Identification of Anticancer Targets in Ovarian Cancer Using Genomic Drug Sensitivity Data
Abstract
1. Introduction
2. Results and Discussion
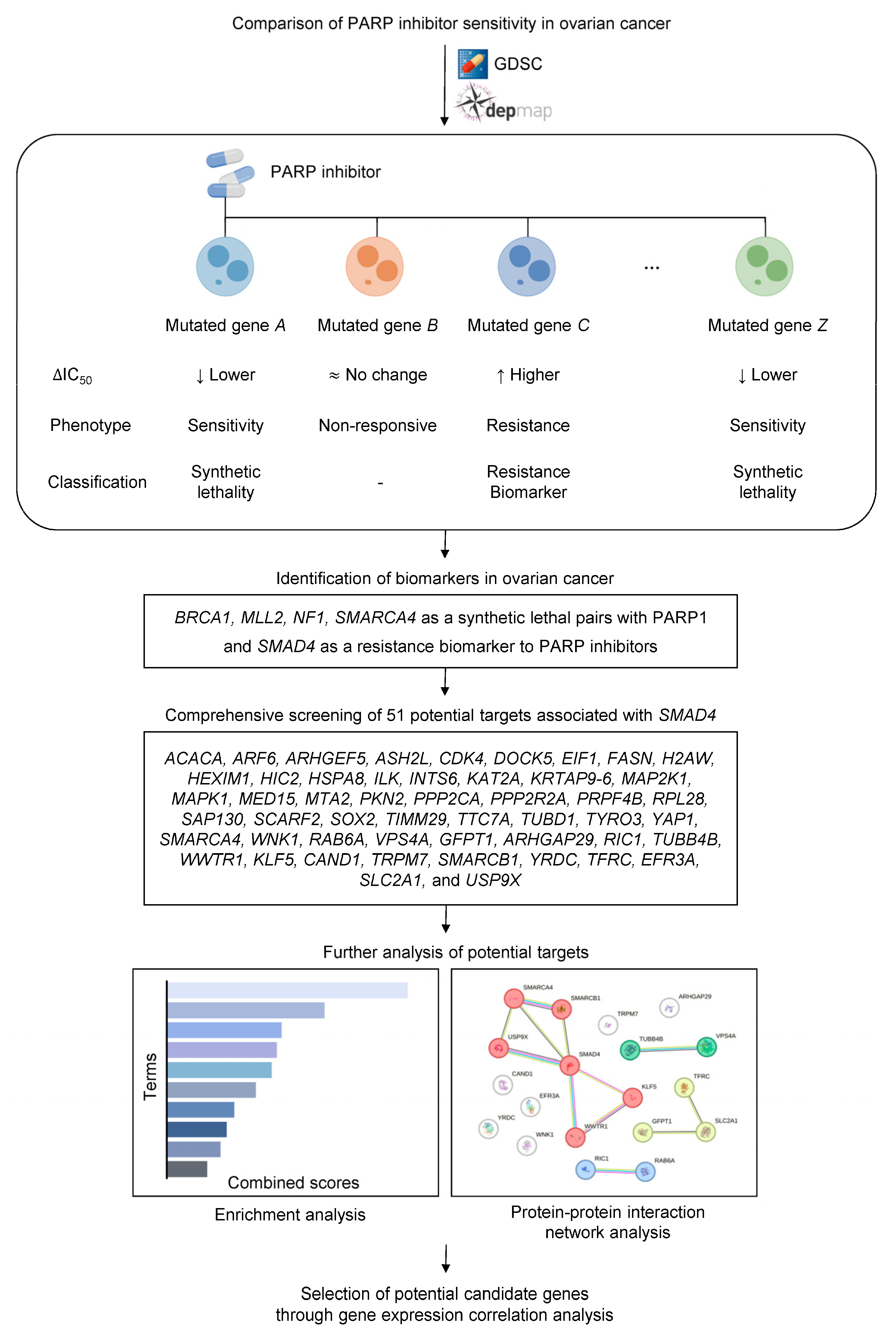
2.1. Identification of Sensitivity and Resistance Biomarkers to PARP Inhibitors
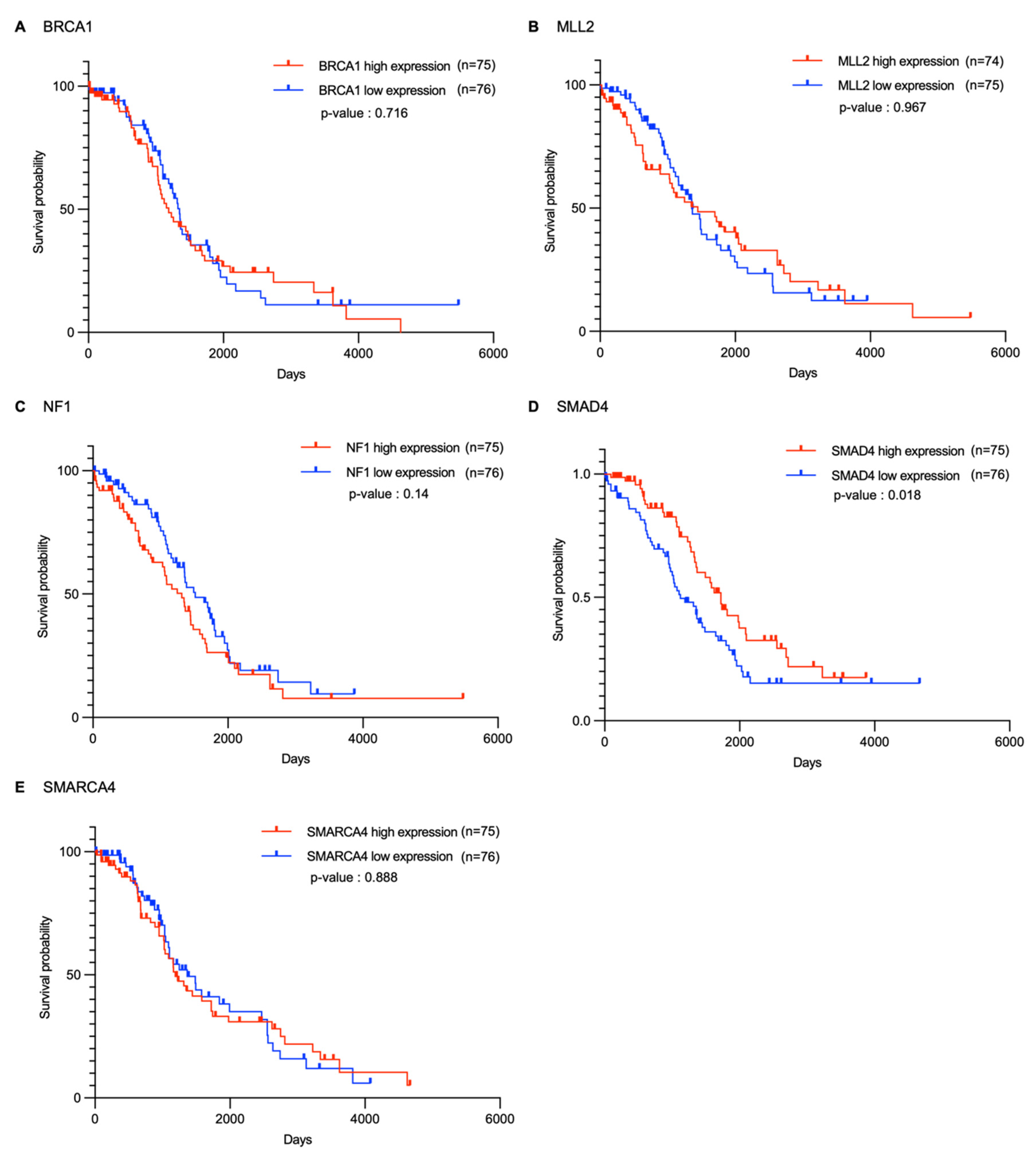
2.2. Survival Analysis of Candidate Genes in Ovarian Cancer
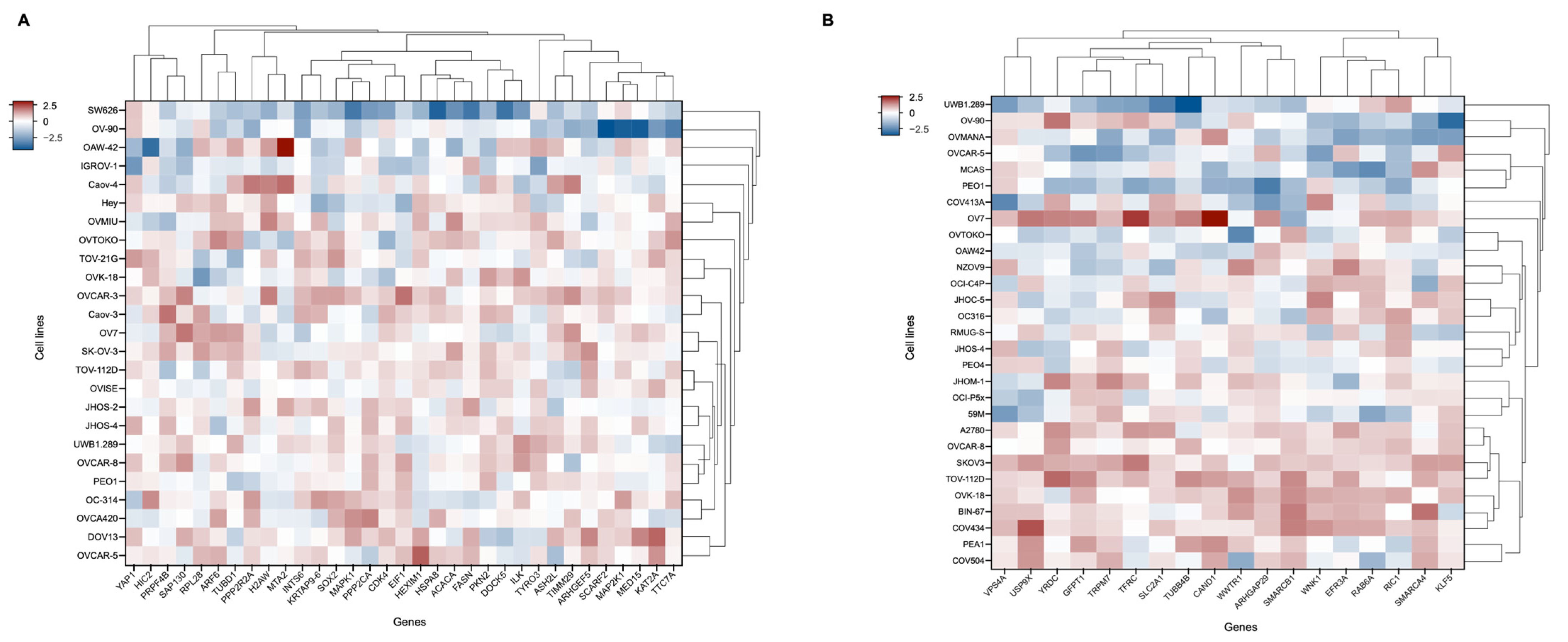
2.3. Gene Dependency Score-Based Identification and Functional Analysis of SMAD4-Mediated Resistance
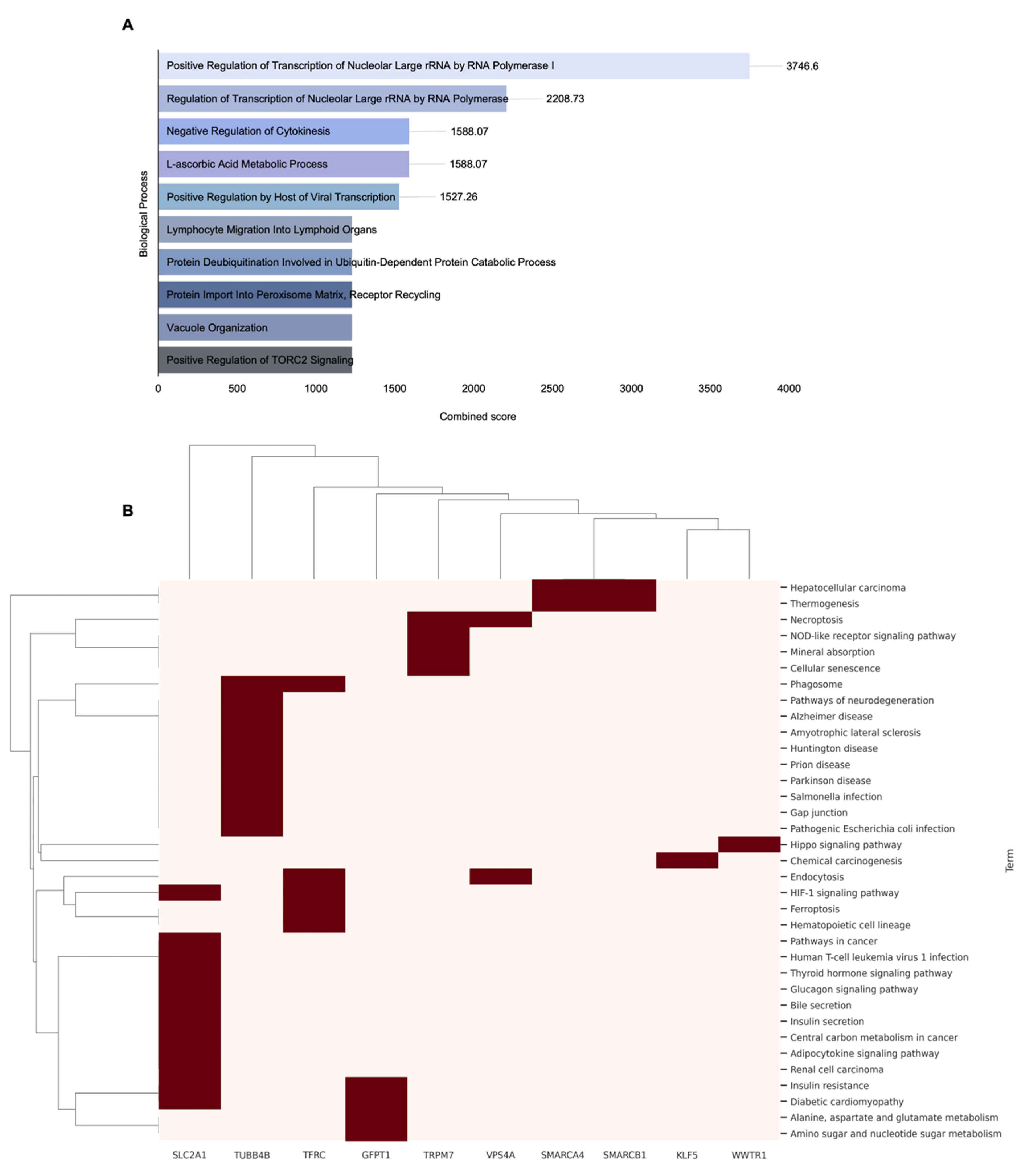
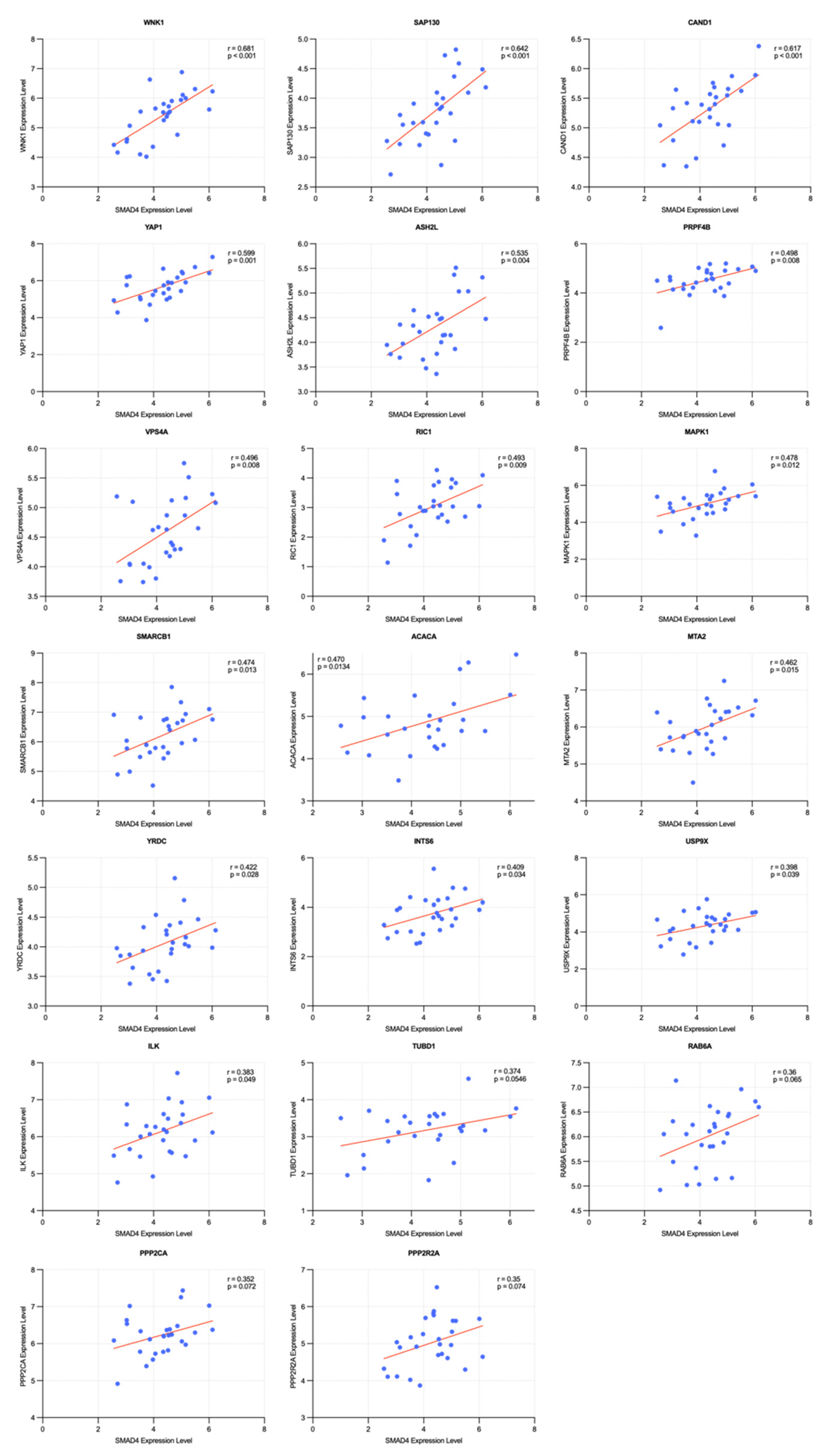
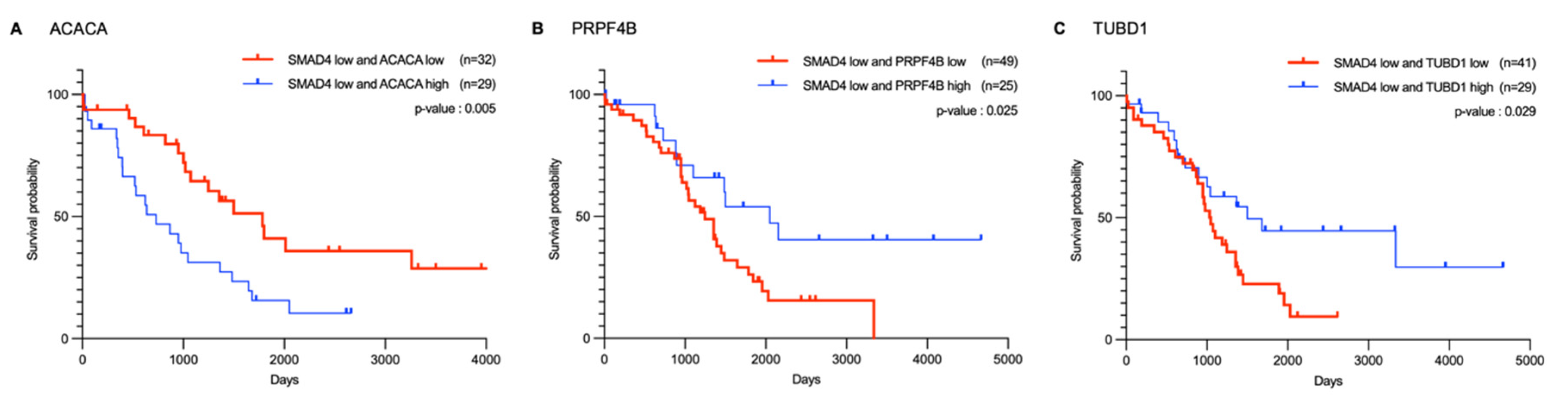
2.4. Correlation and Survival Analysis of SMAD4-Associated Genes
3. Materials and Methods
3.1. Data Acquisition and Target Gene Selection
3.2. Drug Sensitivity Analysis
3.3. Survival Analysis
3.3.1. Kaplan–Meier Estimator
3.3.2. Log-Rank Test
3.4. Gene Dependency Score Analysis
3.5. Enrichment and Functional Analysis
3.5.1. Gene Ontology Biological Process Analysis and Pathway Analysis
3.5.2. Hierarchical Clustering and Visualization
3.5.3. Protein–Protein Interaction Network Analysis
3.6. Correlation Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CI | Confidence Interval |
| DepMap | Cancer Dependency Map |
| FDA | Food and Drug Administration |
| GDSC | The Genomics of Drug Sensitivity in Cancer |
| GO | Gene Ontology |
| HR | Hazard Ratio |
| HRD | Homologous Recombination Deficiency |
| PARP | Poly (ADP-ribose) polymerase |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| MST | Median Survival Time |
| PPI | Protein–Protein Interaction |
| TCGA | The Cancer Genome Atlas |
References
- Reid, B.M.; Permuth, J.B.; Sellers, T.A. Epidemiology of ovarian cancer: A review. Cancer Biol. Med. 2017, 14, 9–32. [Google Scholar] [CrossRef]
- Jones, P.M.; Drapkin, R. Modeling High-Grade Serous Carcinoma: How Converging Insights into Pathogenesis and Genetics are Driving Better Experimental Platforms. Front. Oncol. 2013, 3, 217. [Google Scholar] [CrossRef]
- Gloeckler Ries, L.A.; Reichman, M.E.; Lewis, D.R.; Hankey, B.F.; Edwards, B.K. Cancer survival and incidence from the Surveillance, Epidemiology, and End Results (SEER) program. Oncologist 2003, 8, 541–552. [Google Scholar] [CrossRef]
- Chen, G.M.; Kannan, L.; Geistlinger, L.; Kofia, V.; Safikhani, Z.; Gendoo, D.M.A.; Parmigiani, G.; Birrer, M.; Haibe-Kains, B.; Waldron, L. Consensus on Molecular Subtypes of High-Grade Serous Ovarian Carcinoma. Clin. Cancer Res. 2018, 24, 5037–5047. [Google Scholar] [CrossRef]
