Anti-Amyloid Therapies for Alzheimer’s Disease: Progress, Pitfalls, and the Path Ahead
Abstract
1. Introduction
2. Results
2.1. Clinical Trial Outcomes
2.2. Safety and Risk Management
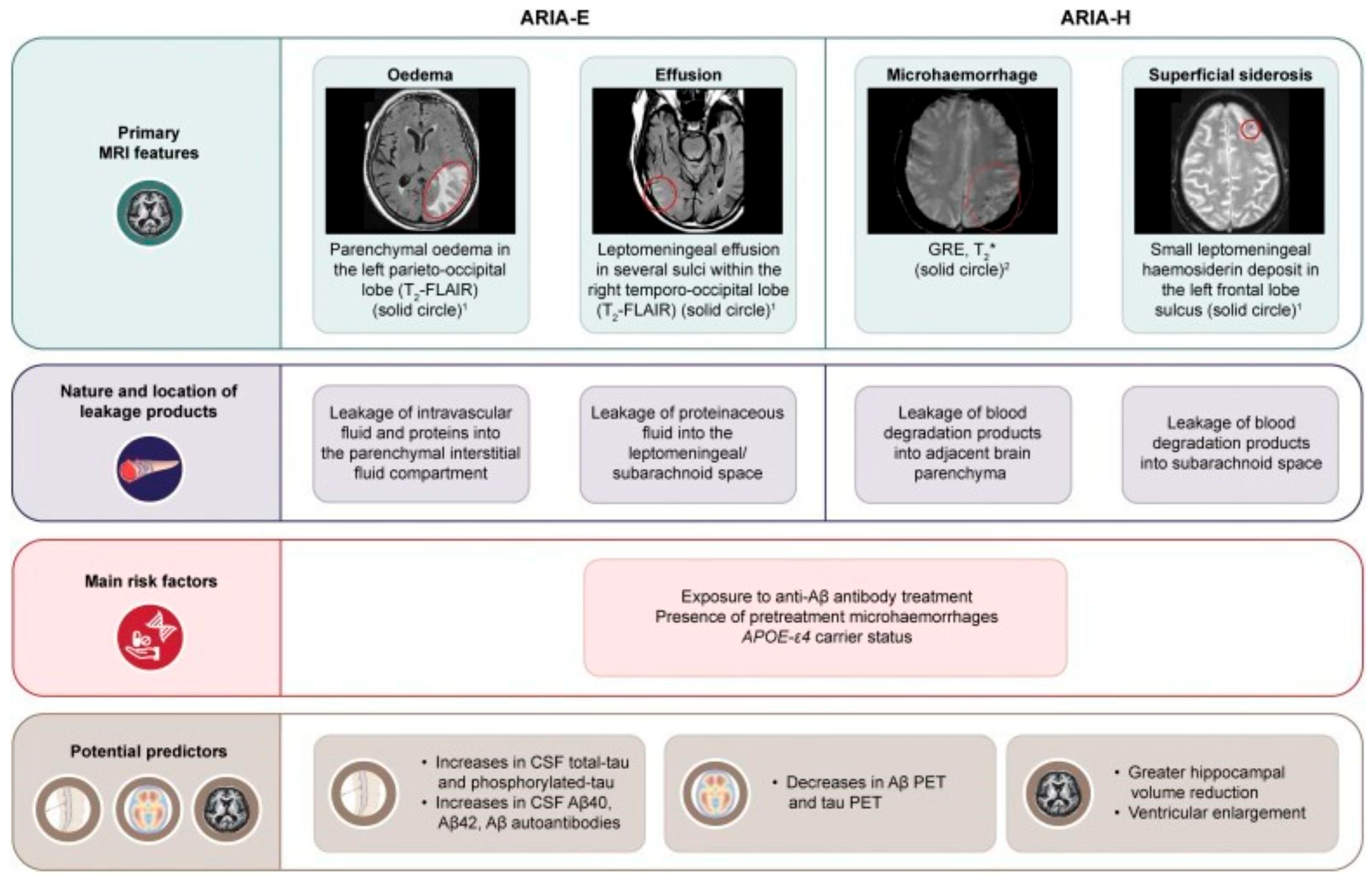
2.2.1. ARIA-E (Edema/Effusion)
2.2.2. Management
2.2.3. ARIA-H (Hemorrhage/Hemosiderosis)
2.2.4. Management
2.2.5. Termination of Therapy
2.2.6. Anticoagulation
2.2.7. Thrombolysis
2.3. Patient and Clinical Decision Making
2.4. Regulatory Divergence and Real-World Access
2.5. Treatment Costs
2.6. Risk Management and Precision Medicine
- Patients with findings suggestive of cerebral amyloid angiopathy on brain MRI, such as the presence of cerebral microhemorrhages (CMH) and cortical superficial siderosis, should be considered to be at a higher risk for ARIA-H and may require more frequent monitoring.
