Octopus minor Antimicrobial Peptide-Loaded Chitosan Nanoparticles Accelerate Dermal Wound Healing
Abstract
1. Introduction
2. Results
2.1. Toxicity of Octominin-CNPs In Vitro and In Vivo
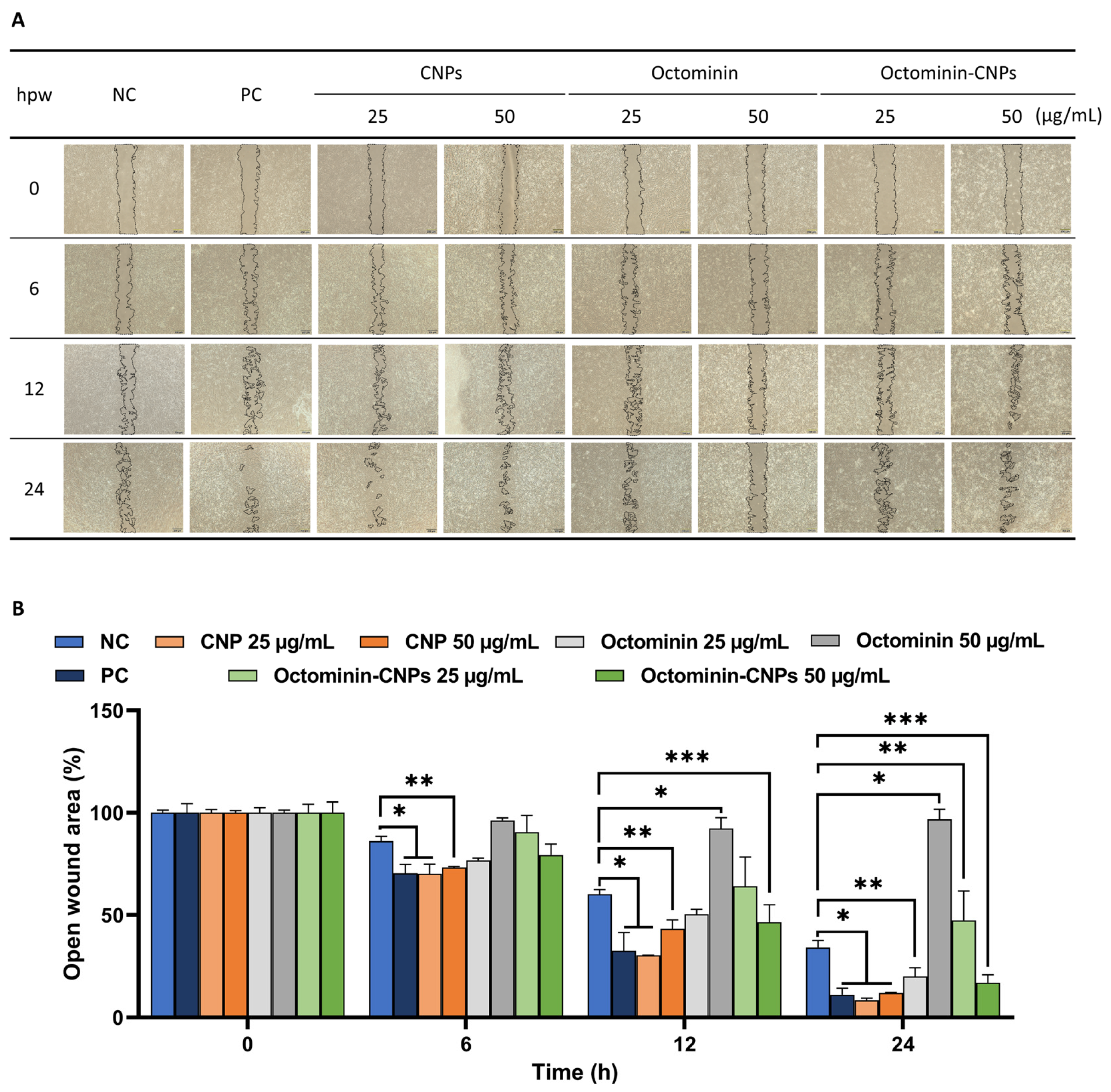
2.2. In Vitro Wound Healing Activity of Octominin-CNPs
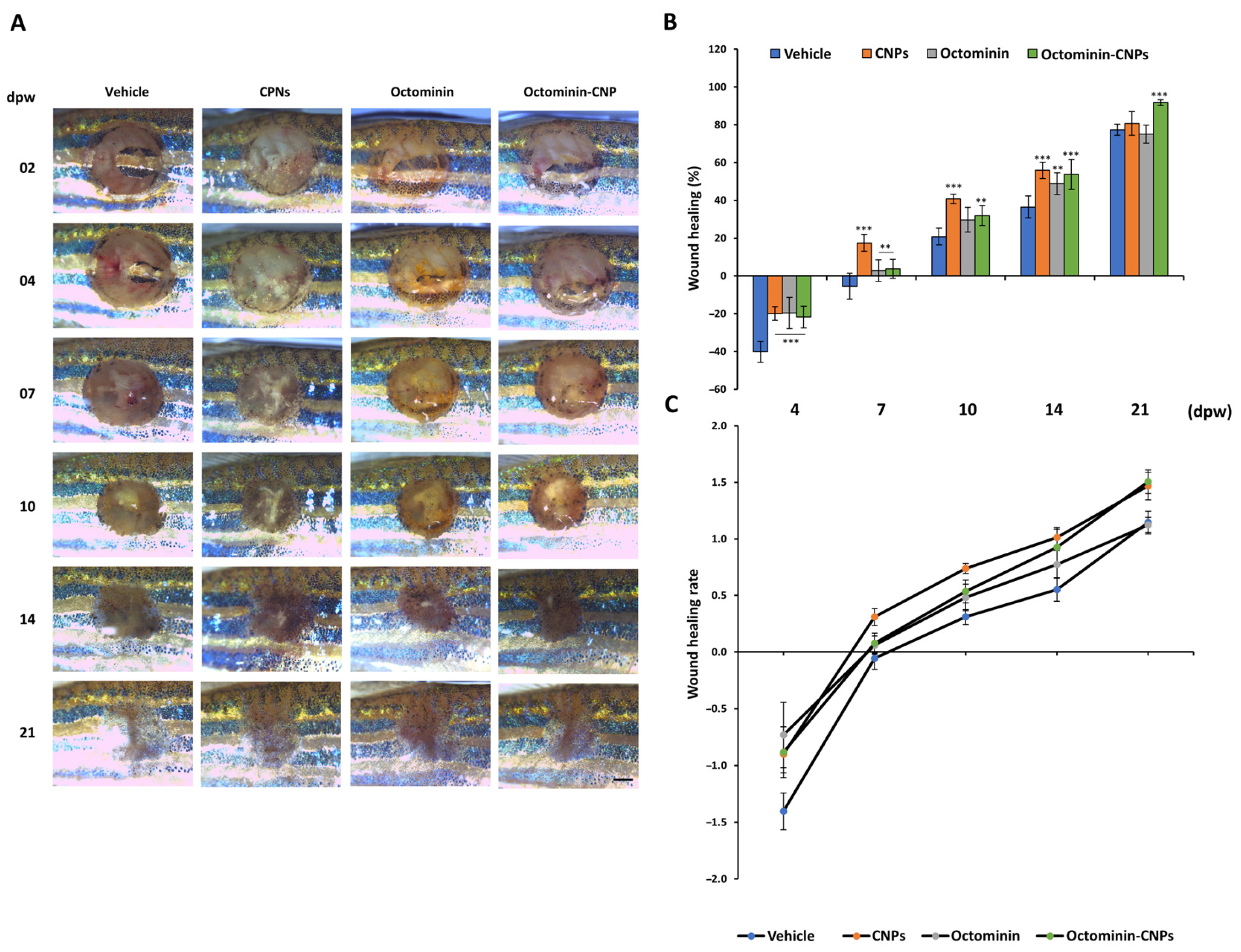
2.3. Enhanced Wound Healing Effects in Octominin-CNPs-Treated Zebrafish
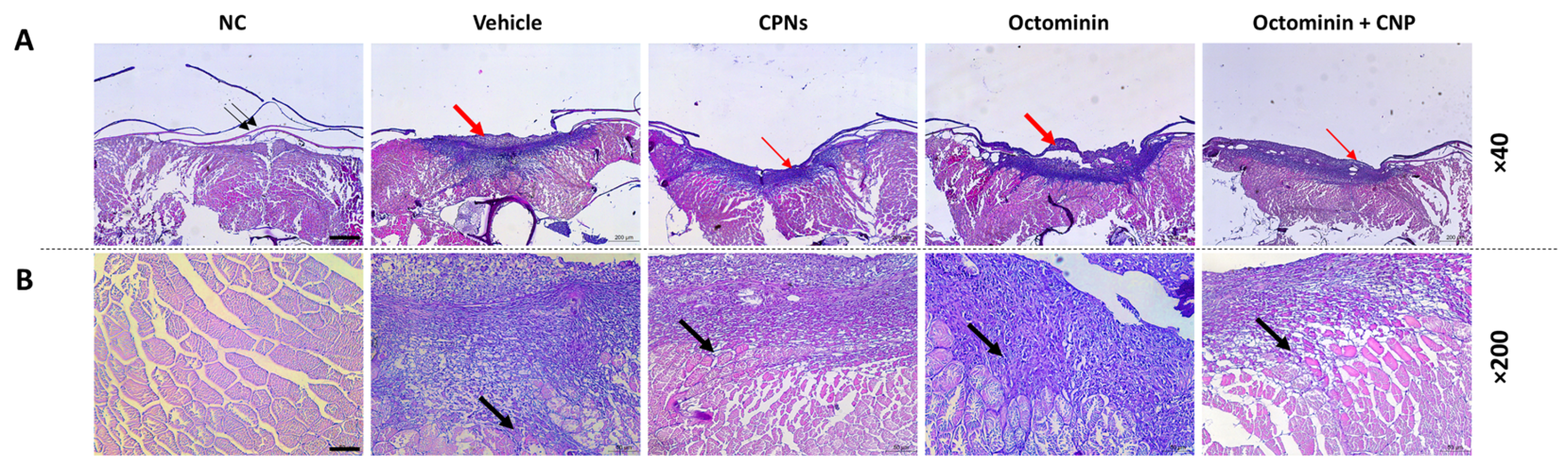
2.4. Re-Epithelialization and Tissue Regeneration of Octominin-CNPs-Treated Zebrafish
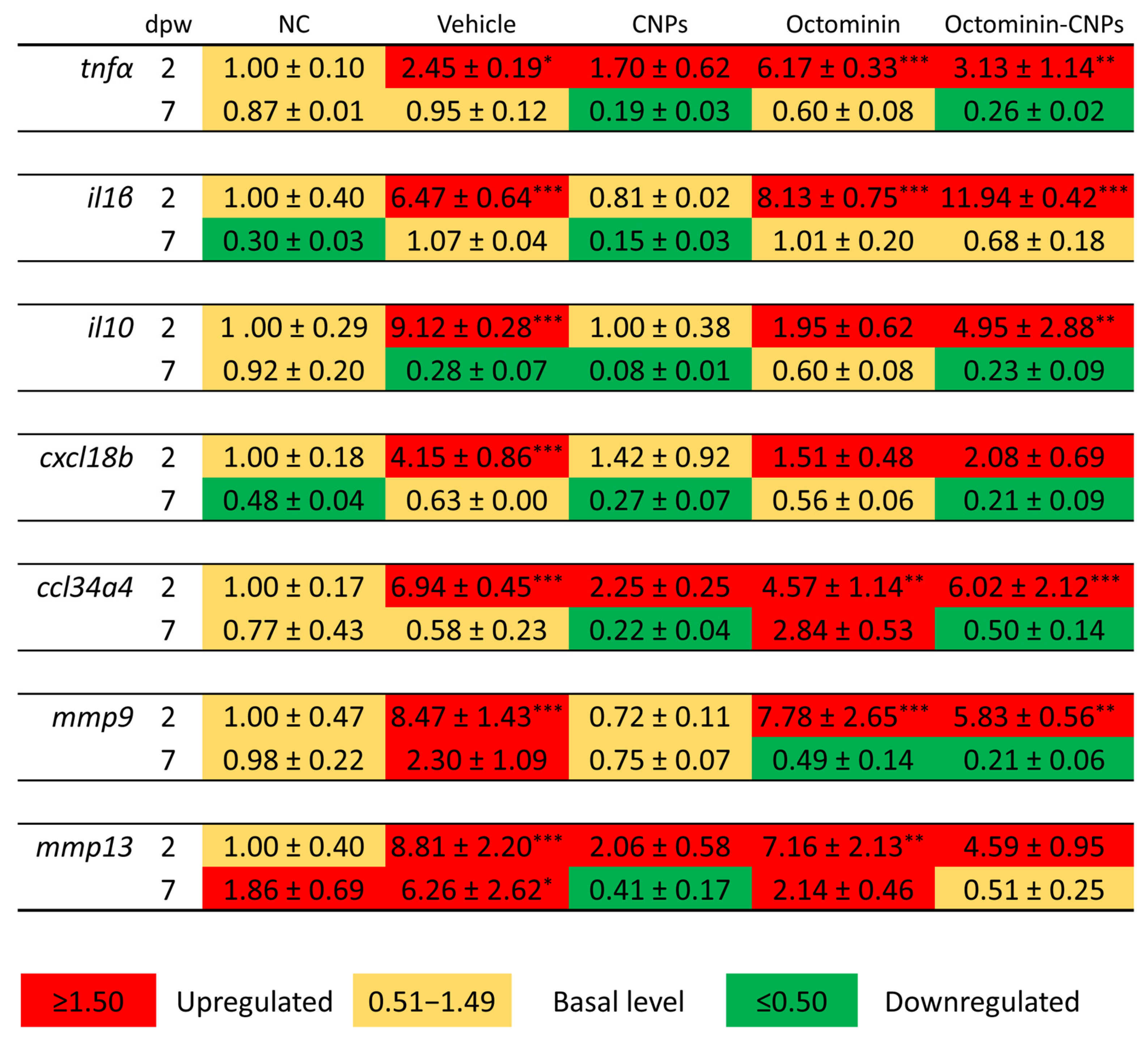
2.5. Octominin-CNPs Treatment Reduced the Pro-Inflammatory Gene Markers in Zebrafish
3. Discussion
4. Materials and Methods
4.1. Raw Materials, Chemicals, and Reagents
4.2. Preparation of Octominin-Encapsulated Chitosan Nanoparticles
4.3. Cells and Zebrafish Maintenance
4.4. In Vitro and In Vivo Toxicity of Octominin-CNPs
4.5. In Vitro Wound Healing Activity Analysis
4.6. Dermal Wound Initiation and Topical Application of the Treatment on Adult Zebrafish
4.7. Morphological Wound Healing Efficacy Analysis of Octominin-CNPs
4.8. Histological Analysis of the Wound Healing Activity of Octominin-CNPs
4.9. mRNA Expression of the Wound Healing Marker Genes in Zebrafish
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef]
- Ana Cristina de Oliveira Gonzalez, T.F.C.; Andrade, Z.d.A.; Medrado, A.R.A.P. Wound healing—A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Talbott, H.E.; Mascharak, S.; Griffin, M.; Wan, D.C.; Longaker, M.T. Wound healing, fibroblast heterogeneity, and fibrosis. Cell Stem Cell 2022, 29, 1161–1180. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; DiPietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Mahlapuu, M.; Björn, C.; Ekblom, J. Antimicrobial peptides as therapeutic agents: Opportunities and challenges. Crit. Rev. Biotechnol. 2020, 40, 978–992. [Google Scholar] [CrossRef]