- Stewart, M.D.; Merino Vega, D.; Arend, R.C.; Baden, J.F.; Barbash, O.; Beaubier, N.; Collins, G.; French, T.; Ghahramani, N.; Hinson, P.; et al. Homologous Recombination Deficiency: Concepts, Definitions, and Assays. Oncologist 2022, 27, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, M.M.; Sundar, R.; Tan, D.S.P.; Jeyasekharan, A.D. Biomarkers for Homologous Recombination Deficiency in Cancer. J. Natl. Cancer Inst. 2018, 110, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Cortesi, L.; Rugo, H.S.; Jackisch, C. An Overview of PARP Inhibitors for the Treatment of Breast Cancer. Target. Oncol. 2021, 16, 255–282. [Google Scholar] [CrossRef] [PubMed]
- Bryant, H.E.; Schultz, N.; Thomas, H.D.; Parker, K.M.; Flower, D.; Lopez, E.; Kyle, S.; Meuth, M.; Curtin, N.J.; Helleday, T. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 2005, 434, 913–917. [Google Scholar] [CrossRef]
- Farmer, H.; McCabe, N.; Lord, C.J.; Tutt, A.N.; Johnson, D.A.; Richardson, T.B.; Santarosa, M.; Dillon, K.J.; Hickson, I.; Knights, C.; et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005, 434, 917–921. [Google Scholar] [CrossRef]
- Huang, A.; Garraway, L.A.; Ashworth, A.; Weber, B. Synthetic lethality as an engine for cancer drug target discovery. Nat. Rev. Drug Discov. 2020, 19, 23–38. [Google Scholar] [CrossRef]
- Hartwell, L.H.; Szankasi, P.; Roberts, C.J.; Murray, A.W.; Friend, S.H. Integrating genetic approaches into the discovery of anticancer drugs. Science 1997, 278, 1064–1068. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Topatana, W.; Juengpanich, S.; Cao, J.; Hu, J.; Zhang, B.; Ma, D.; Cai, X.; Chen, M. Development of synthetic lethality in cancer: Molecular and cellular classification. Signal Transduct. Target. Ther. 2020, 5, 241. [Google Scholar] [CrossRef] [PubMed]
- Daly, G.R.; AlRawashdeh, M.M.; McGrath, J.; Dowling, G.P.; Cox, L.; Naidoo, S.; Vareslija, D.; Hill, A.D.K.; Young, L. PARP Inhibitors in Breast Cancer: A Short Communication. Curr. Oncol. Rep. 2024, 26, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Teyssonneau, D.; Margot, H.; Cabart, M.; Anonnay, M.; Sargos, P.; Vuong, N.S.; Soubeyran, I.; Sevenet, N.; Roubaud, G. Prostate cancer and PARP inhibitors: Progress and challenges. J. Hematol. Oncol. 2021, 14, 51. [Google Scholar] [CrossRef]
- Burger, R.A.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Monk, B.J.; Huang, H.; Mannel, R.S.; Homesley, H.D.; Fowler, J.; Greer, B.E.; et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N. Engl. J. Med. 2011, 365, 2473–2483. [Google Scholar] [CrossRef]
