Short-Term: Cellular Metabolism and Gene Expression During the Onset of Diabetic Kidney Disease: A Diabetes Mellitus Experimental Model
Abstract
1. Introduction
2. Results
2.1. Biochemical Parameters
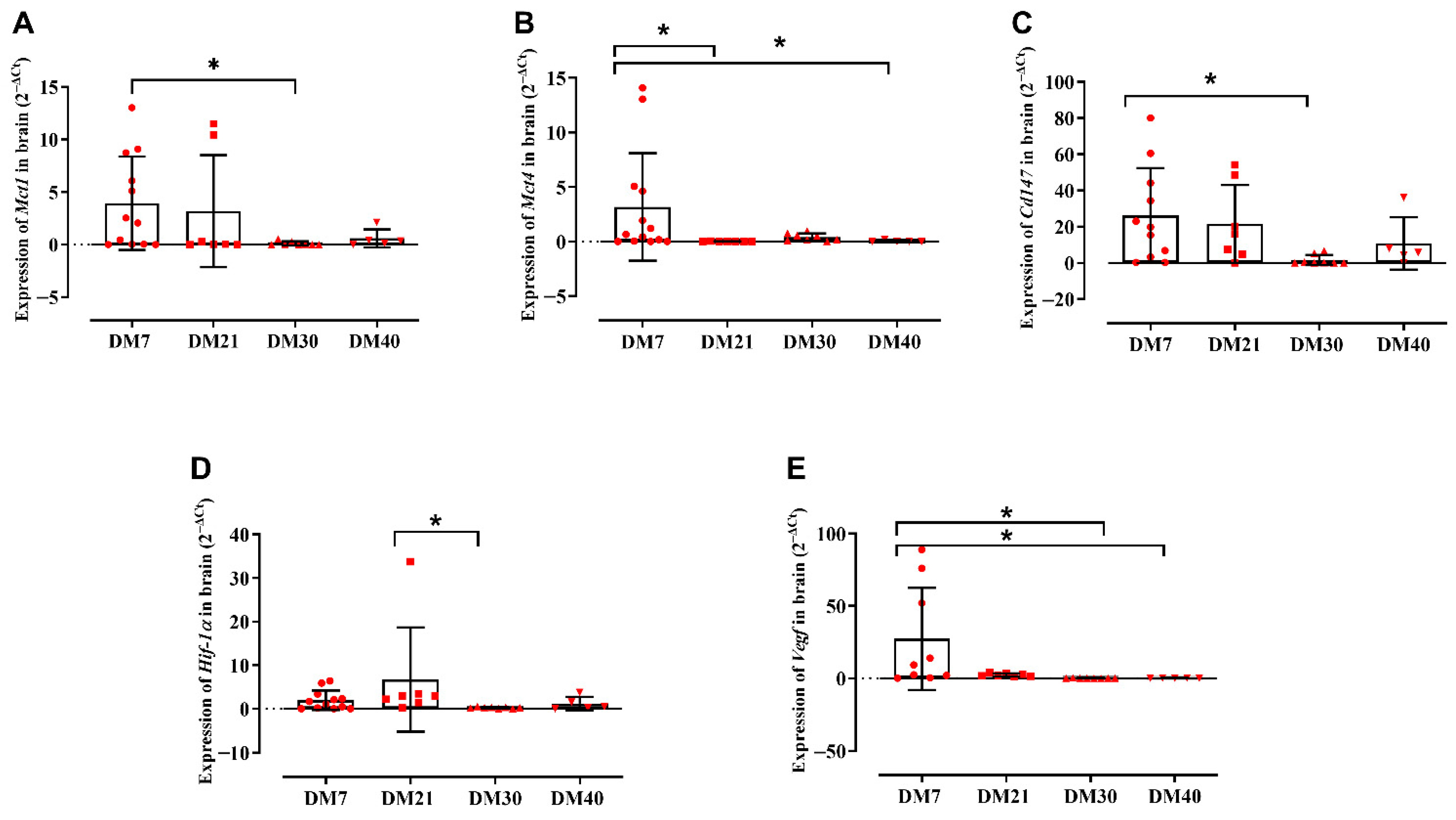
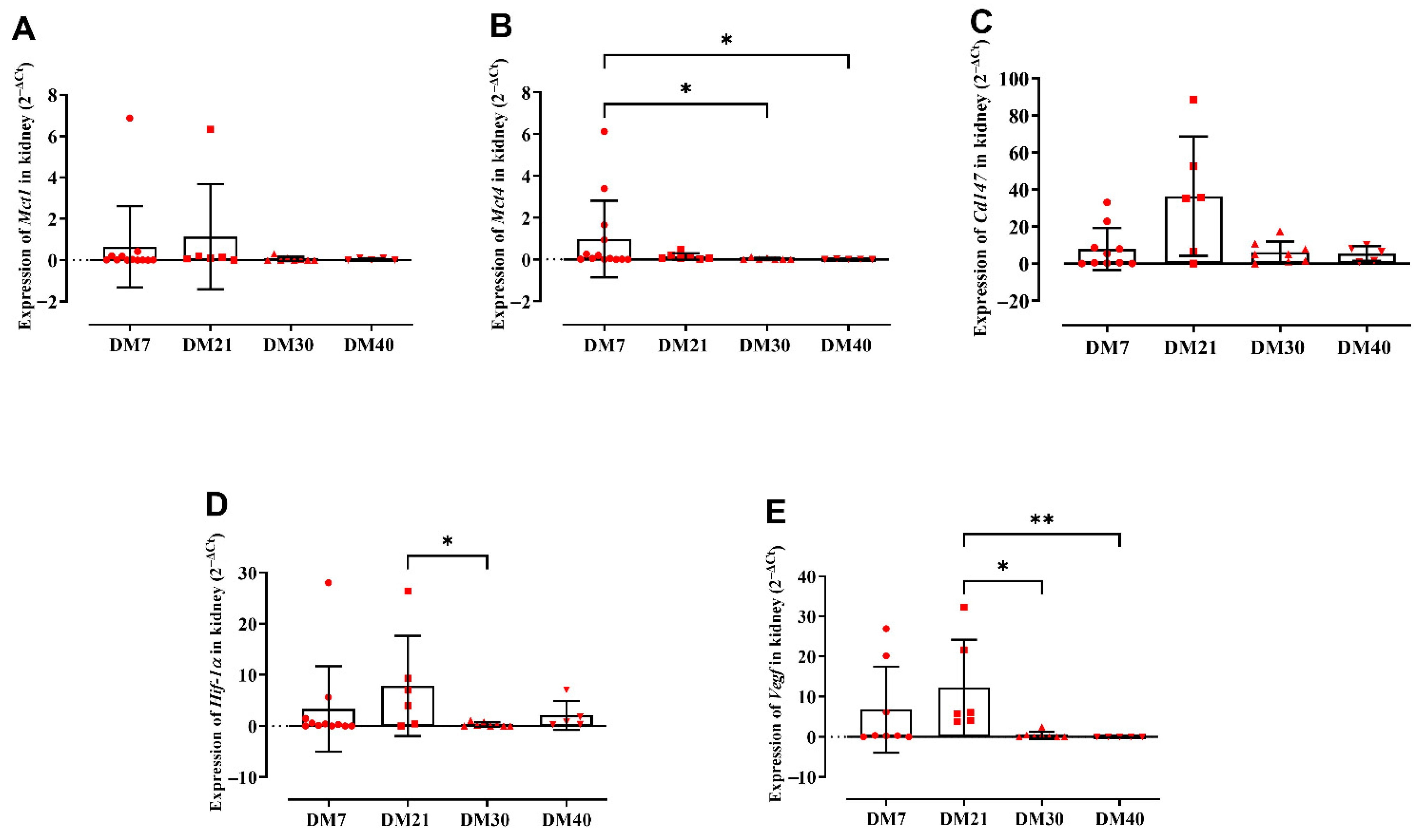
2.2. Gene Expression Profiling of the Analyzed Targets
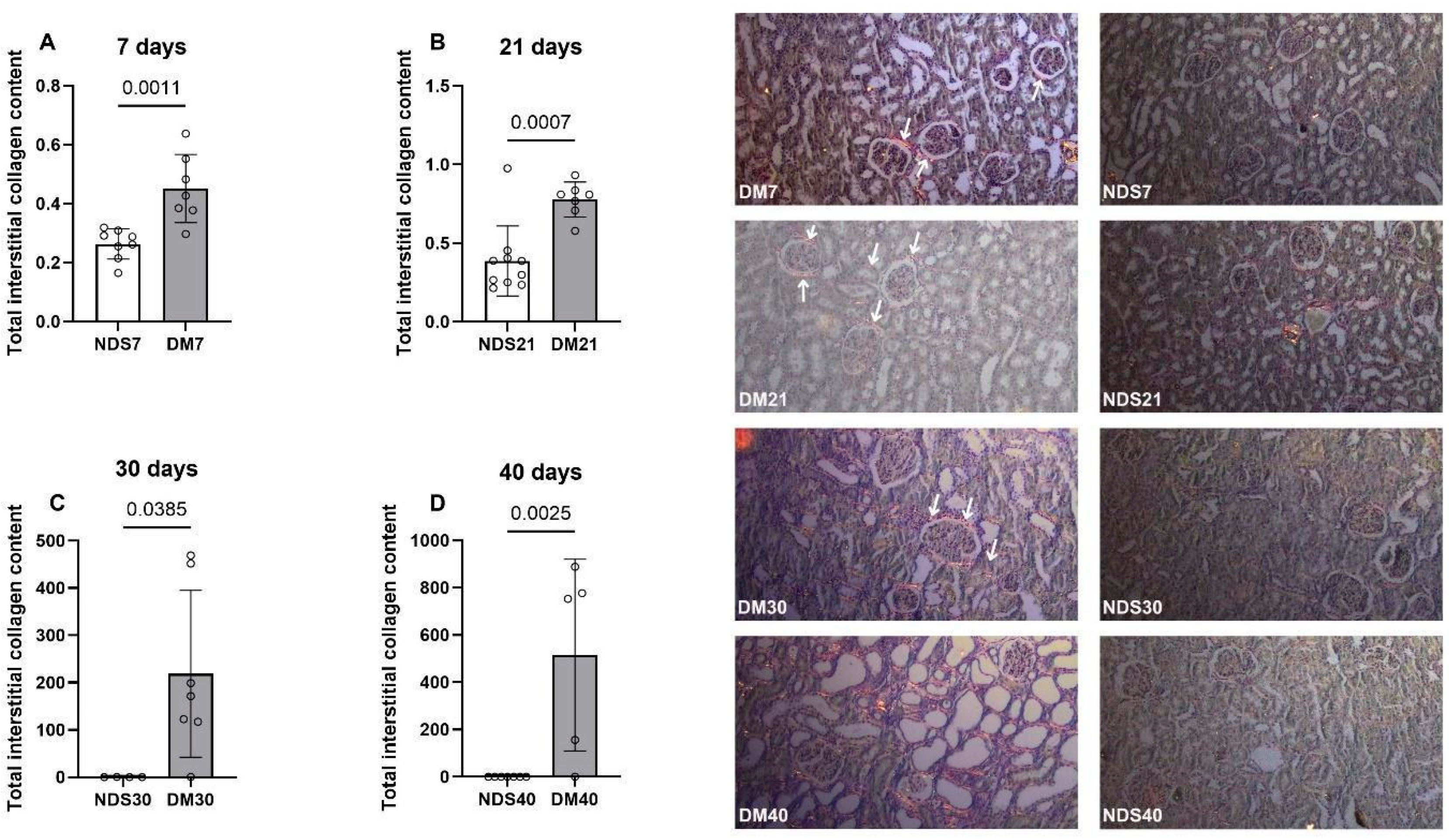
2.3. Picrosirius Analysis of Collagen Deposition in Renal Tissue
3. Discussion
4. Materials and Methods
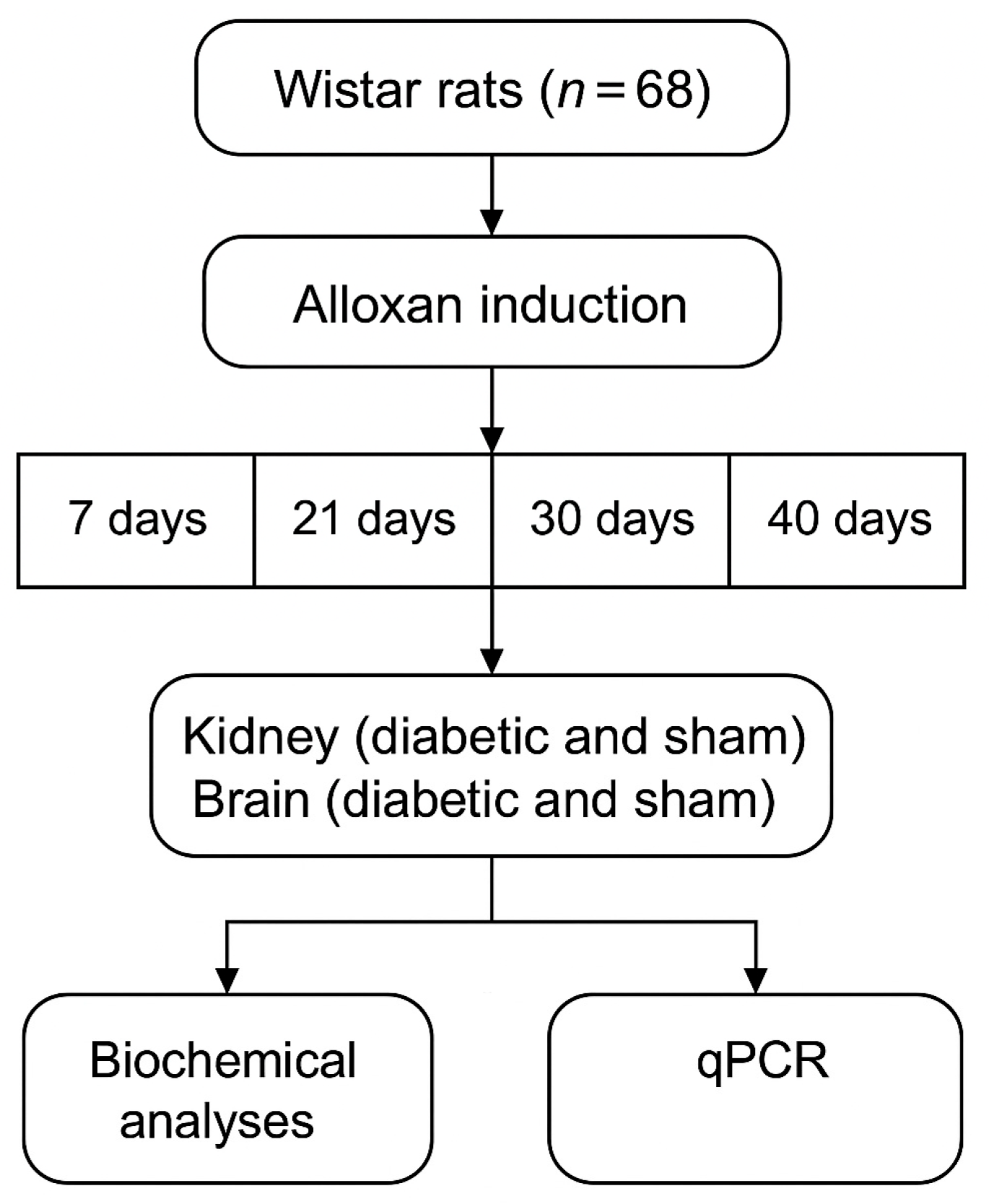
4.1. Experimental Design
4.2. Ethics Committee Approval
4.3. Experimental Induction of Groups
4.4. Sample Collections
4.5. Determination of Biochemical Parameters
4.5.1. Glycemia
4.5.2. Serum Creatinine
