The Phenomenon of Anti-Drug Antibodies in Psoriasis: Mechanisms, Clinical Impact, and Therapeutic Strategies
Abstract
1. Introduction
1.1. Definition of Anti-Drug Antibodies (ADA)
1.2. Type of Blocking Therapy with Therapeutic Antibodies and Their Relevance in Psoriasis (PsO)
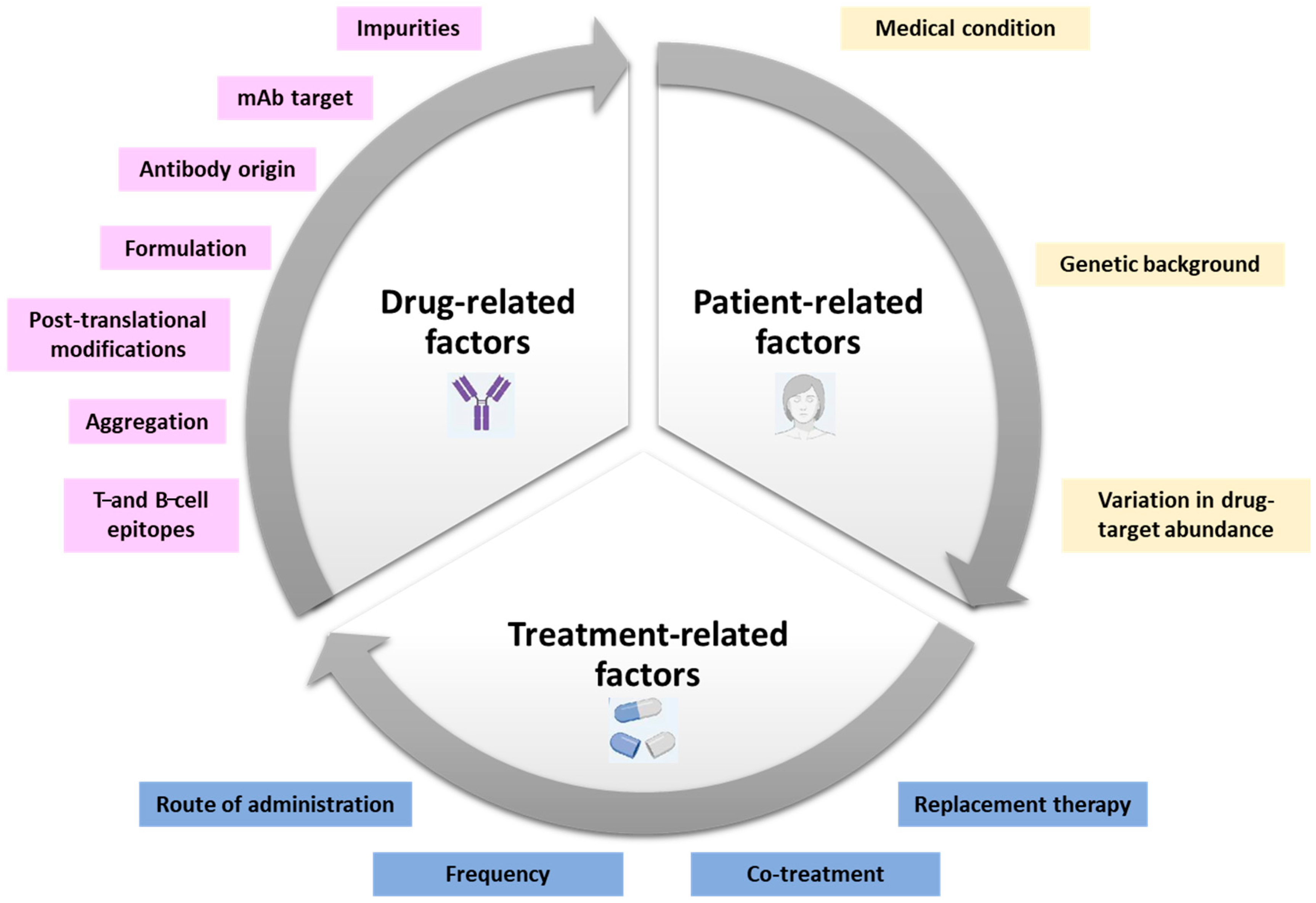
2. Mechanisms of ADA Formation
Factors Influencing ADA Development: Drug-Related Factors, Patient-Related Factors, and Treatment-Related Factors
3. Prevalence of ADAs in Psoriasis
3.1. Summary of Reported ADA Rates for Different Biologics
3.1.1. TNFα Inhibitors (Adalimumab, Infliximab, Certolizumab, and Etanercept)
3.1.2. IL-12/23 Inhibitor (Ustekinumab)
3.1.3. IL-17 Inhibitors (Secukinumab, Brodalumab, and Ixekizumab)
3.1.4. IL-23 Inhibitors (Guselkumab, Risankizumab, and Tildrakizumab)
4. Clinical Consequences of ADA Formation
4.1. Reduced Drug Efficacy and Loss of Response, Drug Neutralization by Anti-Drug Antibodies: Comparison Between Neutralizing Versus Non-Neutralizing ADA
4.2. Increased Drug Clearance and Altered Pharmacokinetics
5. Strategies to Minimize ADA Development
5.1. Combination Therapy with Immunosuppressants (Such as Methotrexate)
5.2. Drug Dose Optimization and Interval Adjustments
6. New Approaches in the Field for Psoriasis and Other Diseases
7. Clinical Management of Patients with ADA
7.1. ADA Monitoring and Testing Methods
7.2. Switching Strategies: Intra-Class Versus Inter-Class Switching
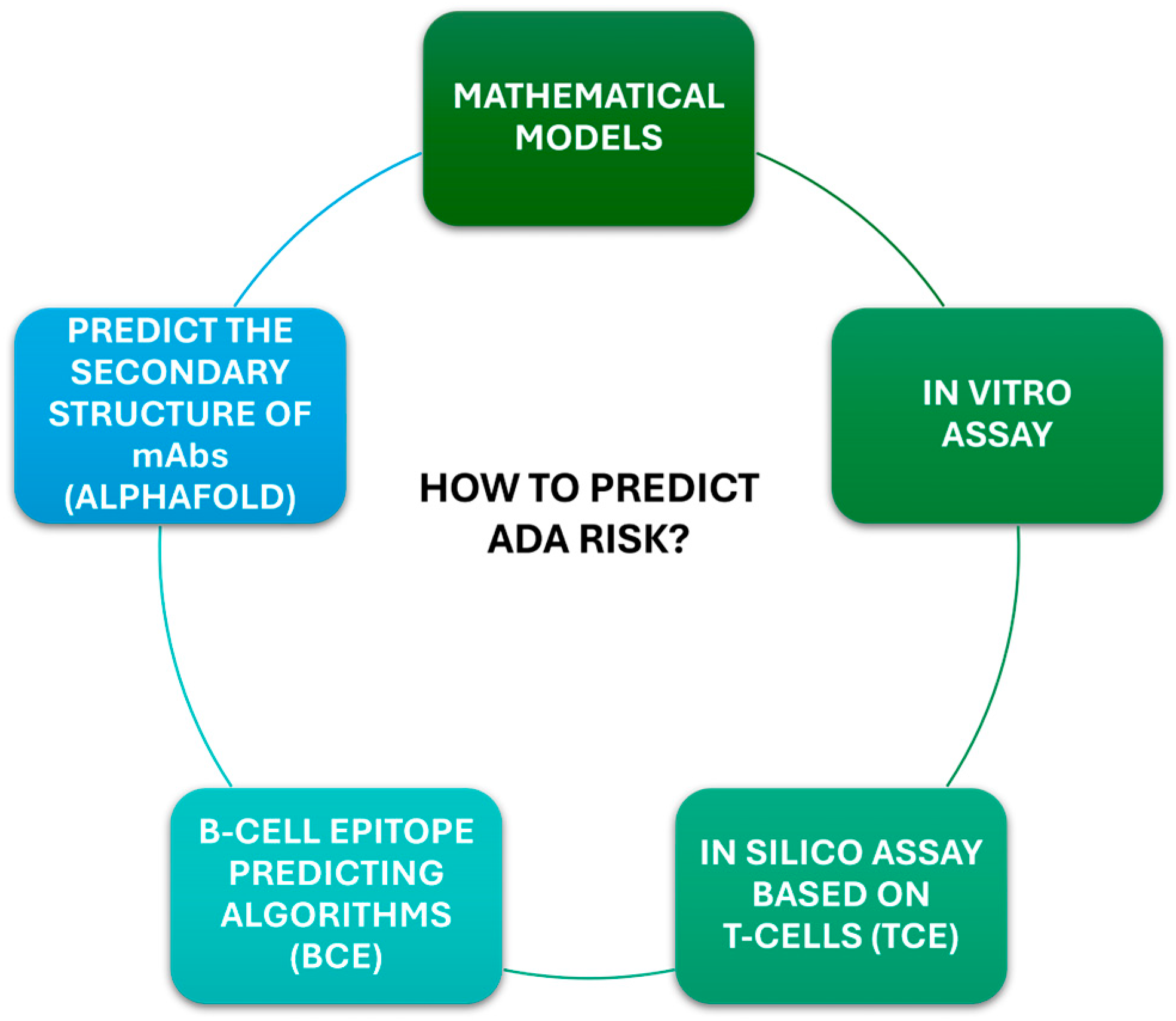
7.3. Emerging Techniques and Biomarkers for Predicting ADA Risk
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Vultaggio, A.; Matucci, A.; Nencini, F.; Pratesi, S.; Parronchi, P.; Rossi, O.; Romagnani, S.; Maggi, E. Anti-infliximab IgE and non-IgE Antibodies and Induction of Infusion-related Severe Anaphylactic Reactions. Allergy 2010, 65, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Strand, V.; Goncalves, J.; Isaacs, J.D. Immunogenicity of Biologic Agents in Rheumatology. Nat. Rev. Rheumatol. 2021, 17, 81–97. [Google Scholar] [CrossRef]
- Van Schouwenburg, P.A.; Rispens, T.; Wolbink, G.J. Immunogenicity of Anti-TNF Biologic Therapies for Rheumatoid Arthritis. Nat. Rev. Rheumatol. 2013, 9, 164–172. [Google Scholar] [CrossRef]
- Garcês, S.; Demengeot, J. The Immunogenicity of Biologic Therapies. In Current Problems in Dermatology; Puig, L., Gulliver, W., Eds.; S. Karger AG: Basel, Switzerland, 2018; Volume 53, pp. 37–48. ISBN 978-3-318-06100-0. [Google Scholar]
- Jochems, A.; Martinez-Feito, A.; Plasencia, C.; Hernández-Breijo, B.; Mezcúa, A.; Villalba, A.; Monjo, I.; Nozal, P.; Balsa, A.; Pascual-Salcedo, M. SAT0141 Optimal Circulating Adalimumab Levels Range Associated with Good Clinical Response in Rheumatoid Arthritis Patients. Ann. Rheum. Dis. 2017, 76, 822. [Google Scholar] [CrossRef]
- Bartelds, G.M. Development of Antidrug Antibodies Against Adalimumab and Association With Disease Activity and Treatment Failure During Long-Term Follow-Up. J. Am. Med. Assoc. 2011, 305, 1460. [Google Scholar] [CrossRef]
- Van Schie, K.A.; Kruithof, S.; Van Schouwenburg, P.A.; Vennegoor, A.; Killestein, J.; Wolbink, G.; Rispens, T. Neutralizing Capacity of Monoclonal and Polyclonal Anti-Natalizumab Antibodies: The Immune Response to Antibody Therapeutics Preferentially Targets the Antigen-Binding Site. J. Allergy Clin. Immunol. 2017, 139, 1035–1037.e6. [Google Scholar] [CrossRef]
- Gunderson, A.J.; Mohammed, J.; Horvath, F.J.; Podolsky, M.A.; Anderson, C.R.; Glick, A.B. CD8+ T Cells Mediate RAS-Induced Psoriasis-Like Skin Inflammation through IFN-γ. J. Investig. Dermatol. 2013, 133, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Huang, L.; Lv, P.; Li, X.; Liu, G.; Chen, Y.; Wang, Z.; Qian, X.; Shen, Y.; Li, Y.; et al. The Role of Th17 Cells in Psoriasis. Immunol. Res. 2020, 68, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, D.; Chamilos, G.; Lande, R.; Gregorio, J.; Meller, S.; Facchinetti, V.; Homey, B.; Barrat, F.J.; Zal, T.; Gilliet, M. Self-RNA–Antimicrobial Peptide Complexes Activate Human Dendritic Cells through TLR7 and TLR8. J. Exp. Med. 2009, 206, 1983–1994. [Google Scholar] [CrossRef]
