RNA Signature as Potential Diagnostic Marker for Differentiation of Pancreatic Cysts: A Pilot Study
Abstract
1. Introduction
2. Results
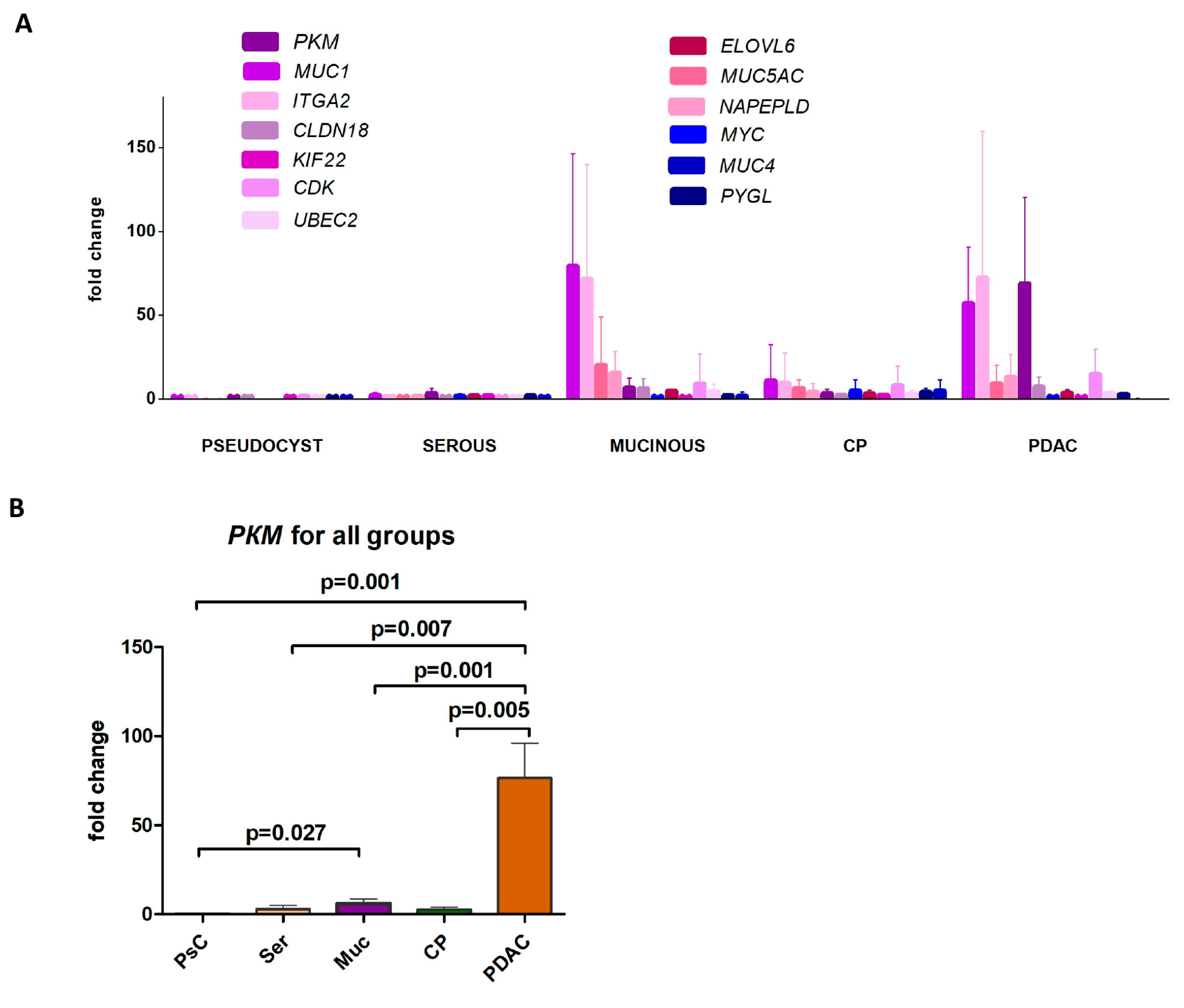
2.1. Gene Expression Profiling in EUS-FNA Samples
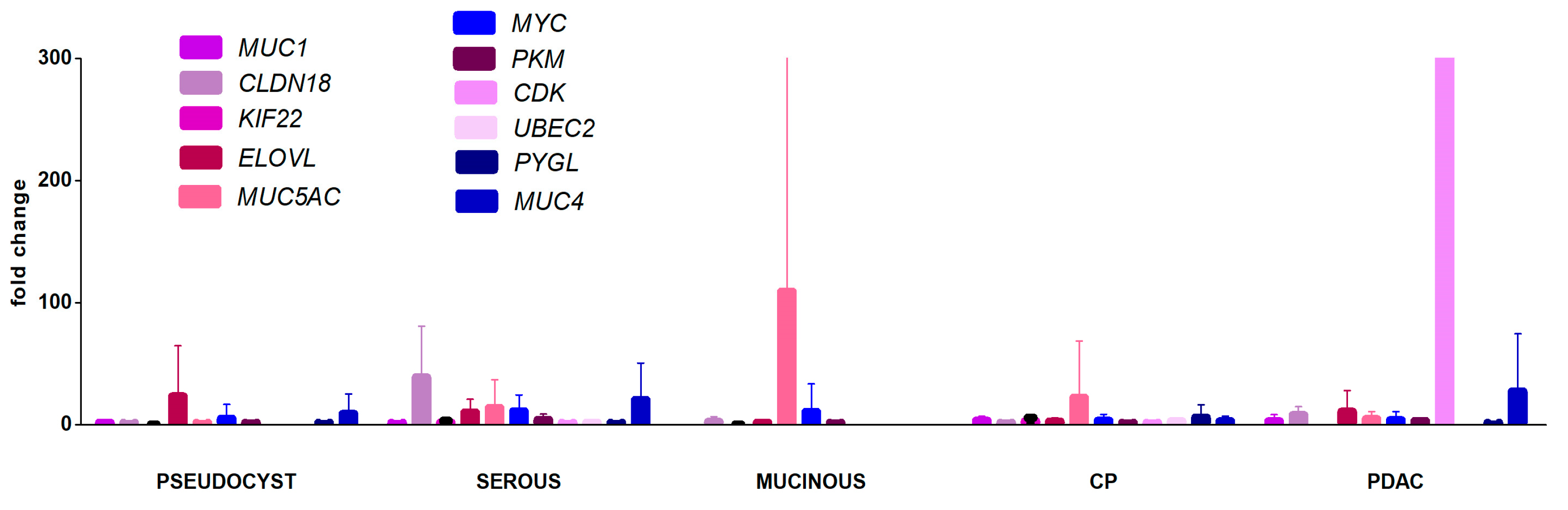
2.2. Gene Expression Profiling in Blood Plasma Samples
3. Discussion
4. Materials and Methods
4.1. Study Cohort
4.2. Sample Preparation
4.3. Reverse Transcription and Real-Time PCR
4.4. mRNA Expression Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EUS-FNA | endoscopic ultrasound-guided fine-needle aspiration |
| CP | chronic pancreatitis |
| SCN | serous cystic neoplasm |
| MCN | mucinous cystic neoplasm |
| IPMN | intraductal papillary mucinous neoplasm |
| PDAC | pancreatic ductal adenocarcinoma |
References
- Zerboni, G.; Signoretti, M.; Crippa, S.; Falconi, M.; Arcidiacono, P.G.; Capurso, G. Systematic review and meta-analysis: Prevalence of incidentally detected pancreatic cystic lesions in asymptomatic individuals. Pancreatology 2019, 19, 2–9. [Google Scholar] [CrossRef]
- Felix, N.; Max, H.C.; Friedrich, K.K.; Pascal, P.; Frederick, L.E.; Eva, K.; Marcus K, D.; Oliver, S.; Peter, M.-S.B.; Thilo, H. Laparoscopic Versus Open Pancreaticoduodenectomy: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Ann. Surg. 2020, 271, 54–66. [Google Scholar] [CrossRef]
- Majumder, S.; Philip, N.A.; Singh Nagpal, S.J.; Takahashi, N.; Mara, K.C.; Kendrick, M.L.; Smyrk, T.C.; Zhang, L.; Levy, M.J.; Gleeson, F.C.; et al. High-Grade Dysplasia in Resected Main-Duct Intraductal Papillary Mucinous Neoplasm (MD-IPMN) is Associated with an Increased Risk of Subsequent Pancreatic Cancer. Off. J. Am. Coll. Gastroenterol. ACG 2019, 114, 524. [Google Scholar] [CrossRef]
- Ohtsuka, T.; Fernandez-Del Castillo, C.; Furukawa, T.; Hijioka, S.; Jang, J.-Y.; Lennon, A.M.; Miyasaka, Y.; Ohno, E.; Salvia, R.; Wolfgang, C.L.; et al. International evidence-based Kyoto guidelines for the management of intraductal papillary mucinous neoplasm of the pancreas. Pancreatology 2024, 24, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Kadayifci, A.; Atar, M.; Wang, J.L.; Forcione, D.G.; Casey, B.W.; Pitman, M.B.; Brugge, W.R. Value of adding GNAS testing to pancreatic cyst fluid KRAS and carcinoembryonic antigen analysis for the diagnosis of intraductal papillary mucinous neoplasms. Dig. Endosc. 2017, 29, 111–117. [Google Scholar] [CrossRef]