4.5.3. Plasma Urea
4.6. Tissue Extraction for Molecular Biology Analysis
4.7. Synthesis of DNA Complementary to Messenger RNA (cDNA)
4.8. q-PCR in Gene Expression
4.9. Picrosirius
4.10. Statistical Analyzes
5. Conclusions
6. Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Full Term |
| AMPK | AMP-activated Protein Kinase |
| CD147 | Cluster of Differentiation 147 |
| CEUA | Ethics Committee on Animal Use |
| CNS | Central Nervous System |
| DKD | Diabetic Kidney Disease |
| DM | Diabetic group |
| EMMPRIN | Extracellular Matrix Metalloproteinase Inducer |
| GAPDH | Glyceraldehyde-3-phosphate Dehydrogenase |
| GBM | Glomerular Basement Membrane |
| HIF | Hypoxia-Inducible Factor |
| MAPK | Mitogen-Activated Protein Kinase |
| MCT | Monocarboxylate Transporters |
| MMP | Matrix Metalloproteinases |
| mTOR | Mechanistic Target of Rapamycin |
| NDS | Non-Diabetic Sham |
| PHD | Prolyl Hydroxylase Domain |
| RAAS | Renin-Angiotensin-Aldosterone System |
| ROS | Reactive Oxygen Species |
| RVLM | Rostral Ventrolateral Medulla |
| SD | Standard Deviation |
| STZ | Streptozotocin |
| VEGF | Vascular Endothelial Growth Factor |
References
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B.; Williams, R.; Colagiuri, S.; Ogurtsova, K.; et al. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas, 11th ed; International Diabetes Federation: Brussels, Belgium, 2025; Available online: https://diabetesatlas.org/data/ (accessed on 24 August 2025).
