Pseudomonas aeruginosa Pathogenicity and Its Interaction with Other Microorganisms During the Skin Wound Healing Process
Abstract
1. Introduction
2. Brief Overview of the Normal Skin Wound Healing Process
3. The Pseudomonas aeruginosa Role in Skin Wound Healing
| Cells | Secreted Molecules | Description | Reference |
|---|---|---|---|
| Pseudomonas aeruginosa | |||
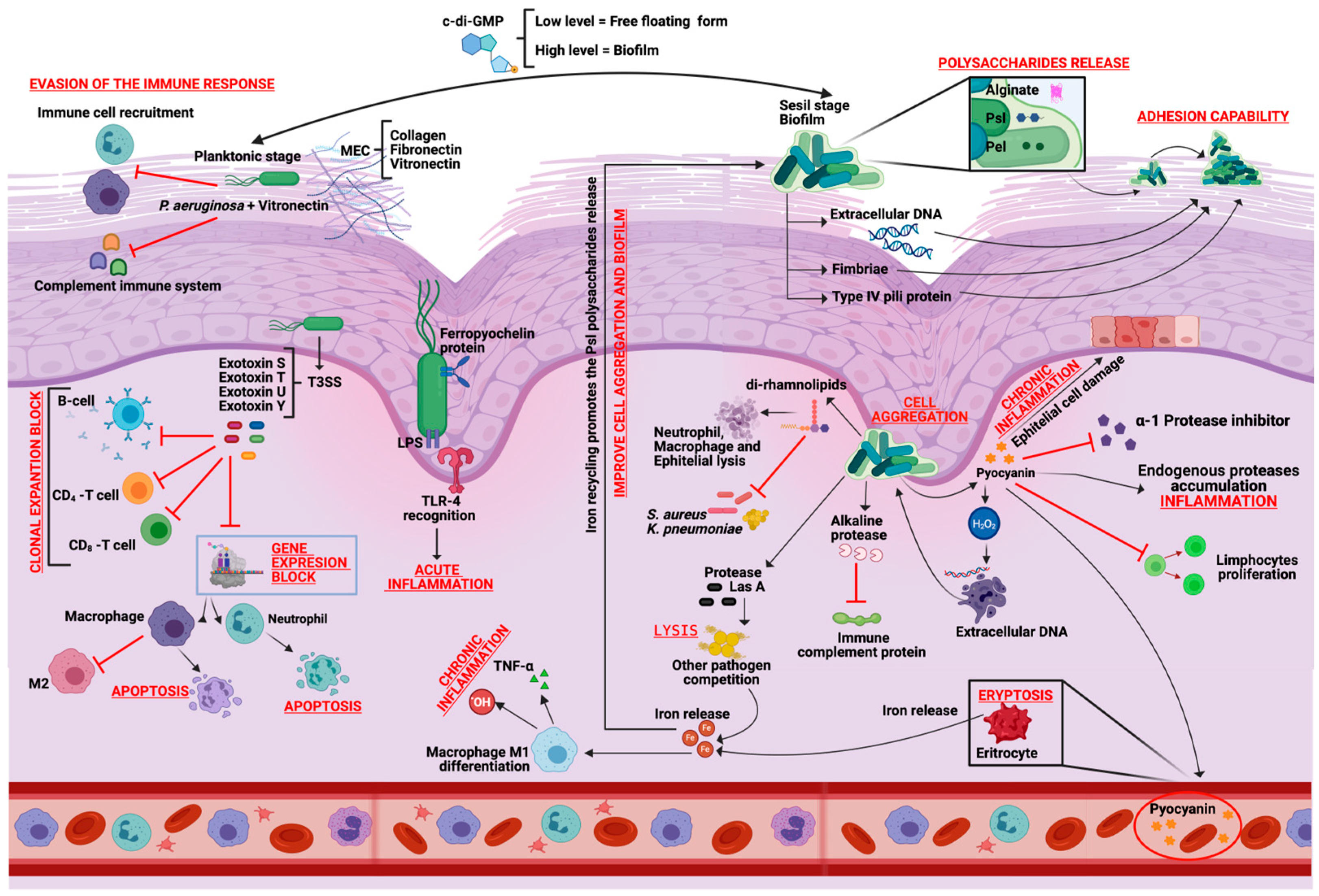
| Planktonic cell | Exotoxin A | Exotoxin that inhibits protein synthesis and causes cytopathic effects in immune cells. | [31] |
| Sessile cells (Biofilm) | Pyocyanin | Virulence factor that induces eryptosis. It has a potential role in biofilm formation by promoting eDNA release due to cell lysis. | [32] |
| Alkaline protease | Extracellular protease, that prevents bacterial elimination by degrading the immune C2 complement protein, can also degrade flagellin and is a known pro-inflammatory responses activator. | [33] | |
| Di-rhamnolipid | Glycolipid biosurfactant that lyse neutrophils, macrophages, and different animal cells rapidly. Acts in swarming motility and shape the biofilm structure, also possess different antimicrobial activity. | [32] | |
| Cyclic diguanosine-5′-monophosphate (c-di-GMP) | Nucleotide on which the lifestyle of P. aeruginosa depends; low levels favor the motility factors expression, promoting the planktonic state and high levels favor the sessile lifestyle by increasing the extracellular matrix components and adhesion factors expression. | [32] | |
| Alginate | Mannuronic acid and glucuronic acid linear polymer, a biofilm component and acts as a cell evasion mechanism, blocking the antibodies and phagocytosis immune action. | [34] | |
| Lectin B | Membrane protein that coats the bacterial cells together and promotes the adhesion of P. aeruginosa to both host cell and exopolysaccharide matrix. On epithelial cells, it inhibits cell migration and proliferation during re-epithelization phase. | [35] | |
| Quinolones | Antimicrobial molecules with selective toxicity to inhibit the synthesis of other bacterial DNA. | [36] | |
| Pel | Polysaccharide involved binding initiation on the surface and maintenance the integrity of biofilm. It crosslinks the eDNA in the biofilm matrix and maintains cell–cell interactions. | [25] | |
| Psl | Polysaccharide involved in cell–cell adhesion. It reduces the immune system attacks because it inhibits opsonization and reduces the neutrophil’s reactive oxygen species (ROS). In addition, it reduces the matrix phagocytosis. | [25] | |
| Acetate | Molecules produced by Pseudomonas aeruginosa, useful to bind to the LPS side chains or alginate by ester bond for preventing the complement immune system activation. | [34] | |
4. Pathogenicity of Pseudomonas aeruginosa in Skin Wound Healing
5. Pseudomonas aeruginosa Biofilm Effect on Skin Wounds
6. Pseudomonas aeruginosa Interaction with the Immune System
7. Interaction of Pseudomonas aeruginosa with Other Pathogens on Skin Wound Healing Process
8. Pseudomonas aeruginosa Skin Wound Infection: Current and Emerging Therapies
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gonzalez, A.C.D.O.; Andrade, Z.A.D.; Costa, T.F.; Medrado, A.R.A.P. Wound healing—A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Tomic-Canic, M.; Burgess, J.L.; O’Neill, K.E.; Strbo, N.; Pastar, I. Skin microbiota and its interplay with wound healing. Am. J. Clin. Dermatol. 2020, 21, 36–43. [Google Scholar] [CrossRef]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef]
