Ochronotic Deposition in Alkaptonuria: Semiquinone-Mediated Oxidative Coupling and Metabolic Drivers of Homogentisic Acid Accumulation
Abstract
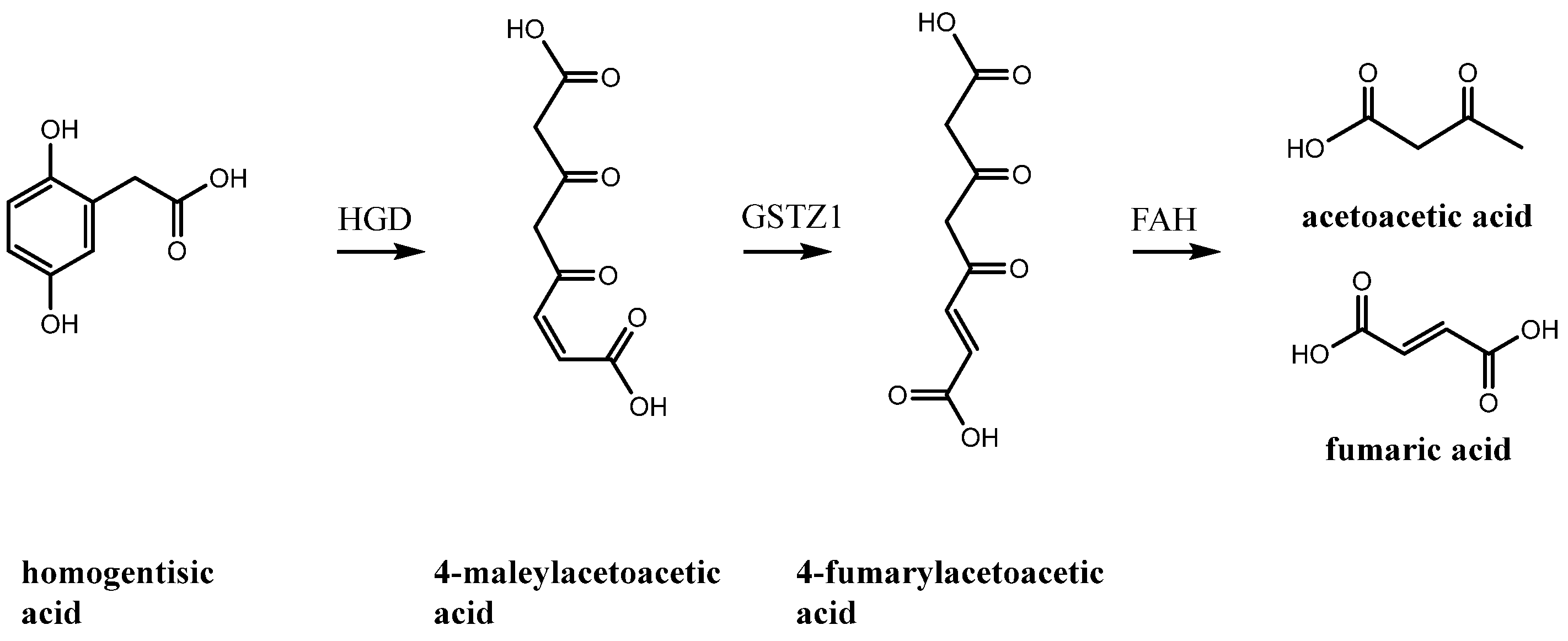
1. Introduction
2. Results and Discussion
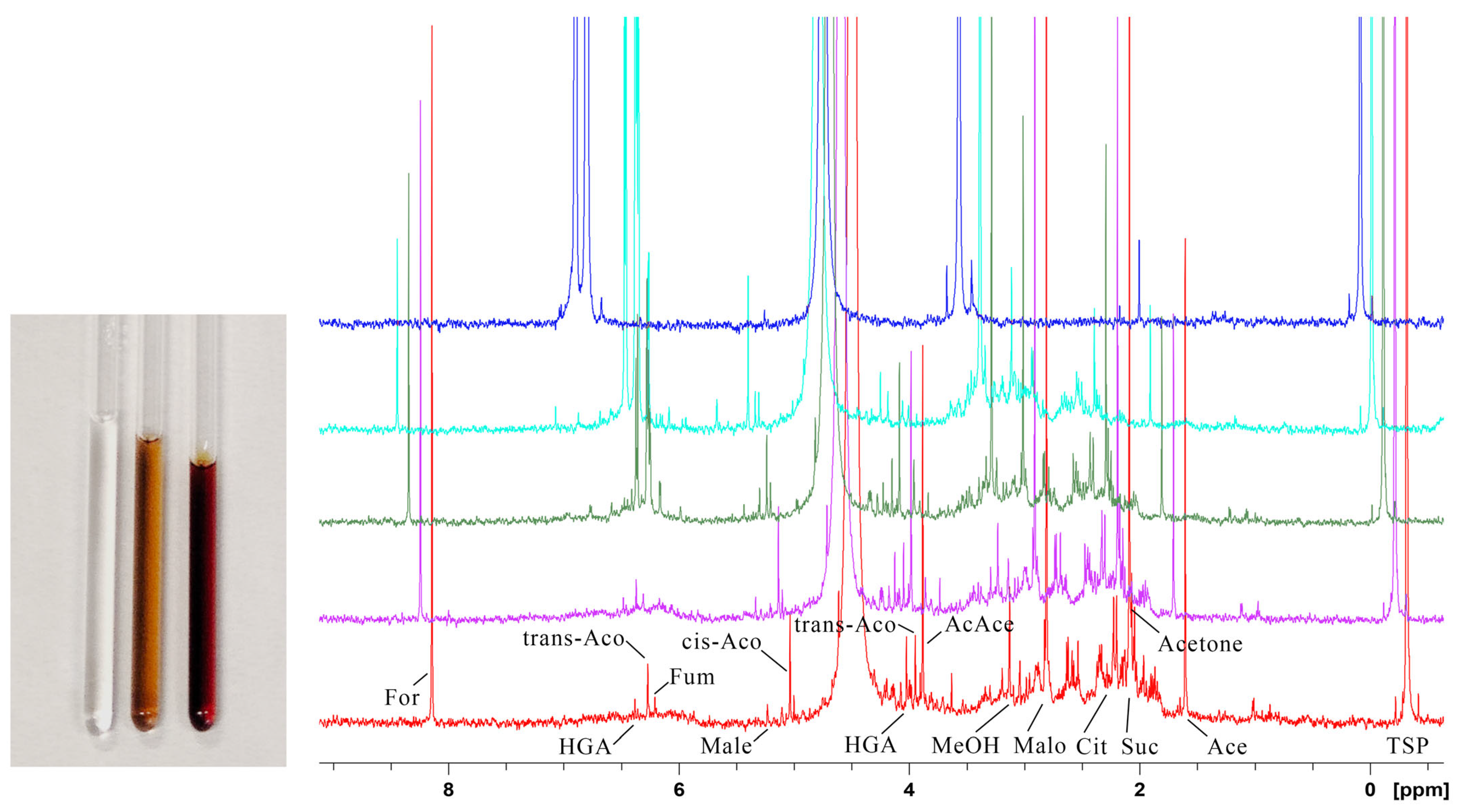
2.1. HGA Discolouration Under Physiological Conditions
2.2. HGA Discolouration Under Non-Neutral Conditions
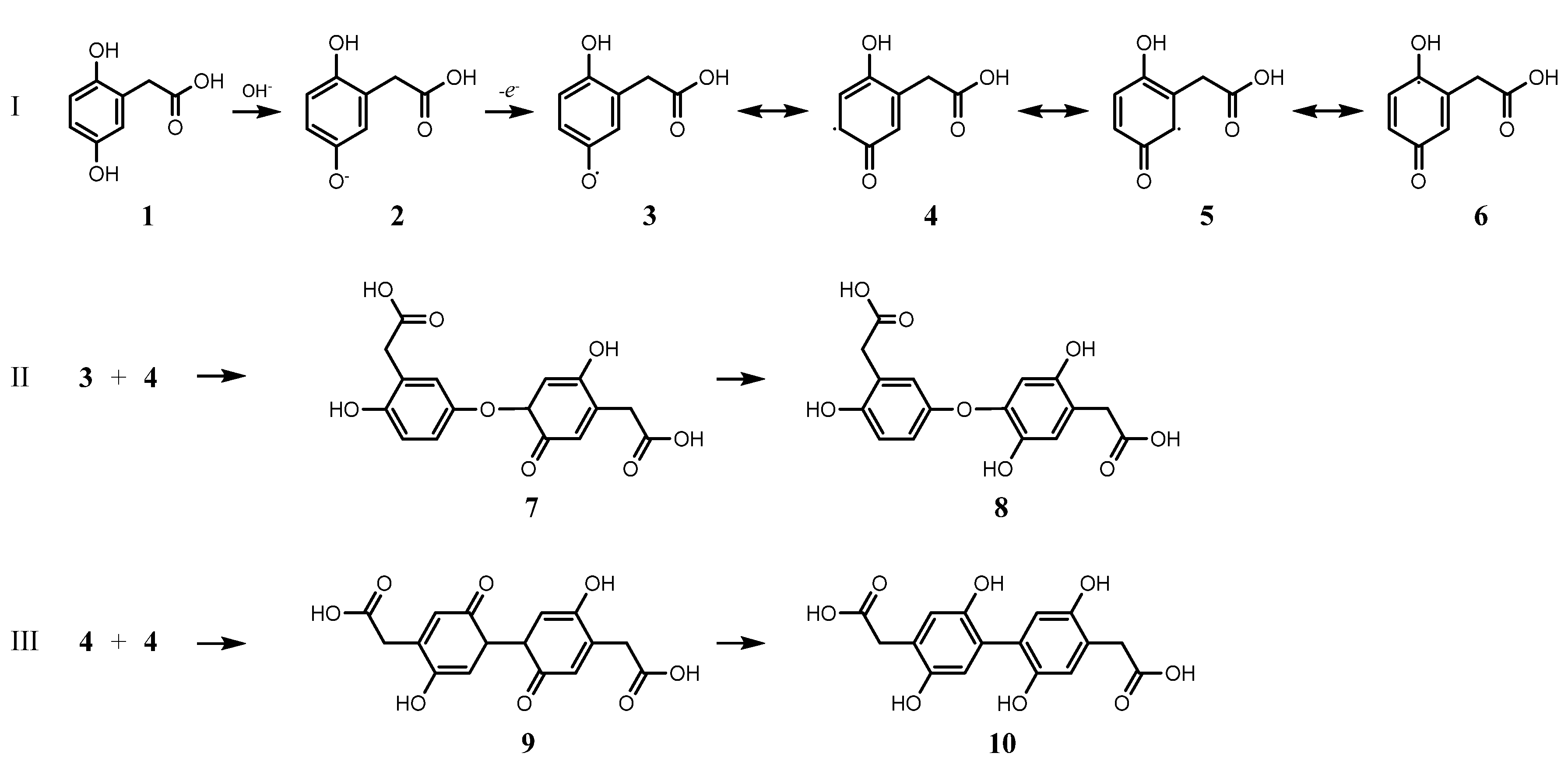
2.3. HGA Polymerisation Mechanism
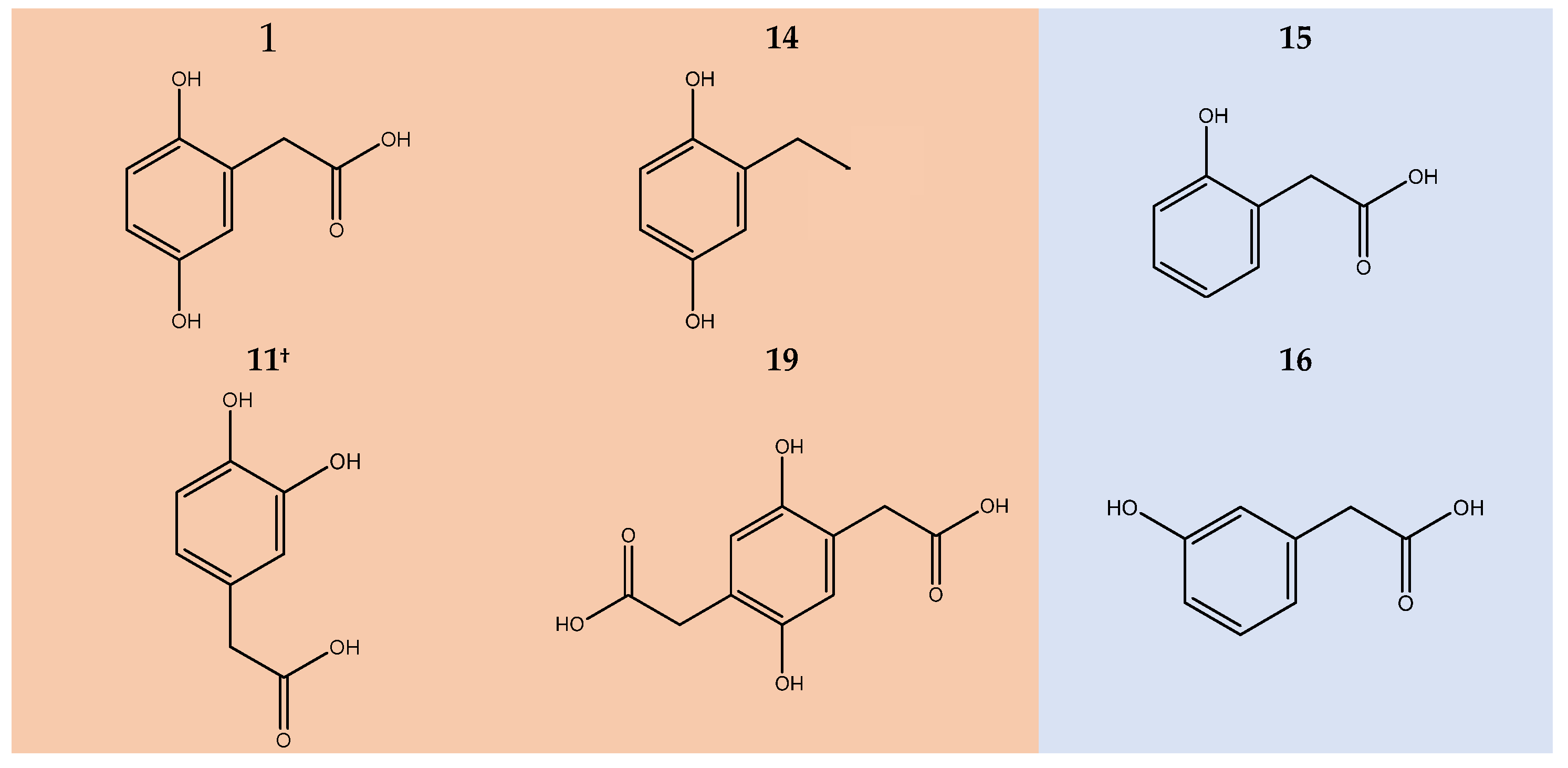
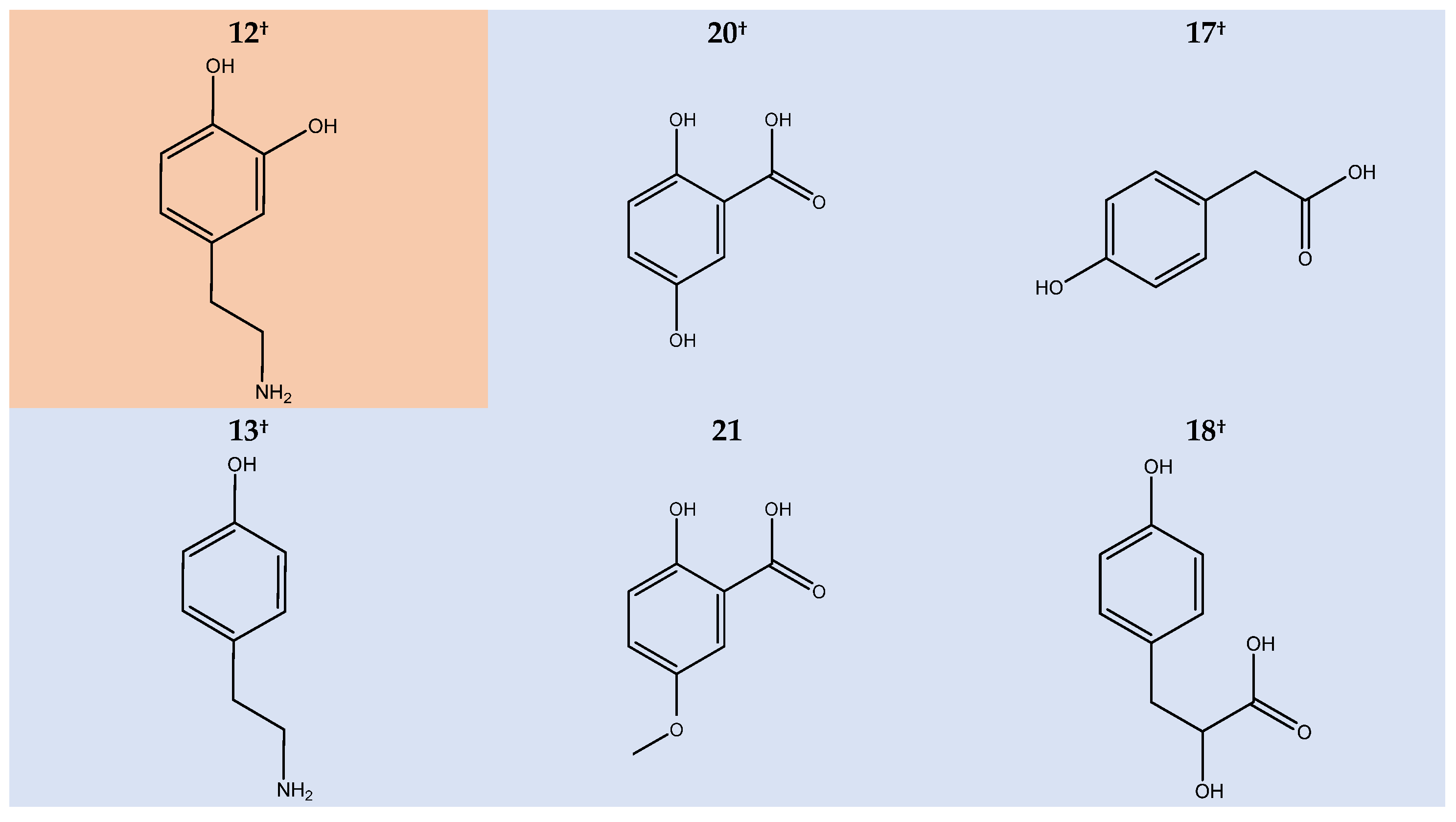
2.4. Discolouration of HGA Similars
2.5. Investigation of Pigment Particles
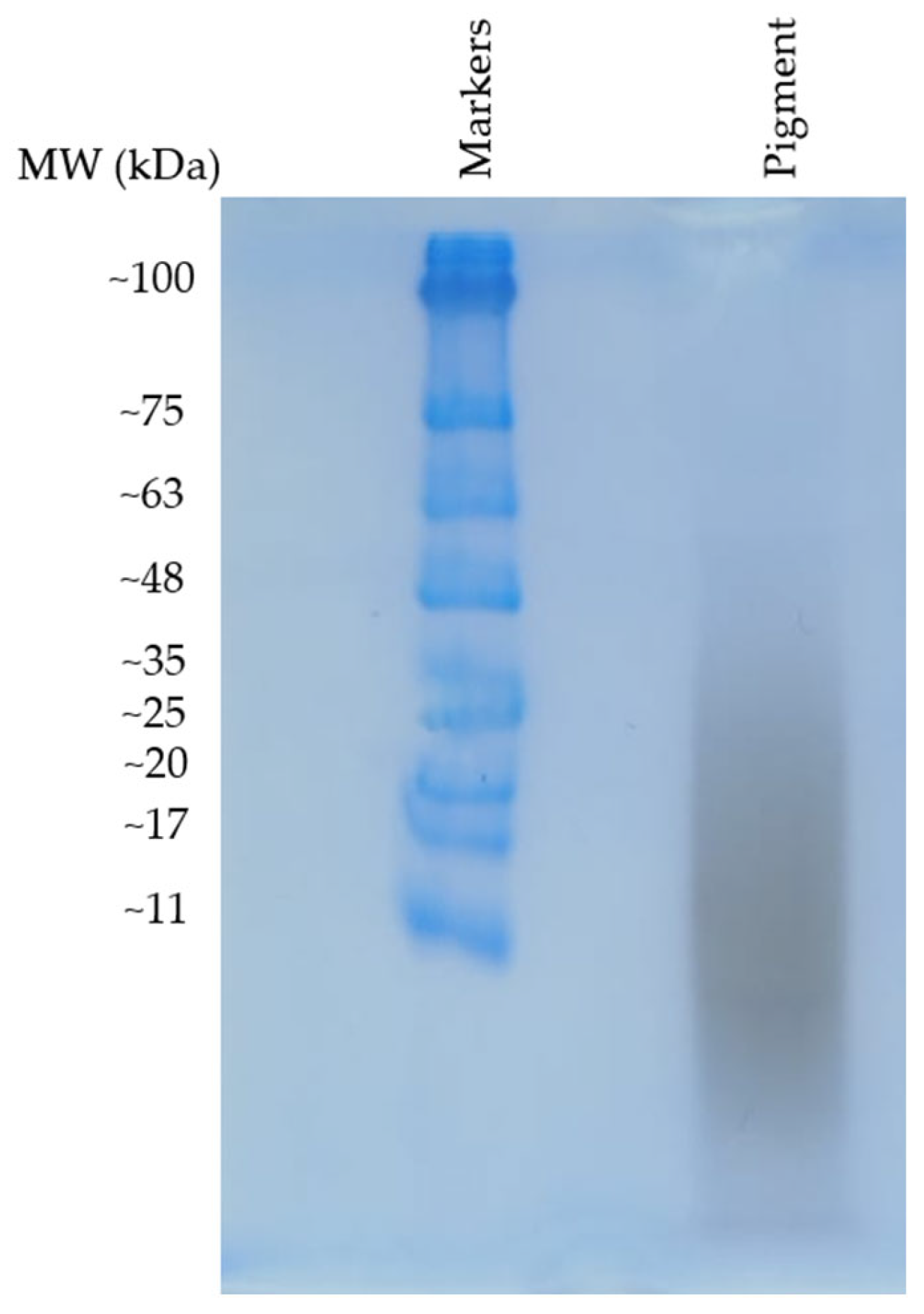
2.5.1. Gel Electrophoresis Separation of Pigment Components
2.5.2. Ultrafiltration Separation of Pigment Components
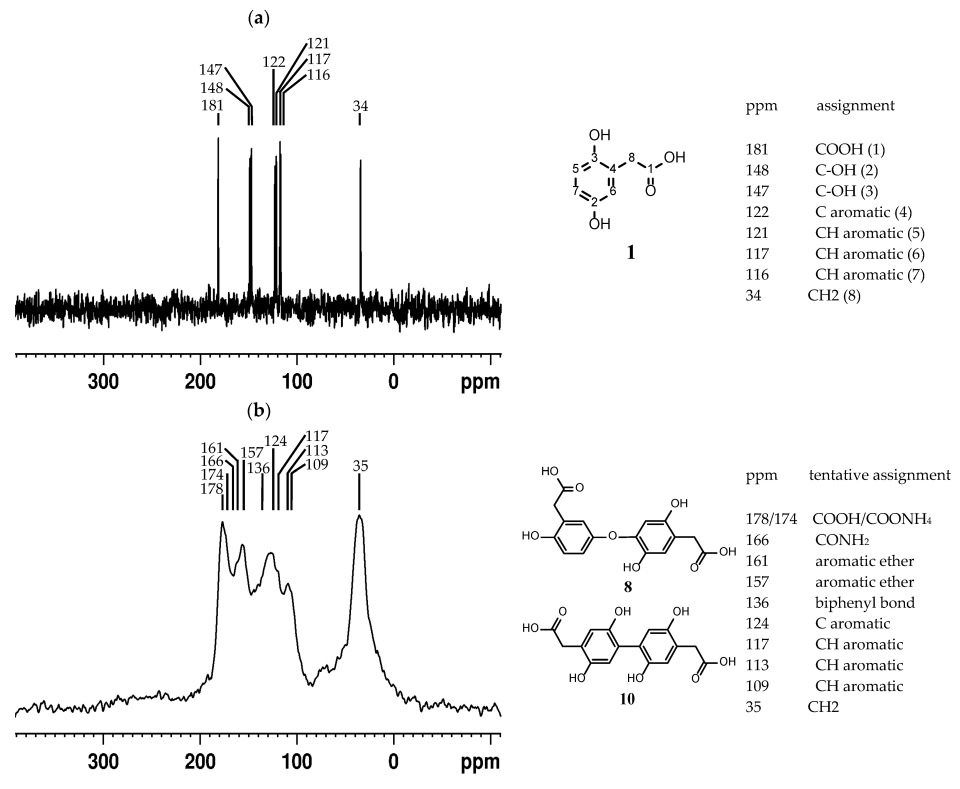
2.5.3. Solid-State NMR of the Pigment
3. Materials and Methods
3.1. Solution NMR
3.2. Compounds
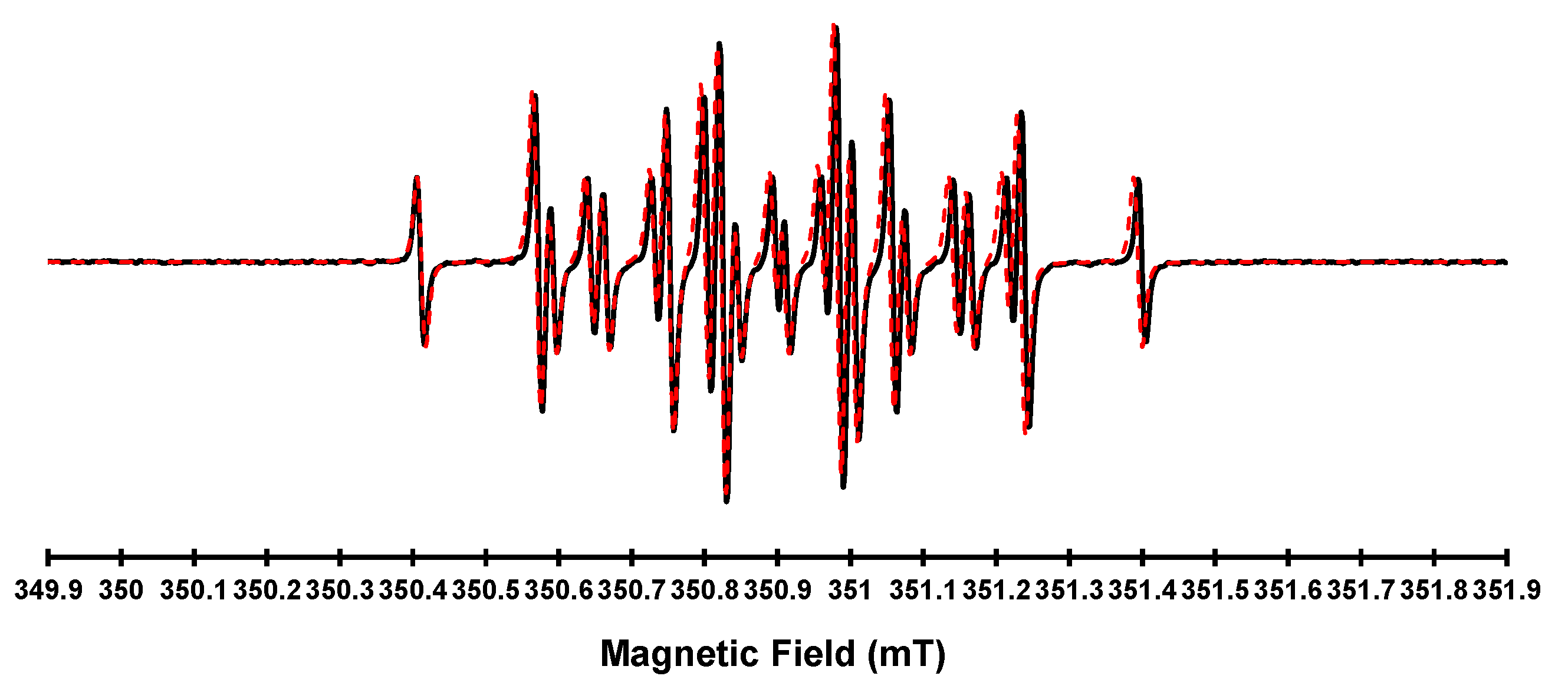
3.3. EPR Spectroscopy
3.4. SDS-PAGE Electrophoresis Buffers