- The presence of CMH should be taken into consideration, as patients with multiple baseline CMHs, particularly those located in cortical or subcortical regions, are at higher risk of ARIA.
2.7. Initial Screening
2.8. Health System Implementation Challenges
3. Future Directions
- 1.
- Combination and sequence strategies: The combination of anti-amyloid therapies with anti-tau agents, microglial modulators, neuroprotective synaptic agents, and lifestyle/digital therapies in trials may enhance benefits, particularly when they are initiated before symptom onset in individuals at high risk.
- 2.
- Adaptive dosing and de-escalation: The current dosing schedules for anti-amyloid treatments follow established protocols that are derived from clinical trial outcomes. Neuroscientists are now exploring adaptive dosing methods, which adjust treatment amounts and administration schedules according to patient-specific factors and treatment outcomes. These methods enable better treatment results, while minimizing ARIA risks and producing cost-effective outcomes. In particular, titration schemes might reduce ARIA incidence in ε4 homozygotes. The finite-course concept of donanemab enables us to study the potential safety of extending maintenance intervals after clearance of the drug [25].
- 3.
- Learning health systems: The U.S. Medicare registry requirement functions as a supportive structure, which enables the development of pragmatic evidence through safety signals (e.g., post-thrombolysis hemorrhage), equity metrics, and patient-reported outcomes that help improve AURs and payer policies [18]. The complete potential of learning health systems in this field depends on solving data quality problems and privacy issues, as well as achieving interoperability and addressing resource limitations.
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Sims, J.R.; Zimmer, J.A.; Evans, C.D.; Lu, M.; Ardayfio, P.; Sparks, J.; Wessels, A.M.; Shcherbinin, S.; Wang, H.; Monkul Nery, E.S.; et al. Donanemab in Early Symptomatic Alzheimer Disease: The TRAILBLAZER-ALZ2 Randomized Clinical Trial. JAMA 2023, 330, 512–527. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Kisunla. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/kisunla (accessed on 21 August 2025).
- Biogen. Biogen to Realign Resources for Alzheimer’s Disease Franchise Biogen. Biogen. 2024. Available online: https://investors.biogen.com/news-releases/news-release-details/biogen-realign-resources-alzheimers-disease-franchise (accessed on 24 August 2025).
- Bracoud, L.; Klein, G.; Lyons, M.; Scelsi, M.A.; Wojtowicz, J.; Bullain, S.; Purcell, D.; Fiebach, J.B.; Barakos, J.; Suhy, J. Validation of 3- and 5-point severity scales to assess ARIA-E. Alzheimer’s Dement. 2023, 15, e12503, Erratum in Alzheimer’s Dement. 2024, 16, e12546. https://doi.org/10.1002/dad2.12546. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Apostolova, L.G.; Rabinovici, G.D.; Atri, A.; Aisen, P.; Greenberg, S.; Hendrix, S.; Selkoe, D.; Weiner, M.; Petersen, R.C.; et al. Lecanemab: Appropriate use recommendations. J. Prev. Alzheimer’s Dis. 2023, 10, 362–377. [Google Scholar] [CrossRef] [PubMed]
- Rabinovici, G.D.; Selkoe, D.J.; Schindler, S.E.; Aisen, P.; Apostolova, L.G.; Atri, A.; Greenberg, S.M.; Hendrix, S.B.; Petersen, R.C.; Weiner, M.; et al. Donanemab: Appropriate use recommendations. J. Prev. Alzheimer’s Dis. 2025, 12, 100150. [Google Scholar] [CrossRef] [PubMed]
- Reish, N.J.; Jamshidi, P.; Stamm, B.; Flanagan, M.E.; Sugg, E.; Tang, M.; Donohue, K.L.; McCord, M.; Krumpelman, C.; Mesulam, M.M.; et al. Multiple cerebral hemorrhages in a patient receiving lecanemab and treated with t-pa for stroke. N. Engl. J. Med. 2023, 388, 478–479. [Google Scholar] [CrossRef] [PubMed]
- Peripheral and Central Nervous System Drugs Advisory Committee. Donanemab for the Treatment of Patients with Early Symptomatic Alzheimer’s disease: Sponsor Briefing Document. Available online: https://www.fda.gov/media/179167/download (accessed on 7 October 2024).
