Curcumin and Acute Myeloid Leukemia: Synergistic Effects with Targeted Therapy
Abstract
1. Introduction
General Considerations on Acute Myeloid Leukemia
2. Curcumin: A Multidimensional Agent
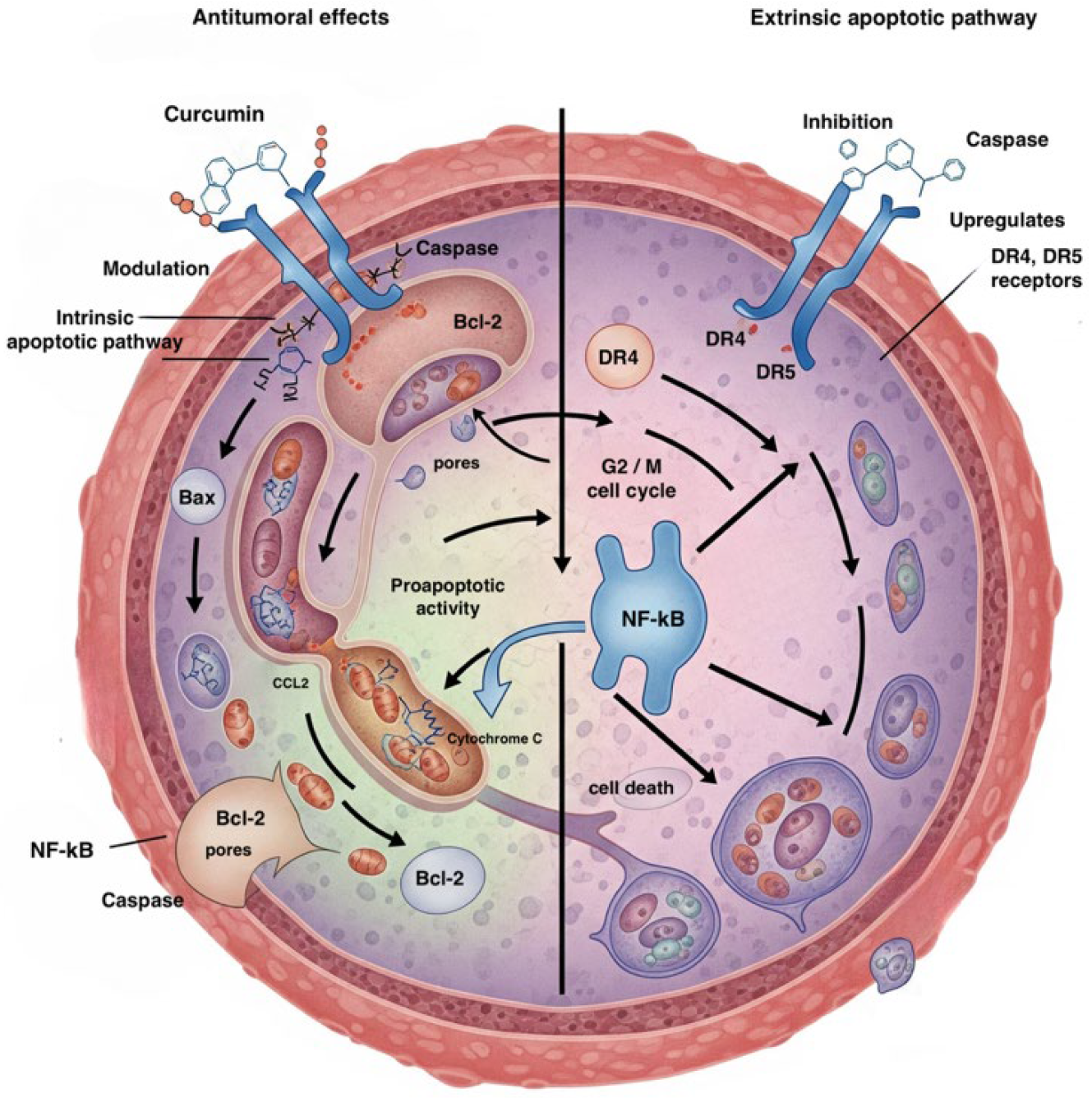
2.1. Overview of Curcumin
2.2. Curcumin and Acute Myeloid Leukemia
3. Curcumin, Combination Therapies, and Synergistic Effects in Acute Myeloid Leukemia
Curcumin, Curcuminoid, and Chemoresistance
| Therapeutic Combination | Cell Model | Mechanism of Action | Main Outcome | References |
|---|---|---|---|---|
| Curcumin + DNR | CD34+ AML (cell lines and primary cells) | ↓ Bcl-2 → ↑ sensitivity to DNR | Synergy in inhibiting proliferation and inducing apoptosis | [38] |
| Curcumin + ATO + lonidamine | Myeloid leukemia cells | ↑ ROS, mitochondrial pathway activation, ΔΨm dissipation | Increased efficacy of ATO and lonidamine | [39] |
| Curcumin + ATO | CD34+ KG-1a | ↑ Bax, ↓ Bcl-2, ↓ PARP | Increased apoptosis | [40] |
| Curcumin + valproic acid | HL-60 (APL) | ↑ Sp1 binding, histone H3/H4 acetylation on Bax promoter | ↑ Bax, ↓ proliferation, apoptosis | [41] |
| Curcumin + thalidomide | AML cell lines | ↓ STAT3, ↓ BCL-XL, anti-angiogenesis | Growth inhibition, apoptosis | [42,43] |
| Curcumin + TRAIL/IL2-TRAIL peptide | K562, MOLT4, HL-60, KG-1, AML PBMCs | ↑ DR4/DR5, ↓ cFLIP, ↑ ROS | 90% efficacy on AML PBMCs, no toxicity to healthy cells | [44,45,46,47,48,49,50,51] |
| Curcumin + naringenin | THP-1 | Cell cycle arrest at S and G2/M, ↑ apoptosis | Increased cytotoxicity vs. curcumin alone | [52] |
| Curcumin + AZA | AML cell lines and patient samples | ↓ DNA methylation, reactivation of tumor suppressor genes | Synergy: ↓ proliferation, ↑ apoptosis, low toxicity to healthy cells | [53,54,55,56,57] |
| Curcumin + Ara-C | AML cells | — | No direct synergy in vitro | [61,62,63,64,65,66] |
| Curcumin + THC | HL60 Ara-C resistant cells | CUR → apoptosis, THC → autophagy | Effective also in Ara-C-resistant primary cells | [67,68,69,70,71,72,73] |
| Therapeutic Combination | In Vivo Model | Mechanism/ Observations | Main Outcome | References |
|---|---|---|---|---|
| Curcumin + Ara-C | AML xenograft mouse model | Effect linked to gut microbiota and barrier integrity, not direct AML cell effect | Improved Ara-C response by strengthening intestinal barrier and reducing bacterial translocation | [64,65,66] |
| MHC + CA | AML in vivo | Ca2+-dependent apoptosis | Effective, no toxicity to healthy cells | [59] |
| Curcumin + CA | AML in vivo | Ca2+-dependent apoptosis without ROS | Selective cytotoxicity | [60] |
4. Future Perspectives: Innovation and Nanotechnologies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Döhner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef] [PubMed]
- Samarkhazan, H.S.; Zehtabcheh, S.; Rahmani Seraji, H.; Beqaj, S.H.; Tayefeh, S.; Mohammadi, M.H.; Aghaei, M. Unveiling the potential of CLL-1: A promising target for AML therapy. Biomark. Res. 2025, 13, 28. [Google Scholar] [CrossRef]
- Bhansali, R.S.; Pratz, K.W.; Lai, C. Recent advances in targeted therapies in acute myeloid leukemia. J. Hematol. Oncol. 2023, 16, 29. [Google Scholar] [CrossRef]
- Chen, Y.F.; Li, J.; Xu, L.L.; Găman, M.A.; Zou, Z.Y. Allogeneic stem cell transplantation in the treatment of acute myeloid leukemia: An overview of obstacles and opportunities. World J. Clin. Cases 2023, 11, 268–291. [Google Scholar] [CrossRef]
- Liu, J.M.; Li, M.; Luo, W.; Sun, H.B. Curcumin attenuates Adriamycin-resistance of acute myeloid leukemia by inhibiting the LncRNA HOTAIR/miR-20a-5p/WT1 axis. Lab. Investig. 2021, 101, 1308–1317. [Google Scholar] [CrossRef]
