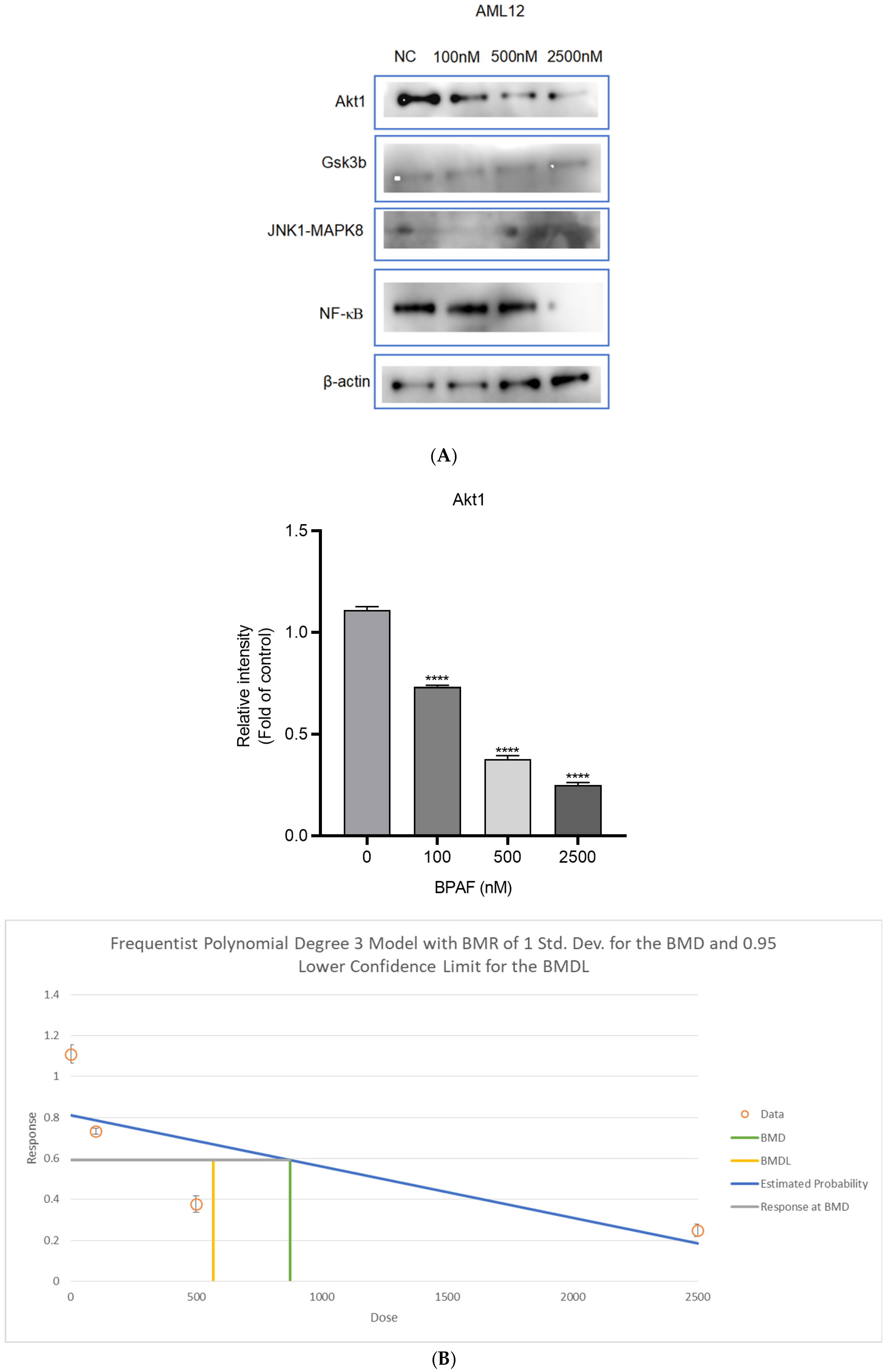
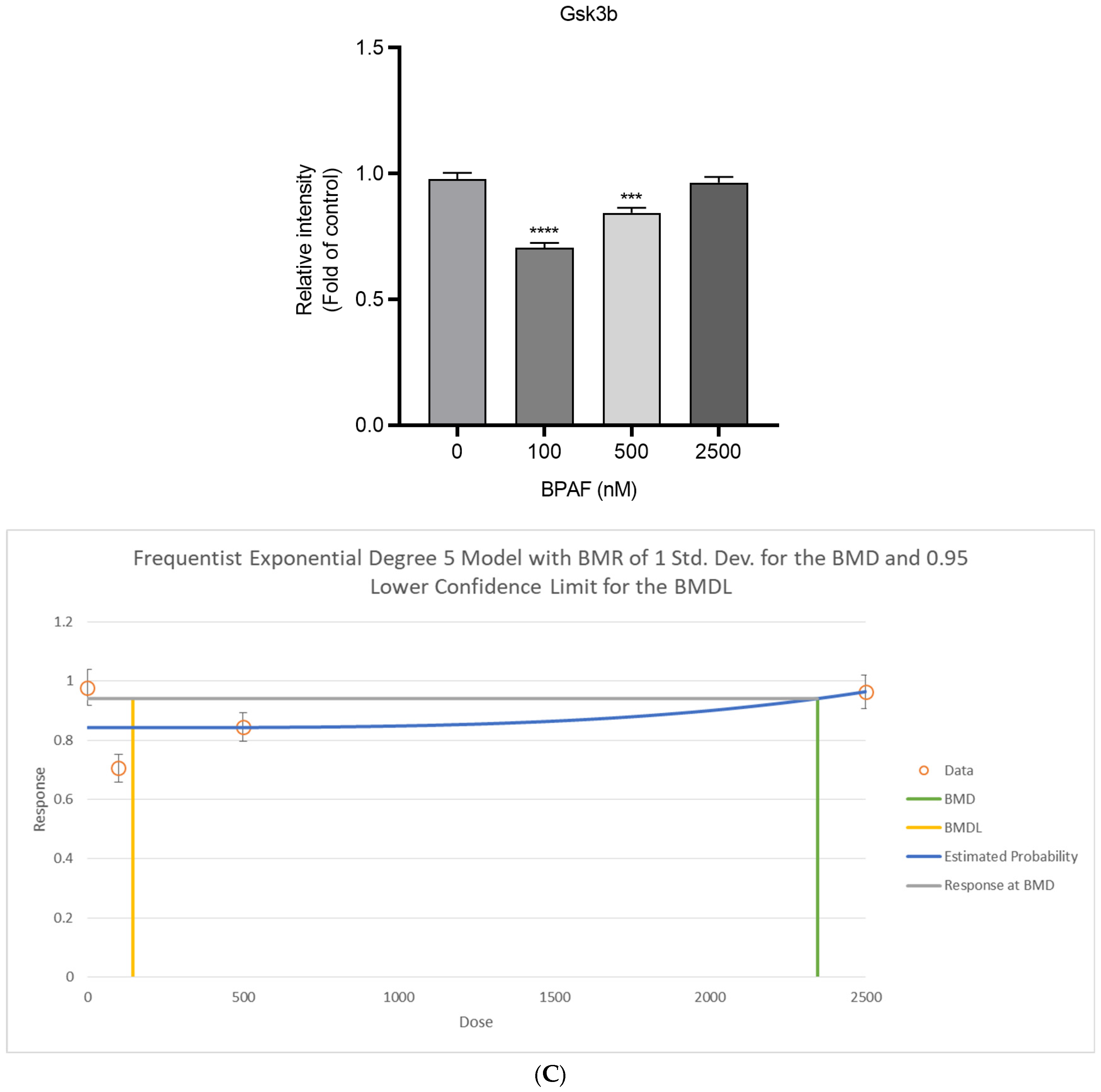
2.3. Succinate Activates Hepatocyte SUCNR1 and Suppresses Akt Signaling
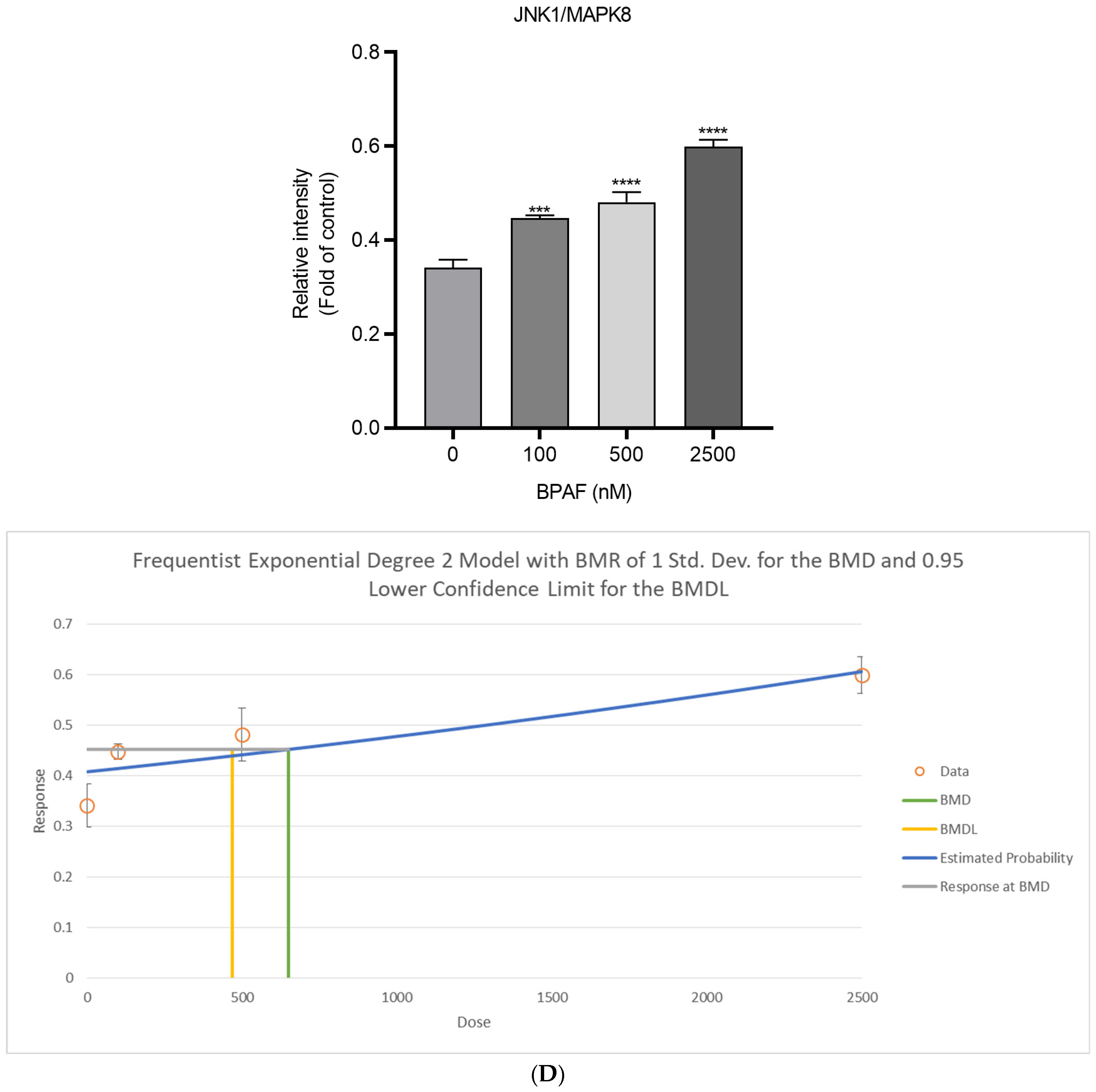
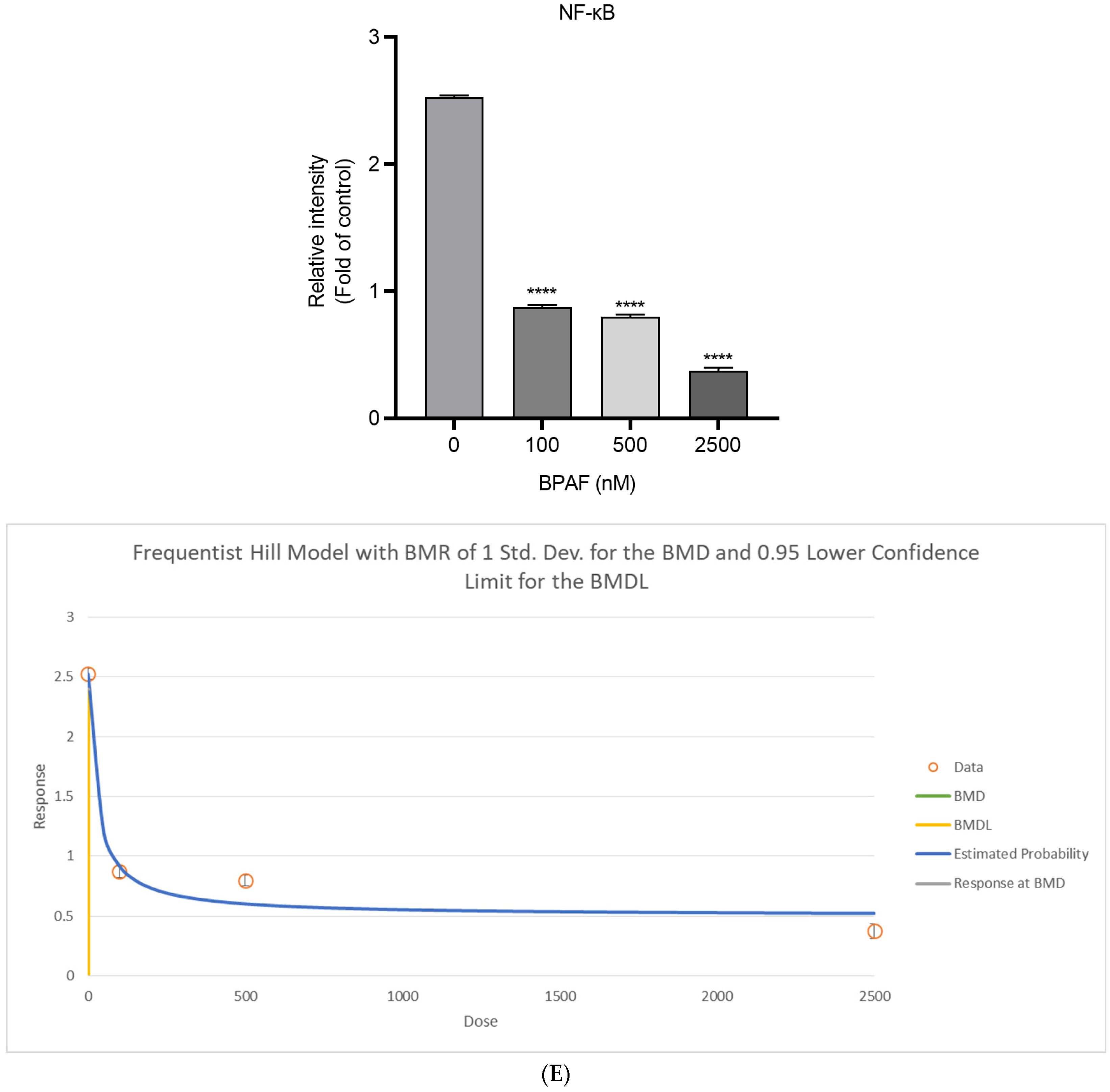
2.4. Lipid Accumulation In Vitro
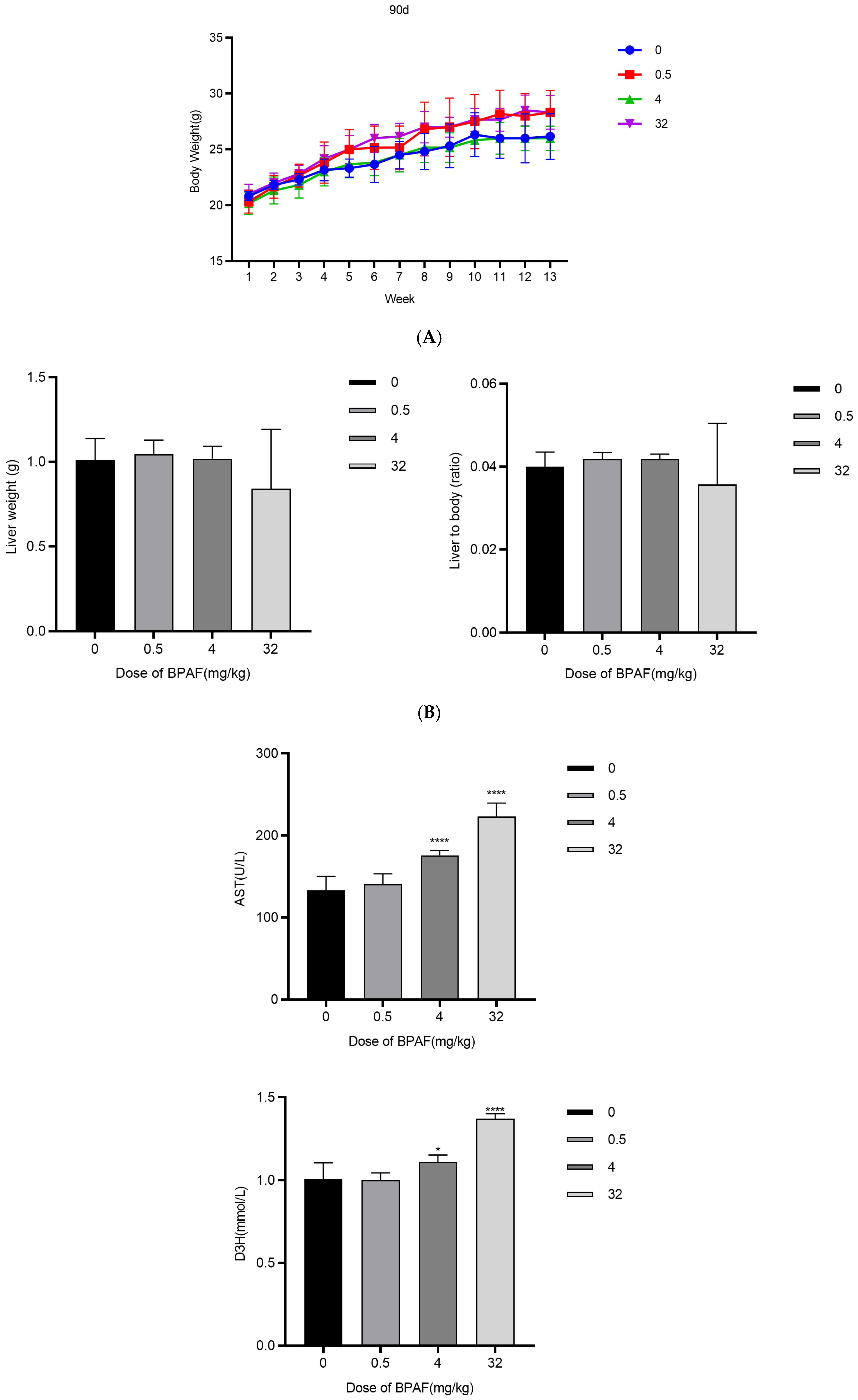
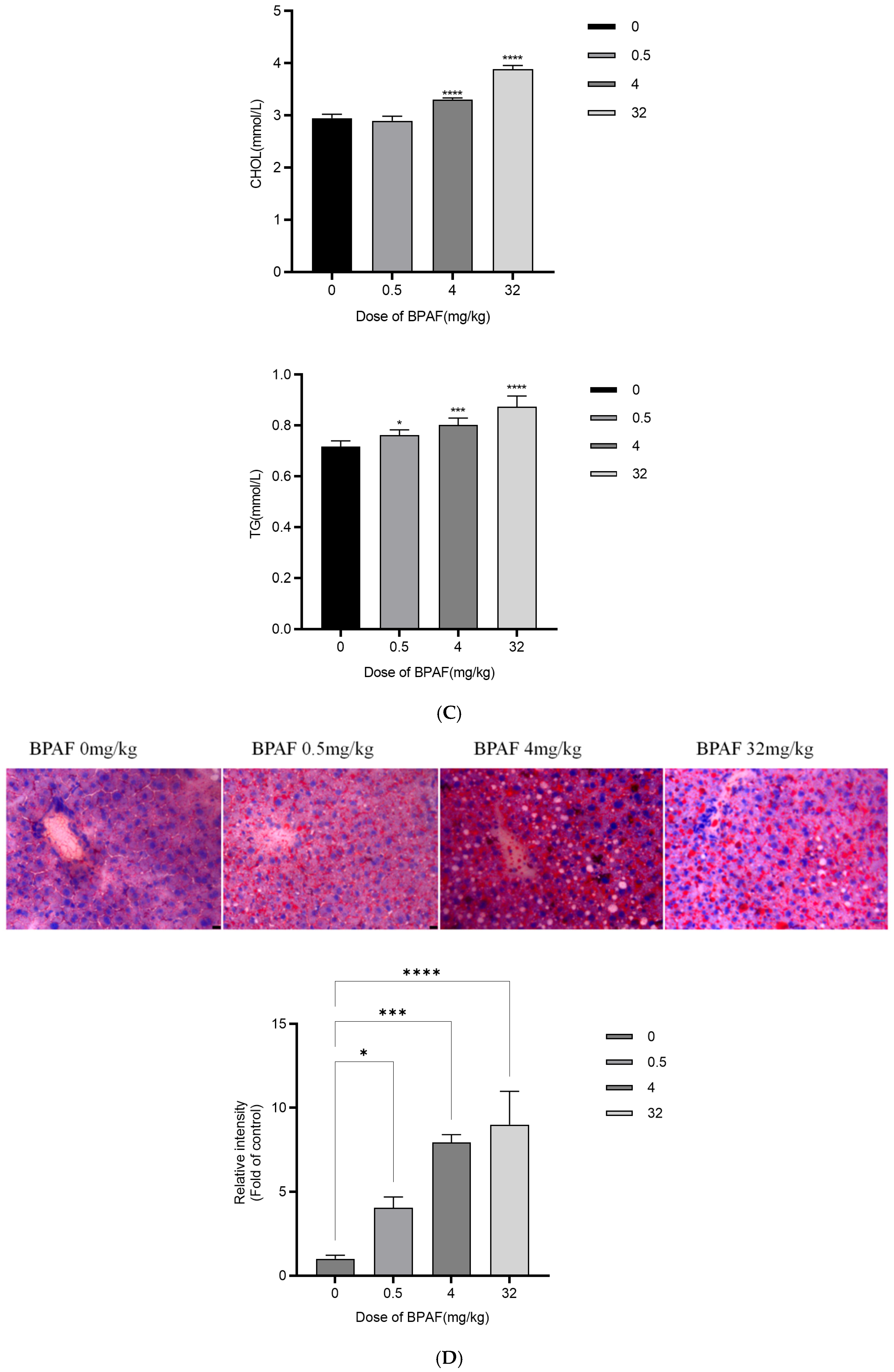
2.5. In Vivo AOP Validation
2.6. Weight-of-Evidence for the AOP
3. Discussion
3.1. Mechanistic Insights
3.2. Comparison with BPA and Other Bisphenols
3.3. Human Relevance and Translational Outlook
3.4. Limitations and Future Directions
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Animals and Treatment
