Exposure to Bisphenol B and S Increases the Risk of Male Reproductive Dysfunction in Middle Age
Abstract
1. Introduction
2. Result
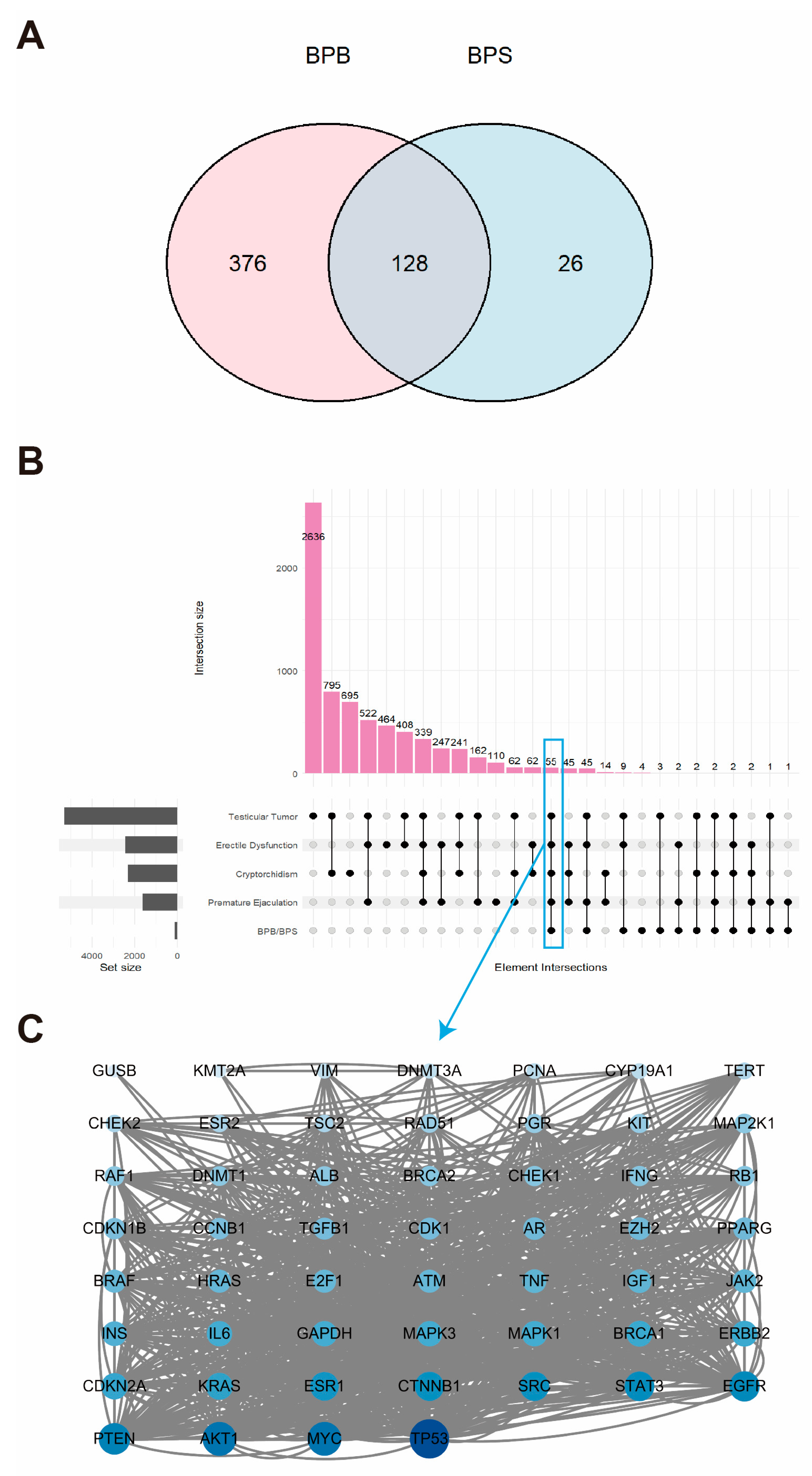
2.1. Landscape of Genes Co-Expressed in BPB and BPS
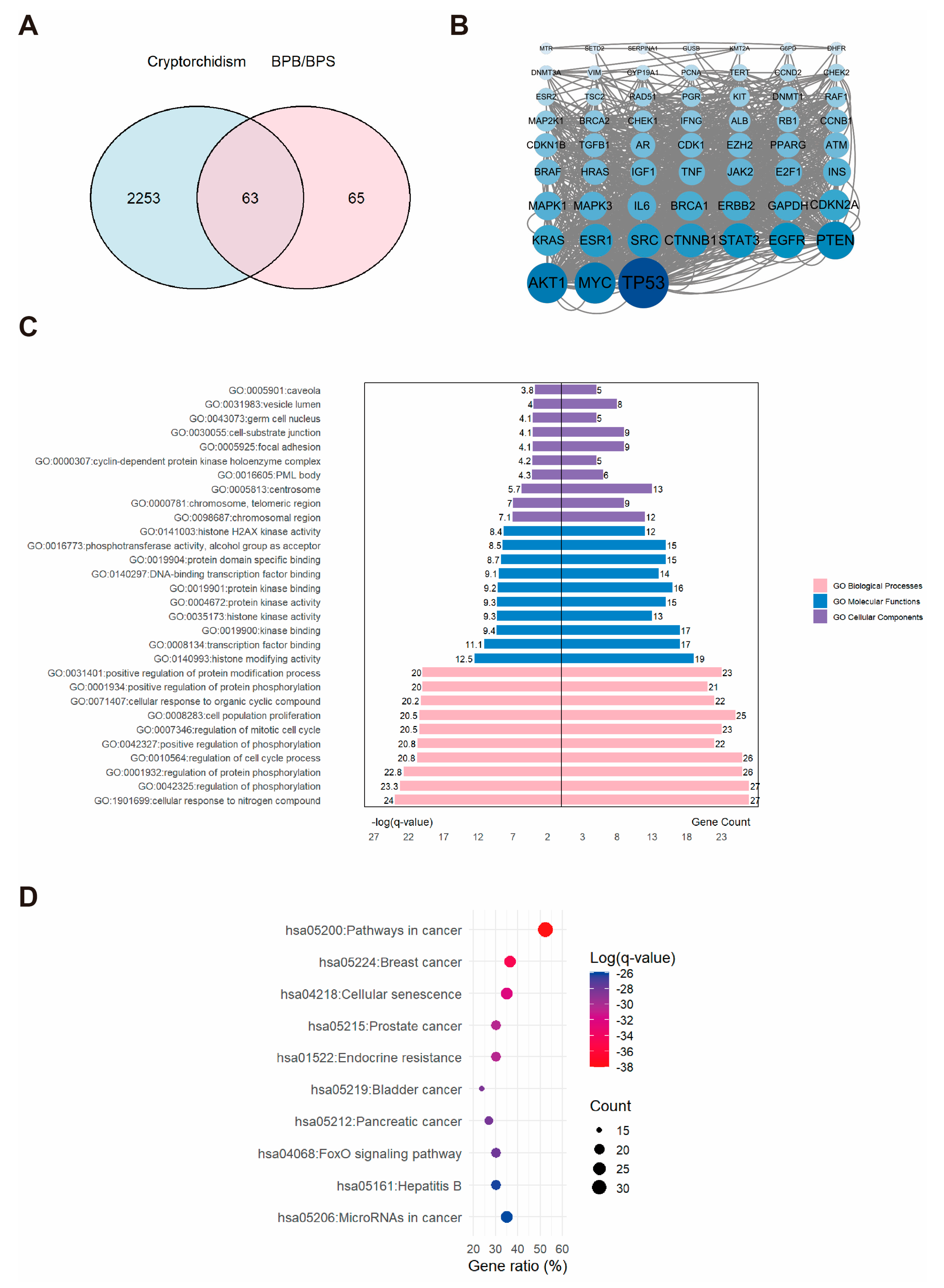
2.2. Network Toxicology Analysis of Potential Target Genes Commons to Both Cryptorchidism and BPs
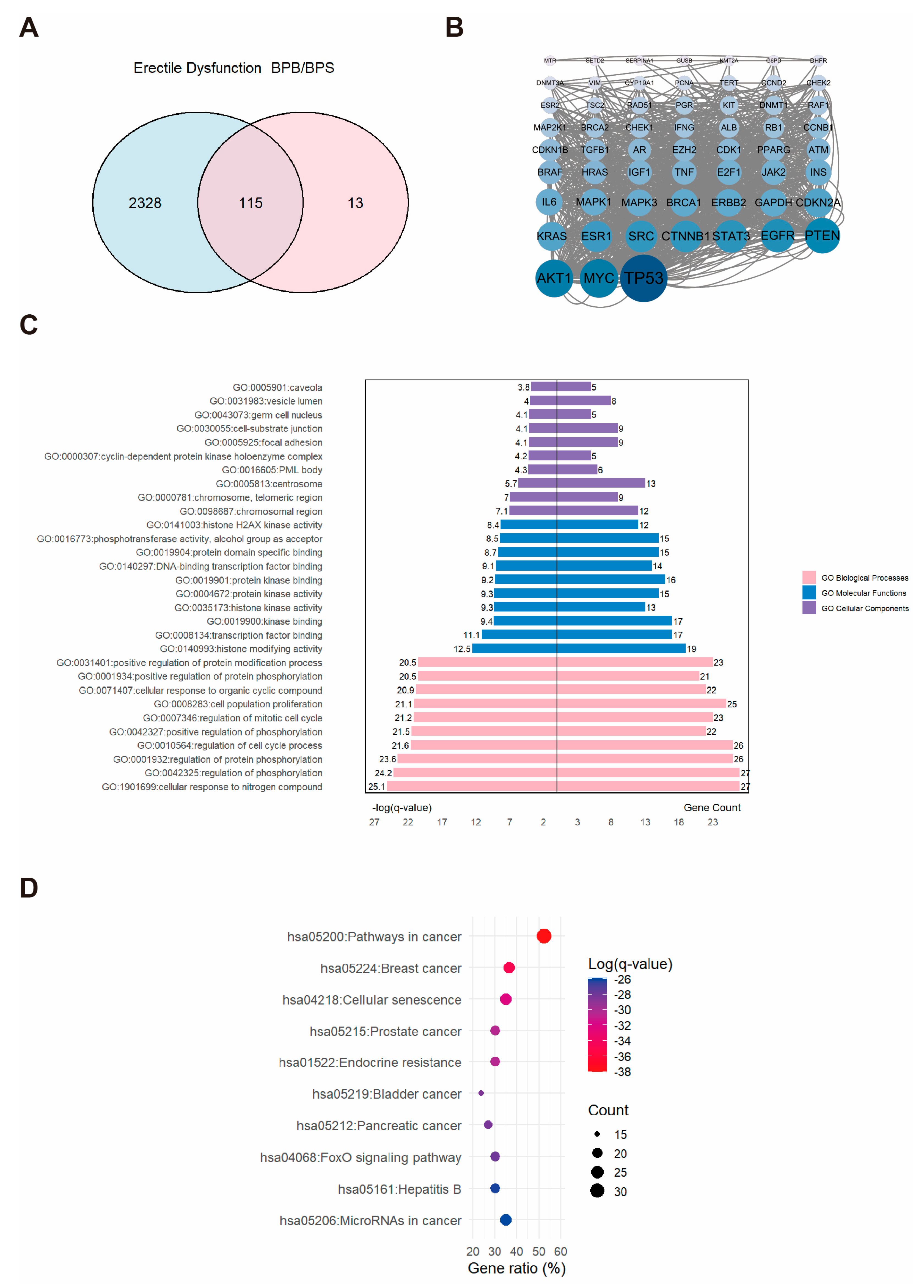
2.3. Network Toxicology Analysis of Potential Target Genes Commons to Both Erectile Dysfunction and BPs
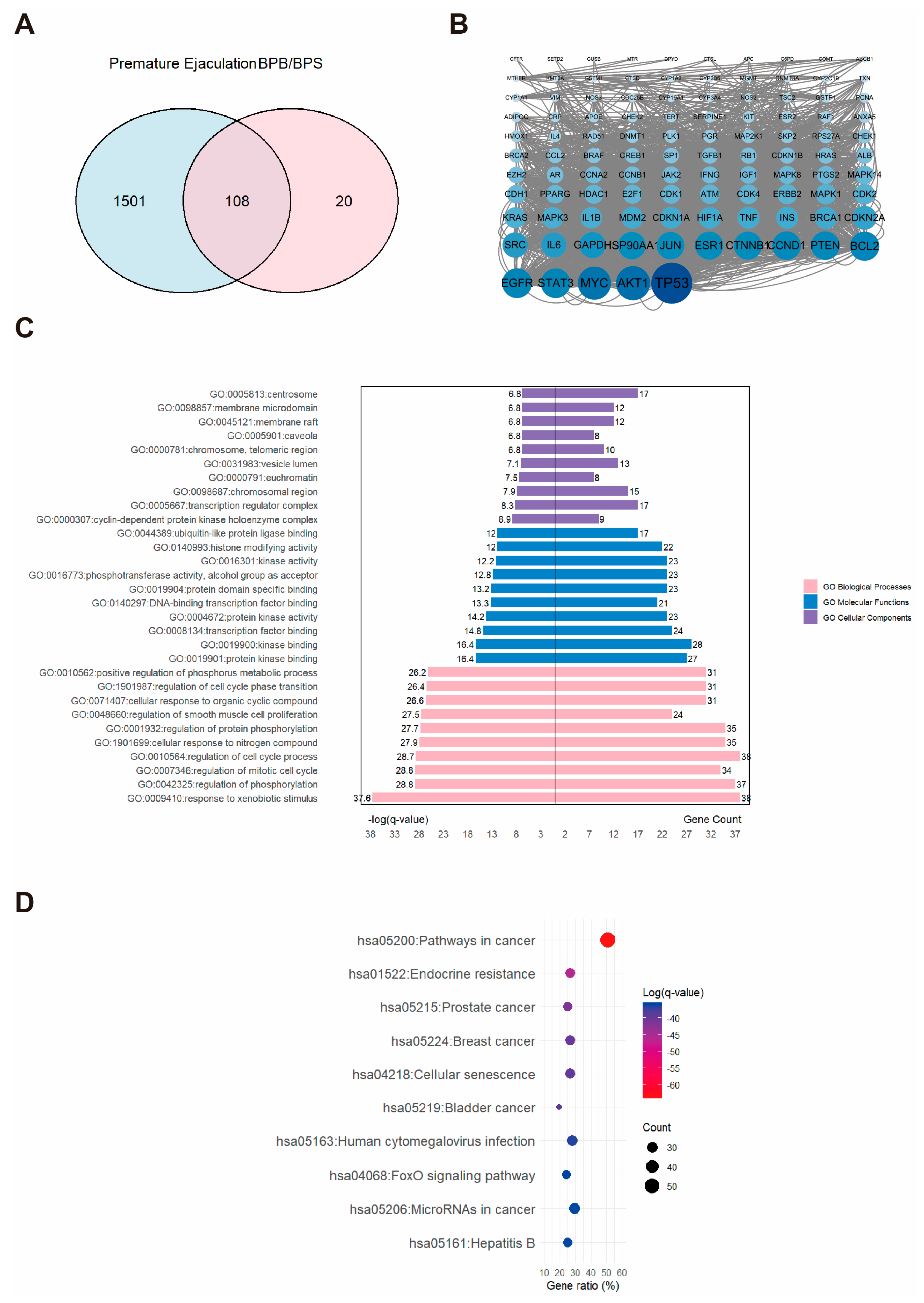
2.4. Network Toxicology Analysis of Potential Target Genes Commons to Both Premature Ejaculation and BPs
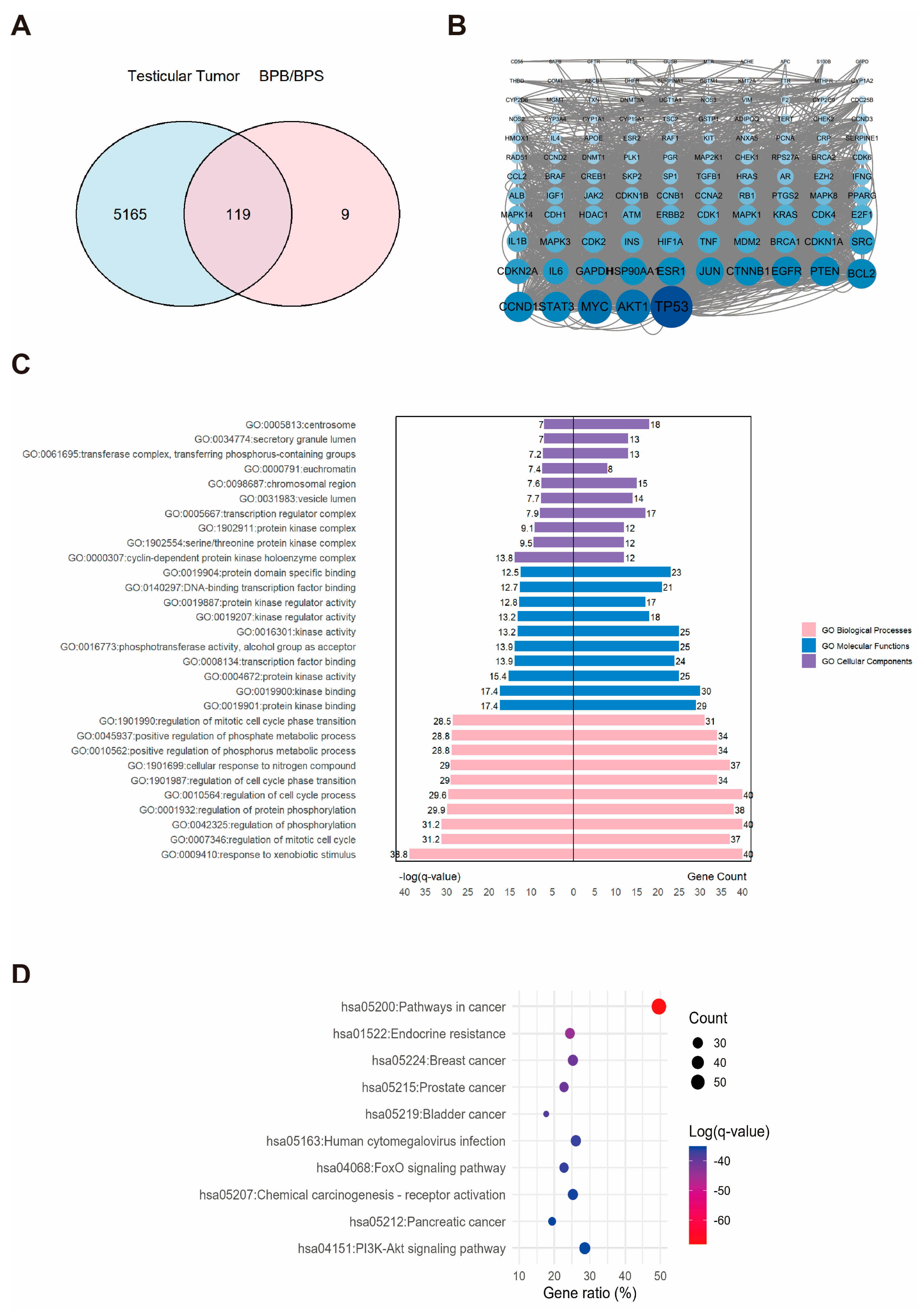
2.5. Network Toxicology Analysis of Potential Target Genes Commons to Both Testicular Tumor and BPs
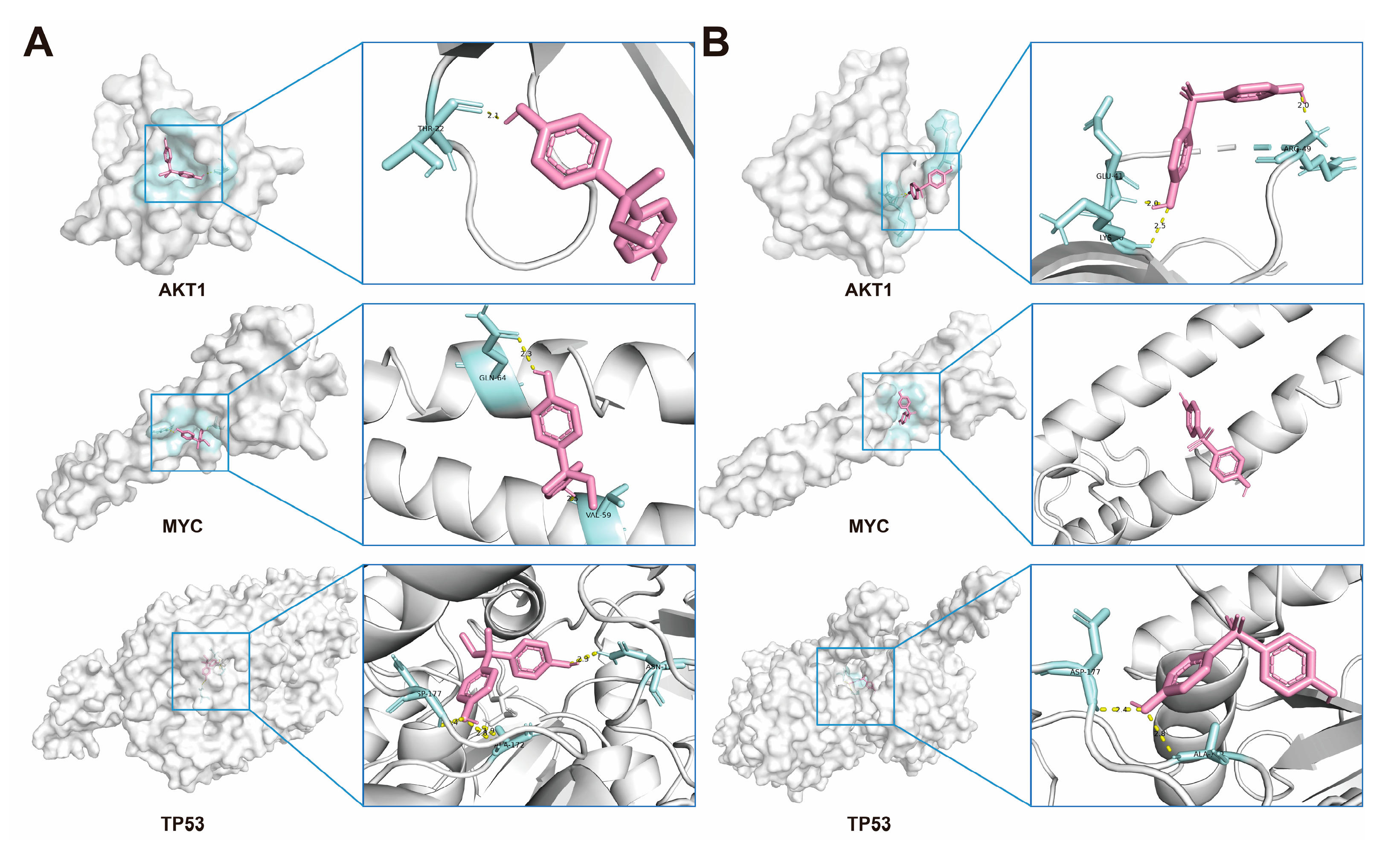
2.6. Molecular Docking of BPs with Potential Target Proteins
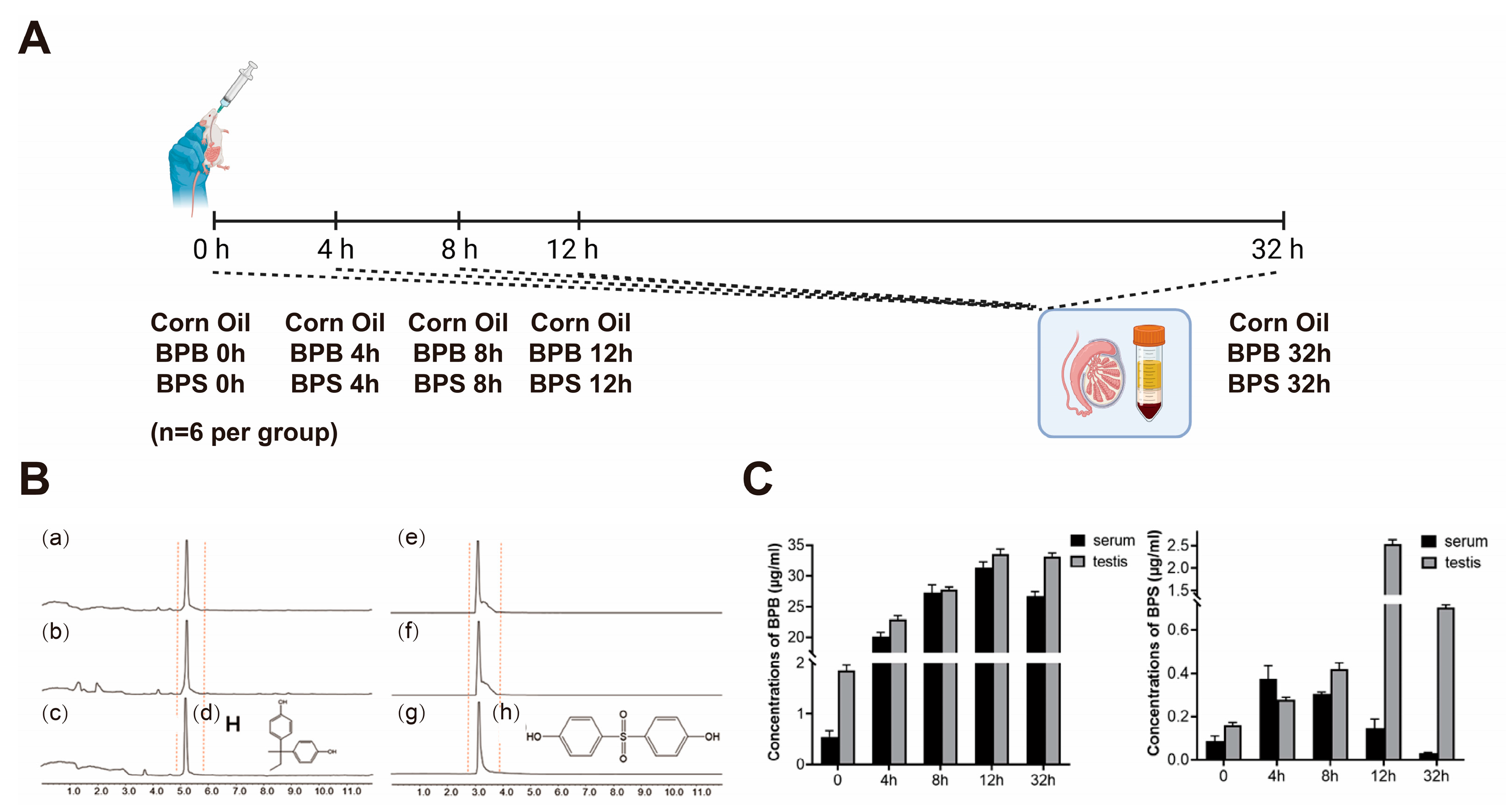
2.7. BPs Concentration in Serum and Testis
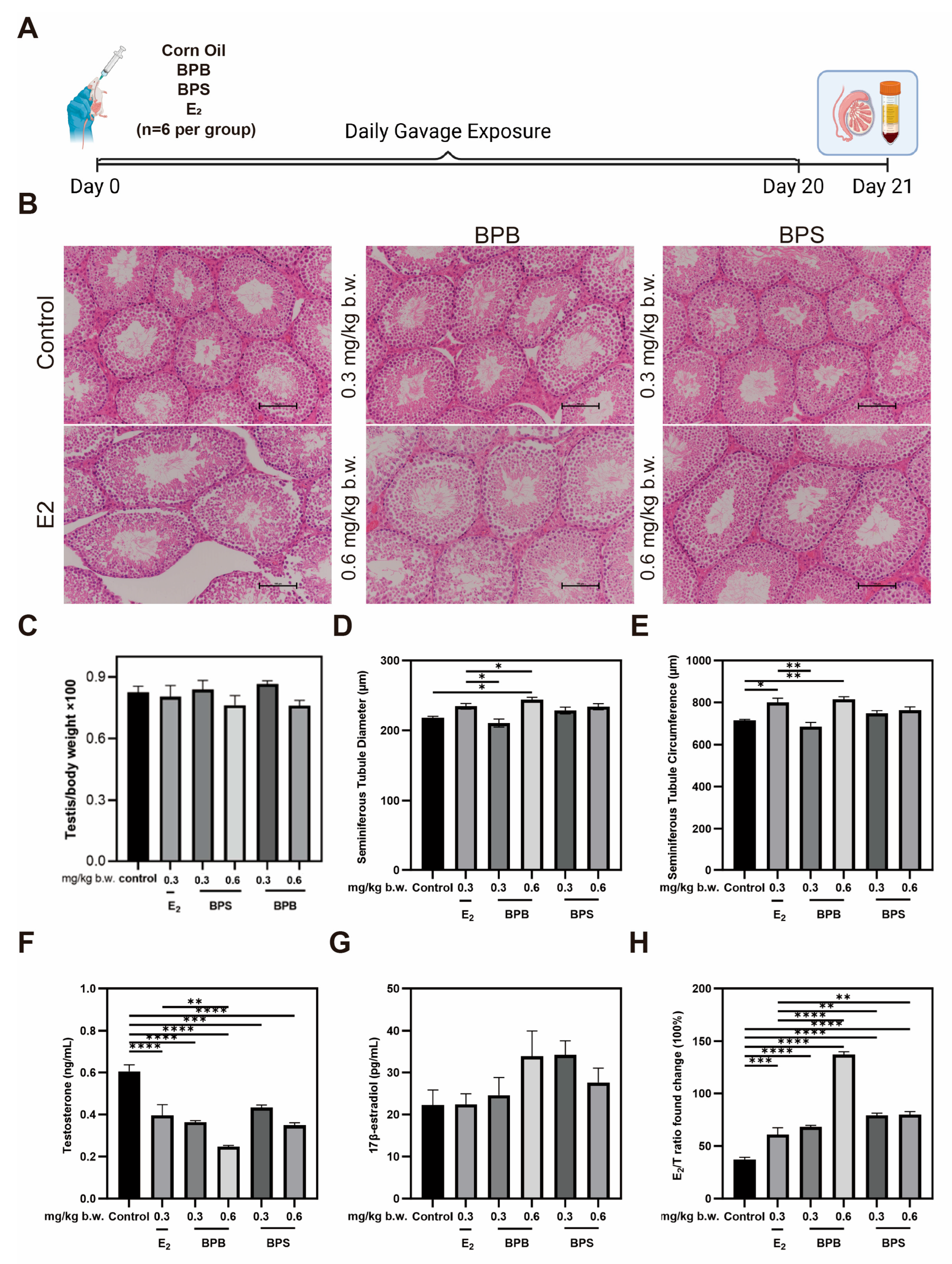
2.8. Serum Hormone Levels and Morphological Changes in the Testis in Middle-Aged Male Mice
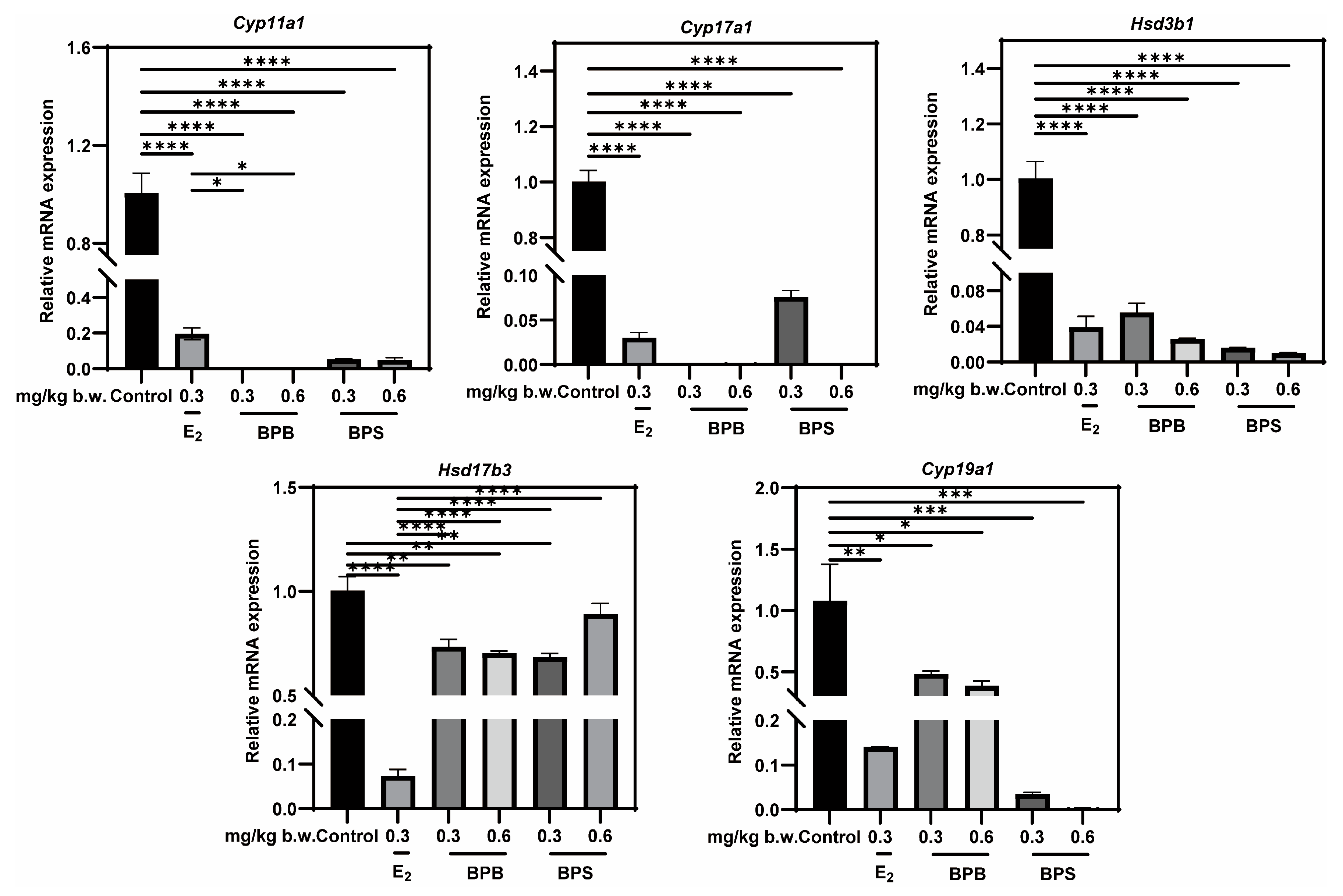
2.9. Identification of Potential Targets of Steroidogenic Enzyme Expression
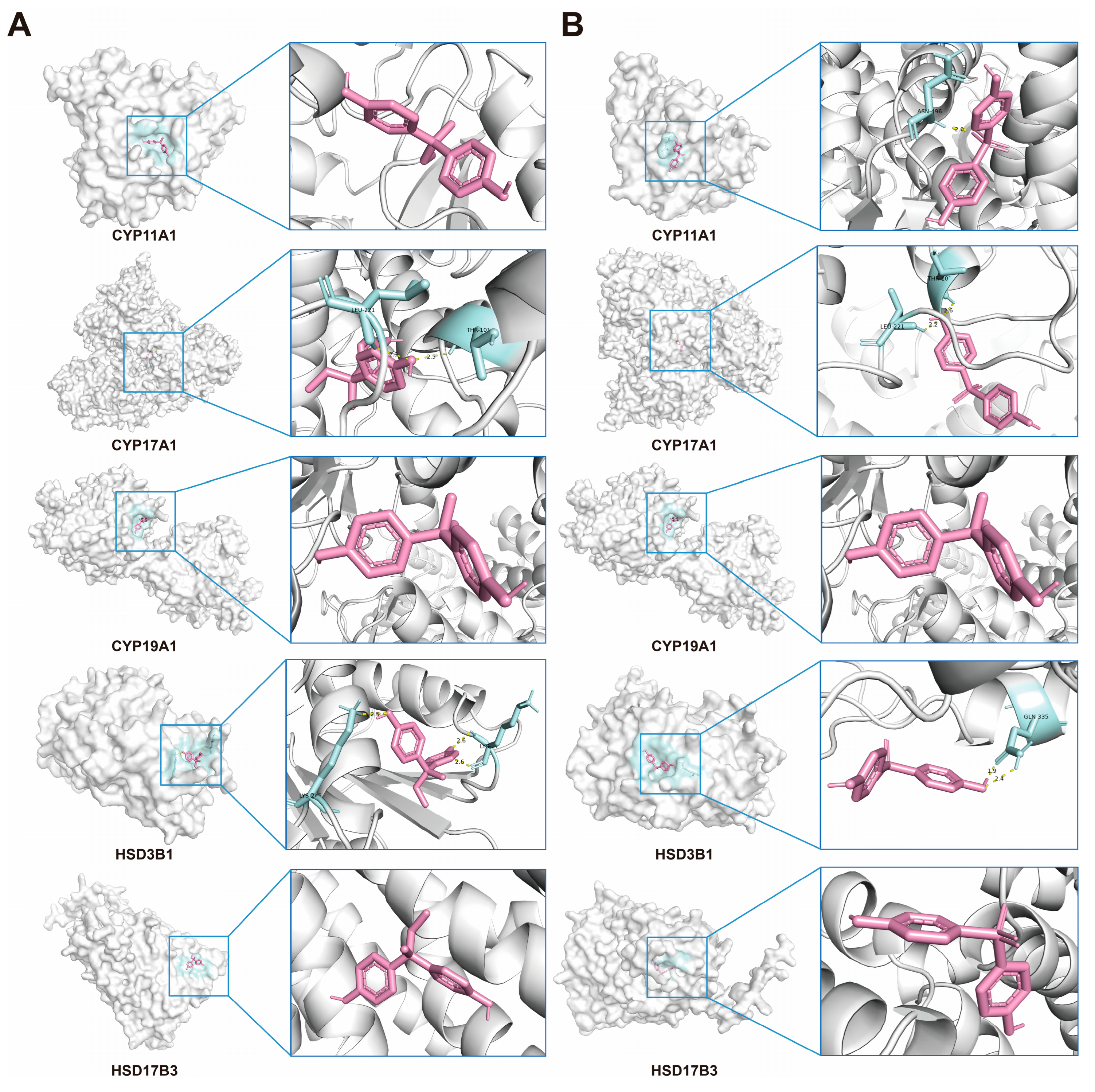
2.10. Verification of Interactions Between BPs and Potential Targets Through Molecular Docking
3. Discussion
4. Materials and Methods
4.1. Animals and Treatment Procedure
4.2. Network Toxicological Analysis of BPB and BPS
4.3. Construction of Protein–Protein Interaction (PPI) Networks
4.4. Bioinformatic Analysis
4.5. Molecular Docking
4.6. Quantitation of BPS or BPB by UPLC-MS/MS from Single Gavage
4.7. Histological Analysis
4.8. ELISA Hormone Assays
4.9. RNA Extraction and Quantitative Real-Time PCR (RT-qPCR)
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adegoke, E.O.; Rahman, M.S.; Pang, M.-G. Bisphenols Threaten Male Reproductive Health via Testicular Cells. Front. Endocrinol. 2020, 11, 624. [Google Scholar] [CrossRef]
- Den Braver-Sewradj, S.P.; Van Spronsen, R.; Hessel, E.V.S. Substitution of Bisphenol A: A Review of the Carcinogenicity, Reproductive Toxicity, and Endocrine Disruption Potential of Alternative Substances. Crit. Rev. Toxicol. 2020, 50, 128–147. [Google Scholar] [CrossRef]