- Urošević, M.; Nikolić, L.; Gajić, I.; Nikolić, V.; Dinić, A.; Miljković, V. Curcumin: Biological Activities and Modern Pharmaceutical Forms. Antibiotics 2022, 11, 135. [Google Scholar] [CrossRef]
- Chota, A.; George, B.P.; Abrahamse, H. Interactions of multi-domain pro-apoptotic and anti-apoptotic proteins in cancer cell death. Oncotarget 2021, 12, 1615–1626. [Google Scholar] [CrossRef]
- Kah, G.; Chandran, R.; Abrahamse, H. Curcumin a Natural Phenol and Its Therapeutic Role in Cancer and Photodynamic Therapy: A Review. Pharmaceutics 2023, 15, 639. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, J.; Jiang, B.; Guo, J. The roles of curcumin in regulating the tumor immunosuppressive microenvironment. Oncol. Lett. 2020, 19, 3059–3070. [Google Scholar] [CrossRef]
- Zoi, V.; Kyritsis, A.P.; Galani, V.; Lazari, D.; Sioka, C.; Voulgaris, S.; Alexiou, G.A. The Role of Curcumin in Cancer: A Focus on the PI3K/Akt Pathway. Cancers 2024, 16, 1554. [Google Scholar] [CrossRef] [PubMed]
- Vieira, B.M.; Ferreira Caetano, M.A.; de Carvalho, M.T.; Dos Santos Arruda, F.; Tomé, F.D.; Fernandes de Oliveira, J.; Soave, D.F.; Pereira, J.X.; Celes, M.R.N. Impacts of Curcumin Treatment on Experimental Sepsis: A Systematic Review. Oxidative Med. Cell. Longev. 2023, 2023, 2252213. [Google Scholar] [CrossRef]
- Delshadi, R.; Bahrami, A.; McClements, D.J.; Moore, M.; Williams, L. Development of nanoparticle-delivery systems for antiviral agents: A review. J. Control. Release 2021, 331, 30–44. [Google Scholar] [CrossRef]
- Dhar, S.; Bhattacharjee, P. Promising role of curcumin against viral diseases emphasizing COVID-19 management: A review on the mechanistic insights with reference to host-pathogen interaction and immunomodulation. J. Funct. Foods 2021, 82, 104503. [Google Scholar] [CrossRef]
- Allegra, A.; Mirabile, G.; Ettari, R.; Pioggia, G.; Gangemi, S. The Impact of Curcumin on Immune Response: An Immunomodulatory Strategy to Treat Sepsis. Int. J. Mol. Sci. 2022, 23, 14710. [Google Scholar] [CrossRef]
- Entezari, M.; Tayari, A.; Paskeh, M.D.A.; Kheirabad, S.K.; Naeemi, S.; Taheriazam, A.; Dehghani, H.; Salimimoghadam, S.; Hashemi, M.; Mirzaei, S.; et al. Curcumin in treatment of hematological cancers: Promises and challenges. J. Tradit. Complement. Med. 2024, 14, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Ning, Y.; Zeng, G.; Zhou, C.; Ding, X. Curcumin promotes cell cycle arrest and apoptosis of acute myeloid leukemia cells by inactivating AKT. Oncol. Rep. 2021, 45, 11. [Google Scholar] [CrossRef] [PubMed]
- Ashour, A.A.; Abdel-Aziz, A.A.H.; Mansour, A.M.; Alpay, S.N.; Huo, L.; Ozpolat, B. Targeting elongation factor-2 kinase (eEF-2K) induces apoptosis in human pancreatic cancer cells. Apoptosis 2014, 19, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Gachpazan, M.; Habbibirad, S.; Kashani, H.; Jamialahmadi, T.; Rahimi, H.R.; Sahebkar, A. Targeting Nuclear Factor-Kappa B Signaling Pathway by Curcumin: Implications for the Treatment of Multiple Sclerosis. Adv. Exp. Med. Biol. 2021, 1291, 41–53. [Google Scholar]
- Zamanian, M.Y.; Alsaab, H.O.; Golmohammadi, M.; Yumashev, A.; Jabba, A.M.; Abid, M.K.; Joshi, A.; Alawadi, A.H.; Jafer, N.S.; Kianifar, F.; et al. NF-κB pathway as a molecular target for curcumin in diabetes mellitus treatment: Focusing on oxidative stress and inflammation. Cell Biochem. Funct. 2024, 42, e4030. [Google Scholar] [CrossRef]
