Antioxidant-Enzyme Profiles in Youth Athletes: Associations of SOD and GPX with Exercise and Implications for Endothelial Health
Abstract
1. Introduction
2. Results
2.1. Participant Characteristics
2.2. Age and Maturation Group Comparisons
2.3. Training Context: Club-Only vs. Additional Extracurricular Sport
Sport-Specific Differences Among Male Athletes (Soccer, Ice-Hockey, Swimming)
2.4. Association of Training Volume/Intensity with Antioxidative Markers
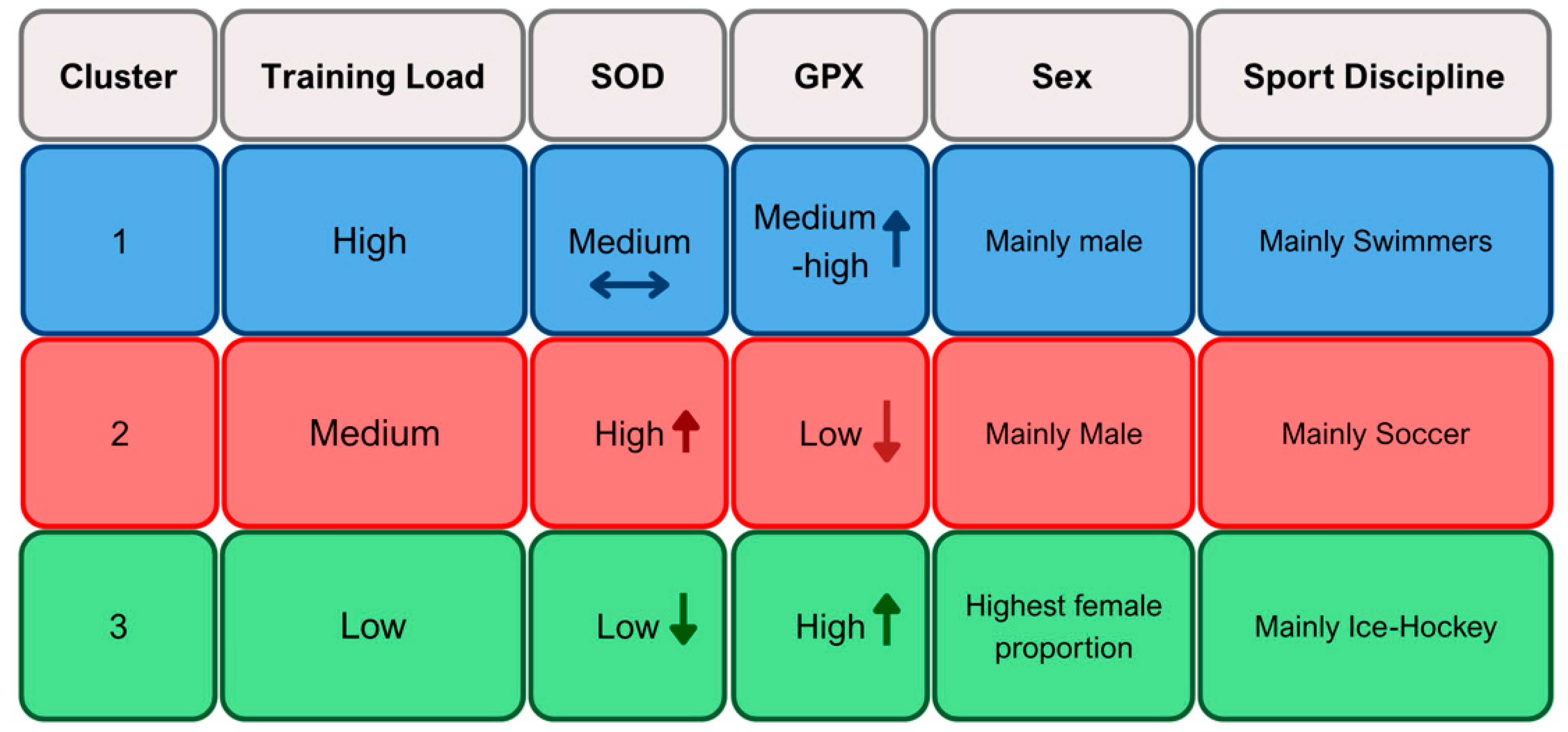
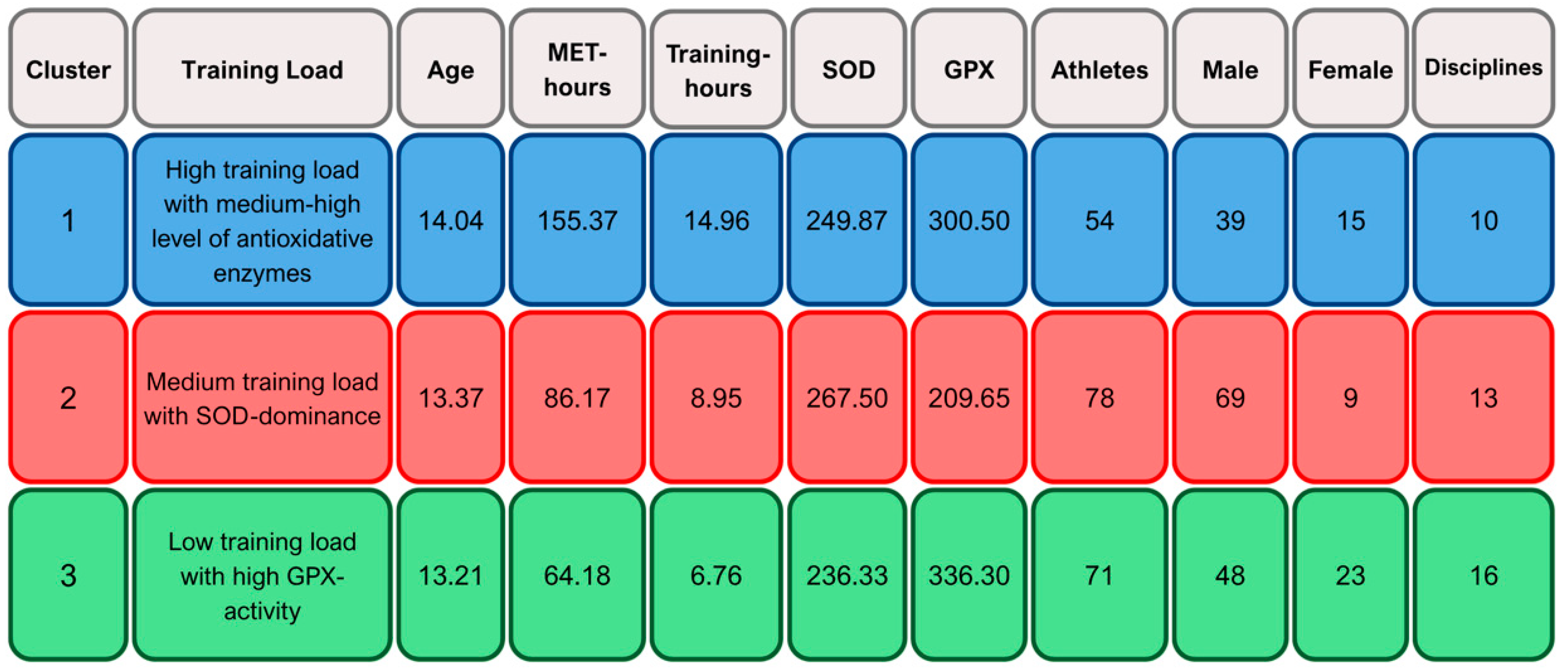
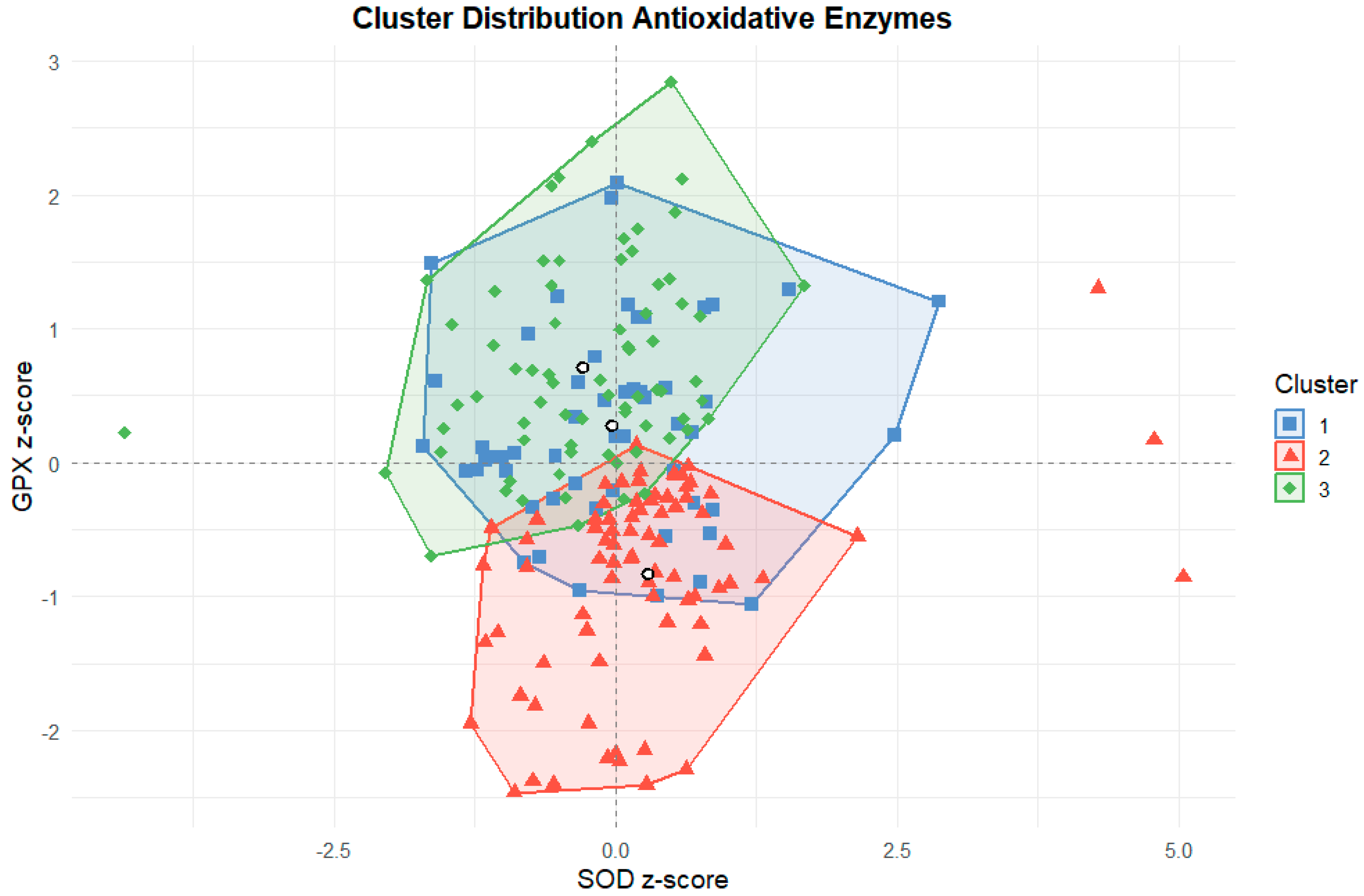
2.5. Cluster-Based Profiles of Training Load and Antioxidant Enzyme Activity
3. Discussion
3.1. Sex Differences
3.2. Maturation and Age
3.3. Training Load
3.4. Sport Discipline
3.5. Antioxidant Clusters and Redox Phenotypes
3.6. Limitations
4. Materials and Methods
4.1. Participants
4.2. Ethical Considerations
4.3. Venous Blood Sampling and Laboratory Analyses
4.4. Physical Activity Assessment (MoMo-PAQ)
4.5. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ROS | reactive oxygen species |
| DNA | deoxyribonucleic acid |
| NO | nitric oxide |
| SOD | superoxide dismutase |
| GPX | glutathione peroxidase |
| O2− | superoxide anion |
| H2O2 | hydrogen peroxide |
| OHOO− | peroxynitrite |
| GSH | reduced glutathione |
| GSSG | glutathione disulfide |
| MAPK | mitogen-activated protein kinase |
| AMPK | AMP-activated protein kinase |
| CaMK | Ca2+/Calmodulin-dependent kinase |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| NF-κB | nuclear factor kappa B |
| KLF2 | Kruppel-like factor 2 |
| ARE | antioxidant response element |
| eNOS | endothelial nitric oxide synthase |
| FoxO3a | forkhead transcription factor 3a |
| SIRT3 | sirtuin 3 |
| PGC-1α | peroxisome proliferator-activated receptor gamma coactivator 1α |
| MuCAYAplus | Munich Cardiovascular Adaptations in Young Athletes Plus |
| MoMo-PAQ | Motorik-Modul-Physical Activity Questionnaire |
| MET | metabolic equivalent of task |
| BSA | body surface area |
| BMI | body mass index |
References
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Chandimali, N.; Bak, S.G.; Park, E.H.; Lim, H.-J.; Won, Y.-S.; Kim, E.-K.; Park, S.-I.; Lee, S.J. Free radicals and their impact on health and antioxidant defenses: A review. Cell Death Discov. 2025, 11, 19. [Google Scholar] [CrossRef]
- Schulz, E.; Gori, T.; Münzel, T. Oxidative stress and endothelial dysfunction in hypertension. Hypertens. Res. 2011, 34, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Dubois-Deruy, E.; Peugnet, V.; Turkieh, A.; Pinet, F. Oxidative Stress in Cardiovascular Diseases. Antioxidants 2020, 9, 864. [Google Scholar] [CrossRef]
