α-Glucosidase Inhibition-Guided Network Pharmacology and Molecular Docking Reveal the Antidiabetic Potential of Cichorium intybus as a Functional Food
Abstract
1. Introduction
2. Results
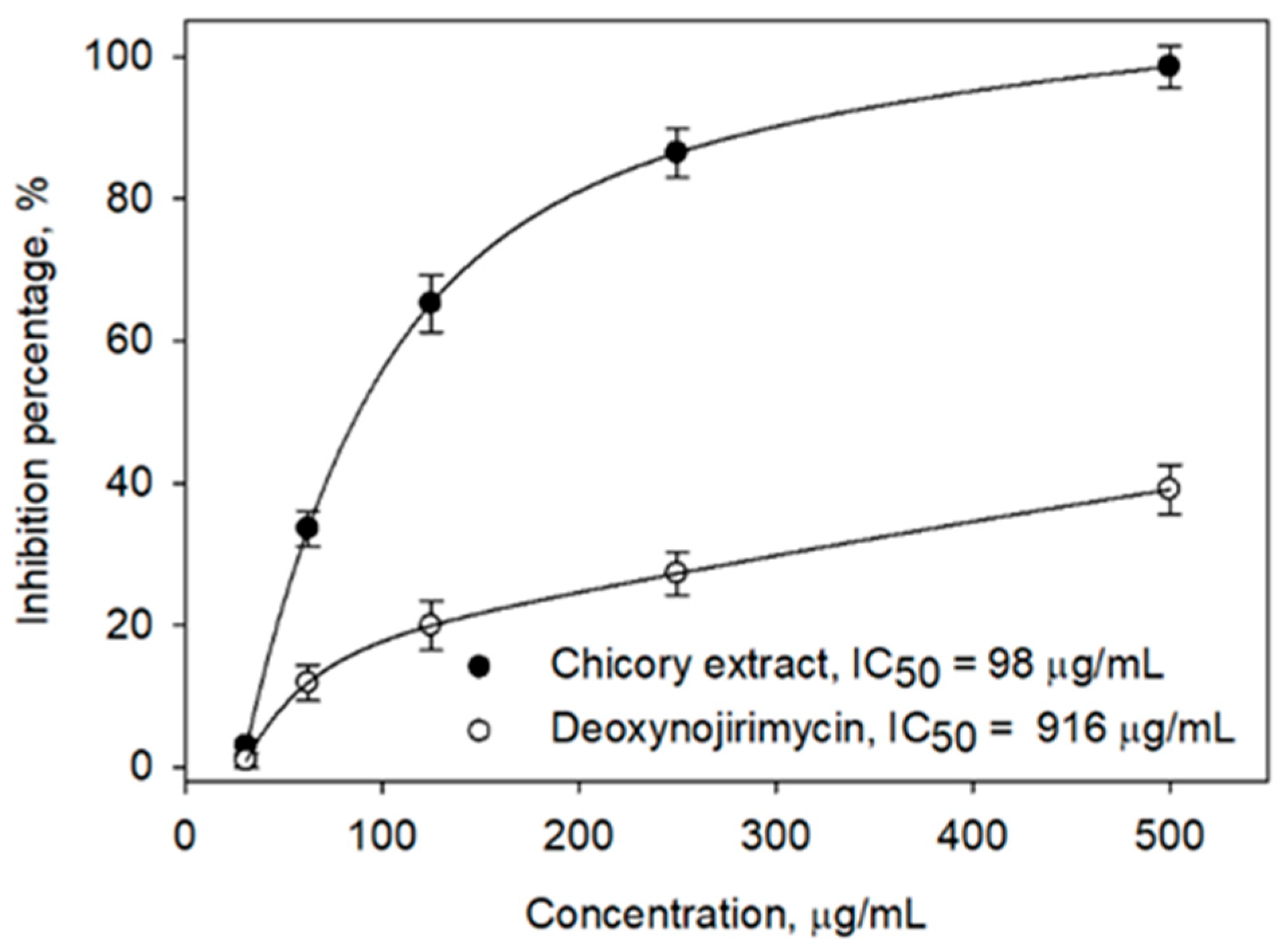
2.1. In Vitro α-Glucosidase Inhibitory Activity
2.2. Network Pharmacology In-Depth Analysis
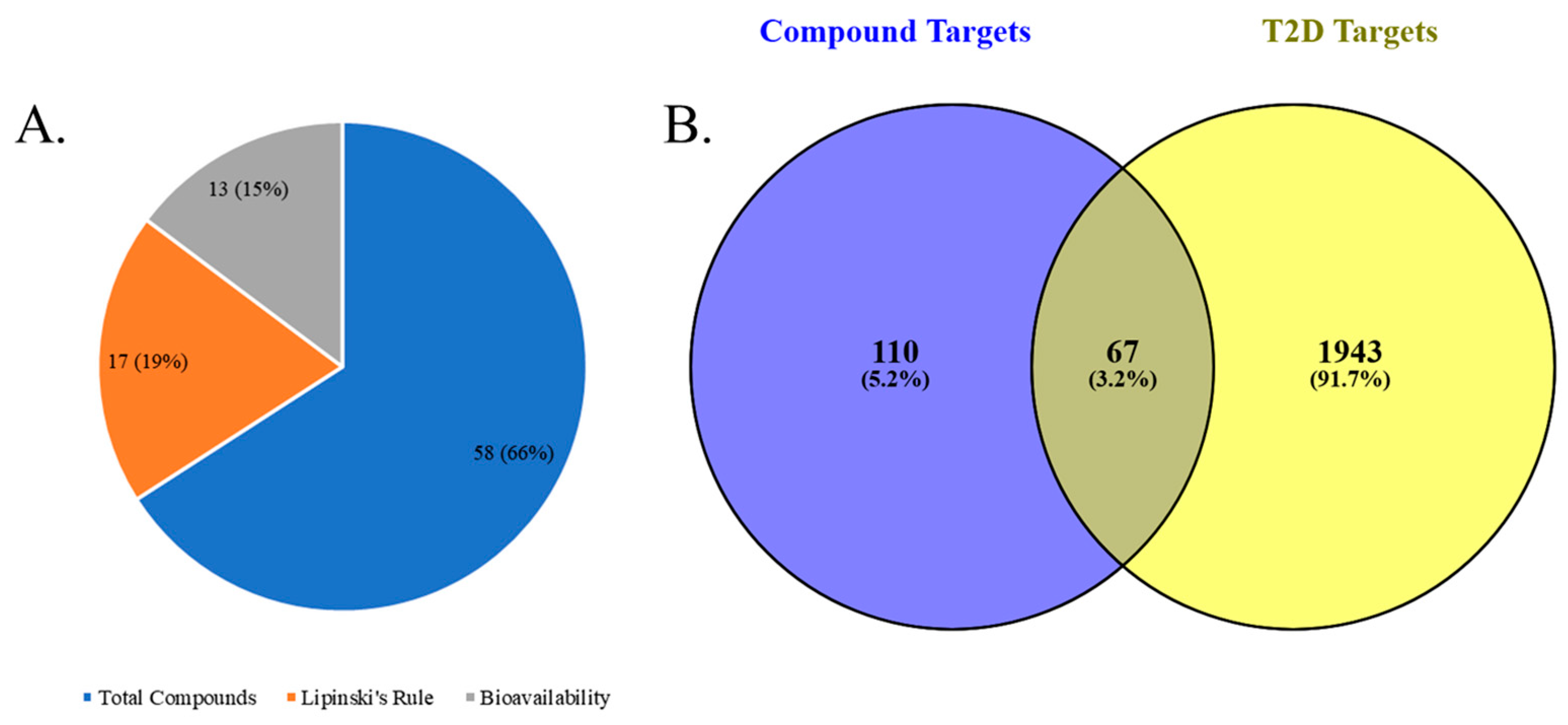