- Daeschlein, G.; von Woedtke, T.; Kindel, E.; Brandenburg, R.; Weltmann, K.D. In vitro susceptibility of multidrug resistant skin and wound pathogens against low temperature atmospheric pressure plasma jet (APPJ) and dielectric barrier discharge plasma (DBD). Plasma Process. Polym. 2014, 11, 175–183. [Google Scholar] [CrossRef]
- Brandenburg, K.S.; Weaver, A.J., Jr.; Karna, S.L.; You, T.; Chen, P.; Van Stryk, S.; Leung, K.P. Formation of Pseudomonas aeruginosa biofilms in full-thickness scald burn wounds in rats. Sci. Rep. 2019, 9, 13627. [Google Scholar] [CrossRef]
- Wood, S.J.; Kuzel, T.M.; Shafikhani, S.H. Pseudomonas aeruginosa: Infections, animal modeling, and therapeutics. Cells 2023, 12, 199. [Google Scholar] [CrossRef]
- Vestweber, P.K.; Wächter, J.; Planz, V.; Jung, N.; Windbergs, M. The interplay of Pseudomonas aeruginosa and Staphylococcus aureus in dual-species biofilms impacts development, antibiotic resistance and virulence of biofilms in in vitro wound infection models. PLoS ONE 2023, 19, e0304491. [Google Scholar] [CrossRef] [PubMed]
- Guzzo, F.; Scognamiglio, M.; Fiorentino, A.; Buommino, E.; D’Abrosca, B. Plant-derived natural products against Pseudomonas aeruginosa and Staphylococcus aureus: Antibiofilm activity and molecular mechanisms. Molecules 2020, 25, 5024. [Google Scholar] [CrossRef]
- Yu, S.; Wei, Q.; Zhao, T.; Guo, Y.; Ma, L.Z. A Survival Strategy for Pseudomonas aeruginosa that uses exopolysaccharides to sequester and store iron to stimulate Psl-dependent biofilm formation. Appl. Environ. Microbiol. 2016, 82, 6403–6413. [Google Scholar] [CrossRef]
- Haschka, D.; Hoffmann, A.; Weiss, G. Iron in immune cell function and host defense. Semin. Cell Dev. Biol. 2021, 115, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Nairz, M.; Weiss, G. Iron in infection and immunity. Mol. Asp. Med. 2020, 75, 100864. [Google Scholar] [CrossRef]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Schäfer, M.; Werner, S. Oxidative stress in normal and impaired wound repair. Pharmacol. Res. 2008, 58, 165–171. [Google Scholar] [CrossRef]
- Peña, O.A.; Martin, P. Cellular and molecular mechanisms of skin wound healing. Nat. Rev. Mol. Cell Biol. 2024, 25, 599–616. [Google Scholar] [CrossRef]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound healing: A cellular perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef] [PubMed]
- Phillipson, M.; Kubes, P. The healing power of neutrophils. Trends Immunol. 2019, 40, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Koh, T.J.; DiPietro, L.A. Inflammation and wound healing: The role of the macrophage. Expert Rev. Mol. Med. 2011, 13, e23. [Google Scholar] [CrossRef]
- Mäemets-Allas, K.; Klaas, M.; Cárdenas-León, C.G.; Arak, T.; Kankuri, E.; Jaks, V. Stimulation with THBS4 activates pathways that regulate proliferation, migration and inflammation in primary human keratinocytes. Biochem. Biophys. Res. Commun. 2023, 642, 97–106. [Google Scholar] [CrossRef]
- Eming, S.A.; Murray, P.J.; Pearce, E.J. Metabolic orchestration of the wound healing response. Cell Metab. 2021, 33, 1726–1743. [Google Scholar] [CrossRef]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tredget, E.E.; Wu, P.Y.G.; Wu, Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE 2008, 3, e1886. [Google Scholar] [CrossRef]
- Uberoi, A.; McCready-Vangi, A.; Grice, E.A. The wound microbiota: Microbial mechanisms of impaired wound healing and infection. Nat. Rev. Microbiol. 2024, 22, 210–225. [Google Scholar] [CrossRef]
- Paulsson, M.; Su, Y.C.; Ringwood, T.; Uddén, F.; Riesbeck, K. Pseudomonas aeruginosa uses multiple receptors for adherence to laminin during infection of the respiratory tract and skin wounds. Sci. Rep. 2019, 9, 18168. [Google Scholar] [CrossRef]
