Regulation of Monocyte Perilipin-2 Expression in Acute and Chronic Coronary Syndromes: Pathogenetic Implications
Abstract
1. Introduction
2. Results
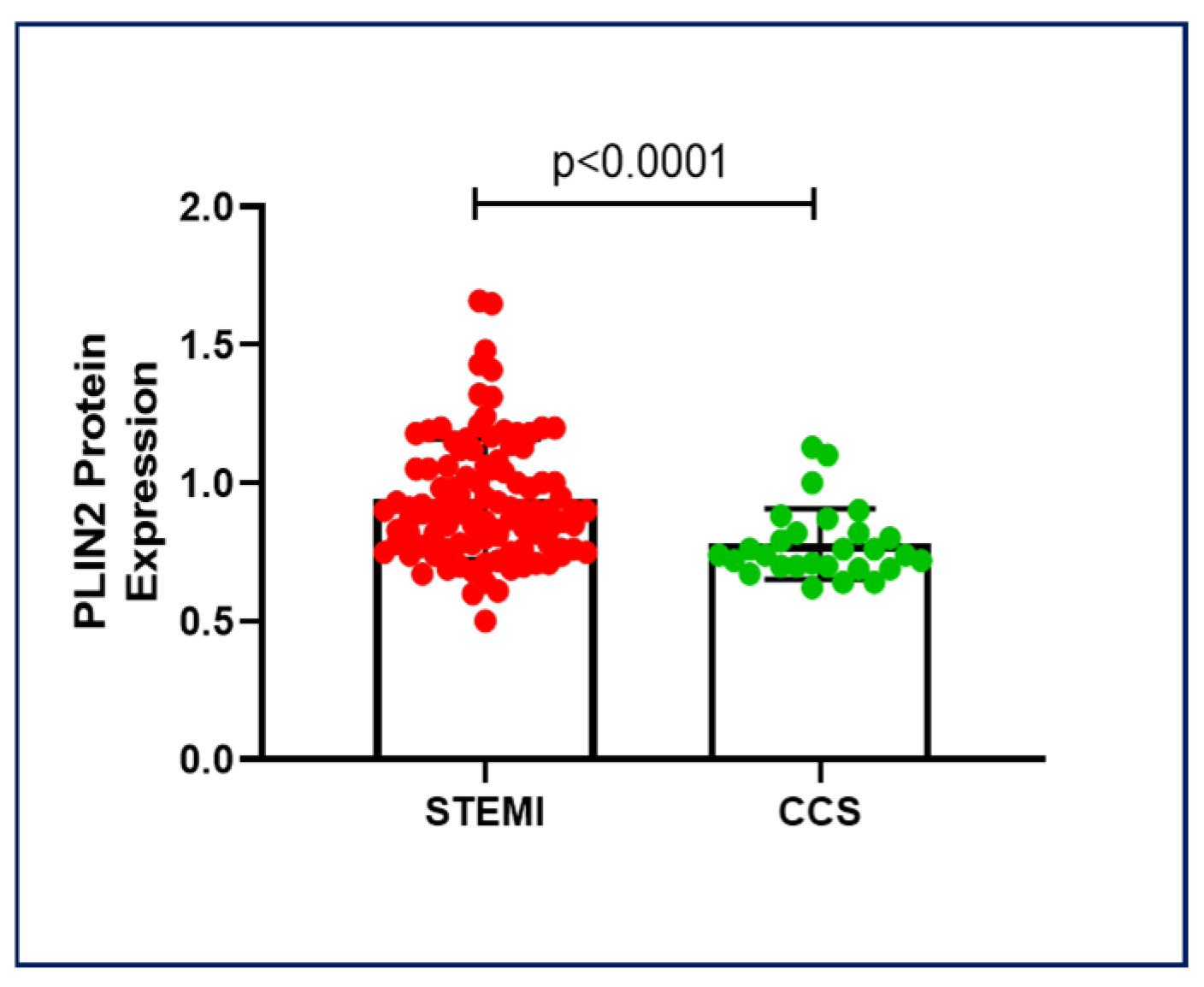
2.1. PLIN2 Protein Expression in Chronic and Acute Coronary Syndromes
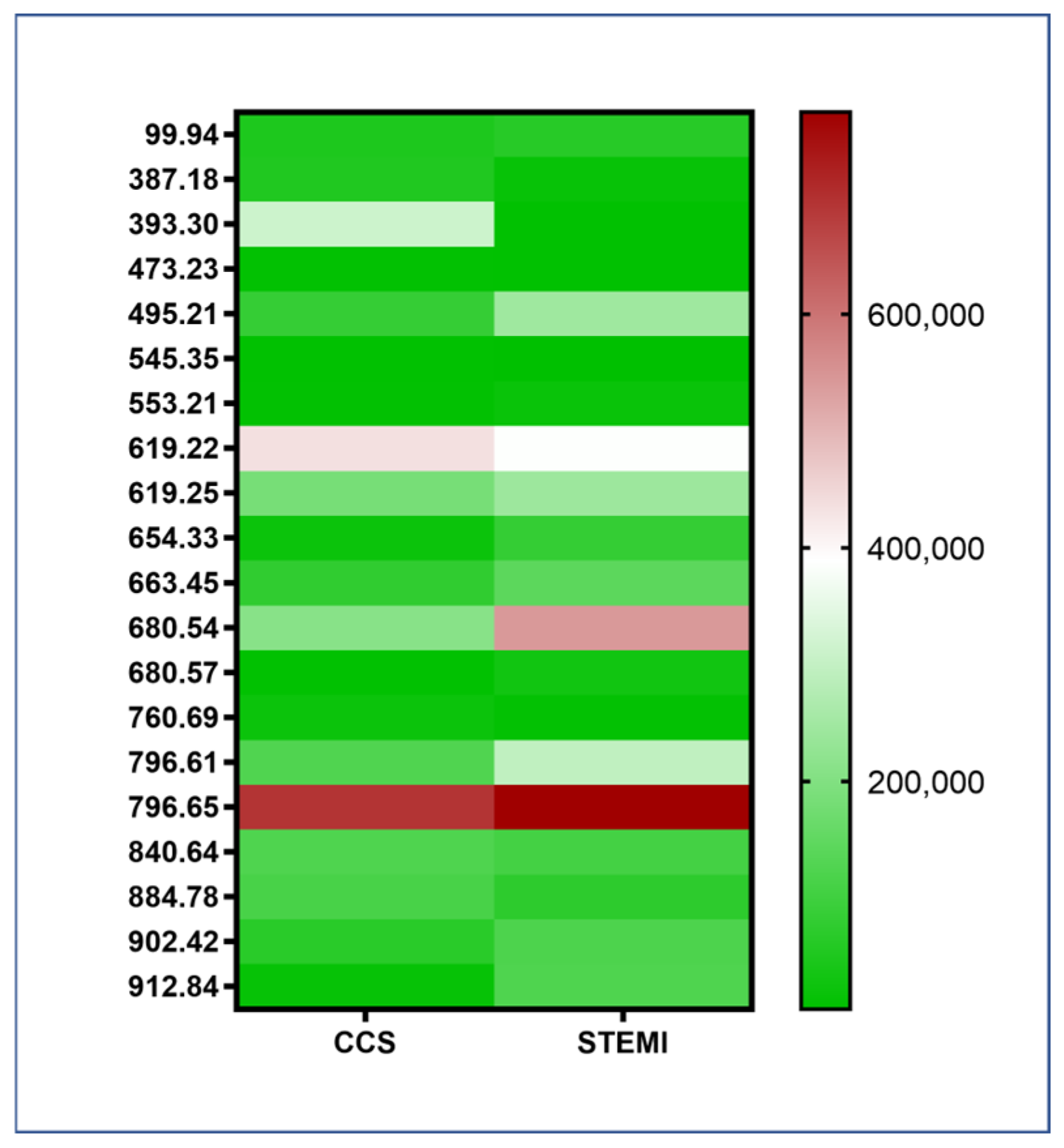
2.2. Lipidomic Panel
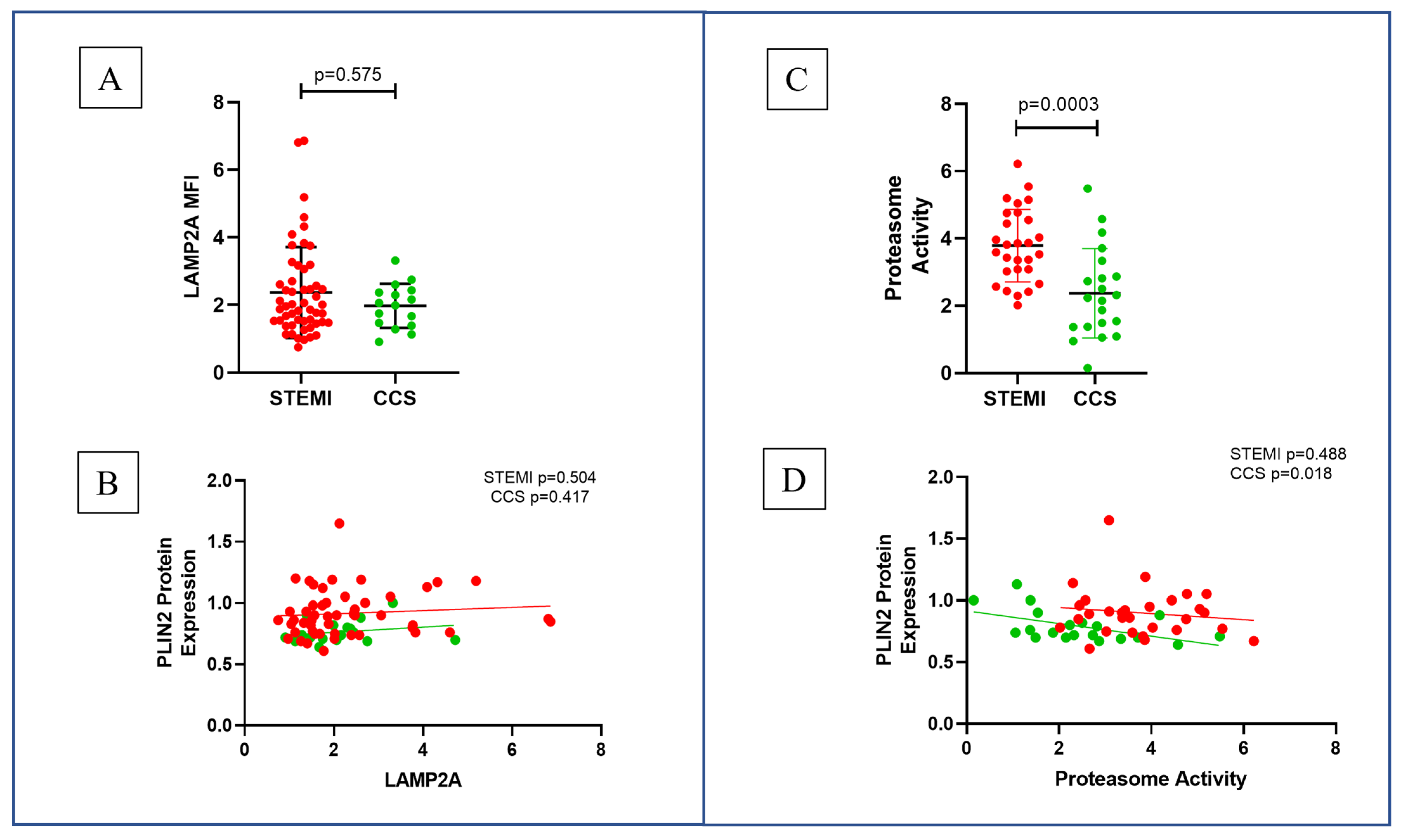
2.3. Proteasome Activity in STEMI and CCS Patients
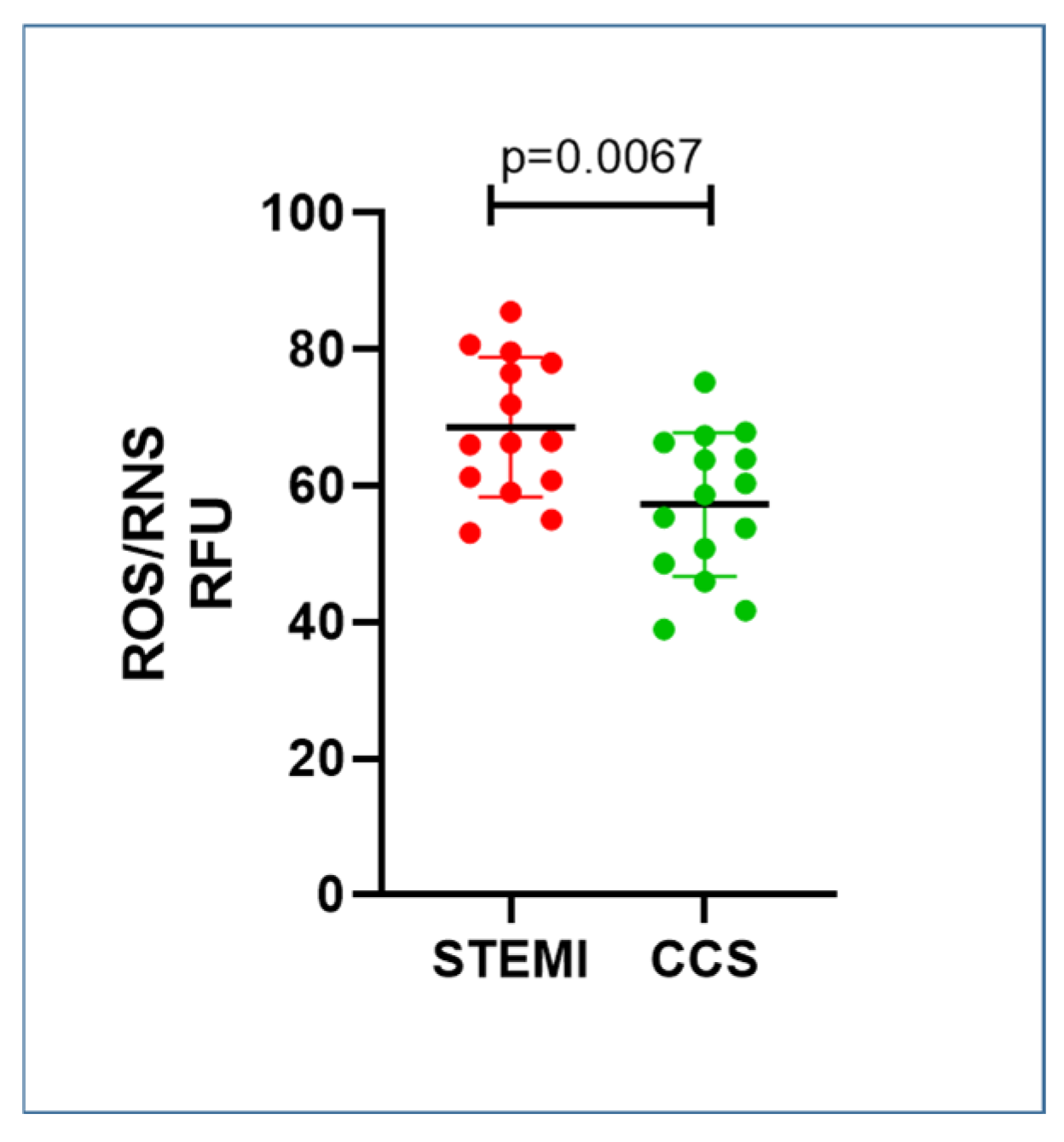
2.4. STEMI Patients Had Higher Levels of Oxidative Stress Compared to CCS
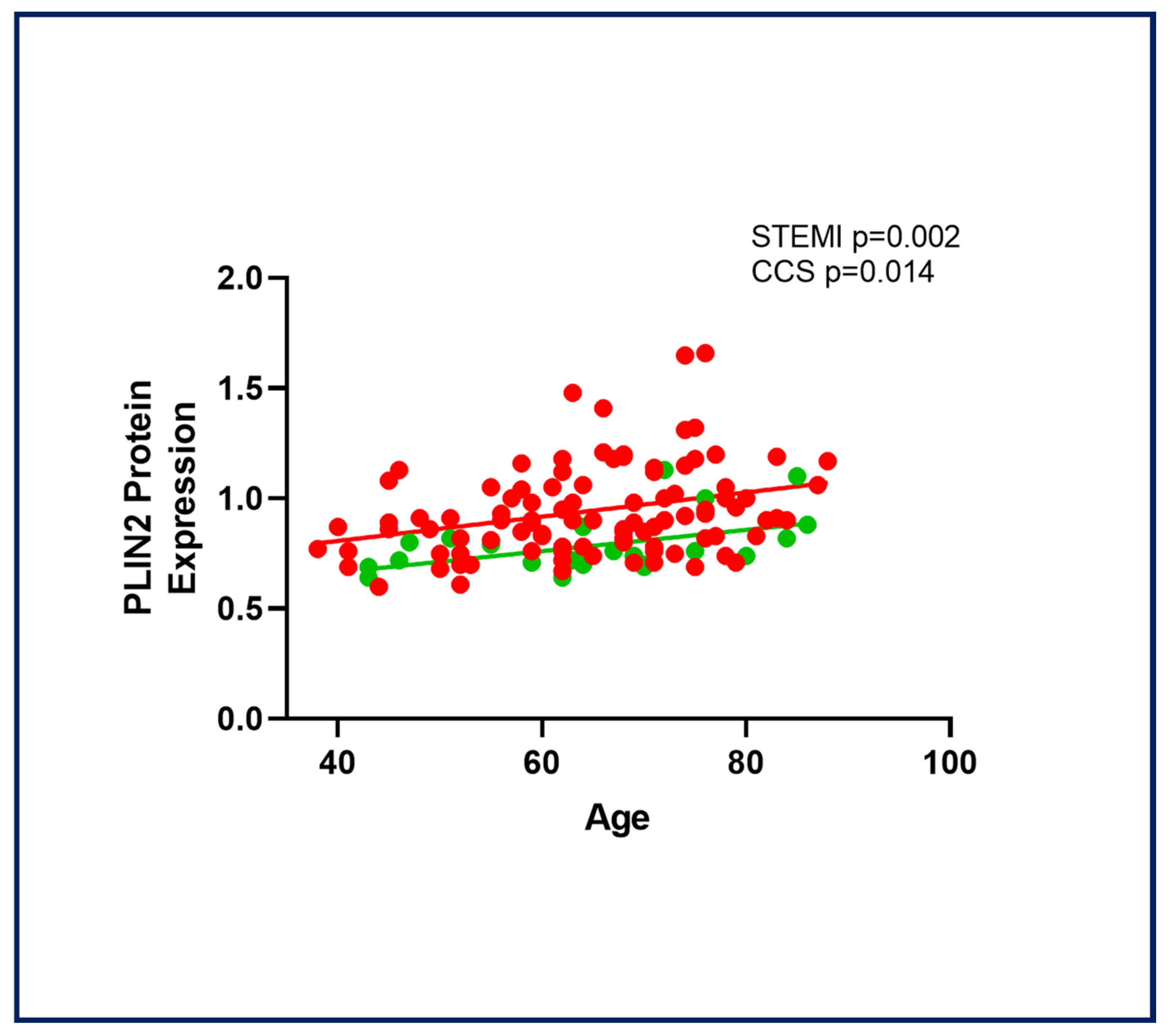
2.5. Age-Related PLIN2 Expression in CAD Patients
3. Discussion
3.1. PLIN2 and Coronary Instability
3.2. PLIN2-Related Molecular Mechanisms
3.3. Clinical Implications
3.4. Study Limitations
4. Materials and Methods
4.1. Study Population
4.2. Monocytes Isolation
4.3. Flow Cytometry Analysis
4.4. Lipidomic Panel
4.5. Proteasome Activity
4.6. Oxidative Stress Quantification
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur. Heart J. 2020, 41, 111–188, Correction in Eur. Heart J. 2020, 41, 4255. [Google Scholar] [CrossRef]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef]
- Goldberg, I.J.; Reue, K.; Abumrad, N.A.; Bickel, P.E.; Cohen, S.; Fisher, E.A.; Galis, Z.S.; Granneman, J.G.; Lewandowski, E.D.; Murphy, R.; et al. Deciphering the Role of Lipid Droplets in Cardiovascular Disease. Circulation 2018, 138, 305–315. [Google Scholar] [CrossRef]
- Son, S.-H.; Goo, Y.-H.; Choi, M.; Saha, P.K.; Oka, K.; Chan, L.C.B.; Paul, A. Enhanced atheroprotection and lesion remodelling by targeting the foam cell and increasing plasma cholesterol acceptors. Cardiovasc. Res. 2015, 109, 294–304. [Google Scholar] [CrossRef]
