Synovial CXCL3+FOSL2+ Macrophages Mediate Inflammation via FOSL2/AP-1 in Rheumatoid Arthritis: A Single-Cell Transcriptome Analysis
Abstract
1. Introduction
2. Results
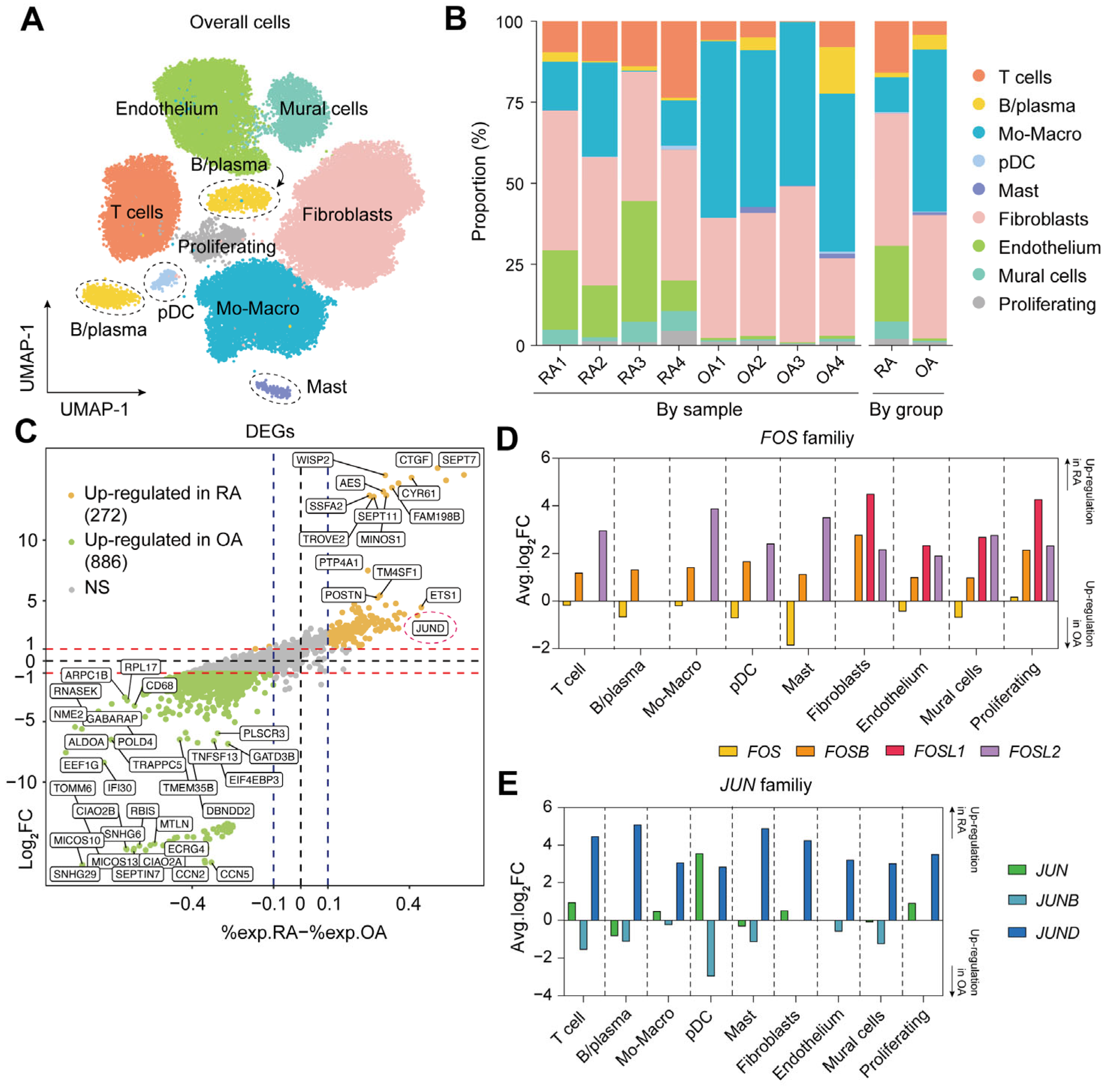
2.1. Single-Cell Landscape of Synovial Tissue in RA
2.2. Elevated Expression of FOSL2 and JUND in RA Synovium
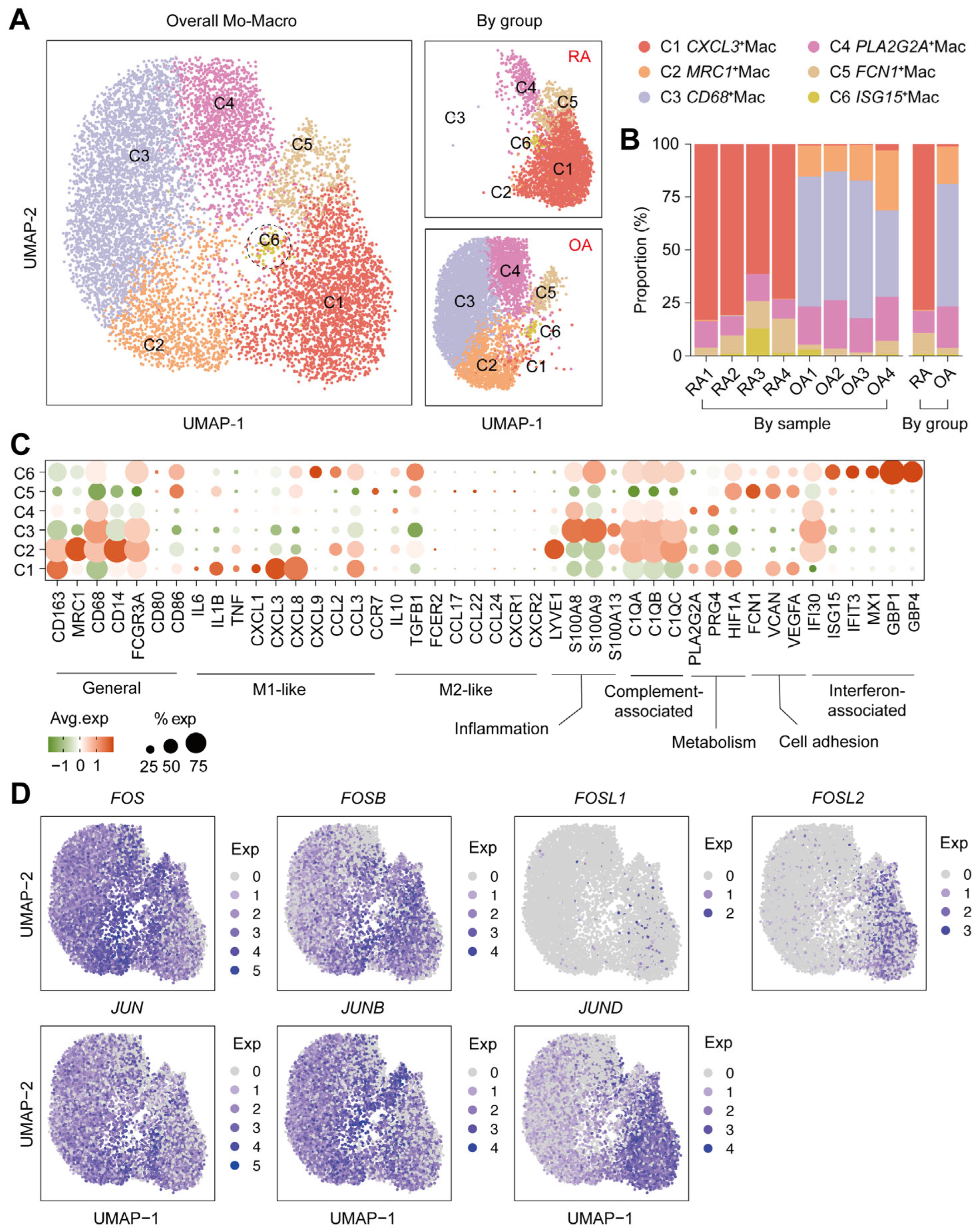
2.3. Immunophenotype Divergence of Macrophages in RA
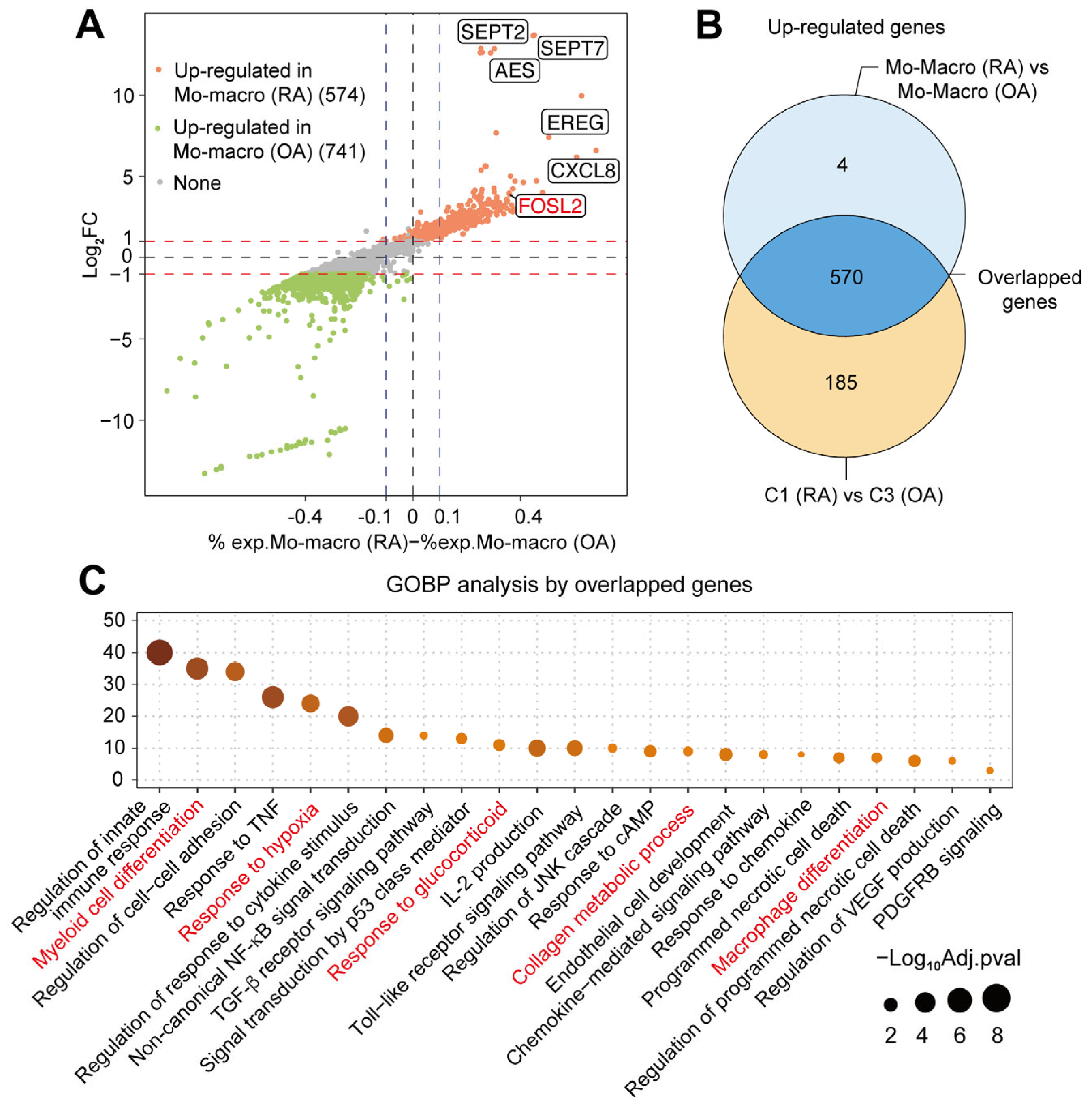
2.4. Multiple Functions of Synovial CXCL3+FOSL2+Mac on RA
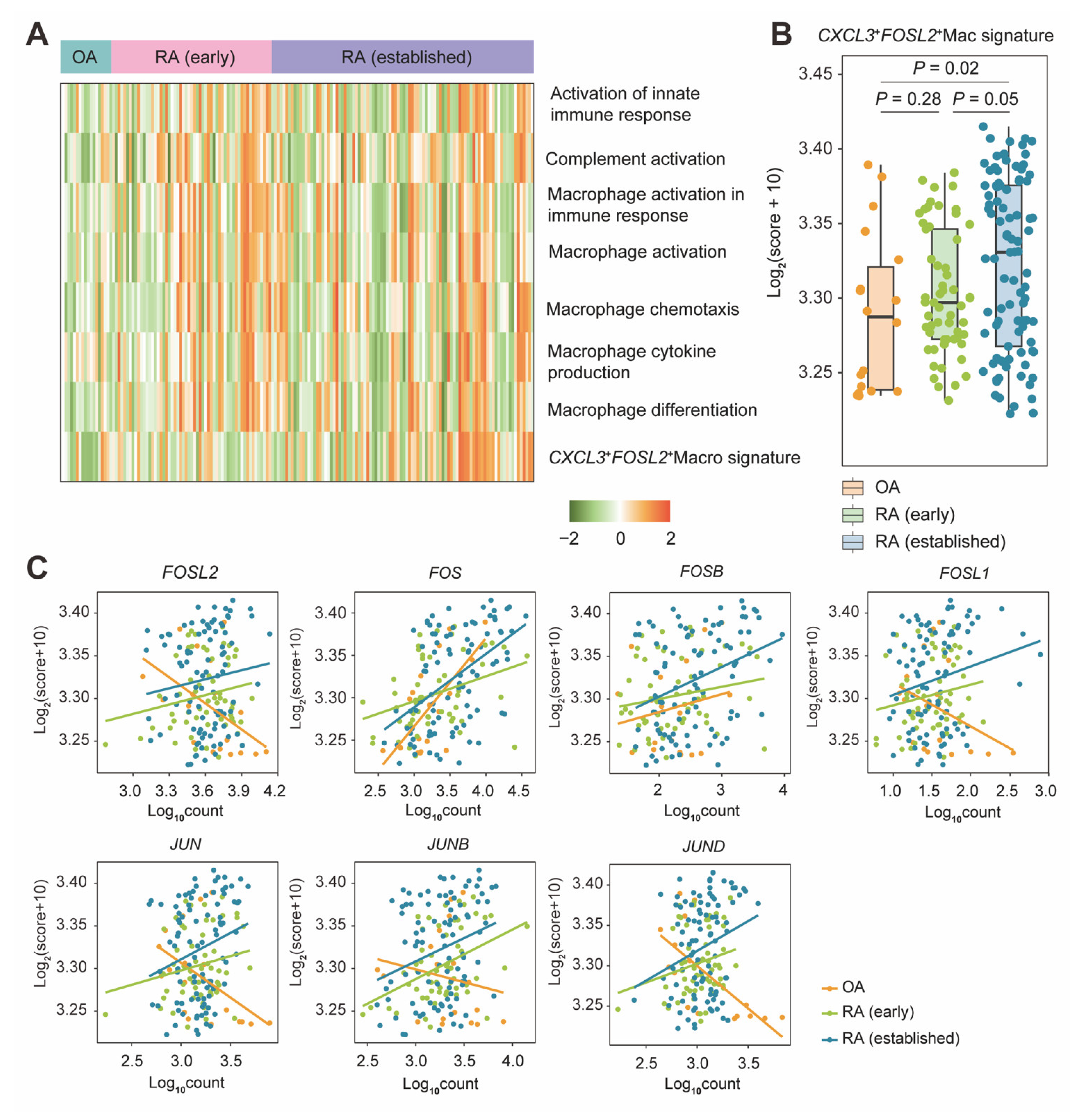
2.5. CXCL3+FOSL2+Mac Signature Enrichment by a Bulk Level Validation
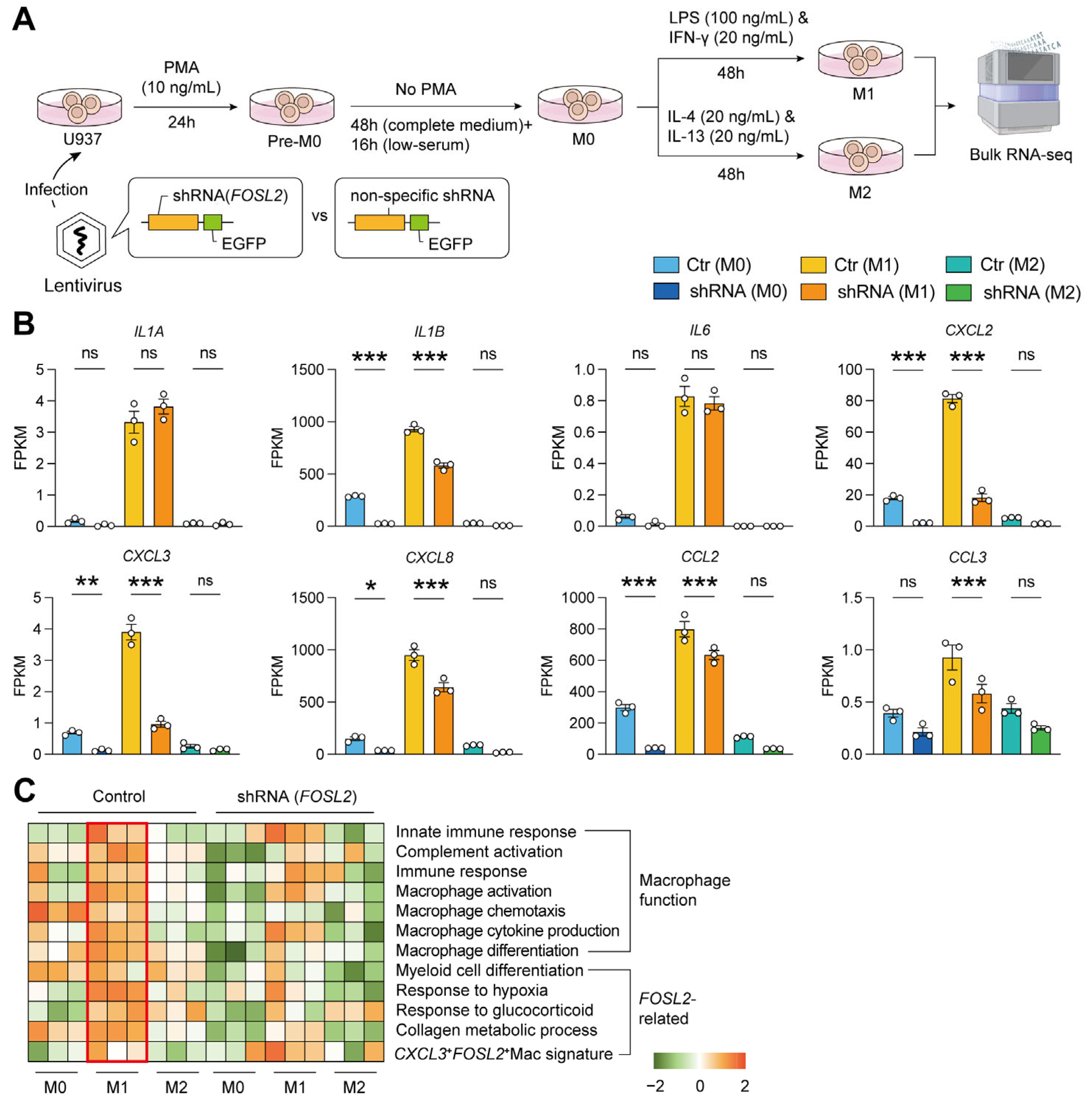
2.6. Validation by M1/M2 Pattern In Vitro
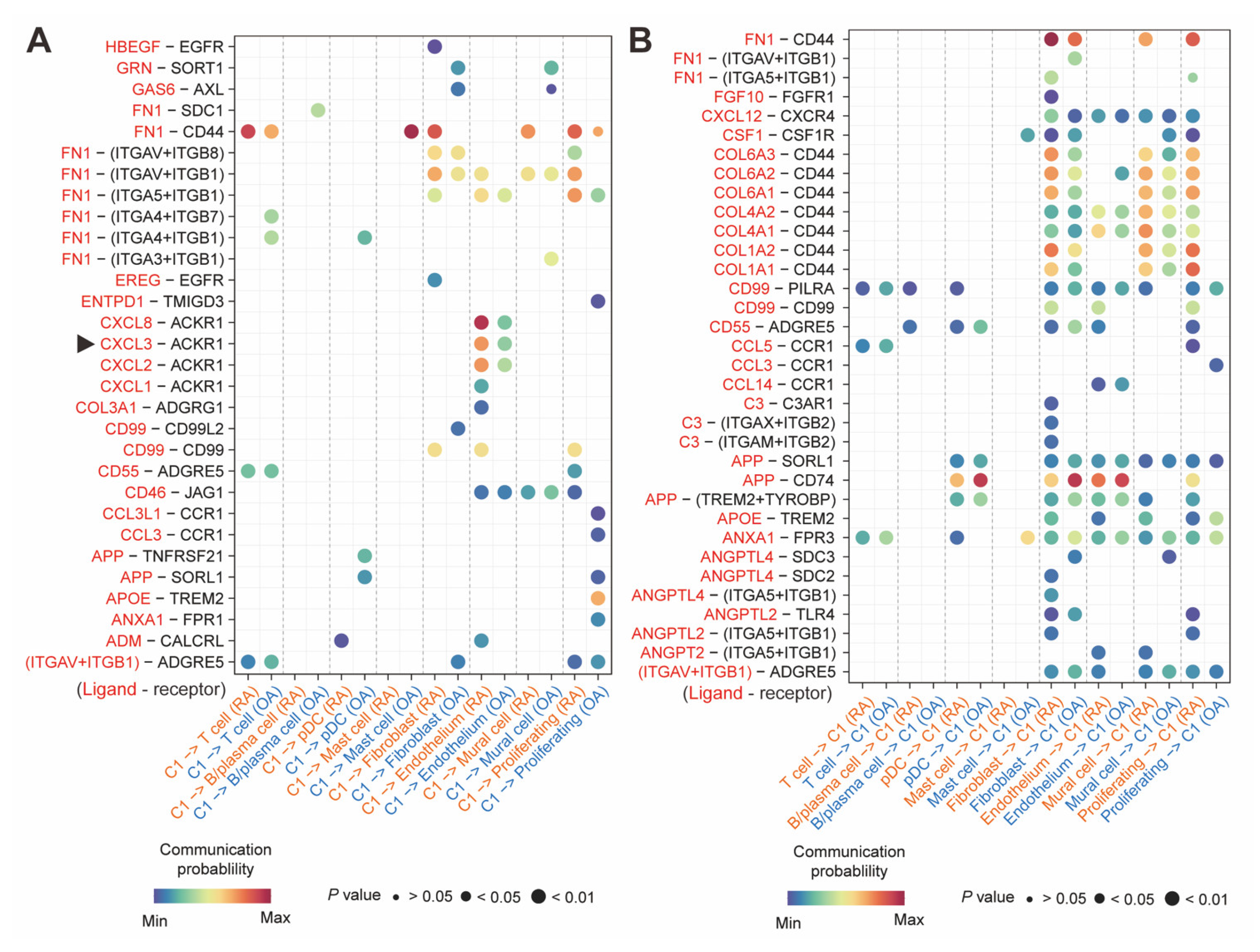
2.7. CXCL3+FOSL2+Mac Interacted with ACKR1+Endothelium
3. Discussion
4. Materials and Methods
4.1. Single-Cell Transcriptome Data Processing
4.2. Cell Annotation
4.3. Differentially Expressed Gene Detection
4.4. Gene Set Enrichment Analysis
4.5. GOBP Analysis
4.6. Intercellular Communication
4.7. The Knockdown of FOSL2
4.8. Macrophage Polarization In Vitro (M1/M2 Pattern)
4.9. The Analysis of Bulk Transcriptome Data
4.10. Statistical Analysis
| Dataset ID | Sequence Types | Platform | Tissues | Constitution |
|---|---|---|---|---|
| GSE200815 | scRNA-seq (10x Genomics) | Illumina NovaSeq 6000 | synovial tissues | Four RA with moderate to high disease activity * |
| GSE248455 | scRNA-seq (10x Genomics) | Illumina NovaSeq 6000 | synovial tissues | Four OA with mild knee pain † |
| GSE89408 | Bulk RNA-seq | GPL11154 Illumina HiSeq 2000 (San Diego, CA, USA) | synovial tissues | 18 OA, 57 early RA, and 93 established RA ‡ |
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AP-1 | activator protein-1 |
| DEG | differentially expressed gene |
| EC | endothelial cell |
| ECM | extracellular matrix |