4.3. Co-Culture Transwell Assays
4.4. Cryo-EM and in Silico Docking
4.5. Succinate Metabolism Detection
4.6. Western Blotting
4.7. Histopathology and Lipid Staining
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, H.; Gao, J.; Liu, Y.; Ding, Y.; Guo, Y.; Wang, Z.; Dong, Z.; Zhang, N. Toxic Effects of Bisphenol AF Exposure on the Reproduction and Liver of Female Marine Medaka (Oryzias melastigma). Animals 2024, 14, 222. [Google Scholar] [CrossRef] [PubMed]
- Zare Jeddi, M.; Hopf, N.B.; Louro, H.; Viegas, S.; Galea, K.S.; Pasanen-Kase, R.; Santonen, T.; Mustieles, V.; Fernandez, M.F.; Verhagen, H.; et al. Developing human biomonitoring as a 21st century toolbox within the European exposure science strategy 2020–2030. Environ. Int. 2022, 168, 107476. [Google Scholar] [CrossRef] [PubMed]
- Villanueva Carmona, T. Metabolic Impact of the Succinate/SUCNR1 Axis in Adipocytes: Insights into Circadian and Leptin Regulation. Ph.D. Dissertation, Universitat Rovira i Virgili, Tarragona, Spain, 2024. Available online: http://hdl.handle.net/10803/691520 (accessed on 27 September 2025).
- Bauset, C.; Gisbert-Ferrandiz, L.; Ortiz-Masia, D.; Coll, S.; Mamie, C.; Scharl, M.; Calatayud, S.; Barrachina, M.D.; Cosín-Roger, J. DOP87 SUCNR1: A novel key protagonist in fistula development. J. Crohn’s Colitis 2020, 14 (Suppl. S1), S126. [Google Scholar] [CrossRef]