- McCarty, T.R.; Paleti, S.; Rustagi, T. Molecular analysis of EUS-acquired pancreatic cyst fluid for KRAS and GNAS mutations for diagnosis of intraductal papillary mucinous neoplasia and mucinous cystic lesions: A systematic review and meta-analysis. Gastrointest. Endosc. 2021, 93, 1019–1033.e5. [Google Scholar] [CrossRef]
- Pflüger, M.J.; Jamouss, K.T.; Afghani, E.; Lim, S.J.; Rodriguez Franco, S.; Mayo, H.; Spann, M.; Wang, H.; Singhi, A.; Lennon, A.M.; et al. Predictive ability of pancreatic cyst fluid biomarkers: A systematic review and meta-analysis. Pancreatology 2023, 23, 868–877. [Google Scholar] [CrossRef]
- Belfrage, H.; Boyd, S.; Louhimo, J.; Kytölä, S.; Johansson, K.; Tenca, A.; Puustinen, L.; Kokkola, A.; Arkkila, P.; Arola, J.; et al. Next-generation sequencing improves diagnostic accuracy of imaging and carcinoembryonic antigen alone for pancreatic cystic neoplasms. Pancreatology 2024, 24, 1322–1331. [Google Scholar] [CrossRef]
- Venkat, S.; Alahmari, A.A.; Feigin, M.E. Drivers of Gene Expression Dysregulation in Pancreatic Cancer. Trends Cancer 2021, 7, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; DePinho, R.A.; Maitra, A. Single-cell RNA sequencing in pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 451–452. [Google Scholar] [CrossRef] [PubMed]
- Seyfedinova, S.S.; Freylikhman, O.A.; Sokolnikova, P.S.; Samochernykh, K.A.; Kostareva, A.A.; Kalinina, O.V.; Solonitsyn, E.G. Fine-needle aspiration technique under endoscopic ultrasound guidance: A technical approach for RNA profiling of pancreatic neoplasms. World J. Gastrointest. Oncol. 2024, 16, 2663–2672. [Google Scholar] [CrossRef]
- Huang, B.; Trujillo, M.A.; Fujikura, K.; Qiu, M.; Chen, F.; Felsenstein, M.; Zhou, C.; Skaro, M.; Gauthier, C.; Macgregor-Das, A.; et al. Molecular characterization of organoids derived from pancreatic intraductal papillary mucinous neoplasms. J. Pathol. 2020, 252, 252–262. [Google Scholar] [CrossRef]
- Melo, S.A.; Luecke, L.B.; Kahlert, C.; Fernandez, A.F.; Gammon, S.T.; Kaye, J.; LeBleu, V.S.; Mittendorf, E.A.; Weitz, J.; Rahbari, N.; et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 2015, 523, 177–182, Correction in Nature 2022, 610, E15–E17. [Google Scholar] [CrossRef]