- Ibberson, C.B.; Whiteley, M. The social life of microbes in chronic infection. Curr. Opin. Microbiol. 2020, 53, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Jennings, L.K.; Storek, K.M.; Ledvina, H.E.; Coulon, C.; Marmont, L.S.; Sadovskaya, I.; Secor, P.R.; Tseng, B.S.; Scian, M.; Filloux, A.; et al. Pel is a cationic exopolysaccharide that cross-links extracellular DNA in the Pseudomonas aeruginosa biofilm matrix. Proc. Natl. Acad. Sci. USA 2015, 112, 11353–11358. [Google Scholar] [CrossRef] [PubMed]
- Moradali, M.F.; Ghods, S.; Rehm, B.H.A. Pseudomonas aeruginosa lifestyle: A paradigm for adaptation, survival, and persistence. Front. Cell. Infect. Microbiol. 2017, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Watters, C.M.; Burton, T.; Dickson, D.B.; Kelly, K.A. The role of Pseudomonas aeruginosa biofilms in delaying wound healing through evasion of innate immunity. Adv. Wound Care 2017, 6, 373–382. [Google Scholar] [CrossRef]
- Qin, S.; Xiao, W.; Zhou, C.; Pu, Q.; Deng, X.; Lan, L.; Liang, H.; Song, X.; Wu, M. Pseudomonas aeruginosa: Pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Signal Transduct. Target. Ther. 2022, 7, 199. [Google Scholar] [CrossRef]
- Hauser, A.R. The type III secretion system of Pseudomonas aeruginosa: Infection by injection. Nat. Rev. Microbiol. 2009, 7, 654–665. [Google Scholar] [CrossRef]
- Hotterbeekx, A.; Kumar-Singh, S.; Goossens, H.; Malhotra-Kumar, S. In vivo and in vitro interactions between Pseudomonas aeruginosa and Staphylococcus spp. Front. Cell. Infect. Microbiol. 2017, 7, 106. [Google Scholar] [CrossRef]
- Morlon-Guyot, J.; Méré, J.; Bonhoure, A.; Beaumelle, B. Processing of Pseudomonas aeruginosa exotoxin A is dispensable for cell intoxication. Infect. Immun. 2009, 77, 3090–3099. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, T.H.; Tolker-Nielsen, T.; Givskov, M. Bacterial biofilm control by perturbation of bacterial signaling processes. Int. J. Mol. Sci. 2017, 18, 1970. [Google Scholar] [CrossRef] [PubMed]
- Casilag, F.; Lorenz, A.; Krueger, J.; Klawonn, F.; Weiss, S.; Häussler, S. The LasB elastase of Pseudomonas aeruginosa acts in concert with alkaline protease AprA to prevent flagellin-mediated immune recognition. Infect. Immun. 2016, 84, 162–171. [Google Scholar] [CrossRef]
- Leid, J.G.; Willson, C.J.; Shirtliff, M.E.; Hassett, D.J.; Parsek, M.R.; Jeffers, A.K. The exopolysaccharide alginate protects Pseudomonas aeruginosa biofilm bacteria from IFN-γ–mediated macrophage killing. J. Immunol. 2005, 175, 7512–7518. [Google Scholar] [CrossRef]
- Thuenauer, R.; Landi, A.; Trefzer, A.; Altmann, S.; Wehrum, S.; Eierhoff, T.; Diedrich, B.; Dengjel, J.; Nyström, A.; Imberty, A.; et al. The Pseudomonas aeruginosa Lectin LecB causes integrin internalization and inhibits epithelial wound healing. mBio 2020, 11, e03260-19. [Google Scholar] [CrossRef]
- Andriole, V.T. The quinolones: Past, present, and future. Clin. Infect. Dis. 2005, 41 (Suppl. S2), S120–S126. [Google Scholar] [CrossRef]
- Fito-Boncompte, L.; Chapalain, A.; Bouffartigues, E.; Chaker, H.; Lesouhaitier, O.; Gicquel, G.; Bazire, A.; Madi, A.; Connil, N.; VérOn, W.; et al. Full virulence of Pseudomonas aeruginosa requires OprF. Infect. Immun. 2011, 79, 1176–1186. [Google Scholar] [CrossRef]
- Bukhari, S.I.; Aleanizy, F.S. Association of OprF mutant and disturbance of biofilm and pyocyanin virulence in Pseudomonas aeruginosa. Saudi Pharm. J. 2020, 28, 196–200. [Google Scholar] [CrossRef]