- Johnson, N.; Johnson, S.F.; Yao, W.; Li, Y.C.; Choi, Y.E.; Bernhardy, A.J.; Wang, Y.; Capelletti, M.; Sarosiek, K.A.; Moreau, L.A.; et al. Stabilization of mutant BRCA1 protein confers PARP inhibitor and platinum resistance. Proc. Natl. Acad. Sci. USA 2013, 110, 17041–17046. [Google Scholar] [CrossRef]
- Sahu, A.D.; Lee, J.S.; Wang, Z.; Zhang, G.; Iglesias-Bartolome, R.; Tian, T.; Wei, Z.; Miao, B.; Nair, N.U.; Ponomarova, O.; et al. Genome-wide prediction of synthetic rescue mediators of resistance to targeted and immunotherapy. Mol. Syst. Biol. 2019, 15, e8323. [Google Scholar] [CrossRef]
- Schaffer, A.A.; Chung, Y.; Kammula, A.V.; Ruppin, E.; Lee, J.S. A systematic analysis of the landscape of synthetic lethality-driven precision oncology. Med 2024, 5, 73–89.e9. [Google Scholar] [CrossRef]
- Sahu, D.; Shi, J.; Segura Rueda, I.A.; Chatrath, A.; Dutta, A. Development of a polygenic score predicting drug resistance and patient outcome in breast cancer. NPJ Precis. Oncol. 2024, 8, 219. [Google Scholar] [CrossRef]
- Levatic, J.; Salvadores, M.; Fuster-Tormo, F.; Supek, F. Mutational signatures are markers of drug sensitivity of cancer cells. Nat. Commun. 2022, 13, 2926. [Google Scholar] [CrossRef]
- Garnett, M.J.; Edelman, E.J.; Heidorn, S.J.; Greenman, C.D.; Dastur, A.; Lau, K.W.; Greninger, P.; Thompson, I.R.; Luo, X.; Soares, J.; et al. Systematic identification of genomic markers of drug sensitivity in cancer cells. Nature 2012, 483, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Guo, M. Synthetic lethality strategies: Beyond BRCA1/2 mutations in pancreatic cancer. Cancer Sci. 2020, 111, 3111–3121. [Google Scholar] [CrossRef] [PubMed]
- Tsujino, T.; Takai, T.; Hinohara, K.; Gui, F.; Tsutsumi, T.; Bai, X.; Miao, C.; Feng, C.; Gui, B.; Sztupinszki, Z.; et al. CRISPR screens reveal genetic determinants of PARP inhibitor sensitivity and resistance in prostate cancer. Nat. Commun. 2023, 14, 252. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, M.; Huang, X.; Wang, L.; Zhang, S.; Liu, H.; Zheng, J. SynLethDB 2.0: A web-based knowledge graph database on synthetic lethality for novel anticancer drug discovery. Database 2022, 2022, baac030. [Google Scholar] [CrossRef]
- Chang, A.; Liu, L.; Ashby, J.M.; Wu, D.; Chen, Y.; O’Neill, S.S.; Huang, S.; Wang, J.; Wang, G.; Cheng, D.; et al. Recruitment of KMT2C/MLL3 to DNA Damage Sites Mediates DNA Damage Responses and Regulates PARP Inhibitor Sensitivity in Cancer. Cancer Res. 2021, 81, 3358–3373. [Google Scholar] [CrossRef]
