Relationship Between Urinary Copper, Zinc, and Cadmium and Kidney Damage Biomarkers in Young People
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. General Evaluation
4.3. Biochemical Analysis
4.4. Biomarkers of Early Kidney Damage Assay
4.5. Determination of Cadmium, Mercury, Copper, and Zinc in Urine
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CKD | Chronic kidney disease |
| Cd | Cadmium |
| Hg | Mercury |
| Zn | Zinc |
| Cu | Copper |
| Cu/Zn-R | Copper–Zinc ratio |
| BMI | Body mass index |
| WtHr | waist-to-height ratio |
| ACR | Albumin–creatinine ratio |
| UCr | Urinary creatinine |
| eGFR | Estimated glomerular filtration rate |
References
- Jiang, C.; Ye, H.; Cui, L.; Pai, P.; Wang, G. Relationship of serum copper and zinc with kidney function and urinary albumin to creatinine ratio: Cross-sectional data from the NHANES 2011–2016. Eur. J. Clin. Nutr. 2022, 76, 1748–1754. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Glenda, C.; Ujjin, G.P.; David Vesey, A. Gender Differences in Zinc and Copper Excretion in Response to Co-Exposure to Low Environmental Concentrations of Cadmium and Lead. Stresses 2020, 1, 3–15. [Google Scholar] [CrossRef]
- Díaz de León-Martínez, L.; Ortega-Romero, M.; Gavilán-García, A.; Barbier, O.C.; Carrizalez-Yáñez, L.; Van-Brusel, E.; Díaz-Barriga, F.; Flores-Ramírez, R. Assessment of biomarkers of early kidney damage and exposure to pollutants in artisanal mercury mining workers from Mexico. Environ. Sci. Pollut. Res. 2022, 29, 13333–13343. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Romero, M.; Jiménez-Córdova, M.I.; Barrera-Hernández, Á.; Sepúlveda-González, M.E.; Narvaez-Morales, J.; Aguilar-Madrid, G.; Juárez-Pérez, C.A.; Del Razo, L.M.; Cruz-Angulo, M.D.C.; Mendez-Hernández, P.; et al. Relationship between urinary biomarkers of early kidney damage and exposure to inorganic toxins in a pediatric population of Apizaco, Tlaxcala, Mexico. J. Nephrol. 2023, 36, 1383–1393. [Google Scholar] [CrossRef] [PubMed]
- Weaver, V.M.; Vargas, G.G.; Silbergeld, E.K.; Rothenberg, S.J.; Fadrowski, J.J.; Rubio-Andrade, M.; Parsons, P.J.; Steuerwald, A.J.; Navas-Acien, A.; Guallar, E. Impact of urine concentration adjustment method on associations between urine metals and estimated glomerular filtration rates (eGFR) in adolescents. Environ. Res. 2014, 132, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Levin-Schwartz, Y.; Politis, M.D.; Gennings, C.; Tamayo-Ortiz, M.; Flores, D.; Amarasiriwardena, C.; Pantic, I.; Tolentino, M.C.; Estrada-Gutierrez, G.; Lamadrid-Figueroa, H.; et al. Nephrotoxic Metal Mixtures and Preadolescent Kidney Function. Children 2021, 8, 673. [Google Scholar] [CrossRef] [PubMed]
- Eom, S.Y.; Yim, D.H.; Huang, M.; Park, C.H.; Kim, G.B.; Yu, S.D.; Choi, B.S.; Park, J.D.; Kim, Y.D.; Kim, H. Copper-zinc imbalance induces kidney tubule damage and oxidative stress in a population exposed to chronic environmental cadmium. Int. Arch. Occup. Environ. Health 2020, 93, 337–344. [Google Scholar]