3.5. Solid State NMR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Bernardini, G.; Braconi, D.; Zatkova, A.; Sireau, N.; Kujawa, M.J.; Introne, W.J.; Spiga, O.; Geminiani, M.; Gallagher, J.A.; Ranganath, L.R.; et al. Alkaptonuria. Nat. Rev. Dis. Primers 2024, 10, 16. [Google Scholar] [CrossRef]
- Mistry, J.B.; Bukhari, M.; Taylor, A.M. Alkaptonuria. Rare Dis. 2013, 1, e27475. [Google Scholar] [CrossRef]
- Phornphutkul, C.; Introne, W.J.; Perry, M.B.; Bernardini, I.; Murphey, M.D.; Fitzpatrick, D.L.; Anderson, P.D.; Huizing, M.; Anikster, Y.; Gerber, L.H.; et al. Natural History of Alkaptonuria. N. Engl. J. Med. 2002, 347, 2111–2121. [Google Scholar] [CrossRef]
- Benedek, T.G. Rudolph Virchow on Ochronosis. Arthritis Rheum. 1966, 9, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Cañoń, J.M.; Peñalva, M.A. Molecular Characterization of a Gene Encoding a Homogentisate Dioxygenase from Aspergillus Nidulans and Identification of Its Human and Plant Homologues. J. Biol. Chem. 1995, 270, 21199–21205. [Google Scholar] [CrossRef] [PubMed]
- Zatkova, A.; Sedlackova, T.; Radvansky, J.; Polakova, H.; Nemethova, M.; Aquaron, R.; Dursun, I.; Usher, J.L.; Kadasi, L. Identification of 11 Novel Homogentisate 1,2 Dioxygenase Variants in Alkaptonuria Patients and Establishment of a Novel LOVD-Based HGD Mutation Database. In JIMD Reports; Springer: Berlin/Heidelberg, Germany, 2012; Volume 4, pp. 55–65. [Google Scholar]