| FC | fold change |
| FPKM | fragments per kilobase of transcript per million mapped reads |
| GEO | Gene Expression Omnibus |
| GOBP | Biological Process of Gene Ontology |
| GSEA | gene set enrichment analysis |
| IL | interleukin |
| JAK | Janus kinase |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| OA | osteoarthritis |
| pDC | plasmacytoid dendritic cell |
| RA | rheumatoid arthritis |
| SMAD | suppressor of mother against decapentaplegic |
| STAT | signal transducers and activators of transcription |
| TNF | tumor-necrosis factor |
| UMAP | uniform manifold approximation and projection |
References
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038, Correction in Lancet 2016, 388, 1984. [Google Scholar] [CrossRef]
- Gravallese, E.M.; Firestein, G.S. Rheumatoid Arthritis—Common Origins, Divergent Mechanisms. N. Engl. J. Med. 2023, 388, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.C.; Keystone, E.C.; van der Heijde, D.; Weinblatt, M.E.; Del Carmen Morales, L.; Reyes Gonzaga, J.; Yakushin, S.; Ishii, T.; Emoto, K.; Beattie, S.; et al. Baricitinib versus Placebo or Adalimumab in Rheumatoid Arthritis. N. Engl. J. Med. 2017, 376, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Burmester, G.R.; Blanco, R.; Charles-Schoeman, C.; Wollenhaupt, J.; Zerbini, C.; Benda, B.; Gruben, D.; Wallenstein, G.; Krishnaswami, S.; Zwillich, S.H.; et al. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: A randomised phase 3 trial. Lancet 2013, 381, 451–460. [Google Scholar] [CrossRef]
- Elliott, M.J.; Maini, R.N.; Feldmann, M.; Kalden, J.R.; Antoni, C.; Smolen, J.S.; Leeb, B.; Breedveld, F.C.; Macfarlane, J.D.; Bijl, H.; et al. Randomised double-blind comparison of chimeric monoclonal antibody to tumour necrosis factor alpha (cA2) versus placebo in rheumatoid arthritis. Lancet 1994, 344, 1105–1110. [Google Scholar] [CrossRef]
