Electrospun Bio-Scaffolds for Mesenchymal Stem Cell-Mediated Neural Differentiation: Systematic Review of Advances and Future Directions
Abstract
1. Introduction
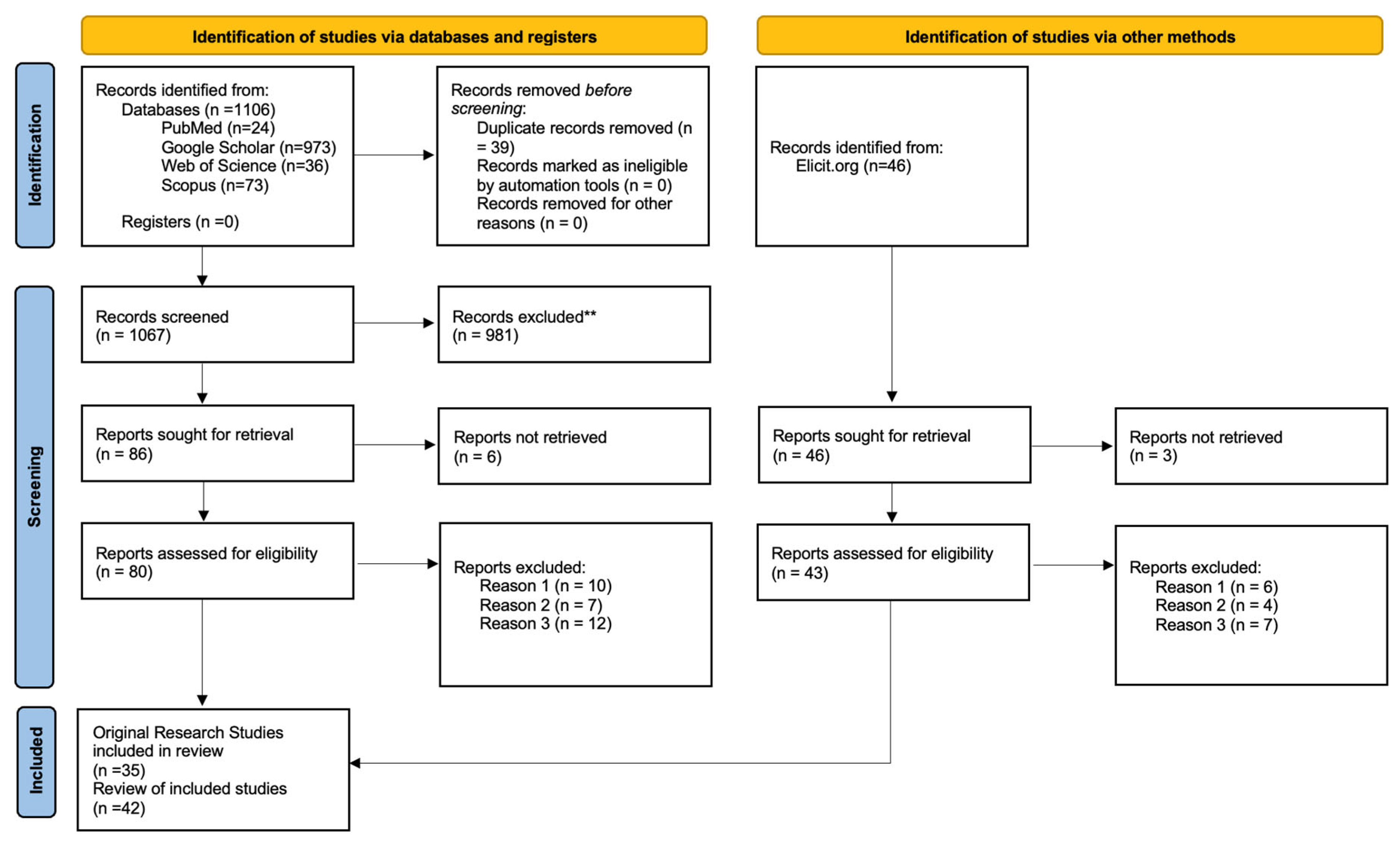
2. Methods
Literature Search Strategy
- Original experimental research or reviews published.
- Use of MSCs derived from bone marrow, adipose tissue, dental pulp, or Wharton’s Jelly.
- Application of electrospun or nanofibrous scaffolds aimed at neural differentiation or CNS regeneration.
- In vitro or in vivo validation of scaffold–MSC interactions.
- Exclusion criteria included the following (**):
- Reason 1: Non-MSC cell sources (e.g., embryonic stem cells, iPSCs).
- Reason 2: Non-electrospun scaffolds (e.g., porous foams, freeze-dried matrices, and 3D-printed).
- Reason 3: Studies not involving the nervous system or neural outcomes.