- Shi, L.-E.; Shang, X.; Nie, K.-C.; Xu, Q.; Chen, N.-B.; Zhu, Z.-Z. Identification of potential crucial genes associated with the pathogenesis and prognosis of pancreatic adenocarcinoma. Oncol. Lett. 2020, 20, 60. [Google Scholar] [CrossRef]
- Zhou, J.; Hui, X.; Mao, Y.; Fan, L. Identification of novel genes associated with a poor prognosis in pancreatic ductal adenocarcinoma via a bioinformatics analysis. Biosci. Rep. 2019, 39, BSR20190625. [Google Scholar] [CrossRef] [PubMed]
- Giulietti, M.; Occhipinti, G.; Principato, G.; Piva, F. Weighted gene co-expression network analysis reveals key genes involved in pancreatic ductal adenocarcinoma development. Cell Oncol. 2016, 39, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-A.; Rong, Y.; Mao, W.; Zhang, L.; Kuang, T.; Lou, W. Gene expression profiling reveals the genomic changes caused by MLN4924 and the sensitizing effects of NAPEPLD knockdown in pancreatic cancer. Cell Cycle 2022, 21, 152–171. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, Y.; Gao, H.; Jia, Y.; Xu, Y.; Wan, X.; Zhang, Z.; Yu, H.; Yan, S. Identification of Key Genes Involved in Pancreatic Ductal Adenocarcinoma with Diabetes Mellitus Based on Gene Expression Profiling Analysis. Pathol. Oncol. Res. 2021, 27, 604730. [Google Scholar] [CrossRef]
- Zhang, R.; Ma, L.; Wei, Y.; Wei, K.; Song, T.; Du, Z.; Feng, Z. KIF22 Promotes Development of Pancreatic Cancer by Regulating the MEK/ERK/P21 Signaling Axis. Biomed. Res. Int. 2022, 2022, 6000925. [Google Scholar] [CrossRef]
- Ji, Q.; Li, H.; Cai, Z.; Yuan, X.; Pu, X.; Huang, Y.; Fu, S.; Chu, L.; Jiang, C.; Xue, J.; et al. PYGL-mediated glucose metabolism reprogramming promotes EMT phenotype and metastasis of pancreatic cancer. Int. J. Biol. Sci. 2023, 19, 1894–1909. [Google Scholar] [CrossRef] [PubMed]
- Shang, M.; Zhang, L.; Chen, X.; Zheng, S. Identification of hub genes and regulators associated with pancreatic ductal adenocarcinoma based on integrated gene expression profile analysis. Discov. Med. 2019, 28, 159–172. [Google Scholar] [PubMed]
- de la Fuente, J.; Majumder, S. Molecular Diagnostics and Testing for Pancreatic Cysts. Curr. Treat. Options Gastroenterol. 2020, 18, 158–171. [Google Scholar] [CrossRef]
