Organ-Specific Extracellular Vesicles in the Treatment of Ischemic Acute Organ Injury: Mechanisms, Successes, and Prospects
Abstract
1. Introduction
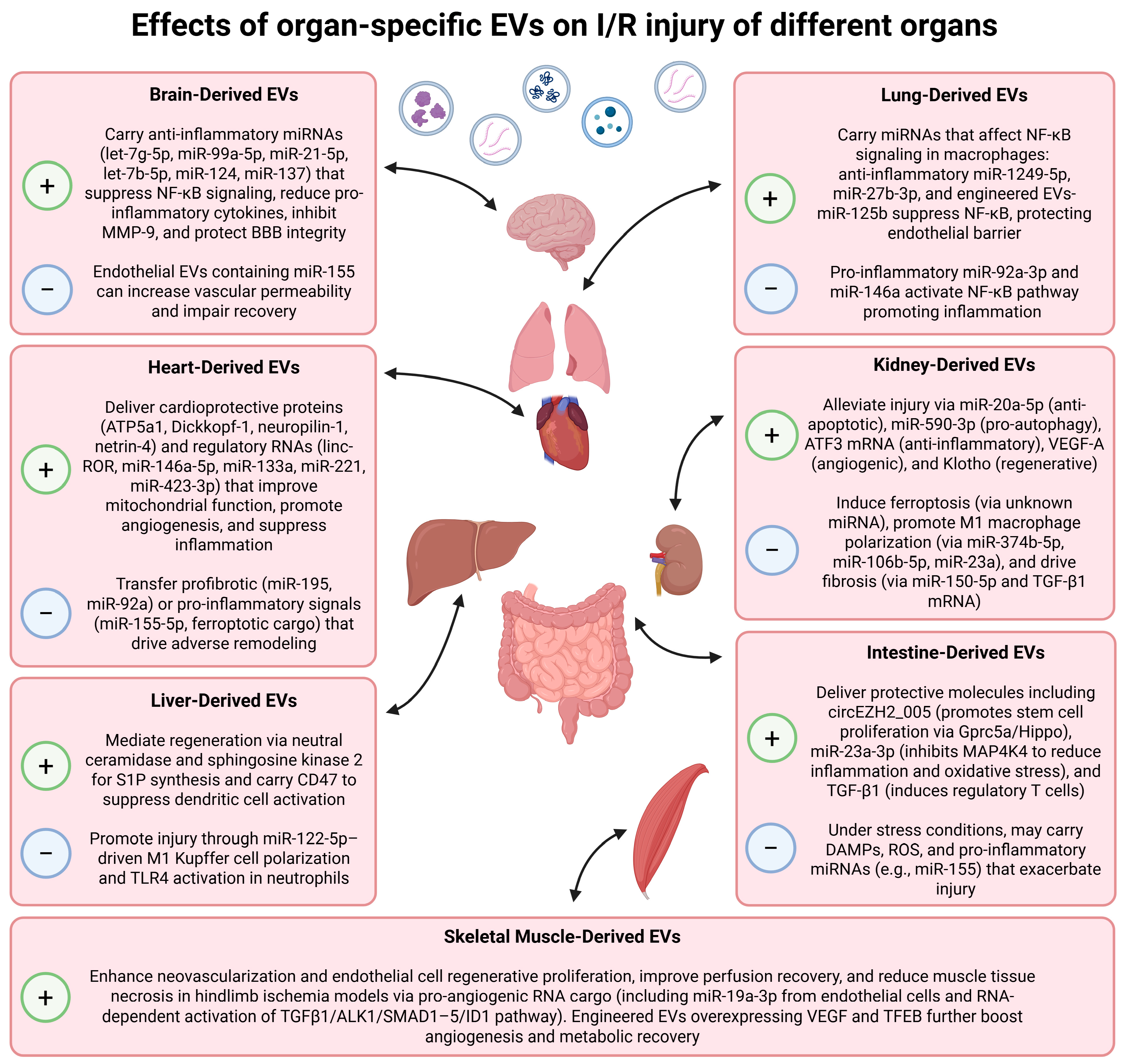
2. Heart-Derived EVs
2.1. Cardiomyocyte-Derived EVs
2.2. Cardiac Fibroblasts-Derived EVs
2.3. Endothelial-Cell-Derived EVs
2.4. Advantages over MSC-EVs
3. Brain-Derived EVs
3.1. Neural Progenitor Cells-Derived EVs
3.2. Astrocyte- and Microglia-Derived EVs
3.3. Endothelial Cells-Derived EVs
4. Kidney-Derived EVs
4.1. EVs Produced by Proximal TECs
4.2. EVs Produced by Podocytes
4.3. Vesicles in Urine
5. Liver-Derived EVs
6. Intestine-Derived EVs
7. Skeletal-Muscle-Derived EVs
8. Lung-Derived EVs
9. Challenges and Future Directions of Therapy Mediated by Organ-Specific EVs
9.1. Biodistribution of Organ-Specific EVs
9.2. Isolation Challenges and Scalable Production Technologies
9.3. Clinical Translation: Current Status of EV-Based Trials
9.4. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eltzschig, H.K.; Eckle, T. Ischemia and Reperfusion—From Mechanism to Translation. Nat. Med. 2011, 17, 1391–1401. [Google Scholar] [CrossRef]
- Yellon, D.M.; Hausenloy, D.J. Myocardial Reperfusion Injury. N. Engl. J. Med. 2007, 357, 1121–1135. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, J.; Lu, Q.; Huang, K.; Yang, B.; Reilly, J.; Jiang, N.; Shu, X.; Shang, L. An Update on the Functional Roles of Long Non-Coding RNAs in Ischemic Injury (Review). Int. J. Mol. Med. 2022, 50, 91. [Google Scholar] [CrossRef]
- Zhang, K.; Li, R.; Chen, X.; Yan, H.; Li, H.; Zhao, X.; Huang, H.; Chen, S.; Liu, Y.; Wang, K.; et al. Renal Endothelial Cell-Targeted Extracellular Vesicles Protect the Kidney from Ischemic Injury. Adv. Sci. 2023, 10, e2204626. [Google Scholar] [CrossRef] [PubMed]
- Nastos, C.; Kalimeris, K.; Papoutsidakis, N.; Tasoulis, M.-K.; Lykoudis, P.M.; Theodoraki, K.; Nastou, D.; Smyrniotis, V.; Arkadopoulos, N. Global Consequences of Liver Ischemia/reperfusion Injury. Oxid. Med. Cell. Longev. 2014, 2014, 906965. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Yu, Y.; Luo, X.; Wang, J.; Lan, X.; Liu, P.; Feng, Y.; Jian, W. Myocardial Ischemia-Reperfusion Injury: Therapeutics from a Mitochondria-Centric Perspective. Cardiology 2021, 146, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Kim, M.; Brown, K.M.; D’Agati, V.D.; Lee, T.H. Paneth Cell-Derived Interleukin-17A Causes Multiorgan Dysfunction after Hepatic Ischemia and Reperfusion Injury. Hepatology 2011, 53, 1662–1675. [Google Scholar] [CrossRef]
- Zhao, Q.; Yan, T.; Chopp, M.; Venkat, P.; Chen, J. Brain–Kidney Interaction: Renal Dysfunction Following Ischemic Stroke. J. Cereb. Blood Flow. Metab. 2020, 40, 246–262. [Google Scholar] [CrossRef]
- Du, S.; Guan, Y.; Xie, A.; Yan, Z.; Gao, S.; Li, W.; Rao, L.; Chen, X.; Chen, T. Extracellular Vesicles: A Rising Star for Therapeutics and Drug Delivery. J. Nanobiotechnol. 2023, 21, 231. [Google Scholar] [CrossRef]
- Beetler, D.J.; Di Florio, D.N.; Bruno, K.A.; Ikezu, T.; March, K.L.; Cooper, L.T., Jr.; Wolfram, J.; Fairweather, D. Extracellular Vesicles as Personalized Medicine. Mol. Aspects Med. 2023, 91, 101155. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Yang, Z.; Wang, B.; Gong, H.; Zhang, K.; Lin, Y.; Sun, M. Extracellular Vesicles: Biological Mechanisms and Emerging Therapeutic Opportunities in Neurodegenerative Diseases. Transl. Neurodegener. 2024, 13, 60. [Google Scholar] [CrossRef]
- Meng, W.; He, C.; Hao, Y.; Wang, L.; Li, L.; Zhu, G. Prospects and Challenges of Extracellular Vesicle-Based Drug Delivery System: Considering Cell Source. Drug Deliv. 2020, 27, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, J.H.; Xie, D.; Kelly, K.J. Renal, but Not Platelet or Skin, Extracellular Vesicles Decrease Oxidative Stress, Enhance Nascent Peptide Synthesis, and Protect from Ischemic Renal Injury. Am. J. Physiol. Renal Physiol. 2023, 325, F164–F176. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Liejendekker, R.; Harding, C.V.; Melief, C.J.; Geuze, H.J. B Lymphocytes Secrete Antigen-Presenting Vesicles. J. Exp. Med. 1996, 183, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Shi, W.; Yu, H.; Feng, Z.; Wei, Z.; He, W.; Gou, X.; Xie, Y. Small Extracellular Vesicles from Human Umbilical Cord Mesenchymal Stem Cells Delivering miR-202-5p Alleviate Renal Ischemia-Reperfusion Injury by Targeting the GOLIM4/PI3K/AKT Axis. Front. Immunol. 2025, 16, 1586174. [Google Scholar] [CrossRef]
- Nojima, H.; Freeman, C.M.; Schuster, R.M.; Japtok, L.; Kleuser, B.; Edwards, M.J.; Gulbins, E.; Lentsch, A.B. Hepatocyte Exosomes Mediate Liver Repair and Regeneration via Sphingosine-1-Phosphate. J. Hepatol. 2016, 64, 60–68. [Google Scholar] [CrossRef]
- EL Andaloussi, S.; Mäger, I.; Breakefield, X.O.; Wood, M.J.A. Extracellular Vesicles: Biology and Emerging Therapeutic Opportunities. Nat. Rev. Drug Discov. 2013, 12, 347–357. [Google Scholar] [CrossRef]
- Fareez, I.M.; Seng, W.Y.; Zaki, R.M.; Shafiq, A.; Izwan, I.M. Molecular and Epigenetic Basis of Extracellular Vesicles Cell Repair Phenotypes in Targeted Organ-Specific Regeneration. Curr. Mol. Med. 2022, 22, 132–150. [Google Scholar] [CrossRef]