3. Results
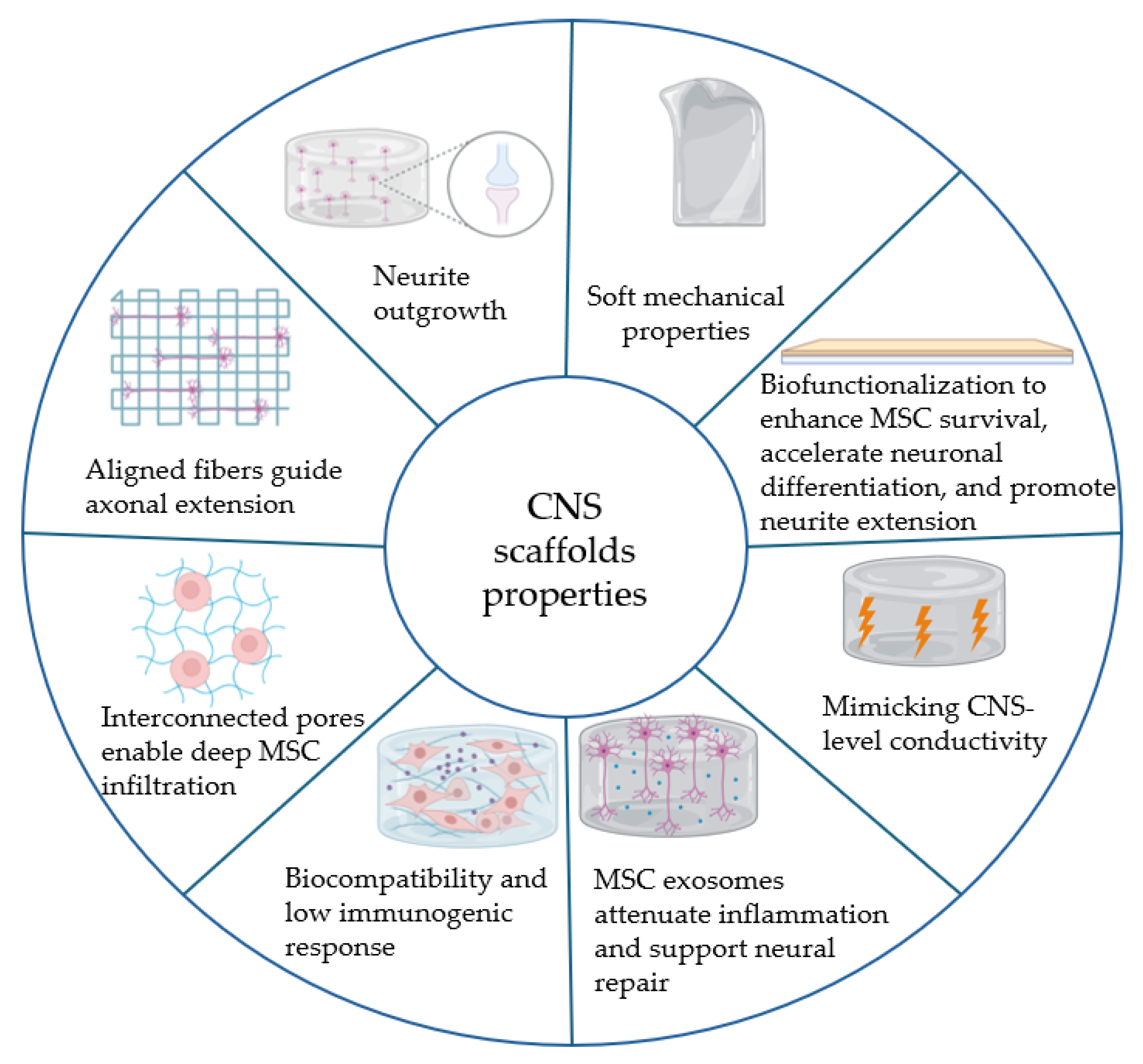
3.1. Electrospun Bio-Scaffolds for Neural Regeneration: Composition and Properties
3.2. Mesenchymal Stem Cells (MSCs) in Neural Regeneration
3.2.1. Bone Marrow MSCs (BM-MSCs)
3.2.2. Adipose-Derived MSCs (AD-MSCs)
3.2.3. Dental Pulp Stem Cells (DPSCs)
3.2.4. Other MSC Sources (Wharton’s Jelly, Conjunctiva, etc.)
3.3. In Vivo and Clinical Trial Evidence
4. Conclusions and Future Steps
Author Contributions
Funding
Data Availability Statements
Conflicts of Interest
Abbreviations
| ACS | American Chemical Society |
| AD | Alzheimer’s Disease |
| AD-MSC | Adipose–Derived Mesenchymal Stem Cell |
| ADSCs | Adipose-Derived Stem Cells |
| AFG | Aligned Fibrin Hydrogel |
| AIS | American Spinal Injury Association Impairment Scale |
| AKT | Protein kinase B |
| AMP | Adenosine Monophosphate |
| ASIA | American Spinal Injury Association Impairment Scale |
| BBB | Blood–Brain Barrier |
| BDNF | Brain–Derived Neurotrophic Factor |
| bFGF | Basic Fibroblast Growth Factor |
| BM | Bone Marrow |
| BM-MSC | Bone Marrow-Derived Mesenchymal Stem Cell |
| BMP | Bone Morphogenetic Protein |
| BMP-2 | Bone Morphogenetic Protein 2 |
| BMSC | Bone Marrow Stromal Cell |
| BMSCs | Bone Marrow Stromal Cells |
| BV2 | Mouse microglial cell line BV2 |
| B27 | B27 supplement (neuronal culture supplement) |
| CD31 | Cluster of Differentiation 31 (PECAM-1) |
| CD86 | Cluster of Differentiation 86 (M1 macrophage marker) |
| CD206 | Cluster of Differentiation 206 (M2 macrophage marker) |
| ChAT | Choline Acetyltransferase |
| CHI | Chitosan |
| CiMSCs | Conjunctiva-derived Mesenchymal Stem Cells |
| CNF | Carbon Nanofiber |
| CNT | Carbon Nanotubes |
| CFO | Cobalt Ferrite |
| CNS | Central Nervous System |
| CNPase | 2′,3′-Cyclic Nucleotide 3′-Phosphodiesterase |
| CSPGs | Chondroitin Sulfate Proteoglycans |
| CPs | Conductive Polymers |
| CRP | C-Reactive Protein |
| DC | Direct Current |
| DF-PEG | Difunctionalized Polyethylene Glycol |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DOPA | 3,4-Dihydroxyphenylalanine |
| DPSC | Dental Pulp Stem Cell |
| ECM | Extracellular Matrix |
| EGF | Epidermal Growth Factor |
| EM | Electromagnetic |
| ES | Electrical Stimulation |
| Exo | Exosomes |
| FBS | Fetal Bovine Serum |
| F-127 | Pluronic F-127 (Poloxamer 407) |
| GA | Glutaraldehyde |
| GAP-43 | Growth Associated Protein-43 |
| GC | Glycol Chitosan |
| GelMA | Gelatin Methacryloyl |
| GDNF | Glial cell line-Derived Neurotrophic Factor |
| GFAP | Glial Fibrillary Acidic Protein |
| GO | Graphene Oxide |
| HB9 | Homeobox 9 transcription factor |
| hUC-MSC | Human Umbilical Cord Mesenchymal Stem Cell |
| IBMX | 3-Isobutyl-1-methylxanthine |
| IGF-1 | Insulin-like Growth Factor 1 |
| iNOS | Inducible Nitric Oxide Synthase |
| iPSCs | Induced Pluripotent Stem Cells |
| IL | Interleukin |
| IL-4 | Interleukin-4 |
| IL-6 | Interleukin-6 |
| IL-10 | Interleukin-10 |
| IMZ | Imidazole |
| IMZ-SPECT | Iomazenil Single-Photon Emission Computed Tomography |
| Iba-1 | Ionized calcium binding adaptor molecule 1 |
| Isl-1 | Insulin gene enhancer binding protein 1 |
| IJMS | International Journal of Molecular Sciences |
| IJN | International Journal of Nanomedicine |
| kPa | kilopascal |
| LDH | Lactate Dehydrogenase |
| LMW | Low Molecular Weight |
| MAP2 | Microtubule-Associated Protein 2 |
| MAPK | Mitogen-Activated Protein Kinase |
| MAFG | Magnetic Aligned Fibrin Hydrogel |
| MBP | Myelin Basic Protein |
| MEP | Motor Evoked Potential |
| mRS | Modified Rankin Scale |
| miRNAs | MicroRNAs |
| miR-7 | MicroRNA-7 |
| mNSS | Modified Neurological Severity Score |
| mRNAs | Messenger RNAs |
| MPa | Megapascal |
| MSC | Mesenchymal Stem Cell |
| MSCs | Mesenchymal Stem Cells |
| MWCNT | Multi-Walled Carbon Nanotubes |
| mTOR | Mechanistic Target Of Rapamycin |
| NEUN | Neuronal Nuclear Antigen (NeuN) |
| Nestin | Intermediate filament protein Nestin |
| NF-L | Neurofilament Light Chain |
| NF-H | Neurofilament Heavy Chain |
| NF200 | Neurofilament 200 |
| NGF | Nerve Growth Factor |
| NGFR | Nerve Growth Factor Receptor |
| NSE | Neuron-Specific Enolase |
| NP | Nanoparticle |
| NSC | Neural Stem Cell |
| NSCs | Neural Stem Cells |
| OE-MSCs | Olfactory Ecto-Mesenchymal Stem Cells |
| OOB | Osteo–Other Bioactive component (core–shell scaffold) |
| PA | Peptide Amphiphile |
| PANI | Polyaniline |
| PC12 | Rat pheochromocytoma cell line PC12 |
| PCL | Polycaprolactone |
| PCL–PDA | PCL-Polydopamine composite |
| PDA | Polydopamine |
| PDGF-AA | Platelet-Derived Growth Factor AA |
| Pen/Strep | Penicillin-Streptomycin |
| PEDOT | Poly(3,4-ethylenedioxythiophene) |
| PI3K | Phosphoinositide 3-kinase |
| PLA | Polylactic Acid |
| PLGA | Poly(lactic-co-glycolic acid) |
| PLLA | Poly(L-lactic acid) |
| PPy | Polypyrrole |
| PRP | Platelet-Rich Plasma |
| PVA | Polyvinyl Alcohol |
| PVDF | Poly(vinylidene fluoride) |
| RA | Retinoic Acid |
| REST | Repressor Element 1-Silencing Transcription Factor |
| REST siRNA | Small interfering RNA targeting REST |
| RGD | Arginine-Glycine-Aspartic Acid peptide motif |
| RNA | Ribonucleic Acid |
| RADA16 | Self-assembling peptide (Ac-(Arg-Ala-Asp-Ala)4-CONH2) |
| SA | Sodium Alginate |
| S-100 | S-100 protein (glial marker) |
| SCI | Spinal Cord Injury |
| SD rat | Sprague-Dawley rat |
| Shh | Sonic Hedgehog |
| SDF | Stromal Cell-Derived Factor |
| SDF-1 | Stromal Cell-Derived Factor-1 |
| SNL | SIM mouse fibroblast feeder layer (SNL 76/7 cells) |
| SPECT | Single Photon Emission Computed Tomography |
| SPION | Superparamagnetic Iron Oxide Nanoparticle |
| TCP | Tissue Culture Polystyrene |
| THPC | Tetrakis(hydroxymethyl)phosphonium Chloride |
| T3 | Triiodothyronine |
| TBI | Traumatic Brain Injury |
| TGF | Transforming Growth Factor |
| TGF-β1 | Transforming Growth Factor-β1 |
| TNF | Tumor Necrosis Factor |
| TNF-α | Tumor Necrosis Factor-α |
| TPU | Thermoplastic Polyurethane |
| TUJ-1 | Class III β-Tubulin (antibody marker) |
| TM-MSCs | Trabecular Meshwork Mesenchymal Stem Cells |
| VEGF | Vascular Endothelial Growth Factor |
| WJ | Wharton’s Jelly |