- Emery, P.; Keystone, E.; Tony, H.P.; Cantagrel, A.; van Vollenhoven, R.; Sanchez, A.; Alecock, E.; Lee, J.; Kremer, J. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: Results from a 24-week multicentre randomised placebo-controlled trial. Ann. Rheum. Dis. 2008, 67, 1516–1523. [Google Scholar] [CrossRef]
- Genovese, M.C.; Becker, J.C.; Schiff, M.; Luggen, M.; Sherrer, Y.; Kremer, J.; Birbara, C.; Box, J.; Natarajan, K.; Nuamah, I.; et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N. Engl. J. Med. 2005, 353, 1114–1123, Correction in N. Engl. J. Med. 2005, 353, 2311. [Google Scholar] [CrossRef]
- Zhang, F.; Jonsson, A.H.; Nathan, A.; Millard, N.; Curtis, M.; Xiao, Q.; Gutierrez-Arcelus, M.; Apruzzese, W.; Watts, G.F.M.; Weisenfeld, D.; et al. Deconstruction of rheumatoid arthritis synovium defines inflammatory subtypes. Nature 2023, 623, 616–624. [Google Scholar] [CrossRef]
- Wu, D.; Huang, Y.; Zhao, J.; Long, W.; Wang, B.; Wang, Y.; Chen, H.; Wu, R. Synovial macrophages drive severe joint destruction in established rheumatoid arthritis. Sci. Rep. 2025, 15, 12111. [Google Scholar] [CrossRef]
- Siouti, E.; Andreakos, E. The many facets of macrophages in rheumatoid arthritis. Biochem. Pharmacol. 2019, 165, 152–169. [Google Scholar] [CrossRef]
- Udalova, I.A.; Mantovani, A.; Feldmann, M. Macrophage heterogeneity in the context of rheumatoid arthritis. Nat. Rev. Rheumatol. 2016, 12, 472–485. [Google Scholar] [CrossRef]
- Hanlon, M.M.; Smith, C.M.; Canavan, M.; Neto, N.G.B.; Song, Q.; Lewis, M.J.; O’Rourke, A.M.; Tynan, O.; Barker, B.E.; Gallagher, P.; et al. Loss of synovial tissue macrophage homeostasis precedes rheumatoid arthritis clinical onset. Sci. Adv. 2024, 10, eadj1252. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Y.; Jin, S.; Wang, M.; Jiao, Y.; Yang, B.; Lu, X.; Ji, X.; Fei, Y.; Yang, H.; et al. Single-cell sequencing of immune cells from anticitrullinated peptide antibody positive and negative rheumatoid arthritis. Nat. Commun. 2021, 12, 4977. [Google Scholar] [CrossRef]