- Larigauderie, G.; Cuaz-Pérolin, C.; Younes, A.B.; Furman, C.; Lasselin, C.; Copin, C.; Jaye, M.; Fruchart, J.; Rouis, M. Adipophilin increases triglyceride storage in human macrophages by stimulation of biosynthesis and inhibition of β-oxidation. FEBS J. 2006, 273, 3498–3510. [Google Scholar] [CrossRef]
- Chen, F.L.; Yang, Z.H.; Wang, X.C.; Liu, Y.; Yang, Y.H.; Li, L.X.; Liang, W.C.; Zhou, W.B.; Hu, R.M. Adipophilin affects the expression of TNF-α, MCP-1, and IL-6 in THP-1 macrophages. Mol. Cell. Biochem. 2009, 337, 193–199. [Google Scholar] [CrossRef]
- Paul, A.; Chang, B.H.-J.; Li, L.; Yechoor, V.K.; Chan, L. Deficiency of Adipose Differentiation-Related Protein Impairs Foam Cell Formation and Protects Against Atherosclerosis. Circ. Res. 2008, 102, 1492–1501. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Zhao, H.; Wang, S.; Sun, X.; Qin, X. Increased ADRP expression in human atherosclerotic lesions correlates with plaque instability. Int. J. Clin. Exp. Med. 2015, 8, 5414–5421. [Google Scholar] [PubMed]
- Niccoli, G.; D’Amario, D.; Borovac, J.A.; Santangelo, E.; Scalone, G.; Fracassi, F.; Vergallo, R.; Vetrugno, V.; Copponi, G.; Severino, A.; et al. Perilipin 2 levels are increased in patients with in-stent neoatherosclerosis: A clue to mechanisms of accelerated plaque formation after drug-eluting stent implantation. Int. J. Cardiol. 2018, 258, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; A Montone, R.; D’Amario, D.; Camilli, M.; Canonico, F.; Santamaria, C.; Iannaccone, G.; Pedicino, D.; Pidone, C.; Galli, M.; et al. Role of perilipin 2 in microvascular obstruction in patients with ST-elevation myocardial infarction. Eur. Heart J. Acute Cardiovasc. Care 2020, 10, 633–642. [Google Scholar] [CrossRef]