- Malavolta, M.; Piacenza, F.; Basso, A.; Giacconi, R.; Costarelli, L.; Mocchegiani, E. Serum copper to zinc ratio: Relationship with aging and health status. Mech. Ageing Dev. 2015, 151, 93–100. [Google Scholar] [PubMed]
- Kunutsor, S.K.; Voutilainen, A.; Laukkanen, J.A. Serum Copper-to-Zinc Ratio and Risk of Chronic Obstructive Pulmonary Disease: A Cohort Study. Lung 2023, 201, 79–84. [Google Scholar]
- Bengtsson, Y.; Demircan, K.; Vallon-Christersson, J.; Malmberg, M.; Saal, L.H.; Rydén, L.; Borg, Å.; Schomburg, L.; Sandsveden, M.; Manjer, J. Serum copper, zinc and copper/zinc ratio in relation to survival after breast cancer diagnosis: A prospective multicenter cohort study. Redox Biol. 2023, 63, 102728. [Google Scholar]
- Li, S.; Yang, C.; Yi, X.; Wei, R.; Aschner, M.; Jiang, Y.; Ou, S.; Yao, C. Effects of Sub-chronic Lead Exposure on Essential Element Levels in Mice. Biol. Trace Elem. Res. 2023, 201, 282–293. [Google Scholar] [CrossRef]
- Satarug, S. Dietary Cadmium Intake and Its Effects on Kidneys. Toxics 2018, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Baker, J.R.; Reilly, P.E.; Moore, M.R.; Williams, D.J. Changes in zinc and copper homeostasis in human livers and kidneys associated with exposure to environmental cadmium. Hum. Exp. Toxicol. 2001, 20, 205–213. [Google Scholar]
- Luo, L.; Xu, J.; Jiang, R.; Yao, B.; Di, J. Association between serum copper, zinc and their ratio and handgrip strength among adults: A study from National Health and Nutrition Examination Survey (NHANES) 2011–2014. Environ. Sci. Pollut. Res. Int. 2023, 30, 29100–29109. [Google Scholar] [PubMed]
- Takao, T.; Yanagisawa, H.; Suka, M.; Yoshida, Y.; Onishi, Y.; Tahara, T.; Kikuchi, T.; Kushiyama, A.; Anai, M.; Takahashi, K.; et al. Synergistic association of the copper/zinc ratio under inflammatory conditions with diabetic kidney disease in patients with type 2 diabetes: The Asahi Diabetes Complications Study. J. Diabetes Investig. 2022, 13, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Orr, S.E.; Bridges, C.C. Chronic Kidney Disease and Exposure to Nephrotoxic Metals. Int. J. Mol. Sci. 2017, 18, 1039. [Google Scholar] [CrossRef] [PubMed]
- Hays, S.M.; Nordberg, M.; Yager, J.W.; Aylward, L.L. Biomonitoring Equivalents (BE) dossier for cadmium (Cd) (CAS No. 7440-43-9). Regul. Toxicol. Pharmacol. 2008, 51, S49–S56. [Google Scholar] [CrossRef]
- Secretaria de Salud 2011. NOM-047-SSA1-2011, NORMA Oficial Mexicana NOM-047-SSA1-2011, Salud Ambiental-Indices Biológicos de Exposición Para el Personal Ocupacionalmente Expuesto a Sustancias Químicas; Diario Oficial de la Federación. 2012. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5249877&fecha=06/06/2012#gsc.tab=0 (accessed on 20 February 2025).