- Zhang, F.; Wei, K.; Slowikowski, K.; Fonseka, C.Y.; Rao, D.A.; Kelly, S.; Goodman, S.M.; Tabechian, D.; Hughes, L.B.; Salomon-Escoto, K.; et al. Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nat. Immunol. 2019, 20, 928–942. [Google Scholar] [CrossRef] [PubMed]
- Alivernini, S.; MacDonald, L.; Elmesmari, A.; Finlay, S.; Tolusso, B.; Gigante, M.R.; Petricca, L.; Di Mario, C.; Bui, L.; Perniola, S.; et al. Distinct synovial tissue macrophage subsets regulate inflammation and remission in rheumatoid arthritis. Nat. Med. 2020, 26, 1295–1306. [Google Scholar] [CrossRef] [PubMed]
- Wagner, E.F. AP-1--Introductory remarks. Oncogene 2001, 20, 2334–2335. [Google Scholar] [CrossRef] [PubMed]
- Chinenov, Y.; Kerppola, T.K. Close encounters of many kinds: Fos-Jun interactions that mediate transcription regulatory specificity. Oncogene 2001, 20, 2438–2452. [Google Scholar] [CrossRef]
- Hannemann, N.; Cao, S.; Eriksson, D.; Schnelzer, A.; Jordan, J.; Eberhardt, M.; Schleicher, U.; Rech, J.; Ramming, A.; Uebe, S.; et al. Transcription factor Fra-1 targets arginase-1 to enhance macrophage-mediated inflammation in arthritis. J. Clin. Investig. 2019, 129, 2669–2684. [Google Scholar] [CrossRef]
- Hosoya, T.; Saito, T.; Baba, H.; Tanaka, N.; Noda, S.; Komiya, Y.; Tagawa, Y.; Yamamoto, A.; Mizoguchi, F.; Kawahata, K.; et al. Chondroprotective effects of CDK4/6 inhibition via enhanced ubiquitin-dependent degradation of JUN in synovial fibroblasts. Rheumatology 2022, 61, 3427–3438. [Google Scholar] [CrossRef]
- Cai, M.; Li, Z.; Wen, X.; Jin, H.; Li, Y.; Wu, H.; Yang, C.; Chen, Z. C3a-C3aR1-mediated interactions between fibroblast-like synoviocytes and macrophages promote the progression of rheumatoid arthritis. Arthritis Rheumatol. 2025. Epub ahead of print. [Google Scholar] [CrossRef]