- Pisano, E.; Pacifico, L.; Perla, F.M.; Liuzzo, G.; Chiesa, C.; Lavorato, M.; Mingrone, G.; Fabrizi, M.; Fintini, D.; Severino, A.; et al. Upregulated monocyte expression of PLIN2 is associated with early arterial injury in children with overweight/obesity. Atherosclerosis 2021, 327, 68–75. [Google Scholar] [CrossRef]
- Masuda, Y.; Itabe, H.; Odaki, M.; Hama, K.; Fujimoto, Y.; Mori, M.; Sasabe, N.; Aoki, J.; Arai, H.; Takano, T. ADRP/adipophilin is degraded through the proteasome-dependent pathway during regression of lipid-storing cells. J. Lipid Res. 2006, 47, 87–98. [Google Scholar] [CrossRef]
- Xu, G.; Sztalryd, C.; Lu, X.; Tansey, J.T.; Gan, J.; Dorward, H.; Kimmel, A.R.; Londos, C. Post-translational Regulation of Adipose Differentiation-related Protein by the Ubiquitin/Proteasome Pathway. J. Biol. Chem. 2005, 280, 42841–42847. [Google Scholar] [CrossRef] [PubMed]
- Morozov, A.V.; Burov, A.V.; Astakhova, T.M.; Spasskaya, D.S.; Margulis, B.A.; Karpov, V.L. Dynamics of the Functional Activity and Expression of Proteasome Subunits during Cellular Adaptation to Heat Shock. Mol. Biol. 2019, 53, 638–647. [Google Scholar] [CrossRef]
- Kaushik, S.; Cuervo, A.M. AMPK-dependent phosphorylation of lipid droplet protein PLIN2 triggers its degradation by CMA. Autophagy 2016, 12, 432–438. [Google Scholar] [CrossRef]
- Van Der Wal, A.C.; E Becker, A. Atherosclerotic plaque rupture—pathologic basis of plaque stability and instability. Cardiovasc. Res. 1999, 41, 334–344. [Google Scholar] [CrossRef]
- Head, T.; Daunert, S.; Goldschmidt-Clermont, P.J. The Aging Risk and Atherosclerosis: A Fresh Look at Arterial Homeostasis. Front. Genet. 2017, 8, 216. [Google Scholar] [CrossRef]