- Yano, K.; Kato, M.; Endo, S.; Igarashi, T.; Wada, R.; Kohno, T.; Zimmermann, A.; Dahmen, H.; Zenke, F.T.; Shiotani, B. PARP inhibition-associated heterochromatin confers increased DNA replication stress and vulnerability to ATR inhibition in SMARCA4-deficient cells. Cell Death Discov. 2025, 11, 31. [Google Scholar] [CrossRef]
- Lee, J.S.; Nair, N.U.; Dinstag, G.; Chapman, L.; Chung, Y.; Wang, K.; Sinha, S.; Cha, H.; Kim, D.; Schperberg, A.V.; et al. Synthetic lethality-mediated precision oncology via the tumor transcriptome. Cell 2021, 184, 2487–2502 e2413. [Google Scholar] [CrossRef]
- Han, D.J.; Kim, S.; Lee, S.Y.; Moon, Y.; Kang, S.J.; Yoo, J.; Jeong, H.Y.; Cho, H.J.; Jeon, J.Y.; Sim, B.C.; et al. Evolutionary dependency of cancer mutations in gene pairs inferred by nonsynonymous-synonymous mutation ratios. Genome Med. 2024, 16, 103. [Google Scholar] [CrossRef]
- Arildsen, N.S.; Jonsson, J.M.; Bartuma, K.; Ebbesson, A.; Westbom-Fremer, S.; Masback, A.; Malander, S.; Nilbert, M.; Hedenfalk, I.A. Involvement of Chromatin Remodeling Genes and the Rho GTPases RhoB and CDC42 in Ovarian Clear Cell Carcinoma. Front. Oncol. 2017, 7, 109. [Google Scholar] [CrossRef]
- Krill-Burger, J.M.; Dempster, J.M.; Borah, A.A.; Paolella, B.R.; Root, D.E.; Golub, T.R.; Boehm, J.S.; Hahn, W.C.; McFarland, J.M.; Vazquez, F.; et al. Partial gene suppression improves identification of cancer vulnerabilities when CRISPR-Cas9 knockout is pan-lethal. Genome Biol. 2023, 24, 192. [Google Scholar] [CrossRef]
- Meyers, R.M.; Bryan, J.G.; McFarland, J.M.; Weir, B.A.; Sizemore, A.E.; Xu, H.; Dharia, N.V.; Montgomery, P.G.; Cowley, G.S.; Pantel, S.; et al. Computational correction of copy number effect improves specificity of CRISPR-Cas9 essentiality screens in cancer cells. Nat. Genet. 2017, 49, 1779–1784. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Hung, J.H.; Yang, T.H.; Hu, Z.; Weng, Z.; DeLisi, C. Gene set enrichment analysis: Performance evaluation and usage guidelines. Brief. Bioinform. 2012, 13, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef]
- Ogretmen, B.; Hannun, Y.A. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat. Rev. Cancer 2004, 4, 604–616. [Google Scholar] [CrossRef]
- Fresno Vara, J.A.; Casado, E.; de Castro, J.; Cejas, P.; Belda-Iniesta, C.; Gonzalez-Baron, M. PI3K/Akt signalling pathway and cancer. Cancer Treat. Rev. 2004, 30, 193–204. [Google Scholar] [CrossRef]