- Mantovani, A.; Sozzani, S.; Locati, M.; Allavena, P.; Sica, A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002, 23, 549–555. [Google Scholar] [CrossRef]
- Li, J.; Liu, P.; Huang, Y.; Wang, Y.; Zhao, J.; Xiong, Z.; Liu, M.; Wu, R. Immunophenotypic Landscape of synovial tissue in rheumatoid arthritis: Insights from ACPA status. Heliyon 2024, 10, e34088. [Google Scholar] [CrossRef]
- Jang, S.; Kwon, E.J.; Lee, J.J. Rheumatoid Arthritis: Pathogenic Roles of Diverse Immune Cells. Int. J. Mol. Sci. 2022, 23, 905. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Gu, M.; Li, X.; Hu, X.; Chen, C.; Kang, Y.; Pan, B.; Chen, W.; Xian, G.; Wu, X.; et al. ITGA5(+) synovial fibroblasts orchestrate proinflammatory niche formation by remodelling the local immune microenvironment in rheumatoid arthritis. Ann. Rheum. Dis. 2025, 84, 232–252. [Google Scholar] [CrossRef] [PubMed]
- Tak, P.P.; Smeets, T.J.; Daha, M.R.; Kluin, P.M.; Meijers, K.A.; Brand, R.; Meinders, A.E.; Breedveld, F.C. Analysis of the synovial cell infiltrate in early rheumatoid synovial tissue in relation to local disease activity. Arthritis Rheum. 1997, 40, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Zec, K.; Schonfeldova, B.; Ai, Z.; Van Grinsven, E.; Pirgova, G.; Eames, H.L.; Berthold, D.L.; Attar, M.; Compeer, E.B.; Arnon, T.I.; et al. Macrophages in the synovial lining niche initiate neutrophil recruitment and articular inflammation. J. Exp. Med. 2023, 220, e20220595. [Google Scholar] [CrossRef]
- Fearon, U.; Canavan, M.; Biniecka, M.; Veale, D.J. Hypoxia, mitochondrial dysfunction and synovial invasiveness in rheumatoid arthritis. Nat. Rev. Rheumatol. 2016, 12, 385–397. [Google Scholar] [CrossRef]