- Li, X.-Q.; Liu, J.-F.; Liu, H.; Meng, Y. Extracellular Vesicles for Ischemia/Reperfusion Injury-Induced Acute Kidney Injury: A Systematic Review and Meta-Analysis of Data from Animal Models. Syst. Rev. 2022, 11, 197. [Google Scholar] [CrossRef]
- Eirin, A.; Lerman, L.O. Mesenchymal Stem/Stromal Cell-Derived Extracellular Vesicles for Chronic Kidney Disease: Are We There Yet? Hypertension 2021, 78, 261–269. [Google Scholar] [CrossRef]
- Shen, H.; Chen, J.; Liu, M.; Zhao, M.; Hu, D.; Xie, F.; Jin, Q.; Xiao, D.; Peng, Z.; Qin, T.; et al. Research Progress of Extracellular Vesicles Derived from Mesenchymal Stem Cells in the Treatment of Neurodegenerative Diseases. Front. Immunol. 2025, 16, 1496304. [Google Scholar] [CrossRef]
- Kahmini, F.R.; Shahgaldi, S. Therapeutic Potential of Mesenchymal Stem Cell-Derived Extracellular Vesicles as Novel Cell-Free Therapy for Treatment of Autoimmune Disorders. Exp. Mol. Pathol. 2021, 118, 104566. [Google Scholar] [CrossRef]
- Linxweiler, J.; Kolbinger, A.; Himbert, D.; Zeuschner, P.; Saar, M.; Stöckle, M.; Junker, K. Organ-Specific Uptake of Extracellular Vesicles Secreted by Urological Cancer Cells. Cancers 2021, 13, 4937. [Google Scholar] [CrossRef] [PubMed]
- French, K.C.; Antonyak, M.A.; Cerione, R.A. Extracellular Vesicle Docking at the Cellular Port: Extracellular Vesicle Binding and Uptake. Semin. Cell Dev. Biol. 2017, 67, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Sluijter, J.P.G.; Verhage, V.; Deddens, J.C.; van den Akker, F.; Doevendans, P.A. Microvesicles and Exosomes for Intracardiac Communication. Cardiovasc. Res. 2014, 102, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Ontoria-Oviedo, I.; Dorronsoro, A.; Sánchez, R.; Ciria, M.; Gómez-Ferrer, M.; Buigues, M.; Grueso, E.; Tejedor, S.; García-García, F.; González-King, H.; et al. Extracellular Vesicles Secreted by Hypoxic AC10 Cardiomyocytes Modulate Fibroblast Cell Motility. Front. Cardiovasc. Med. 2018, 5, 152. [Google Scholar] [CrossRef]
- Martins-Marques, T.; Ribeiro-Rodrigues, T.; de Jager, S.C.; Zuzarte, M.; Ferreira, C.; Cruz, P.; Reis, L.; Baptista, R.; Gonçalves, L.; Sluijter, J.P.; et al. Myocardial Infarction Affects Cx43 Content of Extracellular Vesicles Secreted by Cardiomyocytes. Life Sci. Alliance 2020, 3, e202000821. [Google Scholar] [CrossRef]
- Morelli, M.B.; Shu, J.; Sardu, C.; Matarese, A.; Santulli, G. Cardiosomal microRNAs Are Essential in Post-Infarction Myofibroblast Phenoconversion. Int. J. Mol. Sci. 2019, 21, 201. [Google Scholar] [CrossRef]
- Wang, X.; Morelli, M.B.; Matarese, A.; Sardu, C.; Santulli, G. Cardiomyocyte-Derived Exosomal microRNA-92a Mediates Post-Ischemic Myofibroblast Activation Both in Vitro and Ex Vivo. ESC Heart Fail. 2020, 7, 284–288. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Orbe, J.; Saenz-Pipaon, G.; Abizanda, G.; Gebara, N.; Radulescu, F.; Azcarate, P.M.; Alonso-Perez, L.; Merino, D.; Prosper, F.; et al. Selective Increase of Cardiomyocyte Derived Extracellular Vesicles after Experimental Myocardial Infarction and Functional Effects on the Endothelium. Thromb. Res. 2018, 170, 1–9. [Google Scholar] [CrossRef]
- Chen, G.; Xu, C.; Gillette, T.G.; Huang, T.; Huang, P.; Li, Q.; Li, X.; Li, Q.; Ning, Y.; Tang, R.; et al. Cardiomyocyte-Derived Small Extracellular Vesicles Can Signal eNOS Activation in Cardiac Microvascular Endothelial Cells to Protect against Ischemia/Reperfusion Injury. Theranostics 2020, 10, 11754–11774. [Google Scholar] [CrossRef]
- Chen, C.; Cai, S.; Wu, M.; Wang, R.; Liu, M.; Cao, G.; Dong, M.; Yiu, K.-H. Role of Cardiomyocyte-Derived Exosomal MicroRNA-146a-5p in Macrophage Polarization and Activation. Dis. Markers 2022, 2022, 2948578. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Meng, Q.; Wei, L.; Liu, J.; Li, M.; Liang, X.; Lin, F.; Zhang, Y.; Li, Y.; Liu, Z.; et al. Myocardial Ischemia-Reperfusion Induced Cardiac Extracellular Vesicles Harbour Proinflammatory Features and Aggravate Heart Injury. J. Extracell. Vesicles 2021, 10, e12072. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Wu, Y.; Maimaitijiang, A.; Huang, Q.; Chen, Q. Ferroptotic Cardiomyocyte-Derived Exosomes Promote Cardiac Macrophage M1 Polarization during Myocardial Infarction. PeerJ 2022, 10, e13717. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, Y.; Lin, Y.; Li, S.; Liu, C.; Cai, A.; Li, W.; Zhang, W.; Gao, X.; Ren, Z.; et al. Cardiac Repair Using Regenerating Neonatal Heart Tissue-Derived Extracellular Vesicles. Nat. Commun. 2025, 16, 1292. [Google Scholar] [CrossRef]
- Liu, X.; Meng, Q.; Shi, S.; Geng, X.; Wang, E.; Li, Y.; Lin, F.; Liang, X.; Xi, X.; Han, W.; et al. Cardiac-Derived Extracellular Vesicles Improve Mitochondrial Function to Protect the Heart Against Ischemia/Reperfusion Injury by Delivering ATP5a1. J. Nanobiotechnol. 2024, 22, 385. [Google Scholar] [CrossRef]
- Abrial, M.; Da Silva, C.C.; Pillot, B.; Augeul, L.; Ivanes, F.; Teixeira, G.; Cartier, R.; Angoulvant, D.; Ovize, M.; Ferrera, R. Cardiac Fibroblasts Protect Cardiomyocytes Against Lethal Ischemia-Reperfusion Injury. J. Mol. Cell Cardiol. 2014, 68, 56–65. [Google Scholar] [CrossRef]