- Langley, R.G.; Elewski, B.E.; Lebwohl, M.; Reich, K.; Griffiths, C.E.M.; Papp, K.; Puig, L.; Nakagawa, H.; Spelman, L.; Sigurgeirsson, B.; et al. Secukinumab in Plaque Psoriasis—Results of Two Phase 3 Trials. N. Engl. J. Med. 2014, 371, 326–338. [Google Scholar] [CrossRef]
- Harding, F.A.; Stickler, M.M.; Razo, J.; DuBridge, R. The Immunogenicity of Humanized and Fully Human Antibodies: Residual Immunogenicity Resides in the CDR Regions. mAbs 2010, 2, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Lowes, M.A.; Bowcock, A.M.; Krueger, J.G. Pathogenesis and Therapy of Psoriasis. Nature 2007, 445, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Cargill, M.; Schrodi, S.J.; Chang, M.; Garcia, V.E.; Brandon, R.; Callis, K.P.; Matsunami, N.; Ardlie, K.G.; Civello, D.; Catanese, J.J.; et al. A Large-Scale Genetic Association Study Confirms IL12B and Leads to the Identification of IL23R as Psoriasis-Risk Genes. Am. J. Hum. Genet. 2007, 80, 273–290. [Google Scholar] [CrossRef]
- Tracey, K.J.; Fong, Y.; Hesse, D.G.; Manogue, K.R.; Lee, A.T.; Kuo, G.C.; Lowry, S.F.; Cerami, A. Anti-Cachectin/TNF Monoclonal Antibodies Prevent Septic Shock during Lethal Bacteraemia. Nature 1987, 330, 662–664. [Google Scholar] [CrossRef]
- Kristensen, M.; Chu, C.Q.; Eedy, D.J.; Feldmann, M.; Brennan, F.M.; Breathnach, S.M. Localization of Tumour Necrosis Factor-Alpha (TNF-α) and Its Receptors in Normal and Psoriatic Skin: Epidermal Cells Express the 55-kD but Not the 75-kD TNF Receptor. Clin. Exp. Immunol. 2008, 94, 354–362. [Google Scholar] [CrossRef]
- Hänsel, A.; Günther, C.; Ingwersen, J.; Starke, J.; Schmitz, M.; Bachmann, M.; Meurer, M.; Rieber, E.P.; Schäkel, K. Human Slan (6-Sulfo LacNAc) Dendritic Cells Are Inflammatory Dermal Dendritic Cells in Psoriasis and Drive Strong T 17/T 1 T-Cell Responses. J. Allergy Clin. Immunol. 2011, 127, 787–794.e9. [Google Scholar] [CrossRef]
- Wang, H. Activated Macrophages Are Essential in a Murine Model for T Cell-Mediated Chronic Psoriasiform Skin Inflammation. J. Clin. Investig. 2006, 116, 2105–2114. [Google Scholar] [CrossRef]
- Austin, L.M.; Ozawa, M.; Kikuchi, T.; Walters, I.B.; Krueger, J.G. The Majority of Epidermal T Cells in Psoriasis Vulgaris Lesions Can Produce Type 1 Cytokines, Interferon-γ, Interleukin-2, and Tumor Necrosis Factor-α, Defining TC1 (Cytotoxic T Lymphocyte) and TH1 Effector Populations:1 a Type 1 Differentiation Bias Is Also Measured in Circulating Blood T Cells in Psoriatic Patients. J. Investig. Dermatol. 1999, 113, 752–759. [Google Scholar] [CrossRef]
- Toussirot, Ã.; Aubin, F.; Dumoulin, G. Relationships between Adipose Tissue and Psoriasis, with or without Arthritis. Front. Immunol. 2014, 5, 368. [Google Scholar] [CrossRef]
- Umezawa, Y.; Asahina, A.; Imafuku, S.; Tada, Y.; Sano, S.; Morita, A.; Sakurai, S.; Hoshii, N.; Tilt, N.; Nakagawa, H. Efficacy and Safety of Certolizumab Pegol in Japanese Patients with Moderate to Severe Plaque Psoriasis: 52-Week Results. Dermatol. Ther. 2021, 11, 943–960. [Google Scholar] [CrossRef] [PubMed]
- Van Muijen, M.E.; Van Der Schoot, L.S.; Van Den Reek, J.M.P.A.; De Jong, E.M.G.J. Attitudes and Behaviour Regarding Dose Reduction of Biologics for Psoriasis: A Survey among Dermatologists Worldwide. Arch. Dermatol. Res. 2022, 314, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Atalay, S.; Berends, S.E.; Groenewoud, H.M.M.; Mathot, R.A.A.; Njoo, D.M.; Mommers, J.M.; Ossenkoppele, P.M.; Koetsier, M.I.A.; Berends, M.A.; De Vries, A.; et al. Serum Drug Levels and Anti-Drug Antibodies in the Context of Dose Tapering by Interval Prolongation of Adalimumab, Etanercept and Ustekinumab in Psoriasis Patients: Results of the CONDOR Trial. J. Dermatol. Treat. 2022, 33, 2680–2684. [Google Scholar] [CrossRef]
