Estimating the Contribution of Renal Function to Endothelial Dysfunction and Subclinical Inflammation with a Two-Cohort Study: Living Kidney Donors and Their Transplant Recipients
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
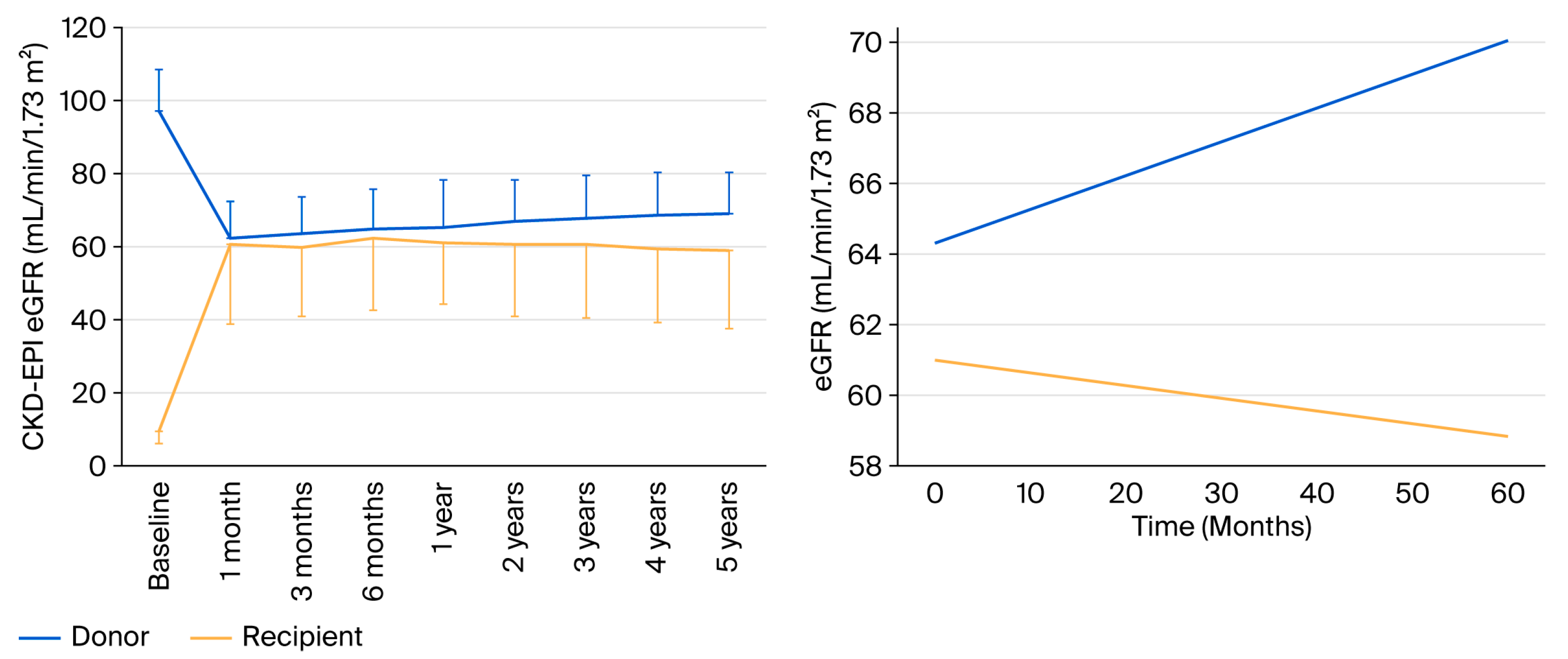
2.2. Renal Function at One Year
2.3. Endothelial Dysfunction and Inflammation Biomarkers
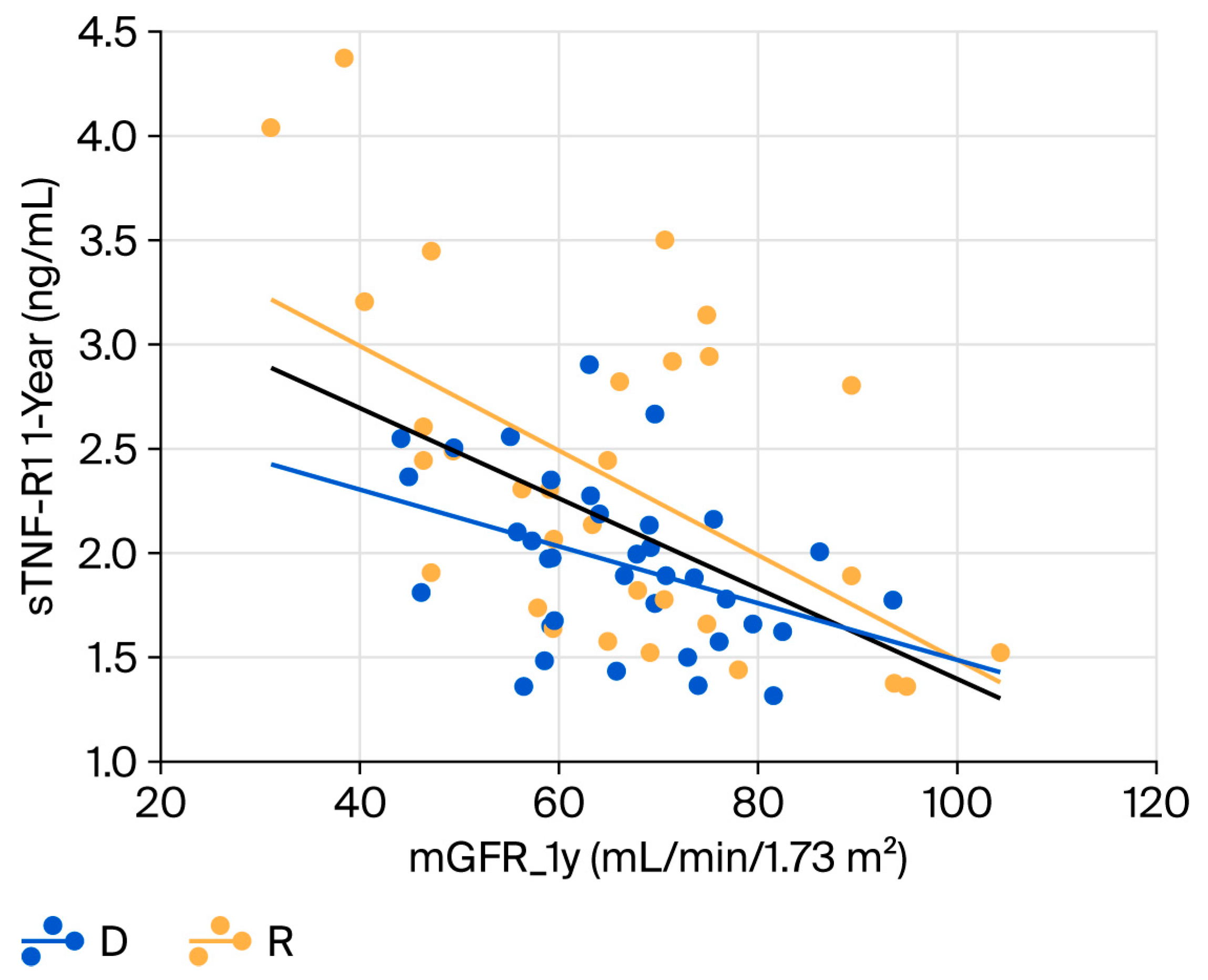
2.4. Relationship Between Renal Function and Biomarkers
2.5. Blood Pressure and Atherosclerotic Burden in the Donor Cohort
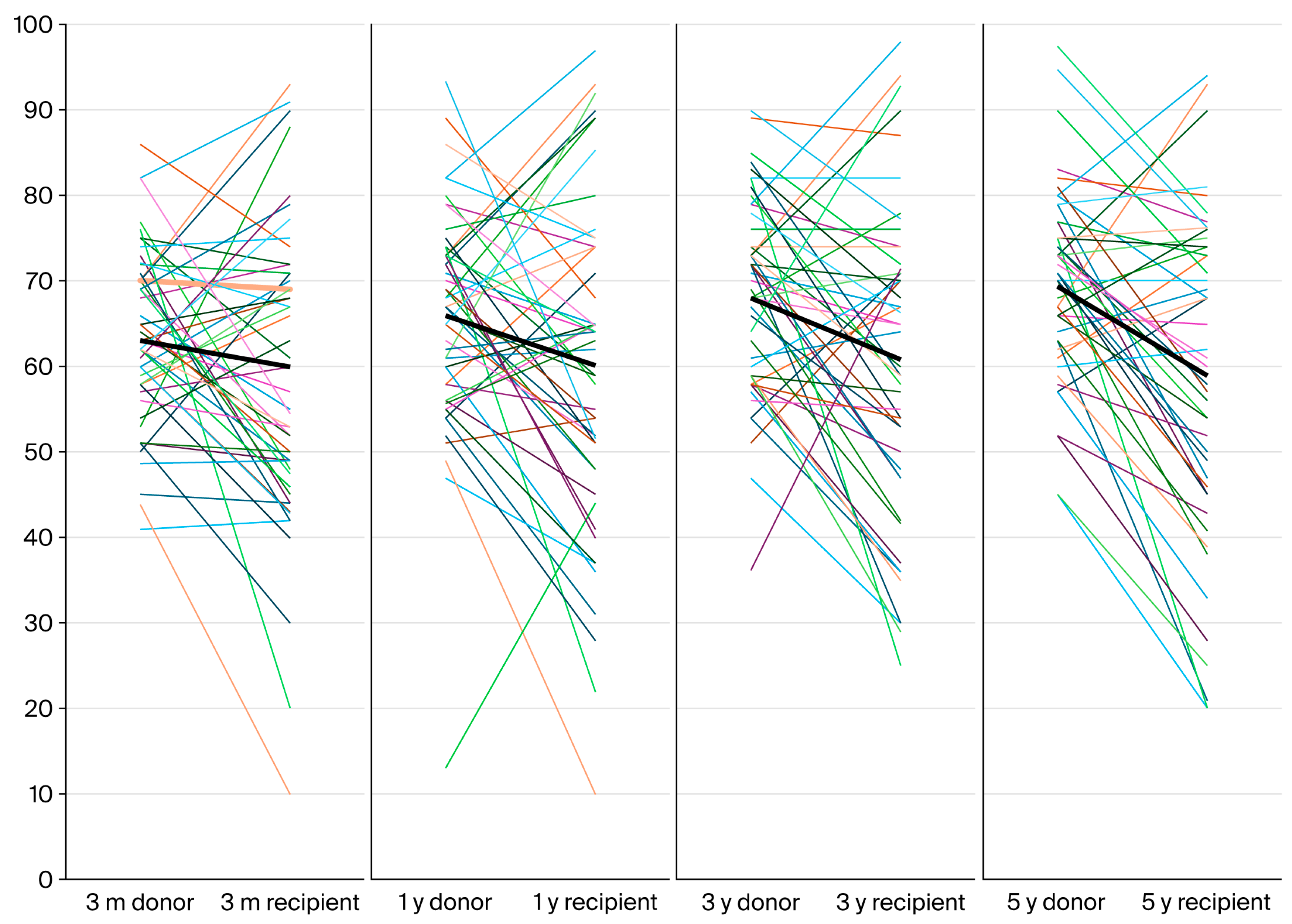
2.6. Follow-Up at 5 Years
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Patients
4.3. Study Procedures
- Glomerular filtration rate before nephrectomy in donors and at one year in donors and recipients was measured by iohexol clearance (mGFR). The protocol for the study was guided by Instituto de Tecnologías Biomédicas, University of La Laguna (Tenerife, Spain) [41]. Iohexol