- Del Bino, S.; Ito, S.; Sok, J.; Wakamatsu, K. 5,6-Dihydroxyindole Eumelanin Content in Human Skin with Varying Degrees of Constitutive Pigmentation. Pigment. Cell Melanoma Res. 2022, 35, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Cao, L.; Fang, J.; Yu, X.; Dai, X.; Miao, X. Ochronotic Arthritis and Ochronotic Achilles Tendon Rupture. Medicine 2019, 98, e16743. [Google Scholar] [CrossRef]
- Roopnarinesingh, R.C.; Donlon, N.E.; Reynolds, J.V. Alkaptonuria: Clinical Manifestations and an Updated Perspective. J. Orthop. Traumatol. 2021, 14, e244240. [Google Scholar] [CrossRef]
- Milella, M.S.; Geminiani, M.; Trezza, A.; Visibelli, A.; Braconi, D.; Santucci, A. Alkaptonuria: From Molecular Insights to a Dedicated Digital Platform. Cells 2024, 13, 1072. [Google Scholar] [CrossRef]
- Hughes, J.H.; Keenan, C.M.; Sutherland, H.; Edwards, H.R.; Wilson, P.J.M.; Ranganath, L.R.; Jarvis, J.C.; Bou-Gharios, G.; Gallagher, J.A. Anatomical Distribution of Ochronotic Pigment in Alkaptonuric Mice Is Associated with Calcified Cartilage Chondrocytes at Osteochondral Interfaces. Calcif. Tissue Int. 2021, 108, 207–218. [Google Scholar] [CrossRef]