- Saeki, N.; Imai, Y. Reprogramming of synovial macrophage metabolism by synovial fibroblasts under inflammatory conditions. Cell Commun. Signal 2020, 18, 188. [Google Scholar] [CrossRef]
- Danks, L.; Sabokbar, A.; Gundle, R.; Athanasou, N.A. Synovial macrophage-osteoclast differentiation in inflammatory arthritis. Ann. Rheum. Dis. 2002, 61, 916–921. [Google Scholar] [CrossRef]
- Gao, P.; Yuan, S.; Wang, Y.; Wang, Y.; Li, X.; Liu, T.; Zheng, Y.; Wang, J.; Liu, D.; Xu, L.; et al. Corydalis decumbens and tetrahydropalmatrubin inhibit macrophages inflammation to relieve rheumatoid arthritis by targeting Fosl2. J. Ethnopharmacol. 2025, 341, 119348. [Google Scholar] [CrossRef]
- Huang, M.; Tabib, T.; Khanna, D.; Assassi, S.; Domsic, R.; Lafyatis, R. Single-cell transcriptomes and chromatin accessibility of endothelial cells unravel transcription factors associated with dysregulated angiogenesis in systemic sclerosis. Ann. Rheum. Dis. 2024, 83, 1335–1344. [Google Scholar] [CrossRef]
- Girbl, T.; Lenn, T.; Perez, L.; Rolas, L.; Barkaway, A.; Thiriot, A.; Del Fresno, C.; Lynam, E.; Hub, E.; Thelen, M.; et al. Distinct Compartmentalization of the Chemokines CXCL1 and CXCL2 and the Atypical Receptor ACKR1 Determine Discrete Stages of Neutrophil Diapedesis. Immunity 2018, 49, 1062–1076.e1066. [Google Scholar] [CrossRef] [PubMed]
- Nakano, K.; Okada, Y.; Saito, K.; Tanikawa, R.; Sawamukai, N.; Sasaguri, Y.; Kohro, T.; Wada, Y.; Kodama, T.; Tanaka, Y. Rheumatoid synovial endothelial cells produce macrophage colony-stimulating factor leading to osteoclastogenesis in rheumatoid arthritis. Rheumatology 2007, 46, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Bartok, B.; Firestein, G.S. Fibroblast-like synoviocytes: Key effector cells in rheumatoid arthritis. Immunol. Rev. 2010, 233, 233–255. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, T.; Nagy, G.; Pap, T. Synovial fibroblasts as potential drug targets in rheumatoid arthritis, where do we stand and where shall we go? Ann. Rheum. Dis. 2022, 81, 1055–1064. [Google Scholar] [CrossRef]