- Bendtzen, K.; Geborek, P.; Svenson, M.; Larsson, L.; Kapetanovic, M.C.; Saxne, T. Individualized Monitoring of Drug Bioavailability and Immunogenicity in Rheumatoid Arthritis Patients Treated with the Tumor Necrosis Factor α Inhibitor Infliximab. Arthritis Rheum. 2006, 54, 3782–3789. [Google Scholar] [CrossRef]
- Jani, M.; Isaacs, J.D.; Morgan, A.W.; Wilson, A.G.; Plant, D.; Hyrich, K.L.; Chinoy, H.; Barton, A. High Frequency of Antidrug Antibodies and Association of Random Drug Levels with Efficacy in Certolizumab Pegol-Treated Patients with Rheumatoid Arthritis: Results from the BRAGGSS Cohort. Ann. Rheum. Dis. 2017, 76, 208–213. [Google Scholar] [CrossRef]
- Arstikyte, I.; Kapleryte, G.; Butrimiene, I.; Venalis, A. Influence of Immunogenicity on the Efficacy of Long-Term Treatment with TNF α Blockers in Rheumatoid Arthritis and Spondyloarthritis Patients. BioMed Res. Int. 2015, 2015, 604872. [Google Scholar] [CrossRef]
- Mojtahed Poor, S.; Henke, M.; Ulshöfer, T.; Köhm, M.; Behrens, F.; Burkhardt, H.; Schiffmann, S. The Role of Antidrug Antibodies in Ustekinumab Therapy and the Impact of Methotrexate. Rheumatology 2023, 62, 3993–3999. [Google Scholar] [CrossRef]
- Wolbink, G.J.; Aarden, L.A.; Dijkmans, B. Dealing with Immunogenicity of Biologicals: Assessment and Clinical Relevance. Curr. Opin. Rheumatol. 2009, 21, 211–215. [Google Scholar] [CrossRef]
- Hsu, L.; Armstrong, A.W. Anti-Drug Antibodies in Psoriasis: A Critical Evaluation of Clinical Significance and Impact on Treatment Response. Expert. Rev. Clin. Immunol. 2013, 9, 949–958. [Google Scholar] [CrossRef]
- Reich, K.; Blauvelt, A.; Armstrong, A.; Langley, R.G.; Fox, T.; Huang, J.; Papavassilis, C.; Liang, E.; Lloyd, P.; Bruin, G. Secukinumab, a Fully Human Anti-Interleukin-17A Monoclonal Antibody, Exhibits Minimal Immunogenicity in Patients with Moderate-to-Severe Plaque Psoriasis. Br. J. Dermatol. 2017, 176, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Bissonnette, R.; Luger, T.; Thaçi, D.; Toth, D.; Messina, I.; You, R.; Guana, A.; Fox, T.; Papavassilis, C.; Gilloteau, I.; et al. Secukinumab Sustains Good Efficacy and Favourable Safety in Moderate-to-Severe Psoriasis after up to 3 Years of Treatment: Results from a Double-Blind Extension Study. Br. J. Dermatol. 2017, 177, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Bagel, J.; Lebwohl, M.; Israel, R.J.; Jacobson, A. Immunogenicity and Skin Clearance Recapture in Clinical Studies of Brodalumab. J. Am. Acad. Dermatol. 2020, 82, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Reich, K.; Jackson, K.; Ball, S.; Garces, S.; Kerr, L.; Chua, L.; Muram, T.M.; Blauvelt, A. Ixekizumab Pharmacokinetics, Anti-Drug Antibodies, and Efficacy through 60 Weeks of Treatment of Moderate to Severe Plaque Psoriasis. J. Investig. Dermatol. 2018, 138, 2168–2173. [Google Scholar] [CrossRef]
- Norden, A.; Moon, J.Y.; Javadi, S.S.; Munawar, L.; Maul, J.-T.; Wu, J.J. Anti-drug Antibodies of IL-23 Inhibitors for Psoriasis: A Systematic Review. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1171–1177. [Google Scholar] [CrossRef]
- Kimball, A.B.; Kerbusch, T.; Aarle, F.; Kulkarni, P.; Li, Q.; Blauvelt, A.; Papp, K.A.; Reich, K.; Montgomery, D. Assessment of the Effects of Immunogenicity on the Pharmacokinetics, Efficacy and Safety of Tildrakizumab. Br. J. Dermatol. 2020, 182, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Anjie, S.I.; Gecse, K.B.; Meloni, C.M.; Vidal-Itriago, A.; Löwenberg, M.; D’Haens, G.R. Immunogenicity and Efficacy of Subcutaneous Infliximab Monotherapy vs. Combination Therapy in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2025; in press. [Google Scholar] [CrossRef]
- Valenzuela, F.; Flores, R. Immunogenicity to Biological Drugs in Psoriasis and Psoriatic Arthritis. Clinics 2021, 76, e3015. [Google Scholar] [CrossRef]