- Bahadar, N.; Bahadar, S.; Sajid, A.; Wahid, M.; Ali, G.; Alghamdi, A.; Zada, H.; Khan, T.; Ullah, S.; Sun, Q. Epigallocatechin gallate and curcumin inhibit Bcl-2: A pharmacophore and docking based approach against cancer. Breast Cancer Res. 2024, 26, 114. [Google Scholar] [CrossRef]
- Singh, S.; Barnes, C.A.; D’Souza, J.S.; Hosur, R.V.; Mishra, P. Curcumin, a potential initiator of apoptosis via direct interactions with Bcl-xL and Bid. Proteins 2022, 90, 455–464. [Google Scholar] [CrossRef]
- Amirsaadat, S.; Jafari-Gharabaghlou, D.; Dadashpour, M.; Zarghami, N. Potential Anti-Proliferative Effect of Nano-Formulated Curcumin Through Modulating MicroRNA-132, Cyclin D1, and hTERT Gene Expression in Breast Cancer Cell Lines. J. Clust. Sci. 2023. [Google Scholar] [CrossRef]
- Mohan, M.; Hussain, M.A.; Khan, F.A.; Anindya, R. Symmetrical and un-symmetrical curcumin analogues as selective COX-1 and COX-2 inhibitor. Eur. J. Pharm. Sci. 2021, 160, 105743. [Google Scholar] [CrossRef]
- Rainey, N.E.; Moustapha, A.; Petit, P.X. Curcumin, a Multi-Faceted Hormetic Agent, Mediates an Intricate Crosstalk between Mitochondrial Turnover, Autophagy, and Apoptosis. Oxidative Med. Cell. Longev. 2020, 2020, 3656419. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Yang, B.; Najafi, M. Targeting of cancer cell death mechanisms by curcumin: Implications to cancer therapy. Basic Clin. Pharmacol. Toxicol. 2021, 129, 397–415. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhuang, Z.; Lu, Y.; Tao, T.; Zhou, Y.; Liu, G.; Wang, H.; Zhang, D.; Wu, L.; Dai, H. Curcumin Mitigates Neuro-Inflammation by Modulating Microglia Polarization Through Inhibiting TLR4 Axis Signaling Pathway Following Experimental Subarachnoid Hemorrhage. Front. Neurosci. 2019, 13, 1223. [Google Scholar] [CrossRef]
- Hsiao, P.C.; Chang, J.H.; Lee, W.J.; Ku, C.C.; Tsai, M.Y.; Yang, S.F.; Chien, M.H. The Curcumin Analogue, EF-24, Triggers p38 MAPK-Mediated Apoptotic Cell Death via Inducing PP2A-Modulated ERK Deactivation in Human Acute Myeloid Leukemia Cells. Cancers 2020, 12, 2163. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Drużga, A.; Katarzyna, J.; Skonieczna-Żydecka, K. Antioxidant Potential of Curcumin- A Meta-Analysis of Randomized Clinical Trials. Antioxidants 2020, 9, 1092. [Google Scholar] [CrossRef]
- Liao, D.; Shangguan, D.; Wu, Y.; Chen, Y.; Liu, N.; Tang, J.; Yao, D.; Shi, Y. Curcumin protects against doxorubicin-induced oxidative stress by regulating the Keap1-Nrf2-ARE and autophagy signaling pathways. Psychopharmacology 2023, 240, 1179–1190. [Google Scholar] [CrossRef]
- Trombetti, S.; Cesaro, E.; Catapano, R.; Sessa, R.; Lo Bianco, A.; Izzo, P.; Grosso, M. Oxidative Stress and ROS-Mediated Signaling in Leukemia: Novel Promising Perspectives to Eradicate Chemoresistant Cells in Myeloid Leukemia. Int. J. Mol. Sci. 2021, 22, 2470. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Al-Ostoot, F.H.; Salah, S.; Khamees, H.A.; Khanum, S.A. Tumor angiogenesis: Current challenges and therapeutic opportunities. Cancer Treat. Res. Commun. 2021, 28, 100422. [Google Scholar] [CrossRef] [PubMed]
- Salemi, M.; Mohammadi, S.; Ghavamzadeh, A.; Nikbakht, M. Anti-Vascular Endothelial Growth Factor Targeting by Curcumin and Thalidomide in Acute Myeloid Leukemia Cells. Asian Pac. J. Cancer Prev. 2017, 18, 3055–3061. [Google Scholar] [PubMed]
- Younes, M.; Mardirossian, R.; Rizk, L.; Fazlian, T.; Khairallah, J.P.; Sleiman, C.; Naim, H.Y.; Rizk, S. The Synergistic Effects of Curcumin and Chemotherapeutic Drugs in Inhibiting Metastatic, Invasive, and Proliferative Pathways. Plants 2022, 11, 2137. [Google Scholar] [CrossRef] [PubMed]
