Developing Up-Scale Allogeneic Chondrocyte Therapies Using Juvenile Donor Cartilage
Abstract
1. Introduction
2. Results
2.1. Participants
2.2. Histological Analysis of Native Infant Cartilage
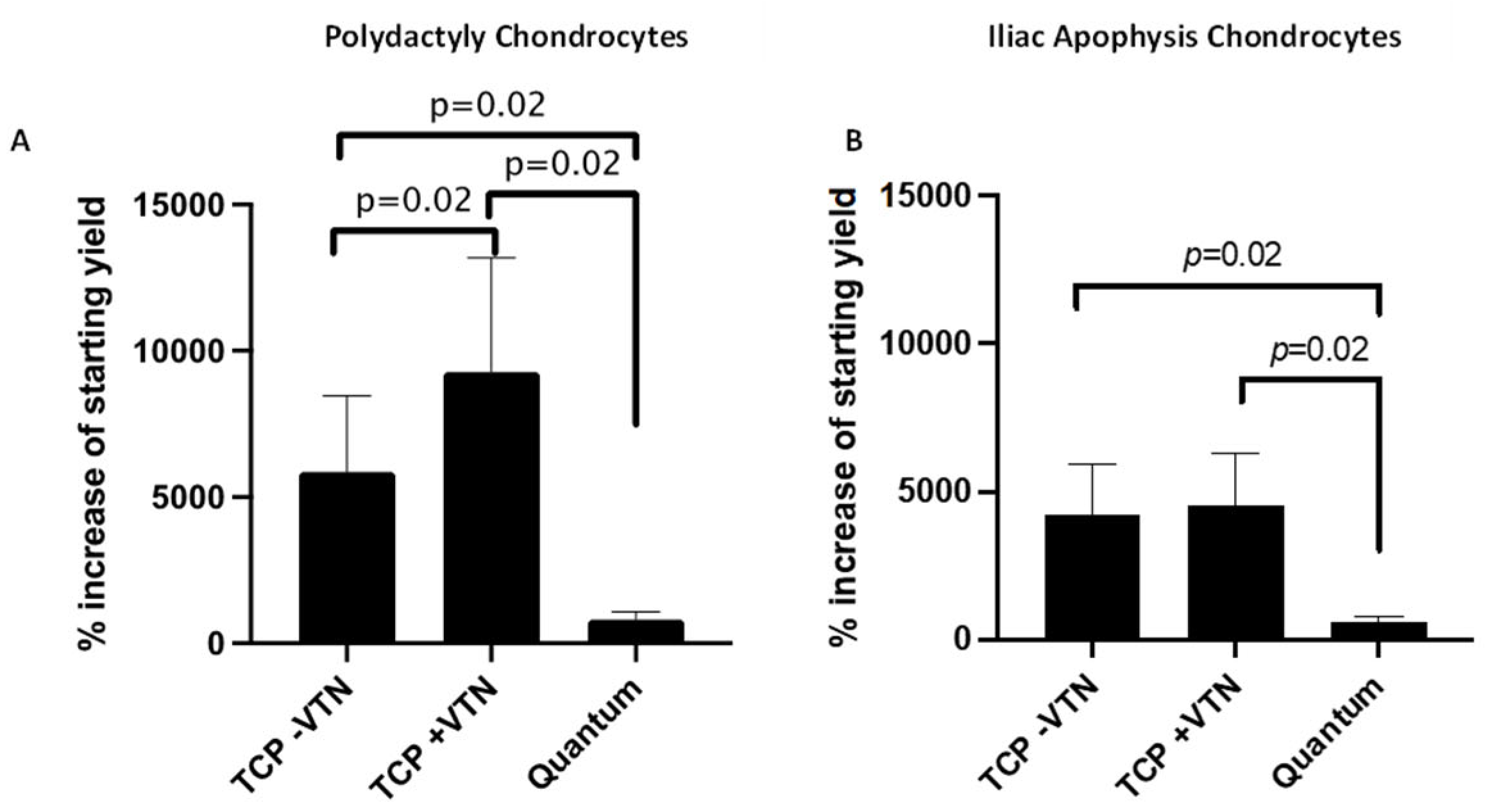
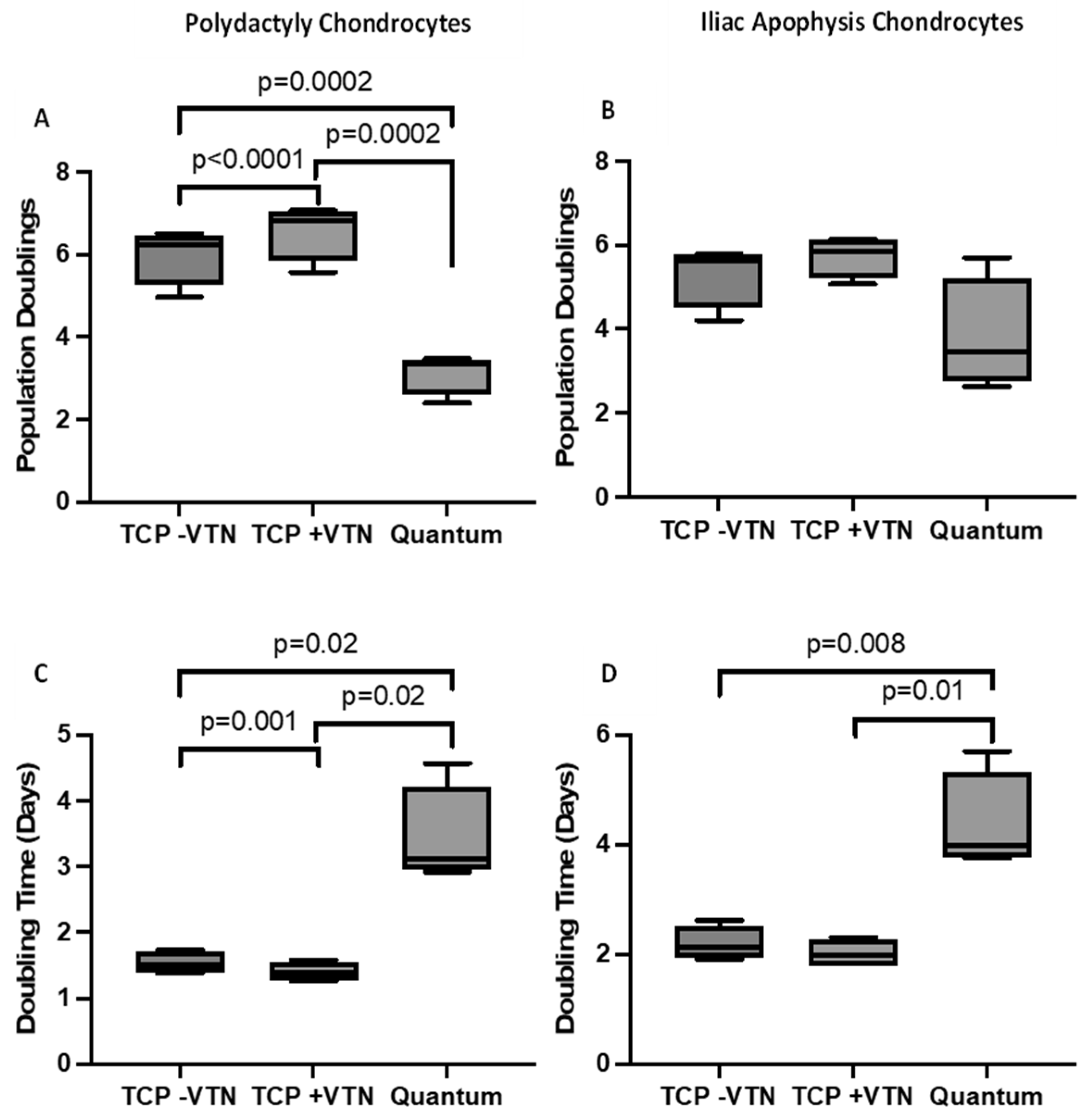
2.3. Chondrocyte Growth and Cell Morphology
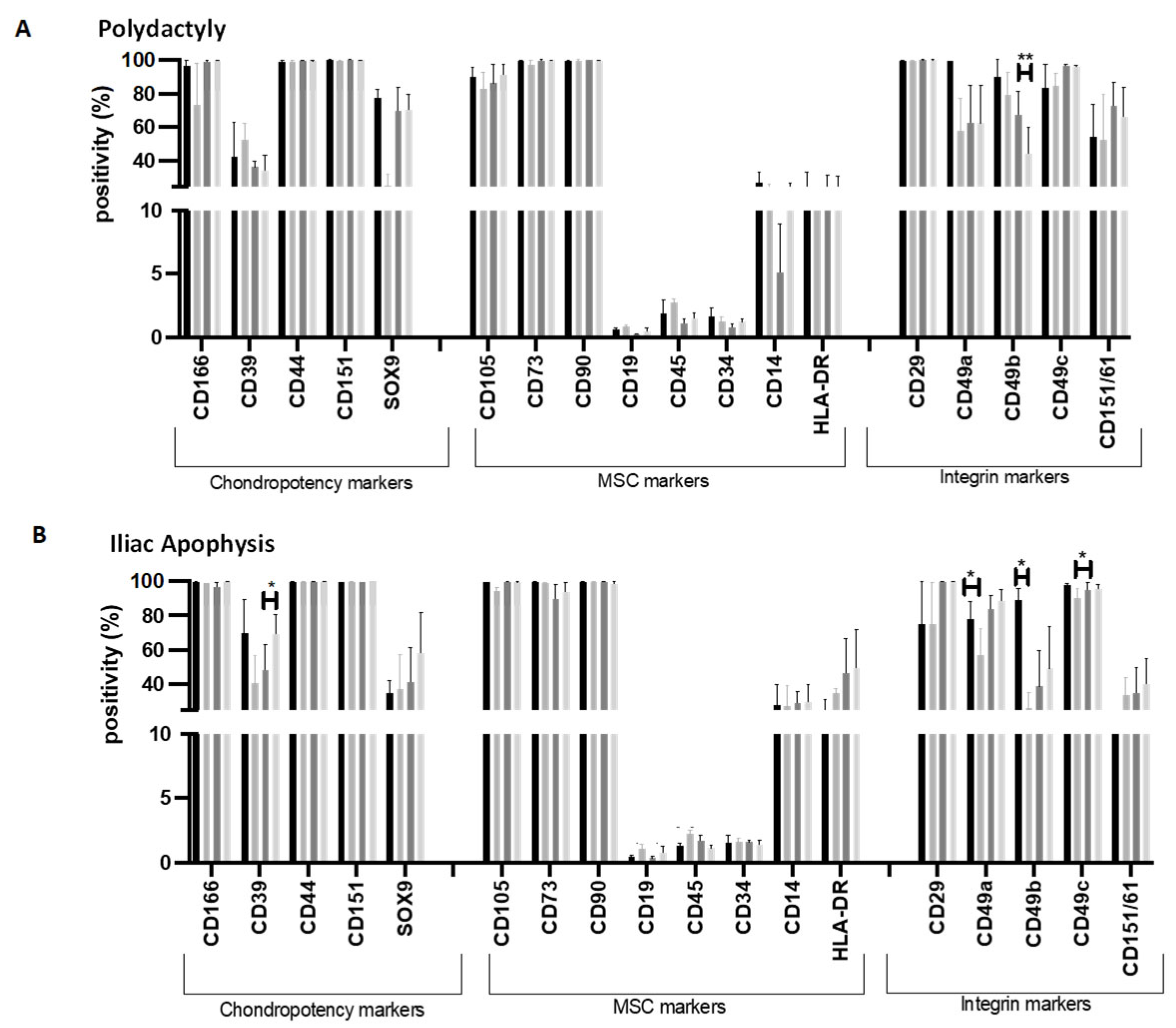
2.4. Flow Cytometry Markers
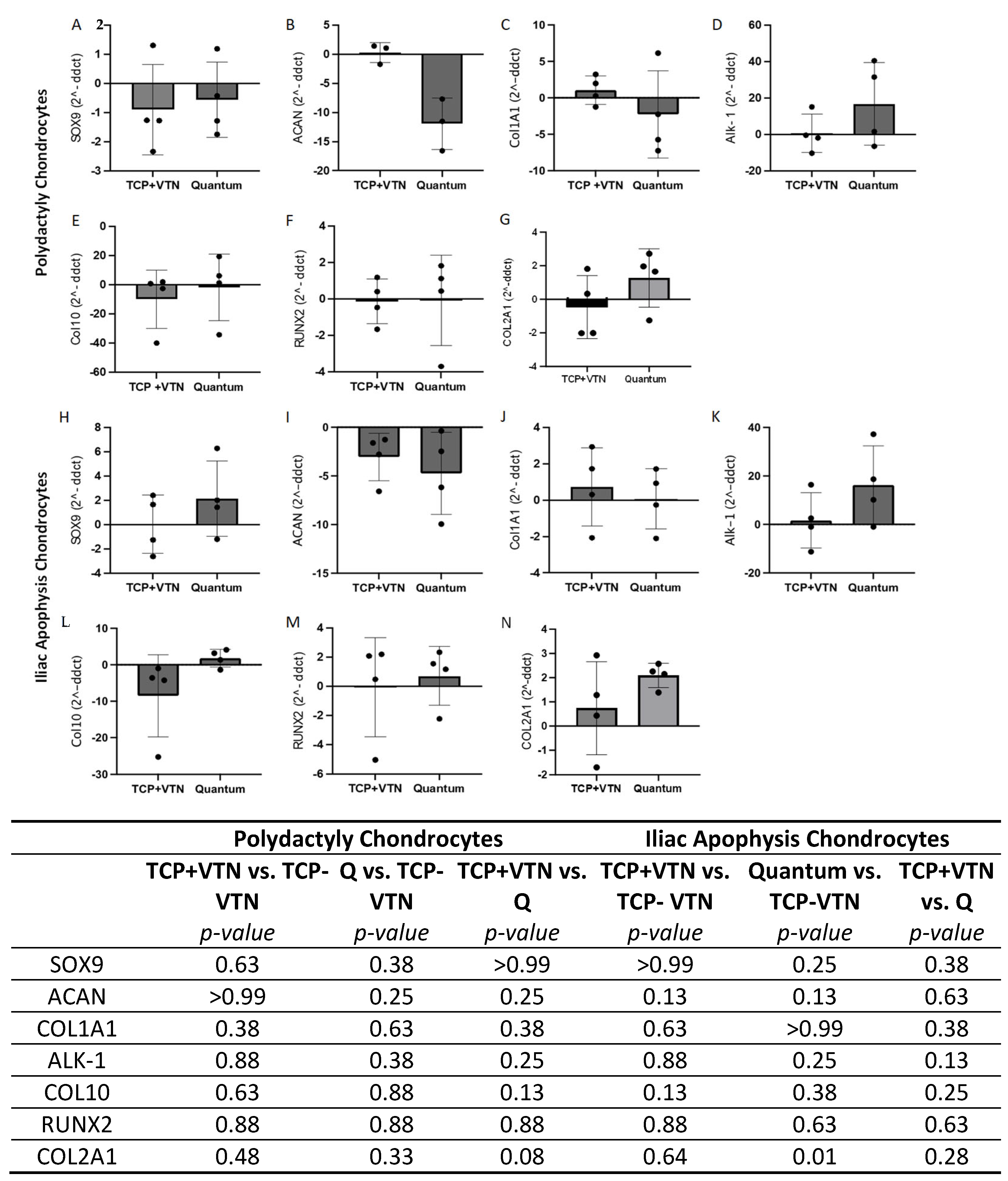
2.5. Gene Expression
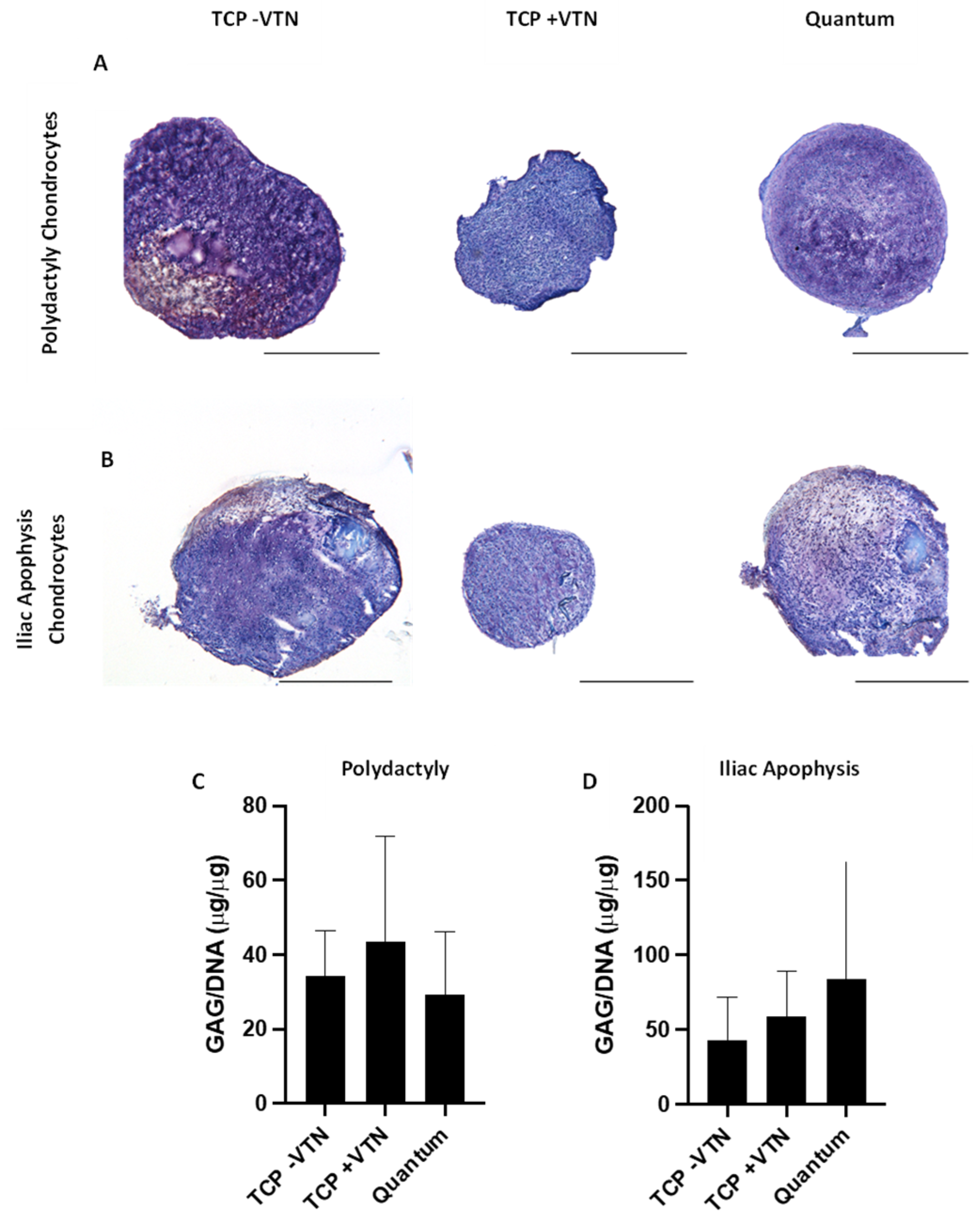
2.6. Chrondrogenesis
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Chondrocyte Isolation and Expansion
4.3. Histological Analysis of the Juvenile Cartilage Tissues
4.4. Up-Scale Chondrocyte Manufacture in the Quantum® Cell Expansion System
4.5. Chondrocyte Parallel ‘Sister’ TCP Culture
4.6. Calculation of Growth Kinetics
4.7. Flow Cytometry Profiling
4.8. Gene Expression Analysis Using RT-qPCR
4.9. Chondrogenic Pellet Culture
4.10. Analysis of Chondrogenic Pellet Glycosaminoglycan (GAG)/DNA Content
4.11. Analysis of Chondrogenic Pellets by Histology
4.12. Statistical Analysis
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muthu, S.; Korpershoek, J.V.; Novais, E.J.; Tawy, G.F.; Hollander, A.P.; Martin, I. Failure of Cartilage Regeneration: Emerging Hypotheses and Related Therapeutic Strategies. Nat. Rev. Rheumatol. 2023, 19, 403–416. [Google Scholar] [CrossRef]
- Curl, W.W.; Krome, J.; Gordon, E.S.; Rushing, J.; Smith, B.P.; Poehling, G.G. Cartilage Injuries: A Review of 31,516 Knee Arthroscopies. Arthroscopy 1997, 13, 456–460. [Google Scholar] [CrossRef] [PubMed]
- Åroøen, A.; Løken, S.; Heir, S.; Alvik, E.; Ekeland, A.; Granlund, O.G.; Engebretsen, L. Articular Cartilage Lesions in 993 Consecutive Knee Arthroscopies. Am. J. Sports Med. 2004, 32, 211–215. [Google Scholar] [CrossRef]