| WJ-MSCs | Wharton’s Jelly Mesenchymal Stem Cells |
| WoS | Web of Science |
References
- Suzuki, H.; Imajo, Y.; Funaba, M.; Ikeda, H.; Nishida, N.; Sakai, T. Current Concepts of Biomaterial Scaffolds and Regenerative Therapy for Spinal Cord Injury. Int. J. Mol. Sci. 2023, 24, 2528. [Google Scholar] [CrossRef]
- Shen, Y.; Cao, X.; Lu, M.; Gu, H.; Li, M.; Posner, D.A. Current Treatments after Spinal Cord Injury: Cell Engineering, Tissue Engineering, and Combined Therapies. Smart Med. 2022, 1, e20220017. [Google Scholar] [CrossRef]
- Sun, Z.; Luan, X.; Sun, Z.; Li, D.; Hu, H.; Xue, Q.; Liu, B.; Yu, Q.; Wei, G.; Zhang, X.; et al. Bioactive Peptide Hydrogel Scaffold with High Fluidity, Thermosensitivity, and Neurotropism in 3D Spatial Structure for Promoted Repair of Spinal Cord Injury. Small 2025, 21, 2406990. [Google Scholar] [CrossRef]
- Tupone, M.G.; Panella, G.; d’Angelo, M.; Castelli, V.; Caioni, G.; Catanesi, M.; Benedetti, E.; Cimini, A. An Update on Graphene-Based Nanomaterials for Neural Growth and Central Nervous System Regeneration. Int. J. Mol. Sci. 2021, 22, 13047. [Google Scholar] [CrossRef]
- Liu, Y.; Xiong, W.; Li, J.; Feng, H.; Jing, S.; Liu, Y.; Zhou, H.; Li, D.; Fu, D.; Xu, C.; et al. Application of Dental Pulp Stem Cells for Bone Regeneration. Front. Med. 2024, 11, 1339573. [Google Scholar] [CrossRef]
- Rahmani, A.; Nadri, S.; Kazemi, H.S.; Mortazavi, Y.; Sojoodi, M. Conductive Electrospun Scaffolds with Electrical Stimulation for Neural Differentiation of Conjunctiva Mesenchymal Stem Cells. Artif. Organs 2019, 43, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Shirian, S.; Ebrahimi-Barough, S.; Saberi, H.; Norouzi-Javidan, A.; Mousavi, S.M.M.; Derakhshan, M.A.; Arjmand, B.; Ai, J. Comparison of Capability of Human Bone Marrow Mesenchymal Stem Cells and Endometrial Stem Cells to Differentiate into Motor Neurons on Electrospun Poly(ε-Caprolactone) Scaffold. Mol. Neurobiol. 2016, 53, 5278–5287. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Pisignano, D.; Xia, Y. Maneuvering the Migration and Differentiation of Stem Cells with Electrospun Nanofibers. Adv. Sci. 2020, 7, 2000735. [Google Scholar] [CrossRef]
- Farrukh, A.; Zhao, S.; Del Campo, A. Microenvironments Designed to Support Growth and Function of Neuronal Cells. Front. Mater. 2018, 5, 62. [Google Scholar] [CrossRef]
- Yim, E.K.F.; Jain, D.; Mattiassi, S.; Goh, E. Extracellular Matrix and Biomimetic Engineering Microenvironment for Neuronal Differentiation. Neural Regen. Res. 2020, 15, 573. [Google Scholar] [CrossRef]
- Eftekhari, B.S.; Eskandari, M.; Janmey, P.A.; Samadikuchaksaraei, A.; Gholipourmalekabadi, M. Surface Topography and Electrical Signaling: Single and Synergistic Effects on Neural Differentiation of Stem Cells. Adv. Funct. Mater. 2020, 30, 1907792. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Huang, W.-Y.; Chen, L.-H.; Liang, N.-W.; Wang, H.-C.; Lu, J.; Wang, X.; Wang, T.-W. Neural Tissue Engineering: The Influence of Scaffold Surface Topography and Extracellular Matrix Microenvironment. J. Mater. Chem. B 2021, 9, 567–584. [Google Scholar] [CrossRef]
- Zhou, J.; Cheng, L.; Sun, X.; Wang, X.; Jin, S.; Li, J.; Wu, Q. Neurogenic Differentiation of Human Umbilical Cord Mesenchymal Stem Cells on Aligned Electrospun Polypyrrole/Polylactide Composite Nanofibers with Electrical Stimulation. Front. Mater. Sci. 2016, 10, 260–269. [Google Scholar] [CrossRef]
- Sun, Z.; Hu, H.; Xu, Y.; Zhang, X.; Zheng, L.; Sun, Z.; Xiao, Y.; Dong, F.; Wei, G.; Zhang, X. Neurophilic Peptide-Reinforced Dual-Fiber-Network Bioactive Hydrogels for Spinal Cord Injury Repair. Chem. Eng. J. 2024, 498, 155301. [Google Scholar] [CrossRef]
- Tang, Y.; Friec, A.L.; Chen, M.; Sun, D. Designed Biomaterial-Enhanced Cell Transplantation for Neural Tissue Engineering. Aggregate 2025, 6, e70022. [Google Scholar] [CrossRef]
- Bagher, Z.; Ebrahimi-Barough, S.; Azami, M.; Safa, M.; Joghataei, M.T. Cellular Activity of WHarton’s JElly-derived Mesenchymal Stem Cells on Electrospun Fibrous and Solvent-cast Film Scaffolds. J. Biomed. Mater. Res. 2016, 104, 218–226. [Google Scholar] [CrossRef]
- Jahani, H.; Jalilian, F.A.; Wu, C.; Kaviani, S.; Soleimani, M.; Abbasi, N.; Ou, K.; Hosseinkhani, H. Controlled Surface Morphology and Hydrophilicity of Polycaprolactone toward Selective Differentiation of Mesenchymal Stem Cells to Neural like Cells. J. Biomed. Mater. Res. 2015, 103, 1875–1881. [Google Scholar] [CrossRef]
- Simitzi, C.; Karali, K.; Ranella, A.; Stratakis, E. Controlling the Outgrowth and Functions of Neural Stem Cells: The Effect of Surface Topography. ChemPhysChem 2018, 19, 1143–1163. [Google Scholar] [CrossRef] [PubMed]
- Fesharaki, M.; Razavi, S.; Behjati, M.; Yarahmadian, R.; Kazemi, M. Differentiation of Human Scalp Adipose-Derived Mesenchymal Stem Cells into Mature Neural Cells on Electrospun Nanofibrous Scaffolds for Nerve Tissue Engineering Applications. Cell J. 2018, 20, 168. [Google Scholar] [CrossRef] [PubMed]
- Mutepfa, A.R.; Hardy, J.G.; Adams, C.F. Electroactive Scaffolds to Improve Neural Stem Cell Therapy for Spinal Cord Injury. Front. Med. Technol. 2022, 4, 693438. [Google Scholar] [CrossRef]
- Boda, S.K.; Chen, S.; Chu, K.; Kim, H.J.; Xie, J. Electrospraying Electrospun Nanofiber Segments into Injectable Microspheres for Potential Cell Delivery. ACS Appl. Mater. Interfaces 2018, 10, 25069–25079. [Google Scholar] [CrossRef]
- Rahimzadegan, M.; Mohammadi, Q.; Shafieian, M.; Sabzevari, O.; Hassannejad, Z. Influence of Reducing Agents on in Situ Synthesis of Gold Nanoparticles and Scaffold Conductivity with Emphasis on Neural Differentiation. Biomater. Adv. 2022, 134, 112634. [Google Scholar] [CrossRef] [PubMed]
- Jedari, B.; Rahmani, A.; Naderi, M.; Nadri, S. MicroRNA-7 Promotes Neural Differentiation of Trabecular Meshwork Mesenchymal Stem Cell on Nanofibrous Scaffold. J. Cell. Biochem. 2020, 121, 2818–2827. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Carlson, M.A.; Li, X.; Siddique, A.; Zhu, W.; Xie, J. Minimally Invasive Delivery of 3D Shape Recoverable Constructs with Ordered Structures for Tissue Repair. ACS Biomater. Sci. Eng. 2021, 7, 2204–2211. [Google Scholar] [CrossRef] [PubMed]