- Kuo, P.F.; Huang, Y.T.; Chuang, M.H.; Jiang, M.Y. Association of low-level heavy metal exposure with risk of chronic kidney disease and long-term mortality. PLoS ONE 2024, 19, e0315688. [Google Scholar]
- Smereczanski, N.M.; Brzoska, M.M. Current Levels of Environmental Exposure to Cadmium in Industrialized Countries as a Risk Factor for Kidney Damage in the General Population: A Comprehensive Review of Available Data. Int. J. Mol. Sci. 2023, 24, 8413. [Google Scholar] [CrossRef]
- Soderland, P.; Lovekar, S.; Weiner, D.E.; Brooks, D.R.; Kaufman, J.S. Chronic kidney disease associated with environmental toxins and exposures. Adv. Chronic Kidney Dis. 2010, 17, 254–264. [Google Scholar] [CrossRef]
- Eom, S.Y.; Seo, M.N.; Lee, Y.S.; Park, K.S.; Hong, Y.S.; Sohn, S.J.; Kim, Y.D.; Choi, B.S.; Lim, J.A.; Kwon, H.J.; et al. Low-Level Environmental Cadmium Exposure Induces Kidney Tubule Damage in the General Population of Korean Adults. Arch. Environ. Contam. Toxicol. 2017, 73, 401–409. [Google Scholar] [CrossRef]
- Yan, H.; Zhai, B.; Feng, R.; Wang, P.; Yang, F.; Zhou, Y. Distribution of blood lead and cadmium levels in healthy children aged 0 to 18 years and analysis of related influencing factors in Henan, China: Data findings from 2017 to 2022. Ital. J. Pediatr. 2024, 50, 43. [Google Scholar] [CrossRef] [PubMed]
- Barregard, L.; Sallsten, G.; Lundh, T.; Mölne, J. Low-level exposure to lead, cadmium and mercury, and histopathological findings in kidney biopsies. Environ. Res. 2022, 211, 113119. [Google Scholar] [CrossRef] [PubMed]
- Smereczański, N.M.; Brzóska, M.M.; Rogalska, J.; Hutsch, T. The Protective Potential of Aronia melanocarpa L. Berry Extract against Cadmium-Induced Kidney Damage: A Study in an Animal Model of Human Environmental Exposure to This Toxic Element. Int. J. Mol. Sci. 2023, 24, 11647. [Google Scholar] [CrossRef]
- Bridges, C.C.L.; Joshee, L.; Zalups, R.K. Multidrug resistance proteins and the renal elimination of inorganic mercury mediated by 2,3-dimercaptopropane-1-sulfonic acid and meso-2,3-dimercaptosuccinic acid. J. Pharmacol. Exp. Ther. 2008, 324, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Desai, G.; Niu, Z.; Luo, W.; Frndak, S.; Shaver, A.L.; Kordas, K. Low-level exposure to lead, mercury, arsenic, and cadmium, and blood pressure among 8–17-year-old participants of the 2009–2016 National Health and Nutrition Examination Survey. Environ. Res. 2021, 197, 111086. [Google Scholar]
- Ovadje, L.; Calys-Tagoe, B.N.; Clarke, E.; Basu, N. Registration status, mercury exposure biomarkers, and neuropsychological assessment of artisanal and small-scale gold miners (ASGM) from the Western Region of Ghana. Environ. Res. 2021, 201, 111639. [Google Scholar] [CrossRef] [PubMed]