- Fernández, M.F.; Arrebola, J.P.; Jiménez-Díaz, I.; Sáenz, J.M.; Molina-Molina, J.M.; Ballesteros, O.; Kortenkamp, A.; Olea, N. Bisphenol A and Other Phenols in Human Placenta from Children with Cryptorchidism or Hypospadias. Reprod. Toxicol. 2016, 59, 89–95. [Google Scholar] [CrossRef]
- Rochester, J.R. Bisphenol A and Human Health: A Review of the Literature. Reprod. Toxicol. 2013, 42, 132–155. [Google Scholar] [CrossRef]
- Zhang, B.; He, Y.; Zhu, H.; Huang, X.; Bai, X.; Kannan, K.; Zhang, T. Concentrations of Bisphenol A and Its Alternatives in Paired Maternal–Fetal Urine, Serum and Amniotic Fluid from an e-Waste Dismantling Area in China. Environ. Int. 2020, 136, 105407. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yang, X.; Tian, Y.; Xu, P.; Yue, H.; Sang, N. Bisphenol B and Bisphenol AF Exposure Enhances Uterine Diseases Risks in Mouse. Environ. Int. 2023, 173, 107858. [Google Scholar] [CrossRef] [PubMed]
- Thoene, M.; Dzika, E.; Gonkowski, S.; Wojtkiewicz, J. Bisphenol S in Food Causes Hormonal and Obesogenic Effects Comparable to or Worse than Bisphenol A: A Literature Review. Nutrients 2020, 12, 532. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Qiu, W.; Zheng, Y.; Xiong, J.; Gao, C.; Hu, S. Occurrence, Distribution, Bioaccumulation, and Ecological Risk of Bisphenol Analogues, Parabens and Their Metabolites in the Pearl River Estuary, South China. Ecotoxicol. Environ. Saf. 2019, 180, 43–52. [Google Scholar] [CrossRef]
- Liang, J.; Li, C.; Dang, Y.; Feng, X.; Ji, X.; Liu, X.; Zhao, X.; Zhang, Q.; Ren, Z.; Wang, Y.; et al. Occurrence of Bisphenol A Analogues in the Aquatic Environment and Their Behaviors and Toxicity Effects in Plants. Environ. Int. 2024, 193, 109105. [Google Scholar] [CrossRef]
- Ullah, H.; Ullah, F.; Rehman, O.; Jahan, S.; Afsar, T.; Al-Disi, D.; Almajwal, A.; Razak, S. Chronic Exposure of Bisphenol S (BPS) Affect Hypothalamic-Pituitary-Testicular Activities in Adult Male Rats: Possible in Estrogenic Mode of Action. Environ. Health Prev. Med. 2021, 26, 31. [Google Scholar] [CrossRef]
- Rochester, J.R.; Bolden, A.L. Bisphenol S and F: A Systematic Review and Comparison of the Hormonal Activity of Bisphenol A Substitutes. Environ. Health Perspect. 2015, 123, 643–650. [Google Scholar] [CrossRef]