- Lock, E.A.; Ellis, M.K.; Gaskin, P.; Robinson, M.; Auton, T.R.; Provan, W.M.; Smith, L.L.; Prisbylla, M.P.; Mutter, L.C.; Lee, D.L. From Toxicological Problem to Therapeutic Use: The Discovery of the Mode of Action of 2-(2-Nitro-4-Trifluoromethylbenzoyl)-1,3-Cyclohexanedione (NTBC), Its Toxicology and Development as a Drug. J. Inherit. Metab. Dis. 1998, 21, 498–506. [Google Scholar] [CrossRef]
- Lock, E.A. The Discovery of the Mode of Action of Nitisinone. Metabolites 2022, 12, 902. [Google Scholar] [CrossRef]
- Wolffenbuttel, B.H.R.; Heiner-Fokkema, M.R.; van Spronsen, F.J. Preventive Use of Nitisinone in Alkaptonuria. Orphanet J. Rare Dis. 2021, 16, 343. [Google Scholar] [CrossRef] [PubMed]
- Ranganath, L.R.; Psarelli, E.E.; Arnoux, J.B.; Braconi, D.; Briggs, M.; Bröijersén, A.; Loftus, N.; Bygott, H.; Cox, T.F.; Davison, A.S.; et al. Efficacy and Safety of Once-Daily Nitisinone for Patients with Alkaptonuria (SONIA 2): An International, Multicentre, Open-Label, Randomised Controlled Trial. Lancet Diabetes Endocrinol. 