- Jung, C.Y.; Yoo, T.H. Pathophysiologic mechanisms and potential biomarkers in diabetic kidney disease. Diabetes Metab. J. 2022, 46, 181–197. [Google Scholar] [CrossRef]
- American Diabetes Association. Standards of Medical Care in Diabetes—2025. Diabetes Care 2025, 48 (Suppl. S1), S1–S200. Available online: https://diabetesjournals.org/care/issue/48/Supplement_1 (accessed on 10 May 2023). [CrossRef]
- Halestrap, A.P.; Price, N.T. The proton-linked monocarboxylate transporter (MCT) family: Structure, function and regulation. Biochem J. 1999, 343, 281–299. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.M.; Geng, K.; Wang, P.; Zhao, Z.Y.; Zhang, L.; Zhang, M.; Xu, Q.; Huang, T.; Feng, Y.; Zhou, W.; et al. MCT4-dependent lactate transport: A novel mechanism for cardiac energy metabolism injury and inflammation in type 2 diabetes mellitus. Cardiovasc. Diabetol. 2024, 23, 96. [Google Scholar] [CrossRef] [PubMed]
- Halestrap, A.P. Monocarboxylic acid transport. Compr. Physiol. 2013, 3, 1611–1643. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Ali, M.M.; Elkhateeb, E.; Soliman, H.A.; Hassan, M.; Farag, M.A.; El-Missiry, M.A.; El-Sayed, M.; El-Sherbiny, M.; Kamel, M.A.; et al. High glucose and advanced glycation end products induce CD147-mediated MMP activity in human adipocytes. Cells 2021, 10, 2098. [Google Scholar] [CrossRef]
- Sivitz, W.I.; Yorek, M.A. ion in diabetes: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2010, 12, 537–577. [Google Scholar] [CrossRef]
- Semenza, G.L. HIF-1 and mechanisms of hypoxia sensing. Curr. Opin. Cell Biol. 2001, 13, 167–171. [Google Scholar] [CrossRef]
- Gupta, N.; Wish, J.B. Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitors: A Potential New Treatment for Anemia in Patients With CKD. Am. J. Kidney Dis. 2017, 69, 815–826. [Google Scholar] [CrossRef]
- Huang, L.E.; Bunn, H.F. Hypoxia-inducible factor and its biomedical relevance. J. Biol. Chem. 2003, 278, 19575–19578. [Google Scholar] [CrossRef]
- Sutter, C.H.; Laughner, E.; Semenza, G.L. Hypoxia-inducible factor 1alpha protein expression is controlled by oxygen-regulated ubiquitination that is disrupted by deletions and missense mutations. Proc. Natl. Acad. Sci. USA 2000, 97, 4748–4753. [Google Scholar] [CrossRef]
- da Veiga, G.L.; da Costa Aguiar Alves, B.; Perez, M.M.; Raimundo, J.R.; de Araújo Encinas, J.F.; Murad, N.; Fonseca, F.L.A.; Azzalis, L.A.; Gehrke, F.S.; Yamashita, E.K.; et al. Kidney diseases: The age of molecular markers. Adv. Exp. Med. Biol. 2021, 1306, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.S.; Kim, Y.S.; Jung, S.H.; Yu, S.Y.; Kim, J.H.; Sun, H.; Kim, J.S.; Park, H.J.; Choi, S.M.; Lim, Y.H.; et al. Lignans from the stems and leaves of Brandisia hancei and their effects on VEGF-induced vascular permeability and migration of HRECs and DLAV formation in zebrafish. Biosci. Biotechnol. Biochem. 2015, 79, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Faes, S.; Uldry, E.; Planche, A.; Santoro, T.; Pythoud, C.; Demartines, N.; Dormond, O.; Allagnat, F.; Bouchet, S.; Morel, P.; et al. Acidic pH reduces VEGF-mediated endothelial cell responses by downregulation of VEGFR-2; relevance for anti-angiogenic therapies. Oncotarget 2016, 7, 86026–86038. [Google Scholar] [CrossRef]
- Guyenet, P.G. The sympathetic control of blood pressure. Nat. Rev. Neurosci. 2006, 7, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Schreihofer, A.M.; Sved, A.F. The Rostral Ventrolateral Medulla and the Sympathetic Control of Blood Pressure. In Central Regulation of Autonomic Functions, 2nd ed.; Loewy, A.D., Ed.; Oxford University Press: Oxford, UK, 2011; pp. 78–97. [Google Scholar]
- Dibona, G.F.; Kopp, U.C. Neural control of renal function. Physiol. Rev. 1997, 77, 75–197. [Google Scholar] [CrossRef]
- Guyenet, P.G.; Koshiya, N. Working model of the sympathetic chemoreflex in rats. Clin. Exp. Hypertens. 1995, 17, 167–179. [Google Scholar] [CrossRef]
- Dampney, R.A.L. Functional organization of central pathways regulating the cardiovascular system. Physiol. Rev. 1994, 74, 323–364. [Google Scholar] [CrossRef]