- Serra, H.; Beausoleil, C.; Habert, R.; Minier, C.; Picard-Hagen, N.; Michel, C. Evidence for Bisphenol B Endocrine Properties: Scientific and Regulatory Perspectives. Environ. Health Perspect. 2019, 127, 106001. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Lai, Y.; Liu, C.-W.; Ru, H. Towards Mass Spectrometry-Based Chemical Exposome: Current Approaches, Challenges, and Future Directions. Toxics 2019, 7, 41. [Google Scholar] [CrossRef]
- Eladak, S.; Grisin, T.; Moison, D.; Guerquin, M.-J.; N’Tumba-Byn, T.; Pozzi-Gaudin, S.; Benachi, A.; Livera, G.; Rouiller-Fabre, V.; Habert, R. A New Chapter in the Bisphenol A Story: Bisphenol S and Bisphenol F Are Not Safe Alternatives to This Compound. Fertil. Steril. 2015, 103, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Rosenmai, A.K.; Dybdahl, M.; Pedersen, M.; Alice Van Vugt-Lussenburg, B.M.; Wedebye, E.B.; Taxvig, C.; Vinggaard, A.M. Are Structural Analogues to Bisphenol A Safe Alternatives? Toxicol. Sci. 2014, 139, 35–47. [Google Scholar] [CrossRef]
- Gonsioroski, A.; Mourikes, V.E.; Flaws, J.A. Endocrine Disruptors in Water and Their Effects on the Reproductive System. Int. J. Mol. Sci. 2020, 21, 1929. [Google Scholar] [CrossRef]
- Peters, A.E.; Ford, E.A.; Roman, S.D.; Bromfield, E.G.; Nixon, B.; Pringle, K.G.; Sutherland, J.M. Impact of Bisphenol A and Its Alternatives on Oocyte Health: A Scoping Review. Hum. Reprod. Update 2024, 30, 653–691. [Google Scholar] [CrossRef]
- Xue, S.; Li, X.; Zhou, S.; Zhang, J.; Sun, K.; Peng, X.; Chen, N.; Dong, M.; Jiang, T.; Chen, Y.; et al. Effects and Mechanisms of Endocrine Disruptor Bisphenol AF on Male Reproductive Health: A Mini Review. Ecotoxicol. Environ. Saf. 2024, 276, 116300. [Google Scholar] [CrossRef] [PubMed]
- Fainberg, J.; Kashanian, J.A. Recent Advances in Understanding and Managing Male Infertility. F1000Research 2019, 8, 670. [Google Scholar] [CrossRef]
- Eisenberg, M.L.; Esteves, S.C.; Lamb, D.J.; Hotaling, J.M.; Giwercman, A.; Hwang, K.; Cheng, Y.-S. Male Infertility. Nat. Rev. Dis. Primers 2023, 9, 49. [Google Scholar] [CrossRef]
- Chen, J.; Fok, K.L.; Chen, H.; Zhang, X.H.; Xu, W.M.; Chan, H.C. Cryptorchidism-Induced CFTR down-Regulation Results in Disruption of Testicular Tight Junctions through up-Regulation of NF- B/COX-2/PGE2. Hum. Reprod. 2012, 27, 2585–2597. [Google Scholar] [CrossRef] [PubMed]
- Komarowska, M.D.; Grubczak, K.; Czerniecki, J.; Hermanowicz, A.; Hermanowicz, J.M.; Debek, W.; Matuszczak, E. Identification of the Bisphenol A (BPA) and the Two Analogues BPS and BPF in Cryptorchidism. Front. Endocrinol. 2021, 12, 694669. [Google Scholar] [CrossRef]