- Hu, R.; Yuan, T.; Wang, H.; Zhao, J.; Shi, L.; Li, Q.; Zhu, C.; Su, N.; Zhang, S. Efficacy, Safety and Immunogenicity of Etanercept Biosimilars versus Reference Biologics in Patients with Rheumatoid Arthritis: A Meta-Analysis. Front. Pharmacol. 2023, 14, 1089272. [Google Scholar] [CrossRef]
- Loeff, F.C.; Tsakok, T.; Dijk, L.; Hart, M.H.; Duckworth, M.; Baudry, D.; Russell, A.; Dand, N.; Van Leeuwen, A.; Griffiths, C.E.M.; et al. Clinical Impact of Antibodies against Ustekinumab in Psoriasis: An Observational, Cross-Sectional, Multicenter Study. J. Investig. Dermatol. 2020, 140, 2129–2137. [Google Scholar] [CrossRef] [PubMed]
- Karle, A.; Spindeldreher, S.; Kolbinger, F. Secukinumab, a Novel Anti–IL-17A Antibody, Shows Low Immunogenicity Potential in Human in Vitro Assays Comparable to Other Marketed Biotherapeutics with Low Clinical Immunogenicity. mAbs 2016, 8, 536–550. [Google Scholar] [CrossRef]
- Gordon, K.B.; Blauvelt, A.; Papp, K.A.; Langley, R.G.; Luger, T.; Ohtsuki, M.; Reich, K.; Amato, D.; Ball, S.G.; Braun, D.K.; et al. Phase 3 Trials of Ixekizumab in Moderate-to-Severe Plaque Psoriasis. N. Engl. J. Med. 2016, 375, 345–356. [Google Scholar] [CrossRef]
- Farahnik, B.; Beroukhim, K.; Abrouk, M.; Nakamura, M.; Zhu, T.H.; Singh, R.; Lee, K.; Bhutani, T.; Koo, J. Brodalumab for the Treatment of Psoriasis: A Review of Phase III Trials. Dermatol. Ther. 2016, 6, 111–124. [Google Scholar] [CrossRef]
- Zhu, Y.; Marini, J.C.; Song, M.; Randazzo, B.; Shen, Y.-K.; Li, S.; Zhou, H. Immunogenicity of Guselkumab Is Not Clinically Relevant in Patients with Moderate-to-Severe Plaque Psoriasis. J. Investig. Dermatol. 2019, 139, 1830–1834.e6. [Google Scholar] [CrossRef] [PubMed]
- Atiqi, S.; Hooijberg, F.; Loeff, F.C.; Rispens, T.; Wolbink, G.J. Immunogenicity of TNF-Inhibitors. Front. Immunol. 2020, 11, 312. [Google Scholar] [CrossRef] [PubMed]
- Bloem, K.; Hernández-Breijo, B.; Martínez-Feito, A.; Rispens, T. Immunogenicity of Therapeutic Antibodies: Monitoring Antidrug Antibodies in a Clinical Context. Ther. Drug Monit. 2017, 39, 327–332. [Google Scholar] [CrossRef]
- Wang, W.; Wang, E.; Balthasar, J. Monoclonal Antibody Pharmacokinetics and Pharmacodynamics. Clin. Pharmacol. Ther. 2008, 84, 548–558. [Google Scholar] [CrossRef]
- Gill, K.L.; Machavaram, K.K.; Rose, R.H.; Chetty, M. Potential Sources of Inter-Subject Variability in Monoclonal Antibody Pharmacokinetics. Clin. Pharmacokinet. 2016, 55, 789–805. [Google Scholar] [CrossRef]
- Ternant, D.; Bejan-Angoulvant, T.; Passot, C.; Mulleman, D.; Paintaud, G. Clinical Pharmacokinetics and Pharmacodynamics of Monoclonal Antibodies Approved to Treat Rheumatoid Arthritis. Clin. Pharmacokinet. 2015, 54, 1107–1123. [Google Scholar] [CrossRef]
- Berkhout, L.C.; l’Ami, M.J.; Ruwaard, J.; Hart, M.H.; Heer, P.O.; Bloem, K.; Nurmohamed, M.T.; Van Vollenhoven, R.F.; Boers, M.; Alvarez, D.F.; et al. Dynamics of Circulating TNF during Adalimumab Treatment Using a Drug-Tolerant TNF Assay. Sci. Transl. Med. 2019, 11, eaat3356. [Google Scholar] [CrossRef]
- Cassotta, A.; Mikol, V.; Bertrand, T.; Pouzieux, S.; Le Parc, J.; Ferrari, P.; Dumas, J.; Auer, M.; Deisenhammer, F.; Gastaldi, M.; et al. A Single T Cell Epitope Drives the Neutralizing Anti-Drug Antibody Response to Natalizumab in Multiple Sclerosis Patients. Nat. Med. 2019, 25, 1402–1407. [Google Scholar] [CrossRef]
- Harris, C.T.; Cohen, S. Reducing Immunogenicity by Design: Approaches to Minimize Immunogenicity of Monoclonal Antibodies. BioDrugs 2024, 38, 205–226. [Google Scholar] [CrossRef]