levels in plasma samples obtained at each center were sent to this validated lab for their determination by HPLC [29]. Results of mGFR were not available to clinicians at the time of kidney donation. Additionally, the estimated glomerular filtration rate by CKD-EPI (eGFR-CKD-EPI) and MDRD-4 (eGFR-MDRD-4) using standard formulas was employed to estimate renal function in both cohorts.
- Urinary albumin to creatinine ratio (UACR) in an early-morning spot sample was determined locally by an immunoturbidimetric assay.
- An oral glucose tolerance test (OGTT) was performed at baseline and at 1 year in the donors. Insulin levels were also determined locally at both time periods to calculate the HOMA-IR.
- Serum samples for the measurement of endothelial dysfunction and low-grade inflammation markers were obtained and stored at each center. At the end of the study, all samples were sent to Hospital Universitari Vall d’Hebron laboratories (Barcelona) for their determination.
- In the donor cohort the following procedures were also performed:
- Ambulatory blood pressure monitoring (ABPM) with an overnight-automated monitor (Spacelab 90207; Spacelabs Healthcare, Snoqualmie, WA, USA) with appropriate cuff sizes for each patient was performed at baseline and at one year.
- Baseline atherosclerotic burden:A carotid ultrasound to determine the number of plaques and intima-media thickness (IMT) was performed in both carotid arteries with a high-frequency (8–12 MHz) linear transducer (ESAOTE, 7300, Florence, Italy). The numbers of carotid plaques in both arteries were added and the mean intima-media thickness (IMT) of both arteries was calculated.Carotid–femoral pulse wave velocity (PWV, m/s) was measured by pulse tonometry (Sphingmocor Atcor, EM3, Sidney, Australia).The ankle–brachial index (ABI) was determined by an automated blood pressure monitor with appropriate cuff sizes (Omron, Kyoto, Japan).