- Raspa, A.; Pugliese, R.; Maleki, M.; Gelain, F. Recent Therapeutic Approaches for Spinal Cord Injury. Biotechnol. Bioeng. 2016, 113, 253–259. [Google Scholar] [CrossRef]
- Ramasubbu, K.; Venkatraman, G.; Ramanathan, G.; Dhanasekar, S.; Rajeswari, V.D. Molecular and Cellular Signalling Pathways for Promoting Neural Tissue Growth—A Tissue Engineering Approach. Life Sci. 2024, 346, 122640. [Google Scholar] [CrossRef]
- Hazeri, Y.; Irani, S.; Zandi, M.; Pezeshki-Modaress, M. Polyvinyl Alcohol/Sulfated Alginate Nanofibers Induced the Neuronal Differentiation of Human Bone Marrow Stem Cells. Int. J. Biol. Macromol. 2020, 147, 946–953. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, S. Potential Role of Growth Factors Controlled Release in Achieving Enhanced Neuronal Trans-Differentiation from Mesenchymal Stem Cells for Neural Tissue Repair and Regeneration. Mol. Neurobiol. 2022, 59, 983–1001. [Google Scholar] [CrossRef]
- Wei, F.; Yang, W.; Wang, H.; Song, S.; Ji, Y.; Chen, Z.; Zhuang, Y.; Dai, J.; Shen, H. Reactive Oxygen Species-Scavenging Biomaterials for Neural Regenerative Medicine. Biomater. Sci. 2025, 13, 343–363. [Google Scholar] [CrossRef]
- Amri, C.; Kim, T.-H.; Lee, J.-H. Recent Developments in Surface Topography-Modulated Neurogenesis. BioChip J. 2021, 15, 334–347. [Google Scholar] [CrossRef]
- Cai, A.; Zheng, Z.-M.; Himmler, M.; Schubert, D.W.; Fuchsluger, T.A.; Weisbach, V.; Horch, R.E.; Arkudas, A. Schwann Cells Promote Myogenic Differentiation of Myoblasts and Adipogenic Mesenchymal Stromal Cells on Poly-ε-Caprolactone-Collagen I-Nanofibers. Cells 2022, 11, 1436. [Google Scholar] [CrossRef]
- Peressotti, S.; Koehl, G.E.; Goding, J.A.; Green, R.A. Self-Assembling Hydrogel Structures for Neural Tissue Repair. ACS Biomater. Sci. Eng. 2021, 7, 4136–4163. [Google Scholar] [CrossRef] [PubMed]
- KarbalaeiMahdi, A.; Shahrousvand, M.; Javadi, H.R.; Ghollasi, M.; Norouz, F.; Kamali, M.; Salimi, A. Neural Differentiation of Human Induced Pluripotent Stem Cells on Polycaprolactone/Gelatin Bi-Electrospun Nanofibers. Mater. Sci. Eng. C 2017, 78, 1195–1202. [Google Scholar] [CrossRef]
- Bonosi, L.; Silven, M.P.; Biancardino, A.A.; Sciortino, A.; Giammalva, G.R.; Scerrati, A.; Sturiale, C.L.; Albanese, A.; Tumbiolo, S.; Visocchi, M.; et al. Stem Cell Strategies in Promoting Neuronal Regeneration after Spinal Cord Injury: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 12996. [Google Scholar] [CrossRef] [PubMed]
- Rahimi Darehbagh, R.; Mahmoodi, M.; Amini, N.; Babahajiani, M.; Allavaisie, A.; Moradi, Y. The Effect of Nanomaterials on Embryonic Stem Cell Neural Differentiation: A Systematic Review. Eur. J. Med. Res. 2023, 28, 576. [Google Scholar] [CrossRef]
- Esmaeili Abdar, Z.; Jafari, R.; Mohammadi, P.; Nadri, S. The Optimal Electrical Stimulation for Neural Differentiation of Conjunctiva Mesenchymal Stem Cells. Int. J. Artif. Organs 2022, 45, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Low, W.C.; Rujitanaroj, P.; Lee, D.; Kuang, J.; Messersmith, P.B.; Chan, J.K.Y.; Chew, S.Y. Mussel-Inspired Modification of Nanofibers for REST siRNA Delivery: Understanding the Effects of Gene-Silencing and Substrate Topography on Human Mesenchymal Stem Cell Neuronal Commitment. Macromol. Biosci. 2015, 15, 1457–1468. [Google Scholar] [CrossRef]
- Niemczyk-Soczynska, B.; Zaszczyńska, A.; Zabielski, K.; Sajkiewicz, P. Hydrogel, Electrospun and Composite Materials for Bone/Cartilage and Neural Tissue Engineering. Materials 2021, 14, 6899. [Google Scholar] [CrossRef]
- Mohammadalizadeh, M.; Dabirian, S.; Akrami, M.; Hesari, Z. SPION Based Magnetic PLGA Nanofibers for Neural Differentiation of Mesenchymal Stem Cells. Nanotechnology 2022, 33, 375101. [Google Scholar] [CrossRef]
- Yang, J.; Jin, H.; Tang, C.; Liu, L. Nanomaterials for the Treatment of Spinal Cord Injury. Appl. Mater. Today 2024, 38, 102193. [Google Scholar] [CrossRef]
- Ghorbani, S.; Tiraihi, T.; Soleimani, M. Differentiation of Mesenchymal Stem Cells into Neuron-like Cells Using Composite 3D Scaffold Combined with Valproic Acid Induction. J. Biomater. Appl. 2018, 32, 702–715. [Google Scholar] [CrossRef]
- Li, Y.; Liao, C.; Tjong, S.C. Electrospun Polyvinylidene Fluoride-Based Fibrous Scaffolds with Piezoelectric Characteristics for Bone and Neural Tissue Engineering. Nanomaterials 2019, 9, 952. [Google Scholar] [CrossRef]
- Pouladzadeh, F.; Katbab, A.A.; Haghighipour, N.; Kashi, E. Carbon Nanotube Loaded Electrospun Scaffolds Based on Thermoplastic Urethane (TPU) with Enhanced Proliferation and Neural Differentiation of Rat Mesenchymal Stem Cells: The Role of State of Electrical Conductivity. Eur. Polym. J. 2018, 105, 286–296. [Google Scholar] [CrossRef]
- Gong, L.; Cao, L.; Shen, Z.; Shao, L.; Gao, S.; Zhang, C.; Lu, J.; Li, W. Materials for Neural Differentiation, Trans-Differentiation, and Modeling of Neurological Disease. Adv. Mater. 2018, 30, 1705684. [Google Scholar] [CrossRef]
- Biazar, E. Application of Polymeric Nanofibers in Medical Designs, Part II: Neural and Cardiovascular Tissues. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 957–970. [Google Scholar] [CrossRef]
- Rasti Boroojeni, F.; Mashayekhan, S.; Abbaszadeh, H.-A.; Ansarizadeh, M.; Khoramgah, M.-S.; Rahimi Movaghar, V. Bioinspired Nanofiber Scaffold for Differentiating Bone Marrow-Derived Neural Stem Cells to Oligodendrocyte-Like Cells: Design, Fabrication, and Characterization. Int. J. Nanomed. 2020, 15, 3903–3920. [Google Scholar] [CrossRef]
- Raspa, A.; Gelain, F. Mimicking Extracellular Matrix via Engineered Nanostructured Biomaterials for Neural Repair. Curr. Neuropharmacol. 2021, 19, 2110–2124. [Google Scholar] [CrossRef]
- Hammam, I.A.; Winters, R.; Hong, Z. Advancements in the Application of Biomaterials in Neural Tissue Engineering: A Review. Biomed. Eng. Adv. 2024, 8, 100132. [Google Scholar] [CrossRef]
- Li, J.; Wu, C.; Zeng, M.; Zhang, Y.; Wei, D.; Sun, J.; Fan, H. Functional Material-Mediated Wireless Physical Stimulation for Neuro-Modulation and Regeneration. J. Mater. Chem. B 2023, 11, 9056–9083. [Google Scholar] [CrossRef] [PubMed]