- Yuan, J.; Dong, X.; Yap, J.; Hu, J. The MAPK and AMPK signalings: Interplay and implication in targeted cancer therapy. J. Hematol. Oncol. 2020, 13, 113. [Google Scholar] [CrossRef]
- Hoxhaj, G.; Manning, B.D. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat. Rev. Cancer 2020, 20, 74–88. [Google Scholar] [CrossRef]
- Arcidiacono, B.; Iiritano, S.; Nocera, A.; Possidente, K.; Nevolo, M.T.; Ventura, V.; Foti, D.; Chiefari, E.; Brunetti, A. Insulin resistance and cancer risk: An overview of the pathogenetic mechanisms. Exp. Diabetes Res. 2012, 2012, 789174. [Google Scholar] [CrossRef]
- Semenza, G.L. HIF-1: Upstream and downstream of cancer metabolism. Curr. Opin. Genet. Dev. 2010, 20, 51–56. [Google Scholar] [CrossRef]
- Semenza, G.L. HIF-1 and mechanisms of hypoxia sensing. Curr. Opin. Cell Biol. 2001, 13, 167–171. [Google Scholar] [CrossRef]
- Chen, W.; Wu, P.; Yu, F.; Luo, G.; Qing, L.; Tang, J. HIF-1alpha Regulates Bone Homeostasis and Angiogenesis, Participating in the Occurrence of Bone Metabolic Diseases. Cells 2022, 11, 3552. [Google Scholar] [CrossRef]
- Montcourrier, P.; Mangeat, P.H.; Valembois, C.; Salazar, G.; Sahuquet, A.; Duperray, C.; Rochefort, H. Characterization of very acidic phagosomes in breast cancer cells and their association with invasion. J. Cell Sci. 1994, 107 Pt 9, 2381–2391. [Google Scholar] [CrossRef]
- Gong, Y.; Fan, Z.; Luo, G.; Yang, C.; Huang, Q.; Fan, K.; Cheng, H.; Jin, K.; Ni, Q.; Yu, X.; et al. The role of necroptosis in cancer biology and therapy. Mol. Cancer 2019, 18, 100. [Google Scholar] [CrossRef]
- Dupont, S.; Mamidi, A.; Cordenonsi, M.; Montagner, M.; Zacchigna, L.; Adorno, M.; Martello, G.; Stinchfield, M.J.; Soligo, S.; Morsut, L.; et al. FAM/USP9x, a deubiquitinating enzyme essential for TGFbeta signaling, controls Smad4 monoubiquitination. Cell 2009, 136, 123–135. [Google Scholar] [CrossRef]
- Harris, D.R.; Mims, A.; Bunz, F. Genetic disruption of USP9X sensitizes colorectal cancer cells to 5-fluorouracil. Cancer Biol. Ther. 2012, 13, 1319–1324. [Google Scholar] [CrossRef] [PubMed]
- Plouffe, S.W.; Lin, K.C.; Moore, J.L., 3rd; Tan, F.E.; Ma, S.; Ye, Z.; Qiu, Y.; Ren, B.; Guan, K.L. The Hippo pathway effector proteins YAP and TAZ have both distinct and overlapping functions in the cell. J. Biol. Chem. 2018, 293, 11230–11240. [Google Scholar] [CrossRef]