- Hulme, C.H.; Perry, J.; McCarthy, H.S.; Wright, K.T.; Snow, M.; Mennan, C.; Roberts, S. Cell Therapy for Cartilage Repair. Emerg. Top. Life Sci. 2021, 5, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Pareek, A.; Carey, J.L.; Reardon, P.J.; Peterson, L.; Stuart, M.J.; Krych, A.J. Long-Term Outcomes after Autologous Chondrocyte Implantation: A Systematic Review at Mean Follow-Up of 11.4 Years. Cartilage 2016, 7, 298–308. [Google Scholar] [CrossRef]
- Ogura, T.; Mosier, B.A.; Bryant, T.; Minas, T. A 20-Year Follow-up After First-Generation Autologous Chondrocyte Implantation. Am. J. Sports Med. 2017, 45, 2751–2761. [Google Scholar] [CrossRef]
- Tierney, L.; Kuiper, J.H.; Roberts, S.; Snow, M.; Williams, M.; Harrington, M.B.; Harrison, P.; Gallacher, P.; Jermin, P.; Wright, K.T. Lower Cell Number, Lateral Defect Location and Milder Grade Are Associated with Improved Autologous Chondrocyte Implantation Outcome. Knee Surg. Sports Traumatol. Arthrosc. 2024, 33, 1308–1320. [Google Scholar] [CrossRef]
- Davies, R.L.; Kuiper, N.J. Regenerative Medicine: A Review of the Evolution of Autologous Chondrocyte Implantation (ACI) Therapy. Bioengineering 2019, 6, 22. [Google Scholar] [CrossRef]
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of Deep Cartilage Defects in the Knee with Autologous Chondrocyte Transplantation. N. Engl. J. Med. 1994, 331, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.; Nagesh, H.Y.; Pandey, V. Allogeneic Chondrocyte Implantation: What Is Stopping It from Being a Standard of Care? J. Arthrosc. Surg. Sports Med. 2021, 3, 34–39. [Google Scholar] [CrossRef]
- Lim, C.L.; Lee, Y.J.; Cho, J.H.; Choi, H.; Lee, B.; Lee, M.C.; Kim, S. Immunogenicity and Immunomodulatory Effects of the Human Chondrocytes, HChonJ. BMC Musculoskelet. Disord. 2017, 18, 199. [Google Scholar] [CrossRef]
- Hulme, C.H.; Garcia, J.K.; Mennan, C.; Perry, J.; Roberts, S.; Norris, K.; Baird, D.; Rix, L.; Banerjee, R.; Meyer, C.; et al. The Upscale Manufacture of Chondrocytes for Allogeneic Cartilage Therapies. Tissue Eng. Part C Methods 2023, 29, 424–437. [Google Scholar] [CrossRef] [PubMed]