- Förstermann, U.; Xia, N.; Li, H. Roles of Vascular Oxidative Stress and Nitric Oxide in the Pathogenesis of Atherosclerosis. Circ. Res. 2017, 120, 713–735. [Google Scholar] [CrossRef]
- Skilton, M.R.; Celermajer, D.S. Endothelial dysfunction and arterial abnormalities in childhood obesity. Int. J. Obes. 2006, 30, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Gu, Y.; Li, Z.; Zhang, L.; Hei, Y. Effects of exercise on different antioxidant enzymes and related indicators: A systematic review and meta-analysis of randomized controlled trials. Sci. Rep. 2025, 15, 12518. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several lines of antioxidant defense against oxidative stress: Antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Markers of oxidative stress in erythrocytes and plasma during aging in humans. Oxidative Med. Cell Longev. 2010, 3, 2–12. [Google Scholar] [CrossRef]
- Fukai, T.; Ushio-Fukai, M. Superoxide dismutases: Role in redox signaling, vascular function, and diseases. Antioxid. Redox Signal. 2011, 15, 1583–1606. [Google Scholar] [CrossRef]
- Scioli, M.G.; Storti, G.; D’Amico, F.; Rodríguez Guzmán, R.; Centofanti, F.; Doldo, E.; Céspedes Miranda, E.M.; Orlandi, A. Oxidative Stress and New Pathogenetic Mechanisms in Endothelial Dysfunction: Potential Diagnostic Biomarkers and Therapeutic Targets. J. Clin. Med. 2020, 9, 1995. [Google Scholar] [CrossRef]
- Choung, B.Y.; Byun, S.J.; Suh, J.G.; Kim, T.Y. Extracellular superoxide dismutase tissue distribution and the patterns of superoxide dismutase mRNA expression following ultraviolet irradiation on mouse skin. Exp. Dermatol. 2004, 13, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Pan, X.; Wei, G.; Hua, Y. Research progress of glutathione peroxidase family (GPX) in redoxidation. Front. Pharmacol. 2023, 14, 1147414. [Google Scholar] [CrossRef] [PubMed]
- Handy, D.E.; Loscalzo, J. The role of glutathione peroxidase-1 in health and disease. Free Radic. Biol. Med. 2022, 188, 146–161. [Google Scholar] [CrossRef]
- Ursini, F.; Maiorino, M. Lipid peroxidation and ferroptosis: The role of GSH and GPx4. Free Radic. Biol. Med. 2020, 152, 175–185. [Google Scholar] [CrossRef]
- Hong, Y.; Boiti, A.; Vallone, D.; Foulkes, N.S. Reactive Oxygen Species Signaling and Oxidative Stress: Transcriptional Regulation and Evolution. Antioxidants 2024, 13, 312. [Google Scholar] [CrossRef]
- Griendling, K.K.; Sorescu, D.; Lassègue, B.; Ushio-Fukai, M. Modulation of Protein Kinase Activity and Gene Expression by Reactive Oxygen Species and Their Role in Vascular Physiology and Pathophysiology. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2175–2183. [Google Scholar] [CrossRef] [PubMed]