- Shamloul, R.; Ghanem, H. Erectile Dysfunction. Lancet 2013, 381, 153–165. [Google Scholar] [CrossRef]
- Akintunde, J.K.; Akintola, T.E.; Aliu, F.H.; Fajoye, M.O.; Adimchi, S.O. Naringin Regulates Erectile Dysfunction by Abolition of Apoptosis and Inflammation through NOS/cGMP/PKG Signalling Pathway on Exposure to Bisphenol-A in Hypertensive Rat Model. Reprod. Toxicol. 2020, 95, 123–136. [Google Scholar] [CrossRef]
- Giuliano, F.; Clément, P. Serotonin and Premature Ejaculation: From Physiology to Patient Management. Eur. Urol. 2006, 50, 454–466. [Google Scholar] [CrossRef] [PubMed]
- Vieiralves, R.R.; Favorito, L.A. Dapoxetine and Premature Ejaculation. Int. Braz. J. Urol. 2023, 49, 511–514. [Google Scholar] [CrossRef]
- Bearelly, P.; Avellino, G.J. The Role of Benign Prostatic Hyperplasia Treatments in Ejaculatory Dysfunction. Fertil. Steril. 2021, 116, 611–617. [Google Scholar] [CrossRef]
- Cannarella, R.; Gül, M.; Rambhatla, A.; Agarwal, A. Temporal Decline of Sperm Concentration: Role of Endocrine Disruptors. Endocrine 2022, 79, 1–16. [Google Scholar] [CrossRef]
- Li, J.; Zheng, H.; Hou, J.; Chen, J.; Zhang, F.; Yang, X.; Jin, F.; Xi, Y. X-Linked RBBP7 Mutation Causes Maturation Arrest and Testicular Tumors. J. Clin. Investig. 2023, 133, e171541. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhu, X.; Xu, K.; Li, Y.; Zhou, J. Network Toxicological and Molecular Docking to Investigate the Mechanisms of Toxicity of Agricultural Chemical Thiabendazole. Chemosphere 2024, 363, 142711. [Google Scholar] [CrossRef]
- Scott, H.M.; Mason, J.I.; Sharpe, R.M. Steroidogenesis in the Fetal Testis and Its Susceptibility to Disruption by Exogenous Compounds. Endocr. Rev. 2009, 30, 883–925. [Google Scholar] [CrossRef]
- Lymperi, S.; Giwercman, A. Endocrine Disruptors and Testicular Function. Metabolism 2018, 86, 79–90. [Google Scholar] [CrossRef]
- Desdoits-Lethimonier, C.; Lesné, L.; Gaudriault, P.; Zalko, D.; Antignac, J.P.; Deceuninck, Y.; Platel, C.; Dejucq-Rainsford, N.; Mazaud-Guittot, S.; Jégou, B. Parallel Assessment of the Effects of Bisphenol A and Several of Its Analogs on the Adult Human Testis. Hum. Reprod. 