- Broxterman, H.J.; Georgopapadakou, N.H. Anticancer therapeutics: “Addictive” targets, multi-targeted drugs, new drug combinations. Drug Resist. Updates 2005, 8, 183–197. [Google Scholar] [CrossRef]
- Jonas, B.A.; Pollyea, D.A. How we use venetoclax with hypomethylating agents for the treatment of newly diagnosed patients with acute myeloid leukemia. Leukemia 2019, 33, 2795–2804. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E. Curcumin Combination Chemotherapy: The Implication and Efficacy in Cancer. Molecules 2019, 24, 2527. [Google Scholar] [CrossRef]
- Rao, J.; Xu, D.R.; Zheng, F.M.; Long, Z.J.; Huang, S.S.; Wu, X.; Zhou, W.H.; Huang, R.W.; Liu, Q. Curcumin reduces expression of Bcl-2, leading to apoptosis in daunorubicin-insensitive CD34+ acute myeloid leukemia cell lines and primary sorted CD34+ acute myeloid leukemia cells. J. Transl. Med. 2011, 9, 71. [Google Scholar] [CrossRef]
- Sánchez, Y.; Simón, G.P.; Calviño, E.; de Blas, E.; Aller, P. Curcumin stimulates reactive oxygen species production and potentiates apoptosis induction by the anti-tumor drugs arsenic trioxide and Lonidamine in human myeloid leukemia cell lines. J. Pharmacol. Exp. Ther. 2010, 335, 114–123. [Google Scholar] [CrossRef]
- Fan, J.X.; Zeng, Y.J.; Wu, J.W.; Li, Z.Q.; Li, Y.M.; Zheng, R.; Weng, G.Y.; Guo, K.Y. Synergistic killing effect of arsenic trioxide combined with Curcumin on KG1a cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2014, 22, 1267–1272. [Google Scholar]
- Chen, J.; Wang, G.; Wang, L.; Kang, J.; Wang, J. Curcumin p38-dependently enhances the anti-cancer activity of valproic acid in human leukemia cells. Eur. J. Pharm. Sci. 2010, 41, 210–218. [Google Scholar] [CrossRef]
- Mohammadi Kian, M.; Salemi, M.; Bahadoran, M.; Haghi, A.; Dashti, N.; Mohammadi, S.; Rostami, S.; Chahardouli, B.; Babakhani, D.; Nikbakht, M. Curcumin Combined with Thalidomide Reduces Expression of STAT3 and Bcl-xL, Leading to Apoptosis in Acute Myeloid Leukemia Cell Lines. Drug Des. Dev. Ther. 2020, 14, 185–194. [Google Scholar] [CrossRef]
- Shehzad, A.; Lee, J.; Lee, Y.S. Curcumin in various cancers. BioFactors 2013, 39, 56–68. [Google Scholar] [CrossRef]
- Surapally, S.; Jayaprakasam, M.; Verma, R.S. Curcumin augments therapeutic efficacy of TRAIL-based immunotoxins in leukemia. Pharmacol. Rep. 2020, 72, 1032–1046. [Google Scholar] [CrossRef]
- Pesakhov, S.; Khanin, M.; Studzinski, G.P.; Danilenko, M. Distinct combinatorial effects of the plant polyphenols curcumin, carnosic acid, and silibinin on proliferation and apoptosis in acute myeloid leukemia cells. Nutr. Cancer 2010, 62, 811–824. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.M.; Lim, J.H.; Lee, T.J.; Park, J.W.; Choi, K.S.; Kwon, T.K. Curcumin sensitizes tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis through reactive oxygen species-mediated upregulation of death receptor 5 (DR5). Carcinogenesis 2005, 26, 1905–1913. [Google Scholar] [CrossRef]
- Park, S.; Cho, D.H.; Andera, L.; Suh, N.; Kim, I. Curcumin enhances TRAIL-induced apoptosis of breast cancer cells by regulating apoptosis-related proteins. Mol. Cell. Biochem. 2013, 383, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Zhang, J.; Arfuso, F.; Chinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; Kumar, A.P.; Ahn, K.S.; Sethi, G. Targeting TNF-related apoptosis-inducing ligand (TRAIL) receptor by natural products as a potential therapeutic approach for cancer therapy. Exp. Biol. Med. 2015, 240, 760–773. [Google Scholar] [CrossRef] [PubMed]
