Genetic Susceptibility to Tuberculosis and the Utility of Polygenic Scores in Population Stratification
Abstract
1. Introduction
2. Host–Pathogen Coevolution
3. Limited Success of Genome-Wide Association Studies (GWAS) in TB
| Reference | Sample Size (Cases/Healthy Controls) | Significant SNPs | Population Ancestry | Year |
|---|---|---|---|---|
| [34] | rs2243639 (SFTPD) | 2014 | ||
| rs2569190 (CD14) | South African Colored (SAC) | |||
| 918/507 | rs34069356 (CTSZ) | |||
| [35] | 410/405 | not statistically significant | South African Colored (SAC) | 2019 |
| [36] | 642/91 | rs2057178 | South African Colored (SAC) population | 2014 |
| [37] | 5530/5607 | rs4733781 (ASAP1) rs10956514 (ASAP1) No associations were observed for rs10956514 and rs11774633 (ASAP1) with TB in Chinese populations [38] | Russian (replication in the African population) | 2015 |
| [39] | Four datasets: young Japanese (60/249), young Thai (137/295), old Japanese (123/685), old Thai (300/295) | rs6071980 | Thai/Japanese | 2012 |
| [40] | 4310/6386 | rs41553512 (HLA-DRB5) | Han Chinese | 2017 |
| [41] | 2237/3122 | rs4331426 (LOC124904262: Intron Variant) No associations were observed for rs4331426 with TB in Chinese populations [38] | Ghanaian/Gambian | 2010 |
| [33] | 8821/13,859 | rs2057178 | Ghanaian/Gambian (Russian/Indonesian) | 2012 |
| [42] | 333/616 | rs17155120 (C10orf90: Intron Variant) | Vietnamese/French/South African | 2021 |
| [43] | 13,692/283,250 | rs557011 | Icelandic/Russian/Croatian | 2016 |
| [44] | 646/1813 | rs9365798 | Korean | 2017 |
| [45] | 616/709 | rs3751112 (RAB17), rs141645096 (DCTN4) | Han Chinese | 2021 |
| [46] | 2175/1827 | rs73226617 (LINC02618: Intron Variant) | Peruvian (Lima) | 2019 |
| [47] | 2949/5090 | rs12437118 (ESRRB), rs6114027 (TGM6: Intron Variant) | Han Chinese | 2018 |
| [48] | 556/650 | rs916943 (AGMO: Intron Variant) | Moroccan/Russian | 2016 |
| [49] | 2653/2537 | rs2273051 (COL4A5) | Indonesian/Russian | 2012 |
| [50] | 4426/84,290 | rs2894257 | European | 2017 |
| [51] | 43 patients with active TB and 49 with latent TB/313 controls | rs62292160 | Chinese Han | 2021 |
4. Genetic Susceptibility to TB Is Caused by a Continuum Ranging from Monogenic to Polygenic Risk
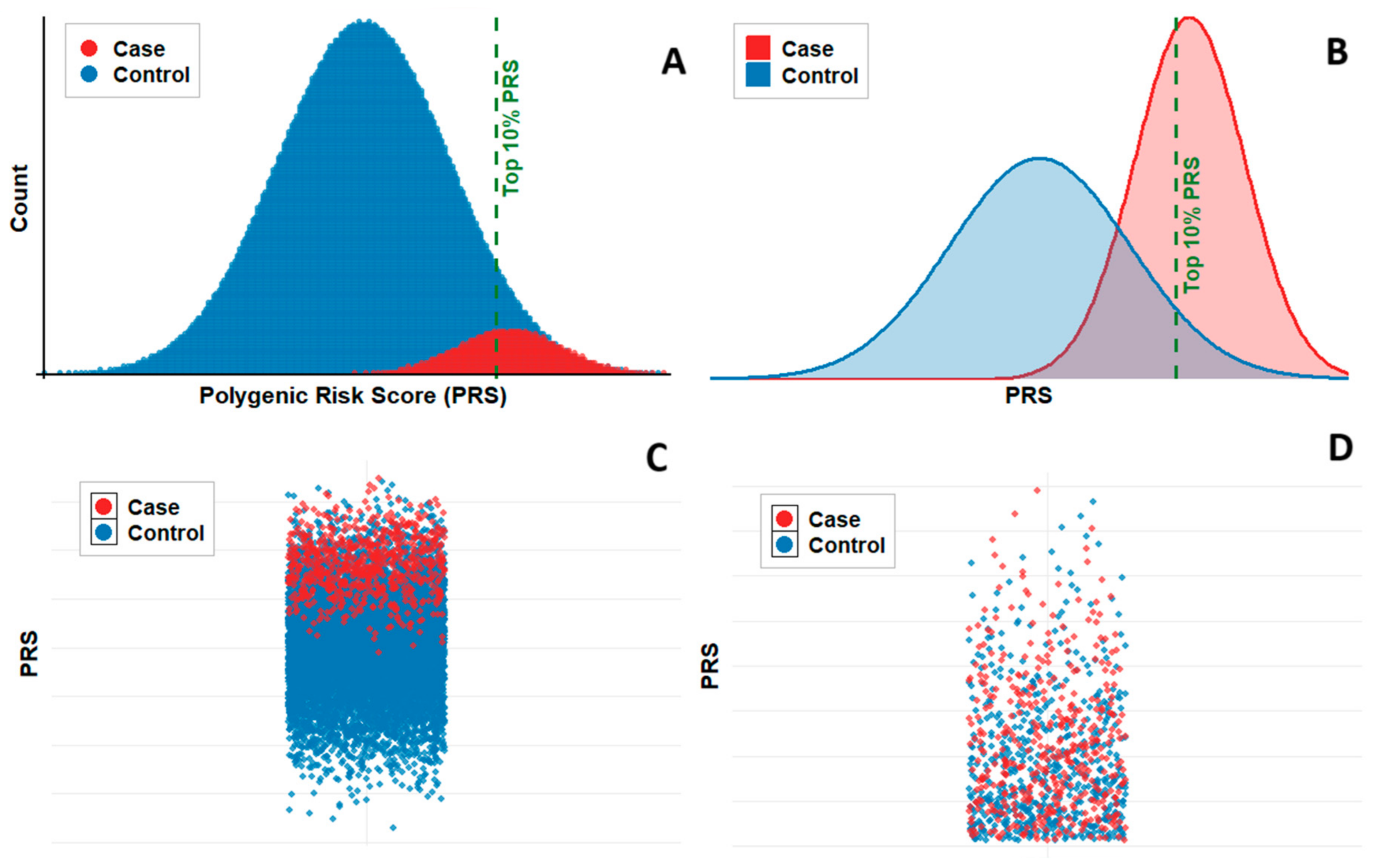
5. The Potential and Challenges of PRS for TB
6. Limited Application of PRSs to Infectious Diseases
7. Shared Immunogenetic Components in PRS Across Infectious Diseases
8. Methodological Challenges in Constructing PRS for Infectious Diseases
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Alene, M.; Yismaw, L.; Assemie, M.A.; Ketema, D.B.; Mengist, B.; Kassie, B.; Birhan, T.Y. Magnitude of asymptomatic COVID-19 cases throughout the course of infection: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0249090. [Google Scholar] [CrossRef] [PubMed]
- Kriz, B.; Hubalek, Z.; Marek, M.; Daniel, M.; Strakova, P.; Betasova, L. Results of the Screening of Tick-Borne Encephalitis Virus Antibodies in Human Sera from Eight Districts Collected Two Decades Apart. Vector-Borne Zoonotic Dis. 2015, 15, 489–493. [Google Scholar] [CrossRef]