- Luo, J.; Dong, B.; Wang, K.; Cai, S.; Liu, T.; Cheng, X.; Lei, D.; Chen, Y.; Li, Y.; Kong, J.; et al. Baicalin inhibits biofilm formation, attenuates the quorum sensing–controlled virulence and enhances Pseudomonas aeruginosa clearance in a mouse peritoneal implant infection model. PLoS ONE 2017, 12, e0176883. [Google Scholar] [CrossRef]
- De Kievit, T.R. Quorum sensing in Pseudomonas aeruginosa biofilms. Environ. Microbiol. 2009, 11, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, A.D.; Hoveizavi, H.; Mohammadian, A.; Farahani, A.; Jenabi, A. Genotyping of multidrug-resistant strains of Pseudomonas aeruginosa isolated from burn and wound infections by ERIC-PCR. Acta Cir. Bras. 2016, 31, 206–211. [Google Scholar] [CrossRef]
- Shrout, J.D.; Chopp, D.L.; Just, C.L.; Hentzer, M.; Givskov, M.; Parsek, M.R. The impact of quorum sensing and swarming motility on Pseudomonas aeruginosa biofilm formation is nutritionally conditional. Mol. Microbiol. 2006, 62, 1264–1277. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Zhang, W.; Zhang, Q. Cyclic-di-GMP signaling and regulation of bacterial virulence. Microbiol. China 2019, 46, 2265–2273. [Google Scholar] [CrossRef]
- Wei, Q.; Zhang, Z.; Luo, J.; Kong, J.; Ding, Y.; Chen, Y.; Wang, K. Insulin treatment enhances Pseudomonas aeruginosa biofilm formation by increasing intracellular cyclic di-GMP levels, leading to chronic wound infection and delayed wound healing. Am. J. Transl. Res. 2019, 11, 3261–3279. [Google Scholar]
- Valentini, M.; Filloux, A. Biofilms and cyclic di-GMP (c-di-GMP) signaling: Lessons from Pseudomonas aeruginosa and other bacteria. J. Biol. Chem. 2019, 294, 7193–7203. [Google Scholar] [CrossRef]
- Trøstrup, H.; Lerche, C.J.; Christophersen, L.J.; Thomsen, K.; Jensen, P.Ø.; Hougen, H.P.; Høiby, N.; Moser, C. Chronic Pseudomonas aeruginosa biofilm infection impairs murine S100A8/A9 and neutrophil effector cytokines—implications for delayed wound closure? Pathog. Dis. 2017, 75, ftx068. [Google Scholar] [CrossRef]
- Karna, S.L.R.; Leung, K.P.; Karna, R.S.; You, T.; Chen, P.; Van Stryk, S.; Brandenburg, K.S. The impact of simultaneous inoculation of Pseudomonas aeruginosa, Staphylococcus aureus, and Candida albicans on burn-wound biofilms and host response in a rat model. Burns 2021, 47, 331–341. [Google Scholar] [CrossRef]
- Mishra, R.; Panda, A.K.; De Mandal, S.; Shakeel, M.; Bisht, S.S.; Khan, J. Natural anti-biofilm agents: Strategies to control biofilm-forming pathogens. Front. Microbiol. 2020, 11, 566325. [Google Scholar] [CrossRef]
- James, G.A.; Swogger, E.; Wolcott, R.; Pulcini, E.; Secor, P.; Sestrich, J.; Costerton, J.W.; Stewart, P.S. Biofilms in chronic wounds. Wound Repair Regen. 2008, 16, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Bjarnsholt, T.; Kirketerp-Møller, K.; Jensen, P.Ø.; Madsen, K.G.; Phipps, R.; Krogfelt, K.; Høiby, N.; Givskov, M. Why chronic wounds will not heal: A novel hypothesis. Wound Repair Regen. 2008, 16, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Malone, M.; Goeres, D.M.; Gosbell, I.B.; Vickery, K.; Jensen, S.; Stoodley, P. Approaches to biofilm-associated infections in chronic wounds. Wound Pract Res. 2017, 25, 78–88. [Google Scholar]
- Pier, G.B. Pseudomonas aeruginosa lipopolysaccharide: A major virulence factor, initiator of inflammation and target for effective immunity. Int. J. Med. Microbiol. 2007, 297, 277–295. [Google Scholar] [CrossRef]
- Cott, C.; Thuenauer, R.; Landi, A.; Kühn, K.; Juillot, S.; Imberty, A.; Madl, J.; Eierhoff, T.; Römer, W. Pseudomonas aeruginosa Lectin LecB inhibits tissue repair processes by triggering β-Catenin degradation. BBA 2016, 1863 Pt A, 1106–1118. [Google Scholar] [CrossRef] [PubMed]