- Dib, L.; Koneva, L.A.; Edsfeldt, A.; Zurke, Y.-X.; Sun, J.; Nitulescu, M.; Attar, M.; Lutgens, E.; Schmidt, S.; Lindholm, M.W.; et al. Lipid-associated macrophages transition to an inflammatory state in human atherosclerosis, increasing the risk of cerebrovascular complications. Nat. Cardiovasc. Res. 2023, 2, 656–672, Erratum in Nat. Cardiovasc. Res. 2023, 2, 853. [Google Scholar] [CrossRef]
- Norman, J.E.; Aung, H.H.; Wilson, D.W.; Rutledge, J.C. Inhibition of perilipin 2 expression reduces pro-inflammatory gene expression and increases lipid droplet size. Food Funct. 2018, 9, 6245–6256. [Google Scholar] [CrossRef]
- Santos-Gallego, C.G.; Picatoste, B.; Badimón, J.J. Pathophysiology of Acute Coronary Syndrome. Curr. Atheroscler. Rep. 2014, 16, 1–9. [Google Scholar] [CrossRef]
- Vergallo, R.; Crea, F. Atherosclerotic Plaque Healing. N. Engl. J. Med. 2020, 383, 846–857. [Google Scholar] [CrossRef]
- Niccoli, G.; Montone, R.A.; Ibanez, B.; Thiele, H.; Crea, F.; Heusch, G.; Bulluck, H.; Hausenloy, D.J.; Berry, C.; Stiermaier, T.; et al. Optimized Treatment of ST-Elevation Myocardial Infarction. Circ. Res. 2019, 125, 245–258. [Google Scholar] [CrossRef]
- Chang, B.H.-J.; Li, L.; Paul, A.; Taniguchi, S.; Nannegari, V.; Heird, W.C.; Chan, L. Protection against Fatty Liver but Normal Adipogenesis in Mice Lacking Adipose Differentiation-Related Protein. Mol. Cell. Biol. 2006, 26, 1063–1076. [Google Scholar] [CrossRef]
- Faleck, D.M.; Ali, K.; Roat, R.; Graham, M.J.; Crooke, R.M.; Battisti, R.; Garcia, E.; Ahima, R.S.; Imai, Y. Adipose differentiation-related protein regulates lipids and insulin in pancreatic islets. Am. J. Physiol. Metab. 2010, 299, E249–E257. [Google Scholar] [CrossRef]
- Orlicky, D.J.; Libby, A.E.; Bales, E.S.; McMahan, R.H.; Monks, J.; La Rosa, F.G.; McManaman, J.L. Perilipin-2 promotes obesity and progressive fatty liver disease in mice through mechanistically distinct hepatocyte and extra-hepatocyte actions. J. Physiol. 2019, 597, 1565–1584. [Google Scholar] [CrossRef] [PubMed]