- Stjernberg, L.; Holmkvist, K.; Berglund, J. A newly detected tick-borne encephalitis (TBE) focus in south-east Sweden: A follow-up study of TBE virus (TBEV) seroprevalence. Scand. J. Infect. Dis. 2008, 40, 4–10. [Google Scholar] [CrossRef]
- Maikova, G.B.; Chernokhaeva, L.L.; Rogova, Y.V.; Kozlovskaya, L.I.; Kholodilov, I.S.; Romanenko, V.V.; Esyunina, M.S.; Ankudinova, A.A.; Kilyachina, A.S.; Vorovitch, M.F.; et al. Ability of inactivated vaccines based on far-eastern tick-borne encephalitis virus strains to induce humoral immune response in originally seropositive and seronegative recipients. J. Med. Virol. 2018, 91, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, A.T.; Petersen, B.W.; Recuenco, S.; Niezgoda, M.; Gómez, J.; Laguna-Torres, V.A.; Rupprecht, C. Evidence of rabies virus exposure among humans in the Peruvian Amazon. Am. J. Trop. Med. Hyg. 2012, 87, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Follmann, E.H.; Ritter, D.G.; Beller, M. Survey of fox trappers in northern Alaska for rabies antibody. Epidemiol. Infect. 1994, 113, 137–141. [Google Scholar] [CrossRef]
- World Health Organization. Global Tuberculosis Report; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- O’Garra, A.; Redford, P.S.; McNab, F.W.; Bloom, C.I.; Wilkinson, R.J.; Berry, M.P.R. The immune response in tuberculosis. Annu. Rev. Immunol. 2013, 31, 475–527. [Google Scholar] [CrossRef]
- Cohen, A.; Mathiasen, V.D.; Schön, T.; Wejse, C. The global prevalence of latent tuberculosis: A systematic review and meta-analysis. Eur. Respir. J. 2019, 54, 1900655. [Google Scholar] [CrossRef]
- Eisenberg, J.N.; Desai, M.A.; Levy, K.; Bates, S.J.; Liang, S.; Naumoff, K.; Scott, J.C. Environmental determinants of infectious disease: A framework for tracking causal links and guiding public health research. Environ. Health Perspect. 2007, 115, 1216–1223. [Google Scholar] [CrossRef]
- Abel, L.; Dessein, A.J. The impact of host genetics on susceptibility to human infectious diseases. Curr. Opin. Immunol. 1997, 9, 509–516. [Google Scholar] [CrossRef]
- Borbour, S.E.; Nakashima, K.; Zhang, J.-B.; Tangada, S.; Hahn, C.-L.; Schenkein, H.A.; Tew, J.G. Tobacco and smoking: Environmental factors that modify the host response (immune system) and have an impact on periodontal health. Crit. Rev. Oral Biol. Med. 1997, 8, 437–460. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.M.; Tsai, F.J.; Lee, Y.L.; Chang, J.H.; Chang, L.T.; Chang, T.Y.; Chung, K.F.; Kuo, H.P.; Lee, K.Y.; Chuang, K.J.; et al. The impact of air pollution on respiratory diseases in an era of climate change: A review of the current evidence. Sci. Total. Environ. 2023, 898, 166340. [Google Scholar] [CrossRef]
- Guarnieri, G.; Olivieri, B.; Senna, G.; Vianello, A. Relative Humidity and Its Impact on the Immune System and Infections. Int. J. Mol. Sci. 2023, 24, 9456. [Google Scholar] [CrossRef]
- Barrea, L.; Muscogiuri, G.; Frias-Toral, E.; Laudisio, D.; Pugliese, G.; Castellucci, B.; Garcia-Velasquez, E.; Savastano, S.; Colao, A. Nutrition and immune system: From the Mediterranean diet to dietary supplementary through the microbiota. Crit. Rev. Food Sci. Nutr. 2020, 61, 3066–3090. [Google Scholar] [CrossRef]