- Sutherland, T.E.; Dyer, D.P.; Allen, J.E. The extracellular matrix and the immune system: A mutually dependent relationship. Science 2023, 379, eabp8964. [Google Scholar] [CrossRef]
- Midwood, K.; Sacre, S.; Piccinini, A.M.; Inglis, J.; Trebaul, A.; Chan, E.; Drexler, S.; Sofat, N.; Kashiwagi, M.; Orend, G.; et al. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat. Med. 2009, 15, 774–780. [Google Scholar] [CrossRef]
- Schaefer, L.; Babelova, A.; Kiss, E.; Hausser, H.J.; Baliova, M.; Krzyzankova, M.; Marsche, G.; Young, M.F.; Mihalik, D.; Gotte, M.; et al. The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J. Clin. Investig. 2005, 115, 2223–2233. [Google Scholar] [CrossRef]
- Floudas, A.; Smith, C.M.; Tynan, O.; Neto, N.; Krishna, V.; Wade, S.M.; Hanlon, M.; Cunningham, C.; Marzaioli, V.; Canavan, M.; et al. Distinct stromal and immune cell interactions shape the pathogenesis of rheumatoid and psoriatic arthritis. Ann. Rheum. Dis. 2022, 81, 1224–1242. [Google Scholar] [CrossRef]
- Philpott, H.T.; Birmingham, T.B.; Blackler, G.; Klapak, J.D.; Knights, A.J.; Farrell, E.C.; Fiset, B.; Walsh, L.A.; Giffin, J.R.; Vasarhelyi, E.M.; et al. Association of Synovial Innate Immune Exhaustion with Worse Pain in Knee Osteoarthritis. Arthritis Rheumatol. 2025, 77, 664–676. [Google Scholar] [CrossRef]
- Nascimento, C.R.; Rodrigues Fernandes, N.A.; Gonzalez Maldonado, L.A.; Junior, C.R. Comparison of monocytic cell lines U937 and THP-1 as macrophage models for in vitro studies. Biochem. Biophys. Rep. 2022, 32, 101383. [Google Scholar] [CrossRef]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef]