2017, 32, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Pirzada, M.; Jahan, S.; Ullah, H.; Shaheen, G.; Rehman, H.; Siddiqui, M.F.; Butt, M.A. Bisphenol A and Its Analogs Bisphenol B, Bisphenol F, and Bisphenol S: Comparative in Vitro and in Vivo Studies on the Sperms and Testicular Tissues of Rats. Chemosphere 2018, 209, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Ikhlas, S.; Ahmad, M. Acute and Sub-Acute Bisphenol-B Exposures Adversely Affect Sperm Count and Quality in Adolescent Male Mice. Chemosphere 2020, 242, 125286. [Google Scholar] [CrossRef]
- Pan, J.; Liu, P.; Yu, X.; Zhang, Z.; Liu, J. The Adverse Role of Endocrine Disrupting Chemicals in the Reproductive System. Front. Endocrinol. 2024, 14, 1324993. [Google Scholar] [CrossRef]
- Kang, H.-J.; Rosenwaks, Z. P53 and Reproduction. Fertil. Steril. 2018, 109, 39–43. [Google Scholar] [CrossRef]
- Dou, Y.; Kawaler, E.A.; Cui Zhou, D.; Gritsenko, M.A.; Huang, C.; Blumenberg, L.; Karpova, A.; Petyuk, V.A.; Savage, S.R.; Satpathy, S.; et al. Proteogenomic Characterization of Endometrial Carcinoma. Cell 2020, 180, 729–748.e26. [Google Scholar] [CrossRef]
- Boublikova, L.; Bakardjieva-Mihaylova, V.; Skvarova Kramarzova, K.; Kuzilkova, D.; Dobiasova, A.; Fiser, K.; Stuchly, J.; Kotrova, M.; Buchler, T.; Dusek, P.; et al. Wilms Tumor Gene 1 (WT1), TP53, RAS/BRAF and KIT Aberrations in Testicular Germ Cell Tumors. Cancer Lett. 2016, 376, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Sun, Y.; Zong, J.; Meng, W.; Yan, J.; Chen, K.; Wang, S.; Guo, D.; Xiao, Z.; Zhou, Q.; et al. METTL3-Dependent m6A Methylation Facilitates Uterine Receptivity and Female Fertility via Balancing Estrogen and Progesterone Signaling. Cell Death Dis. 2023, 14, 349. [Google Scholar] [CrossRef]
- Comparative Toxicokinetics of Bisphenol S in Rats and Mice Following Gavage Administration—PMC. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC7891184/ (accessed on 29 May 2025).
- Li, J.; Sheng, N.; Cui, R.; Feng, Y.; Shao, B.; Guo, X.; Zhang, H.; Dai, J. Gestational and Lactational Exposure to Bisphenol AF in Maternal Rats Increases Testosterone Levels in 23-Day-Old Male Offspring. Chemosphere 2016, 163, 552–561. [Google Scholar] [CrossRef]
- Hu, W.; Dong, T.; Wang, L.; Guan, Q.; Song, L.; Chen, D.; Zhou, Z.; Chen, M.; Xia, Y.; Wang, X. Obesity Aggravates Toxic Effect of BPA on Spermatogenesis. Environ. Int. 2017, 105, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Mruk, D.D.; Wong, C.K.; Cheng, C.Y. Targeting Testis-Specific Proteins to Inhibit Spermatogenesis: Lesson from Endocrine Disrupting Chemicals. Expert Opin. Ther. Targets 2013, 17, 839–855. [Google Scholar] [CrossRef] [PubMed]