- Sheriff, O.L.; Olayemi, O.; Taofeeq, A.O.; Adebayo, A.O.; Salami, A.T.; Ojo, O.A.; Ajiboye, B.O.; Olatunji, L.A.; Olayiwola, G.; Oduola, T.; et al. A New model for Alloxan-induced diabetes mellitus in rats. J. Bangladesh Soc. Physiol. 2019, 14, 56–62. [Google Scholar] [CrossRef]
- Lerco, M.M.; Spadella, C.T.; Machado, J.L.M.; Schellini, S.A.; Padovani, C.R. Caracterização de um modelo experimental de Diabetes Mellitus, induzido pela aloxana em ratos: Estudo clínico e laboratorial. Acta Cir. Bras. 2003, 18, 132–142. [Google Scholar] [CrossRef]
- Raimundo, J.R.S.; da Costa Aguiar Alves, B.; Encinas, J.F.A.; Siqueira, A.M.; de Gois, K.C.; Perez, M.M.; Petri, G.; Dos Santos, J.F.R.; Fonseca, F.L.A.; da Veiga, G.L. Expression of TNFR1, VEGFA, CD147 and MCT1 as early biomarkers of diabetes complications and the impact of aging on this profile. Sci. Rep. 2023, 13, 17927. [Google Scholar] [CrossRef]
- Arcia, C.G.C.; Encinas, J.F.A.; Raimundo, J.R.S.; de Gois, K.C.; da Costa Aguiar Alves, B.; Perez, M.M.; Gascon, T.M.; Fonseca, F.L.A.; da Veiga, G.L. Downregulation of Tnf-α and Cat Expression in a Wistar Rat Diabetic Model during Diabetes Onset. Curr. Diabetes Rev. 2025, 21, e200624231125. [Google Scholar] [CrossRef] [PubMed]
- Abu-Lebdeh, H.S.; Nair, K.S. Protein metabolism in diabetes mellitus. Baillieres Clin. Endocrinol. Metab. 1996, 10, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Felig, P.; Wahren, J.; Sherwin, R.; Palaiologos, G. Amino acid and protein metabolism in diabetes mellitus. Arch. Intern. Med. 1977, 137, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Gerozissis, K. Brain insulin: Regulation, mechanisms of action and functions. Cell Mol. Neurobiol. 2003, 23, 1–25, Erratum in Cell Mol. Neurobiol. 2003, 23, 873–874. [Google Scholar] [CrossRef] [PubMed]
- Plum, L.; Schubert, M.; Brüning, J.C. The role of insulin receptor signaling in the brain. Trends Endocrinol. Metab. 2005, 16, 59–65. [Google Scholar] [CrossRef]
- Silva, M.A.; Oliveira, M.F.; Lima, R.S.; Santos, A.C.; Costa, J.M.; Ribeiro, D.L.; Almeida, M.T.; Nascimento, E.P.; Barreto, J.A.; Fernandes, R.F.; et al. Expression of monocarboxylate transporters in adipose tissues of control and streptozotocin-diabetic rats. FEBS Lett. 2000, 487, 319–322. [Google Scholar] [CrossRef]
- Pierre, K.; Pellerin, L.; Debernardi, R.; Riederer, B.M.; Magistretti, P.J.; Allaman, I.; Chatton, J.Y.; Rouach, N.; Suter, D.; Bouzier-Sore, A.K.; et al. Monocarboxylate transporter 1 (MCT1) expression in the brain: Support for a role in neuronal–glial metabolic interaction. J. Cereb. Blood Flow. Metab. 2007, 27, 375–385. [Google Scholar] [CrossRef]
- Pierre, K.; Pellerin, L. Monocarboxylate transporters in the central nervous system: Distribution, regulation and function. J. Neurochem. 2005, 94, 1–14. [Google Scholar] [CrossRef]
- Pellerin, L.; Bouzier-Sore, A.K. The role of lactate in the energy metabolism of the brain. Behav. Brain Res. 2008, 194, 14–21. [Google Scholar] [CrossRef]
- Shima, T.; Matsui, T.; Jesmin, S.; Kawahara, K.; Hasegawa, T.; Fukami, K.; Yamagishi, S.; Nakamura, K.; Oshima, Y.; Yamamoto, H.; et al. Moderate exercise ameliorates dysregulated hippocampal glycometabolism and memory function in a rat model of type 2 diabetes. Diabetologia 2017, 60, 597–606. [Google Scholar] [CrossRef]
- Shima, T.; Jesmin, S.; Matsui, T.; Soya, M.; Soya, H. Differential effects of type 2 diabetes on brain glycometabolism in rats: Focus on glycogen and monocarboxylate transporter 2. J. Physiol. Sci. 2018, 68, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Wang, L. Oxidative stress and diabetic complications. Nat. Rev. Endocrinol. 2012, 8, 176–186. [Google Scholar] [CrossRef]
- Roohi, T.F.; Faizan, S.; Parray, Z.A.; Baig, M.A.; Mehdi, S.; Kinattingal, N.; Krishna, K.L. Beyond Glucose: The Dual Assault of Oxidative and ER Stress in Diabetic Disorders. High Blood Press Cardiovasc. Prev. 2023, 30, 513–531. [Google Scholar] [CrossRef] [PubMed]