- Hulme, C.H.; Mennan, C.; McCarthy, H.S.; Davies, R.; Lan, T.; Rix, L.; Perry, J.; Wright, K. A Comprehensive Review of Quantum Bioreactor Cell Manufacture: Research and Clinical Applications. Cytotherapy 2023, 25, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Yanke, A.B.; Tilton, A.K.; Wetters, N.G.; Merkow, D.B.; Cole, B.J. DeNovo NT Particulated Juvenile Cartilage Implant. Sports Med. Arthrosc. Rev. 2015, 23, 125–129. [Google Scholar] [CrossRef]
- Farr, J.; Tabet, S.K.; Margerrison, E.; Cole, B.J. Clinical, Radiographic, and Histological Outcomes After Cartilage Repair with Particulated Juvenile Articular Cartilage: A 2-Year Prospective Study. Am. J. Sports Med. 2014, 42, 1417–1425. [Google Scholar] [CrossRef]
- Umair, M.; Ahmad, F.; Bilal, M.; Ahmad, W.; Alfadhel, M. Clinical Genetics of Polydactyly: An Updated Review. Front. Genet. 2018, 9, 447. [Google Scholar] [CrossRef]
- Cavalli, E.; Levinson, C.; Hertl, M.; Broguiere, N.; Brück, O.; Mustjoki, S.; Gerstenberg, A.; Weber, D.; Salzmann, G.; Steinwachs, M.; et al. Characterization of Polydactyly Chondrocytes and Their Use in Cartilage Engineering. Sci. Rep. 2019, 9, 4275. [Google Scholar] [CrossRef]
- Noh, M.J.; Copeland, R.O.; Yi, Y.; Meschter, C.; Hwang, S.; Lim, C.-L.; Yip, V.; Hyun, J.P.; Lee, H.Y.; Lee, K.H. Pre-clinical studies of retrovirally transduced human chondrocytes expressing transforming growth factor-beta-1 (TG-C). Cytotherapy 2010, 12, 384–393. [Google Scholar] [CrossRef]
- Ha, C.W.; Noh, M.J.; Choi, K.B.; Lee, K.H. Initial Phase I Safety of Retrovirally Transduced Human Chondrocytes Expressing Transforming Growth Factor-Beta-1 in Degenerative Arthritis Patients. Cytotherapy 2012, 14, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Cherian, J.J.; Parvizi, J.; Bramlet, D.; Lee, K.H.; Romness, D.W.; Mont, M.A. Preliminary Results of a Phase II Randomized Study to Determine the Efficacy and Safety of Genetically Engineered Allogeneic Human Chondrocytes Expressing TGF-Β1 in Patients with Grade 3 Chronic Degenerative Joint Disease of the Knee. Osteoarthr. Cartil. 2015, 23, 2109–2118. [Google Scholar] [CrossRef]