- Hao, C.; Lu, Z.; Zhao, Y.; Chen, Z.; Shen, C.; Ma, G.; Chen, L. Overexpression of GATA4 Enhances the Antiapoptotic Effect of Exosomes Secreted from Cardiac Colony-Forming Unit Fibroblasts via miRNA221-Mediated Targeting of the PTEN/PI3K/AKT Signaling Pathway. Stem Cell Res. Ther. 2020, 11, 251. [Google Scholar] [CrossRef]
- Liu, N.; Xie, L.; Xiao, P.; Chen, X.; Kong, W.; Lou, Q.; Chen, F.; Lu, X. Cardiac Fibroblasts Secrete Exosome microRNA to Suppress Cardiomyocyte Pyroptosis in Myocardial Ischemia/Reperfusion Injury. Mol. Cell Biochem. 2022, 477, 1249–1260. [Google Scholar] [CrossRef]
- Luo, H.; Li, X.; Li, T.; Zhao, L.; He, J.; Zha, L.; Qi, Q.; Yu, Z. microRNA-423-3p Exosomes Derived from Cardiac Fibroblasts Mediates the Cardioprotective Effects of Ischaemic Post-Conditioning. Cardiovasc. Res. 2019, 115, 1189–1204. [Google Scholar] [CrossRef]
- Davidson, S.M.; Riquelme, J.A.; Zheng, Y.; Vicencio, J.M.; Lavandero, S.; Yellon, D.M. Endothelial Cells Release Cardioprotective Exosomes That May Contribute to Ischaemic Preconditioning. Sci. Rep. 2018, 8, 15885. [Google Scholar] [CrossRef]
- Penna, C.; Femminò, S.; Tapparo, M.; Lopatina, T.; Fladmark, K.E.; Ravera, F.; Comità, S.; Alloatti, G.; Giusti, I.; Dolo, V.; et al. The Inflammatory Cytokine IL-3 Hampers Cardioprotection Mediated by Endothelial Cell-Derived Extracellular Vesicles Possibly via Their Protein Cargo. Cells 2020, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Zhang, W.; Yin, Y.; Wei, Z.; Chen, F.; Zhao, J.; Sun, X.; Mu, D.; Xie, J.; Xu, B. Extracellular Vesicles Derived from Krüppel-Like Factor 2-Overexpressing Endothelial Cells Attenuate Myocardial Ischemia-Reperfusion Injury by Preventing Ly6C Monocyte Recruitment. Theranostics 2020, 10, 11562–11579. [Google Scholar] [CrossRef] [PubMed]
- Gollmann-Tepeköylü, C.; Pölzl, L.; Graber, M.; Hirsch, J.; Nägele, F.; Lobenwein, D.; Hess, M.W.; Blumer, M.J.; Kirchmair, E.; Zipperle, J.; et al. miR-19a-3p Containing Exosomes Improve Function of Ischaemic Myocardium upon Shock Wave Therapy. Cardiovasc. Res. 2020, 116, 1226–1236. [Google Scholar] [CrossRef] [PubMed]
- Akbar, N.; Digby, J.E.; Cahill, T.J.; Tavare, A.N.; Corbin, A.L.; Saluja, S.; Dawkins, S.; Edgar, L.; Rawlings, N.; Ziberna, K.; et al. Endothelium-Derived Extracellular Vesicles Promote Splenic Monocyte Mobilization in Myocardial Infarction. JCI Insight 2017, 2, e93344. [Google Scholar] [CrossRef]
- Liu, B.; Lee, B.W.; Nakanishi, K.; Villasante, A.; Williamson, R.; Metz, J.; Kim, J.; Kanai, M.; Bi, L.; Brown, K.; et al. Cardiac Recovery via Extended Cell-Free Delivery of Extracellular Vesicles Secreted by Cardiomyocytes Derived from Induced Pluripotent Stem Cells. Nat. Biomed. Eng. 2018, 2, 293–303. [Google Scholar] [CrossRef]
- Nawaz, M.; Tangruksa, B.; Heydarkhan-Hagvall, S.; Kohl, F.; Gonzalez-King Garibotti, H.; Jing, Y.; Payandeh, Z.; Reyahi, A.; Jennbacken, K.; Wiseman, J.; et al. Targeted Delivery of mRNA to the Heart via Extracellular Vesicles or Lipid Nanoparticles. bioRxiv 2025. [Google Scholar] [CrossRef]
- Tian, T.; Cao, L.; He, C.; Ye, Q.; Liang, R.; You, W.; Zhang, H.; Wu, J.; Ye, J.; Tannous, B.A.; et al. Targeted Delivery of Neural Progenitor Cell-Derived Extracellular Vesicles for Anti-Inflammation After Cerebral Ischemia. Theranostics 2021, 11, 6507–6521. [Google Scholar] [CrossRef]
- Zhang, L.; Graf, I.; Kuang, Y.; Zheng, X.; Haupt, M.; Majid, A.; Kilic, E.; Hermann, D.M.; Psychogios, M.-N.; Weber, M.S.; et al. Neural Progenitor Cell-Derived Extracellular Vesicles Enhance Blood-Brain Barrier Integrity by NF-κB (Nuclear Factor-κB)-Dependent Regulation of ABCB1 (ATP-Binding Cassette Transporter B1) in Stroke Mice. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 1127–1145. [Google Scholar] [CrossRef]
- Hira, K.; Ueno, Y.; Tanaka, R.; Miyamoto, N.; Yamashiro, K.; Inaba, T.; Urabe, T.; Okano, H.; Hattori, N. Astrocyte-Derived Exosomes Treated with a Semaphorin 3A Inhibitor Enhance Stroke Recovery via Prostaglandin D2 Synthase. Stroke 2018, 49, 2483–2494. [Google Scholar] [CrossRef]
- Zhang, D.; Cai, G.; Liu, K.; Zhuang, Z.; Jia, K.; Pei, S.; Wang, X.; Wang, H.; Xu, S.; Cui, C.; et al. Microglia Exosomal miRNA-137 Attenuates Ischemic Brain Injury Through Targeting Notch1. Aging 2021, 13, 4079–4095. [Google Scholar] [CrossRef]
- Mohamud Yusuf, A.; Hagemann, N.; Zhang, X.; Zafar, M.; Hussner, T.; Bromkamp, C.; Martiny, C.; Tertel, T.; Börger, V.; Schumacher, F.; et al. Acid Sphingomyelinase Deactivation Post-Ischemia Promotes Brain Angiogenesis and Remodeling by Small Extracellular Vesicles. Basic Res. Cardiol. 2022, 117, 43. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Zhou, S.; Sun, C.; Cheng, D.; Zhang, Y.; Li, X.; Zhang, L.; Zhao, J.; Xu, D.; Bai, Y. Brain Endothelial Cell-Derived Exosomes Induce Neuroplasticity in Rats with Ischemia/Reperfusion Injury. ACS Chem. Neurosci. 2020, 11, 2201–2213. [Google Scholar] [CrossRef] [PubMed]