- Salian, S.; Doshi, T.; Vanage, G. Perinatal Exposure of Rats to Bisphenol A Affects the Fertility of Male Offspring. Life Sci. 2009, 85, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.; Terekeci, H.; Sandal, S.; Kelestimur, F. Endocrine Disrupting Chemicals: Exposure, Effects on Human Health, Mechanism of Action, Models for Testing and Strategies for Prevention. Rev. Endocr. Metab. Disord. 2020, 21, 127–147. [Google Scholar] [CrossRef]
- Vander Borght, M.; Wyns, C. Fertility and Infertility: Definition and Epidemiology. Clin. Biochem. 2018, 62, 2–10. [Google Scholar] [CrossRef]
- Levine, H.; Jørgensen, N.; Martino-Andrade, A.; Mendiola, J.; Weksler-Derri, D.; Jolles, M.; Pinotti, R.; Swan, S.H. Temporal Trends in Sperm Count: A Systematic Review and Meta-Regression Analysis of Samples Collected Globally in the 20th and 21st Centuries. Hum. Reprod. Update 2023, 29, 157–176. [Google Scholar] [CrossRef]
- Kahn, L.G.; Philippat, C.; Nakayama, S.F.; Slama, R.; Trasande, L. Endocrine-Disrupting Chemicals: Implications for Human Health. Lancet Diabetes Endocrinol. 2020, 8, 703–718. [Google Scholar] [CrossRef]
- Bräuner, E.V.; Lim, Y.-H.; Koch, T.; Uldbjerg, C.S.; Gregersen, L.S.; Pedersen, M.K.; Frederiksen, H.; Petersen, J.H.; Coull, B.A.; Andersson, A.-M.; et al. Endocrine Disrupting Chemicals and Risk of Testicular Cancer: A Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 2021, 106, e4834–e4860. [Google Scholar] [CrossRef]
- Faja, F.; Esteves, S.; Pallotti, F.; Cicolani, G.; Di Chiano, S.; Delli Paoli, E.; Lenzi, A.; Lombardo, F.; Paoli, D. Environmental Disruptors and Testicular Cancer. Endocrine 2022, 78, 429–435. [Google Scholar] [CrossRef]
- Sharpe, R.M. Endocrine Disruption and Male Reproductive Disorders: Unanswered Questions. Hum. Reprod. 2024, 39, 1879–1888. [Google Scholar] [CrossRef]
- Gao, H.; Yang, B.-J.; Li, N.; Feng, L.-M.; Shi, X.-Y.; Zhao, W.-H.; Liu, S.-J. Bisphenol A and Hormone-Associated Cancers: Current Progress and Perspectives. Medicine 2015, 94, e211. [Google Scholar] [CrossRef]
- Lacouture, A.; Lafront, C.; Peillex, C.; Pelletier, M.; Audet-Walsh, É. Impacts of Endocrine-Disrupting Chemicals on Prostate Function and Cancer. Environ. Res. 2022, 204, 112085. [Google Scholar] [CrossRef] [PubMed]