- Zhang, S.; Cui, W. Sox2, a key factor in the regulation of pluripotency and neural differentiation. World J. Stem Cells 2014, 6, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Gao, B.; Li, R.; Li, W.; Chen, W.; Yu, Z.; Zhang, J. Expression levels of resistant genes affect cervical cancer prognosis. Mol. Med. Rep. 2017, 15, 2802–2806. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhu, Z.; Zhou, J.; Shi, M.; Wang, N.; Yu, F.; Xu, L. Disulfidptosis-related gene expression reflects the prognosis of drug-resistant cancer patients and inhibition of MYH9 reverses sorafenib resistance. Transl. Oncol. 2024, 49, 102091. [Google Scholar] [CrossRef]
- Yang, W.; Soares, J.; Greninger, P.; Edelman, E.J.; Lightfoot, H.; Forbes, S.; Bindal, N.; Beare, D.; Smith, J.A.; Thompson, I.R.; et al. Genomics of Drug Sensitivity in Cancer (GDSC): A resource for therapeutic biomarker discovery in cancer cells. Nucleic Acids Res. 2013, 41, D955–D961. [Google Scholar] [CrossRef] [PubMed]
- Kalliokoski, T.; Kramer, C.; Vulpetti, A.; Gedeck, P. Comparability of mixed IC(5)(0) data—A statistical analysis. PLoS ONE 2013, 8, e61007. [Google Scholar] [CrossRef] [PubMed]
- Tomczak, K.; Czerwinska, P.; Wiznerowicz, M. The Cancer Genome Atlas (TCGA): An immeasurable source of knowledge. Contemp. Oncol. 2015, 19, A68–A77. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D.G. The logrank test. BMJ 2004, 328, 1073. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Chen, E.Y.; Tan, C.M.; Kou, Y.; Duan, Q.; Wang, Z.; Meirelles, G.V.; Clark, N.R.; Ma’ayan, A. Enrichr: Interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinform. 2013, 14, 128. [Google Scholar] [CrossRef]
- Reimand, J.; Isserlin, R.; Voisin, V.; Kucera, M.; Tannus-Lopes, C.; Rostamianfar, A.; Wadi, L.; Meyer, M.; Wong, J.; Xu, C.; et al. Pathway enrichment analysis and visualization of omics data using g:Profiler, GSEA, Cytoscape and EnrichmentMap. Nat. Protoc. 2019, 14, 482–517. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Franceschini, A.; Kuhn, M.; Simonovic, M.; Roth, A.; Minguez, P.; Doerks, T.; Stark, M.; Muller, J.; Bork, P.; et al. The STRING database in 2011: Functional interaction networks of proteins, globally integrated and scored. Nucleic Acids Res. 2011, 39, D561–D568. [Google Scholar] [CrossRef]



| Gene | Drug | Wild-Type (n) | IC50 (WT*) ± SD | Mutant (n) | IC50 (MT†) ± SD |
|---|---|---|---|---|---|
| BRCA1 | Niraparib | 24 | 149.2 ± 125.8 | 4 | 36.8 ± 14.1 |
| Olaparib | 29 | 297.3 ± 495.8 | 4 | 77.9 ± 84.7 | |
| Rucaparib | 27 | 163.3 ± 344.9 | 4 | 28.1 ± 10.2 | |
| Talazoparib | 29 | 247.2 ± 688.1 | 4 | 12.2 ± 15.7 | |