- Díaz, S.M.; Muñoz-Guerrero, M.N.; Palma-Parra, M.; Becerra-Arias, C.; Fernández-Niño, J.A. Exposure to Mercury in Workers and the Population Surrounding Gold Mining Areas in the Mojana Region, Colombia. Int. J. Environ. Res. Public Health 2018, 15, 2337. [Google Scholar] [CrossRef]
- Castilhos, Z.; Rodrigues-Filho, S.; Cesar, R.; Rodrigues, A.P.; Villas-Bôas, R.; de Jesus, I.; Lima, M.; Faial, K.; Miranda, A.; Brabo, E.; et al. Human exposure and risk assessment associated with mercury contamination in artisanal gold mining areas in the Brazilian Amazon. Environ. Sci. Pollut. Res. Int. 2015, 22, 11255–11264. [Google Scholar] [PubMed]
- Lee, E.K.; Shin, Y.J.; Park, E.Y.; Kim, N.D.; Moon, A.; Kwack, S.J.; Son, J.Y.; Kacew, S.; Lee, B.M.; Bae, O.N.; et al. Selenium-binding protein 1: A sensitive urinary biomarker to detect heavy metal-induced nephrotoxicity. Arch. Toxicol. 2017, 91, 1635–1648. [Google Scholar]
- Mezzaroba, L.; Alfieri, D.F.; Colado Simão, A.N.; Vissoci Reiche, E.M. The role of zinc, copper, manganese and iron in neurodegenerative diseases. Neurotoxicology 2019, 74, 230–241. [Google Scholar]
- Poddalgoda, D.; Macey, K.; Hancock, S. Derivation of biomonitoring equivalents (BE values) for zinc. Regul. Toxicol. Pharmacol. 2019, 106, 178–186. [Google Scholar] [PubMed]
- Prohaska, J.R. Impact of copper deficiency in humans. Ann. N. Y. Acad. Sci. 2014, 1314, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.M.; Komprdová, K.; Lörinczová, K.; Kuta, J.; Přibylová, P.; Scheringer, M.; Šebejová, L.; Piler, P.; Zvonař, M.; Klánová, J. Human biomonitoring of essential and toxic trace elements (heavy metals and metalloids) in urine of children, teenagers, and young adults from a Central European Cohort in the Czech Republic. J. Expo. Sci. Environ. Epidemiol. 2024. [Google Scholar] [CrossRef]
- Paiva, A.M.; Barros, B.; Azevedo, R.; Oliveira, M.; Alves, S.; Esteves, F.; Fernandes, A.; Vaz, J.; Alves, M.J.; Slezakova, K.; et al. Biomonitoring of firefighters’ exposure to priority pollutant metal(loid)s during wildland fire combat missions: Impact on urinary levels and health risks. Sci. Total Environ. 2024, 953, 176105. [Google Scholar] [PubMed]
- Saravanabhavan, G.; Werry, K.; Walker, M.; Haines, D.; Malowany, M.; Khoury, C. Human biomonitoring reference values for metals and trace elements in blood and urine derived from the Canadian Health Measures Survey 2007–2013. Int. J. Hyg. Environ. Health 2017, 220, 189–200. [Google Scholar]
- Li, B.; Tan, Y.; Sun, W.; Fu, Y.; Miao, L.; Cai, L. The role of zinc in the prevention of diabetic cardiomyopathy and nephropathy. Toxicol. Mech. Methods 2013, 23, 27–33. [Google Scholar]