- Zefferino, R.; Di Gioia, S.; Conese, M. Molecular links between endocrine, nervous and immune system during chronic stress. Brain Behav. 2020, 11, e01960. [Google Scholar] [CrossRef] [PubMed]
- Brodin, P.; Jojic, V.; Gao, T.; Bhattacharya, S.; Angel, C.J.L.; Furman, D.; Shen-Orr, S.; Dekker, C.L.; Swan, G.E.; Butte, A.J.; et al. Variation in the human immune system is largely driven by non-heritable influences. Cell 2015, 160, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Fox, G.J.; Orlova, M.; Schurr, E. Tuberculosis in Newborns: The Lessons of the “Lübeck Disaster” (1929–1933). PLoS Pathog. 2016, 12, e1005271. [Google Scholar] [CrossRef]
- Hill, H.G. Der erbeinfluss bei der tuberkulose (zwillingstuberkulose II). Eugen. Rev. 1937, 29, 207. [Google Scholar]
- Comstock, G. Tuberculosis in twins: A re-analysis of the Prophit survey. Am. Rev. Respir. Dis. 1978, 117, 621–624. [Google Scholar] [PubMed]
- Ndong Sima, C.A.A.; Smith, D.; Petersen, D.C.; Schurz, H.; Uren, C.; Möller, M. The immunogenetics of tuberculosis (TB) susceptibility. Immunogenetics 2022, 75, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Kwon, N.; Goo, G.-Y.; Cho, S.-I. Inadequate housing and pulmonary tuberculosis: A systematic review. BMC Public Health 2022, 22, 622. [Google Scholar] [CrossRef]
- Cardona, P.-J. Pathogenesis of tuberculosis and other mycobacteriosis. Enfermedades Infecc. Microbiol. Clin. 2018, 36, 38–46. [Google Scholar] [CrossRef]
- Schurz, H.; Naranbhai, V.; Yates, T.A.; Gilchrist, J.J.; Parks, T.; Dodd, P.J.; Möller, M.; Hoal, E.G.; Morris, A.P.; Hill, A.V.; et al. Multi-ancestry meta-analysis of host genetic susceptibility to tuberculosis identifies shared genetic architecture. eLife 2024, 13, e84394. [Google Scholar] [CrossRef]
- Riojas, M.A.; McGough, K.J.; Rider-Riojas, C.J.; Rastogi, N.; Hazbón, M.H. Phylogenomic analysis of the species of the mycobacterium tuberculosis complex demonstrates that mycobacterium africanum, mycobacterium bovis, mycobacterium caprae, mycobacterium microti and mycobacterium pinnipedii are later heterotypic synonyms of mycobacterium tuberculosis. Int. J. Syst. Evol. Microbiol. 2018, 68, 324–332. [Google Scholar] [CrossRef]
- Müller, S.J.; Schurz, H.; Tromp, G.; van der Spuy, G.D.; Hoal, E.G.; van Helden, P.D.; Owusu-Dabo, E.; Meyer, C.G.; Muntau, B.; Thye, T.; et al. A multi-phenotype genome-wide association study of clades causing tuberculosis in a Ghanaian- and South African cohort. Genomics 2021, 113, 1802–1815. [Google Scholar] [CrossRef]
- Correa-Macedo, W.; Cambri, G.; Schurr, E. The Interplay of Human and Mycobacterium Tuberculosis Genomic Variability. Front. Genet. 2019, 10, 865. [Google Scholar] [CrossRef]
- Salie, M.; van der Merwe, L.; Möller, M.; Daya, M.; van der Spuy, G.D.; van Helden, P.D.; Martin, M.P.; Gao, X.-J.; Warren, R.M.; Carrington, M.; et al. Associations between human leukocyte antigen class I variants and the mycobacterium tuberculosis subtypes causing disease. J. Infect. Dis. 2013, 209, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Comas, I.; Luo, D.; Lu, B.; Wu, J.; Wei, L.; Yang, C.; Liu, Q.; Gan, M.; Sun, G.; et al. Southern East Asian origin and coexpansion of Mycobacterium tuberculosis Beijing family with Han Chinese. Proc. Natl. Acad. Sci. USA 2015, 112, 8136–8141. [Google Scholar] [CrossRef] [PubMed]