- Kang, D.S.; Yang, J.H.; Kim, H.S.; Koo, B.K.; Lee, C.M.; Ahn, Y.-S.; Jung, J.-H.; Seo, Y.R. Application of the adverse outcome pathway framework to risk assessment for predicting carcinogenicity of chemicals. J. Cancer Prev. 2018, 23, 126–133. [Google Scholar] [CrossRef]
- Huc, L.; Lemarie, A.; Guéraud, F.; Héliès-Toussaint, C. Low concentrations of bisphenol A induce lipid accumulation mediated by the production of reactive oxygen species in the mitochondria of HepG2 cells. Toxicol. Vitr. 2012, 26, 709–717. [Google Scholar] [CrossRef]
- Sabadell-Basallote, J.; Astiarraga, B.; Castaño, C.; Ejarque, M.; Repollés-de-Dalmau, M.; Quesada, I.; Blanco, J.; Núñez-Roa, C.; Rodríguez-Peña, M.M.; Martínez, L.; et al. SUCNR1 regulates insulin secretion and glucose elevates the succinate response in people with prediabetes. J. Clin. Investig. 2024, 134, e173214. [Google Scholar] [CrossRef]
- Budtz-Jørgensen, E.; Keiding, N.; Grandjean, P. Benchmark dose calculation from epidemiological data. Biometrics 2001, 57, 698–706. [Google Scholar] [CrossRef]
- OECD. Guidance Document on Developing and Assessing Adverse Outcome Pathways (Online Version). OECD Adverse Outcome Pathways Knowledge Base; OECD: Paris, France, 2023; Available online: https://aopwiki.org (accessed on 27 September 2025).
- Villeneuve, D.L.; Crump, D.; Garcia-Reyero, N.; Hecker, M.; Hutchinson, T.H.; LaLone, C.A.; Landesmann, B.; Lettieri, T.; Munn, S.; Nepelska, M.; et al. Adverse outcome pathway (AOP) development I: Strategies and principles. Toxicol. Sci. 2014, 142, 312–320. [Google Scholar] [CrossRef] [PubMed]
- OECD. Update on Modular AOP Development and IATA Integration. OECD Environment; Health and Safety Series, ENV/CBC/MONO(2023)28; OECD Publishing: Paris, France, 2023; Available online: https://one.oecd.org/document/ENV/CBC/MONO(2023)28/en/pdf (accessed on 27 September 2025).