- Lecluse, L.L.A.; Driessen, R.J.B.; Spuls, P.I.; De Jong, E.M.G.J.; Stapel, S.O.; Van Doorn, M.B.A.; Bos, J.D.; Wolbink, G.-J. Extent and Clinical Consequences of Antibody Formation Against Adalimumab in Patients With Plaque Psoriasis. Arch. Dermatol. 2010, 146, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Adişen, E.; Aral, A.; Aybay, C.; Gürer, M.A. Anti-infliximab Antibody Status and Its Relation to Clinical Response in Psoriatic Patients: A Pilot Study. J. Dermatol. 2010, 37, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Reich, K.; Sorbe, C.; Griese, L.; Reich, J.L.K.; Augustin, M. The Value of Subcutaneous vs. Oral Methotrexate: Real-world Data from the German Psoriasis Registry PsoBest. Br. J. Dermatol. 2021, 184, 765–767. [Google Scholar] [CrossRef]
- Drach, M.; Papageorgiou, K.; Maul, J.-T.; Djamei, V.; Yawalkar, N.; Häusermann, P.; Anzengruber, F.; Navarini, A.A. Effectiveness of Methotrexate in Moderate to Severe Psoriasis Patients: Real-World Registry Data from the Swiss Dermatology Network for Targeted Therapies (SDNTT). Arch. Dermatol. Res. 2019, 311, 753–760. [Google Scholar] [CrossRef]
- Vultaggio, A.; Perlato, M.; Nencini, F.; Vivarelli, E.; Maggi, E.; Matucci, A. How to Prevent and Mitigate Hypersensitivity Reactions to Biologicals Induced by Anti-Drug Antibodies? Front. Immunol. 2021, 12, 765747. [Google Scholar] [CrossRef]
- Affleck, A.G.; Williams, H. Is Leflunomide Effective in the Treatment of Psoriasis in a Patient Who Is Unable to Benefit From Standard First- and Second-Line Therapies and Needs an Affordable Treatment Option? Arch. Dermatol. 2008, 144, 1642–1643. [Google Scholar] [CrossRef]
- Vaisman-Mentesh, A.; Gutierrez-Gonzalez, M.; DeKosky, B.J.; Wine, Y. The Molecular Mechanisms That Underlie the Immune Biology of Anti-Drug Antibody Formation Following Treatment With Monoclonal Antibodies. Front. Immunol. 2020, 11, 1951. [Google Scholar] [CrossRef]
- De Spéville, B.D.; Moreno, V. Antidrug Antibodies and Drug Development: Challenges in the Immunotherapy Era. Clin. Cancer Res. 2021, 27, 2669–2671. [Google Scholar] [CrossRef]
- Sauna, Z.E.; Lagassé, D.; Pedras-Vasconcelos, J.; Golding, B.; Rosenberg, A.S. Evaluating and Mitigating the Immunogenicity of Therapeutic Proteins. Trends Biotechnol. 2018, 36, 1068–1084. [Google Scholar] [CrossRef] [PubMed]
- Bissonnette, R.; Papp, K.; Maari, C.; Yao, Y.; Robbie, G.; White, W.I.; Le, C.; White, B. A Randomized, Double-Blind, Placebo-Controlled, Phase I Study of MEDI-545, an Anti–Interferon-Alfa Monoclonal Antibody, in Subjects with Chronic Psoriasis. J. Am. Acad. Dermatol. 2010, 62, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Zettlitz, K.A.; Lorenz, V.; Landauer, K.; Münkel, S.; Herrmann, A.; Scheurich, P.; Pfizenmaier, K.; Kontermann, R. ATROSAB, a Humanized Antagonistic Anti-Tumor Necrosis Factor Receptor One-Specific Antibody. mAbs 2010, 2, 639–647. [Google Scholar] [CrossRef]
- Dapavo, P.; Vujic, I.; Fierro, M.T.; Quaglino, P.; Sanlorenzo, M. The Infliximab Biosimilar in the Treatment of Moderate to Severe Plaque Psoriasis. J. Am. Acad. Dermatol. 2016, 75, 736–739. [Google Scholar] [CrossRef]
- Weinblatt, M.; Schiff, M.; Goldman, A.; Kremer, J.; Luggen, M.; Li, T.; Chen, D.; Becker, J.-C. Selective Costimulation Modulation Using Abatacept in Patients with Active Rheumatoid Arthritis While Receiving Etanercept: A Randomised Clinical Trial. Ann. Rheum. Dis. 2007, 66, 228–234. [Google Scholar] [CrossRef]
- Fischer, J.A.A.; Hueber, A.J.; Wilson, S.; Galm, M.; Baum, W.; Kitson, C.; Auer, J.; Lorenz, S.H.; Moelleken, J.; Bader, M.; et al. Combined Inhibition of Tumor Necrosis Factor α and Interleukin-17 As a Therapeutic Opportunity in Rheumatoid Arthritis: Development and Characterization of a Novel Bispecific Antibody. Arthritis Rheumatol. 2015, 67, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Dimasi, N.; Fleming, R.; Hay, C.; Woods, R.; Xu, L.; Wu, H.; Gao, C. Development of a Trispecific Antibody Designed to Simultaneously and Efficiently Target Three Different Antigens on Tumor Cells. Mol. Pharm. 2015, 12, 3490–3501. [Google Scholar] [CrossRef]
- Conrad, C.; Gilliet, M. Psoriasis: From Pathogenesis to Targeted Therapies. Clin. Rev. Allergy Immunol. 2018, 54, 102–113. [Google Scholar] [CrossRef]