- Venkat, P.; Cui, C.; Chopp, M.; Zacharek, A.; Wang, F.; Landschoot-Ward, J.; Shen, Y.; Chen, J. MiR-126 Mediates Brain Endothelial Cell Exosome Treatment-Induced Neurorestorative Effects After Stroke in Type 2 Diabetes Mellitus Mice. Stroke 2019, 50, 2865–2874. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, B.; Zhao, T.; Quan, X.; Han, Y.; Cheng, Y.; Chen, Y.; Shen, X.; Zheng, Y.; Zhao, Y. Comparative Study of Extracellular Vesicles Derived from Mesenchymal Stem Cells and Brain Endothelial Cells Attenuating Blood-Brain Barrier Permeability via Regulating Caveolin-1-Dependent ZO-1 and Claudin-5 Endocytosis in Acute Ischemic Stroke. J. Nanobiotechnol. 2023, 21, 70. [Google Scholar] [CrossRef]
- Zagrean, A.-M.; Hermann, D.M.; Opris, I.; Zagrean, L.; Popa-Wagner, A. Multicellular Crosstalk Between Exosomes and the Neurovascular Unit After Cerebral Ischemia. Therapeutic Implications. Front. Neurosci. 2018, 12, 811. [Google Scholar] [CrossRef]
- Wang, X.; Kim, C.S.; Adams, B.C.; Wilkinson, R.; Hill, M.M.; Shah, A.K.; Mohamed, A.; Dutt, M.; Ng, M.S.Y.; Ungerer, J.P.J.; et al. Human Proximal Tubular Epithelial Cell-Derived Small Extracellular Vesicles Mediate Synchronized Tubular Ferroptosis in Hypoxic Kidney Injury. Redox Biol. 2024, 70, 103042. [Google Scholar] [CrossRef]
- Dominguez, J.M., 2nd; Dominguez, J.H.; Xie, D.; Kelly, K.J. Human Extracellular Microvesicles from Renal Tubules Reverse Kidney Ischemia-Reperfusion Injury in Rats. PLoS ONE 2018, 13, e0202550. [Google Scholar] [CrossRef]
- Dominguez, J.H.; Liu, Y.; Gao, H.; Dominguez, J.M., 2nd; Xie, D.; Kelly, K.J. Renal Tubular Cell-Derived Extracellular Vesicles Accelerate the Recovery of Established Renal Ischemia Reperfusion Injury. J. Am. Soc. Nephrol. 2017, 28, 3533–3544. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, H.; Xu, K.; Ling, Z.; Huang, Y.; Hu, Q.; Lu, K.; Liu, C.; Wang, Y.; Liu, N.; et al. Hypoxia Preconditioned Renal Tubular Epithelial Cell-Derived Extracellular Vesicles Alleviate Renal Ischaemia-Reperfusion Injury Mediated by the HIF-1α/Rab22 Pathway and Potentially Affected by microRNAs. Int. J. Biol. Sci. 2019, 15, 1161–1176. [Google Scholar] [CrossRef]
- Yu, W.; Zeng, H.; Chen, J.; Fu, S.; Huang, Q.; Xu, Y.; Xu, A.; Lan, H.-Y.; Tang, Y. miR-20a-5p Is Enriched in Hypoxia-Derived Tubular Exosomes and Protects against Acute Tubular Injury. Clin. Sci. 2020, 134, 2223–2234. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhou, X.; Yao, Q.; Liu, Y.; Zhang, H.; Dong, Z. HIF-1-Mediated Production of Exosomes during Hypoxia Is Protective in Renal Tubular Cells. Am. J. Physiol.-Ren. Physiol. 2017, 313, F906. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, C.; Du, Y.; Yang, X.; Liu, M.; Yang, W.; Lei, G.; Wang, G. Exosomal Transfer of microRNA-590-3p Between Renal Tubular Epithelial Cells After Renal Ischemia-Reperfusion Injury Regulates Autophagy by Targeting TRAF6. Chin. Med. J. 2022, 135, 2467. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-H.; Lai, P.-F.; Lan, Y.-F.; Cheng, C.-F.; Zhong, W.-B.; Lin, Y.-F.; Chen, T.-W.; Lin, H. Exosomal ATF3 RNA Attenuates Pro-Inflammatory Gene MCP-1 Transcription in Renal Ischemia-Reperfusion. J. Cell. Physiol. 2014, 229, 1202–1211. [Google Scholar] [CrossRef]
- Zhong, X.; Tang, T.-T.; Shen, A.-R.; Cao, J.-Y.; Jing, J.; Wang, C.; Zhu, X.-X.; Wen, Y.; Li, Z.-L.; Wang, B.; et al. Tubular Epithelial Cells-Derived Small Extracellular Vesicle-VEGF-A Promotes Peritubular Capillary Repair in Ischemic Kidney Injury. npj Regen. Med. 2022, 7, 73. [Google Scholar] [CrossRef]
- Ding, C.; Zheng, J.; Wang, B.; Li, Y.; Xiang, H.; Dou, M.; Qiao, Y.; Tian, P.; Ding, X.; Xue, W. Exosomal MicroRNA-374b-5p from Tubular Epithelial Cells Promoted M1 Macrophages Activation and Worsened Renal Ischemia/Reperfusion Injury. Front. Cell Dev. Biol. 2020, 8, 587693. [Google Scholar] [CrossRef]
- Li, X.; Zhong, Y.; Yue, R.; Xie, J.; Zhang, Y.; Lin, Y.; Li, H.; Xu, Y.; Zheng, D. Inhibition of MiR-106b-5p Mediated by Exosomes Mitigates Acute Kidney Injury by Modulating Transmissible Endoplasmic Reticulum Stress and M1 Macrophage Polarization. J. Cell. Mol. Med. 2023, 27, 2876–2889. [Google Scholar] [CrossRef]
- Li, Z.L.; Lv, L.L.; Tang, T.T.; Wang, B.; Feng, Y.; Zhou, L.T.; Cao, J.Y.; Tang, R.N.; Wu, M.; Liu, H.; et al. HIF-1α Inducing Exosomal microRNA-23a Expression Mediates the Cross-Talk Between Tubular Epithelial Cells and Macrophages in Tubulointerstitial Inflammation. Kidney Int. 2019, 95, 388–404. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, S.; Li, W.; Ruan, Y.; Yuan, R.; Ning, J.; Jiang, K.; Xie, J.; Yao, X.; Li, H.; et al. Tubular Cell-Derived Exosomal miR-150-5p Contributes to Renal Fibrosis Following Unilateral Ischemia-Reperfusion Injury by Activating Fibroblast In Vitro and In Vivo. Int. J. Biol. Sci. 2021, 17, 4021–4033. [Google Scholar] [CrossRef]
- Guan, H.; Peng, R.; Mao, L.; Fang, F.; Xu, B.; Chen, M. Injured Tubular Epithelial Cells Activate Fibroblasts to Promote Kidney Fibrosis Through miR-150-Containing Exosomes. Exp. Cell Res. 2020, 392, 112007. [Google Scholar] [CrossRef]