- Meek, M.E.; Palermo, C.M.; Bachman, A.N.; North, C.M.; Lewis, R.J. New developments in the evolution and application of the WHO/IPCS framework on mode of action/species concordance analysis. J. Appl. Toxicol. 2014, 34, 595–606. [Google Scholar] [CrossRef]
- Kim, S.; Grenert, J.P.; Patterson, C.; Correia, M.A. CHIP−/−-mouse liver: Adiponectin-AMPK-FOXO-activation overrides CYP2E1-elicited JNK1-activation, delaying onset of NASH: Therapeutic implications. Sci. Rep. 2016, 6, 29423. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Wang, C.; Xia, W.-R.; Zheng, J.-Y.; Yang, J.; Liu, B.; Liu, J.-Q.; Liu, L.-F. Succinate induces synovial angiogenesis in rheumatoid arthritis through metabolic remodeling and HIF-1α/VEGF axis. Free Radic. Biol. Med. 2018, 126, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Littlewood-Evans, A.; Sarret, S.; Apfel, V.; Loesle, P.; Dawson, J.; Zhang, J.; Muller, A.; Tigani, B.; Kneuer, R.; Patel, S.; et al. GPR91 senses extracellular succinate released from inflammatory macrophages and exacerbates rheumatoid arthritis. J. Exp. Med. 2016, 213, 1655–1662. [Google Scholar] [CrossRef] [PubMed]
- Kaneto, H.; Katakami, N.; Matsuhisa, M.; Matsuoka, T.-A. Involvement of oxidative stress and the JNK pathway in glucose toxicity. Rev. Diabet. Stud. 2004, 1, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Perniss, A.; Boonen, B.; Tonack, S.; Thiel, M.; Poharkar, K.; Alnouri, M.W.; Keshavarz, M.; Papadakis, T.; Wiegand, S.; Pfeil, U.; et al. A succinate/SUCNR1-brush cell defense program in the tracheal epithelium. Sci. Adv. 2023, 9, eadg8842. [Google Scholar] [CrossRef]
- Paini, A.; Leonard, J.A.; Joossens, E.; Bessems, J.; Desalegn, A.; Dorne, J.L.; Gajewska, M.; Kienzler, A.; Lumen, A.; Paini, M.; et al. Next generation PBPK models in chemical risk assessment. Arch. Toxicol. 2021, 95, 2897–2916. [Google Scholar]
- Cortada-Garcia, J.; Haggarty, J.; Moses, T.; Daly, R.; Arnold, S.A.; Burgess, K. On-line untargeted metabolomics monitoring of an Escherichia coli succinate fermentation process. Biotechnol. Bioeng. 2022, 119, 2757–2769. [Google Scholar] [CrossRef]
- Tarantino, G.; Finelli, C.; Scopacasa, F.; Pasanisi, F.; Contaldo, F.; Capone, D. JNKs, insulin resistance and inflammation: A possible link between NAFLD and coronary artery disease. World J. Gastroenterol. 2011, 17, 3785–3794. [Google Scholar] [CrossRef]
- van den Hooff, G.P.; Bussel, E.F.; Gacesa, S.; Biter, L.U.; van der Meij, B.S.; Stassen, P.M.; Stumpel, M.P.; van Zon, S.N.; van der Kallen, C.J.; Koonen, D.P.; et al. The association of circulating succinate with tumor staging and prognostic factors in patients with oral squamous cell carcinoma. J. Clin. Med. 2022, 11, 6901. [Google Scholar] [CrossRef]
- Yamada, T.; Kuroda, K.; Kato, T.; Koyama, T.; Sakai, N.; Sato, Y.; Takahashi, M.; Nakamura, M.; Yoshida, R.; Ito, S.; et al. Adverse Outcome Pathway (AOP): A Framework for Toxicity Prediction. Yakugaku Zasshi 2020, 140, 481–484. [Google Scholar] [CrossRef]
- Mills, E.L.; Harmon, C.; Jedrychowski, M.P.; Xiao, H.; Garrity, R.; Tran, N.V.; Bradshaw, G.A.; Fu, A.; Szpyt, J.; Reddy, A.; et al. UCP1 governs liver extracellular succinate and inflammatory pathogenesis. Nat. Metab. 2021, 3, 604–617. [Google Scholar] [CrossRef]
- Hirosumi, J.; Tuncman, G.; Chang, L.; Görgün, C.Z.; Uysal, K.T.; Maeda, K.; Karin, M.; Hotamisligil, G.S. A central role for JNK in obesity and insulin resistance. Nature 2002, 420, 333–336. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, J.; Wang, F.; Zhou, Y.; Jiang, J.; Ksimu, S.; Li, J.Z.; Niu, J.; Wang, Q. Tumoral PD-1-CD8- T cells are partially exhausted and predict favorable outcome in triple-negative breast cancer. Clin. Sci. 2020, 134, 711–726. [Google Scholar] [CrossRef]
- Bruinen de Bruin, Y.; Franco, A.; Ahrens, A.; Morris, A.; Verhagen, H.; Kephalopoulos, S.; Dulio, V.; Slobodnik, J.; Sijm, D.T.H.M.; Vermeire, T.; et al. Enhancing the use of exposure science across EU chemical policies as part of the European Exposure Science Strategy 2020–2030. Toxicol. Sci. 2021, 182, 213–225. [Google Scholar] [CrossRef]
- US Environmental Protection Agency. Benchmark Dose Technical Guidance (EPA/100/R-22/001); US Environmental Protection Agency: Washington, DC, USA, 2022.