- Greenberg, S.M.; Aparicio, H.J.; Furie, K.L.; Goyal, M.S.; Hinman, J.D.; Kozberg, M.; Leonard, A.; Fisher, M.J.; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; et al. Vascular Neurology Considerations for Antiamyloid Immunotherapy: A Science Advisory From the American Heart Association. Stroke 2025, 56, e30–e38. [Google Scholar] [CrossRef] [PubMed]
- Honig, L.S.; Sabbagh, M.N.; van Dyck, C.H.; Sperling, R.A.; Hersch, S.; Matta, A.; Giorgi, L.; Gee, M.; Kanekiyo, M.; Li, D.; et al. Updated safety results from phase 3 lecanemab study in early Alzheimer’s disease. Alzheimer’s Res. Ther. 2024, 16, 105. [Google Scholar] [CrossRef] [PubMed]
- Biogen. Available online: https://www.leqembihcp.com/about-leqembi/study-2-clarity-ad (accessed on 21 August 2025).
- Government U.K. Donanemab Licensed for Early Stages of Alzheimer’s Disease in Adult Patients Who Have One or No Copies of Apolipoprotein E4 Gene. Available online: https://www.gov.uk/government/news/donanemab-licensed-for-early-stages-of-alzheimers-disease-in-adult-patients-who-have-one-or-no-copies-of-apolipoprotein-e4-gene (accessed on 21 August 2025).
- Hampel, H.; Elhage, A.; Cho, M.; Apostolova, L.G.; Nicoll, J.A.R.; Atri, A. Amyloid-related imaging abnormalities (ARIA): Radiological, biological and clinical characteristics. Brain 2023, 146, 4414–4424. [Google Scholar] [CrossRef] [PubMed]
- Mittler, B.; Cambi, X.; Biskach, M.; Reisman, J.; Wang, Y.; Berlowitz, D.; Morin, P.; Miller, D.R.; Brandao-Viruet, K.; Clare, S.J.; et al. Patient journey and decision processes for anti-amyloid therapy in Alzheimer’s disease. J. Neurol. 2025, 272, 341. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, S.; Lan, G.; Lai, Y.J.; Wang, Q.H.; Chen, Y.; Xiao, Z.S.; Chen, X.; Bu, X.L.; Liu, Y.H.; et al. Diagnostic accuracy of plasma p-tau217/Aβ42 for Alzheimer’s disease in clinical and community cohorts. Alzheimer’s Dement. 2025, 21, e70038. [Google Scholar] [CrossRef] [PubMed]
- Medel Sánchez, A.; Ortiz Hernández, A.; Moreno Moreno, R.A.; Salas López, D.; Madrigal Gómez, L.E.; Dominguez Ibarra, A.K.; Gutiérrez Rojas, B.A.; Garcia Navarro, C.O.; Moreno Becerril, G.T.; Montelongo Quevedo, M.; et al. Aducanumab in Alzheimer’s Disease: A Comparative Study of Its Effects on Dementia and Mild Cognitive Impairment. Cureus 2024, 16, e75907. [Google Scholar] [CrossRef] [PubMed]
- Centers for Medicare and Medicaid Services. Available online: https://www.cms.gov/newsroom/fact-sheets/medicare-coverage-policy-monoclonal-antibodies-directed-against-amyloid-treatment-alzheimers-disease (accessed on 21 August 2025).
- Yoon, C.H.; Groff, C.; Criss, O. Lecanemab: A Second in Class Therapy for the Management of Early Alzheimer’s Disease. Innov. Pharm 2024, 15. [Google Scholar] [CrossRef] [PubMed]
- Eli Lilly. Lilly’s Kisunla™ (donanemab-azbt) Approved by the FDA for the Treatment of Early Symptomatic Alzheimer’s Disease. Available online: https://investor.lilly.com/news-releases/news-release-details/lillys-kisunlatm-donanemab-azbt-approved-fda-treatment-early (accessed on 21 August 2025).
- ICER. ICER Publishes Final Evidence Report on Lecanemab for Alzheimer’s Disease. Available online: https://icer.org/news-insights/press-releases/icer-publishes-final-evidence-report-on-lecanemab-for-alzheimers-disease/ (accessed on 21 August 2025).
- Fujirebio. Fujirebio Receives Marketing Clearance for Lumipulse® G pTau 217/β-Amyloid 1-42 Plasma Ratio In-Vitro Diagnostic Test as an Aid to Identify Patients with Amyloid Pathology Associated with Alzheimer’s Disease. Available online: https://www.fujirebio.com/en-us/news-events/fujirebio-receives-marketing-clearance-for-lumipulser-g-ptau-217bamyloid-142-plasma-0 (accessed on 21 August 2025).
