Fusobacterium Nucleatum in Colorectal Cancer: Relationship Among Immune Modulation, Potential Biomarkers and Therapeutic Implications
Abstract
1. Introduction
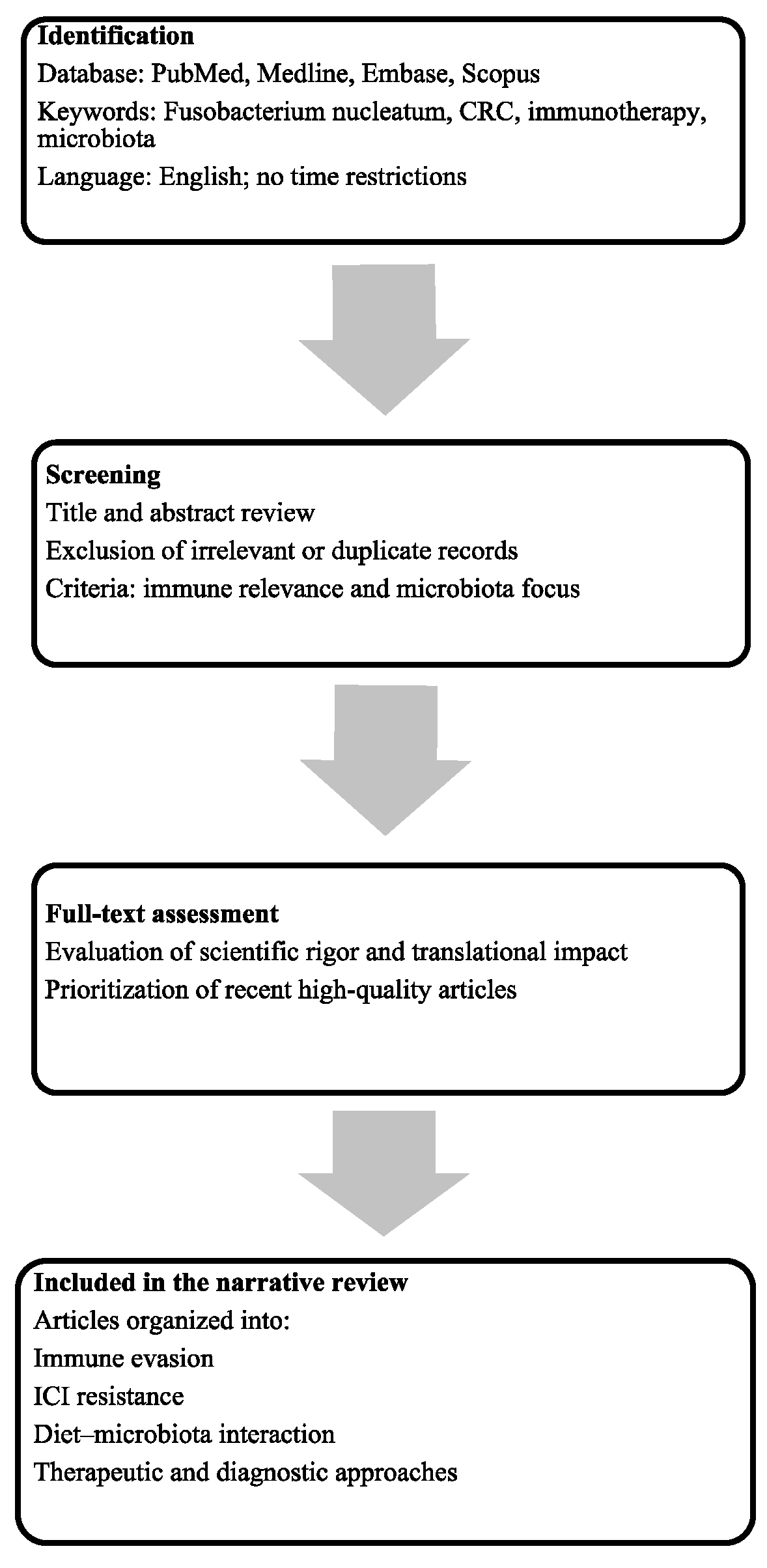
2. Materials and Methods
3. Microbiota and Cancer
4. Taxonomy and Subspecies Diversity of Fn
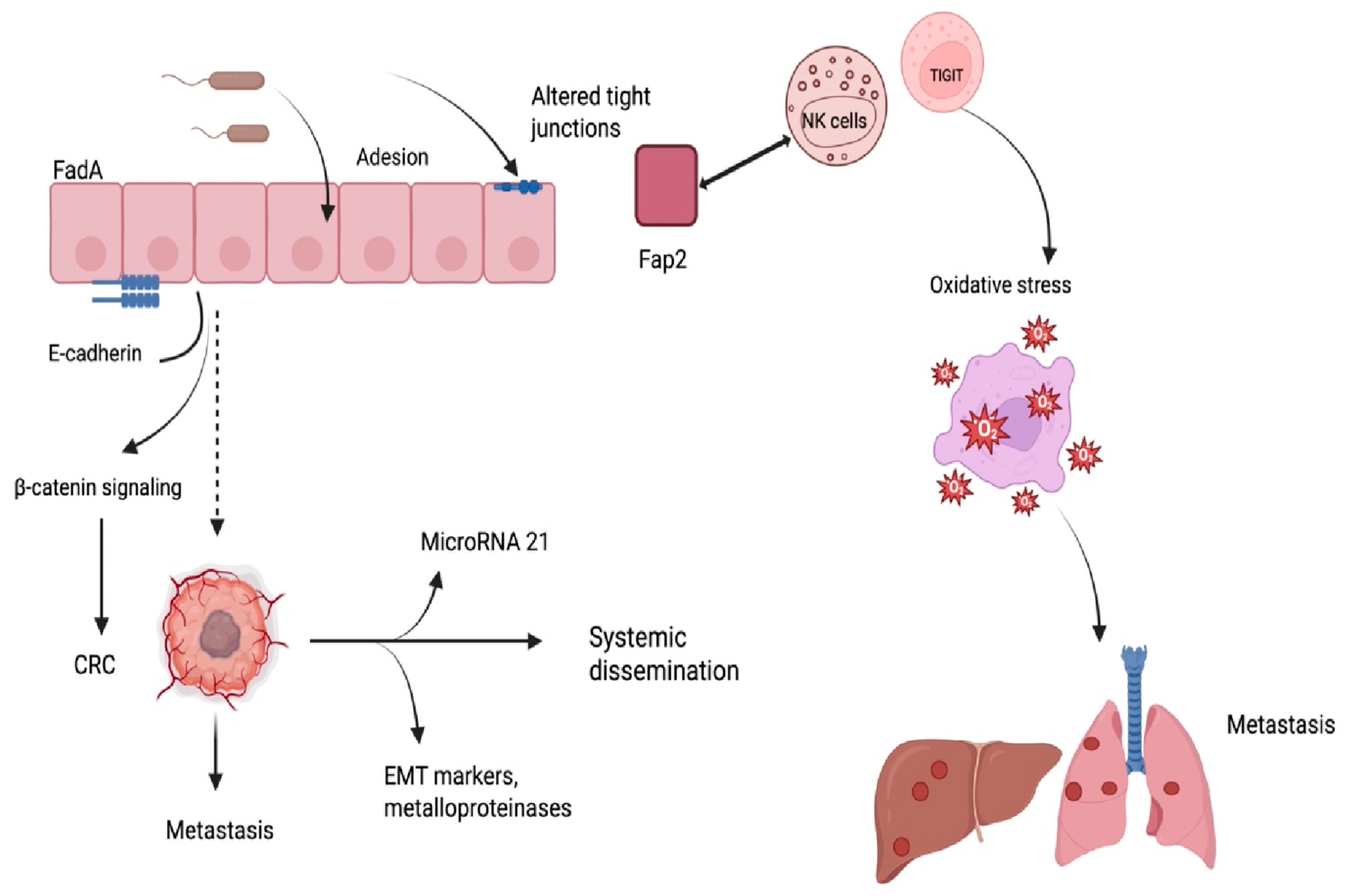
5. Microbial Colonization and Tissue Tropism
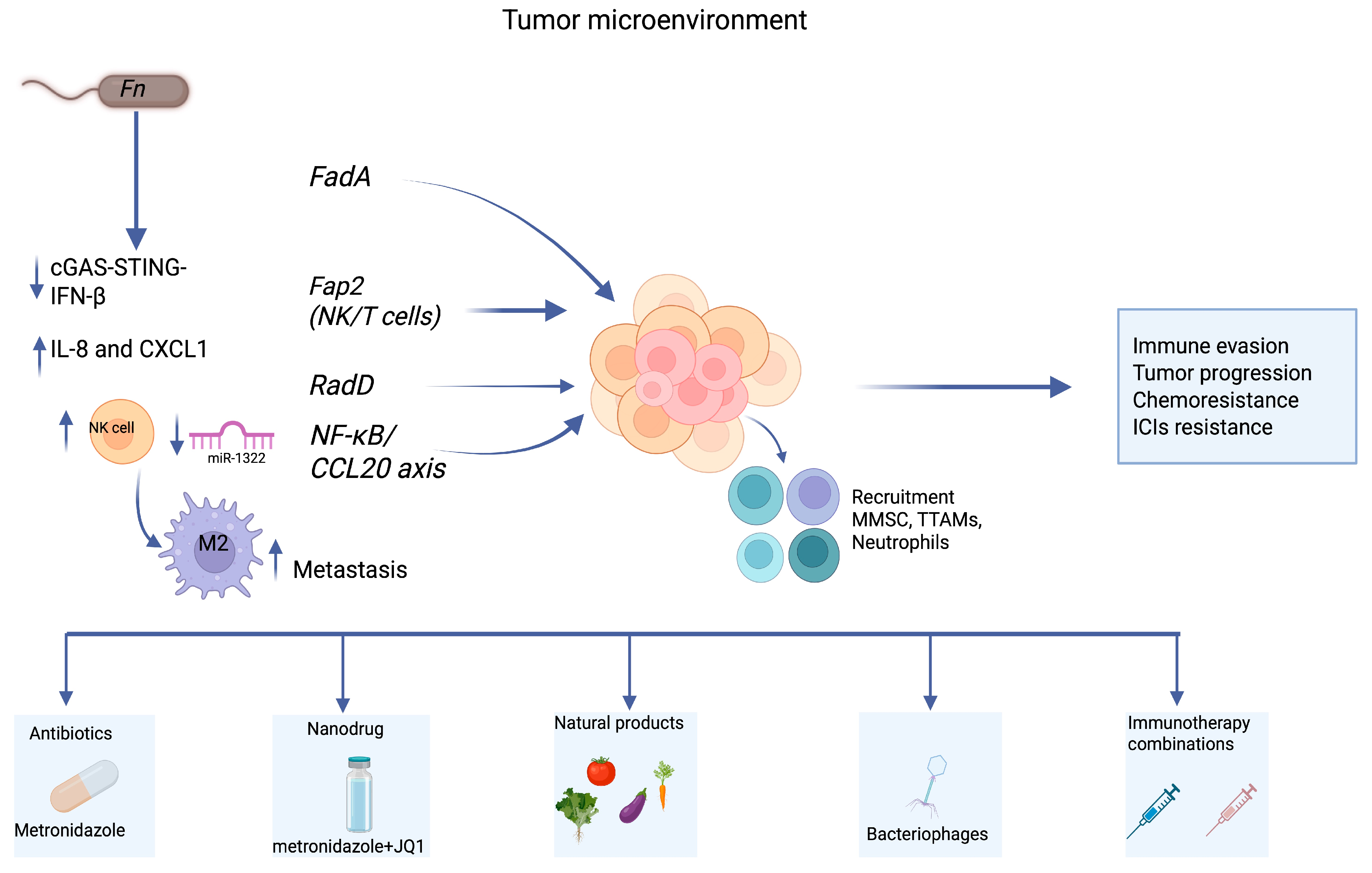
6. Immune Modulation and Tumor Immune Evasion
7. Clinical Implications and Immunotherapy Resistance
8. Therapeutic Targeting Strategies
9. Biomarker Value and Diagnostic Applications
10. Fecal Detection of Fn
11. Evidence Gaps and Conflicting Findings
12. Future Directions: Ethical Considerations and Trial Design
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Torre, L.A.; Soerjomataram, I.; Hayes, R.B.; Bray, F.; Weber, T.K.; Jemal, A. Global patterns and trends in colorectal cancer incidence in young adults. Gut 2019, 68, 2179–2185. [Google Scholar] [CrossRef]
- Zheng, E.; Włodarczyk, M.; Węgiel, A.; Osielczak, A.; Możdżan, M.; Biskup, L.; Grochowska, A.; Wołyniak, M.; Gajewski, D.; Porc, M.; et al. Navigating through novelties concerning mCRC treatment—The role of immunotherapy, chemotherapy, and targeted therapy in mCRC. Front. Surg. 2024, 11, 1398289. [Google Scholar] [CrossRef] [PubMed]
- Morris, V.K.; Kennedy, E.B.; Baxter, N.N.; Benson, A.B., 3rd; Cercek, A.; Cho, M.; Ciombor, K.K.; Cremolini, C.; Davis, A.; Deming, D.A.; et al. Treatment of metastatic colorectal cancer: ASCO guideline. J. Clin. Oncol. 2023, 41, 678–700. [Google Scholar] [CrossRef]
- Jiang, S.S.; Xie, Y.L.; Xiao, X.Y.; Kang, Z.R.; Lin, X.L.; Zhang, L.; Li, C.S.; Qian, Y.; Xu, P.P.; Leng, X.X.; et al. Fusobacterium nucleatum-derived succinic acid induces tumor resistance to immunotherapy in colorectal cancer. Cell Host Microbe 2023, 31, 781–797.e9. [Google Scholar] [CrossRef]
- Qu, R.; Zhang, Y.; Ma, Y.; Zhou, X.; Sun, L.; Jiang, C.; Zhang, Z.; Fu, W. Role of the gut microbiota and its metabolites in tumorigenesis or development of colorectal cancer. Adv. Sci. 2023, 10, 2205563. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Liu, X.; Liu, Z.; Pan, C.; Zhang, X.; Zhao, Z.; Sun, H. Fusobacterium nucleatum in tumors: From tumorigenesis to tumor metastasis and tumor resistance. Cancer Biol. Ther. 2024, 25, 2306676. [Google Scholar] [CrossRef]