- Najt, C.P.; Senthivinayagam, S.; Aljazi, M.B.; Fader, K.A.; Olenic, S.D.; Brock, J.R.L.; Lydic, T.A.; Jones, A.D.; Atshaves, B.P. Liver-specific loss of Perilipin 2 alleviates diet-induced hepatic steatosis, inflammation, and fibrosis. Am. J. Physiol. Liver Physiol. 2016, 310, G726–G738. [Google Scholar] [CrossRef]
- McManaman, J.L.; Bales, E.S.; Orlicky, D.J.; Jackman, M.; MacLean, P.S.; Cain, S.; Crunk, A.E.; Mansur, A.; Graham, C.E.; Bowman, T.; et al. Perilipin-2-null mice are protected against diet-induced obesity, adipose inflammation, and fatty liver disease. J. Lipid Res. 2013, 54, 1346–1359. [Google Scholar] [CrossRef]
- Jin, Y.; Tan, Y.; Chen, L.; Liu, Y.; Ren, Z. Reactive Oxygen Species Induces Lipid Droplet Accumulation in HepG2 Cells by Increasing Perilipin 2 Expression. Int. J. Mol. Sci 2018, 19, 3445. [Google Scholar] [CrossRef]
- Mazzola, A.; Cianti, R.; Bini, L.; Armini, A.; Eberini, I.; Pompella, G.; Capecchi, P.L.; Natale, M.; Abbracchio, M.P.; Laghi-Pasini, F. Using peripheral blood mononuclear cells to determine proteome profiles in human cardiac failure. Eur. J. Heart Fail. 2008, 10, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Georgiopoulos, G.; Makris, N.; Laina, A.; Theodorakakou, F.; Briasoulis, A.; Trougakos, I.P.; Dimopoulos, M.-A.; Kastritis, E.; Stamatelopoulos, K. Cardiovascular Toxicity of Proteasome Inhibitors: Underlying Mechanisms and Management Strategies. JACC CardioOncology 2023, 5, 1–21. [Google Scholar] [CrossRef]