- Kang, H.K.; Kim, C.; Seo, C.H.; Park, Y. The therapeutic applications of antimicrobial peptides (AMPs): A patent review. J. Microbiol. 2017, 55, 1–12. [Google Scholar] [CrossRef]
- Bahar, A.A.; Ren, D. Antimicrobial peptides. Pharmaceuticals 2013, 6, 1543–1575. [Google Scholar] [CrossRef]
- Zhang, Q.-Y.; Yan, Z.-B.; Meng, Y.-M.; Hong, X.-Y.; Shao, G.; Ma, J.-J.; Cheng, X.-R.; Liu, J.; Kang, J.; Fu, C.-Y. Antimicrobial peptides: Mechanism of action, activity and clinical potential. Mil. Med. Res. 2021, 8, 48. [Google Scholar] [CrossRef]
- Zheng, S.; Tu, Y.; Li, B.; Qu, G.; Li, A.; Peng, X.; Li, S.; Shao, C. Antimicrobial peptide biological activity, delivery systems and clinical translation status and challenges. J. Transl. Med. 2025, 23, 292. [Google Scholar] [CrossRef]
- Thapa, R.K.; Diep, D.B.; Tønnesen, H.H. Topical antimicrobial peptide formulations for wound healing: Current developments and future prospects. Acta Biomater. 2020, 103, 52–67. [Google Scholar] [CrossRef]
- Hulla, J.; Sahu, S.; Hayes, A. Nanotechnology: History and future. Hum. Exp. Toxicol. 2015, 34, 1318–1321. [Google Scholar] [CrossRef]
- Tang, Z.; Ma, Q.; Chen, X.; Chen, T.; Ying, Y.; Xi, X.; Wang, L.; Ma, C.; Shaw, C.; Zhou, M. Recent Advances and Challenges in Nanodelivery Systems for Antimicrobial Peptides (AMPs). Antibiotics 2021, 10, 990. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.d.