4.4. Treatments
4.5. Biomarkers of Endothelial Dysfunction and Chronic Inflammation
- Endothelial dysfunction: Circulating levels of soluble VCAM (vascular cell adhesion molecule), soluble ICAM (intercellular adhesion molecule), soluble E-selectin and PTX-3 (pentraxin) were determined by the microfluidics-based quantitative immunoassay, ELLA® (Protein Simple, CA, USA) [42]. The serum concentration of PECAM (platelet/endothelial cell adhesion molecule) was determined by ELISA (Novus Biologicals, CO, USA). Determination of vWF (antigen of von Willebrand factor) serum levels was performed on an AcuStar instrument (Instrumentation Laboratories, Bedford, MA, USA), by using the HemosIL AcuStar VWF:Ag chemiluminescent cartridge reagent kit [43].
- Chronic inflammation: usPCR (ultrasensitive C-reactive protein) was determined by nephelometry. Circulating levels of IL-6 (interleukin 6), sTNFR1 and sTNFR2 (soluble tumor necrosis factor receptors 1 and 2) were determined using the microfluidics-based quantitative immunoassay, ELLA® (Protein Simple, CA, USA) [42]. The serum concentration of sTWEAK (soluble TNF-like weak inducer of apoptosis) was determined by ELISA (DuoSet, Minneapolis, MN, USA) [44].
4.6. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Best, P.J.M.; Reddan, D.N.; Berger, P.B.; Szczech, L.A.; McCullough, P.A.; Califf, R.M. Cardiovascular disease and chronic kidney disease: Insights and an update. Am. Heart J. 2004, 148, 230–242. [Google Scholar] [CrossRef] [PubMed]
- KDIGO_2012_CKD_GL.pdf. Available online: https://kdigo.org/wp-content/uploads/2017/02/KDIGO_2012_CKD_GL.pdf (accessed on 11 April 2022).
- Matsushita, K.; Van Der Velde, M.; Astor, B.C.; Woodward, M.; Levey, A.S.; De Jong, P.E.; Coresh, J.; Gansevoort, R.T. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 2010, 375, 2073–2081. [Google Scholar] [CrossRef]
- Ninomiya, T.; Kiyohara, Y.; Tokuda, Y.; Doi, Y.; Arima, H.; Harada, A.; Ohashi, Y.; Ueshima, H. Impact of kidney disease and blood pressure on the development of cardiovascular disease: An overview from the Japan Arteriosclerosis Longitudinal Study. Circulation 2008, 118, 2694–2701. [Google Scholar] [CrossRef]
- Van Biesen, W.; De Bacquer, D.; Verbeke, F.; Delanghe, J.; Lameire, N.; Vanholder, R. The glomerular filtration rate in an apparently healthy population and its relationship with cardiovascular mortality during 10 years. Eur. Heart J. 2007, 28, 478–483. [Google Scholar] [CrossRef]
- Kang, M.; Kwon, S.; Lee, J.; Shin, J.-I.; Kim, Y.C.; Park, J.Y.; Bae, E.; Kim, E.Y.; Kim, D.K.; Lim, C.S.; et al. Albuminuria within the Normal Range Can Predict All-Cause Mortality and Cardiovascular Mortality. Kidney360 2022, 3, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Moody, W.E.; Edwards, N.C.; Madhani, M.; Chue, C.D.; Steeds, R.P.; Ferro, C.J.; Townend, J.N. Endothelial dysfunction and cardiovascular disease in early-stage chronic kidney disease: Cause or association? Atherosclerosis 2012, 223, 86–94. [Google Scholar] [CrossRef]
- Moody, W.E.; Chue, C.D.; Inston, N.G.; Edwards, N.C.; Steeds, R.P.; Ferro, C.J.; Townend, J.N. Understanding the effects of chronic kidney disease on cardiovascular risk: Are there lessons to be learnt from healthy kidney donors? J. Hum. Hypertens. 2012, 26, 141–148. [Google Scholar] [CrossRef]