- Moots, R.J.; Xavier, R.M.; Mok, C.C.; Rahman, M.U.; Tsai, W.-C.; Al-Maini, M.H.; Pavelka, K.; Mahgoub, E.; Kotak, S.; Korth-Bradley, J.; et al. The Impact of Anti-Drug Antibodies on Drug Concentrations and Clinical Outcomes in Rheumatoid Arthritis Patients Treated with Adalimumab, Etanercept, or Infliximab: Results from a Multinational, Real-World Clinical Practice, Non-Interventional Study. PLoS ONE 2017, 12, e0175207. [Google Scholar] [CrossRef]
- Bourdage, J.S.; Cook, C.A.; Farrington, D.L.; Chain, J.S.; Konrad, R.J. An Affinity Capture Elution (ACE) Assay for Detection of Anti-Drug Antibody to Monoclonal Antibody Therapeutics in the Presence of High Levels of Drug. J. Immunol. Methods 2007, 327, 10–17. [Google Scholar] [CrossRef]
- Schmidt, E.; Hennig, K.; Mengede, C.; Zillikens, D.; Kromminga, A. Immunogenicity of Rituximab in Patients with Severe Pemphigus. Clin. Immunol. 2009, 132, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Bloem, K.; Van Leeuwen, A.; Verbeek, G.; Nurmohamed, M.T.; Wolbink, G.J.; Van Der Kleij, D.; Rispens, T. Systematic Comparison of Drug-Tolerant Assays for Anti-Drug Antibodies in a Cohort of Adalimumab-Treated Rheumatoid Arthritis Patients. J. Immunol. Methods 2015, 418, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-M.C.; Fang, L.; Zhou, L.; Wang, J.; Ahn, H.-Y. A Survey of Applications of Biological Products for Drug Interference of Immunogenicity Assays. Pharm. Res. 2012, 29, 3384–3392. [Google Scholar] [CrossRef]
- Lofgren, J.A.; Wala, I.; Koren, E.; Swanson, S.J.; Jing, S. Detection of Neutralizing Anti-Therapeutic Protein Antibodies in Serum or Plasma Samples Containing High Levels of the Therapeutic Protein. J. Immunol. Methods 2006, 308, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Vincent, F.B.; Morand, E.F.; Murphy, K.; Mackay, F.; Mariette, X.; Marcelli, C. Antidrug Antibodies (ADAb) to Tumour Necrosis Factor (TNF)-Specific Neutralising Agents in Chronic Inflammatory Diseases: A Real Issue, a Clinical Perspective. Ann. Rheum. Dis. 2013, 72, 165–178. [Google Scholar] [CrossRef]
- De, A.; Chakraborty, D.; Ahmed, S.S. Intra-Class Interleukin-(IL)-17 Blocker Switching: Ixekizumab as a Solution for Secukinumab Non-Responders in Secondary Biologic Failure in Psoriasis. Cureus 2025, 17, e78954. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, X.; Li, W.; Sun, Y.; Liu, H. Switching Biologic Agent in Patients with Psoriasis: A Systematic Review and Meta-Analysis. J. Dermatol. Treat. 2025, 36, 2521082. [Google Scholar] [CrossRef]
- Bagit, A.; Maliyar, K.; Georgakopoulos, J.R.; Rankin, B.; Rimke, A.; Mufti, A.; Le, H.; Vender, R.; Prajapati, V.H.; Yeung, J. Real-World Effectiveness and Safety of Risankizumab in Patients with Plaque Psoriasis in Whom Guselkumab Failed Recently: A Multicenter Retrospective Study of Switching within the Interleukin-23 Inhibitor Class. JAAD Int. 2023, 12, 136–138. [Google Scholar] [CrossRef]
- Georgakopoulos, J.R.; Phung, M.; Ighani, A.; Yeung, J. Efficacy and Safety of Switching to Ixekizumab in Secukinumab Nonresponders with Plaque Psoriasis: A Multicenter Retrospective Study of Interleukin 17A Antagonist Therapies. J. Am. Acad. Dermatol. 2018, 79, 155–157. [Google Scholar] [CrossRef] [PubMed]
- Piaserico, S.; Cazzaniga, S.; Chimenti, S.; Giannetti, A.; Maccarone, M.; Picardo, M.; Peserico, A.; Naldi, L. Efficacy of Switching between Tumor Necrosis Factor-Alfa Inhibitors in Psoriasis: Results from the Italian Psocare Registry. J. Am. Acad. Dermatol. 2014, 70, 257–262.e3. [Google Scholar] [CrossRef]
- Kerdel, F.; Zaiac, M. An Evolution in Switching Therapy for Psoriasis Patients Who Fail to Meet Treatment Goals. Dermatol. Ther. 2015, 28, 390–403. [Google Scholar] [CrossRef]
- Ruiz-Villaverde, R.; Rodriguez-Fernandez-Freire, L.; Armario-Hita, J.C.; Pérez-Gil, A.; Chinchay, F.V.; Galán-Gutiérrez, M. Guselkumab as a Switching Strategy after anti-TNFα, anti-IL17, or anti-IL12/23 Therapies in Moderate-to-severe Psoriasis. Dermatol. Ther. 2022, 35, e15760. [Google Scholar] [CrossRef]