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Piras, A.M.; Sandreschi, S.; Maisetta, G.; Esin, S.; Batoni, G.; Chiellini, F. Chitosan nanoparticles for the linear release of model cationic peptide. Pharm. Res. 2015, 32, 2259–2265. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Rashidpour, A.; Almajano, M.P.; Metón, I. Chitosan-based drug delivery system: Applications in fish biotechnology. Polymers 2020, 12, 1177. [Google Scholar] [CrossRef] [PubMed]
- Piras, A.M.; Maisetta, G.; Sandreschi, S.; Gazzarri, M.; Bartoli, C.; Grassi, L.; Esin, S.; Chiellini, F.; Batoni, G. Chitosan nanoparticles loaded with the antimicrobial peptide temporin B exert a long-term antibacterial activity in vitro against clinical isolates of Staphylococcus epidermidis. Front. Microbiol. 2015, 6, 372. [Google Scholar] [CrossRef]
- Nikapitiya, C.; Dananjaya, S.H.S.; Chandrarathna, H.; De Zoysa, M.; Whang, I. Octominin: A novel synthetic anticandidal peptide derived from defense protein of Octopus minor. Mar. Drugs 2020, 18, 56. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Nagahawatta, D.P.; Yang, H.-W.; Oh, J.Y.; Jayawardena, T.U.; Jeon, Y.-J.; De Zoysa, M.; Whang, I.; Ryu, B. Octominin inhibits LPS-induced chemokine and pro-inflammatory cytokine secretion from Raw 264.7 macrophages via blocking TLRs/NF-κB signal transduction. Biomolecules 2020, 10, 511. [Google Scholar] [CrossRef]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial peptides: Classification, design, application and research progress in multiple fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef]
- Zhang, L.-j.; Gallo, R.L. Antimicrobial peptides. Curr. Biol. 2016, 26, R14–R19. [Google Scholar] [CrossRef]