- Young, A.; Wu, W.; Sun, W.; Benjamin Larman, H.; Wang, N.; Li, Y.S.; Shyy, J.Y.; Chien, S.; García-Cardeña, G. Flow activation of AMP-activated protein kinase in vascular endothelium leads to Krüppel-like factor 2 expression. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1902–1908, Erratum in Arterioscler. Thromb. Vasc. Biol. 2010, 30, e325. [Google Scholar] [CrossRef]
- Erickson, J.R.; He, B.J.; Grumbach, I.M.; Anderson, M.E. CaMKII in the cardiovascular system: Sensing redox states. Physiol. Rev. 2011, 91, 889–915. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Mendoza, N.; Morales-González, Á.; Madrigal-Santillán, E.O.; Madrigal-Bujaidar, E.; Álvarez-González, I.; García-Melo, L.F.; Anguiano-Robledo, L.; Fregoso-Aguilar, T.; Morales-Gonzalez, J.A. Antioxidant and Adaptative Response Mediated by Nrf2 during Physical Exercise. Antioxidants 2019, 8, 196. [Google Scholar] [CrossRef]
- Lingappan, K. NF-κB in Oxidative Stress. Curr. Opin. Toxicol. 2018, 7, 81–86. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Sukhorukov, V.N.; Kalmykov, V.A.; Grechko, A.V.; Shakhpazyan, N.K.; Orekhov, A.N. The Role of KLF2 in the Regulation of Atherosclerosis Development and Potential Use of KLF2-Targeted Therapy. Biomedicines 2022, 10, 254. [Google Scholar] [CrossRef]
- Muthusamy, V.R.; Kannan, S.; Sadhaasivam, K.; Gounder, S.S.; Davidson, C.J.; Boeheme, C.; Hoidal, J.R.; Wang, L.; Rajasekaran, N.S. Acute exercise stress activates Nrf2/ARE signaling and promotes antioxidant mechanisms in the myocardium. Free Radic. Biol. Med. 2012, 52, 366–376. [Google Scholar] [CrossRef]
- Inoue, N.; Ramasamy, S.; Fukai, T.; Nerem, R.M.; Harrison, D.G. Shear stress modulates expression of Cu/Zn superoxide dismutase in human aortic endothelial cells. Circ. Res. 1996, 79, 32–37. [Google Scholar] [CrossRef]
- Hitomi, Y.; Watanabe, S.; Kizaki, T.; Sakurai, T.; Takemasa, T.; Haga, S.; Ookawara, T.; Suzuki, K.; Ohno, H. Acute exercise increases expression of extracellular superoxide dismutase in skeletal muscle and the aorta. Redox Rep. 2008, 13, 213–216. [Google Scholar] [CrossRef]
- Kops, G.J.; Dansen, T.B.; Polderman, P.E.; Saarloos, I.; Wirtz, K.W.; Coffer, P.J.; Huang, T.T.; Bos, J.L.; Medema, R.H.; Burgering, B.M. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature 2002, 419, 316–321. [Google Scholar] [CrossRef]
- Radák, Z.; Asano, K.; Inoue, M.; Kizaki, T.; Oh-Ishi, S.; Suzuki, K.; Taniguchi, N.; Ohno, H. Superoxide dismutase derivative reduces oxidative damage in skeletal muscle of rats during exhaustive exercise. J. Appl. Physiol. 1995, 79, 129–135. [Google Scholar] [CrossRef]