- US Environmental Protection Agency. BMDS 3.2 User Manual: Section 4.4 Joint Covariate Model; US Environmental Protection Agency: Washington, DC, USA, 2022.
- European Food Safety Authority, Scientific Committee. Guidance on the calculation of benchmark dose (BMD) values. EFSA J. 2017, 15, e04658. [Google Scholar] [CrossRef]
- Organisation for Economic Co-Operation and Development. Users’ Handbook Supplement to the Guidance Document for Developing and Assessing AOPs (Version 3.7, ENV/JM/MONO(2022)25); OECD Publishing: Paris, France, 2022. [Google Scholar]

| Component | Criterion | Score | Description |
|---|---|---|---|
| Molecular Initiating Event (MIE) | Essentiality | 4/4 | BPAF binding to SUCNR1 is essential for initiating the adverse outcome pathway. |
| Key Event 1 (KE1) | Biological Plausibility | 3/4 | Macrophage succinate release is biologically plausible and supported by experimental data. |
| Key Event 2 (KE2) | Biological Plausibility | 3/4 | Succinate activation of hepatocyte SUCNR1 is biologically plausible and supported by experimental data. |
| Key Event 3 (KE3) | Biological Plausibility | 3/4 | Inhibition of Akt signaling in hepatocytes is biologically plausible and supported by experimental data. |
| Key Event 4 (KE4) | Biological Plausibility | 3/4 | Activation of JNK signaling is biologically plausible and supported by experimental data. |
| Key Event 5 (KE5) | Biological Plausibility | 3/4 | Increased lipid accumulation in hepatocytes is biologically plausible and supported by experimental data. |
| Adverse Outcome (AO) | Biological Plausibility | 4/4 | Hepatic steatosis is a well-documented adverse outcome with strong biological plausibility. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, N.; Leng, J.; Zhang, H.; Xu, J.; Yu, X.; Qian, K.; Zheng, Z.; Ying, M.; Tao, G.; Xiao, P.; et al. Bisphenol AF Induces Hepatic Steatosis via Succinate–SUCNR1-Mediated Macrophage–Hepatocyte Interactions: An Adverse Outcome Pathway Study in Male C57BL/6 Mice. Int. J. Mol. Sci. 2025, 26, 9720. https://doi.org/10.3390/ijms26199720
Wang N, Leng J, Zhang H, Xu J, Yu X, Qian K, Zheng Z, Ying M, Tao G, Xiao P, et al. Bisphenol AF Induces Hepatic Steatosis via Succinate–SUCNR1-Mediated Macrophage–Hepatocyte Interactions: An Adverse Outcome Pathway Study in Male C57BL/6 Mice. International Journal of Molecular Sciences. 2025; 26(19):9720. https://doi.org/10.3390/ijms26199720
Chicago/Turabian StyleWang, Ning, Jing Leng, Huimin Zhang, Jing Xu, Xiaoqi Yu, Kelei Qian, Zhiqing Zheng, Mengchao Ying, Gonghua Tao, Ping Xiao, and et al. 2025. "Bisphenol AF Induces Hepatic Steatosis via Succinate–SUCNR1-Mediated Macrophage–Hepatocyte Interactions: An Adverse Outcome Pathway Study in Male C57BL/6 Mice" International Journal of Molecular Sciences 26, no. 19: 9720. https://doi.org/10.3390/ijms26199720
APA StyleWang, N., Leng, J., Zhang, H., Xu, J., Yu, X., Qian, K., Zheng, Z., Ying, M., Tao, G., Xiao, P., & Hong, X. (2025). Bisphenol AF Induces Hepatic Steatosis via Succinate–SUCNR1-Mediated Macrophage–Hepatocyte Interactions: An Adverse Outcome Pathway Study in Male C57BL/6 Mice. International Journal of Molecular Sciences, 26(19), 9720. https://doi.org/10.3390/ijms26199720