- Woo, W.A.; Waite, A.; Rustin, M.H.A.; McBride, S.R. Switching Biological Agents for Psoriasis in Secondary Care: A Single-Centre, Retrospective, Open-Label Study. Br. J. Dermatol. 2014, 170, 989–990. [Google Scholar] [CrossRef]
- Hyrich, K.; Symmons, D.; Watson, K.; Silman, A. Baseline Comorbidity Levels in Biologic and Standard DMARD Treated Patients with Rheumatoid Arthritis: Results from a National Patient Register. Ann. Rheum. Dis. 2006, 65, 895–898. [Google Scholar] [CrossRef]
- Karampetsou, M.P.; Liossis, S.-N.C.; Sfikakis, P.P. TNF- Antagonists beyond Approved Indications: Stories of Success and Prospects for the Future. QJM Int. J. Med. 2010, 103, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Krieckaert, C.L.; Lems, W.F. Are We Ready for Therapeutic Drug Monitoring of Biologic Therapeutics? Arthritis Res. Ther. 2011, 13, 120. [Google Scholar] [CrossRef] [PubMed]
- Krishna, M.; Nadler, S.G. Immunogenicity to Biotherapeutics—The Role of Anti-Drug Immune Complexes. Front. Immunol. 2016, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Kraev, K.; Hristov, B.; Uchikov, P.; Kraeva, M.; Geneva-Popova, M.; Popova, S.; Basheva-Kraeva, Y.; Stoyanova, N.S.; Mitkova-Hristova, V. Prognostic Models of Drug-Induced Neutralizing Antibody Formation in Patients with Rheumatoid Arthritis, Psoriatic Arthritis, Ankylosing Spondylitis Treated with TNF-α Blockersockers. Folia Med. 2024, 66, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Mattei, A.E.; Gutierrez, A.H.; Seshadri, S.; Tivin, J.; Ardito, M.; Rosenberg, A.S.; Martin, W.D.; De Groot, A.S. In Silico Methods for Immunogenicity Risk Assessment and Human Homology Screening for Therapeutic Antibodies. mAbs 2024, 16, 2333729. [Google Scholar] [CrossRef]
- Sun, R.; Qian, M.G.; Zhang, X. T and B Cell Epitope Analysis for the Immunogenicity Evaluation and Mitigation of Antibody-Based Therapeutics. mAbs 2024, 16, 2324836. [Google Scholar] [CrossRef]
- Ukalovic, D.; Leeb, B.F.; Rintelen, B.; Eichbauer-Sturm, G.; Spellitz, P.; Puchner, R.; Herold, M.; Stetter, M.; Ferincz, V.; Resch-Passini, J.; et al. Prediction of Ineffectiveness of Biological Drugs Using Machine Learning and Explainable AI Methods: Data from the Austrian Biological Registry BioReg. Arthritis Res. Ther. 2024, 26, 44. [Google Scholar] [CrossRef]
- Magee, C.; Jethwa, H.; FitzGerald, O.M.; Jadon, D.R. Biomarkers Predictive of Treatment Response in Psoriasis and Psoriatic Arthritis: A Systematic Review. Ther. Adv. Musculoskelet. Dis. 2021, 13, 1–14. [Google Scholar] [CrossRef]
| Class/Target. | Drugs (mAbs) | Mechanism of Action | Clinical Notes |
|---|---|---|---|
| anti-TNFα | Infliximab, Adalimumab, Golimumab, Certolizumab pegol | Neutralization of TNFα: reduced inflammation | First to be introduced; effective but with risk of opportunistic infections |
| anti-IL-12/23 (p40) | Ustekinumab | Blocks p40 subunit shared by IL-12 and IL-23 | Acts on both Th1 and Th17 pathways |
| anti-IL-23 (p19 selective) | Guselkumab, Risankizumab, Tildrakizumab | Selective blockade of IL-23 p19 subunit | High specificity and excellent efficacy and safety |
| anti-IL-17A | Secukinumab, Ixekizumab | Neutralize IL-17A | Rapid clinical efficacy, risk of mucocutaneous candidiasis |
| anti-IL-17RA | Brodalumab | Blocks IL-17 receptor A (IL-17RA): inhibits multiple isoforms | Very potent but with warning for suicidal ideation risk |
| Others in development/less widely used | Sonelokimab (nanobody anti-IL-17A/F), Bimekizumab (anti-IL-17A/F) | Block multiple IL-17 isoforms | In late-stage development or already approved in some countries |
| Biologics | Key Trials/Program | Phase Population | Reported Treatment-Emergent ADA | Reported Clinical Impact of ADA | Source (Main) |
|---|---|---|---|---|---|