- Elokda, A.S.; Shields, R.K.; Nielsen, D.H. Effects of a maximal graded exercise test on glutathione as a marker of acute oxidative stress. J. Cardiopulm. Rehabil. 2005, 25, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Gounder, S.S.; Kannan, S.; Devadoss, D.; Miller, C.J.; Whitehead, K.J.; Odelberg, S.J.; Firpo, M.A.; Paine, R., 3rd; Hoidal, J.R.; Abel, E.D.; et al. Impaired transcriptional activity of Nrf2 in age-related myocardial oxidative stress is reversible by moderate exercise training. PLoS ONE 2012, 7, e45697, Erratum in PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Brandauer, J.; Andersen, M.A.; Kellezi, H.; Risis, S.; Frøsig, C.; Vienberg, S.G.; Treebak, J.T. AMP-activated protein kinase controls exercise training- and AICAR-induced increases in SIRT3 and MnSOD. Front. Physiol. 2015, 6, 85. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Deminice, R.; Ozdemir, M.; Yoshihara, T.; Bomkamp, M.P.; Hyatt, H. Exercise-induced oxidative stress: Friend or foe? J. Sport Health Sci. 2020, 9, 415–425. [Google Scholar] [CrossRef]
- Powers, S.K.; Jackson, M.J. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef]
- Yan, Z.; Spaulding, H.R. Extracellular superoxide dismutase, a molecular transducer of health benefits of exercise. Redox Biol. 2020, 32, 101508. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Liao, Y.; Zhu, C.; Zou, Z. GPX4, ferroptosis, and diseases. Biomed. Pharmacother. 2024, 174, 116512. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhou, Y.; Wu, Y.; Lang, Y.; Mao, Y.; Pan, G.; Gao, Z. Long-term aerobic exercise improves learning memory capacity and effects on oxidative stress levels and Keap1/Nrf2/GPX4 pathway in the hippocampus of APP/PS1 mice. Front. Neurosci. 2024, 18, 1505650. [Google Scholar] [CrossRef]
- Haferanke, J.; Baumgartner, L.; Willinger, L.; Oberhoffer-Fritz, R.; Schulz, T. Molecular Mechanisms of Vascular Tone in Exercising Pediatric Populations: A Comprehensive Overview on Endothelial, Antioxidative, Metabolic and Lipoprotein Signaling Molecules. Int. J. Mol. Sci. 2025, 26, 1027. [Google Scholar] [CrossRef] [PubMed]
- Matusik, P.; Prokopowicz, Z.; Norek, B.; Olszanecka-Glinianowicz, M.; Chudek, J.; Malecka-Tendera, E. Oxidative/Antioxidative status in obese and sport trained children: A comparative study. Biomed. Res. Int. 2015, 2015, 315747. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, N.; Erel, O.; Hazer, M.; Bağci, C.; Namiduru, E.; Gül, E. Biochemical assessments of retinol, alpha-tocopherol, pyridoxal--5-phosphate oxidative stress index and total antioxidant status in adolescent professional basketball players and sedentary controls. Int. J. Adolesc. Med. Health 2007, 19, 177–186. [Google Scholar] [CrossRef]