- Kammerl, I.E.; Hardy, S.; Flexeder, C.; Urmann, A.; Peierl, J.; Wang, Y.; Vosyka, O.; Frankenberger, M.; Milger, K.; Behr, J.; et al. Activation of immune cell proteasomes in peripheral blood of smokers and COPD patients: Implications for therapy. Eur. Respir. J. 2021, 59, 2101798. [Google Scholar] [CrossRef] [PubMed]
- Bramasole, L.; Meiners, S. Profiling Proteasome Activities in Peripheral Blood—A Novel Biomarker Approach. J. Cell. Immunol. 2022, 4, 171–179. [Google Scholar] [CrossRef]
- Castiello, D.S.; Buongiorno, F.; Manzi, L.; Narciso, V.; Forzano, I.; Florimonte, D.; Sperandeo, L.; Canonico, M.E.; Avvedimento, M.; Paolillo, R.; et al. Procedural and Antithrombotic Therapy Optimization in Patients with Atrial Fibrillation Undergoing Percutaneous Coronary Intervention: A Narrative Review. J. Cardiovasc. Dev. Dis. 2025, 12, 142. [Google Scholar] [CrossRef]
- Boden, W.E.; De Caterina, R.; Kaski, J.C.; Merz, N.B.; Berry, C.; Marzilli, M.; Pepine, C.J.; Barbato, E.; Stefanini, G.; Prescott, E.; et al. Myocardial ischaemic syndromes: A new nomenclature to harmonize evolving international clinical practice guidelines. Eur. Hear. J. 2024, 45, 3701–3706. [Google Scholar] [CrossRef] [PubMed]
- Vrints, C.; Andreotti, F.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; Chiariello, G.A.; et al. 2024 ESC Guidelines for the management of chronic coronary syndromes. Eur. Hear. J. 2024, 45, 3415–3537. [Google Scholar] [CrossRef] [PubMed]
| Overall | STEMI | CCS | p-Value | |
|---|---|---|---|---|
| Number of Patients | 167 | 122 | 45 | |
| Sex (M/F) | 138/29 | 100/22 | 38/7 | p = 0.8202 |
| Age (Median—IQR) | 68 (59–74) | 67 (57–74) | 69 (61–76) | p = 0.2495 |
| Weight (Mean ± SD) | 77.4 ± 14.2 | 78.4 ± 15.1 | 76.0 ± 11.7 | p = 0.3559 |
| Height (Mean ± SD) | 1.71 ± 0.1 | 1.71 ± 0.1 | 1.71 ± 0.1 | p = 0.8072 |