- Borges, F.T.; Melo, S.A.; Özdemir, B.C.; Kato, N.; Revuelta, I.; Miller, C.A.; Gattone, V.H.; LeBleu, V.S.; Kalluri, R. TGF-β1-Containing Exosomes from Injured Epithelial Cells Activate Fibroblasts to Initiate Tissue Regenerative Responses and Fibrosis. J. Am. Soc. Nephrol. JASN 2013, 24, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Munkonda, M.N.; Akbari, S.; Landry, C.; Sun, S.; Xiao, F.; Turner, M.; Holterman, C.E.; Nasrallah, R.; Hébert, R.L.; Kennedy, C.R.J.; et al. Podocyte-Derived Microparticles Promote Proximal Tubule Fibrotic Signaling via p38 MAPK and CD36. J. Extracell. Vesicles 2018, 7, 1432206. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, X.; Zhou, Y.; Wen, X.; Xu, J.; He, M.; Chen, J.; Jia, N.; Liu, Y. Extracellular Vesicles Play a Central Role in Linking Podocyte Injury to Mesangial Activation in Glomerular Disease. Theranostics 2025, 15, 5121–5137. [Google Scholar] [CrossRef] [PubMed]
- Erdbrügger, U.; Blijdorp, C.J.; Bijnsdorp, I.V.; Borràs, F.E.; Burger, D.; Bussolati, B.; Byrd, J.B.; Clayton, A.; Dear, J.W.; Falcón-Pérez, J.M.; et al. Urinary Extracellular Vesicles: A Position Paper by the Urine Task Force of the International Society for Extracellular Vesicles. J. Extracell. Vesicles 2021, 10, e12093. [Google Scholar] [CrossRef]
- Grange, C.; Papadimitriou, E.; Dimuccio, V.; Pastorino, C.; Molina, J.; O’Kelly, R.; Niedernhofer, L.J.; Robbins, P.D.; Camussi, G.; Bussolati, B. Urinary Extracellular Vesicles Carrying Klotho Improve the Recovery of Renal Function in an Acute Tubular Injury Model. Mol. Ther. 2024, 32, 4158–4159. [Google Scholar] [CrossRef]
- Gracia, T.; Wang, X.; Su, Y.; Norgett, E.E.; Williams, T.L.; Moreno, P.; Micklem, G.; Karet Frankl, F.E. Urinary Exosomes Contain MicroRNAs Capable of Paracrine Modulation of Tubular Transporters in Kidney. Sci. Rep. 2017, 7, 40601. [Google Scholar] [CrossRef]
- Yuan, Z.; Ye, L.; Feng, X.; Zhou, T.; Zhou, Y.; Zhu, S.; Jia, C.; Li, H.; Qiu, D.; Li, K.; et al. YAP-Dependent Induction of CD47-Enriched Extracellular Vesicles Inhibits Dendritic Cell Activation and Ameliorates Hepatic Ischemia-Reperfusion Injury. Oxid. Med. Cell. Longev. 2021, 2021, 6617345. [Google Scholar] [CrossRef]
- Yang, M.-Q.; Du, Q.; Goswami, J.; Varley, P.R.; Chen, B.; Wang, R.-H.; Morelli, A.E.; Stolz, D.B.; Billiar, T.R.; Li, J.; et al. Interferon Regulatory Factor 1-Rab27a Regulated Extracellular Vesicles Promote Liver Ischemia/Reperfusion Injury. Hepatology 2018, 67, 1056–1070. [Google Scholar] [CrossRef]
- Liu, L.; Xiao, F.; Sun, J.; Wang, Q.; Wang, A.; Zhang, F.; Li, Z.; Wang, X.; Fang, Z.; Qiao, Y. Hepatocyte-Derived Extracellular Vesicles miR-122-5p Promotes Hepatic Ischemia Reperfusion Injury by Regulating Kupffer Cell Polarization. Int. Immunopharmacol. 2023, 119, 110060. [Google Scholar] [CrossRef]
- Calleri, A.; Roggio, D.; Navarro-Tableros, V.; De Stefano, N.; Pasquino, C.; David, E.; Frigatti, G.; Rigo, F.; Antico, F.; Caropreso, P.; et al. Protective Effects of Human Liver Stem Cell-Derived Extracellular Vesicles in a Mouse Model of Hepatic Ischemia-Reperfusion Injury. Stem Cell Rev. Rep. 2021, 17, 459–470. [Google Scholar] [CrossRef]
- De Stefano, N.; Navarro-Tableros, V.; Roggio, D.; Calleri, A.; Rigo, F.; David, E.; Gambella, A.; Bassino, D.; Amoroso, A.; Patrono, D.; et al. Human Liver Stem Cell-Derived Extracellular Vesicles Reduce Injury in a Model of Normothermic Machine Perfusion of Rat Livers Previously Exposed to a Prolonged Warm Ischemia. Transpl. Int. 2021, 34, 1607–1617. [Google Scholar] [CrossRef]
- Nie, H.-Y.; Ge, J.; Huang, G.-X.; Liu, K.-G.; Yue, Y.; Li, H.; Lin, H.-G.; Zhang, T.; Yan, H.-F.; Xu, B.-X.; et al. New Insights into the Intestinal Barrier through “Gut-Organ” Axes and a Glimpse of the Microgravity’s Effects on Intestinal Barrier. Front. Physiol. 2024, 15, 1465649. [Google Scholar] [CrossRef]
- Chen, Y.; Pu, W.; Maswikiti, E.P.; Tao, P.; Li, X.; Wang, D.; Gu, B.; Yu, Y.; Gao, L.; Zhao, C.; et al. Intestinal Congestion and Reperfusion Injury: Damage Caused to the Intestinal Tract and Distal Organs. Biosci. Rep. 2021, 41, BSR20211560. [Google Scholar] [CrossRef]
- Wang, S.; Luo, J.; Wang, H.; Chen, T.; Sun, J.; Xi, Q.; Zhang, Y. Extracellular Vesicles: A Crucial Player in the Intestinal Microenvironment and Beyond. Int. J. Mol. Sci. 2024, 25, 3478. [Google Scholar] [CrossRef]
- Appiah, M.G.; Park, E.J.; Darkwah, S.; Kawamoto, E.; Akama, Y.; Gaowa, A.; Kalsan, M.; Ahmad, S.; Shimaoka, M. Intestinal Epithelium-Derived Luminally Released Extracellular Vesicles in Sepsis Exhibit the Ability to Suppress TNF-a and IL-17A Expression in Mucosal Inflammation. Int. J. Mol. Sci. 2020, 21, 8445. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; Manson, J.J. What Is the Clinical Relevance of TNF Inhibitor Immunogenicity in the Management of Patients with Rheumatoid Arthritis? Front. Immunol. 2020, 11, 589. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhou, B.; Yang, X.; Zhao, J.; Hu, J.; Ding, Y.; Zhan, S.; Yang, Y.; Chen, J.; Zhang, F.; et al. Exosomal circEZH2_005, an Intestinal Injury Biomarker, Alleviates Intestinal Ischemia/reperfusion Injury by Mediating Gprc5a Signaling. Nat. Commun. 2023, 14, 5437. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zheng, X.G.; Wu, Y.L.; Wang, A.P.; Wang, C.H.; Chen, W.X.; Zhong, S.; Yang, H. Intestinal Epithelial Cell-Derived Exosomes Package microRNA-23a-3p Alleviate Gut Damage after Ischemia/Reperfusion via Targeting MAP4K4. Biomed. Pharmacother. 2022, 149, 112810. [Google Scholar] [CrossRef]