- Alshammari, E.; Shafi, S.; Nurmi-Lawton, J.; Taylor, A.; Lanham-New, S.; Ferns, G. Altered antioxidant and trace-element status in adolescent female gymnasts. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 291–298. [Google Scholar] [CrossRef]
- Zivkovic, V.; Lazarevic, P.; Djuric, D.; Cubrilo, D.; Macura, M.; Vusletic, M.; Barudzic, N.; Nesic, M.; Jakovljevic, V. Alteration in basal redox state of young male soccer players after a six-month training programme. Acta Physiol. Hung. 2013, 100, 64–76. [Google Scholar] [CrossRef]
- Samsonov, A.; Urlacher, S.S. Oxidative Stress in Children and Adolescents: Insights Into Human Biology. Am. J. Hum. Biol. 2025, 37, e24200. [Google Scholar] [CrossRef]
- Mendoza-Núñez, V.M.; Beristain-Pérez, A.; Pérez-Vera, S.P.; Altamirano-Lozano, M.A. Age-related sex differences in glutathione peroxidase and oxidative DNA damage in a healthy Mexican population. J. Womens Health 2010, 19, 919–926. [Google Scholar] [CrossRef]
- Kendall, B.; Eston, R. Exercise-induced muscle damage and the potential protective role of estrogen. Sports Med. 2002, 32, 103–123. [Google Scholar] [CrossRef]
- Barp, J.; Araújo, A.S.; Fernandes, T.R.; Rigatto, K.V.; Llesuy, S.; Belló-Klein, A.; Singal, P. Myocardial antioxidant and oxidative stress changes due to sex hormones. Braz. J. Med. Biol. Res. 2002, 35, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Alkazemi, D.; Rahman, A.; Habra, B. Alterations in glutathione redox homeostasis among adolescents with obesity and anemia. Sci. Rep. 2021, 11, 3034. [Google Scholar] [CrossRef] [PubMed]
- Avloniti, A.; Chatzinikolaou, A.; Deli, C.K.; Vlachopoulos, D.; Gracia-Marco, L.; Leontsini, D.; Draganidis, D.; Jamurtas, A.Z.; Mastorakos, G.; Fatouros, I.G. Exercise-Induced Oxidative Stress Responses in the Pediatric Population. Antioxidants 2017, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, A.; Zeyrek, D.; Atas, A.; Erel, O. Paraoxonase activity in athletic adolescents. Pediatr. Exerc. Sci. 2010, 22, 93–104. [Google Scholar] [CrossRef]
- Alvear-Vasquez, F.; Vidal-Espinoza, R.; Gomez-Campos, R.; de Campos, L.F.C.C.; Lazari, E.; Guzmán-Luján, J.F.; Pablos-Monzó, A.; Cossio-Bolaños, M. Body surface area is a predictor of maturity status in school children and adolescents. BMC Pediatr. 2023, 23, 410. [Google Scholar] [CrossRef]
- Steinbacher, P.; Eckl, P. Impact of oxidative stress on exercising skeletal muscle. Biomolecules 2015, 5, 356–377. [Google Scholar] [CrossRef]
- Carlsohn, A.; Rohn, S.; Bittmann, F.; Raila, J.; Mayer, F.; Schweigert, F.J. Exercise increases the plasma antioxidant capacity of adolescent athletes. Ann. Nutr. Metab. 2008, 53, 96–103. [Google Scholar] [CrossRef]