- Lu, J.; Wei, W.; Zheng, D. Fusobacterium nucleatum in colorectal cancer: Ally mechanism and targeted therapy strategies. Research 2025, 8, 0640. [Google Scholar] [CrossRef]
- Guo, H. Interactions between the tumor microbiota and breast cancer. Front. Cell. Infect. Microbiol. 2024, 14, 1499203. [Google Scholar] [CrossRef]
- Incognito, G.G.; Ronsini, C.; Palmara, V.; Romeo, P.; Vizzielli, G.; Restaino, S.; La Verde, M.; De Tommasi, O.; Palumbo, M.; Cianci, S. The interplay between cervicovaginal microbiota diversity, Lactobacillus profiles and human papillomavirus in cervical cancer: A systematic review. Healthcare 2025, 13, 599. [Google Scholar] [CrossRef] [PubMed]
- Occhipinti, S.; Incognito, G.G.; Palumbo, M. The influence of the vaginal ecosystem on vaginitis, bacterial vaginosis, and sexually transmitted diseases: An epidemiological study and literature review. Arch. Gynecol. Obstet. 2025, 311, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.C.; Guo, F.; Yu, Y.; Sun, T.; Ma, D.; Han, J.; Qian, Y.; Kryczek, I.; Sun, D.; Nagarsheth, N.; et al. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell 2017, 170, 548–563.e16. [Google Scholar] [CrossRef]
- Krieger, M.; Guo, M.; Merritt, J. Reexamining the role of Fusobacterium nucleatum subspecies in clinical and experimental studies. Gut Microbes 2024, 16, 2415490. [Google Scholar] [CrossRef]
- Queen, J.; Cing, Z.; Minsky, H.; Nandi, A.; Southward, T.; Ferri, J.; McMann, M.; Iyadorai, T.; Vadivelu, J.; Roslani, A.; et al. Fusobacterium nucleatum is enriched in invasive biofilms in colorectal cancer. NPJ Biofilms Microbiomes 2025, 11, 81. [Google Scholar] [CrossRef]
- Sivertsen, A.; Forni, D.; Molteni, C.; Bivand, J.; Dimmen, G.; Bruvold, T.S.; Sironi, M.; Kommedal, Ø. Reassessing taxonomy and virulence in the Fusobacterium nucleatum group-rebuttal of Fusobacterium animalis clades “Fna C1” and “Fna C2,” genome announcement for Fusobacterium watanabei, and description of Fusobacterium paranimalis sp. nov. mBio 2025, 16, e0094125. [Google Scholar] [CrossRef]
- Zepeda-Rivera, M.; Minot, S.S.; Bouzek, H.; Wu, H.; Blanco-Míguez, A.; Manghi, P.; Jones, D.S.; LaCourse, K.D.; Wu, Y.; McMahon, E.F.; et al. A distinct Fusobacterium nucleatum clade dominates the colorectal cancer niche. Nature 2024, 628, 424–432. [Google Scholar] [CrossRef]