- Jeffery Marano, R.; Branski, L.K.; Boehning, D.F.; McCauley, R.L. Mitochondrial dysfunction and reactive oxygen species in thermal injury: A review. J. Burn. Care Res. 2015, 36, e1–e9. [Google Scholar] [CrossRef]
- Trøstrup, H.; Thomsen, K.; Christophersen, L.J.; Hougen, H.P.; Bjarnsholt, T.; Jensen, P.Ø.; Kirkby, N.; Calum, H.; Høiby, N.; Moser, C. Pseudomonas aeruginosa biofilm aggravates skin inflammatory response in BALB/c mice in a novel chronic wound model. Wound Repair Regen. 2013, 21, 292–299. [Google Scholar] [CrossRef]
- Pena, R.T.; Blasco, L.; Ambroa, A.; González-Pedrajo, B.; Fernández-García, L.; López, M.; Bou, G.; García-Contreras, R.; Wood, T.K.; Tomás, M. Relationship between quorum sensing and secretion systems. Front Microbiol. 2019, 10, 1100. [Google Scholar] [CrossRef]
- Nakagami, G.; Minematsu, T.; Harada, Y.; Shigematsu, T.; Ohta, Y.; Sanada, H. Risk factors and prediction model for intractable pressure ulcers. Wound Repair Regen. 2011, 19, 505–515. [Google Scholar] [CrossRef]
- Weaver AJJr Brandenburg, K.S.; Karna, S.L.R.; You, T.; Chen, P.; Leung, K.P. Differential macrophage polarization and cytokine profiles in response to oral and wound biofilm isolates of Pseudomonas aeruginosa. Microorganisms 2019, 7, 676. [Google Scholar] [CrossRef]
- Parameswaran, N.; Patial, S. Tumor necrosis factor-α signaling in macrophages. Crit. Rev. Eukaryot. Gene Expr. 2010, 20, 87–103. [Google Scholar] [CrossRef]
- Fernando, M.R.; Reyes, J.L.; Iannuzzi, J.; Leung, G.; McKay, D.M. The pro-inflammatory cytokine, interleukin-6, enhances the polarization of alternatively activated macrophages. PLoS ONE. 2014, 9, e94188. [Google Scholar] [CrossRef]
- Xing, Z.; Zganiacz, A.; Santosuosso, M. Role of IL-12 in protection and immunopathology of tuberculosis. Cell Mol. Biol. 2000, 46, 537–546. [Google Scholar]
- Ryan, R.P. Cyclic di-GMP signaling and the regulation of bacterial virulence. Microbiology 2013, 159 Pt 7, 1286–1297. [Google Scholar] [CrossRef]
- Wynn, T.A.; Barron, L. Macrophages: Master regulators of inflammation and fibrosis. Sem. Liver Dis. 2010, 30, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, K.; Hagio, T.; Matsuoka, S. The role of neutrophil elastase in acute lung injury. Eur. J. Pharmacol. 2002, 451, 1–10. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Anju, V.T.; Busi, S.; Imchen, M.; Kumavath, R.; Mohan, M.S.; Salim, S.A.; Subhaswaraj, P.; Dyavaiah, M. Polymicrobial infections and biofilms: Clinical Significance and Eradication Strategies. Antibiotics 2022, 11, 1731. [Google Scholar] [CrossRef]
- Nguyen, T.; Soulika, A.M. The dynamics of the skin’s immune system and microbial environment. Clin. Dermatol. 2019, 37, 619–627. [Google Scholar] [CrossRef]
- Qin, Z.; Yang, L.; Qu, D.; Molin, S.; Tolker-Nielsen, T. Pseudomonas aeruginosa and Staphylococcus aureus interaction in biofilm development and disease. Mol. Microbiol. 2009, 71, 442–455. [Google Scholar] [CrossRef]
- Singh, V.; Sharma, J.; Kumari, A.; Halliwell, R.F.; Goyal, R. Quorum sensing and virulence regulation in polymicrobial infections of Pseudomonas aeruginosa. Microb. Pathog. 2017, 111, 14–20. [Google Scholar] [CrossRef]
- Hotterbeekx, A.; Kumar-Singh, S.; Goossens, H.; Malhotra-Kumar, S. Interaction of Pseudomonas aeruginosa with other pathogens and the host. Int. J. Med. Microbiol. 2017, 307, 409–419. [Google Scholar] [CrossRef]