- Gagneux, S.; DeRiemer, K.; Van, T.; Kato-Maeda, M.; de Jong, B.C.; Narayanan, S.; Nicol, M.; Niemann, S.; Kremer, K.; Gutierrez, M.C.; et al. Variable host–pathogen compatibility in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 2006, 103, 2869–2873. [Google Scholar] [CrossRef]
- Otchere, I.D.; Coscollá, M.; Sánchez-Busó, L.; Asante-Poku, A.; Brites, D.; Loiseau, C.; Meehan, C.; Osei-Wusu, S.; Forson, A.; Laryea, C.; et al. Comparative genomics of Mycobacterium africanum Lineage 5 and Lineage 6 from Ghana suggests distinct ecological niches. Sci. Rep. 2018, 8, 11269. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.M.; Baker, R.E.; Proulx, M.K.; Mishra, B.B.; Long, J.E.; Park, S.W.; Lee, H.-N.; Kiritsy, M.C.; Bellerose, M.M.; Olive, A.J.; et al. Host-pathogen genetic interactions underlie tuberculosis susceptibility in genetically diverse mice. eLife 2022, 11, e74419. [Google Scholar] [CrossRef] [PubMed]
- Thye, T.; Owusu-Dabo, E.; Vannberg, F.O.; Van Crevel, R.; Curtis, J.; Sahiratmadja, E.; Balabanova, Y.; Ehmen, C.; Muntau, B.; Ruge, G.; et al. Common variants at 11p13 are associated with susceptibility to tuberculosis. Nat. Genet. 2012, 44, 257–259. [Google Scholar] [CrossRef]
- Daya, M.; van der Merwe, L.; Gignoux, C.R.; van Helden, P.D.; Möller, M.; Hoal, E.G. Using multi-way admixture mapping to elucidate TB susceptibility in the South African Coloured population. BMC Genom. 2014, 15, 1021. [Google Scholar] [CrossRef]
- Schurz, H.; Kinnear, C.J.; Gignoux, C.; Wojcik, G.; van Helden, P.D.; Tromp, G.; Henn, B.; Hoal, E.G.; Möller, M. A sex-stratified genome-wide association study of tuberculosis using a multi-ethnic genotyping array. Front. Genet. 2019, 9, 678. [Google Scholar] [CrossRef]
- Chimusa, E.R.; Zaitlen, N.; Daya, M.; Möller, M.; van Helden, P.D.; Mulder, N.J.; Price, A.L.; Hoal, E.G. Genome-wide association study of ancestry-specific TB risk in the South African coloured population. Human Mol. Genet. 2013, 23, 796–809. [Google Scholar] [CrossRef]
- Curtis, J.; Luo, Y.; Zenner, H.L.; Cuchet-Lourenço, D.; Wu, C.; Lo, K.; Maes, M.; Alisaac, A.; Stebbings, E.; Liu, J.Z.; et al. Susceptibility to tuberculosis is associated with variants in the ASAP1 gene encoding a regulator of dendritic cell migration. Nat. Genet. 2015, 47, 523–527. [Google Scholar] [CrossRef]
- Miao, R.; Huang, S.; Li, C.; Ding, S.; Wang, R.; Xu, K.; Yang, C.; Xu, F.; Ge, H. An HLA class II locus, previously identified by a genome-wide association study, is also associated with susceptibility to pulmonary tuberculosis in a Chinese population. Infect. Genet. Evol. 2018, 64, 164–167. [Google Scholar] [CrossRef]
- Mahasirimongkol, S.; Yanai, H.; Mushiroda, T.; Promphittayarat, W.; Wattanapokayakit, S.; Phromjai, J.; Yuliwulandari, R.; Wichukchinda, N.; Yowang, A.; Yamada, N.; et al. Genome-wide association studies of tuberculosis in Asians identify distinct at-risk locus for young tuberculosis. J. Human Genet. 2012, 57, 363–367. [Google Scholar] [CrossRef]
- Qi, H.; Zhang, Y.-B.; Sun, L.; Chen, C.; Xu, B.; Xu, F.; Liu, J.-W.; Liu, J.-C.; Jiao, W.-W.; Shen, C.; et al. Discovery of susceptibility loci associated with tuberculosis in Han Chinese. Human Mol. Genet. 2017, 26, 4752–4763. [Google Scholar] [CrossRef] [PubMed]