- Guo, Y.; Walsh, A.M.; Fearon, U.; Smith, M.D.; Wechalekar, M.D.; Yin, X.; Cole, S.; Orr, C.; McGarry, T.; Canavan, M.; et al. CD40L-Dependent Pathway Is Active at Various Stages of Rheumatoid Arthritis Disease Progression. J. Immunol. 2017, 198, 4490–4501. [Google Scholar] [CrossRef]
- Liberzon, A.; Subramanian, A.; Pinchback, R.; Thorvaldsdóttir, H.; Tamayo, P.; Mesirov, J.P. Molecular signatures database (MSigDB) 3.0. Bioinformatics 2011, 27, 1739–1740. [Google Scholar] [CrossRef]
| Genes | Avg.log2FC * | % exp in RA † | % exp in OA ‡ | Adjusted p Values § |
|---|---|---|---|---|
| JUND | 3.78 | 0.907 | 0.477 | 0 |
| FOSL2 | 3.21 | 0.427 | 0.158 | 0 |
| FOSB | 1.98 | 0.792 | 0.668 | 0 |
| JUN | 0.41 | 0.753 | 0.805 | 6.5 × 10−52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Yang, J.; Chen, M.; Chen, X.; Cao, S. Synovial CXCL3+FOSL2+ Macrophages Mediate Inflammation via FOSL2/AP-1 in Rheumatoid Arthritis: A Single-Cell Transcriptome Analysis. Int. J. Mol. Sci. 2025, 26, 9718. https://doi.org/10.3390/ijms26199718
Wu Y, Yang J, Chen M, Chen X, Cao S. Synovial CXCL3+FOSL2+ Macrophages Mediate Inflammation via FOSL2/AP-1 in Rheumatoid Arthritis: A Single-Cell Transcriptome Analysis. International Journal of Molecular Sciences. 2025; 26(19):9718. https://doi.org/10.3390/ijms26199718
Chicago/Turabian StyleWu, Yiwei, Jinming Yang, Mengke Chen, Xiaoxiang Chen, and Shan Cao. 2025. "Synovial CXCL3+FOSL2+ Macrophages Mediate Inflammation via FOSL2/AP-1 in Rheumatoid Arthritis: A Single-Cell Transcriptome Analysis" International Journal of Molecular Sciences 26, no. 19: 9718. https://doi.org/10.3390/ijms26199718
APA StyleWu, Y., Yang, J., Chen, M., Chen, X., & Cao, S. (2025). Synovial CXCL3+FOSL2+ Macrophages Mediate Inflammation via FOSL2/AP-1 in Rheumatoid Arthritis: A Single-Cell Transcriptome Analysis. International Journal of Molecular Sciences, 26(19), 9718. https://doi.org/10.3390/ijms26199718