- Saroj, S.D.; Shashidhar, R.; Karani, M.; Bandekar, J.R. Acyl homoserine lactones interfere with Streptococcus pyogenes hemolytic activity by modulating sag operon expression. J. Med. Microbiol. 2017, 66, 775–783. [Google Scholar] [CrossRef]
- Murray, E.J.; Dubern, J.-F.; Chan, W.C.; Chhabra, S.R.; Williams, P. A Pseudomonas aeruginosa PQS quorum-sensing system inhibitor with anti-staphylococcal activity sensitizes polymicrobial biofilms to tobramycin. Cell Chem. Biol. 2022, 29, 1187–1199.e6. [Google Scholar] [CrossRef]
- Korgaonkar, A.; Trivedi, U.; Rumbaugh, K.P.; Whiteley, M. Community surveillance enhances Pseudomonas aeruginosa virulence during polymicrobial infection. Proc. Natl. Acad. Sci. USA 2013, 110, 1059–1064. [Google Scholar] [CrossRef]
- Vacca, I. Modified sugar compound can clear intestinal colonization by UPEC. Nat. Rev. Microbiol. 2017, 15, 449. [Google Scholar] [CrossRef]
- Déziel, E.; Lépine, F.; Milot, S.; He, J.; Mindrinos, M.N.; Tompkins, R.G.; Rahme, L.G. Analysis of Pseudomonas aeruginosa 4-Hydroxy-2-Alkylquinolines (HAQs) reveals a role for 4-Hydroxy-2-Heptylquinoline in cell-to-cell communication. Proc. Natl. Acad. Sci. USA 2004, 101, 1339–1344. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, M.M.; Rumbaugh, K.P.; Whiteley, M. Metabolite cross-feeding enhances virulence in a model polymicrobial infection. PLoS Pathog. 2016, 12, e1005465. [Google Scholar] [CrossRef]
- Scoffield, J.A.; Duan, D.; Zhu, F.; Wu, H. A commensal Streptococcus hijacks a Pseudomonas aeruginosa exopolysaccharide to promote biofilm formation. PLoS Pathog. 2017, 13, e1006300. [Google Scholar] [CrossRef] [PubMed]
- Turner, K.H.; Wessel, A.K.; Palmer, G.C.; Murray, J.L.; Whiteley, M. Essential genome of Pseudomonas aeruginosa in cystic fibrosis sputum. Proc. Natl. Acad. Sci. USA 2014, 111, 5109–5114. [Google Scholar] [CrossRef] [PubMed]
- Livermore, D.M. Multiple mechanisms of antimicrobial resistance in Pseudomonas aeruginosa: Our worst nightmare? Clin. Infect. Dis. 2002, 34, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Mensa, J.; Barberán, J.; Soriano, A.; Llinares, P.; Grau, S. Guía de terapéutica antimicrobiana 2018; Antares: Barcelona, Spain, 2018. [Google Scholar]
- Limoli, D.H.; Jones, C.J.; Wozniak, D.J. Bacterial extracellular polysaccharides in biofilm formation and function. Nat. Rev. Microbiol. 2015, 13, 461–469. [Google Scholar] [CrossRef]
- Lin, L.; Nonejuie, P.; Munguia, J.; Hollands, A.; Olson, J.; Dam, Q.; Kumaraswamy, M.; Rivera, H.; Corriden, R.; Rohde, M.; et al. Azithromycin synergizes with cationic antimicrobial peptides to exert bactericidal and therapeutic activity against highly multidrug-resistant Gram-negative bacterial pathogens. EBioMedicine 2015, 2, 690–698. [Google Scholar] [CrossRef]
- Hagihara, M.; Aitken, S.L.; Hasegawa, Y.; Nicolau, D.P. In vitro evaluation of azithromycin combinations with ceftolozane/tazobactam and other antibiotics against Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2025, 69, e01234-24. [Google Scholar] [CrossRef]
- Nambiar, S.; Laessig, K.A.; Toerner, J.G.; Farley, J.; Cox, E. Antibacterial drug development: Challenges, recent developments, and future considerations. Clin. Pharmacol. Ther. 2019, 106, 1298–1311. [Google Scholar] [CrossRef]
- Chung, P.Y.; Khanum, R. Antimicrobial peptides as potential anti-biofilm agents against multidrug-resistant bacteria. J. Microbiol. Immunol. Infect. 2017, 50, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Shimizu, T.; Yokoyama, T.; Takabe, W.; Takahashi, T. Lipoxygenase-derived oxylipins modulate keratinocyte migration via PI3K/Akt signaling. J. Dermatol. Sci. 2019, 93, 180–189. [Google Scholar] [CrossRef]