- Pavan, A.R.; Bernardes da Silva, G.D.; Hartmann Jornada, D.; Chiba, D.; dos Santos Fernandes, G.F.; Chin, C.M.; dos Santos, J.L. Unraveling the Anticancer Effect of Curcumin and Resveratrol. Nutrients 2016, 8, 628. [Google Scholar] [CrossRef]
- Madhumathi, J.; Sridevi, S.; Verma, R.S. Novel TNF-related Apoptotic-inducing Ligand-based Immunotoxin for Therapeutic Targeting of CD25 Positive Leukemia. Target. Oncol. 2016, 11, 535–547. [Google Scholar] [CrossRef]
- Madhumathi, J.; Sridevi, S.; Verma, R.S. CD25 targeted therapy of chemotherapy resistant leukemic stem cells using DR5 specific TRAIL peptide. Stem Cell Res. 2017, 19, 65–75. [Google Scholar] [CrossRef]
- Shi, D.; Xu, Y.; Du, X.; Chen, X.; Zhang, X.; Lou, J.; Li, M.; Zhuo, J. Co-treatment of THP-1 cells with naringenin and curcumin induces cell cycle arrest and apoptosis via numerous pathways. Mol. Med. Rep. 2015, 12, 8223–8228. [Google Scholar] [CrossRef]
- Martìn, I.; Navarro, B.; Solano, C.; Calabuig, M.; Hernández-Boluda, J.C.; Amat, P.; Remigia, M.J.; García, F.; Villamón, E.; Tormo, M. Synergistic Antioncogenic Activity of Azacitidine and Curcumin in Myeloid Leukemia Cell Lines and Patient Samples. Anticancer Res. 2019, 39, 4757–4766. [Google Scholar] [CrossRef]
- Su, Y.; Xu, H.; Xu, Y.; Yu, J.; Xian, Y.; Luo, Q. Azacytidine inhibits the proliferation of human promyelocytic leukemia cells (HL60) by demethylation of MGMT, DAPK, and p16 genes. Hematology 2012, 17, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Gambari, R.; del Senno, L.; Barbieri, R.; Viola, L.; Tripodi, M.; Raschellà, G.; Fantoni, A. Human leukemia K-562 cells: Induction of erythroid differentiation by 5-azacytidine. Cell Differ. 1984, 14, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.N.; Luna-Moran, A.; Richard, N.; Belka, I.; Brady, H.; MacBeth, K.J. Azacitidine induces differentiation of acute myeloid leukemia cell lines along the granulocytic/monocytic lineage. Cancer Res. 2010, 70, 191. [Google Scholar] [CrossRef]
- Hassan, H.E.; Keita, J.A.; Narayan, L.; Brady, S.M.; Frederick, R.; Carlson, S.; Glass, K.C.; Natesan, S.; Buttolph, T.; Fandy, T.E. The combination of dimethoxycurcumin with a DNA methylation inhibitor enhances gene re-expression of promoter-methylated genes and antagonizes their cytotoxic effect. Epigenetics 2016, 11, 740–749. [Google Scholar] [CrossRef]
- Trachtenberg, A.; Muduli, S.; Sidoryk, K.; Cybulski, M.; Danilenko, M. Synergistic Cytotoxicity of Methyl 4-Hydroxycinnamate and Carnosic Acid to Acute Myeloid Leukemia Cells via Calcium-Dependent Apoptosis Induction. Front. Pharmacol. 2019, 10, 507. [Google Scholar] [CrossRef]
- Pesakhov, S.; Nachliely, M.; Barvish, Z.; Aqaqe, N.; Schwartzman, B.; Voronov, E.; Sharoni, Y.; Studzinski, G.P.; Fishman, D.; Danilenko, M. Cancer-selective cytotoxic Ca2+ overload in acute myeloid leukemia cells and attenuation of disease progression in mice by synergistically acting polyphenols curcumin and carnosic acid. Oncotarget 2016, 7, 31847–31861. [Google Scholar] [CrossRef]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef]
- Liu, J.; Luo, W.; Chen, Q.; Chen, X.; Zhou, G.; Sun, H. Curcumin sensitizes response to cytarabine in acute myeloid leukemia by regulating intestinal microbiota. Cancer Chemother. Pharmacol. 2022, 89, 243–253. [Google Scholar] [CrossRef]
- Ferrara, F.; Schiffer, C.A. Acute myeloid leukaemia in adults. Lancet 2013, 381, 484–495. [Google Scholar] [CrossRef]