- Thye, T.; Vannberg, F.O.; Wong, S.H.; Owusu-Dabo, E.; Osei, I.; Gyapong, J.; Hill, A.V.S. Genome-wide association analyses identifies a susceptibility locus for tuberculosis on chromosome 18q11.2. Nat. Genet. 2010, 42, 739–741. [Google Scholar] [CrossRef] [PubMed]
- Quistrebert, J.; Orlova, M.; Kerner, G.; Ton, L.T.; Luong, N.T.; Danh, N.T.; Vincent, Q.B.; Jabot-Hanin, F.; Seeleuthner, Y.; Bustamante, J.; et al. Genome-wide association study of resistance to Mycobacterium tuberculosis infection identifies a locus at 10q26.2 in three distinct populations. PLoS Genet. 2021, 17, e1009392. [Google Scholar] [CrossRef]
- Sveinbjornsson, G.; Gudbjartsson, D.F.; Halldorsson, B.V.; Kristinsson, K.G.; Gottfredsson, M.; Barrett, J.C.; Gudmundsson, L.J.; Blondal, K.; Gylfason, A.; Gudjonsson, S.A.; et al. HLA class II sequence variants influence tuberculosis risk in populations of European ancestry. Nat. Genet. 2016, 48, 318–322. [Google Scholar] [CrossRef]
- Hong, E.P.; Go, M.J.; Kim, H.-L.; Park, J.W. Risk prediction of pulmonary tuberculosis using genetic and conventional risk factors in adult Korean population. PLoS ONE 2017, 12, e0174642. [Google Scholar] [CrossRef]
- Li, M.; Hu, Y.; Zhao, B.; Chen, L.; Huang, H.; Huai, C.; Zhang, X.; Zhang, J.; Zhou, W.; Shen, L.; et al. A next generation sequencing combined genome-wide association study identifies novel tuberculosis susceptibility loci in Chinese population. Genomics 2021, 113, 2377–2384. [Google Scholar] [CrossRef]
- Luo, Y.; Suliman, S.; Asgari, S.; Amariuta, T.; Baglaenko, Y.; Martínez-Bonet, M.; Ishigaki, K.; Gutierrez-Arcelus, M.; Calderon, R.; Lecca, L.; et al. Early progression to active tuberculosis is a highly heritable trait driven by 3q23 in Peruvians. Nat. Commun. 2019, 10, 3765. [Google Scholar] [CrossRef]
- Zheng, R.; Li, Z.; He, F.; Liu, H.; Chen, J.; Chen, J.; Xie, X.; Zhou, J.; Chen, H.; Wu, X.; et al. Genome-wide association study identifies two risk loci for tuberculosis in Han Chinese. Nat. Commun. 2018, 9, 4072. [Google Scholar] [CrossRef] [PubMed]
- Grant, A.V.; Sabri, A.; Abid, A.; Abderrahmani Rhorfi, I.; Benkirane, M.; Souhi, H.; Naji Amrani, H.; Alaoui-Tahiri, K.; Gharbaoui, Y.; Lazrak, F.; et al. A genome-wide association study of pulmonary tuberculosis in Morocco. Human Genet. 2016, 135, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Png, E.; Alisjahbana, B.; Sahiratmadja, E.; Marzuki, S.; Nelwan, R.; Balabanova, Y.; Nikolayevskyy, V.; Drobniewski, F.; Nejentsev, S.; Adnan, I.; et al. A genome wide association study of pulmonary tuberculosis susceptibility in Indonesians. BMC Med. Genet. 2012, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Hromatka, B.S.; Kiefer, A.K.; Eriksson, N.; Noble, S.M.; Tung, J.Y.; Hinds, D.A. Genome-wide association and HLA region fine-mapping studies identify susceptibility loci for multiple common infections. Nat. Commun. 2017, 8, 599. [Google Scholar] [CrossRef]
- Ai, J.-W.; Zhang, H.; Zhou, Z.; Weng, S.; Huang, H.; Wang, S.; Shao, L.; Gao, Y.; Wu, J.; Ruan, Q.; et al. Gene expression pattern analysis using dual-color RT-MLPA and integrative genome-wide association studies of eQTL for tuberculosis suscepitibility. Respir. Res. 2021, 22, 23. [Google Scholar] [CrossRef]