- Naganuma, T.; Takemoto, Y.; Taiyou, O.; Hirokazu, T.; Masaki, M.; Maeda, S.; Nakatani, T. Risk of cardiovascular disease in kidney donors as a chronic kidney disease cohort. Mol. Med. Rep. 2012, 5, 7–11. [Google Scholar] [CrossRef]
- Wolfe, R.A.; Ashby, V.B.; Milford, E.L.; Ojo, A.O.; Ettenger, R.E.; Agodoa, L.Y.; Held, P.J.; Port, F.K. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N. Engl. J. Med. 1999, 341, 1725–1730. [Google Scholar] [CrossRef]
- Arend, S.M.; Mallat, M.J.; Westendorp, R.J.; van der Woude, F.J.; van Es, L.A. Patient survival after renal transplantation; more than 25 years follow-up. Nephrol. Dial. Transplant. 1997, 12, 1672–1679. [Google Scholar] [CrossRef]
- Garg, A.X.; Muirhead, N.; Knoll, G.; Yang, R.C.; Prasad, G.V.R.; Thiessen-Philbrook, H.; Rosas-Arellano, M.; Housawi, A.; Boudville, N. Proteinuria and reduced kidney function in living kidney donors: A systematic review, meta-analysis, and meta-regression. Kidney Int. 2006, 70, 1801–1810. [Google Scholar] [CrossRef] [PubMed]
- Boudville, N.; Prasad, G.V.R.; Knoll, G.; Muirhead, N.; Thiessen-Philbrook, H.; Yang, R.C.; Rosas-Arellano, M.P.; Housawi, A.; Garg, A.X. Meta-analysis: Risk for hypertension in living kidney donors. Ann. Intern. Med. 2006, 145, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, F.; Cervantes, C.E.; Crews, D.C.; Blanck, J.; Al Ammary, F.; Ng, D.K.; Purnell, T.S. Examining Post-Donation Outcomes in Hispanic/Latinx Living Kidney Donors in the United States: A Systematic Review. Am. J. Transplant. 2022, 22, 1737–1753. [Google Scholar] [CrossRef] [PubMed]
- Bellos, I.; Pergialiotis, V. Risk of pregnancy complications in living kidney donors: A systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 270, 35–41. [Google Scholar] [CrossRef]
- Grupper, A.; Angel, Y.; Baruch, A.; Schwartz, I.F.; Schwartz, D.; Nakache, R.; Goykhman, Y.; Katz, P.; Nachmany, I.; Lubezky, N.; et al. Long term metabolic and renal outcomes of kidney donors compared to controls with excellent kidney function. BMC Nephrol. 2019, 20, 30. [Google Scholar] [CrossRef]
- Lee, H.; Eum, S.H.; Ko, E.J.; Cho, H.J.; Yang, C.W.; Chung, B.H. Alterations in the Mineral Bone Metabolism of Living Kidney Donors After Uni-Nephrectomy: Prospective Observational Study. Front. Med. 2021, 8, 741944. [Google Scholar] [CrossRef]
- Segev, D.L.; Muzaale, A.D.; Caffo, B.S.; Mehta, S.H.; Singer, A.L.; Taranto, S.E.; McBride, M.A.; Montgomery, R.A. Perioperative mortality and long-term survival following live kidney donation. JAMA 2010, 303, 959–966. [Google Scholar] [CrossRef]
- Garg, A.X.; Meirambayeva, A.; Huang, A.; Kim, J.; Prasad, G.V.R.; Knoll, G.; Boudville, N.; Lok, C.; McFarlane, P.; Karpinski, M.; et al. Cardiovascular disease in kidney donors: Matched cohort study. BMJ 2012, 344, e1203. [Google Scholar] [CrossRef]
- Mjøen, G.; Hallan, S.; Hartmann, A.; Foss, A.; Midtvedt, K.; Øyen, O.; Reisæter, A.; Pfeffer, P.; Jenssen, T.; Leivestad, T.; et al. Long-term risks for kidney donors. Kidney Int. 2014, 86, 162–167, Erratum in Kidney Int. 2015, 88, 1452. [Google Scholar] [CrossRef]
- Mjoen, G.; Reisaeter, A.; Hallan, S.; Line, P.-D.; Hartmann, A.; Midtvedt, K.; Foss, A.; Dahle, D.O.; Holdaas, H. Overall and cardiovascular mortality in Norwegian kidney donors compared to the background population. Nephrol. Dial. Transplant. 2012, 27, 443–447. [Google Scholar] [CrossRef]