- Bierman-Duquette, R.D.; Safarians, G.; Huang, J.; Rajput, B.; Chen, J.Y.; Wang, Z.Z.; Seidlits, S.K. Engineering Tissues of the Central Nervous System: Interfacing Conductive Biomaterials with Neural Stem/Progenitor Cells. Adv. Healthc. Mater. 2022, 11, 2101577. [Google Scholar] [CrossRef]
- Farokhi, M.; Mottaghitalab, F.; Saeb, M.R.; Shojaei, S.; Zarrin, N.K.; Thomas, S.; Ramakrishna, S. Conductive Biomaterials as Substrates for Neural Stem Cells Differentiation towards Neuronal Lineage Cells. Macromol. Biosci. 2021, 21, 2000123. [Google Scholar] [CrossRef]
- Nekounam, H.; Samadian, H.; Golmohammadi, H.; Asghari, F.; Shokrgozar, M.A.; Ahadian, S.; Majidi, R.F. Carbon Nanofibers Fabrication, Surface Modifications, and Application as the Innovative Substrate for Electrical Stimulation of Neural Cell Differentiation. Surf. Interfaces 2023, 40, 102926. [Google Scholar] [CrossRef]
- Guo, B.; Ma, P.X. Conducting Polymers for Tissue Engineering. Biomacromolecules 2018, 19, 1764–1782. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Álvarez, Z.; Edelbrock, A.N.; Sato, K.; Stupp, S.I. Bioactive Nanofibers Induce Neural Transdifferentiation of Human Bone Marrow Mesenchymal Stem Cells. ACS Appl. Mater. Interfaces 2018, 10, 41046–41055. [Google Scholar] [CrossRef]
- Patel, R.; Santhosh, M.; Dash, J.K.; Karpoormath, R.; Jha, A.; Kwak, J.; Patel, M.; Kim, J.H. Ile-Lys-Val-ala-Val (IKVAV) Peptide for Neuronal Tissue Engineering. Polym. Adv. Technol. 2019, 30, 4–12. [Google Scholar] [CrossRef]
- Sahab Negah, S.; Khooei, A.; Samini, F.; Gorji, A. Laminin-Derived Ile-Lys-Val-Ala-Val: A Promising Bioactive Peptide in Neural Tissue Engineering in Traumatic Brain Injury. Cell Tissue Res. 2018, 371, 223–236. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Li, X.; Liang, Z.; Wang, Z.; Shahzad, K.A.; Xu, M.; Tan, F. Local Delivery of Dual Stem Cell-Derived Exosomes Using an Electrospun Nanofibrous Platform for the Treatment of Traumatic Brain Injury. ACS Appl. Mater. Interfaces 2024, 16, 37497–37512. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, S.; Wang, R.; Che, Y.; Han, C.; Feng, W.; Wang, C.; Zhao, W. Electrospun Nanofiber/Hydrogel Composite Materials and Their Tissue Engineering Applications. J. Mater. Sci. Technol. 2023, 162, 157–178. [Google Scholar] [CrossRef]
- Shelke, N.B.; Lee, P.; Anderson, M.; Mistry, N.; Nagarale, R.K.; Ma, X.; Yu, X.; Kumbar, S.G. Neural Tissue Engineering: Nanofiber-hydrogel Based Composite Scaffolds. Polym. Adv. Technol. 2016, 27, 42–51. [Google Scholar] [CrossRef]
- Teleanu, R.I.; Gherasim, O.; Gherasim, T.G.; Grumezescu, V.; Grumezescu, A.M.; Teleanu, D.M. Nanomaterial-Based Approaches for Neural Regeneration. Pharmaceutics 2019, 11, 266. [Google Scholar] [CrossRef]
- Bakhtiary, N.; Pezeshki-Modaress, M.; Najmoddin, N. Wet-Electrospinning of Nanofibrous Magnetic Composite 3-D Scaffolds for Enhanced Stem Cells Neural Differentiation. Chem. Eng. Sci. 2022, 264, 118144. [Google Scholar] [CrossRef]
- Sykova, E.; Cizkova, D.; Kubinova, S. Mesenchymal Stem Cells in Treatment of Spinal Cord Injury and Amyotrophic Lateral Sclerosis. Front. Cell Dev. Biol. 2021, 9, 695900, Erratum in Front. Cell Dev. Biol. 2021, 9, 770243. [Google Scholar] [CrossRef]
- Valentino, C.; Vigani, B.; Sandri, G.; Ferrari, F.; Rossi, S. Current Status of Polysaccharides-Based Drug Delivery Systems for Nervous Tissue Injuries Repair. Pharmaceutics 2023, 15, 400. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Meng, Z.; He, Z.; Ma, P.; Hou, Z.; Kim, K.; Lu, J.; Yang, K.; Wang, G.; Wang, X. Engineering Neuroregenerative Microenvironment via Aligned Hydrogel-Assisted Magnetic Stimulation for Complete Spinal Cord Injury Repair. Eng. Regen. 2024, 5, 139–152. [Google Scholar] [CrossRef]
- Pei, Y.; Huang, L.; Wang, T.; Yao, Q.; Sun, Y.; Zhang, Y.; Yang, X.; Zhai, J.; Qin, L.; Xue, J.; et al. Bone Marrow Mesenchymal Stem Cells Loaded into Hydrogel/Nanofiber Composite Scaffolds Ameliorate Ischemic Brain Injury. Mater. Today Adv. 2023, 17, 100349. [Google Scholar] [CrossRef]
- Pinar, E.; Sahin, A.; Unal, S.; Gunduz, O.; Harman, F.; Kaptanoglu, E. The Effect of Polycaprolactone/Graphene Oxide Electrospun Scaffolds on the Neurogenic Behavior of Adipose Stem Cells. Eur. Polym. J. 2022, 165, 111000. [Google Scholar] [CrossRef]
- Borah, R.; Das, J.M.; Upadhyay, J. Surface Functionalized Polyaniline Nanofibers:Chitosan Nanocomposite for Promoting Neuronal-like Differentiation of Primary Adipose Derived Mesenchymal Stem Cells and Urease Activity. ACS Appl. Bio Mater. 2022, 5, 3193–3211. [Google Scholar] [CrossRef]
- Huang, L.; Fu, C.; Xiong, F.; He, C.; Wei, Q. Stem Cell Therapy for Spinal Cord Injury. Cell Transplant. 2021, 30, 0963689721989266. [Google Scholar] [CrossRef]
- Schepici, G.; Gugliandolo, A.; Mazzon, E. Serum-Free Cultures: Could They Be a Future Direction to Improve Neuronal Differentiation of Mesenchymal Stromal Cells? Int. J. Mol. Sci. 2022, 23, 6391. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, W.; Bai, Z.; Huang, S.; Jiang, K.; Liu, H.; Liu, L. Mimicking Bone Matrix through Coaxial Electrospinning of Core-Shell Nanofibrous Scaffold for Improving Neurogenesis Bone Regeneration. Biomater. Adv. 2023, 145, 213246. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, E.; Soleimani, M.; Ghiass, M.A.; Hatamie, S.; Vakilian, S.; Zomorrod, M.S.; Sadeghzadeh, N.; Vossoughi, M.; Hosseinzadeh, S. Magnetoelectric Nanocomposite Scaffold for High Yield Differentiation of Mesenchymal Stem Cells to Neural-like Cells. J. Cell. Physiol. 2019, 234, 13617–13628. [Google Scholar] [CrossRef]
- Ruan, H.; Xiao, R.; Jiang, X.; Zhao, B.; Wu, K.; Shao, Z.; Zhang, Z.; Duan, H.; Song, Y. Biofunctionalized Self-Assembly of Peptide Amphiphile Induces the Differentiation of Bone Marrow Mesenchymal Stem Cells into Neural Cells. Mol. Cell. Biochem. 2019, 450, 199–207. [Google Scholar] [CrossRef]