- Shen, Y.; Yin, Z.; Lv, Y.; Luo, J.; Shi, W.; Fang, J.; Shi, X. Plasma element levels and risk of chronic kidney disease in elderly populations (>/= 90 Years old). Chemosphere 2020, 254, 126809. [Google Scholar] [CrossRef]
- Brewer, G.J. Risks of copper and iron toxicity during aging in humans. Chem. Res. Toxicol. 2010, 23, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Meng, W.; Kuang, H.; Chen, X.; Zhu, X.; Wang, L.; Tan, H.; Xu, Y.; Ding, P.; Xiang, M.; et al. Association of urinary exposure to multiple metal(loid)s with kidney function from a national cross-sectional study. Sci. Total Environ. 2023, 882, 163100. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.; Trombetta, L.D. Low level mancozeb exposure causes copper bioaccumulation in the renal cortex of rats leading to tubular injury. Environ. Toxicol. Pharmacol. 2023, 100, 104148. [Google Scholar] [CrossRef] [PubMed]
- Gerhardsson, L.; Englyst, V.; Lundström, N.G.; Sandberg, S.; Nordberg, G. Cadmium, copper and zinc in tissues of deceased copper smelter workers. J. Trace Elem. Med. Biol. 2002, 16, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Nishijo, M.; Ujjin, P.; Moore, M.R. Chronic exposure to low-level cadmium induced zinc-copper dysregulation. J. Trace Elem. Med. Biol. 2018, 46, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Pizent, A.; Jurasović, J.; Telisman, S. Serum calcium, zinc, and copper in relation to biomarkers of lead and cadmium in men. J. Trace Elem. Med. Biol. 2003, 17, 199–205. [Google Scholar] [CrossRef]
- Osredkar, J.S.N. Copper and zinc, biological role and significance of copper/zinc imbalance. J. Clin. Toxicol. 2011, 3, 0495. [Google Scholar] [CrossRef]
- Lin, Y.S.; Ho, W.C.; Caffrey, J.L.; Sonawane, B. Low serum zinc is associated with elevated risk of cadmium nephrotoxicity. Environ. Res. 2014, 134, 33–38. [Google Scholar] [CrossRef]
- Carpenter, W.E.; Lam, D.; Toney, G.M.; Weintraub, N.L.; Qin, Z. Zinc, copper, and blood pressure: Human population studies. Med. Sci. Monit. 2013, 19, 1–8. [Google Scholar]
- Satarug, S.; Vesey, D.A.; Gobe, G.C. Kidney cadmium toxicity, diabetes and high blood pressure: The Perfect Storm. Tohoku J. Exp. Med. 2017, 241, 65–87. [Google Scholar] [CrossRef]
- Ortega-Romero, M.; Méndez-Hernández, P.; Cruz-Angulo, M.D.C.; Hernández-Sánchez, A.M.; Álvarez-Elías, A.C.; Muñoz-Arizpe, R.; Sales-Heredia, F.; Aguilar-Madrid, G.; Juárez-Perez, C.A.; Soto, V. Chronic Kidney Disease in Children Aged 6–15 Years and Associated Risk Factors in Apizaco, Tlaxcala, Mexico, a Pilot Study. Nephron 2019, 143, 264–273. [Google Scholar] [CrossRef]