- Li, B.; Wei, Z.; Wang, Z.; Xu, F.; Yang, J.; Lin, B.; Chen, Y.; Wenren, H.; Wu, L.; Guo, X.; et al. Fusobacterium nucleatum induces oxaliplatin resistance by inhibiting ferroptosis through E-cadherin/β-catenin/GPX4 axis in colorectal cancer. Free Radic. Biol. Med. 2024, 220, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Carbone, L.; Incognito, G.G.; Incognito, D.; Marano, L. Clinical implications of epithelial-to-mesenchymal transition (EMT) in cancer. Adv. Exp. Med. Biol. 2024, 1413, 455–470. [Google Scholar] [CrossRef]
- Carbone, L.; Incognito, G.G.; Incognito, D.; Nibid, L.; Caruso, G.; Berretta, M.; Taffon, C.; Palumbo, M.; Perrone, G.; Roviello, F.; et al. Clinical implications of epithelial-to-mesenchymal transition in cancers which potentially spread to peritoneum. Clin. Transl. Oncol. 2025, 27, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Scano, A.; Fais, S.; Ciappina, G.; Genovese, M.; Granata, B.; Montopoli, M.; Consolo, P.; Carroccio, P.; Muscolino, P.; Ottaiano, A.; et al. Oxidative stress by H2O2 as a potential inductor in the switch from commensal to pathogen in oncogenic bacterium Fusobacterium nucleatum. Antioxidants 2025, 14, 323. [Google Scholar] [CrossRef] [PubMed]
- Galaski, J.; Rishiq, A.; Liu, M.; Bsoul, R.; Bergson, A.; Lux, R.; Bachrach, G.; Mandelboim, O. Fusobacterium nucleatum subsp. nucleatum RadD binds Siglec-7 and inhibits NK cell-mediated cancer cell killing. iScience 2024, 27, 110157. [Google Scholar] [CrossRef]
- Luo, M. Fusobacterium nucleatum: A novel regulator of antitumor immune checkpoint blockade therapy in colorectal cancer. Am. J. Cancer Res. 2024, 14, 3962–3975. [Google Scholar] [CrossRef]
- Vargas-Castellanos, E.; Rincón-Riveros, A. Microsatellite instability in the tumor microenvironment: The role of inflammation and the microbiome. Cancer Med. 2025, 14, 70603. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Fan, L.; Lin, Y.; Shen, W.; Qi, Y.; Zhang, Y.; Chen, Z.; Wang, L.; Long, Y.; Hou, T.; et al. Fusobacterium nucleatum promotes colorectal cancer metastasis through miR-1322/CCL20 axis and M2 polarization. Gut Microbes 2021, 13, 1980347. [Google Scholar] [CrossRef]
- Shigematsu, Y.; Saito, R.; Amori, G.; Kanda, H.; Takahashi, Y.; Takeuchi, K.; Takahashi, S.; Inamura, K. Fusobacterium nucleatum, immune responses, and metastatic organ diversity in colorectal cancer liver metastasis. Cancer Sci. 2024, 115, 3248–3255. [Google Scholar] [CrossRef]
- González-Montero, J.; Rojas, C.I.; Burotto, M. Predictors of Response to Immunotherapy in Colorectal Cancer. Oncologist 2024, 29, 824–832. [Google Scholar] [CrossRef]