- Möller, M.; Kinnear, C.J. Human global and population-specific genetic susceptibility to Mycobacterium tuberculosis infection and disease. Curr. Opin. Pulm. Med. 2020, 26, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Harishankar, M.; Selvaraj, P.; Bethunaickan, R. Influence of genetic polymorphism towards pulmonary tuberculosis susceptibility. Front. Med. 2018, 5, 213. [Google Scholar] [CrossRef]
- Azad, A.K.; Sadee, W.; Schlesinger, L.S. Innate immune gene polymorphisms in tuberculosis. Infect. Immun. 2012, 80, 3343–3359. [Google Scholar] [CrossRef]
- Boisson-Dupuis, S.; Ramirez-Alejo, N.; Li, Z.; Patin, E.; Rao, G.; Kerner, G.; Lim, C.K.; Krementsov, D.N.; Hernandez, N.; Ma, C.S.; et al. Tuberculosis and impaired IL-23–dependent IFN-γ immunity in humans homozygous for a common TYK2 missense variant. Sci. Immunol. 2018, 3, eaau8714. [Google Scholar] [CrossRef]
- Koo, H.-K.; Min, J.; Kim, H.W.; Ko, Y.; Oh, J.Y.; Jeong, Y.-J.; Kang, H.H.; Kang, J.Y.; Lee, S.-S.; Seo, M.; et al. Cluster analysis categorizes five phenotypes of pulmonary tuberculosis. Sci. Rep. 2022, 12, 10084. [Google Scholar] [CrossRef]
- Stein, C.M.; Baker, A.R. Tuberculosis as a complex trait: Impact of genetic epidemiological study design. Mamm. Genome 2010, 22, 91–99. [Google Scholar] [CrossRef]
- Bruiners, N.; Schurz, H.; Daya, M.; Salie, M.; van Helden, P.D.; Kinnear, C.J.; Hoal, E.G.; Möller, M.; van Pittius, N.C.G. A regulatory variant in the C1Q gene cluster is associated with tuberculosis susceptibility and C1qA plasma levels in a South African population. Immunogenetics 2020, 72, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Areeshi, M.Y.; Mandal, R.K.; Dar, S.A.; Jawed, A.; Wahid, M.; Lohani, M.; Panda, A.K.; Mishra, B.N.; Akhter, N.; Haque, S. IL-10-1082 A > G (rs1800896) polymorphism confers susceptibility to pulmonary tuberculosis in Caucasians but not in Asians and Africans: A meta-analysis. Biosci. Rep. 2017, 37. [Google Scholar] [CrossRef] [PubMed]
- Lyadova, I.V.; Tsiganov, E.N.; Kapina, M.A.; Shepelkova, G.S.; Sosunov, V.V.; Radaeva, T.V.; Majorov, K.B.; Shmitova, N.S.; Ham, H.-J.V.D.; Ganusov, V.V.; et al. In mice, tuberculosis progression is associated with intensive inflammatory response and the accumulation of Gr-1dim cells in the lungs. PLoS ONE 2010, 5, e10469. [Google Scholar] [CrossRef]
- Stepanikova, I.; Bateman, L.B.; Oates, G.R. Systemic Inflammation in Midlife: Race, Socioeconomic Status, and Perceived Discrimination. Am. J. Prev. Med. 2017, 52, S63–S76. [Google Scholar] [CrossRef]
- Sobhy, S.; Babiker, Z.; Zamora, J.; Khan, K.; Kunst, H. Maternal and perinatal mortality and morbidity associated with tuberculosis during pregnancy and the postpartum period: A systematic review and meta-analysis. BJOG Int. J. Obstet. Gynaecol. 2016, 124, 727–733. [Google Scholar] [CrossRef]
- Motulsky, A.G. Metabolic polymorphisms and the role of infectious diseases in human evolution. Human Biol. 1960, 32, 28–62. Available online: http://www.jstor.org/stable/41448424 (accessed on 24 September 2025).