- Cripps, S.M.; Marshall, S.A.; Mattiske, D.M.; Ingham, R.Y.; Pask, A.J. Estrogenic Endocrine Disruptor Exposure Directly Impacts Erectile Function. Commun. Biol. 2024, 7, 403. [Google Scholar] [CrossRef] [PubMed]
- Rouiller-Fabre, V.; Guerquin, M.J.; N’Tumba-Byn, T.; Muczynski, V.; Moison, D.; Tourpin, S.; Messiaen, S.; Habert, R.; Livera, G. Nuclear Receptors and Endocrine Disruptors in Fetal and Neonatal Testes: A Gapped Landscape. Front. Endocrinol. 2015, 6, 58. [Google Scholar] [CrossRef]
- Huang, Q.; Chen, Q. Mediating Roles of PPARs in the Effects of Environmental Chemicals on Sex Steroids. PPAR Res. 2017, 2017, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Stanojević, M.; Sollner Dolenc, M. Mechanisms of Bisphenol A and Its Analogs as Endocrine Disruptors via Nuclear Receptors and Related Signaling Pathways. Arch. Toxicol. 2025, 99, 2397–2417. [Google Scholar] [CrossRef]
- Goldberg, M.S.; Zapata-Marin, S.; Labrèche, F.; Ho, V.; Lavigne, E.; Valois, M.-F.; Parent, M.-E. Ambient Exposures to Selected Volatile Organic Compounds and the Risk of Prostate Cancer in Montreal. Environ. Epidemiol. 2022, 6, e231. [Google Scholar] [CrossRef]
- Xi, W.; Lee, C.K.F.; Yeung, W.S.B.; Giesy, J.P.; Wong, M.H.; Zhang, X.; Hecker, M.; Wong, C.K.C. Effect of Perinatal and Postnatal Bisphenol A Exposure to the Regulatory Circuits at the Hypothalamus–Pituitary–Gonadal Axis of CD-1 Mice. Reprod. Toxicol. 2011, 31, 409–417. [Google Scholar] [CrossRef]
- Shi, M.; Sekulovski, N.; MacLean, J.A.; Hayashi, K. Effects of Bisphenol A Analogues on Reproductive Functions in Mice. Reprod. Toxicol. 2017, 73, 280–291. [Google Scholar] [CrossRef]
- Ferrini, R.L.; Barrett-Connor, E. Sex Hormones and Age: A Cross-Sectional Study of Testosterone and Estradioi and Their Bioavailable Fractions in Community-Dwelling Men. Am. J. Epidemiol. 1998, 147, 750–754. [Google Scholar] [CrossRef]
- Davidson, J.M.; Chen, J.J.; Crapo, L.; Gray, G.D.; Greenleaf, W.J.; Catania, J.A. Hormonal Changes and Sexual Function in Aging Men*. J. Clin. Endocrinol. Metab. 1983, 57, 71–77. [Google Scholar] [CrossRef]