| Infliximab | Pivotal psoriasis trials and multiple observational cohorts | Phase 3_moderate-to-severe plaque psoriasis | High variability. Clinically meaningful ADA rates were reported, with antibodies reported in many patients in observational series (rates higher than fully human mAbs). | ADAs are frequently associated with lower serum drug levels and an increased risk of loss of response and infusion reactions. Concomitant immunosuppression reduces ADA development. | Anjie et al. [36] |
| Adalimumab | Pivotal psoriasis trials and biosimilar comparators | Phase 3_large psoriasis populations | Variable (5–20% reported in different studies and assays); biosimilar studies report similar ADA levels. | ADAs (especially neutralizing) can reduce trough levels and are related to diminished efficacy in some patients. Assay/definition dependent. | Valenzuela et al. [37] |
| Etanercept | Pivotal trials (e.g., etanercept registration trials) and long-term cohort studies | Phase 3_moderate-to-severe psoriasis | Low to modest ADA detection. Many assays find non-neutralizing antibodies or low clinical relevance. | When present, ADAs are often non-neutralizing and less linked to loss of response than with monoclonal TNF agents. | Hu et al. [38] |
| Ustekinumab | PHOENIX/ACCEPT biosimilar trials and pooled analyses | Phase 3_moderate-to-severe psoriasis | Low (typically single digit %; assay dependent). | Most studies report low ADA rates. When ADAs occur, they can be associated with lower drug levels, but clinical impact is often limited and assay dependent. Improvements in assay methods have changed reported rates over time. | Loeff et al. [39] |
| Secukinumab | ERASURE/FIXTURE pooled safety programs | Phase 3_moderate-to-severe psoriasis | Very low (~0.4%) ADA reported in pooled Phase 3 analyses. | ADA incidence is very low. Pooled analyses report no clear association with loss of efficacy or altered pharmacokinetics. | Karle et al. [40] |
| Ixekizumab | UNCOVER-1, UNCOVER-2, and UNCOVER-3 (phase 3 program) | Phase 3_moderate-to-severe psoriasis | Low-to-moderate. Neutralizing ADAs reported in pooled analyses. 5–8% had neutralizing ADA. | High-titer neutralizing ADAs are linked to reduced serum concentrations and, in some cases, loss of efficacy. Most patients remain ADA-negative, and efficacy is maintained. | Gordon et al. [41] |
| Brodalumab | AMAGINE-1/AMAGINE-2/AMAGINE-3 Phase 3 program and pooled analyses | Phase 3_moderate-to-severe psoriasis | Very low ADA rates were reported in trials. | Low immunogenicity. When ADAs appear, they have not been a major driver of loss of efficacy in pooled studies data. | Farahnik et al. [42] |
| Guselkumab | VOYAGE-1/VOYAGE-2, and other phase 3 trials | Phase 3_moderate-to-severe psoriasis | Low ADA rates were reported in VOYAGE pooled data. | ADAs are reported but generally not clinically meaningful (no consistent effect on efficacy or pharmacokinetics). | Zhu et al. [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mennella, A.; Frasca, L. The Phenomenon of Anti-Drug Antibodies in Psoriasis: Mechanisms, Clinical Impact, and Therapeutic Strategies. Int. J. Mol. Sci. 2025, 26, 9616. https://doi.org/10.3390/ijms26199616
Mennella A, Frasca L. The Phenomenon of Anti-Drug Antibodies in Psoriasis: Mechanisms, Clinical Impact, and Therapeutic Strategies. International Journal of Molecular Sciences. 2025; 26(19):9616. https://doi.org/10.3390/ijms26199616
Chicago/Turabian StyleMennella, Anna, and Loredana Frasca. 2025. "The Phenomenon of Anti-Drug Antibodies in Psoriasis: Mechanisms, Clinical Impact, and Therapeutic Strategies" International Journal of Molecular Sciences 26, no. 19: 9616. https://doi.org/10.3390/ijms26199616
APA StyleMennella, A., & Frasca, L. (2025). The Phenomenon of Anti-Drug Antibodies in Psoriasis: Mechanisms, Clinical Impact, and Therapeutic Strategies. International Journal of Molecular Sciences, 26(19), 9616. https://doi.org/10.3390/ijms26199616