| Veliparib | 28 | 505.3 ± 677.0 | 4 | 280.7 ± 240.6 | |
| MLL2 | Niraparib | 26 | 141.4 ± 123.8 | 2 | 25.5 ± 3.5 |
| Olaparib | 30 | 291.0 ± 473.7 | 3 | 67.8 ± 43.4 | |
| Rucaparib | 26 | 165.4 ± 351.6 | 3 | 30.7 ± 15.3 | |
| Talazoparib | 30 | 239.3 ± 677.5 | 3 | 12.5 ± 16.7 | |
| Veliparib | 29 | 516.5 ± 661.2 | 3 | 97.3 ± 49.3 | |
| NF1 | Niraparib | 24 | 149.4 ± 125.4 | 4 | 35.5 ± 26.7 |
| Olaparib | 29 | 288.9 ± 481.3 | 4 | 138.9 ± 163.2 | |
| Rucaparib | 27 | 162.1 ± 345.3 | 4 | 35.8 ± 2.1 | |
| Talazoparib | 28 | 233.0 ± 700.9 | 4 | 13.4 ± 12.3 | |
| Veliparib | 28 | 517.2 ± 676.5 | 4 | 197.5 ± 65.7 | |
| SMAD4 | Niraparib | 25 | 124.0 ± 117.8 | 3 | 209.3 ± 166.9 |
| Olaparib | 29 | 171.9 ± 148.0 | 4 | 987.1 ± 1110.1 | |
| Rucaparib | 27 | 81.3 ± 58.2 | 4 | 581.5 ± 855.7 | |
| Talazoparib | 29 | 123.1 ± 337.1 | 4 | 911.5 ± 1641.8 | |
| Veliparib | 28 | 331.5 ± 211.7 | 4 | 1497.0 ± 1501.3 | |
| SMARCA4 | Niraparib | 25 | 144.2 ± 125.5 | 3 | 40.6 ± 24.9 |
| Olaparib | 30 | 291.7 ± 473.5 | 3 | 61.6 ± 10.3 | |
| Rucaparib | 28 | 154.2 ± 340.7 | 3 | 67.8 ± 28.6 | |
| Talazoparib | 30 | 240.3 ± 677.2 | 3 | 2.8 ± 1.3 | |
| Veliparib | 29 | 503.4 ± 667.3 | 3 | 223.8 ± 148.1 |
| Group | Cell Lines |
|---|---|
| Wild-type | PEO1, FU-OV-1, DOV13, OAW42, OVK18, UWB1.289, Caov-3, OVTOKO, OV-17R, OVCA433, OVCAR-4, JHOS-3, Caov-4, TOV-21G, OAW28, JHOS-4, SK-OV-3, OVCAR-8, OV-56, OVCAR-3, OVMIU, OVCA420, TOV-112D, OC-314, OVCAR-5, JHOS-2, OVISE, Hey, OV-7 |
| SMAD4 Mutant | IGROV-1, OVKATE, SW626, OV-90 |
| High SMAD4 Expression | OAW28, SK-OV-3, JHOS-4, JHOM-1, JHOC-5, NZOV9, PEA1, A2780, BIN-67, COV504, TOV-112D, OC-316, OVCAR-8, FU-OV-1, COV434, OVK18, OV-7, OVSAHO |
| Low SMAD4 Expression | JHOM-2B, OVTOKO, MCAS, OCI-P5x, 59M, OAW42, UWB1.289, OCI-C4P, OCI-C5x, OVKATE, COV413A, OVCAR-5, OVCAR-4, PEO1, OV-90, PEO4, OVMANA, RMUG-S |
| Gene | p-Value | GDS in Mutant Cell Lines | GDS in Wild-Type Cell Lines |
|---|---|---|---|
| MAPK1 | 0.0004 | −0.9643 | −0.1284 |
| INTS6 | 0.0007 | −1.7723 | −0.6206 |
| TTC7A | 0.0008 | −0.5756 | −0.1820 |
| MTA2 | 0.0018 | −0.8225 | −0.2730 |
| SOX2 | 0.0034 | −0.5008 | −0.1705 |
| MAP2K1 | 0.0053 | −0.5004 | −0.1118 |
| PPP2R2A | 0.0060 | −0.5252 | −0.1287 |
| RPL28 | 0.0083 | −1.3475 | −0.8033 |
| TYRO3 | 0.0093 | −0.6591 | −0.3882 |
| KAT2A | 0.0093 | −0.5809 | −0.2899 |
| ARHGEF5 | 0.0095 | −0.6281 | −0.3619 |
| DOCK5 | 0.0098 | −0.5625 | −0.1139 |
| HSPA8 | 0.0109 | −1.4931 | −0.6527 |