- Kondo, M.; Kameishi, S.; Kim, K.; Metzler, N.F.; Maak, T.G.; Hutchinson, D.T.; Wang, A.A.; Maehara, M.; Sato, M.; Grainger, D.W.; et al. Safety and Efficacy of Human Juvenile Chondrocyte-Derived Cell Sheets for Osteochondral Defect Treatment. NPJ Regen. Med. 2021, 6, 65. [Google Scholar] [CrossRef]
- Maehara, M.; Sato, M.; Toyoda, E.; Takahashi, T.; Okada, E.; Kotoku, T. Characterization of Polydactyly-Derived Chondrocyte Sheets versus Adult Chondrocyte Sheets for Articular Cartilage Repair. Inflamm. Regen. 2017, 37, 22. [Google Scholar] [CrossRef]
- Hamahashi, K.; Toyoda, E.; Ishihara, M.; Mitani, G.; Takagaki, T.; Kaneshiro, N.; Maehara, M.; Takahashi, T.; Okada, E.; Watanabe, A.; et al. Polydactyly-Derived Allogeneic Chondrocyte Cell-Sheet Transplantation with High Tibial Osteotomy as Regenerative Therapy for Knee Osteoarthritis. NPJ Regen. Med. 2022, 7, 71. [Google Scholar] [CrossRef] [PubMed]
- Kreuz, P.C.; Gentili, C.; Samans, B.; Martinelli, D.; Krüger, J.P.; Mittelmeier, W.; Endres, M.; Cancedda, R.; Kaps, C. Scaffold-Assisted Cartilage Tissue Engineering Using Infant Chondrocytes from Human Hip Cartilage. Osteoarthr. Cartil. 2013, 21, 1997–2005. [Google Scholar] [CrossRef]
- Richards, J.A.; Nelson, L.M.; Caborn, D.N.M. Long-Term Results of Particulated Juvenile Allograft Cartilage Implantation: A Case Report with Eleven-Year Second-Look Arthroscopy and Review of Literature. J. Cartil. Jt. Preserv. 2023, 3, 100113. [Google Scholar] [CrossRef]
- Karnovsky, S.C.; DeSandis, B.; Haleem, A.M.; Sofka, C.M.; O’Malley, M.; Drakos, M.C. Comparison of Juvenile Allogenous Articular Cartilage and Bone Marrow Aspirate Concentrate Versus Microfracture with and Without Bone Marrow Aspirate Concentrate in Arthroscopic Treatment of Talar Osteochondral Lesions. Foot Ankle Int. 2018, 39, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Aiyegbusi, O.L.; Macpherson, K.; Elston, L.; Myles, S.; Washington, J.; Sungum, N.; Briggs, M.; Newsome, P.N.; Calvert, M.J. Patient and Public Perspectives on Cell and Gene Therapies: A Systematic Review. Nat. Commun. 2020, 11, 6265. [Google Scholar] [CrossRef]