- Haugen, A.J.; Hallan, S.; Langberg, N.E.; Dahle, D.O.; Pihlstrøm, H.; Birkeland, K.I.; Reisæter, A.V.; Midtvedt, K.; Hartmann, A.; Holdaas, H.; et al. Increased risk of ischemic heart disease after kidney donation. Nephrol. Dial. Transplant. 2022, 37, 928–936. [Google Scholar] [CrossRef]
- Schiffrin, E.L.; Lipman, M.L.; Mann, J.F.E. Chronic kidney disease: Effects on the cardiovascular system. Circulation 2007, 116, 85–97. [Google Scholar] [CrossRef]
- Endemann, D.H.; Schiffrin, E.L. Endothelial dysfunction. J. Am. Soc. Nephrol. 2004, 15, 1983–1992. [Google Scholar] [CrossRef]
- Zhang, J. Biomarkers of endothelial activation and dysfunction in cardiovascular diseases. Rev. Cardiovasc. Med. 2022, 23, 73. [Google Scholar] [CrossRef] [PubMed]
- Mihai, S.; Codrici, E.; Popescu, I.D.; Enciu, A.-M.; Albulescu, L.; Necula, L.G.; Mambet, C.; Anton, G.; Tanase, C. Inflammation-Related Mechanisms in Chronic Kidney Disease Prediction, Progression, and Outcome. J. Immunol. Res. 2018, 2018, 2180373. [Google Scholar] [CrossRef] [PubMed]
- Valentín, M.O.; Hernández, D.; Crespo, M.; Mahillo, B.; Beneyto, I.; Martínez, I.; Kanter, J.; Calderari, E.; Gil-Vernet, S.; Sánchez, S.; et al. Live donor kidney transplantation. Situation analysis and roadmap. Nefrologia 2021, 42, 85–93. [Google Scholar] [CrossRef]
- Garg, N.; Poggio, E.D.; Mandelbrot, D. The Evaluation of Kidney Function in Living Kidney Donor Candidates. Kidney360 2021, 2, 1523–1530. [Google Scholar] [CrossRef] [PubMed]
- González-Rinne, A.; Luis-Lima, S.; Escamilla, B.; Negrín-Mena, N.; Ramírez, A.; Morales, A.; Vega, N.; García, P.; Cabello, E.; Marrero-Miranda, D.; et al. Impact of errors of creatinine and cystatin C equations in the selection of living kidney donors. Clin. Kidney J. 2019, 12, 748–755. [Google Scholar] [CrossRef]
- Caballo, C.; Palomo, M.; Cases, A.; Galán, A.M.; Molina, P.; Vera, M.; Bosch, X.; Escolar, G.; Diaz-Ricart, M. NFκB in the Development of Endothelial Activation and Damage in Uremia: An In Vitro Approach. PLoS ONE 2012, 7, e43374. [Google Scholar] [CrossRef]
- Ristagno, G.; Fumagalli, F.; Bottazzi, B.; Mantovani, A.; Olivari, D.; Novelli, D.; Latini, R. Pentraxin 3 in Cardiovascular Disease. Front. Immunol. 2019, 10, 823. [Google Scholar] [CrossRef]
- van Gennip, A.C.E.; Broers, N.J.H.; ter Meulen, K.J.; Canaud, B.; Christiaans, M.H.L.; Cornelis, T.; Gelens, M.A.C.J.; Hermans, M.M.H.; Konings, C.J.A.M.; van der Net, J.B.; et al. Endothelial dysfunction and low-grade inflammation in the transition to renal replacement therapy. PLoS ONE 2019, 14, e0222547. [Google Scholar] [CrossRef]
- Troncoso, M.F.; Ortiz-Quintero, J.; Garrido-Moreno, V.; Sanhueza-Olivares, F.; Guerrero-Moncayo, A.; Chiong, M.; Castro, P.F.; García, L.; Gabrielli, L.; Corbalán, R.; et al. VCAM-1 as a predictor biomarker in cardiovascular disease. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2021, 1867, 166170. [Google Scholar] [CrossRef]
- Chen, J.; Hamm, L.L.; Mohler, E.R.; Hudaihed, A.; Arora, R.; Chen, C.S.; He, J. Interrelationship of Multiple Endothelial Dysfunction Biomarkers with Chronic Kidney Disease. PLoS ONE 2015, 10, e0132047. [Google Scholar]
- Yilmaz, B.A.; Caliskan, Y.; Yilmaz, A.; Ozkok, A.; Bilge, A.K.; Deniz, G. Cardiovascular-Renal Changes After Kidney Donation One-year Follow-up Study. Transplantation 2015, 99, 760–764. [Google Scholar] [CrossRef]