- Palmqvist, S.; Warmenhoven, N.; Anastasi, F.; Pilotto, A.; Janelidze, S.; Tideman, P.; Stomrud, E.; Mattsson-Carlgren, N.; Smith, R.; Ossenkoppele, R.; et al. Plasma phospho-tau217 for Alzheimer’s disease diagnosis in primary and secondary care using a fully automated platform. Nat. Med. 2025, 31, 2036–2043. [Google Scholar] [CrossRef] [PubMed]
- Canadian Agency for Drugs and Technologies in Health. Health System Readiness for Disease-Modifying Therapies for Alzheimer Disease: Health Systems; Report No.: CM0003; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2024. [Google Scholar]
- Zimmer, J.A.; Ardayfio, P.; Wang, H.; Khanna, R.; Evans, C.D.; Lu, M.; Sparks, J.; Andersen, S.; Lauzon, S.; Nery, E.S.M.; et al. Amyloid-Related Imaging Abnormalities with Donanemab in Early Symptomatic Alzheimer Disease: Secondary Analysis of the TRAILBLAZER-ALZ and ALZ 2 Randomized Clinical Trials. JAMA Neurol. 2025, 82, 461–469. [Google Scholar] [CrossRef] [PubMed]
| Clarity-Ad (Lecanemab) | Trailblazer-Alz 2 (Donanemab) | |
|---|---|---|
| Study Design | Phase 3, double-blind, placebo-controlled | Phase 3, double-blind, placebo-controlled |
| Inclusion Criteria |
|
|
| N(Participants) | N = 1759 (859 patients, 875 placebo) | N = 1736 (860 patients, 878 placebo) |
| Treatment Duration | 18 months | 76 weeks |
| Dosage | Lecanemab 10 mg/kg every 2 weeks | Donanemab (700 mg for the first 3 doses and 1400 mg thereafter) every 4 weeks |
| Efficacy Outcomes | ||
| Primary Endpoint | The change in the score on the Clinical Dementia Rating–Sum of Boxes (CDR-SB)18 from baseline at 18 months. | The change in the iADRS score from baseline to 76 weeks in either the low-/medium-tau population or combined (low/medium and high tau) population. |
| Change in Primary Endpoint | 1.21 in the lecanemab group and 1.66 in the placebo group (difference, −0.45; 95% confidence interval [CI], −0.67 to −0.23; p < 0.001) | −6.02 (95% CI, −7.01 to −5.03) in the donanemab group and −9.27 (95% CI, −10.23 to −8.31) in the placebo group (difference, 3.25 [95% CI, 1.88–4.62]; p < 0.001), representing a 35.1% (95% CI, 19.90–50.23%) slowing of disease progression |
| Key Secondary Endpoints | Mean differences between the two groups in the change from baseline favoring lecanemab were as follows: Adjusted mean change from baseline at 18 months was as follows:
| Least squares mean changes were as follows:
|
| Safety Data | ||
| ARIA-E Incidence APOE e4 carrier | 12.6% with lecanemab,1.7% with placebo. Heterozygote 10.9% Homozygote 32.6% | 24.0% with donanemab, 2.1% with placebo Heterozygote 22.8% Homozygote 40.6% |
| ARIA-H Incidence APOE e4 carrier | 17.3% with lecanemab, 9.0% with placebo Heterozygote 14% Homozygote 39% | 31.4% with donanemab, 13.6% with placebo Heterozygote 32.3% Homozygote 50.3% |
| Serious Adverse Events | 14.0% with lecanemab, 11.3% with placebo | 17.4% with donanemab, 15.8% with placebo |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papaliagkas, V. Anti-Amyloid Therapies for Alzheimer’s Disease: Progress, Pitfalls, and the Path Ahead. Int. J. Mol. Sci. 2025, 26, 9529. https://doi.org/10.3390/ijms26199529
Papaliagkas V. Anti-Amyloid Therapies for Alzheimer’s Disease: Progress, Pitfalls, and the Path Ahead. International Journal of Molecular Sciences. 2025; 26(19):9529. https://doi.org/10.3390/ijms26199529
Chicago/Turabian StylePapaliagkas, Vasileios. 2025. "Anti-Amyloid Therapies for Alzheimer’s Disease: Progress, Pitfalls, and the Path Ahead" International Journal of Molecular Sciences 26, no. 19: 9529. https://doi.org/10.3390/ijms26199529
APA StylePapaliagkas, V. (2025). Anti-Amyloid Therapies for Alzheimer’s Disease: Progress, Pitfalls, and the Path Ahead. International Journal of Molecular Sciences, 26(19), 9529. https://doi.org/10.3390/ijms26199529