- Moradipour, P.; Abbasi, E.; Bagheri, F.; Zhaleh, H.; Behbood, L.; Hosseinzadeh, L.; Arkan, E. Fabrication of 3D Oriented Carbon Nanofiber by Two-Nuzzle Electrospinning as a Cell Scaffold. Cell Tissue Bank 2023, 24, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Dolatyar, B.; Zeynali, B.; Shabani, I.; Parvaneh Tafreshi, A. High-Efficient Serum-Free Differentiation of Trabecular Meshwork Mesenchymal Stem Cells into Schwann-like Cells on Polylactide Electrospun Nanofibrous Scaffolds. Neurosci. Lett. 2023, 813, 137417. [Google Scholar] [CrossRef] [PubMed]
- Habibizadeh, M.; Nadri, S.; Fattahi, A.; Rostamizadeh, K.; Mohammadi, P.; Andalib, S.; Hamidi, M.; Forouzideh, N. Surface Modification of Neurotrophin-3 Loaded PCL/Chitosan Nanofiber/Net by Alginate Hydrogel Microlayer for Enhanced Biocompatibility in Neural Tissue Engineering. J. Biomed. Mater. Res. 2021, 109, 2237–2254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Han, S.; Liang, L.; Chen, Y.; Sun, B.; Liang, N.; Feng, Z.; Zhou, H.; Sun, C.; Liu, H.; et al. Ultrasonic-Driven Electrical Signal-Iron Ion Synergistic Stimulation Based on Piezotronics Induced Neural Differentiation of Mesenchymal Stem Cells on FeOOH/PVDF Nanofibrous Hybrid Membrane. Nano Energy 2021, 87, 106192. [Google Scholar] [CrossRef]
- De, I.; Sharma, P.; Singh, M. Emerging Approaches of Neural Regeneration Using Physical Stimulations Solely or Coupled with Smart Piezoelectric Nano-Biomaterials. Eur. J. Pharm. Biopharm. 2022, 173, 73–91. [Google Scholar] [CrossRef]


| Biomaterial (Type/Composition) | Fiber Diameter/Orientation | MSC Source | Growth Factors/Stimulation | In Vitro/In Vivo | MSC Adhesion & Proliferation | Neural-like Differentiation | Mechanical Testing | Reference |
|---|---|---|---|---|---|---|---|---|
| IKVAV-PA (self-assembling peptide amphiphile with IKVAV motif) | 10.4 ± 1.7 nm; random orientation | Human BM-MSCs (ATCC, P5) | DMEM/F12 + N2/B27 + 20 ng/mL bFGF + 20 ng/mL EGF | In vitro (14 days) | 80–90% viability at 24 h; slowed proliferation | Upregulation of Nestin and GFAP at 1 wk; β-III tubulin, MAP2 and NEUN at 2 wk; neuron-like morphology | Storage modulus G′ ≈ 1500 Pa | [54] |
| CRP composite hydrogel (2% chitosan + RADA16 peptide nanofibers + PPFLMLLKGSTR) | RADA16 nanofibers 1–4 µm; random orientation | Rat BM-MSCs | None (scaffold presents neuro-affinity peptide) | In vitro and in vivo (rat spinal cord injury) | >95% viability; stable proliferation; 97.5% scratch closure at 24 h | Not assessed on MSCs; focus on NSC differentiation and functional recovery in vivo | None reported | [3] |
| IKVAV-PA hydrogels (C16H31O–A4G3D2–IKVAV) | 2–5 nm diameter; 100 nm–1.8 µm length; random network | Rat BM-MSCs (Sprague–Dawley, P3) | None (scaffold provides IKVAV cue) | In vitro only | >95% viability (days 1, 3, 5); proliferation comparable to control | ~50% NSE+ neuron-like and ~30% GFAP+ glial-like at 7 days; neuron-like morphology | None reported | [72] |
| Hybrid PCL/gelatin scaffold (PCL + gelatin + 2% PAG + 2% T3-NPs) | Gelatin/PAG: 263 nm; PCL/T3-NPs: 750 ± 72 nm; random | Rat BM-MSCs → NSCs | Scaffold releases T3 (~25 ng/mL); medium with bFGF, PDGF-AA, heregulin | In vitro only | >95% viability (days 1–3); increased attachment on 2% NP scaffold (days 5–7) | Efficient oligodendrocyte-like differentiation: PDGFR-α+, Olig2+, MBP+; high O4/O1/MOG expression | None reported | [46] |
| Hydrogel/nanofiber composite (GC/DF-PEG hydrogel + GelMA/PCL core–shell fibers) | Core–shell: 10–300 nm Ø × 100 µm length; random | Rat BM-MSCs | None (oxygen-glucose deprivation stress model) | In vitro and in vivo (rat MCAO model) | >95% viability; enhanced migration; sphere-like aggregation | Paracrine neuroprotection and angiogenesis: ↑ SH-SY5Y viability, neurite outgrowth; ↑ HUVEC tube formation | None reported | [65] |
| Plasma-treated carbon nanofibers (CNF/Plasma O2) | 157.5 ± 28.6 nm; random orientation | Human adipose-derived MSCs | Electrical stimulation: 1.5 mA, 500 Hz, 10 min/day; DMEM/F12 + 1% FBS | In vitro only | >90% viability; <7% toxicity; slight proliferative effect | Increased GFAP, MAP2, Nestin and TUJ1 expression under electrical stimulation | None reported | [52] |
| TPU/MWNT composite scaffolds (TPU + 1.5–3.5 wt% MWNT) | 220–350 nm (↓ to 220 nm at 2.5%); random orientation | Rat adipose-derived MSCs | DMEM/F12 + 10% FBS + bFGF + EGF + retinoic acid + BHA; ± EM stimulation | In vitro only | Enhanced adhesion and flattening; increased proliferation with CNT content and EM stimulation | Upregulation of β-III tubulin and MAP2; downregulation of Nestin; accelerated neural differentiation | Tensile strength 5.7 → 20.6 MPa; Young’s modulus 1.8 → 9.8 MPa; decreased elongation | [43] |
| Collagen-coated PCL nanofibers (electrospun PCL with immobilized collagen) | 400–500 nm; random orientation | WJ-MSCs | Pre-induction (24 h): DMEM/F12, 20% FBS, 2% B27, FGF2, IBMX, 2-ME; induction (7 d): DMEM/F12, B27, Shh, RA; maturation (7 d): DMEM/F12, B27, GDNF, BDNF | In vitro (15 d) | Increased adhesion, spreading, and proliferation (days 1–5) vs. controls | Increased motor neuron markers (Islet-1, Pax6, HB9, ChAT); decreased Nestin; and motor neuron maturation | None reported | [16] |
| Electrospun PCL scaffold (single-polymer PCL) | 200–300 nm; random orientation | Human bone marrow and endometrial MSCs | Pre-induction (24 h): DMEM/F12, FBS, B27, FGF2, IBMX, 2-ME; induction (7 d): DMEM/F12, B27, Shh, RA; maturation (7 d): DMEM/F12, B27, GDNF, BDNF | In vitro only | Enhanced adhesion, viability, and proliferation (days 3–5) vs. TCP | Increased motor neuron markers (Islet-1, NF-H, Pax6, HB9, β-III tubulin, ChAT) on PCL vs. TCP | None reported | [7] |
| Electrospun PCL nanofibers (8% PCL; untreated vs. O2-plasma treated) | 400–1500 nm; random or aligned; contact angle 130–134° vs. <80° | hMSCs | DMEM/F12 + BDNF + bFGF + NT-3 + NGF + IBMX (15 d) | In vitro (15 d) | Increased adhesion, spreading, and proliferation on p-PCL vs. TCP; random > aligned | Decreased Nestin; increased MAP2 expression; β-tubulin III positive; cells align along fibers | Tensile stress aligned: 24.11→20.97 MPa; random: 1.85→1.68 MPa; strain aligned: 51.4→47.2%; random: 363.8→247.4% | [17] |