- Heinlin, J.; Isbary, G.; Stolz, W.; Zeman, F.; Landthaler, M.; Shimizu, T.; Steffes, B.; Nosenko, T.; Zimmermann, J.; Karrer, S. Plasma applications in medicine with a special focus on dermatology. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Su, M.; Hasan, N.; Lee, J.; Kwak, D.; Kim, D.Y.; Kim, K.; Lee, E.H.; Jung, J.H.; Yoo, J.-W. Nitric oxide-releasing thermoresponsive pluronic F127/alginate hydrogel for enhanced antibacterial activity and accelerated healing of infected wounds. Pharmaceutics 2020, 12, 926. [Google Scholar] [CrossRef]
- Fila, G.; Krychowiak, M.; Rychlowski, M.; Bielawski, K.P.; Grinholc, M. Antimicrobial blue light photoinactivation of Pseudomonas aeruginosa: Quorum sensing signaling molecules, biofilm formation and pathogenicity. J. Biophotonics 2018, 11, e201800079. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.-H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef] [PubMed]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive oxygen species (ROS) and wound healing: The functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. Wound J. 2017, 14, 89–96. [Google Scholar] [CrossRef]
- Miliotis, S.; Nicolalde, B.; Ortega, M.; Yepez, J.; Caicedo, A. Forms of extracellular mitochondria and their impact in health. Mitochondrion 2019, 48, 16–30. [Google Scholar] [CrossRef]
- Balcázar, M.; Cañizares, S.; Borja, T.; Pontón, P.; Bisiou, S.; Carabasse, E.; Bacilieri, A.; Canavese, C.; Diaz, R.F.; Cabrera, F.; et al. Bases for treating skin aging with artificial mitochondrial transfer/transplant (AMT/T). Front. Bioeng. Biotechnol. 2020, 8, 919. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Fu, Z.; Zou, L. Mechanism and potential of extracellular vesicles derived from mesenchymal stem cells for the treatment of infectious diseases. Front. Microbiol. 2021, 12, 761338. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, T.; Saleem, A.; Farooq, N.; Dar, L.A.; Nazir, J.; Saleem, S.; Ismail, S.; Gugjoo, M.B.; Shiekh, P.A.; Ahmad, S.M. Extracellular vesicles derived from mesenchymal stem cells—A novel therapeutic tool in infectious diseases. Inflamm. Regen. 2023, 43, 17. [Google Scholar] [CrossRef] [PubMed]
- Martins-Green, M.; Kim, J.; Aziz, K. The impact of the skin microbiome and oxidative stress on the initiation and development of cutaneous chronic wounds. Antioxidants 2025, 14, 682. [Google Scholar] [CrossRef]

| Cells | Secreted Molecules | Description | Reference | |
|---|---|---|---|---|
| Immune System | ||||
| M1 macrophages | Pro-inflammatory cytokines | Tumor Necrosis Factor (TNFα) | Cytokine released immediately after any damage by exposure to bacterial LPS. Acts through two transmembrane receptors: TNF receptor 1 (TNFR1) induces programmed cell death and TNF receptor 2 (TNFR2) is responsible for cell proliferation. Therefore, depending on the cell type, TNFR1 and TNFR2 may have distinct roles in signal transduction and gene expression. | [58] |
| IL-6 | Interleukin that promotes T and B lymphocytes differentiation and maturation, stimulates immunoglobulin release by B cells and pro-inflammatory cytokines inhibition such as TNF-α that participates in the macrophage M1 to M2 maturation. | [59] | ||
| IL-12 | Interleukin that activates T CD4+ (H1) type 1 cells and stimulates the NK cells and T CD8+ lymphocytes production. | [60] | ||
| ROS/NOS | Reactive oxygen and nitrogen species that cause significant cell structures damage causing cell lysis. | [61] | ||
| M2 macrophages | Anti-inflammatory cytokines | TGF-β | Interleukin that protects the collagen expression by some protease activity inhibition. | [62] |