- Nirenjen, S.; Narayanan, J.; Tamilanban, T.; Subramaniyan, V.; Chitra, V.; Fuloria, N.K.; Wong, L.S.; Ramachawolran, G.; Sekar, M.; Gupta, G.; et al. Exploring the contribution of pro-inflammatory cytokines to impaired wound healing in diabetes. Front. Immunol. 2023, 14, 1216321. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, Y.; Ding, H.; Liu, Y.; Zhang, J.; Chen, X.; Li, Q.; Zhou, Y.; Huang, Z.; Feng, S.; et al. Highly glycosylated CD147 promotes hemorrhagic transformation after rt-PA treatment in diabetes: A novel therapeutic target? J. Neuroinflammation 2019, 16, 72. [Google Scholar] [CrossRef]
- Zhang, H.; Qian, J.; Gu, Y.; Chen, L.; Li, X.; Gao, B.; Wang, J.; Liu, Y.; Xu, X.; Sun, Y.; et al. HIF-1α Regulates the Expression of CD147 in a Mitogen-Activated Protein Kinase-Dependent Manner. Mol. Med. Rep. 2016, 14, 4440–4448. [Google Scholar] [CrossRef]
- Park, J.H.; He, M.; Choi, K.Y.; Jung, Y.; Min, D.S.; Kim, S.; Lee, H.; Song, J.; Kwon, Y.; Han, J.; et al. Phospholipase D1 protein coordinates dynamic assembly of HIF-1α-PHD-VHL to regulate HIF-1α stability. Oncotarget 2014, 5, 5764–5773. [Google Scholar] [CrossRef]
- Zhang, H.; Qian, J.; Gu, Y.; Chen, L.; Li, X.; Gao, B.; Wang, J.; Liu, Y.; Xu, X.; Sun, Y.; et al. Hypoxia-inducible Factor-1α regulates vascular endothelial growth factor and angiogenesis through multiple pathways. Mol. Med. Rep. 2015, 11, 3488–3496. [Google Scholar] [CrossRef]
- Mi, D.H.; Fang, H.J.; Zheng, G.H.; Zhang, Y.; Liu, X.; Wang, J.; Chen, L.; Li, Q.; Xu, T.; Huang, W.; et al. DPP-4 inhibitors promote proliferation and migration of rat brain microvascular endothelial cells under hypoxic/high-glucose conditions, potentially through the SIRT1/HIF-1/VEGF pathway. CNS Neurosci. Ther. 2019, 25, 323–332. [Google Scholar] [CrossRef]
- Tesfaye, S.; Malik, R.; Harris, N.; Wiggins, C.; Ward, J.D.; Sharma, A.K.; Bradbury, A.W.; Boulton, A.J.M.; Benbow, S.J.; Selmi, F.; et al. Arterio-venous shunting and proliferating new vessels in acute painful neuropathy of rapid glycaemic control (insulin neuritis). Diabetologia 1996, 39, 329–335. [Google Scholar] [CrossRef]
- Yao, X.; Wang, Y.; Zhang, D.; Liu, Y.; Zhao, Y.; Li, Y.; Chen, L.; Xu, X.; Sun, Y.; Gao, C.; et al. Chronic bilateral renal denervation attenuates renal injury in a transgenic rat model of diabetic nephropathy. Am. J. Physiol. Physiol. 2014, 307, F251–F262. [Google Scholar] [CrossRef]
- Gambaryan, S.; Tsikas, D.; Becker, S.; Smolenski, A.; Walter, U.; Eigenthaler, M.; Friebe, A.; Hofmann, F.; Feil, R.; Lohmann, S.M.; et al. Regulation of the renin-angiotensin-aldosterone system by cyclic nucleotides and phosphodiesterases. Front. Endocrinol. 2023, 14, 1239492. [Google Scholar] [CrossRef] [PubMed]
- Pizon, A.F.; Rajzer, M.; Wojciechowska, W.; Drożdż, T.; Drożdż, D.; Rojek, M.; Gruszka, K.; Czarnecka, D. Plasma renin activity, serum aldosterone concentration and selected organ damage indices in essential arterial hypertension. Arch. Med. Sci. 2021, 17, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Faria, A.; Persaud, S. Cardiac oxidative stress in diabetes: Mechanisms and therapeutic potential. Pharmacol. Ther. 2017, 172, 50–62. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Liu, Y.; Wang, J.; Chen, Y.; Zhao, H.; Sun, W.; Zhou, Q.; Yang, L.; Xu, Y.; et al. The sex steroid precursor dehydroepiandrosterone prevents nonalcoholic steatohepatitis by activating the AMPK pathway mediated by GPR30. Redox Biol. 2021, 42, 102187. [Google Scholar] [CrossRef]
- Yao, Y.; Siregar, M.; Lubis, Z.; Siregar, G.A.; Nasution, S.; Lubis, I.; Harahap, E.; Siregar, R.P.; Siregar, F.P.; Siregar, R.; et al. Activated AMP-activated protein kinase prevents hepatic steatosis, oxidative stress and inflammation in primary chicken hepatocytes. Front. Physiol. 2022, 13, 974825. [Google Scholar] [CrossRef]