- Mennan, C.; Garcia, J.; Roberts, S.; Hulme, C.; Wright, K. A Comprehensive Characterisation of Large- Scale Expanded Human Bone Marrow and Umbilical Cord Mesenchymal Stem Cells. Stem Cell Res. Ther. 2019, 4, 99. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Park, K.H. Regulation and Function of SOX9 during Cartilage Development and Regeneration. Semin. Cancer Biol. 2020, 67, 12–23. [Google Scholar] [CrossRef]
- Futrega, K.; Robey, P.G.; Klein, T.J.; Crawford, R.W.; Doran, M.R. A single day of TGF-β1 exposure activates chondrogenic and hypertrophic differentiation pathways in bone marrow-derived stromal cells. Commun. Biol. 2021, 4, 29. [Google Scholar] [CrossRef]
- Lai, B.; Jiang, H.; Gao, Y.; Zhou, X. Skeletal Ciliopathy: Pathogenesis and Related Signaling Pathways. Mol. Cell. Biochem. 2024, 479, 811–823. [Google Scholar] [CrossRef]
- Vonk, L.A. Potency Assay Considerations for Cartilage Repair, Osteoarthritis and Use of Extracellular Vesicles. In Potency Assays for Advanced Stem Cell Therapy Medicinal Products; Springer: Cham, Switzerland, 2023; pp. 59–80. [Google Scholar]
- Roberts, S.; Manage, J. Microscopic Methods for the Analysis of Engineered Tissues. In Biopolymer Methods in Tissue Engineering; Hollander, A., Hatton, P., Eds.; Humana: Louisville, KY, USA, 2004; Volume 238, pp. 171–196. [Google Scholar]
- Harrison, P.; Hopkins, T.; Hulme, C.H.; McCarthy, H.S.; Wright, K.T. Chondrocyte Isolation and Expansion. In Methods in Molecular Biology—“Cartilage Tissue Engineering”; Humana: New York, NY, USA, 2022. [Google Scholar]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. Int. Soc. Cell. Ther. Position Statement Cytother. 