- Dékány, M.; Nemeskéri, V.; Györe, I.; Harbula, I.; Malomsoki, J.; Pucsok, J. Antioxidant status of interval-trained athletes in various sports. Int. J. Sports Med. 2006, 27, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Margaritelis, N.V.; Paschalis, V.; Theodorou, A.A.; Kyparos, A.; Nikolaidis, M.G. Antioxidants in Personalized Nutrition and Exercise. Adv. Nutr. 2018, 9, 813–823. [Google Scholar] [CrossRef]
- Miranda-Vilela, A.L.; Lordelo, G.S.; Akimoto, A.K.; Alves, P.C.Z.; Pereira, L.C.d.S.; Klautau-Guimarães, M.d.N.; Grisolia, C.K. Genetic polymorphisms influence runners’ responses to the dietary ingestion of antioxidant supplementation based on pequi oil (Caryocar brasiliense Camb.): A before-after study. Genes Nutr. 2011, 6, 369–395. [Google Scholar] [CrossRef]
- Haferanke, J.; Baumgartner, L.; Willinger, L.; Schulz, T.; Mühlbauer, F.; Engl, T.; Weberruß, H.; Hofmann, H.; Wasserfurth, P.; Köhler, K.; et al. The MuCAYAplus Study—Influence of Physical Activity and Metabolic Parameters on the Structure and Function of the Cardiovascular System in Young Athletes. CJC Open 2024, 6, 1549–1557. [Google Scholar] [CrossRef]
- Bös, K.; Opper, E.; Worth, A.; Oberge, J.; Romahn, N.; Wagner, M.; Woll, A. Motorik-Modul: Motorische Leistungsfähigkeit und körperlich-sportliche Aktivität von Kindern und Jugendlichen in Deutschland. Foss. Newsl. 2007, 7, 1–2. [Google Scholar]
- Jekauc, D.; Voelkle, M.; Wagner, M.O.; Mewes, N.; Woll, A. Reliability, validity, and measurement invariance of the German version of the physical activity enjoyment scale. J. Pediatr. Psychol. 2013, 38, 104–115. [Google Scholar] [CrossRef] [PubMed]
| N | Total (M ± SD) | N | Boys (M ± SD) | N | Girls (M ± SD) | p | |
|---|---|---|---|---|---|---|---|
| Age (yrs) | 203 | 13.49 ± 1.64 | 156 | 13.61 ± 1.63 | 47 | 13.09 ± 1.61 | 0.058 |
| Body mass (kg) | 203 | 52.51 ± 12.33 | 156 | 53.44 ± 12.58 | 47 | 49.44 ± 11.03 | 0.051 |
| Body height (cm) | 203 | 165.58 ± 12.72 | 156 | 166.76 ± 13.29 | 47 | 161.66 ± 9.74 | 0.005 |
| BMI (kg/m2) | 203 | 18.85 ± 2.27 | 156 | 18.90 ± 2.14 | 47 | 18.68 ± 2.69 | 0.556 |
| BSA (m2) | 203 | 1.54 ± 0.24 | 156 | 1.56 ± 0.24 | 47 | 1.48 ± 0.20 | 0.022 |
| Training duration (h/week) | 203 | 9.78 ± 4.14 | 156 | 9.78 ± 3.71 | 47 | 9.79 ± 5.38 | 0.996 |
| Training intensity (MET-h/week) | 203 | 96.89 ± 46.07 | 156 | 96.28 ± 40.51 | 47 | 98.90 ± 61.52 | 0.785 |
| SOD (U/mL) | 203 | 251.91 ± 54.38 | 156 | 259.43 ± 54.02 | 47 | 226.93 ± 48.22 | <0.001 |
| GPX (U/L) | 203 | 278.11 ± 82.16 | 156 | 276.11 ± 85.93 | 47 | 284.76 ± 68.58 | 0.528 |
| N | Under Median (n = 101; 72 Boys, 29 Girls) (M ± SD) | N | Over Median (n = 102; 84 Boys; 18 Girls) (M ± SD) | p | |
|---|---|---|---|---|---|