- Lee, J.; Park, E.J.; Yuki, Y.; Ahmad, S.; Mizuguchi, K.; Ishii, K.J.; Shimaoka, M.; Kiyono, H. Profiles of microRNA Networks in Intestinal Epithelial Cells in a Mouse Model of Colitis. Sci. Rep. 2015, 5, 18174. [Google Scholar] [CrossRef]
- Jiang, L.; Shen, Y.; Guo, D.; Yang, D.; Liu, J.; Fei, X.; Yang, Y.; Zhang, B.; Lin, Z.; Yang, F.; et al. EpCAM-Dependent Extracellular Vesicles from Intestinal Epithelial Cells Maintain Intestinal Tract Immune Balance. Nat. Commun. 2016, 7, 13045. [Google Scholar] [CrossRef]
- Hiatt, W.R. Medical Treatment of Peripheral Arterial Disease and Claudication. N. Engl. J. Med. 2001, 344, 1608–1621. [Google Scholar] [CrossRef]
- Rice, T.W.; Lumsden, A.B. Optimal Medical Management of Peripheral Arterial Disease. Vasc. Endovascular Surg. 2006, 40, 312–327. [Google Scholar] [CrossRef]
- Ranghino, A.; Cantaluppi, V.; Grange, C.; Vitillo, L.; Fop, F.; Biancone, L.; Deregibus, M.C.; Tetta, C.; Segoloni, G.P.; Camussi, G. Endothelial Progenitor Cell-Derived Microvesicles Improve Neovascularization in a Murine Model of Hindlimb Ischemia. Int. J. Immunopathol. Pharmacol. 2012, 25, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Leroyer, A.S.; Ebrahimian, T.G.; Cochain, C.; Récalde, A.; Blanc-Brude, O.; Mees, B.; Vilar, J.; Tedgui, A.; Levy, B.I.; Chimini, G.; et al. Microparticles from Ischemic Muscle Promotes Postnatal Vasculogenesis. Circulation 2009, 119, 2808–2817. [Google Scholar] [CrossRef] [PubMed]
- Cavallari, C.; Ranghino, A.; Tapparo, M.; Cedrino, M.; Figliolini, F.; Grange, C.; Giannachi, V.; Garneri, P.; Deregibus, M.C.; Collino, F.; et al. Serum-Derived Extracellular Vesicles (EVs) Impact on Vascular Remodeling and Prevent Muscle Damage in Acute Hind Limb Ischemia. Sci. Rep. 2017, 7, 8180. [Google Scholar] [CrossRef]
- Xing, Z.; Zhao, C.; Wu, S.; Yang, D.; Zhang, C.; Wei, X.; Wei, X.; Su, H.; Liu, H.; Fan, Y. Hydrogel Loaded with VEGF/TFEB-Engineered Extracellular Vesicles for Rescuing Critical Limb Ischemia by a Dual-Pathway Activation Strategy. Adv. Healthc. Mater. 2022, 11, e2100334. [Google Scholar] [CrossRef]
- Quan, C.; Wang, M.; Chen, H.; Zhang, H. Extracellular Vesicles in Acute Respiratory Distress Syndrome: Recent Developments from Bench to Bedside. Int. Immunopharmacol. 2021, 100, 108118. [Google Scholar] [CrossRef]
- Mahida, R.Y.; Matsumoto, S.; Matthay, M.A. Extracellular Vesicles: A New Frontier for Research in Acute Respiratory Distress Syndrome. Am. J. Respir. Cell Mol. Biol. 2020, 63, 15–24. [Google Scholar] [CrossRef]
- Fujita, Y.; Kosaka, N.; Araya, J.; Kuwano, K.; Ochiya, T. Extracellular Vesicles in Lung Microenvironment and Pathogenesis. Trends Mol. Med. 2015, 21, 533–542. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, S.; Yang, Y.; Yao, J.-Q.; Tang, W.-F.; Lyon, C.J.; Hu, T.Y.; Wan, M.-H. Extracellular Vesicles in the Pathogenesis and Treatment of Acute Lung Injury. Mil. Med. Res. 2022, 9, 61. [Google Scholar] [CrossRef]
- Liu, F.; Peng, W.; Chen, J.; Xu, Z.; Jiang, R.; Shao, Q.; Zhao, N.; Qian, K. Exosomes Derived From Alveolar Epithelial Cells Promote Alveolar Macrophage Activation Mediated by miR-92a-3p in Sepsis-Induced Acute Lung Injury. Front. Cell Infect. Microbiol. 2021, 11, 646546. [Google Scholar] [CrossRef]
- Zheng, L.; Su, J.; Zhang, Z.; Jiang, L.; Wei, J.; Xu, X.; Lv, S. Salidroside Regulates Inflammatory Pathway of Alveolar Macrophages by Influencing the Secretion of miRNA-146a Exosomes by Lung Epithelial Cells. Sci. Rep. 2020, 10, 20750. [Google Scholar] [CrossRef]
- Zhu, M.; Ma, X.; Huang, J.; Lu, F.-G.; Chen, Y.; Hu, J.; Cheng, L.; Zhang, B.; Liu, W.; Li, L. Extracellular Vesicle-Derived miR-1249-5p Regulates Influenza A Virus-Induced Acute Lung Injury in RAW246.7 Cells Through Targeting SLC4A1. Microbes Infect. 2022, 24, 104998. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Zhou, J.; Liu, Y.; Xia, R.; Li, Q.; Yan, L.; Chen, Q.; Chen, X.; Jiang, Y.; Chao, G.; et al. Epithelium- and Endothelium-Derived Exosomes Regulate the Alveolar Macrophages by Targeting RGS1 Mediated Calcium Signaling-Dependent Immune Response. Cell Death Differ. 2021, 28, 2238–2256. [Google Scholar] [CrossRef] [PubMed]
- Kadota, T.; Fujita, Y.; Araya, J.; Watanabe, N.; Fujimoto, S.; Kawamoto, H.; Minagawa, S.; Hara, H.; Ohtsuka, T.; Yamamoto, Y.; et al. Human Bronchial Epithelial Cell-Derived Extracellular Vesicle Therapy for Pulmonary Fibrosis via Inhibition of TGF-β-WNT Crosstalk. J. Extracell. Vesicles 2021, 10, e12124. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Kadota, T.; Kaneko, R.; Hirano, Y.; Fujimoto, S.; Watanabe, N.; Kizawa, R.; Ohtsuka, T.; Kuwano, K.; Ochiya, T.; et al. Mitigation of Acute Lung Injury by Human Bronchial Epithelial Cell-Derived Extracellular Vesicles via ANXA1-Mediated FPR Signaling. Commun. Biol. 2024, 7, 514. [Google Scholar] [CrossRef]