- Kerner, G.; Laval, G.; Patin, E.; Boisson-Dupuis, S.; Abel, L.; Casanova, J.-L.; Quintana-Murci, L. Human ancient DNA analyses reveal the high burden of tuberculosis in Europeans over the last 2000 years. Am. J. Human Genet. 2021, 108, 517–524. [Google Scholar] [CrossRef]
- Gorman, J.A.; Hundhausen, C.; Kinsman, M.; Arkatkar, T.; Allenspach, E.J.; Clough, C.; West, S.E.; Thomas, K.; Eken, A.; Khim, S.; et al. The Tyk2-p1104A autoimmune protective variant limits coordinate signals required to generate specialized t cell subsets. Front. Immunol. 2019, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Boisson-Dupuis, S.; Bustamante, J. Mycobacterial diseases in patients with inborn errors of immunity. Curr. Opin. Immunol. 2021, 72, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Peñafiel Vicuña, A.K.; Yamazaki Nakashimada, M.; León Lara, X.; Mendieta Flores, E.; Nuñez Núñez, M.E.; Lona-Reyes, J.C.; Nieto, L.H.; Vázquez, M.G.R.; Santos, J.B.; Iñiguez, Á.L.; et al. Mendelian Susceptibility to Mycobacterial Disease: Retrospective Clinical and Genetic Study in Mexico. J. Clin. Immunol. 2022, 43, 123–135. [Google Scholar] [CrossRef]
- Bustamante, J. Mendelian susceptibility to mycobacterial disease: Recent discoveries. Human Genet. 2020, 139, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.W.; Mak, T.S.-H.; O’Reilly, P.F. Tutorial: A guide to performing polygenic risk score analyses. Nat. Protoc. 2020, 15, 2759–2772. [Google Scholar] [CrossRef]
- Kachuri, L.; Chatterjee, N.; Hirbo, J.; Schaid, D.J.; Martin, I.; Kullo, I.J.; Kenny, E.E.; Pasaniuc, B.; Witte, J.S.; Ge, T. Principles and methods for transferring polygenic risk scores across global populations. Nat. Rev. Genet. 2023, 25, 8–25. [Google Scholar] [CrossRef]
- Khera, A.V.; Chaffin, M.; Aragam, K.G.; Haas, M.E.; Roselli, C.; Choi, S.H.; Natarajan, P.; Lander, E.S.; Lubitz, S.A.; Ellinor, P.T.; et al. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat. Genet. 2018, 50, 1219–1224. [Google Scholar] [CrossRef]
- Lennon, N.J.; Kottyan, L.C.; Kachulis, C.; Abul-Husn, N.S.; Arias, J.; Belbin, G.; Below, J.E.; Berndt, S.I.; Chung, W.K.; Cimino, J.J.; et al. Selection, optimization and validation of ten chronic disease polygenic risk scores for clinical implementation in diverse US populations. Nat. Med. 2024, 30, 480–487. [Google Scholar] [CrossRef]
- Herold, K.C.; Gitelman, S.E.; Gottlieb, P.A.; Knecht, L.A.; Raymond, R.; Ramos, E.L. Teplizumab: A Disease-Modifying Therapy for Type 1 Diabetes That Preserves β-Cell Function. Diabetes Care 2023, 46, 1848–1856. [Google Scholar] [CrossRef]
- Akil, A.A.-S.; Yassin, E.; Al-Maraghi, A.; Aliyev, E.; Al-Malki, K.; Fakhro, K.A. Diagnosis and treatment of type 1 diabetes at the dawn of the personalized medicine era. J. Transl. Med. 2021, 19, 137. [Google Scholar] [CrossRef] [PubMed]
- Sharp, S.A.; Rich, S.S.; Wood, A.R.; Jones, S.E.; Beaumont, R.N.; Harrison, J.W.; Schneider, D.A.; Locke, J.M.; Tyrrell, J.; Weedon, M.N.; et al. Development and standardization of an improved type 1 diabetes genetic risk score for use in newborn screening and incident diagnosis. Diabetes Care 2019, 42, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-R.; Chen, Y.-L.; Wu, C.-W.; Chen, L.-C.; Chang, L.-Y.; Chen, J.-Y.; Huang, Y.-T.; Wang, J.-Y.; Shih, J.-Y.; Yu, C.-J. Toll-like receptor and matrix metalloproteinase single-nucleotide polymorphisms, haplotypes, and polygenic risk score differentiated between tuberculosis disease and infection. Int. J. Infect. Dis. 2022, 125, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Bodro, M.; Cervera, C.; Linares, L.; Suárez, B.; Llopis, J.; Sanclemente, G.; Casadó-Llombart, S.; Fernández-Ruiz, M.; Fariñas, M.C.; Cantisan, S.; et al. Polygenic Innate Immunity Score to Predict the Risk of Cytomegalovirus Infection in CMV D+/R- Transplant Recipients. A Prospective Multicenter Cohort Study. Front. Immunol. 2022, 13, 897912. [Google Scholar] [CrossRef]