| BMI (Mean ± SD) | 26.4 ± 4.0 | 26.7 ± 4.25 | 25.8 ± 3.23 | p = 0.2397 |
| Risk factors [n (%)] | ||||
| Dyslipidemia | 81 (49) | 55 (45) | 26 (59) | p = 0.1588 |
| Hypertension | 104 (63) | 73 (60) | 31 (70) | p = 0.2761 |
| Smoker | 85 (52) | 65 (54) | 20 (45) | p = 0.3818 |
| Family History of IHD | 53 (32) | 35 (29) | 18 (41) | p = 0.1867 |
| Diabetes | 39 (24) | 26 (21) | 13 (30) | p = 0.3039 |
| Obesity | 18 (11) | 14 (12) | 4 (9) | p = 0.7827 |
| Pharmacological treatment (at the time of blood sampling) [n (%)] | ||||
| Aspirin | 55 (35) | 27 (24) | 28 (62) | p < 0.0001 * |
| Antiplatelet Drug | 16 (10) | 10 (9) | 6 (13) | p = 0.5598 |
| Anticoagulants | 21 (13) | 12 (11) | 9 (20) | p = 0.1242 |
| Beta-blockers | 43 (27) | 23 (20) | 20 (44) | p = 0.0030 * |
| Diuretics | 29 (18) | 14 (12) | 15 (33) | p = 0.0033 * |
| ACEi | 32 (20) | 23 (20) | 9 (20) | p > 0.9999 |
| Sartans | 33 (21) | 20 (18) | 13 (29) | p = 0.1324 |
| Statins | 56 (35) | 37 (32) | 19 (42) | p = 0.2688 |
| Ca-antagonists | 29 (18) | 19 (17) | 10 (22) | p = 0.4955 |
| Insulin | 10 (6) | 6 (5) | 4 (9) | p = 0.4721 |
| OHA | 29 (18) | 20 (18) | 9 (20) | p = 0.8204 |
| Laboratory assay (Mean ± SD) | ||||
| Glucose Level (mg/dL) | 122 ± 51.7 | 128 ± 56.2 | 105 ± 31.3 | p = 0.0009 * |
| TC (mg/dL) | 166 ± 38.9 | 169 ± 41.2 | 160 ± 32.5 | p = 0.4627 |
| LDL-C (mg/dL) | 101 ± 33.1 | 103 ± 35.0 | 95 ± 26.0 | p = 0.2688 |
| HDL-C (mg/dL) | 42 ± 10.9 | 40 ± 10.0 | 48 ± 11.4 | p = 0.0001 * |
| TG (mg/dL) | 124 ± 86.1 | 131 ± 97.0 | 106 ± 45.9 | p = 0.2154 |
| Hemoglobin (g/dL) | 14 ± 8.44 | 14 ± 1.90 | 13 ± 1.87 | p = 0.1319 |
| Platelets (109/L) | 233 ± 70.63 | 237 ± 74.22 | 222 ± 59.01 | p = 0.4214 |
| WBC (109/L) | 9.11 ± 3.9 | 9.84 ± 4.1 | 7.19 ± 2.6 | p < 0.0001 * |