| Age (yrs) | 101 | 12.14 ± 1.17 | 102 | 14.83 ± 0.64 | <0.001 |
| Body mass (kg) | 101 | 45.00 ± 9.95 | 102 | 59.95 ± 9.69 | <0.001 |
| Body height (cm) | 101 | 157.38 ± 10.40 | 102 | 173.70 ± 9.09 | <0.001 |
| BMI (kg/m2) | 101 | 17.95 ± 2.19 | 102 | 19.75 ± 1.99 | <0.001 |
| BSA (m2) | 101 | 1.39 ± 0.19 | 102 | 1.69 ± 0.17 | <0.001 |
| Training duration (h/week) | 101 | 8.78 ± 3.87 | 102 | 10.77 ± 4.18 | <0.001 |
| Training intensity (MET-h/week) | 101 | 84.71 ± 41.41 | 102 | 108.95 ± 47.45 | <0.001 |
| SOD (U/mL) | 101 | 252.32 ± 55.33 | 102 | 251.50 ± 53.69 | 0.914 |
| GPX (U/L) | 101 | 269.86 ± 85.79 | 102 | 286.29 ± 77.97 | 0.155 |
| N | Under Median (n = 101; 71 Boys, 30 Girls) (M ± SD) | N | Over Median (n = 102; 85 Boys; 17 Girls) (M ± SD) | p | |
|---|---|---|---|---|---|
| Age (yrs) | 101 | 12.43 ± 1.49 | 102 | 14.54 ± 0.98 | <0.001 |
| Body mass (kg) | 101 | 42.24 ± 6.62 | 102 | 62.65 ± 7.23 | <0.001 |
| Body height (cm) | 101 | 155.52 ± 8.23 | 102 | 175.54 ± 7.42 | <0.001 |
| BMI (kg/m2) | 101 | 17.39 ± 1.79 | 102 | 20.30 ± 1.71 | <0.001 |
| BSA (m2) | 101 | 1.34 ± 0.13 | 102 | 1.74 ± 0.12 | <0.001 |
| Training duration (h/week) | 101 | 9.24 ± 4.18 | 102 | 10.32 ± 4.05 | 0.064 |
| Training intensity (MET-h/week) | 101 | 91.02 ± 47.13 | 102 | 102.70 ± 44.47 | 0.071 |
| SOD (U/mL) | 101 | 248.62 ± 57.11 | 102 | 255.17 ± 51.61 | 0.393 |
| GPX (U/L) | 101 | 268.18 ± 80.60 | 102 | 287.95 ± 82.90 | 0.087 |
| N | Only Club (n = 120; 89 Boys, 31 Girls) (M ± SD) | N | Club + Extra (n = 83; 67 Boys, 16 Girls) (M ± SD) | p | |
|---|---|---|---|---|---|
| Age (yrs) | 120 | 13.52 ± 1.56 | 83 | 13.46 ± 1.75 | 0.818 |
| Body mass (kg) | 120 | 51.78 ± 12.08 | 83 | 53.57 ± 12.68 | 0.309 |
| Body height (cm) | 120 | 83 | 166.22 ± 13.45 | 0.556 | |
| BMI (kg/m2) | 120 | 18.71 ± 2.36 | 83 | 19.06 ± 2.15 | 0.288 |
| BSA (m2) | 120 | 1.53 ± 0.23 | 83 | 1.56 ± 0.24 | 0.358 |
| Training duration (h/week) | 120 | 9.09 ± 3.97 | 83 | 10.79 ± 4.19 | 0.004 |
| Training intensity (MET-h/week) | 120 | 92.84 ± 47.02 | 83 | 102.73 ± 44.30 | 0.133 |
| SOD (U/mL) | 120 | 249.42 ± 57.20 | 83 | 255.50 ± 50.15 | 0.435 |
| GPX (U/L) | 120 | 284.64 ± 82.13 | 83 | 268.68 ± 81.78 | 0.174 |
| N | Soccer (M ± SD) | N | Ice-Hockey (M ± SD) | N | Swimming (M ± SD) | p | |
|---|---|---|---|---|---|---|---|
| Age (yrs) | 46 | 13.40 ± 1.95 | 50 | 13.89 ± 1.30 | 27 | 13.07 ± 1.63 | 0.098 |
| Body mass (kg) | 46 | 49.71 ± 14.01 | 50 | 55.41 ± 10.78 | 27 | 50.42 ± 11.54 | 0.107 |
| Body height (cm) | 46 | 164.21 ± 15.23 | 50 | 168.36 ± 11.40 | 27 | 165.80 ± 12.88 | 0.210 |
| BMI (kg/m2) | 46 | 18.38 ± 2.33 | 50 | 19.35 ± 1.96 | 27 | 18.56 ± 2.09 | 0.073 |
| BSA (m2) | 46 | 1.51 ± 0.27 | 50 | 1.60 ± 0.20 | 27 | 1.50 ± 0.22 | 0.108 |