- Gu, Z.; Sun, M.; Liu, J.; Huang, Q.; Wang, Y.; Liao, J.; Shu, T.; Tao, M.; Mao, G.; Pei, Z.; et al. Endothelium-Derived Engineered Extracellular Vesicles Protect the Pulmonary Endothelial Barrier in Acute Lung Injury. Adv. Sci. 2024, 11, e2306156. [Google Scholar] [CrossRef]
- Li, Z.; Bu, Y.; Wang, C.; Yu, Y.; Han, L.; Liu, C.; Chen, G.; Li, C.; Zhang, Y.; Cao, H.; et al. Extracellular Vesicle-Packaged GBP2 from Macrophages Aggravates Sepsis-Induced Acute Lung Injury by Promoting Ferroptosis in Pulmonary Vascular Endothelial Cells. Redox Biol. 2025, 82, 103614. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, L.; Li, H.; Ren, W.; Zhuo, R.; Feng, C.; He, Y.; Hu, Y.; Ye, C. Alveolar Macrophage-Derived Exosomal tRF-22-8BWS7K092 Activates Hippo Signaling Pathway to Induce Ferroptosis in Acute Lung Injury. Int. Immunopharmacol. 2022, 107, 108690. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, C.; Cai, D.; Zhu, R.; Cao, Y. Exosomal miR-155-5p Drives Widespread Macrophage M1 Polarization in Hypervirulent Klebsiella Pneumoniae-Induced Acute Lung Injury via the MSK1/p38-MAPK Axis. Cell Mol. Biol. Lett. 2023, 28, 92. [Google Scholar] [CrossRef]
- Yang, J.; Huang, X.; Yu, Q.; Wang, S.; Wen, X.; Bai, S.; Cao, L.; Zhang, K.; Zhang, S.; Wang, X.; et al. Extracellular Vesicles Derived from M2-like Macrophages Alleviate Acute Lung Injury in a miR-709-Mediated Manner. J. Extracell. Vesicles 2024, 13, e12437. [Google Scholar] [CrossRef]
- Kang, M.; Jordan, V.; Blenkiron, C.; Chamley, L.W. Biodistribution of Extracellular Vesicles Following Administration into Animals: A Systematic Review. J. Extracell. Vesicles 2021, 10, e12085. [Google Scholar] [CrossRef]
- Bruno, S.; Pasquino, C.; Herrera Sanchez, M.B.; Tapparo, M.; Figliolini, F.; Grange, C.; Chiabotto, G.; Cedrino, M.; Deregibus, M.C.; Tetta, C.; et al. HLSC-Derived Extracellular Vesicles Attenuate Liver Fibrosis and Inflammation in a Murine Model of Non-Alcoholic Steatohepatitis. Mol. Ther. 2020, 28, 479–489. [Google Scholar] [CrossRef]
- Royo, F.; Cossío, U.; Ruiz de Angulo, A.; Llop, J.; Falcon-Perez, J.M. Modification of the Glycosylation of Extracellular Vesicles Alters Their Biodistribution in Mice. Nanoscale 2019, 11, 1531–1537. [Google Scholar] [CrossRef] [PubMed]
- Jaffery, A.; Cortes, A.C.; Minamiguchi, K.; Anfossi, S.; Avritscher, R. Abstract 6583: Optimized Production, Biodistribution, and Targeted Delivery of T Cell-Derived Extracellular Vesicles for Liver Cancer Therapy. Cancer Res. 2025, 85, 6583. [Google Scholar] [CrossRef]
- Yang, S.; Liu, P.; Gao, T.; Song, D.; Zhao, X.; Li, Y.; Wu, J.; Wang, L.; Wang, Z.; Hao, J.; et al. Every Road Leads to Rome: Therapeutic Effect and Mechanism of the Extracellular Vesicles of Human Embryonic Stem Cell-Derived Immune and Matrix Regulatory Cells Administered to Mouse Models of Pulmonary Fibrosis through Different Routes. Stem Cell Res. Ther. 2022, 13, 163. [Google Scholar] [CrossRef] [PubMed]
- Tolomeo, A.M.; Zuccolotto, G.; Malvicini, R.; De Lazzari, G.; Penna, A.; Franco, C.; Caicci, F.; Magarotto, F.; Quarta, S.; Pozzobon, M.; et al. Biodistribution of Intratracheal, Intranasal, and Intravenous Injections of Human Mesenchymal Stromal Cell-Derived Extracellular Vesicles in a Mouse Model for Drug Delivery Studies. Pharmaceutics 2023, 15, 548. [Google Scholar] [CrossRef]
- Roefs, M.T.; Heusermann, W.; Brans, M.A.D.; Snijders Blok, C.; Lei, Z.; Vader, P.; Sluijter, J.P.G. Evaluation and Manipulation of Tissue and Cellular Distribution of Cardiac Progenitor Cell-Derived Extracellular Vesicles. Front. Pharmacol. 2022, 13, 1052091. [Google Scholar] [CrossRef]
- Mentkowski, K.I.; Lang, J.K. Exosomes Engineered to Express a Cardiomyocyte Binding Peptide Demonstrate Improved Cardiac Retention In Vivo. Sci. Rep. 2019, 9, 10041. [Google Scholar] [CrossRef]
- Wiklander, O.P.B.; Nordin, J.Z.; O’Loughlin, A.; Gustafsson, Y.; Corso, G.; Mäger, I.; Vader, P.; Lee, Y.; Sork, H.; Seow, Y.; et al. Extracellular Vesicle In Vivo Biodistribution Is Determined by Cell Source, Route of Administration and Targeting. J. Extracell. Vesicles 2015, 4, 26316. [Google Scholar] [CrossRef]
- Lai, C.P.; Mardini, O.; Ericsson, M.; Prabhakar, S.; Maguire, C.; Chen, J.W.; Tannous, B.A.; Breakefield, X.O. Dynamic Biodistribution of Extracellular Vesicles In Vivo Using a Multimodal Imaging Reporter. ACS Nano 2014, 8, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Brezgin, S.; Parodi, A.; Kostyusheva, A.; Ponomareva, N.; Lukashev, A.; Sokolova, D.; Pokrovsky, V.S.; Slatinskaya, O.; Maksimov, G.; Zamyatnin, A.A., Jr.; et al. Technological Aspects of Manufacturing and Analytical Control of Biological Nanoparticles. Biotechnol. Adv. 2023, 64, 108122. [Google Scholar] [CrossRef] [PubMed]
- Ansari, F.J.; Tafti, H.A.; Amanzadeh, A.; Rabbani, S.; Shokrgozar, M.A.; Heidari, R.; Behroozi, J.; Eyni, H.; Uversky, V.N.; Ghanbari, H. Comparison of the Efficiency of Ultrafiltration, Precipitation, and Ultracentrifugation Methods for Exosome Isolation. Biochem. Biophys. Rep. 2024, 38, 101668. [Google Scholar] [CrossRef] [PubMed]