2020, 8, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Bernini, A.; Petricci, E.; Atrei, A.; Baratto, M.C.; Manetti, F.; Santucci, A. A Molecular Spectroscopy Approach for the Investigation of Early Phase Ochronotic Pigment Development in Alkaptonuria. Sci. Rep. 2021, 11, 22562. [Google Scholar] [CrossRef] [PubMed]
- Hegedus, Z.L. The Probable Involvement of Soluble and Deposited Melanins, Their Intermediates and the Reactive Oxygen Side-Products in Human Diseases and Aging. Toxicology 2000, 145, 85–101. [Google Scholar] [CrossRef]
- Tokuhara, Y.; Shukuya, K.; Tanaka, M.; Mouri, M.; Ohkawa, R.; Fujishiro, M.; Takahashi, T.; Okubo, S.; Yokota, H.; Kurano, M.; et al. Detection of Novel Visible-Light Region Absorbance Peaks in the Urine after Alkalization in Patients with Alkaptonuria. PLoS ONE 2014, 9, e86606. [Google Scholar] [CrossRef]
- de Vries, S.; Albracht, S.P.; Berden, J.A.; Slater, E.C. A New Species of Bound Ubisemiquinone Anion in QH2: Cytochrome c Oxidoreductase. J. Biol. Chem. 1981, 256, 11996–11998. [Google Scholar] [CrossRef]