- Schwartz, G.J.; Furth, S.L. Glomerular filtration rate measurement and estimation in chronic kidney disease. Pediatr. Nephrol. 2007, 22, 1839–1848. [Google Scholar] [PubMed]
- Graul, R.J.; Stanley, R.L. Specific gravity adjustment of urine analysis results. Am. Ind. Hyg. Assoc. J. 1982, 43, 863. [Google Scholar] [PubMed]
| Characteristic | n = 914 |
|---|---|
| Age(years), median (IQR) | 13 (12; 15) |
| Sex, n (%) Female Male | 503 (55.03) 411 (44.97) |
| BMI, n (%) Underweight Normal Overweight Obese | 20 (2.19) 608 (66.52) 171 (18.71) 115 (12.58) |
| WHtR, median (IQR) | 0.47 (0.44; 0.52) |
| Smoking status | |
| Nonsmoker | 879 (96.17) |
| Former smoker | 22 (2.41) |
| Current smoker | 13 (1.42) |
| Poverty, n (%) Yes No | 525 (57.44) 389 (42.56) |
| Renal parameters, median (IQR) | |
| eGFR (ml/min por 1.73 m2) | 103.75 (92.04; 121.52) |
| ACR (mg/g-creatinine) | 4.94 (<LOD; 22.53) |
| Early kidney biomarker (ng/mL) a median (IQR) | |
| NGAL | 5.31 (1.59; 14.92) |
| KIM-1 | 0.25 (0.11; 0.52) |
| α-1MG OPN | 27.94 (5.53; 79.66) 59.78 (23.45; 126.68) |
| Cys-C | 1.20 (0.44; 3.31) |
| CLU | 132.03 (62.86; 264.99) |
| Urinary Metals (ng/mL) a median (IQR) | |
| Cadmium | 0.013 (0.004; 0.036) |
| Mercury | 0.013 (0.003; 0.043) |
| Zinc | 617.06 (413.99; 818.01) |
| Copper | 24.73 (17.90; 39.07) |
| Copper/Zinc ratio | 0.044 (0.029; 0.070) |
| Description | NGAL Rho | KIM-1 Rho | α-1MG Rho | OPN Rho | Cys-C Rho | CLU Rho | eGFR Rho |
|---|---|---|---|---|---|---|---|
| Cadmium | 0.082 * | 0.085 * | 0.042 | 0.093 * | 0.065 * | 0.015 | 0.027 |
| Mercury | 0.044 | 0.023 | 0.042 | 0.060 | 0.053 | 0.069 * | 0.014 |
| Zinc | −0.097 * | 0.001 | −0.034 | 0.050 | −0.058 | 0.001 | −0.046 |
| Copper | 0.117 ** | 0.104 * | 0.154 ** | 0.106 * | 0.105 * | 0.175 ** | −0.074 * |
| Copper/Zinc Ratio | 0.169 ** | 0.095 * | 0.173 ** | 0.060 | 0.148 ** | 0.163 ** | −0.019 |
| Description | Log-Zinc β (95% CI) | Log-Copper β (95% CI) | Log-Mercury β (95% CI) | Log-Cu/Zn-R β (95% CI) |
|---|---|---|---|---|
| Cadmium n = 914 | 0.017 (−0.002; 0.035) | 0.017 (−0.009; 0.043) | 0.159 (0.089; 0.228) | 0.0003 (−0.026; 0.026) |
| <LOD n = 839 | Ref. | Ref. | Ref. | Ref. |
| ≥0.069 n = 75 | 0.169 (0.050; 0.287) | −0.069 (−0.198; 0.061) | 0.981 (0.509; 1.453) | −0.237 (−0.352; −0.123) |
| Description | Log-NGAL β (95% CI) | Log-KIM-1 β (95% CI) | Log-α-1MG β (95% CI) | Log-OPN β (95% CI) | Log-Cys-C β (95% CI) | Log-CLU β (95% CI) | Log-eGFR β (95% CI) | ACR (≥30 vs. <30) OR (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Log-Cadmium n = 914 | 0.035 (−0.022; 0.092) | 0.044 (−0.001; 0.089) | 0.033 (−0.045; 0.112) | 0.079 (0.035; 0.123) | 0.064 (0.001; 0.126) | 0.001 (−0.036; 0.039) | 0.001 (−0.005; 0.008) | 0.974 (0.896; 1.06) |