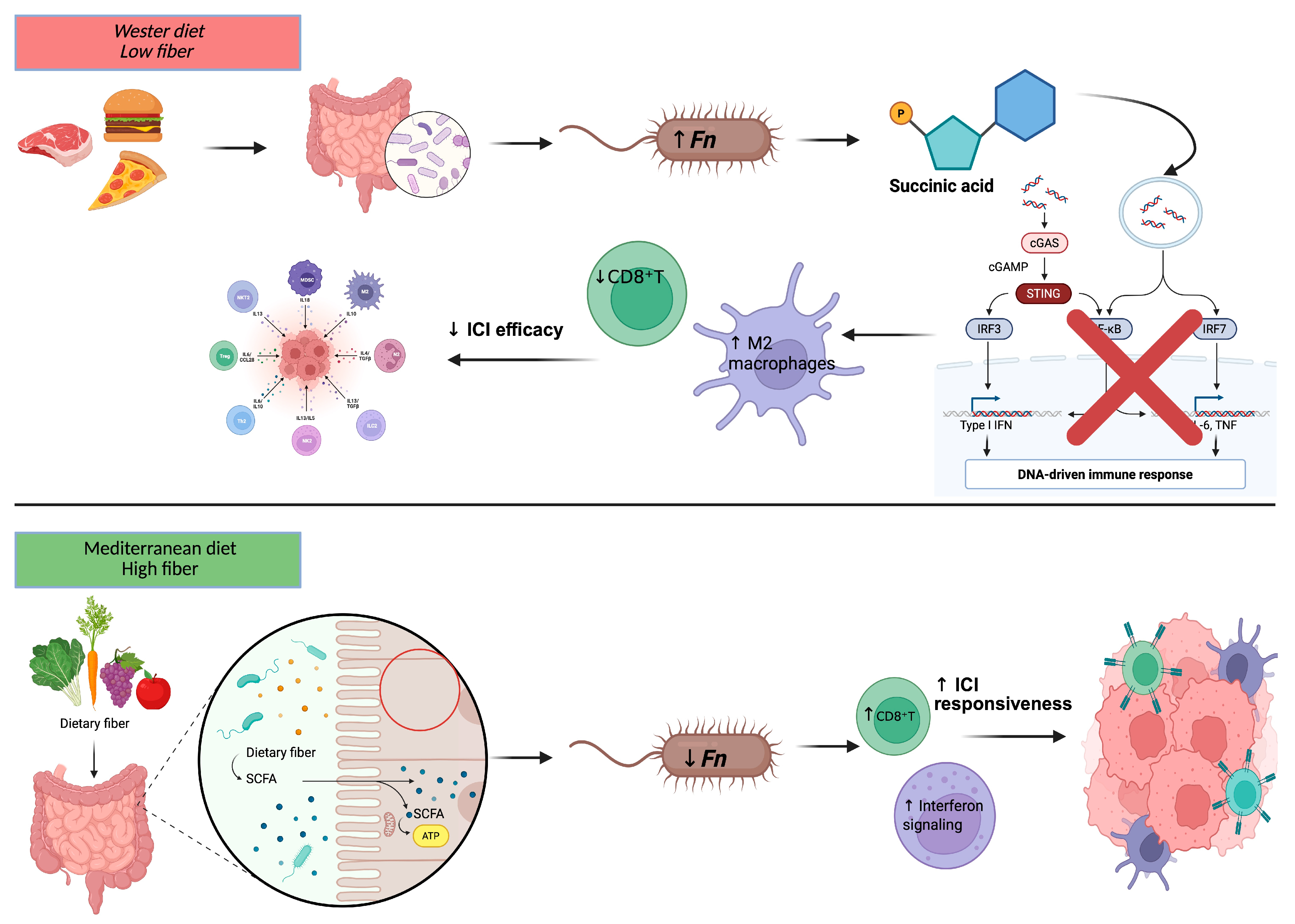
- Wang, K.; Lo, C.H.; Mehta, R.S.; Nguyen, L.H.; Wang, Y.; Ma, W.; Ugai, T.; Kawamura, H.; Ugai, S.; Takashima, Y.; et al. An empirical dietary pattern associated with the gut microbial features in relation to colorectal cancer risk. Gastroenterology 2024, 167, 1371–1383.e4. [Google Scholar] [CrossRef] [PubMed]
- Abu-Ghazaleh, N.; Chua, W.J.; Gopalan, V. Intestinal microbiota and its association with colon cancer and red/processed meat consumption. J. Gastroenterol. Hepatol. 2021, 36, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Nawab, S.; Bao, Q.; Ji, L.H.; Luo, Q.; Fu, X.; Fan, S.; Deng, Z.; Ma, W. The pathogenicity of Fusobacterium nucleatum modulated by dietary fibers: A possible missing link between the dietary composition and the risk of colorectal cancer. Microorganisms 2023, 11, 2004. [Google Scholar] [CrossRef]
- Farinetti, A.; Zurlo, V.; Manenti, A.; Coppi, F.; Mattioli, A.V. Mediterranean diet and colorectal cancer: A systematic review. Nutrition 2017, 43–44, 83–88. [Google Scholar] [CrossRef]
- Szczyrek, M.; Bitkowska, P.; Chunowski, P.; Czuchryta, P.; Krawczyk, P.; Milanowski, J. Diet, microbiome, and cancer immunotherapy: A comprehensive review. Nutrients 2021, 13, 2217. [Google Scholar] [CrossRef]
- Hanus, M.; Parada-Venegas, D.; Landskron, G.; Wielandt, A.M.; Hurtado, C.; Alvarez, K.; Hermoso, M.A.; López-Köstner, F.; De la Fuente, M. Immune system, microbiota, and microbial metabolites: The unresolved triad in colorectal cancer microenvironment. Front. Immunol. 2021, 12, 612826. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Z.; Wu, R.; Mao, M.; Ji, Y.; Wang, X.; Dou, S.; Yan, M.; Chen, W. Fusobacterium nucleatum-derived outer membrane vesicles promote immunotherapy resistance via changes in tryptophan metabolism in tumour-associated macrophages. J. Extracell. Vesicles 2025, 14, e70070. [Google Scholar] [CrossRef]
- Ding, T.; Chen, Q.; Liu, H.; Zhang, H.; Sun, Y.; Zhao, L.; Gao, Y.; Wei, Q. Single-cell RNA sequencing analysis reveals the distinct features of colorectal cancer with or without Fusobacterium nucleatum infection in PD-L1 blockade therapy. Heliyon 2024, 10, e37511. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Wang, X.; Ma, X.; Xu, M.; Li, J. Potential of natural products and gut microbiome in tumor immunotherapy. Chin. Med. 2024, 19, 1032. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, M.; Wu, Y.; Xin, Y.; Gao, L.; Elsabahy, M.; Wang, X.; Zhang, J.; Qu, X.; Gao, H. Multifunctional nanodrug for simultaneously combating chemoresistance and immunosuppression in Fusobacterium nucleatum-associated colorectal cancer. Acta Biomater. 2025, 185, 67–82. [Google Scholar] [CrossRef]