- Adebamowo, S.N.; Adeyemo, A.; Adebayo, A.; Achara, P.; Alabi, B.; Bakare, R.A.; Famooto, A.O.; Obende, K.; Offiong, R.; Olaniyan, O.; et al. Genome, HLA and polygenic risk score analyses for prevalent and persistent cervical human papillomavirus (HPV) infections. Eur. J. Human Genet. 2024, 32, 708–716. [Google Scholar] [CrossRef]
- Pare, G.; Neupane, B.; Eskandarian, S.; Harris, E.; Halstead, S.; Gresh, L.; Kuan, G.; Balmaseda, A.; Villar, L.; Rojas, E.; et al. Genetic risk for dengue hemorrhagic fever and dengue fever in multiple ancestries. EBioMedicine 2020, 51, 102584. [Google Scholar] [CrossRef]
- Domdom, M.-A.; Brest, P.; Grosjean, I.; Roméo, B.; Landi, M.T.; Gal, J.; Klionsky, D.J.; Hofman, P.; Mograbi, B. A multifactorial score including autophagy for prognosis and care of COVID-19 patients. Autophagy 2020, 16, 2276–2281. [Google Scholar] [CrossRef]
- Ben-Moshe, N.B.; Hen-Avivi, S.; Levitin, N.; Yehezkel, D.; Oosting, M.; Joosten, L.A.B.; Netea, M.G.; Avraham, R. Predicting bacterial infection outcomes using single cell RNA-sequencing analysis of human immune cells. Nat. Commun. 2019, 10, 3266. [Google Scholar] [CrossRef]
- Vilhjálmsson, B.J.; Yang, J.; Finucane, H.K.; Gusev, A.; Lindström, S.; Ripke, S.; Genovese, G.; Loh, P.-R.; Bhatia, G.; Do, R.; et al. Modeling Linkage Disequilibrium Increases Accuracy of Polygenic Risk Scores. Am. J. Human Genet. 2015, 97, 576–592. [Google Scholar] [CrossRef] [PubMed]
- Mak, T.S.H.; Porsch, R.M.; Choi, S.W.; Zhou, X.; Sham, P.C. Polygenic scores via penalized regression on summary statistics. Genet. Epidemiol. 2017, 41, 469–480. [Google Scholar] [CrossRef] [PubMed]
| Cancer | 746 PGS |
| Cardiovascular disease | 413 PGS |
| Digestive system disorder | 430 PGS |
| Immune system disorder | 232 PGS |
| Infectious disease | 8 PGS (https://www.pgscatalog.org/trait/EFO_0005741/) (accessed on 1 August 2025) |
| COVID-19 | 3 PGS |
| Tuberculosis | 0 PGS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dashian, M.A.; Shipulin, G.A.; Deviatkin, A.A. Genetic Susceptibility to Tuberculosis and the Utility of Polygenic Scores in Population Stratification. Int. J. Mol. Sci. 2025, 26, 9544. https://doi.org/10.3390/ijms26199544
Dashian MA, Shipulin GA, Deviatkin AA. Genetic Susceptibility to Tuberculosis and the Utility of Polygenic Scores in Population Stratification. International Journal of Molecular Sciences. 2025; 26(19):9544. https://doi.org/10.3390/ijms26199544
Chicago/Turabian StyleDashian, Mariia A., German A. Shipulin, and Andrei A. Deviatkin. 2025. "Genetic Susceptibility to Tuberculosis and the Utility of Polygenic Scores in Population Stratification" International Journal of Molecular Sciences 26, no. 19: 9544. https://doi.org/10.3390/ijms26199544
APA StyleDashian, M. A., Shipulin, G. A., & Deviatkin, A. A. (2025). Genetic Susceptibility to Tuberculosis and the Utility of Polygenic Scores in Population Stratification. International Journal of Molecular Sciences, 26(19), 9544. https://doi.org/10.3390/ijms26199544