- Jayathilaka, E.H.T.T.; Nikapitiya, C.; De Zoysa, M.; Whang, I. Antimicrobial peptide octominin-encapsulated chitosan nanoparticles enhanced antifungal and antibacterial activities. Int. J. Mol. Sci. 2022, 23, 15882. [Google Scholar] [CrossRef]
- Raza, Z.A.; Khalil, S.; Ayub, A.; Banat, I.M. Recent developments in chitosan encapsulation of various active ingredients for multifunctional applications. Carbohydr. Res. 2020, 492, 108004. [Google Scholar] [CrossRef]
- Shariatinia, Z. Carboxymethyl chitosan: Properties and biomedical applications. Int. J. Biol. Macromol. 2018, 120, 1406–1419. [Google Scholar] [CrossRef]
- Sun, T.; Zhan, B.; Zhang, W.; Qin, D.; Xia, G.; Zhang, H.; Peng, M.; Li, S.-A.; Zhang, Y.; Gao, Y. Carboxymethyl chitosan nanoparticles loaded with bioactive peptide OH-CATH30 benefit nonscar wound healing. Int. J. Nanomed. 2018, 13, 5771. [Google Scholar] [CrossRef] [PubMed]
- Ramos, R.; Silva, J.P.; Rodrigues, A.C.; Costa, R.; Guardão, L.; Schmitt, F.; Soares, R.; Vilanova, M.; Domingues, L.; Gama, M. Wound healing activity of the human antimicrobial peptide LL37. Peptides 2011, 32, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Wang, D.; An, C.; Xu, H.; Zhao, Q.; Shi, Y.; Song, N.; Deng, B.; Guo, X.; Rao, J.; et al. The antimicrobial peptide YD attenuates inflammation via miR-155 targeting CASP12 during liver fibrosis. Acta Pharm. Sin. B 2021, 11, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.; Slanchev, K.; Kraus, C.; Knyphausen, P.; Eming, S.; Hammerschmidt, M. Adult zebrafish as a model system for cutaneous wound-healing research. J. Investig. Dermatol. 2013, 133, 1655–1665. [Google Scholar] [CrossRef]