- Jünemann, S.; Heathcote, P.; Rich, P.R. On the Mechanism of Quinol Oxidation in Thebc1 Complex. J. Biol. Chem. 1998, 273, 21603–21607. [Google Scholar] [CrossRef]
- Jeoung, J.H.; Bommer, M.; Lin, T.Y.; Dobbek, H. Visualizing the Substrate-, Superoxo-, Alkylperoxo-, and Product-Bound States at the Nonheme Fe(II) Site of Homogentisate Dioxygenase. Proc. Natl. Acad. Sci. USA 2013, 110, 12625–12630. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, R.; Xiang, J.; Kang, H.; Liu, Z.; Huang, Y. Dissolution Mechanism of Cellulose in DMAc/LiCl: Revisiting through Molecular Interactions (Solution NMR Evidence). J. Phys. Chem. B 2014, 118, 9507–9514. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Okumura, Y.; Seno, M.; Sato, T. Isotactic-Rich Poly(N-Vinyl-2-Pyrrolidone): 1D/2D Solution NMR Assignments (Triad/Tetrad). RSC Adv. 2014, 4, 53079–53089. [Google Scholar] [CrossRef]
- Sasaki, A.; Konishi, T.; Kobayashi, K.; Wada, M.; Kusumi, R. Complete 1H and 13C NMR Assignment of a Cellulose Oligomer in LiCl/DMSO (Solution-State, High Resolution). Cellulose 2024, 31, 7895–7904. [Google Scholar] [CrossRef]