- Rusdiana, R.; Widjaja, S.S.; Amelia, R. The Correlation between Serum Vascular Endothelial Growth Factor and Lipid Profile in Type 2 Diabetes Mellitus. Open Access Maced. J. Med. Sci. 2020, 8, 5402. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, Y.; Liu, J.; Chen, H.; Wang, Q.; Li, M.; Zhou, T.; Huang, L.; Zhao, Y.; Feng, Z.; et al. Hyperglycemia disrupted the integrity of the blood-brain barrier following diffuse axonal injury through the sEH/NF-κB pathway. Immun. Inflamm. Dis. 2023, 11, e1105. [Google Scholar] [CrossRef] [PubMed]
- Alleboina, S.; Kumar, A.; Reddy, P.H.; Sharma, R.K.; Mishra, R.; Singh, S.; Patel, A.; Gupta, V.; Rao, M.; Joshi, M.; et al. Inhibition of protein kinase C beta phosphorylation activates nuclear factor-kappa B and improves postischemic recovery in type 1 diabetes. Exp. Biol. Med. 2020, 245, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Sivaskandarajah, G.A.; Jeansson, M.; Maezawa, Y.; Eremina, V.; Baelde, H.J.; Quaggin, S.E.; Haraldsson, B.; Sorensen, C.M.; Peti-Peterdi, J.; Satchell, S.C.; et al. Vegfa Protects the Glomerular Microvasculature in Diabetes. Diabetes 2012, 61, 1839–1850. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Tabata, H.; Hirakawa, Y.; Kodama, T.; Suzuki, Y.; Ohta, H.; Kondo, M.; Maruyama, S.; Yokoyama, H.; Yamada, Y.; et al. Amelioration of renal alterations in obese type 2 diabetic mice by vasohibin-1, a negative feedback regulator of angiogenesis. Am. J. Physiol. Renal Physiol. 2011, 301, F625–F633. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, Y.; Li, H.; Chen, J.; Wang, L.; Liu, Q.; Zhao, M.; Sun, Y.; Xu, T.; Huang, Y.; et al. Esaxerenone Inhibits Renal Angiogenesis and Endothelial-Mesenchymal Transition via the VEGFA and TGF-β1 Pathways in Aldosterone-Infused Mice. Int. J. Mol. Sci. 2023, 24, 11766. [Google Scholar] [CrossRef]
- Bohuslavova, R.; Cerychova, R.; Nepomucka, K.; Pavlinkova, G. Renal injury is accelerated by global hypoxia-inducible factor 1 alpha deficiency in a mouse model of STZ-induced diabetes. BMC Endocr. Disord. 2017, 17, 48. [Google Scholar] [CrossRef]
- Wang, Y.F.; Ma, S.R.; Wang, W.M.; Zhang, W.F.; Sun, Z.J.; Liu, B.; Chen, Y.; Yu, G.T.; Yang, C.; Fan, Z.P.; et al. Inhibition of survivin reduces HIF-1α, TGF-β1 and TFE3 in salivary adenoid cystic carcinoma. PLoS ONE 2014, 9, e114051. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, S.; Zou, J.; Zhong, Y.; Ding, X. Silencing HIF-1α aggravates growth inhibition and necrosis of proximal renal tubular epithelial cell under hypoxia. Ren. Fail. 2016, 38, 1726–1734. [Google Scholar] [CrossRef]
- Yu, W.; Li, Y.; Wang, Z.; Liu, H.; Zhang, J.; Chen, X.; Zhao, Y.; Huang, Q.; Feng, Y.; Xu, L.; et al. Transcriptomic changes in human renal proximal tubular cells revealed under hypoxic conditions by RNA sequencing. Int. J. Mol. Med. 2016, 38, 894–902. [Google Scholar] [CrossRef]
- Li, Y.; Qi, Y.; Kim, M.S.; Xu, K.Z.Y.; Huang, T.H.W.; Rong, X.; Murray, M.; Yamahara, J. Increased renal collagen cross-linking and lipid accumulation in nephropathy of Zucker diabetic fatty rats. Diabetes Metab. Res. Rev. 2008, 24, 498–506. [Google Scholar] [CrossRef]
- Khalil, I.A.; El-Sayed, M.I.; Elberry, A.A.; El-Sherbiny, G.A.; Abdel Ghaffar, S.K.; El-Beshbishy, H.A. Protective effect of carvedilol on renal fibrosis in streptozotocin-induced diabetic rats. Eur. J. Pharmacol. 2014, 738, 14–23. [Google Scholar]
- He, W.; Tan, R.J.; Li, Y.; Wang, D.; Nie, J.; Hou, F.F.; Liu, Y. Role of connective tissue growth factor in diabetic nephropathy. J. Am. Soc. Nephrol. 2013, 24, 411–426. [Google Scholar] [CrossRef]
- Sun, X.; He, Z.; Guo, L.; Zhang, W.; Wang, H.; Yang, L. Inhibition of Wnt/β-catenin pathway alleviates renal fibrosis in streptozotocin-induced diabetic rats. Mol. Med. Rep. 2018, 18, 5739–5746. [Google Scholar] [CrossRef]
- Santos, L.R.M.; Lima, D.R.; da Silva, S.A.; Oliveira, R.A.; Costa, M.A.; Ferreira, T.L.; Almeida, M.C.; Nascimento, J.P.; Barbosa, F.S.; Rocha, V.C.; et al. Renal fibrosis and inflammation are attenuated by pomegranate peel extract in streptozotocin-induced diabetic rats. Phytomedicine 2019, 61, 152847. [Google Scholar] [CrossRef]