- Consolo, P.; Giorgi, C.; Crisafulli, C.; Fiorica, F.; Pinton, P.; Maurea, N.; Missiroli, S.; Quagliariello, V.; Mantoan, B.; Ottaiano, A.; et al. Investigating the impact of Fusobacterium nucleatum on oxidative stress, chemoresistance, and inflammation in inflammatory bowel disease and colorectal cancer: Rationale and design of a clinical trial. Int. J. Mol. Sci. 2025, 26, 7823. [Google Scholar] [CrossRef] [PubMed]
- Galasso, L.; Termite, F.; Mignini, I.; Esposto, G.; Borriello, R.; Vitale, F.; Nicoletti, A.; Paratore, M.; Ainora, M.E.; Gasbarrini, A.; et al. Unraveling the role of Fusobacterium nucleatum in colorectal cancer: Molecular mechanisms and pathogenic insights. Cancers 2025, 17, 368. [Google Scholar] [CrossRef]
- Chen, Y.W.; Camacho, M.I.; Chen, Y.; Bhat, A.H.; Chang, C.; Peluso, E.A.; Wu, C.; Das, A.; Ton-That, H. Genetic Determinants of Hydrogen Sulfide Biosynthesis in Fusobacterium nucleatum Are Required for Bacterial Fitness, Antibiotic Sensitivity, and Virulence. mBio 2022, 13, e0193622. [Google Scholar] [CrossRef]
- Peng, B.J.; Cao, C.Y.; Li, W.; Zhou, Y.J.; Zhang, Y.; Nie, Y.Q.; Cao, Y.W.; Li, Y.Y. Diagnostic Performance of Intestinal Fusobacterium Nucleatum in Colorectal Cancer: A Meta-Analysis. Chin. Med. J. 2018, 131, 1349–1356. [Google Scholar] [CrossRef]
- Janati, A.I.; Karp, I.; Laprise, C.; Sabri, H.; Emami, E. Detection of Fusobaterium nucleatum in feces and colorectal mucosa as a risk factor for colorectal cancer: A systematic review and meta-analysis. Syst. Rev. 2020, 9, 276. [Google Scholar] [CrossRef]
- Li, D.H.; Li, Z.W.; Sun, Q.; Wang, L.; Ning, S.B. Lower fecal microbiota transplantation ameliorates ulcerative colitis by eliminating oral-derived Fusobacterium nucleatum and virulence factor. Gut Pathog. 2024, 16, 42. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, X.; Cao, Y.; Fang, J.Y.; Hong, J.; Chen, H. Fecal Fusobacterium nucleatum for the diagnosis of colorectal tumor: A systematic review and meta-analysis. Cancer Med. 2019, 8, 480–491. [Google Scholar] [CrossRef]