- Singhi, A.D.; Koay, E.J.; Chari, S.T.; Maitra, A. Early Detection of Pancreatic Cancer: Opportunities and Challenges. Gastroenterology 2019, 156, 2024–2040. [Google Scholar] [CrossRef] [PubMed]
- Nikiforova, M.N.; Wald, A.I.; Spagnolo, D.M.; Melan, M.A.; Grupillo, M.; Lai, Y.-T.; Brand, R.E.; O’Broin-Lennon, A.M.; McGrath, K.; Park, W.G.; et al. A Combined DNA/RNA-based Next-Generation Sequencing Platform to Improve the Classification of Pancreatic Cysts and Early Detection of Pancreatic Cancer Arising From Pancreatic Cysts. Ann. Surg. 2023, 278, e789–e797. [Google Scholar] [CrossRef] [PubMed]
- Jury, R.P.; Thibodeau, B.J.; Fortier, L.E.; Geddes, T.J.; Ahmed, S.; Pruetz, B.L.; Farinola, M.A.; Wilson, G.D. Gene expression changes associated with the progression of intraductal papillary mucinous neoplasms. Pancreas 2012, 41, 611–618. [Google Scholar] [CrossRef]
- Ziaziaris, W.A.; Lim, C.S.H.; Sioson, L.; Gill, A.J.; Samra, J.S.; Sahni, S.; Mittal, A. Gene Expression Profiling of Pancreatic Ductal Adenocarcinoma Arising From Intraductal Papillary Mucinous Neoplasms of the Pancreas. Cancer Med. 2024, 13, e70499. [Google Scholar] [CrossRef]
- Xu, F.; Liu, F.; Zhao, H.; An, G.; Feng, G. Prognostic Significance of Mucin Antigen MUC1 in Various Human Epithelial Cancers. Medicine 2015, 94, e2286. [Google Scholar] [CrossRef]
- Gao, T.; Cen, Q.; Lei, H. A review on development of MUC1-based cancer vaccine. Biomed. Pharmacother. 2020, 132, 110888. [Google Scholar] [CrossRef]
- Roy, L.D.; Sahraei, M.; Subramani, D.B.; Besmer, D.; Nath, S.; Tinder, T.L.; Bajaj, E.; Shanmugam, K.; Lee, Y.Y.; Hwang, S.I.L.; et al. MUC1 enhances invasiveness of pancreatic cancer cells by inducing epithelial to mesenchymal transition. Oncogene 2011, 30, 1449–1459. [Google Scholar] [CrossRef]
- Sierzega, M.; Młynarski, D.; Tomaszewska, R.; Kulig, J. Semiquantitative immunohistochemistry for mucin (MUC1, MUC2, MUC3, MUC4, MUC5AC, and MUC6) profiling of pancreatic ductal cell adenocarcinoma improves diagnostic and prognostic performance. Histopathology 2016, 69, 582–591. [Google Scholar] [CrossRef]
- Wang, S.; You, L.; Dai, M.; Zhao, Y. Mucins in pancreatic cancer: A well-established but promising family for diagnosis, prognosis and therapy. J. Cell Mol. Med. 2020, 24, 10279–10289. [Google Scholar] [CrossRef]