| <LOD n = 839 ≥0.069 n = 75 | Ref. −0.319 (−0.764; 0.126) | Ref. −0.057 (−0.406; 0.291) | Ref −0.236 (−0.82; 0.344) | Ref. 0.431 (0.151; 0.712) | Ref. −0.104 (−0.524; 0.32) | Ref. −0.259 (−0.513; −0.005) | Ref. 0.0325 (−0.015; 0.080) | Ref. 0.525 (0.256; 1.076) |
| Log-Mercury n = 914 | 0.030 (−0.016; 0.076) | 0.017 (−0.022; 0.056) | 0.051 (−0.011; 0.11) | 0.050 (0.008; 0.092) | 0.056 (−0.001; 0.11) | 0.027 (−0.008; 0.06) | 0.002 (−0.004; 0.007) | 0.963 (0.889; 1.043) |
| <LOD n = 837 ≥0.079 n = 77 | Ref. −0.060 (−0.390; 0.269) | Ref. −0.012 (−0.271; 0.248) | Ref. 0.371 (−0.093; 0.83) | Ref. 0.531 (0.254; 0.809) | Ref. 0.522 (0.147; 0.897) | Ref. 0.008 (−0.26; 0.274) | Ref. −0.014 (0.054; 0.027) | Ref. 0.603 (0.307; 1.184) |
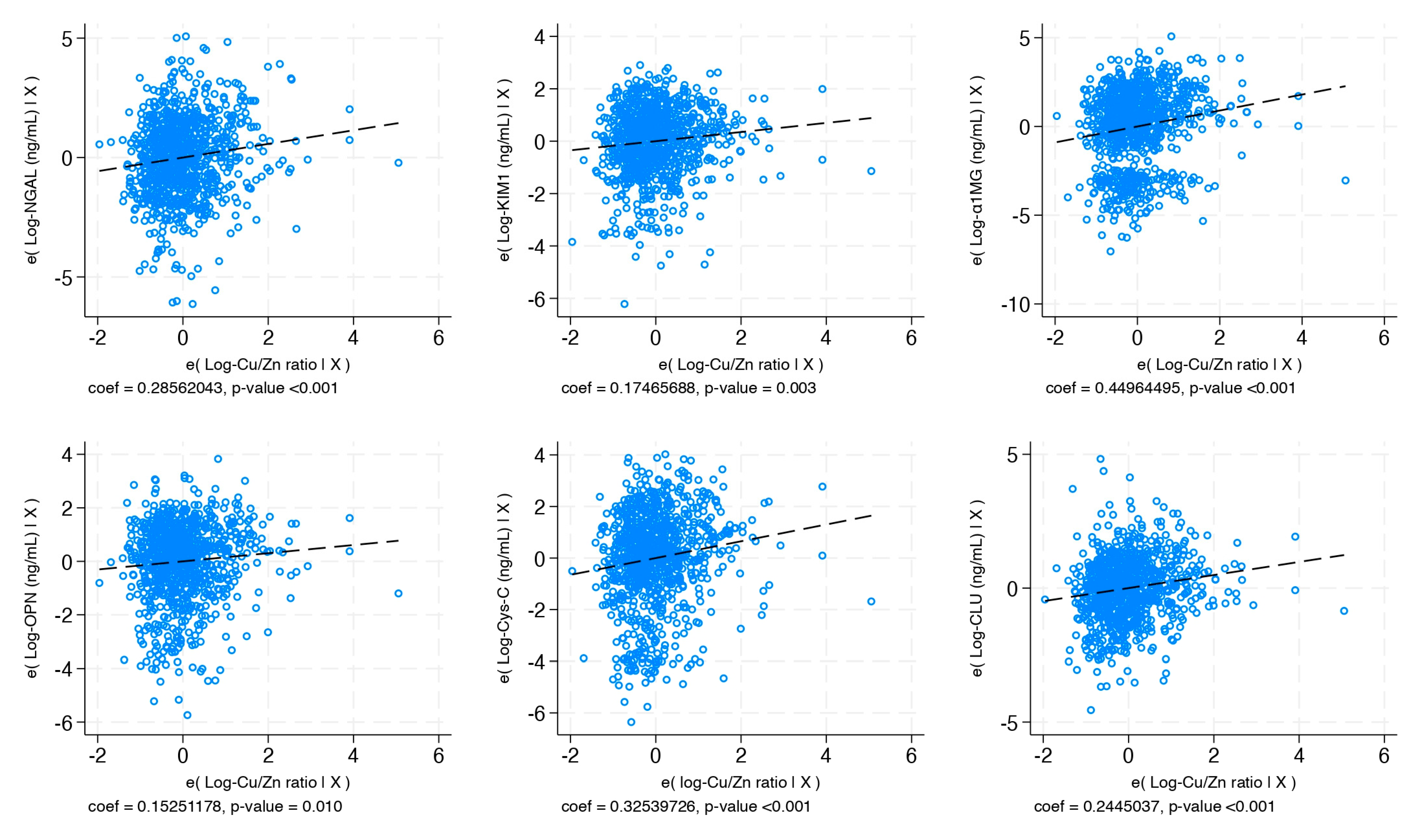
| Log-Copper/Zinc Ratio n = 914 | 0.286 (0.146; 0.425) | 0.175 (0.061; 0.288) | 0.450 (0.250; 0.649) | 0.153 (0.037; 0.268) | 0.325 (0.170; 0.481) | 0.245 (0.142; 0.347) | −0.018 (−0.03; −0.002) | 1.420 (1.160; 1.740) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega-Romero, M.; Lima, E.R.; Barbier, O.C.; Aztatzi-Aguilar, O.G.; Rubio-Gutiérrez, J.C.; Morales, J.N.; García, M.E.; Barrera-Hernández, Á.; Jiménez-Córdova, M.I.; Del Razo, L.M.; et al. Relationship Between Urinary Copper, Zinc, and Cadmium and Kidney Damage Biomarkers in Young People. Int. J. Mol. Sci. 2025, 26, 7980. https://doi.org/10.3390/ijms26167980
Ortega-Romero M, Lima ER, Barbier OC, Aztatzi-Aguilar OG, Rubio-Gutiérrez JC, Morales JN, García ME, Barrera-Hernández Á, Jiménez-Córdova MI, Del Razo LM, et al. Relationship Between Urinary Copper, Zinc, and Cadmium and Kidney Damage Biomarkers in Young People. International Journal of Molecular Sciences. 2025; 26(16):7980. https://doi.org/10.3390/ijms26167980
Chicago/Turabian StyleOrtega-Romero, Manolo, Elodia Rojas Lima, Olivier C. Barbier, Octavio Gamaliel Aztatzi-Aguilar, Juan Carlos Rubio-Gutiérrez, Juana Narváez Morales, Mariela Esparza García, Ángel Barrera-Hernández, Mónica I. Jiménez-Córdova, Luz María Del Razo, and et al. 2025. "Relationship Between Urinary Copper, Zinc, and Cadmium and Kidney Damage Biomarkers in Young People" International Journal of Molecular Sciences 26, no. 16: 7980. https://doi.org/10.3390/ijms26167980
APA StyleOrtega-Romero, M., Lima, E. R., Barbier, O. C., Aztatzi-Aguilar, O. G., Rubio-Gutiérrez, J. C., Morales, J. N., García, M. E., Barrera-Hernández, Á., Jiménez-Córdova, M. I., Del Razo, L. M., Mendez-Hernández, P., & Medeiros, M. (2025). Relationship Between Urinary Copper, Zinc, and Cadmium and Kidney Damage Biomarkers in Young People. International Journal of Molecular Sciences, 26(16), 7980. https://doi.org/10.3390/ijms26167980