- Zinellu, A.; Mangoni, A.A. A systematic review and meta-analysis of the effect of statin treatment on sVCAM-1 and sICAM-1. Expert Rev. Clin. Pharmacol. 2022, 15, 601–620. [Google Scholar] [CrossRef]
- Bhatraju, P.K.; Zelnick, L.R.; Shlipak, M.; Katz, R.; Kestenbaum, B. Association of Soluble TNFR-1 Concentrations with Long-Term Decline in Kidney Function: The Multi-Ethnic Study of Atherosclerosis. J. Am. Soc. Nephrol. 2018, 29, 2713–2721. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.K.; Coca, S.G.; Estrella, M.M.; Appel, L.J.; Coresh, J.; Philbrook, H.T.; Obeid, W.; Fried, L.F.; Heerspink, H.J.; Ix, J.H.; et al. Longitudinal TNFR1 and TNFR2 and Kidney Outcomes: Results from AASK and VA NEPHRON-D. J. Am. Soc. Nephrol. 2022, 33, 996–1010. [Google Scholar] [CrossRef] [PubMed]
- Gentil-Govantes, M.A.; Pereira-Palomo, P. Assessing and selecting a living kidney donor. Nefrologia 2010, 30 (Suppl. S2), 47–59. [Google Scholar]
- Andrés, A. Indications and contraindications of living-donor kidney transplantation. Nefrologia 2010, 30 (Suppl. S2), 30–38. [Google Scholar] [PubMed]
- Luis-Lima, S.; Gaspari, F.; Porrini, E.; García-González, M.; Batista, N.; Bosa-Ojeda, F.; Oramas, J.; Carrara, F.; González-Posada, J.M.; Marrero, D.; et al. Measurement of glomerular filtration rate: Internal and external validations of the iohexol plasma clearance technique by HPLC. Clin. Chim. Acta 2014, 430, 84–85. [Google Scholar] [CrossRef]
- Leligdowicz, A.; Conroy, A.L.; Hawkes, M.; Zhong, K.; Lebovic, G.; Matthay, M.A.; Kain, K.C. Validation of two multiplex platforms to quantify circulating markers of inflammation and endothelial injury in severe infection. PLoS ONE 2017, 12, e0175130. [Google Scholar] [CrossRef] [PubMed]
- Verfaillie, C.J.; De Witte, E.; Devreese, K.M.J. Validation of a new panel of automated chemiluminescence assays for von Willebrand factor antigen and activity in the screening for von Willebrand disease. Int. J. Lab. Hematol. 2013, 35, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Dereke, J.; Nilsson, J.; Nilsson, C.; Strevens, H.; Landin-Olsson, M.; Hillman, M. Soluble CD163 and TWEAK in early pregnancy gestational diabetes and later glucose intolerance. PLoS ONE 2019, 14, e0216728. [Google Scholar] [CrossRef] [PubMed]


| Variable | Donors | Recipients |
|---|---|---|
| Age (years) | 52 ± 12 | 48 ± 14 |
| Sex (male/female) | 20/30 | 29/21 |
| Race (Caucasian/Hispanic) | 46/4 | 47/3 |
| Height (cm) | 165 ± 9 | 168 ± 10 |
| Weight (kg) | 72 ± 13 | 74 ± 16 |
| BMI (kg/m2) | 26.0 ± 3.8 | 26.2 ± 5.2 |
| Hypertension (no/yes) | 42/8 | 7/43 |
| Diabetes (no/yes) | 50/0 | 44/6 |
| Smoker (never/past/current) | 32/8/10 | 30/14/6 |
| Office systolic blood pressure (mm Hg) | 126.4 ± 14.8 | 137.6 ± 17.9 |
| Office diastolic blood pressure (mm Hg) | 73.2 ± 9.4 | 83.1 ± 12.4 |
| Mean office blood pressure (mm Hg) | 91.0 ± 10.0 | 101.3 ± 13.0 |
| Serum glucose (mg/dL) | 91 ± 6 | 125 ± 42 |
| Total cholesterol (mg/dL) | 219 ± 41 | 194 ± 38 |
| LDL cholesterol (mg/dL) | 140 ± 36 | 125 ± 35 |
| Triglycerides (mg/dL) | 113 ± 37 | 109 ± 68 |
| Cause of ESRD | ||
| GN/CTIN/ADPKD/DN/vascular/others | 12/9/7/3/5/14 | |
| Pre-emptive/HD/PD | 30/15/5 | |
| Time on RRT (mo.) | 11 ± 4 |
| Donors | Recipients | |||
|---|---|---|---|---|
| Baseline | 1 Year | Baseline | 1 Year | |