- Cheng, C.; Yuan, F.; Chen, X.; Zhang, W.; Zhao, X.; Jiang, Z.; Zhou, H.; Zhou, G.; Cao, S. Inhibition of Nrf2-mediated glucose metabolism by brusatol synergistically sensitizes acute myeloid leukemia to Ara-C. Biomed. Pharmacother. 2021, 142, 111652. [Google Scholar] [CrossRef]
- Saland, E.; Boutzen, H.; Castellano, R.; Pouyet, L.; Griessinger, E.; Larrue, C.; de Toni, F.; Scotland, S.; David, M.; Danet-Desnoyers, G. A robust and rapid xenograft model to assess efficacy of chemotherapeutic agents for human acute myeloid leukemia. Blood Cancer J. 2015, 5, e297. [Google Scholar] [CrossRef]
- Hosseini, M.; Rezvani, H.R.; Aroua, N.; Bosc, C.; Farge, T.; Saland, E.; Guyonnet-Dupérat, V.; Zaghdoudi, S.; Jarrou, L.; Larrue, C.; et al. Targeting Myeloperoxidase Disrupts Mitochondrial Redox Balance and Overcomes Cytarabine Resistance in Human Acute Myeloid Leukemia. Cancer Res. 2019, 79, 5191–5203. [Google Scholar] [CrossRef] [PubMed]
- Hueso, T.; Ekpe, K.; Mayeur, C.; Gatse, A.; Joncquel-Chevallier Curt, M.; Gricourt, G.; Rodriguez, C.; Burdet, C.; Ulmann, G.; Neut, C. Impact and consequences of intensive chemotherapy on intestinal barrier and microbiota in acute myeloid leukemia: The role of mucosal strengthening. Gut Microbes 2020, 12, 1800897. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.H.; Chiou, S.S.; Weng, J.P.; Lin, P.C. Curcumin and tetrahydrocurcumin induce cell death in Ara-C-resistant acute myeloid leukemia. Phytother. Res. 2019, 33, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.C.; Lai, C.S.; Badmaev, V.; Nagabhushanam, K.; Ho, C.T.; Pan, M.H. Tetrahydrocurcumin, a major metabolite of curcumin, induced autophagic cell death through coordinative modulation of PI3K/Akt-mTOR and MAPK signaling pathways in human leukemia HL-60 cells. Mol. Nutr. Food Res. 2011, 55, 1646–1654. [Google Scholar] [CrossRef]
- Wu, J.C.; Tsai, M.L.; Lai, C.S.; Wang, Y.J.; Ho, C.T.; Pan, M.H. Chemopreventative effects of tetrahydrocurcumin on human diseases. Food Funct. 2014, 5, 12–17. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Deb, L.; Prasad, S. Curcumin differs from tetrahydrocurcumin for molecular targets, signaling pathways and cellular responses. Molecules 2014, 20, 185–205. [Google Scholar] [CrossRef]
- Chen, L.; Guo, P.; Zhang, Y.; Li, X.; Jia, P.; Tong, J.; Li, J. Autophagy is an important event for low-dose cytarabine treatment in acute myeloid leukemia cells. Leuk. Res. 2017, 60, 44–52. [Google Scholar] [CrossRef]
- De Vries, J.F.; Falkenburg, J.H.; Willemze, R.; Barge, R.M.Y. The mechanisms of Ara-C-induced apoptosis of resting B-chronic lymphocytic leukemia cells. Haematologica 2006, 91, 912–919. [Google Scholar]
- Pan, S.T.; Li, Z.L.; He, Z.; Qiu, J.; Zhou, S. Molecular mechanisms for tumour resistance to chemotherapy. Clin. Exp. Pharmacol. Physiol. 2016, 43, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Bordoloi, D.; Kunnumakkara, A.B. The Potential of Curcumin: A Multitargeting Agent in Cancer Cell Chemosensitization. Role Nutraceuticals Cancer Chemosensitizat. 2018, 2, 31–60. [Google Scholar]
- Zoi, V.; Galani, V.; Lianos, G.D.; Voulgaris, S.; Kyritsis, A.P.; Alexiou, G.A. The Role of Curcumin in Cancer Treatment. Biomedicines 2021, 9, 1086. [Google Scholar] [CrossRef] [PubMed]
- Bučević Popović, V.; Farhat, E.K.; Banjari, I.; Jeličić Kadić, A.; Puljak, L. Bioavailability of Oral Curcumin in Systematic Reviews: A Methodological Study. Pharmaceuticals 2024, 17, 164. [Google Scholar] [CrossRef]
- Hegde, M.; Girisa, S.; Chetty, B.B.; Vishwa, R.; Kunnumakkara, A.B. Curcumin Formulations for Better Bioavailability: What We Learned from Clinical Trials Thus Far? ACS Omega 2023, 8, 10713–10746. [Google Scholar] [CrossRef]