- Naomi, R.; Bahari, H.; Yazid, M.D.; Embong, H.; Othman, F. Zebrafish as a model system to study the mechanism of cutaneous wound healing and drug discovery: Advantages and challenges. Pharmaceuticals 2021, 14, 1058. [Google Scholar] [CrossRef]
- Edirisinghe, S.L.; Nikapitiya, C.; Kim, C.H.; Castex, M.; Leclercq, E.; De Zoysa, M. Dietary supplementation with a multi-strain yeast fraction enhances skin wound healing in zebrafish (Danio rerio). Aquac. Rep. 2023, 32, 101741. [Google Scholar] [CrossRef]
- Edirisinghe, S.L.; Rajapaksha, D.C.; Nikapitiya, C.; Oh, C.; Lee, K.-A.; Kang, D.-H.; De Zoysa, M. Spirulina maxima derived marine pectin promotes the in vitro and in vivo regeneration and wound healing in zebrafish. Fish. Shellfish. Immunol. 2020, 107, 414–425. [Google Scholar] [CrossRef]
- Dias, M.K.H.M.; Madusanka, D.M.D.; Han, E.J.; Kim, H.-S.; Jeon, Y.-J.; Jee, Y.; Kim, K.-N.; Lee, K.; Fernando, I.P.S.; Ahn, G. Sargassum horneri (Turner) C. Agardh ethanol extract attenuates fine dust-induced inflammatory responses and impaired skin barrier functions in HaCaT keratinocytes. J. Ethnopharmacol. 2021, 273, 114003. [Google Scholar] [CrossRef]
- Akdis, M.; Aab, A.; Altunbulakli, C.; Azkur, K.; Costa, R.A.; Crameri, R.; Duan, S.; Eiwegger, T.; Eljaszewicz, A.; Ferstl, R.; et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor β, and TNF-α: Receptors, functions, and roles in diseases. J. Allergy Clin. Immunol. 2016, 138, 984–1010. [Google Scholar] [CrossRef] [PubMed]
- Torraca, V.; Otto, N.A.; Tavakoli-Tameh, A.; Meijer, A.H. The inflammatory chemokine Cxcl18b exerts neutrophil-specific chemotaxis via the promiscuous chemokine receptor Cxcr2 in zebrafish. Dev. Comp. Immunol. 2017, 67, 57–65. [Google Scholar] [CrossRef]