| PCL/gelatin/PRP nanofibrous scaffold (70:30 PCL/gelatin + PRP) | 189 ± 56 nm; random | Human scalp adipose-MSCs (P3) | DMEM/F12 + 10% FBS + insulin + indomethacin + IBMX | In vitro only | Gelatin ↑ prolif. vs. PCL; PRP coating further enhances this effect | Nestin and NEUN (early) and MAP2 & TAU (mature) expression; GFAP absent; no difference between scaffolds | None reported | [19] |
| Wet-electrospun PLA scaffold (15% w/v PLA + gelatin/alginate/MWCNT) | Random non-woven with internal pores | WJ-MSCs (P4) | 1 mM valproic acid in DMEM/F12 + 10% FBS | In vitro (21 d) | Live/dead: >95% viability; MTT: 91% vs. 76% a 24 h | Nestin, MAP2, and NSE positive; ↑ NeuroD1 & Nestin; ↓ Sox2 | None reported | [41] |
| PCL-gelatin NF microspheres (electrosprayed NF segments) | 150–450 µm (varia con voltaggio); random | Rat BM-MSCs | None reported | In vitro only | Adhesion and proliferation increased on nanofibers compared to solid microspheres (p < 0.05) | β-III tubulin+ extended neurites on NF microspheres; few cells on solid microspheres | None reported | [21] |
| Magnetic-responsive aligned fibrin hydrogel (5 wt% fibrinogen + PEO + 10 mg/mL Fe3O4 MNPs) | Aligned (rotor at 50 rpm); diameter not reported | Rat embryonic (E18) NSCs | Magnetic field 200 mT, 3 h/day + EGF (20 ng/mL) & bFGF (10 ng/mL) | In vitro and in vivo | Viability maintained; adhesion and proliferation aligned | ↑ Tuj1, NSE, MBP, MOG in vitro; in vivo: ↑ Tuj1+ aligned axons, ↑ Nestin+/Tuj1+ NSC, ↑ synaptophysin and NF200; axonal continuity; improved BBB, CatWalk, and MEP recovery | None reported | [64] |
| 3D oriented carbon nanofiber scaffold (two-nozzle PAN) | Pre-carboniz. 217–343 nm; post 259–797 nm; best at 300 rpm | Mouse BM-MSCs and PC12 | None reported | In vitro only | MTT: ↑ viability 24→48 h; LDH/Caspase-3: non-toxic; adhesion superior vs. 2D | Not evaluated (no differentiation marker reported) | None reported | [73] |
| PLA electrospun scaffold (plasma-treated) | 565 ± 18 nm; random | TM-MSCs (trabecular meshwork MSCs) | Neurosphere induction (bFGF, EGF, B27, NEAA) + Schwann induction (forskolin, PDGF-AA, heregulin-β, bFGF) | In vitro (14 d) | High viability; ↑ adhesion vs. TCP; proliferation maintained | ↑ S100B, GAP43, GFAP, SOX10; MBP ↑ with serum, ↓ in KOSR; Schwann-like bipolar/tripolar morphology | None reported | [74] |
| Electrospun PCL/chitosan + in situ Au-NPs (THPC + formaldehyde) | 70.8 ± 12.7 nm; random | MSCs (not specified) | Pre-induction (24 h): DMEM/F12 + 20% FBS + 2% B27 + 10 ng/mL FGF2 + 250 µM IBMX + 100 µM 2-ME; induction (9 d): DMEM/F12 + 0.2% B27 + 100 ng/mL SHH + 0.01 ng/mL RA | In vitro only | None reported | 57% β-III tubulin+ vs. 26% su TCP; ↑ β-III tubulin protein (p < 0.05) | Tensile strength 14.47 ± 2.32 MPa; Young’s modulus 127.67 ± 34.51 kPa; and ultimate strain 44.77 ± 6.24% | [22] |
| Electrospun PCL–PDA nanofibers (dual exosome delivery) | Random porous; diameter similar to PCL NF | hUC-MSC-Exo and mouse NSC-Exo | Local release of dual exosomes (100 µg/mL each) | In vitro (BV2 and PC12) and in vivo | None reported | ↓ M1 (CD86, TNF-α, iNOS) and ↑ M2 (CD206, TGF-β, IL-4) in BV2; ↑ migrazione and neuritogenesi PC12; in vivo: ↓ mNSS and miss-step, ↑ rotarod; ↑ GAP-43 and DCX, ↓ GFAP and Iba-1 | None reported | [57] |
| Magnetoelectric PVDF/GO/CFO nanofibrous scaffold | 266.9 ± 189.7 nm; random | Human adipose-MSCs (P3) | EM field 1 mT, 50 Hz, 8 h/day; no exogenous factors. | In vitro only | Viability maintained; ↑ proliferation over 21 d (MTT) | ↑ Nestin, β-III tubulin & NSE genes; NGFR p75 IF; cell alignment under EM | Tensile strength 6.01 ± 1.02 MPa; strain 12.35 ± 2.89%; Young’s modulus 71.31 ± 6.34 MPa | [71] |
| PLLA/PCL hybrid nanofibrous scaffold (1:1) | ~1.1 µm; allineate (collector 3000 rpm) | TM-MSCs (trabecular meshwork MSCs) | miR-7 overexpression via lentivirus (MOI 15) | In vitro only | Uniform adhesion; viability maintained for 21 d | ↑ MAP-2, Nestin and GFAP mRNA a 21 d vs. control; IF conferma ↑ MAP-2 and Nestin | None reported | [23] |
| Core–shell nanofibrous scaffold: core = Mg-doped mesoporous bioactive glass + OOB; shell = silk fibroin + NGF | Random; diameter not reported | Primary bone-derived MSCs (BMSCs) | NGF (7.2 µg in shell); neurobasal + B27, EGF 20 ng/mL, FGF 20 ng/mL for 7 d | In vitro and in vivo (mouse cranial defect) | Viability ↑ day 1–7 | Neurite-like morphology; ↑ β-III tubulin, MAP2, NSE in vitro; new neurons in Haversian canals in vivo | None reported | [70] |
| 0.05% GelMA-coated PCL/0.5% Pluronic F-127 scaffold: PCL (80 kDa) + Pluronic core; 0.05% GelMA coating | Aligned along expansion axis | Rat BMSCs | DMEM + 10% FBS (no added GFs) | In vitro only | ↑ adhesion and proliferation day 1–14; viability maintained after minimally invasive delivery | n.a. for MSCs (hNSCs tested separately) | Cyclic compression (50%, 70%, 90% strain; Instron 5640, 9 mm/min): full recovery after 100× 90% cycles; modulus from 0–30% strain; pore-size recovery measured | [24] |
| PCL nanofibers + DOPA-melanin coating for REST siRNA | Random: 545 ± 9 nm (PCL-RF), 553 ± 13 nm (DM-RF); aligned: 567 ± 12 nm (PCL-AF), 574 ± 13 nm (DM-AF) | Human fetal BM-MSCs | REST siRNA (2–4 µg); neural medium DMEM + 1% FBS + 1% N2 + 1% B27 | In vitro only | None reported | ↑ Tuj1 (d 7–21); MAP2 by d 14 only on aligned; ↓ GFAP; minimal glial (O4/Olig2); synapsin not detected | None reported | [37] |
| PCL/GEL bi-electrospun nanofibers: PCL (80 kDa)/Type A Gelatin | PCL: 836 ± 50 nm; PCL/GEL: 407 ± 30 nm; orientation not reported | Human iPSCs (SNL feeder) | Neural induction: DMEM/F12 + 0.5 mM IBMX, forskolin; FBS: 10% (d 1–5), 5% (d 6–10), 2% (d 11–14) | In vitro only | ↑ viability on PCL/GEL > PCL > TCPS (d 1–5); >95% at d 3 | ↑ NSE, MAP2, βIII-tubulin, Olig2, GFAP vs. TCPS; ICC confirmation | Tensile (10 mm/min): PCL σu = 3.4 ± 0.2 MPa, εu = 70%; PCL/GEL σu = 3.2 ± 0.2 MPa, εu = 25%; Young’s modulus ↑ for PCL/GEL | [33] |
| PCL-SA nanofiber–hydrogel composite: PCL lattice (600–900 nm) + LMW sulfated alginate (0.004 wt% laminin) | 600–900 nm; aligned & random | hMSCs (P5) | Laminin (0.004 wt%); NGF 50 ng/mL in induction medium | In vitro only | DNA content ↑ ~2× vs. PCL (d 7 and 14); viability > 95% | ↑ S-100 expression (d 7, 21); neurite extensions | Tensile (20 × 10 mm; Instron 5544, 10 mm/min): modulus 20–35 MPa; max load ↑; suture pull-out 18–20 N; LMW SA > HMW SA; aligned > random | [59] |