- Zhou, L.; Zuo, Z.; Chow, M.S.S. Effect of curcumin on renal fibrosis in streptozotocin-induced diabetic rats. Int. J. Clin. Exp. Pathol. 2015, 8, 4193–4203. [Google Scholar]
- Barbagallo, F.D.G.; Bosco, G.; Di Marco, M.; Piro, S.; Purrello, F.; Malaguarnera, M.; Musumeci, M.; Di Mauro, M.; Scicali, R. Evaluation of glycemic status and subclinical atherosclerosis in familial hypercholesterolemia subjects with or without LDL receptor mutation. Cardiovasc. Diabetol. 2025, 24, 126. [Google Scholar] [CrossRef]
- Fajarwati, I.; Solihin, D.; Wresdiyati, T.; Batubara, I. Administration of alloxan and streptozotocin in Sprague Dawley rats and the challenges in producing diabetes model. IOP Conf. Ser. Earth Environ. Sci. 2023, 1174, 012035. [Google Scholar] [CrossRef]
- Ighodaro, O.; Adeosun, A.; Asejeje, F.; Soetan, O.; Kassim, O. Time course effects of 5,5-dihydroxyl pyrimidine-2,4,6-trione (alloxan) as a diabetogenic agent in animal model. Alex. J. Med. 2018, 54, 705–710. [Google Scholar] [CrossRef]
- Podell, B.; Ackart, D.; Richardson, M.; DiLisio, J.; Pulford, B.; Basaraba, R. A model of type 2 diabetes in the guinea pig using sequential diet-induced glucose intolerance and streptozotocin treatment. Dis. Model. Mech. 2017, 10, 151–160. [Google Scholar] [CrossRef]
- Boicean, A.; Ichim, C.; Sasu, S.-M.; Todor, S.B. Key Insights into Gut Alterations in Metabolic Syndrome. J. Clin. Med. 2025, 14, 2678. [Google Scholar] [CrossRef]
- Popa, M.L.; Ichim, C.; Anderco, P.; Todor, S.B.; Pop-Lodromanean, D. MicroRNAs in the Diagnosis of Digestive Diseases: A Comprehensive Review. J. Clin. Med. 2025, 14, 2054. [Google Scholar] [CrossRef]
| Variables | Times | NDS Average (±SD) | DM Average (±SD) (n = 13) | p Value |
|---|---|---|---|---|
| Plasma glucose (mg/dL) | 7 days | 190.63 (±56.78) | 599.58 (±139.29) * | <0.0001 |
| 21 days | 181.33 (±34.70) | 777.20 (±233.74) * | <0.0001 | |
| 30 days | 169.18 (±32.09) | 763.74 (±221.40)* | <0.0001 | |
| 40 days | 195.50 (±38.02) | 763.28 (±77.92) * | <0.0007 | |
| Plasma creatinine (mg/dL) | 7 days | 0.42 (±0.06) | 0.80 (±1.24) * | 0.04 |
| 21 days | 0.46 (±0) | 1.99 (±4.02) | 0.41 | |
| 30 days | 0.86 (±0.12) | 1.23 (±0.45) * | 0.03 | |
| 40 days | 1.13 (±0.42) | 1.72 (±0.73) | 0.18 | |
| Plasma urea (mg/dL) | 7 days | 57.43 (±14.71) | 97.94 (±28.27) * | <0.0002 |
| 21 days | 68.31 (±5.08) | 153.38 (±44.13) * | <0.0001 | |
| 30 days | 79.62 (±7.15) | 170.20 (±21.00) * | <0.0007 | |
| 40 days | 82.52 (±12.00) | 173.10 (±42.71) * | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Encinas, J.; Veiga, G.; Raimundo, J.; Perez, M.; Petri, G.; Cavalheiro, R.; Reis, P.; Maifrino, L.; Alves, B.; Fonseca, F. Short-Term: Cellular Metabolism and Gene Expression During the Onset of Diabetic Kidney Disease: A Diabetes Mellitus Experimental Model. Int. J. Mol. Sci. 2025, 26, 9676. https://doi.org/10.3390/ijms26199676
Encinas J, Veiga G, Raimundo J, Perez M, Petri G, Cavalheiro R, Reis P, Maifrino L, Alves B, Fonseca F. Short-Term: Cellular Metabolism and Gene Expression During the Onset of Diabetic Kidney Disease: A Diabetes Mellitus Experimental Model. International Journal of Molecular Sciences. 2025; 26(19):9676. https://doi.org/10.3390/ijms26199676
Chicago/Turabian StyleEncinas, Jéssica, Glaucia Veiga, Joyce Raimundo, Matheus Perez, Giuliana Petri, Renan Cavalheiro, Pedro Reis, Laura Maifrino, Beatriz Alves, and Fernando Fonseca. 2025. "Short-Term: Cellular Metabolism and Gene Expression During the Onset of Diabetic Kidney Disease: A Diabetes Mellitus Experimental Model" International Journal of Molecular Sciences 26, no. 19: 9676. https://doi.org/10.3390/ijms26199676
APA StyleEncinas, J., Veiga, G., Raimundo, J., Perez, M., Petri, G., Cavalheiro, R., Reis, P., Maifrino, L., Alves, B., & Fonseca, F. (2025). Short-Term: Cellular Metabolism and Gene Expression During the Onset of Diabetic Kidney Disease: A Diabetes Mellitus Experimental Model. International Journal of Molecular Sciences, 26(19), 9676. https://doi.org/10.3390/ijms26199676