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Garcia, J.; Mennan, C.; McCarthy, H.S.; Roberts, S.; Richardson, J.B.; Wright, K.T. Chondrogenic Potency Analyses of Donor-Matched Chondrocytes and Mesenchymal Stem Cells Derived from Bone Marrow, Infrapatellar Fat Pad, and Subcutaneous Fat. Stem Cells Int. 2016, 2016, 6969726. [Google Scholar] [CrossRef] [PubMed]
- Ahlberg, E.; Jenmalm, M.C.; Tingö, L. Evaluation of Five Column-Based Isolation Kits and Their Ability to Extract MiRNA from Human Milk. J. Cell Mol. Med. 2021, 25, 7973–7979. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Farndale, R.W.; Buttle, D.J.; Barrett, A.J. Improved Quantitation and Discrimination of Sulphated Glycosaminoglycans by Use of Dimethylmethylene Blue. Biochim. Biophys. Acta-Gen. Subj. 1986, 883, 173–177. [Google Scholar] [CrossRef]
- Farndale, R.W.; Sayers, C.A.; Barrett, A.J. A Direct Spectrophotometric Microassay for Sulfated Glycosaminoglycans in Cartilage Cultures. Connect. Tissue Res. 1982, 9, 247–248. [Google Scholar] [CrossRef]
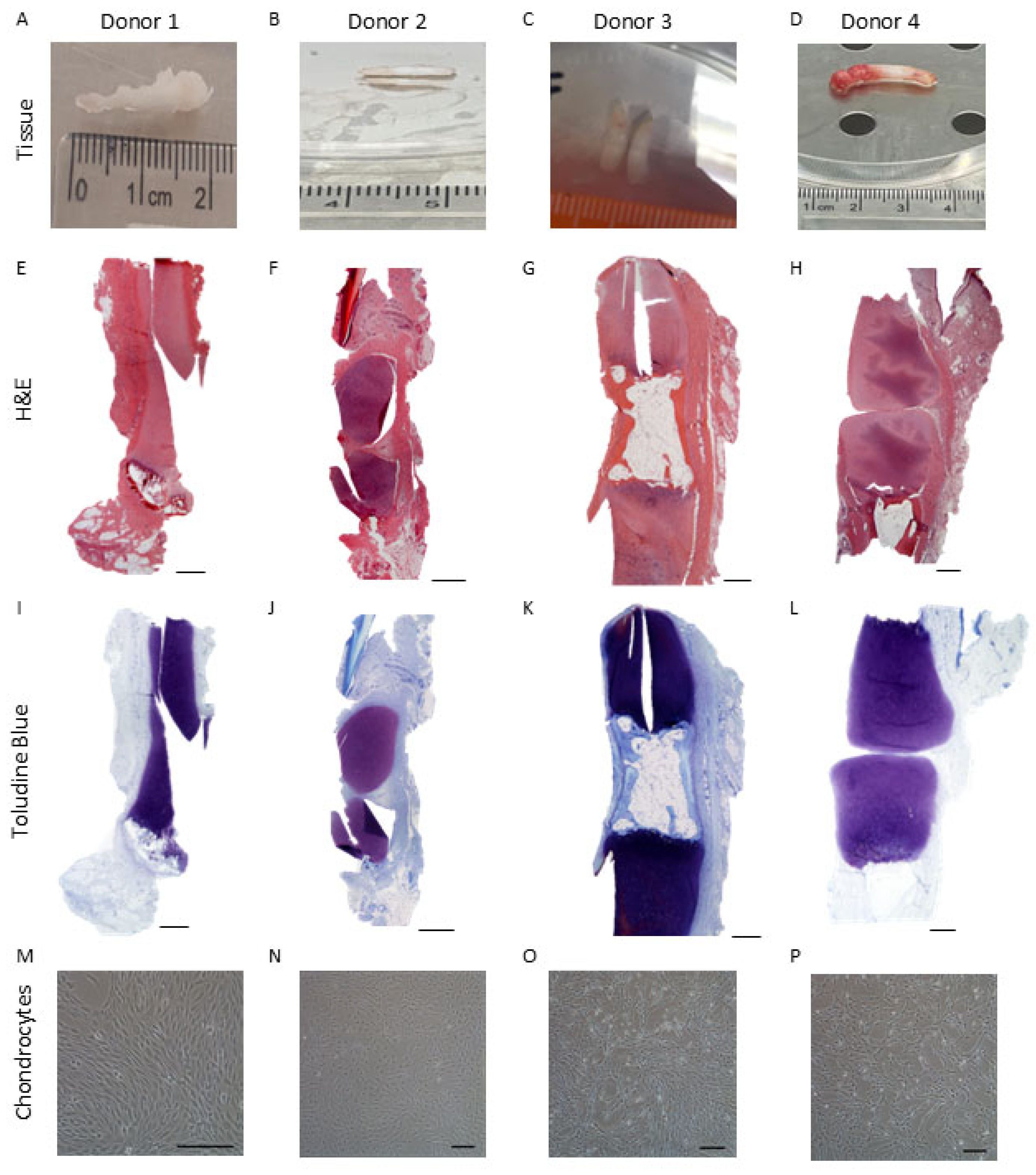
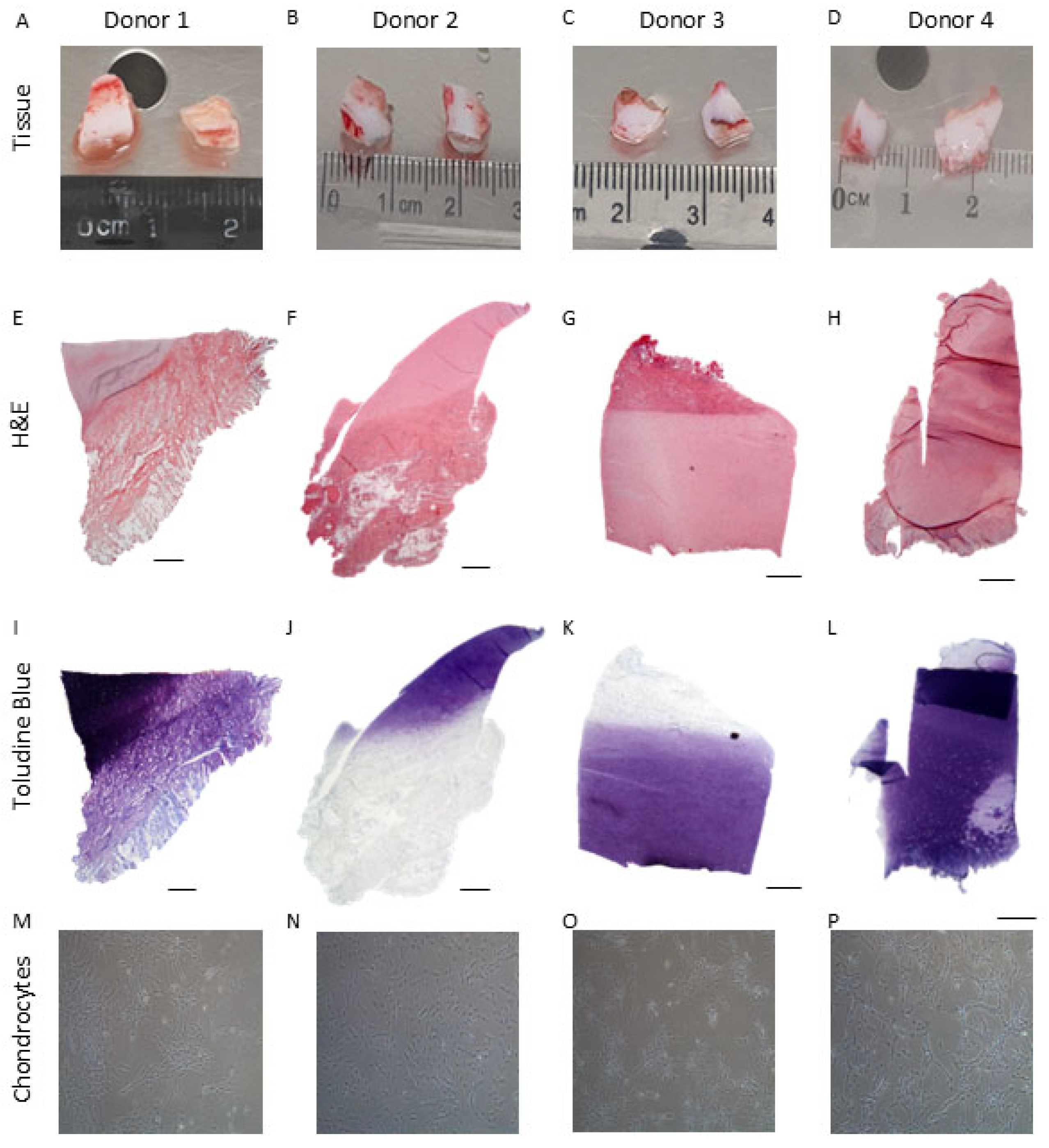
| Sample Type | Side | Donor | Gender | Age (Years, Months) |
|---|---|---|---|---|
| Polydactyly Digit | Right | 1 | F | 1 y 2 mo |
| Polydactyly Digit | 2 | M | 0 y 9 mo | |
| Polydactyly Digit | Right | 3 | M | 3 y 2 mo |
| Polydactyly Digit | Left | 4 | M | 1 y 9 mo |
| Iliac Apophysis | Right | 1 | F | 2 y 0 mo |
| Iliac Apophysis | Left | 2 | F | 2 y 2 mo |
| Iliac Apophysis | Right | 3 | F | 1 y 3 mo |
| Iliac Apophysis | Left | 4 | F | 2 y 0 mo |
| Donor | 1 | 2 | 3 | 4 | Mean | SD | |
|---|---|---|---|---|---|---|---|
| Tissue digested (g) | 0.23 | 0.069 | 0.041 | 0.015 | 0.089 | 0.097 | |
| Cell yield (×106) | 0.62 | 0.17 | 0.11 | 0.25 | 0.29 | 0.23 | |
| Passage for Quantum® and matched TCP expansion | 1 | 2 | 6 | 3 | 3 | 2.2 | |
| TCP vitronectin coated | Cells seeded (×106) | 0.875 | 0.875 | 0.875 | 0.875 | 0.875 | 0.0 |