- Helwa, I.; Cai, J.; Drewry, M.D.; Zimmerman, A.; Dinkins, M.B.; Khaled, M.L.; Seremwe, M.; Dismuke, W.M.; Bieberich, E.; Stamer, W.D.; et al. A Comparative Study of Serum Exosome Isolation Using Differential Ultracentrifugation and Three Commercial Reagents. PLoS ONE 2017, 12, e0170628. [Google Scholar] [CrossRef]
- Patel, G.K.; Khan, M.A.; Zubair, H.; Srivastava, S.K.; Khushman, M. ’d; Singh, S.; Singh, A.P. Comparative Analysis of Exosome Isolation Methods Using Culture Supernatant for Optimum Yield, Purity and Downstream Applications. Sci. Rep. 2019, 9, 5335. [Google Scholar] [CrossRef]
- Mendonca, A.; Acharjee, A.; Kumar, J.S.; Sundaresan, S. Comparative Analysis of Exosomes Isolated by Ultracentrifugation and Total Exosome Isolation Reagent: A Biophysical and Physicochemical Study. J. Nanopart. Res. 2025, 27, 28. [Google Scholar] [CrossRef]
- Wei, R.; Zhao, L.; Kong, G.; Liu, X.; Zhu, S.; Zhang, S.; Min, L. Combination of Size-Exclusion Chromatography and Ultracentrifugation Improves the Proteomic Profiling of Plasma-Derived Small Extracellular Vesicles. Biol. Proced. Online 2020, 22, 12. [Google Scholar] [CrossRef]
- Takov, K.; Yellon, D.M.; Davidson, S.M. Comparison of Small Extracellular Vesicles Isolated from Plasma by Ultracentrifugation or Size-Exclusion Chromatography: Yield, Purity and Functional Potential. J. Extracell. Vesicles 2019, 8, 1560809. [Google Scholar] [CrossRef]
- Busatto, S.; Vilanilam, G.; Ticer, T.; Lin, W.-L.; Dickson, D.W.; Shapiro, S.; Bergese, P.; Wolfram, J. Tangential Flow Filtration for Highly Efficient Concentration of Extracellular Vesicles from Large Volumes of Fluid. Cells 2018, 7, 273. [Google Scholar] [CrossRef]
- Visan, K.S.; Lobb, R.J.; Ham, S.; Lima, L.G.; Palma, C.; Edna, C.P.Z.; Wu, L.-Y.; Gowda, H.; Datta, K.K.; Hartel, G.; et al. Comparative Analysis of Tangential Flow Filtration and Ultracentrifugation, Both Combined with Subsequent Size Exclusion Chromatography, for the Isolation of Small Extracellular Vesicles. J. Extracell. Vesicles 2022, 11, e12266. [Google Scholar] [CrossRef]
- Zhang, H.; Lyden, D. Asymmetric-Flow Field-Flow Fractionation Technology for Exomere and Small Extracellular Vesicle Separation and Characterization. Nat. Protoc. 2019, 14, 1027–1053. [Google Scholar] [CrossRef]
- Zhang, H.; Freitas, D.; Kim, H.S.; Fabijanic, K.; Li, Z.; Chen, H.; Mark, M.T.; Molina, H.; Martin, A.B.; Bojmar, L.; et al. Identification of Distinct Nanoparticles and Subsets of Extracellular Vesicles by Asymmetric Flow Field-Flow Fractionation. Nat. Cell Biol. 2018, 20, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.; Law, S.Q.K.; Shojaee, M.; Hall, A.S.; Bhuiyan, S.; Lim, M.B.L.; Silva, A.; Kong, K.J.W.; Schoppet, M.; Blyth, C.; et al. First-in-Human Clinical Trial of Allogeneic, Platelet-Derived Extracellular Vesicles as a Potential Therapeutic for Delayed Wound Healing. J. Extracell. Vesicles 2023, 12, e12332. [Google Scholar] [CrossRef] [PubMed]
- Golikov, M.V.; Valuev-Elliston, V.T.; Smirnova, O.A.; Ivanov, A.V. Physiological Media in Studies of Cell Metabolism. Mol. Biol. 2022, 56, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Li, C.; Qi, X.; Xu, R.; Dong, L.; Jiang, Y.; Gong, Q.; Wang, D.; Cheng, R.; Zhang, C.; et al. Extracellular Vesicles from NMN Preconditioned Mesenchymal Stem Cells Ameliorated Myocardial Infarction via miR-210-3p Promoted Angiogenesis. Stem Cell Rev. Rep. 2023, 19, 1051–1066. [Google Scholar] [CrossRef]
- Liang, X.; Gupta, D.; Xie, J.; Van Wonterghem, E.; Van Hoecke, L.; Hean, J.; Niu, Z.; Ghaeidamini, M.; Wiklander, O.P.B.; Zheng, W.; et al. Engineering of Extracellular Vesicles for Efficient Intracellular Delivery of Multimodal Therapeutics Including Genome Editors. Nat. Commun. 2025, 16, 4028. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pevzner, I.B.; Andrianova, N.V.; Lomakina, A.K.; Cherkesova, K.S.; Semenchenko, E.D.; Plotnikov, E.Y. Organ-Specific Extracellular Vesicles in the Treatment of Ischemic Acute Organ Injury: Mechanisms, Successes, and Prospects. Int. J. Mol. Sci. 2025, 26, 9709. https://doi.org/10.3390/ijms26199709
Pevzner IB, Andrianova NV, Lomakina AK, Cherkesova KS, Semenchenko ED, Plotnikov EY. Organ-Specific Extracellular Vesicles in the Treatment of Ischemic Acute Organ Injury: Mechanisms, Successes, and Prospects. International Journal of Molecular Sciences. 2025; 26(19):9709. https://doi.org/10.3390/ijms26199709
Chicago/Turabian StylePevzner, Irina B., Nadezda V. Andrianova, Anna K. Lomakina, Kseniia S. Cherkesova, Elizaveta D. Semenchenko, and Egor Y. Plotnikov. 2025. "Organ-Specific Extracellular Vesicles in the Treatment of Ischemic Acute Organ Injury: Mechanisms, Successes, and Prospects" International Journal of Molecular Sciences 26, no. 19: 9709. https://doi.org/10.3390/ijms26199709
APA StylePevzner, I. B., Andrianova, N. V., Lomakina, A. K., Cherkesova, K. S., Semenchenko, E. D., & Plotnikov, E. Y. (2025). Organ-Specific Extracellular Vesicles in the Treatment of Ischemic Acute Organ Injury: Mechanisms, Successes, and Prospects. International Journal of Molecular Sciences, 26(19), 9709. https://doi.org/10.3390/ijms26199709