- Vermeulen, A.; Kaufman, J.M.; Goemaere, S.; Pottelberg, I.V. Estradiol in Elderly Men. Aging Male 2002, 5, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Tian, Y.; Wu, X.; Yang, X.; Xu, P.; Zhu, H.; Sang, N. Exploration of the Damage and Mechanisms of BPS Exposure on the Uterus and Ovary of Adult Female Mice. Sci. Total Environ. 2023, 868, 161660. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Martínez, Y.; Caravaca, M.; Soto-Meca, A. Determination of Very Low Concentration of Bisphenol A in Toys and Baby Pacifiers Using Dispersive Liquid–Liquid Microextraction by In Situ Ionic Liquid Formation and High-Performance Liquid Chromatography. Pharmaceuticals 2020, 13, 301. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Gao, J.; Dou, X.; Peng, D.; Zhang, Y.; Li, H.; Zhu, T.; Jiang, H.; Zhang, X. The Relationship between Vascular Endothelial Growth Factor and Spermatogenesis Disturbance in an Experimentally-Induced Unilateral Cryptorchidism Murine Model. Mol. Biol. Rep. 2020, 47, 3605–3613. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
| BPB | BPS | ||
|---|---|---|---|
| Protein Name | Affinity (kcal/mol) | Protein Name | Affinity (kcal/mol) |
| AKT1 | −6.0 | AKT1 | −4.5 |
| MYC | −5.9 | MYC | −5.6 |
| TP53 | −7.3 | TP53 | −7.6 |
| BPB | BPS | ||
|---|---|---|---|
| Protein Name | Affinity (kcal/mol) | Protein Name | Affinity (kcal/mol) |
| CYP11A1 | −6.9 | CYP11A1 | −2.2 |
| CYP17A1 | −6.7 | CYP17A1 | −7.7 |
| CYP19A1 | −6.6 | CYP19A1 | −5.8 |
| HSD3B1 | −5.4 | HSD3B1 | −4.9 |
| HSD17B3 | −5.1 | HSD17B3 | −5.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, S.; Ni, H.; Xiao, Y.; Du, J.; Han, Y.; Wang, W.; Tang, S.; Yu, M. Exposure to Bisphenol B and S Increases the Risk of Male Reproductive Dysfunction in Middle Age. Int. J. Mol. Sci. 2025, 26, 9507. https://doi.org/10.3390/ijms26199507
Zhao S, Ni H, Xiao Y, Du J, Han Y, Wang W, Tang S, Yu M. Exposure to Bisphenol B and S Increases the Risk of Male Reproductive Dysfunction in Middle Age. International Journal of Molecular Sciences. 2025; 26(19):9507. https://doi.org/10.3390/ijms26199507
Chicago/Turabian StyleZhao, Sen, Heliang Ni, Yuan Xiao, Jing Du, Yudong Han, Wenying Wang, Shuang Tang, and Mingxi Yu. 2025. "Exposure to Bisphenol B and S Increases the Risk of Male Reproductive Dysfunction in Middle Age" International Journal of Molecular Sciences 26, no. 19: 9507. https://doi.org/10.3390/ijms26199507
APA StyleZhao, S., Ni, H., Xiao, Y., Du, J., Han, Y., Wang, W., Tang, S., & Yu, M. (2025). Exposure to Bisphenol B and S Increases the Risk of Male Reproductive Dysfunction in Middle Age. International Journal of Molecular Sciences, 26(19), 9507. https://doi.org/10.3390/ijms26199507