| Cells harvested (×106) | 11.78 | 4.17 | 9.10 | 11.01 | 9.02 | 3.4 | |
| Increased cells Yield (×106) | 10.9 | 3.3 | 8.2 | 10.1 | 8.13 | 3.4 | |
| Days in culture | 9 | 7 | 10 | 11 | 9.3 | 1.7 | |
| Doubling time (days) | 1.3 | 1.3 | 1.5 | 1.6 | 1.4 | 0.2 | |
| Population doublings | 7.1 | 5.6 | 6.7 | 7.0 | 6.6 | 0.7 | |
| TCP-not vitronectin coated | Cells seeded (×106) | 0.875 | 0.875 | 0.875 | 0.875 | 0.875 | 0.0 |
| Cells harvested (×106) | 8.01 | 2.74 | 6.24 | 7.04 | 6.01 | 2.3 | |
| Increased cells Yield (×106) | 7.1 | 1.87 | 5.37 | 6.17 | 5.13 | 2.3 | |
| Days in culture | 9 | 7 | 10 | 11 | 9.25 | 1.7 | |
| Doubling time (days) | 1.4 | 1.4 | 1.6 | 1.7 | 1.5 | 0.2 | |
| Population doublings | 6.5 | 5.0 | 6.2 | 6.3 | 6.0 | 0.7 | |
| Quantum®- vitronectin coated | Cells seeded (×106) | 10 | 10 | 10 | 10 | 10 | 0.0 |
| Cells harvested (×106) | 96 | 53 | 107 | 110.8 | 91.7 | 26.6 | |
| Increased cells Yield (×106) | 86 | 43 | 97 | 100.8 | 81.7 | 26.6 | |
| Days in culture | 10 | 11 | 10 | 11 | 10.5 | 0.6 | |
| Doubling time (days) | 3.1 | 4.6 | 2.9 | 3.2 | 3.4 | 0.8 | |
| Population doublings | 3.3 | 2.4 | 3.4 | 3.5 | 3.1 | 0.5 | |
| Donor | 1 | 2 | 3 | 4 | Mean | SD | |
|---|---|---|---|---|---|---|---|
| Tissue digested (g) | 0.140 | 0.117 | 0.748 | 0.138 | 0.286 | 0.31 | |
| Cell yield (×106) | 1.05 | 1.17 | 0.99 | 0.625 | 0.959 | 0.23 | |
| Passage for Quantum® and matched TCP expansion | 3 | 3 | 3 | 3 | 3 | 0.0 | |
| TCP vitronectin coated | Cells seeded (×106) | 0.875 | 0.875 | 0.875 | 0.875 | 0.875 | 0.0 |
| Cells harvested (×106) | 6.02 | 6.15 | 4.32 | 2.95 | 4.86 | 1.5 | |
| Increased cells Yield (×106) | 5.15 | 5.28 | 3.45 | 2.08 | 3.99 | 1.5 | |
| Days in culture | 11 | 11 | 13 | 11 | 11.5 | 1.0 | |
| Doubling time (days) | 1.8 | 1.8 | 2.3 | 2.2 | 2.0 | 0.3 | |
| Population doublings | 6.1 | 6.1 | 5.6 | 5.1 | 5.7 | 0.5 | |
| TCP-not vitronectin coated | Cells seeded (×106) | 0.875 | 0.875 | 0.875 | 0.875 | 0.875 | 0 |