- Tomishima, K.; Sai, J.K.; Kanazawa, R.; Miura, H.; Shimizu, R.; Sato, K.; Ishii, S.; Saito, H.; Ito, T.; Fukumura, Y.; et al. Impact of MUC1 Expression on the Progression of Intraductal Papillary Mucinous Neoplasm With Worrisome Features During Follow-up. Pancreas 2017, 46, 1127–1132. [Google Scholar] [CrossRef]
- Sinha, J.; Cao, Z.; Dai, J.; Tang, H.; Partyka, K.; Hostetter, G.; Simeone, D.M.; Feng, Z.; Allen, P.J.; Brand, R.E.; et al. A Gastric Glycoform of MUC5AC Is a Biomarker of Mucinous Cysts of the Pancreas. PLoS ONE 2016, 11, e0167070. [Google Scholar] [CrossRef]
- Carrara, S.; Cangi, M.G.; Arcidiacono, P.G.; Perri, F.; Petrone, M.C.; Mezzi, G.; Boemo, C.; Talarico, A.; Cin, E.D.; Grassini, G.; et al. Mucin expression pattern in pancreatic diseases: Findings from EUS-guided fine-needle aspiration biopsies. Am. J. Gastroenterol. 2011, 106, 1359–1363. [Google Scholar] [CrossRef] [PubMed]
- Okajima, Y.; Matsuzaka, T.; Miyazaki, S.; Motomura, K.; Ohno, H.; Sharma, R.; Shimura, T.; Istiqamah, N.; Han, S.-I.; Mizunoe, Y.; et al. Morphological and functional adaptation of pancreatic islet blood vessels to insulin resistance is impaired in diabetic db/db mice. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166339. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.-Y.; Yang, Y.-C.; Wang, H.-P.; Tien, Y.-W.; Shun, C.-T.; Huang, H.-Y.; Hsiao, M.; Hua, K.-T. Pyruvate kinase M2 promotes pancreatic ductal adenocarcinoma invasion and metastasis through phosphorylation and stabilization of PAK2 protein. Oncogene 2018, 37, 1730–1742. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Mi, L.; Li, J.; Li, T.; Chen, J.; Dionigi, G.; Guan, H.; Sun, H. Pan-Cancer Analysis of the Oncogenic and Prognostic Role of PKM2: A Potential Target for Survival and Immunotherapy. Biomed. Res. Int. 2023, 2023, 3375109. [Google Scholar] [CrossRef]
- Zheng, H.; Tan, J.; Qin, F.; Zheng, Y.; Yang, X.; Liu, Z.; Cai, W.; Qin, X.; Liao, H. PKM2 modulates chemotherapy sensitivity by regulating autophagy and predicts the prognosis and immunity in pancancer. Sci. Rep. 2025, 15, 14626. [Google Scholar] [CrossRef]
- Primer Designing Tool. Available online: https://www.ncbi.nlm.nih.gov/tools/primer-blast/ (accessed on 4 August 2025).