- Dias, M.K.H.M.; Jayathilaka, E.H.T.T.; Jayasinghe, J.N.C.; Tennakoon, N.; Nikapitiya, C.; Whang, I.; De Zoysa, M. Exploring the proteomic landscape and immunomodulatory functions of Edwardsiella piscicida derived extracellular vesicles. J. Microbiol. Biotechnol. 2025, 35, e2410001. [Google Scholar] [CrossRef]
- Dias, M.K.H.M.; Jayathilaka, E.H.T.T.; De Zoysa, M. Isolation, characterization, and immunomodulatory effects of extracellular vesicles isolated from fish pathogenic Aeromonas hydrophila. Fish. Shellfish. Immunol. 2024, 152, 109787. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dias, M.K.H.M.; Edirisinghe, S.L.; Zoysa, M.D.; Whang, I. Octopus minor Antimicrobial Peptide-Loaded Chitosan Nanoparticles Accelerate Dermal Wound Healing. Int. J. Mol. Sci. 2025, 26, 9701. https://doi.org/10.3390/ijms26199701
Dias MKHM, Edirisinghe SL, Zoysa MD, Whang I. Octopus minor Antimicrobial Peptide-Loaded Chitosan Nanoparticles Accelerate Dermal Wound Healing. International Journal of Molecular Sciences. 2025; 26(19):9701. https://doi.org/10.3390/ijms26199701
Chicago/Turabian StyleDias, Mawalle Kankanamge Hasitha Madhawa, Shan Lakmal Edirisinghe, Mahanama De Zoysa, and Ilson Whang. 2025. "Octopus minor Antimicrobial Peptide-Loaded Chitosan Nanoparticles Accelerate Dermal Wound Healing" International Journal of Molecular Sciences 26, no. 19: 9701. https://doi.org/10.3390/ijms26199701
APA StyleDias, M. K. H. M., Edirisinghe, S. L., Zoysa, M. D., & Whang, I. (2025). Octopus minor Antimicrobial Peptide-Loaded Chitosan Nanoparticles Accelerate Dermal Wound Healing. International Journal of Molecular Sciences, 26(19), 9701. https://doi.org/10.3390/ijms26199701