- Van Rossum, B.J.; Förster, H.; De Groot, H.J.M. High-Field and High-Speed CP-MAS13C NMR Heteronuclear Dipolar-Correlation Spectroscopy of Solids with Frequency-Switched Lee–Goldburg Homonuclear Decoupling. J. Magn. Reson. 1997, 124, 516–519. [Google Scholar] [CrossRef]
- Harris, R.K.; Becker, E.D.; Cabral De Menezes, S.M.; Goodfellow, R.; Granger, P. NMR Nomenclature. Nuclear Spin Properties and Conventions for Chemical Shifts (IUPAC Recommendations 2001). Pure Appl. Chem. 2001, 73, 1795–1818. [Google Scholar] [CrossRef]


| Stacking Gel | Vol. | Running Gel | Vol. |
|---|---|---|---|
| H2O | 1.49 mL | H2O | 1.20 mL |
| 0.5 m Tris-HCl pH 6.8 | 0.63 mL | 1.5 m Tris-HCl pH 8.8 | 1.25 mL |
| 10% (m/v) SDS | 25 µL | 10% (m/v) SDS | 50 µL |
| Acrylamide/Bis-acrylamide (30%/0.8% w/v) | 0.34 mL | Acrylamide/Bis-acrylamide (30%/0.8% w/v) | 2.50 mL |
| 10% (m/v) ammonium persulfate | 25 µL | 10% (m/v) ammonium persulfate | 50 µL |
| TEMED | 2.5 µL | TEMED | 5 µL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grasso, D.; Balloni, V.; Baratto, M.C.; Mucci, A.; Santucci, A.; Bernini, A. Ochronotic Deposition in Alkaptonuria: Semiquinone-Mediated Oxidative Coupling and Metabolic Drivers of Homogentisic Acid Accumulation. Int. J. Mol. Sci. 2025, 26, 9674. https://doi.org/10.3390/ijms26199674
Grasso D, Balloni V, Baratto MC, Mucci A, Santucci A, Bernini A. Ochronotic Deposition in Alkaptonuria: Semiquinone-Mediated Oxidative Coupling and Metabolic Drivers of Homogentisic Acid Accumulation. International Journal of Molecular Sciences. 2025; 26(19):9674. https://doi.org/10.3390/ijms26199674
Chicago/Turabian StyleGrasso, Daniela, Valentina Balloni, Maria Camilla Baratto, Adele Mucci, Annalisa Santucci, and Andrea Bernini. 2025. "Ochronotic Deposition in Alkaptonuria: Semiquinone-Mediated Oxidative Coupling and Metabolic Drivers of Homogentisic Acid Accumulation" International Journal of Molecular Sciences 26, no. 19: 9674. https://doi.org/10.3390/ijms26199674
APA StyleGrasso, D., Balloni, V., Baratto, M. C., Mucci, A., Santucci, A., & Bernini, A. (2025). Ochronotic Deposition in Alkaptonuria: Semiquinone-Mediated Oxidative Coupling and Metabolic Drivers of Homogentisic Acid Accumulation. International Journal of Molecular Sciences, 26(19), 9674. https://doi.org/10.3390/ijms26199674