- Liu, K.; Yang, X.; Zeng, M.; Yuan, Y.; Sun, J.; He, P.; Sun, J.; Xie, Q.; Chang, X.; Zhang, S.; et al. The Role of Fecal Fusobacterium nucleatum and Pks+Escherichia coli as Early Diagnostic Markers of Colorectal Cancer. Dis. Markers 2021, 2021, 1171239. [Google Scholar] [CrossRef]
- Wang, H.F.; Li, L.F.; Guo, S.H.; Zeng, Q.Y.; Ning, F.; Liu, W.L.; Zhang, G. Evaluation of Antibody Level against Fusobacterium nucleatum in the Serological Diagnosis of Colorectal Cancer. Sci. Rep. 2016, 6, 33440. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Y.; Guo, P.; Wei, Y. Construction and validation of a novel angiogenesis pattern to predict prognosis and immunotherapy efficacy in colorectal cancer. Aging 2023, 15, 12413–12450. [Google Scholar] [CrossRef] [PubMed]
- Farhadi Rad, H.; Tahmasebi, H.; Javani, S.; Hemati, M.; Zakerhamidi, D.; Hosseini, M.; Alibabaei, F.; Banihashemian, S.Z.; Oksenych, V.; Eslami, M. Microbiota and Cytokine Modulation: Innovations in Enhancing Anticancer Immunity and Personalized Cancer Therapies. Biomedicines 2024, 12, 2776. [Google Scholar] [CrossRef] [PubMed]
- Mo, S.; Wang, H.; Han, L.; Xiang, W.; Dai, W.; Zhao, P.; Pei, F.; Su, Z.; Ma, C.; Li, Q.; et al. Fecal Multidimensional Assay for Non-Invasive Detection of Colorectal Cancer: Fecal Immunochemical Test, Stool DNA Mutation, Methylation, and Intestinal Bacteria Analysis. Front. Oncol. 2021, 11, 643136. [Google Scholar] [CrossRef]
- Liang, J.Q.; Wong, S.H.; Szeto, C.H.; Chu, E.S.H.; Lau, H.C.; Chen, Y.; Fang, J.; Yu, J.; Sung, J.J.Y. Fecal Microbial DNA Markers Serve for Screening Colorectal Neoplasm in Asymptomatic Subjects. J. Gastroenterol. Hepatol. 2021, 36, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Zhang, Y.; Xiang, L.; You, Y.; Duan, Y.; Zhao, Y.; Li, S.; Wu, R.; Zhang, J.; Zhou, L.; et al. Fusobacterium nucleatum-triggered neutrophil extracellular traps facilitate colorectal carcinoma progression. J. Exp. Clin. Cancer Res. 2023, 42, 236. [Google Scholar] [CrossRef]
- Tito, R.Y.; Verbandt, S.; Aguirre Vazquez, M.; Lahti, L.; Verspecht, C.; Lloréns-Rico, V.; Vieira-Silva, S.; Arts, J.; Falony, G.; Dekker, E.; et al. Microbiome confounders and quantitative profiling challenge predicted microbial targets in colorectal cancer development. Nat. Med. 2024, 30, 1339–1348. [Google Scholar] [CrossRef]