| Training duration (h/week) | 46 | 9.77 ± 3.24 | 50 | 8.48 ± 3.18 | 27 | 12.90 ± 4.41 | <0.001 |
| Training intensity (MET-h/week) | 46 | 97.18 ± 32.53 | 50 | 80.25 ± 28.93 | 27 | 139.05 ± 52.85 | <0.001 |
| SOD (U/mL) | 46 | 264.71 ± 61.68 | 50 | 268.96 ± 58.69 | 27 | 245.77 ± 39.64 | 0.216 |
| GPX (U/L) | 46 | 271.54 ± 82.75 | 50 | 292.06 ± 89.11 | 27 | 283.96 ± 78.97 | 0.473 |
| β | Std. β | p | R2 | |
|---|---|---|---|---|
| MET-h/week | ||||
| SOD (U/mL) | −0.035 | −0.030 | 0.680 | 0.070 |
| GPX (U/L) | −0.031 | −0.018 | 0.810 | 0.028 |
| Hours/week | ||||
| SOD (U/mL) | 0.045 | 0.003 | 0.962 | 0.070 |
| GPX (U/L) | −0.363 | −0.018 | 0.802 | 0.028 |
| Boys | Girls | |||||||
|---|---|---|---|---|---|---|---|---|
| β | Std. β | p | R2 | β | Std. β | p | R2 | |
| MET-h/week | ||||||||
| SOD (U/mL) | −0.079 | −0.059 | 0.483 | 0.018 | 0.047 | 0.060 | 0.709 | 0.017 |
| GPX (U/L) | −0.028 | −0.013 | 0.873 | 0.030 | −0.027 | −0.025 | 0.879 | 0.014 |
| Hours/week | ||||||||
| SOD (U/mL) | −0.380 | −0.026 | 0.754 | 0.016 | 1.029 | 0.114 | 0.479 | 0.025 |
| GPX (U/L) | −0.781 | −0.034 | 0.683 | 0.031 | 0.506 | 0.040 | 0.807 | 0.015 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haferanke, J.; Freilinger, S.; Baumgartner, L.; Engl, T.; Dettenhofer, M.; Huber, S.; Mühlbauer, F.; Oberhoffer, R.; Schulz, T. Antioxidant-Enzyme Profiles in Youth Athletes: Associations of SOD and GPX with Exercise and Implications for Endothelial Health. Int. J. Mol. Sci. 2025, 26, 9532. https://doi.org/10.3390/ijms26199532
Haferanke J, Freilinger S, Baumgartner L, Engl T, Dettenhofer M, Huber S, Mühlbauer F, Oberhoffer R, Schulz T. Antioxidant-Enzyme Profiles in Youth Athletes: Associations of SOD and GPX with Exercise and Implications for Endothelial Health. International Journal of Molecular Sciences. 2025; 26(19):9532. https://doi.org/10.3390/ijms26199532
Chicago/Turabian StyleHaferanke, Jonas, Sebastian Freilinger, Lisa Baumgartner, Tobias Engl, Maximilian Dettenhofer, Stefanie Huber, Frauke Mühlbauer, Renate Oberhoffer, and Thorsten Schulz. 2025. "Antioxidant-Enzyme Profiles in Youth Athletes: Associations of SOD and GPX with Exercise and Implications for Endothelial Health" International Journal of Molecular Sciences 26, no. 19: 9532. https://doi.org/10.3390/ijms26199532
APA StyleHaferanke, J., Freilinger, S., Baumgartner, L., Engl, T., Dettenhofer, M., Huber, S., Mühlbauer, F., Oberhoffer, R., & Schulz, T. (2025). Antioxidant-Enzyme Profiles in Youth Athletes: Associations of SOD and GPX with Exercise and Implications for Endothelial Health. International Journal of Molecular Sciences, 26(19), 9532. https://doi.org/10.3390/ijms26199532