| Creatinine (mg/dL) | 0.75 ± 0.15 | 1.08 ± 0.22 | 6.01 ± 2.62 | 1.36 ± 0.41 |
| eGFR CKD-EPI | 98 ± 13 | 66 ± 11 | 10 ± 4 | 64 ± 17 |
| eGFR MDRD-4 | 95 ± 16 | 61 ± 10 | 10 ± 4 | 56 ± 15 |
| mGFR | 93 ± 17 | 65 ± 12 | n.a. | 57 ± 13 |
| Donors | Recipients | |||
|---|---|---|---|---|
| Baseline | 1 Year | Baseline | 1 Year | |
| VCAM-1 [ng/mL] | 635 ± 197 | 718 ± 213 * | 1208 ± 417 | 943 ± 313 * |
| ICAM-1 [ng/mL] | 382 ± 91 | 407 ± 101 | 430± 159 | 433 ± 109 |
| E-selectin [ng/mL] | 29 ± 10 | 30 ± 11 | 37 ± 19 | 34 ± 14 ** |
| PECAM-1 [ng/mL] | 73 ± 13 | 80 ± 14 | 79 ± 23 | 71 ±15 * |
| vWF [%] | 96 ± 36 | 103 ± 35 | 181 ± 53 | 167 ± 57 |
| PTX-3 [ng/mL] | 2.7 ± 1.8 | 3.4 ± 2.1 | 3.7 ± 3.1 | 3.0 ± 2.3 |
| UACR [mg/g] | 5.9 ± 6.3 | 10.5 ± 24.0 | - | - |
| IL-6 [pg/mL] | 4.6 ± 5.7 | 6.1 ± 10.8 | 7.7 ± 9.0 | 6.1 ± 3.6 |
| TNFR1 [ng/mL] | 1.4 ± 0.9 | 1.9 ± 0.4 * | 10.2 ± 5.6 | 2.4 ± 0.8 * |
| TNFR2 [ng/mL] | 2.8 ± 1.2 | 3.8 ± 0.7 * | 12.2 ± 4.0 | 4.8 ± 1.9 * |
| TWEAK [pg/mL] | 544 ± 499 | 493 ± 100 | 437 ± 107 | 465 ± 122 |
| hsCRP [mg/dL] | 0.36 ± 1.04 | 0.31 ± 0.39 | 0.41 ± 0.69 | 0.36 ± 0.41 |
| Time Point | Office BP | ABPM-Day | ABPM-Night | ABPM-Pattern | |||
|---|---|---|---|---|---|---|---|
| SBP | DBP | SBP | DPB | SBP | DPB | Non-Dipper (%) | |
| Baseline | 124 ± 15 | 76 ± 9 | 122 ± 13 | 76 ± 9 | 109 ± 11 | 66 ± 8 | 18% |
| 1 year | 129 ± 15 | 77 ± 10 | 120 ± 10 | 77 ± 8 | 108 ± 9 | 66 ± 8 | 34% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres, I.B.; Burballa, C.; González-Posada, J.M.; Hernández, D.; Porrini, E.; Perurena, J.; Cortina, V.; Perelló, M.; Redondo-Pachón, D.; González-Rine, A.; et al. Estimating the Contribution of Renal Function to Endothelial Dysfunction and Subclinical Inflammation with a Two-Cohort Study: Living Kidney Donors and Their Transplant Recipients. Int. J. Mol. Sci. 2025, 26, 9535. https://doi.org/10.3390/ijms26199535
Torres IB, Burballa C, González-Posada JM, Hernández D, Porrini E, Perurena J, Cortina V, Perelló M, Redondo-Pachón D, González-Rine A, et al. Estimating the Contribution of Renal Function to Endothelial Dysfunction and Subclinical Inflammation with a Two-Cohort Study: Living Kidney Donors and Their Transplant Recipients. International Journal of Molecular Sciences. 2025; 26(19):9535. https://doi.org/10.3390/ijms26199535
Chicago/Turabian StyleTorres, Irina B., Carla Burballa, José M. González-Posada, Domingo Hernández, Esteban Porrini, Janire Perurena, Vicente Cortina, Manel Perelló, Dolores Redondo-Pachón, Ana González-Rine, and et al. 2025. "Estimating the Contribution of Renal Function to Endothelial Dysfunction and Subclinical Inflammation with a Two-Cohort Study: Living Kidney Donors and Their Transplant Recipients" International Journal of Molecular Sciences 26, no. 19: 9535. https://doi.org/10.3390/ijms26199535
APA StyleTorres, I. B., Burballa, C., González-Posada, J. M., Hernández, D., Porrini, E., Perurena, J., Cortina, V., Perelló, M., Redondo-Pachón, D., González-Rine, A., Cabello, M., Pérez-Sáez, M. J., Crespo, M., Bestard, O., Serón, D., & Moreso, F. (2025). Estimating the Contribution of Renal Function to Endothelial Dysfunction and Subclinical Inflammation with a Two-Cohort Study: Living Kidney Donors and Their Transplant Recipients. International Journal of Molecular Sciences, 26(19), 9535. https://doi.org/10.3390/ijms26199535