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X. A Review of Curcumin and Its Derivatives as Anticancer Agents. Int. J. Mol. Sci. 2019, 20, 1033. [Google Scholar] [CrossRef]
- Bertoncini-Silva, C.; Vlad, A.; Ricciarelli, R.; Fassini, P.G.; Suen, V.M.M.; Zingg, J. Enhancing the Bioavailability and Bioactivity of Curcumin for Disease Prevention and Treatment. Antioxidants 2024, 13, 331. [Google Scholar] [CrossRef]
- Nirachonkul, W.; Ogonoki, S.; Thumvijit, T.; Chiampanichayakul, S.; Panyajai, P.; Anuchapreeda, S.; Tima, S.; Chiampanichayakul, S. CD123-Targeted Nano-Curcumin Molecule Enhances Cytotoxic Efficacy in Leukemic Stem Cells. Nanomaterials 2021, 11, 2974. [Google Scholar] [CrossRef]
- Sun, D.; Zhou, J.K.; Zhao, L.; Zheng, Z.; Li, J.; Pu, W.; Liu, S.; Liu, X.; Liu, S.J.; Zheng, Y.; et al. Novel Curcumin Liposome Modified with Hyaluronan Targeting CD44 Plays an Anti-Leukemic Role in Acute Myeloid Leukemia in Vitro and in Vivo. ACS Appl. Mater. Interfaces 2017, 9, 16857–16868. [Google Scholar] [CrossRef]
- Hafez Ghoran, S.; Calcaterra, A.; Abbasi, M.; Taktaz, F.; Nieselt, K.; Babaei, E. Curcumin-Based Nanoformulations: A Promising Adjuvant towards Cancer Treatment. Molecules 2022, 27, 5236. [Google Scholar] [CrossRef] [PubMed]
- Tima, S.; Anuchapreeda, S.; Ampasavate, C.; Berkland, C.; Okonogi, S. Stable curcumin-loaded polymeric micellar formulation for enhancing cellular uptake and cytotoxicity to FLT3 overexpressing EoL-1 leukemic cells. Eur. J. Pharm. Biopharm. 2017, 114, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.K.; Chakraborty, D.; Sarkar, I.; Khan, T.; Sa, G. New insights into therapeutic activity and anticancer properties of curcumin. J. Exp. Pharmacol. 2017, 9, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Oyeyode, M.; Tempel, M.; Lakowski, T.M.; Davie, J.R. DNA intercalating drugs: Mechanisms of action in cancer treatment. Adv. Biol. Regul. 2025, 98, 101115. [Google Scholar] [CrossRef]
- Hilmer, S.N.; Cogger, V.C.; Muller, M.; Le Couteur, D.G. The hepatic pharmacokinetics of doxorubicin and liposomal doxorubicin. Drug Metab. Dispos. 2004, 32, 794–799. [Google Scholar] [CrossRef]
- Gabizon, A.; Shmeeda, H.; Barenholz, Y. Pharmacokinetics of pegylated liposomal Doxorubicin: Review of animal and human studies. Clin. Pharmacokinet. 2003, 42, 419–436. [Google Scholar] [CrossRef]
- Minotti, G.; Menna, P.; Salvatorelli, E.; Cairo, G.; Gianni, L. Anthracyclines: Molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol. Rev. 2004, 56, 185–229. [Google Scholar] [CrossRef]
- Fernandez, H.F.; Sun, Z.; Yao, X.; Litzow, M.R.; Luger, S.; Paietta, E.; Racevskis, J.; Dewald, G.; Ketterling, R.; Bennett, J.; et al. Anthracycline dose intensification in acute myeloid leukemia. N. Engl. J. Med. 2009, 361, 1249–1259. [Google Scholar] [CrossRef]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2013, 65, 157–170. [Google Scholar] [CrossRef]
- Chueahongthong, F.; Chiampanichayakul, S.; Viriyaadhammaa, N.; Dejkriengkraikul, P.; Okonogi, S.; Berkland, C.; Anuchapreeda, S. Cytotoxicity of Doxorubicin-Curcumin Nanoparticles Conjugated with Two Different Peptides (CKR and EVQ) against FLT3 Protein in Leukemic Stem Cells. Polymers 2024, 16, 2498. [Google Scholar] [CrossRef]
- Sharma, R.A.; Gescher, A.J.; Steward, W.P. Curcumin: The story so far. Eur. J. Cancer 2005, 41, 1955–1968. [Google Scholar] [CrossRef]