- Dmitrieva, R.I.; Lelyavina, T.A.; Komarova, M.Y.; Galenko, V.L.; Ivanova, O.A.; Tikanova, P.A.; Khromova, N.V.; Golovkin, A.S.; Bortsova, M.A.; Sergushichev, A.; et al. Skeletal Muscle Resident Progenitor Cells Coexpress Mesenchymal and Myogenic Markers and Are Not Affected by Chronic Heart Failure-Induced Dysregulations. Stem Cells Int. 2019, 2019, 5690345. [Google Scholar] [CrossRef]
- James, B.L.; Zaidi, S.N.; Bs, N.; R, V.B.; Dokhe, Y.; Shetty, V.; Pillai, V.; Kuriakose, M.A.; Suresh, A. Reference gene evaluation for normalization of gene expression studies with lymph tissue and node-derived stromal cells of patients with oral squamous cell carcinoma. Oncol. Lett. 2024, 28, 540. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, J.-J.; Ge, W.-L.; Chen, L.; Yuan, H.; Meng, L.-D.; Huang, X.-M.; Shen, P.; Miao, Y.; Jiang, K.-R. YY1 inhibits the migration and invasion of pancreatic ductal adenocarcinoma by downregulating the FER/STAT3/MMP2 signaling pathway. Cancer Lett. 2019, 463, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Ge, W.-L.; Shi, G.-D.; Huang, X.-M.; Zong, Q.-Q.; Chen, Q.; Meng, L.-D.; Miao, Y.; Zhang, J.-J.; Jiang, K.-R. Optimization of internal reference genes for qPCR in human pancreatic cancer research. Transl. Cancer Res. 2020, 9, 2962–2971. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Study Cohorts | ||||||
|---|---|---|---|---|---|---|---|
| IPMN, MCN n = 7 | SCN n = 3 | Pseudocyst n = 6 | Adenocarcinoma n = 10 | Chronic Pancreatitis n = 5 | p | ||
| Patient gender, n (%) | Female | 3 (42.8) | 3 (100) | 2 (33.3) | 7 (70.0) | 1 (20.0) | 0.126 |
| Male | 4 (57.2) | 0 | 4 (66.6) | 3 (30.0) | 4 (80.0) | 0.126 | |
| Patient age (year), mean [range] | 72.4 [56–83] | 54.3 [37–70] | 51.7 [42–57] | 61.1 [44–84] | 63.8 [51–77] | 0.977 | |
| Source of specimen within pancreas, n (%) | Head\uncinate | 6 (85.7) | 0 | 4 (66.7) | 8 (80) | 5 (100) | 0.020 * |
| Body | 0 | 3 (100) | 0 | 0 | 0 | n/a | |
| Tail | 1 (14.3) | 0 | 2 (33.3) | 0 | 0 | n/a | |
| Neck\body\tail | 0 | 0 | 0 | 2 (20) | 0 | n/a | |
| Neoplasm diameter (mm), median [range] | 25 [10–38] | 40 [24–60] | 85 [27–150] | 35 [18–39] | - | 0.784 | |
| Stage of cancer if applicable, n (%) | I–II | - | - | - | 5 (50.0) | - | n/a |
| III–IV | - | - | - | 5 (50.0) | - | n/a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freylikhman, O.; Seyfedinova, S.; Kamalova, V.; Vatian, A.; Boukhanovsky, A.; Kostareva, A.; Solonitsyn, E.; Kalinina, O. RNA Signature as Potential Diagnostic Marker for Differentiation of Pancreatic Cysts: A Pilot Study. Int. J. Mol. Sci. 2025, 26, 9680. https://doi.org/10.3390/ijms26199680
Freylikhman O, Seyfedinova S, Kamalova V, Vatian A, Boukhanovsky A, Kostareva A, Solonitsyn E, Kalinina O. RNA Signature as Potential Diagnostic Marker for Differentiation of Pancreatic Cysts: A Pilot Study. International Journal of Molecular Sciences. 2025; 26(19):9680. https://doi.org/10.3390/ijms26199680
Chicago/Turabian StyleFreylikhman, Olga, Sabina Seyfedinova, Valeriia Kamalova, Aleksandra Vatian, Alexander Boukhanovsky, Anna Kostareva, Evgenii Solonitsyn, and Olga Kalinina. 2025. "RNA Signature as Potential Diagnostic Marker for Differentiation of Pancreatic Cysts: A Pilot Study" International Journal of Molecular Sciences 26, no. 19: 9680. https://doi.org/10.3390/ijms26199680
APA StyleFreylikhman, O., Seyfedinova, S., Kamalova, V., Vatian, A., Boukhanovsky, A., Kostareva, A., Solonitsyn, E., & Kalinina, O. (2025). RNA Signature as Potential Diagnostic Marker for Differentiation of Pancreatic Cysts: A Pilot Study. International Journal of Molecular Sciences, 26(19), 9680. https://doi.org/10.3390/ijms26199680