| Lymphocytes (109/L) | 1.96 ± 0.97 | 1.99 ± 1.07 | 1.88 ± 0.62 | p = 0.9947 |
| Neutrophil (109/L) | 7.10 ± 3.28 | 7.91 ± 3.23 | 4.91 ± 2.26 | p < 0.0001 * |
| Monocytes (109/L) | 0.57 ± 0.28 | 0.61 ± 0.30 | 0.46 ± 0.16 | p = 0.0028 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canonico, F.; Laborante, R.; Pidone, C.; Vinci, R.; Galli, M.; Pisano, E.; Bonanni, A.; Di Sario, M.; Severino, A.; Lisi, L.; et al. Regulation of Monocyte Perilipin-2 Expression in Acute and Chronic Coronary Syndromes: Pathogenetic Implications. Int. J. Mol. Sci. 2025, 26, 9550. https://doi.org/10.3390/ijms26199550
Canonico F, Laborante R, Pidone C, Vinci R, Galli M, Pisano E, Bonanni A, Di Sario M, Severino A, Lisi L, et al. Regulation of Monocyte Perilipin-2 Expression in Acute and Chronic Coronary Syndromes: Pathogenetic Implications. International Journal of Molecular Sciences. 2025; 26(19):9550. https://doi.org/10.3390/ijms26199550
Chicago/Turabian StyleCanonico, Francesco, Renzo Laborante, Chiara Pidone, Ramona Vinci, Mattia Galli, Eugenia Pisano, Alice Bonanni, Marianna Di Sario, Anna Severino, Lucia Lisi, and et al. 2025. "Regulation of Monocyte Perilipin-2 Expression in Acute and Chronic Coronary Syndromes: Pathogenetic Implications" International Journal of Molecular Sciences 26, no. 19: 9550. https://doi.org/10.3390/ijms26199550
APA StyleCanonico, F., Laborante, R., Pidone, C., Vinci, R., Galli, M., Pisano, E., Bonanni, A., Di Sario, M., Severino, A., Lisi, L., Pedicino, D., Liuzzo, G., Ruscica, M., Crea, F., Patti, G., & D’Amario, D. (2025). Regulation of Monocyte Perilipin-2 Expression in Acute and Chronic Coronary Syndromes: Pathogenetic Implications. International Journal of Molecular Sciences, 26(19), 9550. https://doi.org/10.3390/ijms26199550