- Cao, L.J.; Peng, X.L.; Xue, W.Q.; Zhang, R.; Zhang, J.B.; Zhou, T.; Wu, Z.Y.; Li, G.R.; Wang, T.M.; He, Y.Q.; et al. A Fecal-Based Test for the Detection of Advanced Adenoma and Colorectal Cancer: A Case-Control and Screening Cohort Study. BMC Med. 2021, 19, 250. [Google Scholar] [CrossRef]
- Amitay, E.L.; Krilaviciute, A.; Brenner, H. Systematic review: Gut microbiota in fecal samples and detection of colorectal neoplasms. Gut Microbes 2018, 9, 293–307. [Google Scholar] [CrossRef]
- Yu, J.; Feng, Q.; Wong, S.H.; Zhang, D.; Yi Liang, Q.; Qin, Y.; Tang, L.; Zhao, H.; Stenvang, J.; Li, Y.; et al. Metagenomic Analysis of Faecal Microbiome as a Tool towards Targeted Non-Invasive Biomarkers for Colorectal Cancer. Gut 2017, 66, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Parhi, L.; Alon-Maimon, T.; Sol, A.; Nejman, D.; Shhadeh, A.; Fainsod-Levi, T.; Yajuk, O.; Isaacson, B.; Abed, J.; Maalouf, N.; et al. Breast cancer colonization by Fusobacterium nucleatum accelerates tumor growth and metastatic progression. Nat. Commun. 2020, 11, 3259. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Cho, N.Y.; Kang, G.H. Prognostic and clinicopathological significance of Fusobacterium nucleatum in colorectal cancer: A systemic review and meta-analysis. J. Pathol. Transl. Med. 2022, 56, 144–151. [Google Scholar] [CrossRef] [PubMed]

| Mechanism | Molecular Pathway | Role in CRC | Evidence |
|---|---|---|---|
| FadA | E-cadherin/β-catenin/TCF4 | Adhesion, internalization, oncogenic signaling | Preclinical |
| Fap2 | Gal-GalNAc binding; TIGIT receptor | Tumor colonization, lymphocyte inhibition, immune evasion | Preclinical and human |
| RadD | Autotransporter protein | Biofilm formation, lymphocyte killing, immune modulation | Preclinical |
| Fn-derived succinic acid | cGAS–STING–IFN-β | Inhibits DC activation, T-cell priming; PD-1 resistance | Preclinical |
| miR-21 | Upregulated by Fn | Invasion, proliferation, metastasis | Preclinical and clinical |
| NF-κB/CCL20 | NF-κB activation; ↓ miR-1322 | TAM recruitment, M2 polarization, metastatic niche | Preclinical |
| Cytokines/NETs | IL-8, CXCL1, NETs | Angiogenesis, EMT, metastasis | Preclinical and translational |
| Strategy | Mechanism/Target | Preclinical Evidence | Clinical Status/ Limitations |
|---|---|---|---|
| Antibiotics (e.g., metronidazole) | Reduce intratumoral Fn; modulate TLR4–MYD88 | Reduced Fn load and tumor growth in murine CRC | No CRC-specific trials; microbiota disruption risk |
| Bacteriophages | Selective Fn lysis | Effective killing in vitro | Early preclinical; no in vivo CRC data |