| PPy/PLA composite nanofiber film: PPy nanoparticles embedded in PLA | 315.2 ± 3.7 nm; random & aligned | hUC-MSC | DC electrical stimulation 100 mV/mm, 30 min/day × 5 d | In vitro only | ↑ adhesion and proliferation vs. PLA film over 5 d | Alignment + ES ↑ NF-L and nestin (~3× vs. TCP); neurite-like outgrowth on aligned + ES | Anisotropic: Along fibers: E = 45.2 MPa, σmax = 4.9 MPa, εβ = 30.7%; ⟂ fibers: E = 14.1 MPa, σmax = 1.3 MPa, εβ = 60% | [13] |
| PVA/SA electrospun nanofibers (30 wt% SA) | Random; 169 ± 34 nm (30 wt%), 289 ± 66 nm (20 wt%), 488 ± 176 nm (10 wt%) vs. PVA 584 ± 179 nm | hBM-MSCs | β-Carotene 5 and 20 µM in DMEM + 10% FBS for 4 d | In vitro only | Viability ↑ 24 h–21 d (p ≤ 0.005) | MAP2 expression and ICC positive at d 7 and 14; neuritic morphology | None reported | [27] |
| Aligned PCL–collagen I nanofibers (Mb/ADSC ± Schwann cells 1:1:0.5) | Aligned; diameter not reported | Human myoblasts + ADSCs ± Schwann cells | Myogenic medium: DMEM/Ham’s F12 + 2% horse serum + 1 ng/mL bFGF + 0.4 µg/mL dexamethasone + L-glutamine + pen/strep | In vitro only | Good viability over 28 d; no differences in WST-8 or live/dead indices between co-cultures | SC co-cultures ↑ myotube fusion index and MYH2/MYOG vs. Mb/ADSC; aligned multinucleated myotubes | None reported | [31] |
| Magnetic PLGA nanofibers (0%, 5%, 10% SPION): PLGA (75/25) + SPION | <100 nm; aligned via 3000 rpm rotating collector | hAD-MSC | None; SPION provides magnetic stimulus | In vitro only | Viability and proliferation on 5% and 10% vs. control; 10% > 0% | TUJ-1 ↑ 3.8× (10%) and 1.8× (5%); NSE ↑ 6.3× and 1.2× vs. 0%; ICC confirms ↑ with SPION | Tensile strength 4.08→5.85 MPa (0→10%); elongation ↓; modulus ↑ (20 mm/min) | [39] |
| Polyaniline–Chitosan nanocomposite (4 wt% PAni in CHI; GA-functionalized) | ~35 ± 7 nm (TEM); random | Primary AD-MSC | bFGF and EGF, 10 ng/mL each | In vitro only | Improved viability, adhesion and spreading vs. non-functionalized | >85% βIII-tubulin+; ~40% GFAP+; pronounced neurite-like projections after 14 d | E = 16–26 kPa; UTS = 347–445 kPa (functionalized) vs. E = 18 kPa; UTS = 379 kPa (non-functionalized) | [67] |
| PCL/chitosan nanofiber/net + alginate hydrogel microlayer (NT-3 loaded) | Random; ~275 nm thick fibers and ~20 nm ultrathin NFN | Human conjunctiva MSCs (CJMSCs) | NT-3 burst 69% @ 3 d; sustained 90% @ 21 d + DMEM + 10% FBS | In vitro (neural assays); in vivo (subcutaneous) | Alginate coat ↑ CJMSC proliferation vs. unmodified; entrapment design best | RT-PCR: Nestin ↑ 6×; MAP-2 ↑ 5.4×; β-III tubulin ↑ 8.8×; ICC: ↑ Nestin and MAP-2; SEM: neuron-like morphology | PCL/chitosan mat (no coat): thickness 50.3 µm; tensile modulus 53.0 ± 25.9 MPa; UTS 33.1 ± 18.3 MPa; strain at break 1.56 ± 1.08%; yield 0.22 ± 0.07 MPa | [75] |
| PCL + graphene oxide (0.1 wt% GO) composite nanofibers | PCL: 485 ± 162 nm; PCL+GO: 628 ± 238 nm; random | Human subcutaneous and epidural ADSCs | DMEM + 1% pen/strep, 0.5 mM IBMX, 10 ng/mL BDNF, EGF, bFGF + 20% NSC supplement | In vitro only | GO enhances attachment, proliferation and infiltration vs. PCL alone | Nestin and GFAP expressed in all groups; PCL+GO directs spontaneous MAP2 & CNPase differentiation in epidural ADSCs even without induction medium | None reported | [66] |
| PCL–PPy conductive nanofiber scaffold | PCL: 492 nm; PCL–PPy: 423 nm; random | CJMSCs | Electrical stimulation 115 V/m, 100 Hz, 1 min/day × 3 days; no added GFs | In vitro only | Viability on PCL–PPy > PCL > TCP at days 3/5/7; ES further enhances viability | qPCR (1 min/day): Nestin ~127×; β-tubulin ~30×; MAP-2 ~52× vs. non-stimulated | Tensile: PCL UTS 25 MPa → PCL–PPy UTS 10 MPa; elongation 60% → 35% (STM-50, 5 mm/min) | [36] |
| FeOOH/PVDF piezotronic hybrid membrane | PVDF ~600 nm; FeOOH nanorods 80–100 nm × 600–800 nm; random 3D network | Rat BMSCs | Ultrasonic 400 W, 8 min twice daily; no exogenous GFs | In vitro only | CCK-8: viability PVDF ≈ 82% TCP; FeOOH/PVDF slightly lower; US ↑ proliferation | qPCR (21 d + US): Nestin ↑ 89×, Tuj1 ↑ 128×, MAP2 ↑ 220×; no GFAP; GAD65 ↑ 30×, ChAT ↑ 5.5×, DβH ↑ 21×; ICC: Nestin, Tuj1, MAP2; Ca2+ imaging: GABA transients (~1.8×) | PFM: d33 PVDF 26.8 pC/N; FeOOH/PVDF 27.2 pC/N; ultrasonic piezo-voltage ~2 V | [76] |
| PCL/gelatin (70:30)/SPION 3D scaffold (wet-electrospun at 350 mT) | 605 ± 169 nm; random network under 350 mT | Human olfactory ecto-MSCs (OE-MSCs) | None (no exogenous GFs) | In vitro only | MTT: proliferation on 350 mT > 500 mT scaffolds over 7 d (p < 0.05) | RT-PCR (14 d): Nestin ↓; MAP2 ↑ (p < 0.05); ICC: β-III tubulin and MAP2; Ca2+ imaging: GABA transients confirm functional neurons | Tensile: UTS 0.13 ± 0.06 MPa; Young’s 0.50 ± 0.10 MPa; elongation ~29.6% | [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruccolo, L.; Evangelista, A.; Benazzo, M.; Conti, B.; Pisani, S. Electrospun Bio-Scaffolds for Mesenchymal Stem Cell-Mediated Neural Differentiation: Systematic Review of Advances and Future Directions. Int. J. Mol. Sci. 2025, 26, 9528. https://doi.org/10.3390/ijms26199528
Ruccolo L, Evangelista A, Benazzo M, Conti B, Pisani S. Electrospun Bio-Scaffolds for Mesenchymal Stem Cell-Mediated Neural Differentiation: Systematic Review of Advances and Future Directions. International Journal of Molecular Sciences. 2025; 26(19):9528. https://doi.org/10.3390/ijms26199528
Chicago/Turabian StyleRuccolo, Luigi, Aleksandra Evangelista, Marco Benazzo, Bice Conti, and Silvia Pisani. 2025. "Electrospun Bio-Scaffolds for Mesenchymal Stem Cell-Mediated Neural Differentiation: Systematic Review of Advances and Future Directions" International Journal of Molecular Sciences 26, no. 19: 9528. https://doi.org/10.3390/ijms26199528
APA StyleRuccolo, L., Evangelista, A., Benazzo, M., Conti, B., & Pisani, S. (2025). Electrospun Bio-Scaffolds for Mesenchymal Stem Cell-Mediated Neural Differentiation: Systematic Review of Advances and Future Directions. International Journal of Molecular Sciences, 26(19), 9528. https://doi.org/10.3390/ijms26199528