| Cells harvested (×106) | 4.73 | 3.98 | 4.83 | 1.61 | 3.79 | 1.5 | |
| Increased cells Yield (×106) | 3.86 | 3.1 | 4.0 | 0.7 | 3.7 | 1.5 | |
| Days in culture | 11 | 11 | 13 | 11 | 11.5 | 1.0 | |
| Doubling time (days) | 1.9 | 2.0 | 2.3 | 2.6 | 2.2 | 0.3 | |
| Population doublings | 5.8 | 5.5 | 5.8 | 4.2 | 5.3 | 0.8 | |
| Quantum®- vitronectin coated | Cells seeded (×106) | 10 | 10 | 10 | 10 | 10 | 0 |
| Cells harvested (×106) | 62 | 90 | 76 | 43 | 67.8 | 20.1 | |
| Increased cells Yield (×106) | 52 | 80 | 66 | 33 | 57.8 | 20.1 | |
| Days in culture | 11 | 12 | 11 | 12 | 11.5 | 0.6 | |
| Doubling time (days) | 4.2 | 3.8 | 3.8 | 5.7 | 4.4 | 0.9 | |
| Population doublings | 2.6 | 3.2 | 2.9 | 2.1 | 2.7 | 0.5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hulme, C.H.; Perry, J.; McCarthy, H.S.; Lan, T.; Ranasinghe, T.; Kiely, N.; Freeman, R.; Wright, J.; Wright, K.T. Developing Up-Scale Allogeneic Chondrocyte Therapies Using Juvenile Donor Cartilage. Int. J. Mol. Sci. 2025, 26, 9566. https://doi.org/10.3390/ijms26199566
Hulme CH, Perry J, McCarthy HS, Lan T, Ranasinghe T, Kiely N, Freeman R, Wright J, Wright KT. Developing Up-Scale Allogeneic Chondrocyte Therapies Using Juvenile Donor Cartilage. International Journal of Molecular Sciences. 2025; 26(19):9566. https://doi.org/10.3390/ijms26199566
Chicago/Turabian StyleHulme, Charlotte H., Jade Perry, Helen S. McCarthy, Tian Lan, Thavisha Ranasinghe, Nigel Kiely, Robert Freeman, Jonathan Wright, and Karina T. Wright. 2025. "Developing Up-Scale Allogeneic Chondrocyte Therapies Using Juvenile Donor Cartilage" International Journal of Molecular Sciences 26, no. 19: 9566. https://doi.org/10.3390/ijms26199566
APA StyleHulme, C. H., Perry, J., McCarthy, H. S., Lan, T., Ranasinghe, T., Kiely, N., Freeman, R., Wright, J., & Wright, K. T. (2025). Developing Up-Scale Allogeneic Chondrocyte Therapies Using Juvenile Donor Cartilage. International Journal of Molecular Sciences, 26(19), 9566. https://doi.org/10.3390/ijms26199566