| Nanodrug delivery (e.g., metronidazole and JQ1) | Overcome chemoresistance and immunosuppression | Enhanced oxaliplatin efficacy, ↑ CD8+ T cells in mice | Preclinical only; no human data |
| Probiotics/FMT | Restore microbiota balance; ↓ Fn colonization | Reduced Fn in colitis/CRC models | Limited translational studies; safety unproven |
| Natural products (inulin, anthocyanins, ginsenosides) | Enhance SCFA-producers; immunomodulation; ↓ Fn inflammation | ↑ CD8+ T-cell infiltration, ↓ tumor burden in models | Preclinical and observational; no randomized trials |
| Immune checkpoint modulation | Block Fn mediated TIGIT; reverse immunosuppression | Improved PD-1/PD-L1 blockade in mice | Conceptual; no targeted clinical trials |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Incognito, D.; Ciappina, G.; Gelsomino, C.; Picone, A.; Consolo, P.; Scano, A.; Franchina, T.; Maurea, N.; Quagliariello, V.; Berretta, S.; et al. Fusobacterium Nucleatum in Colorectal Cancer: Relationship Among Immune Modulation, Potential Biomarkers and Therapeutic Implications. Int. J. Mol. Sci. 2025, 26, 9710. https://doi.org/10.3390/ijms26199710
Incognito D, Ciappina G, Gelsomino C, Picone A, Consolo P, Scano A, Franchina T, Maurea N, Quagliariello V, Berretta S, et al. Fusobacterium Nucleatum in Colorectal Cancer: Relationship Among Immune Modulation, Potential Biomarkers and Therapeutic Implications. International Journal of Molecular Sciences. 2025; 26(19):9710. https://doi.org/10.3390/ijms26199710
Chicago/Turabian StyleIncognito, Dalila, Giuliana Ciappina, Claudia Gelsomino, Antonio Picone, Pierluigi Consolo, Alessandra Scano, Tindara Franchina, Nicola Maurea, Vincenzo Quagliariello, Salvatore Berretta, and et al. 2025. "Fusobacterium Nucleatum in Colorectal Cancer: Relationship Among Immune Modulation, Potential Biomarkers and Therapeutic Implications" International Journal of Molecular Sciences 26, no. 19: 9710. https://doi.org/10.3390/ijms26199710
APA StyleIncognito, D., Ciappina, G., Gelsomino, C., Picone, A., Consolo, P., Scano, A., Franchina, T., Maurea, N., Quagliariello, V., Berretta, S., Ottaiano, A., & Berretta, M. (2025). Fusobacterium Nucleatum in Colorectal Cancer: Relationship Among Immune Modulation, Potential Biomarkers and Therapeutic Implications. International Journal of Molecular Sciences, 26(19), 9710. https://doi.org/10.3390/ijms26199710