| HIC2 | 0.0121 | −0.5542 | −0.0774 |
| PKN2 | 0.0124 | −0.8481 | −0.4446 |
| SCARF2 | 0.0145 | −0.5807 | −0.1586 |
| MED15 | 0.0159 | −0.6711 | −0.1594 |
| ARF6 | 0.0171 | −0.6359 | −0.2516 |
| CDK4 | 0.0186 | −1.4800 | −0.6004 |
| TIMM29 | 0.0197 | −0.8811 | −0.5807 |
| HEXIM1 | 0.0218 | −0.6848 | −0.3736 |
| YAP1 | 0.0261 | −1.2623 | −0.5317 |
| ASH2L | 0.0264 | −0.6810 | −0.3158 |
| TUBD1 | 0.0283 | −0.7341 | −0.4012 |
| H2AW | 0.0299 | −0.5787 | −0.3424 |
| EIF1 | 0.0357 | −1.6192 | −1.0654 |
| PPP2CA | 0.0452 | −1.5073 | −0.7478 |
| PRPF4B | 0.0455 | −0.9152 | −0.6036 |
| ACACA | 0.0462 | −0.6231 | −0.3011 |
| SAP130 | 0.0465 | −0.7302 | −0.4637 |
| KRTAP9-6 | 0.0468 | −0.6083 | −0.3868 |
| ILK | 0.0478 | −0.8788 | −0.4457 |
| FASN | 0.0497 | −0.5177 | −0.2311 |
| Gene | p-Value | GDS in Low Expression Cell Lines | GDS in High Expression Cell Lines |
|---|---|---|---|
| SMARCA4 | 0.0019 | −0.6337 | −0.2506 |
| WNK1 | 0.0025 | −0.7793 | −0.4229 |
| RAB6A | 0.0044 | −0.5010 | −0.1044 |
| VPS4A | 0.0047 | −0.7433 | −0.3425 |
| GFPT1 | 0.0124 | −0.7171 | −0.4088 |
| ARHGAP29 | 0.0129 | −0.5395 | −0.1669 |
| RIC1 | 0.0135 | −0.7341 | −0.3076 |
| TUBB4B | 0.0147 | −0.5456 | −0.3078 |
| WWTR1 | 0.0152 | −0.5619 | −0.3043 |
| KLF5 | 0.0154 | −0.6163 | −0.2499 |
| CAND1 | 0.0157 | −0.5319 | −0.2209 |
| TRPM7 | 0.0199 | −0.7870 | −0.4315 |
| SMARCB1 | 0.0240 | −0.8087 | −0.4964 |
| YRDC | 0.0340 | −2.2461 | −1.4869 |
| TFRC | 0.0342 | −0.9311 | −0.5985 |
| EFR3A | 0.0422 | −0.6048 | −0.3122 |
| SLC2A1 | 0.0458 | −0.5958 | −0.3070 |
| USP9X | 0.0491 | −0.6929 | −0.3983 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, Y.; Ryu, J.Y. Identification of Anticancer Targets in Ovarian Cancer Using Genomic Drug Sensitivity Data. Int. J. Mol. Sci. 2025, 26, 9530. https://doi.org/10.3390/ijms26199530
Son Y, Ryu JY. Identification of Anticancer Targets in Ovarian Cancer Using Genomic Drug Sensitivity Data. International Journal of Molecular Sciences. 2025; 26(19):9530. https://doi.org/10.3390/ijms26199530
Chicago/Turabian StyleSon, Yebin, and Jae Yong Ryu. 2025. "Identification of Anticancer Targets in Ovarian Cancer Using Genomic Drug Sensitivity Data" International Journal of Molecular Sciences 26, no. 19: 9530. https://doi.org/10.3390/ijms26199530
APA StyleSon, Y., & Ryu, J. Y. (2025). Identification of Anticancer Targets in Ovarian Cancer Using Genomic Drug Sensitivity Data. International Journal of Molecular Sciences, 26(19), 9530. https://doi.org/10.3390/ijms26199530