| IL-14 | Interleukin that inhibits the pro-inflammatory cytokines synthesis such as TNF-alpha and IL-6. | [60] | ||
| IL-10 | Interleukin that inhibits the pro-inflammatory cytokines synthesis such as IFN-γ, IL-2, IL-3, and TNFα. | [60] | ||
| Arginase | Enzyme responsible for NOS synthesis regulation and tissue regeneration. | [62] | ||
| Neutrophils | Elastases | Protease that is released as a defense mechanism to remove NOS and ROS tissue degradation products. | [63] | |
| Pseudomonas aeruginosa | Exotoxin A | Modifies macrophage gene expression and inhibits the maturation of M1 macrophages to M2. | [56] | |
| Alginate | Inhibits bacterial uptake during phagocytosis in Macrophages and Neutrophils. | [34] | ||
| Exotoxin A | Modify gene expression to cause apoptosis in Neutrophils. | [56] | ||
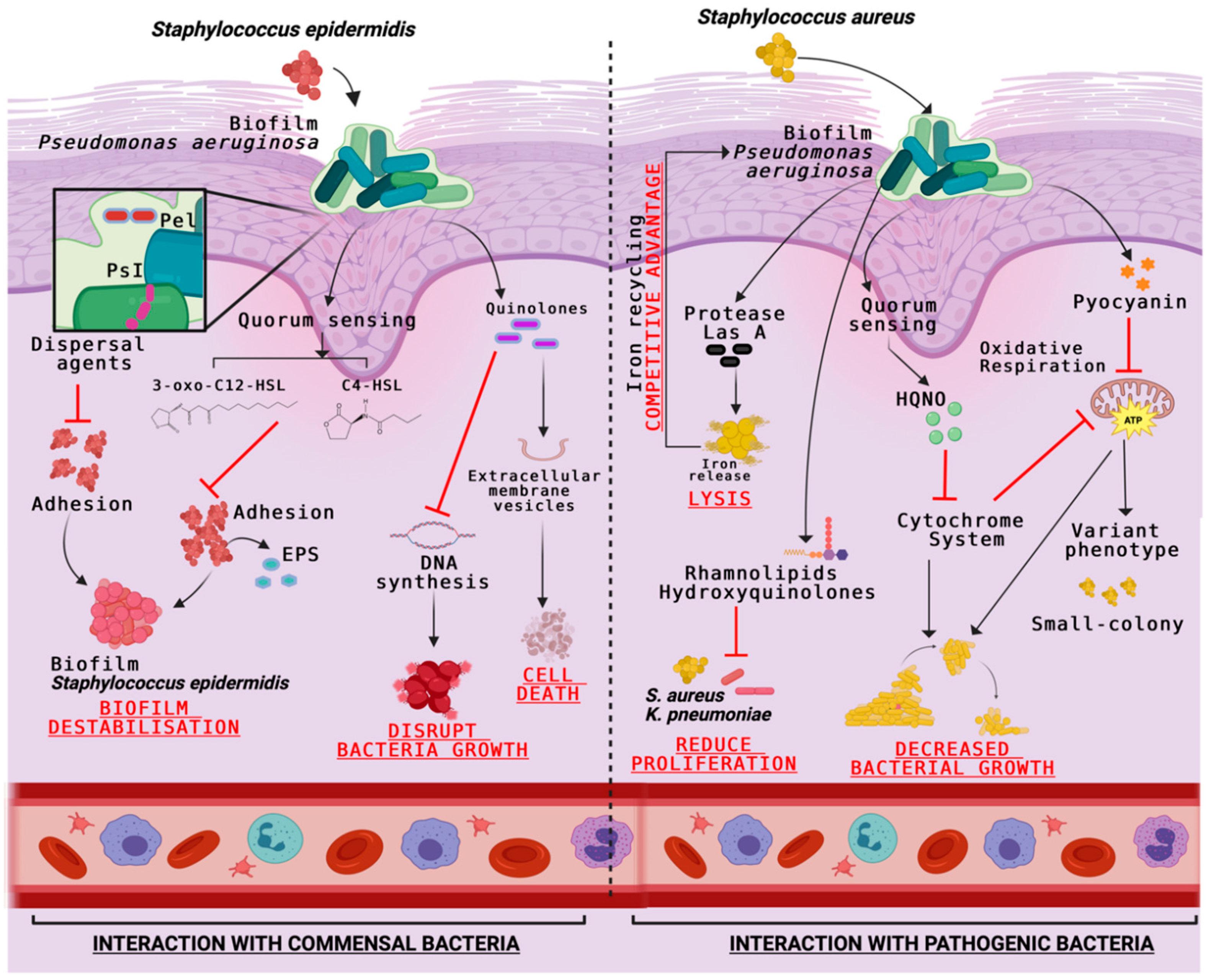
| Pseudomonas aeruginosa Secreted Molecules | Cells Interaction with | Description | Reference |
|---|---|---|---|
| Quinolones | Staphylococcus epidermidis | Molecule packaged in extracellular membrane vesicles (MVs) to cause cell lysis. | [67] |
| Pel and PsI | Both acts as dispersing agents to inhibit biofilm formation and adhesion. | [67] | |
| 3-Oxo-C12-HSL | Inhibits the bacterial growth and EPS secretion that hinders the initial adhesion and the biofilms formation. | [67] | |
| 4-hydroxy-2-heptylquinoline N-oxide (HQNO) | Staphylococcus aureus | Quinolone signal system component acts as an inhibitor of S. aureus electron transport chain (ETC). Prolonged exposure to this compound leads to the small colony variants selection. | [68] |
| Pyocyanin | Increases the H2O2 formation and leads to cell lysis. | [68] | |
| Las A | Protease that lyses S. aureus cells. | [68] | |
| Pyocyanin | Streptococcus spp. | Increases the H2O2 formation and leads to cell lysis. | [69] |
| Acyl homoserine lactone (AHL) | Streptococcus pyogenes | Modifies hemolytic activity and reduces pathogenicity of S. pyogenes. | [70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamberla, I.; Pupiales, C.; Chiliquinga, A.J.; Sulca-Villamarín, T.; Plasencia, A.; Cabrera Aulestia, F.; Díaz, R.F.; Caicedo, A.; Barba, P.M. Pseudomonas aeruginosa Pathogenicity and Its Interaction with Other Microorganisms During the Skin Wound Healing Process. Int. J. Mol. Sci. 2025, 26, 9677. https://doi.org/10.3390/ijms26199677
Yamberla I, Pupiales C, Chiliquinga AJ, Sulca-Villamarín T, Plasencia A, Cabrera Aulestia F, Díaz RF, Caicedo A, Barba PM. Pseudomonas aeruginosa Pathogenicity and Its Interaction with Other Microorganisms During the Skin Wound Healing Process. International Journal of Molecular Sciences. 2025; 26(19):9677. https://doi.org/10.3390/ijms26199677
Chicago/Turabian StyleYamberla, Inti, Carla Pupiales, Andrea Jazmín Chiliquinga, Tania Sulca-Villamarín, Alejandra Plasencia, Francisco Cabrera Aulestia, Ramiro F. Díaz, Andrés Caicedo, and Pedro Miguel Barba. 2025. "Pseudomonas aeruginosa Pathogenicity and Its Interaction with Other Microorganisms During the Skin Wound Healing Process" International Journal of Molecular Sciences 26, no. 19: 9677. https://doi.org/10.3390/ijms26199677
APA StyleYamberla, I., Pupiales, C., Chiliquinga, A. J., Sulca-Villamarín, T., Plasencia, A., Cabrera Aulestia, F., Díaz, R. F., Caicedo, A., & Barba, P. M. (2025). Pseudomonas aeruginosa Pathogenicity and Its Interaction with Other Microorganisms During the Skin Wound Healing Process. International Journal of Molecular Sciences, 26(19), 9677. https://doi.org/10.3390/ijms26199677