- Tima, S.; Okonogi, S.; Ampasavate, C.; Pickens, C.; Berkland, C.; Anuchapreeda, S. Development and Characterization of FLT3-Specific Curcumin-Loaded Polymeric Micelles as a Drug Delivery System for Treating FLT3-Overexpressing Leukemic Cells. J. Pharm. Sci. 2016, 105, 3645–3657. [Google Scholar] [CrossRef] [PubMed]
- Chueahongthong, F.; Tima, S.; Chiampanichayakul, S.; Dejkriengkraikul, P.; Okonogi, S.; Sasarom, M.; Rodwattanagul, S.; Berkland, C.; Anuchapreeda, S. Doxorubicin-Loaded Polymeric Micelles Conjugated with CKR- and EVQ-FLT3 Peptides for Cytotoxicity in Leukemic Stem Cells. Pharmaceutics 2022, 14, 2115. [Google Scholar] [CrossRef]
- Elsayed Ebeid, F.S. Safety and Efficacy of Curcumin in Children with Acute Lymphoblastic Leukemia. ClinicalTrials.gov ID NCT05045443. 2024. Available online: https://clinicaltrials.gov/study/NCT05045443 (accessed on 1 August 2024).
- Caimi, P. Curcumin and Cholecalciferol in Treating Patients with Previously Untreated Stage 0-II Chronic Lymphocytic Leukemia or Small Lymphocytic Lymphoma. ClinicalTrials.gov ID NCT02100423. 2024. Available online: https://clinicaltrials.gov/study/NCT02100423 (accessed on 1 August 2024).
- Papież, M.A.; Krzyściak, W.; Szade, K.; Bukowska-Strakovà, K.; Kozakowska, M.; Hajduk, K.; Bystrowska, B.; Dulak, J.; Jozkowicz, A. Curcumin enhances the cytogenotoxic effect of etoposide in leukemia cells through induction of reactive oxygen species. Drug Des. Dev. Ther. 2016, 4, 557–570. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.R.; Lu, F.; Lu, T.; Dong, W.; Li, P.; Liu, N.; Ma, D.; Ji, C. Inactivation of FoxM1 transcription factor contributes to curcumin-induced inhibition of survival, angiogenesis, and chemosensitivity in acute myeloid leukemia cells. J. Mol. Med. 2014, 12, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.J.; Liu, F.; Wu, M.; Wu, X.; Zhang, D.; Yuan, Q.; Zhou, L.; Wu, Z. Curcumin combined with arsenic trioxide in the treatment of acute myeloid leukemia: Network pharmacology analysis and experimental validation. J. Cancer Res. Clin. Oncol. 2023, 149, 219–230. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, S.; Cui, Y.; Jiang, J.; Luo, J.; Gao, Y.; Zeng, Y. Curcumin combined with arsenic trioxide enhances autophagy and immune surveillance to inhibit immune escape in acute myeloid leukemia. Int. Immunopharmacol. 2025, 159, 114966. [Google Scholar] [CrossRef]
- Sudarshan, K.; Yarlagadda, S.; Sengupta, S. Recent Advances in the Synthesis of Diarylheptanoids. Chem. Asian J. 2024, 19, e202400380. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badagliacca, R.; Fazio, M.; Stagno, F.; Mirabile, G.; Gerace, D.; Allegra, A. Curcumin and Acute Myeloid Leukemia: Synergistic Effects with Targeted Therapy. Int. J. Mol. Sci. 2025, 26, 9700. https://doi.org/10.3390/ijms26199700
Badagliacca R, Fazio M, Stagno F, Mirabile G, Gerace D, Allegra A. Curcumin and Acute Myeloid Leukemia: Synergistic Effects with Targeted Therapy. International Journal of Molecular Sciences. 2025; 26(19):9700. https://doi.org/10.3390/ijms26199700
Chicago/Turabian StyleBadagliacca, Rita, Manlio Fazio, Fabio Stagno, Giuseppe Mirabile, Demetrio Gerace, and Alessandro Allegra. 2025. "Curcumin and Acute Myeloid Leukemia: Synergistic Effects with Targeted Therapy" International Journal of Molecular Sciences 26, no. 19: 9700. https://doi.org/10.3390/ijms26199700
APA StyleBadagliacca, R., Fazio, M., Stagno, F., Mirabile, G., Gerace, D., & Allegra, A. (2025). Curcumin and Acute Myeloid Leukemia: Synergistic Effects with Targeted Therapy. International Journal of Molecular Sciences, 26(19), 9700. https://doi.org/10.3390/ijms26199700