2.2.1. Screening and Selection of Bioactive Compounds in Chicory
2.2.2. Determination of Compound Targets, Disease Targets, and Overlapping Genes
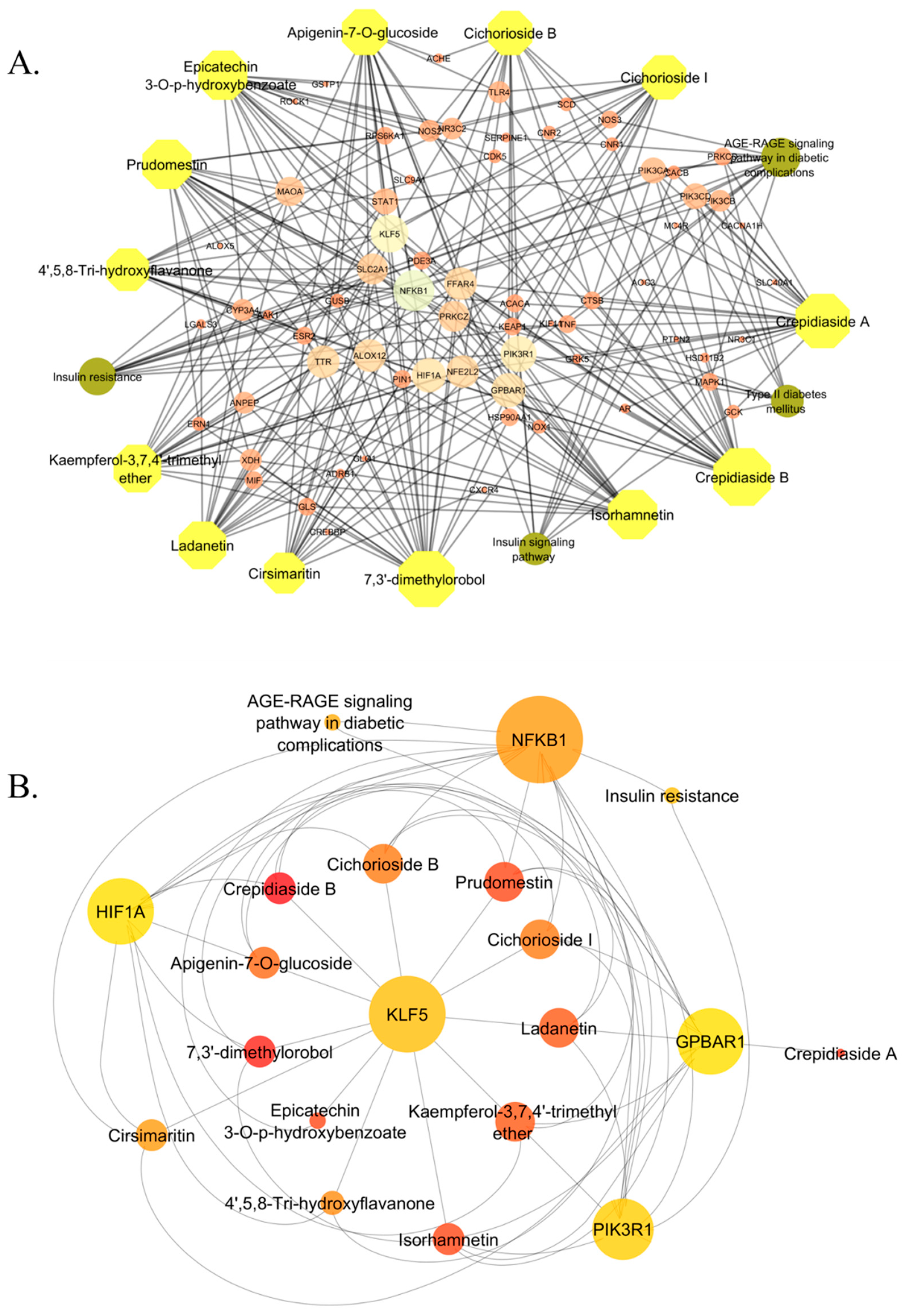
2.2.3. Protein–Protein Network Analysis
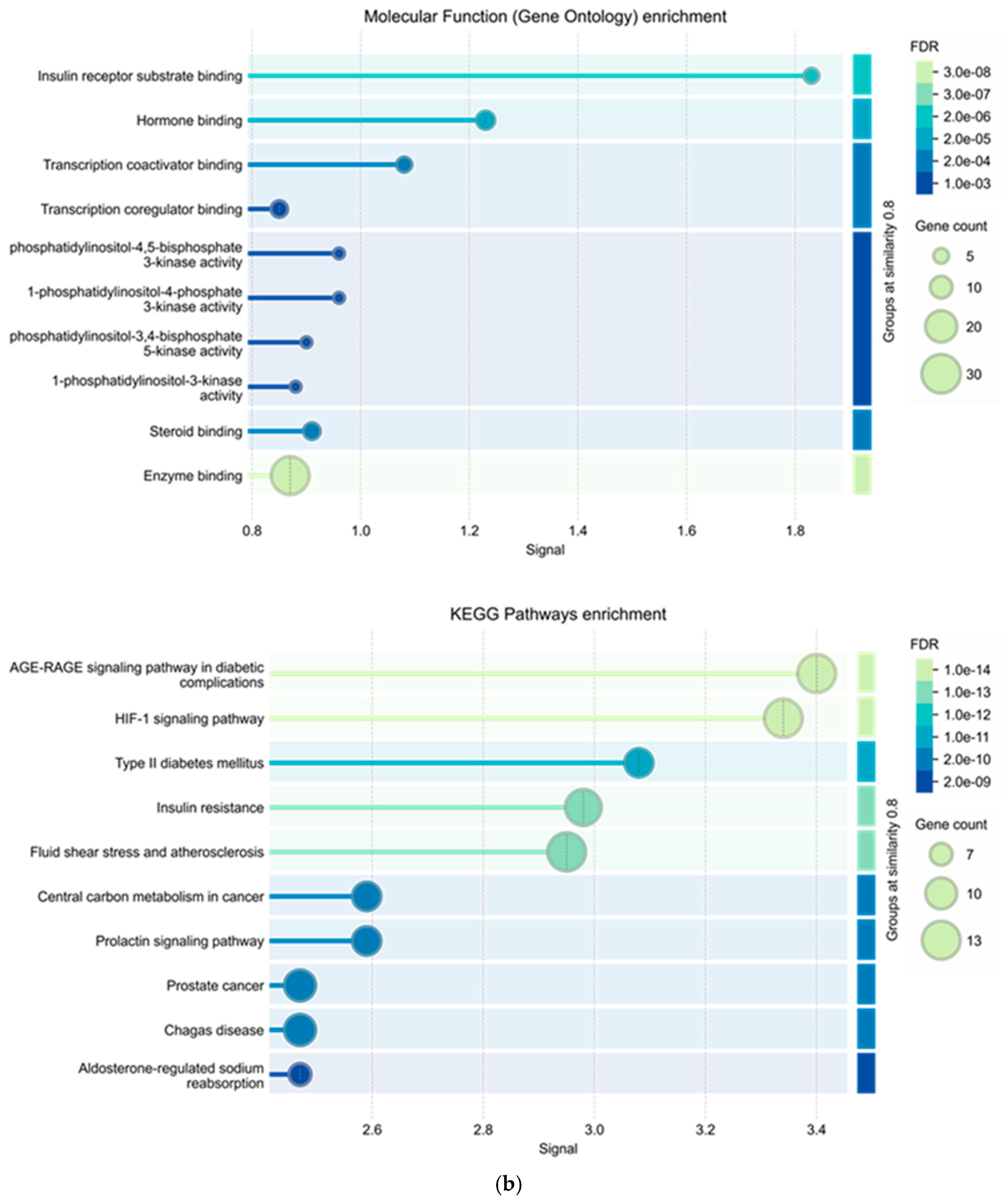
2.2.4. Gene Ontology Enrichment and KEGG Pathway Analyses
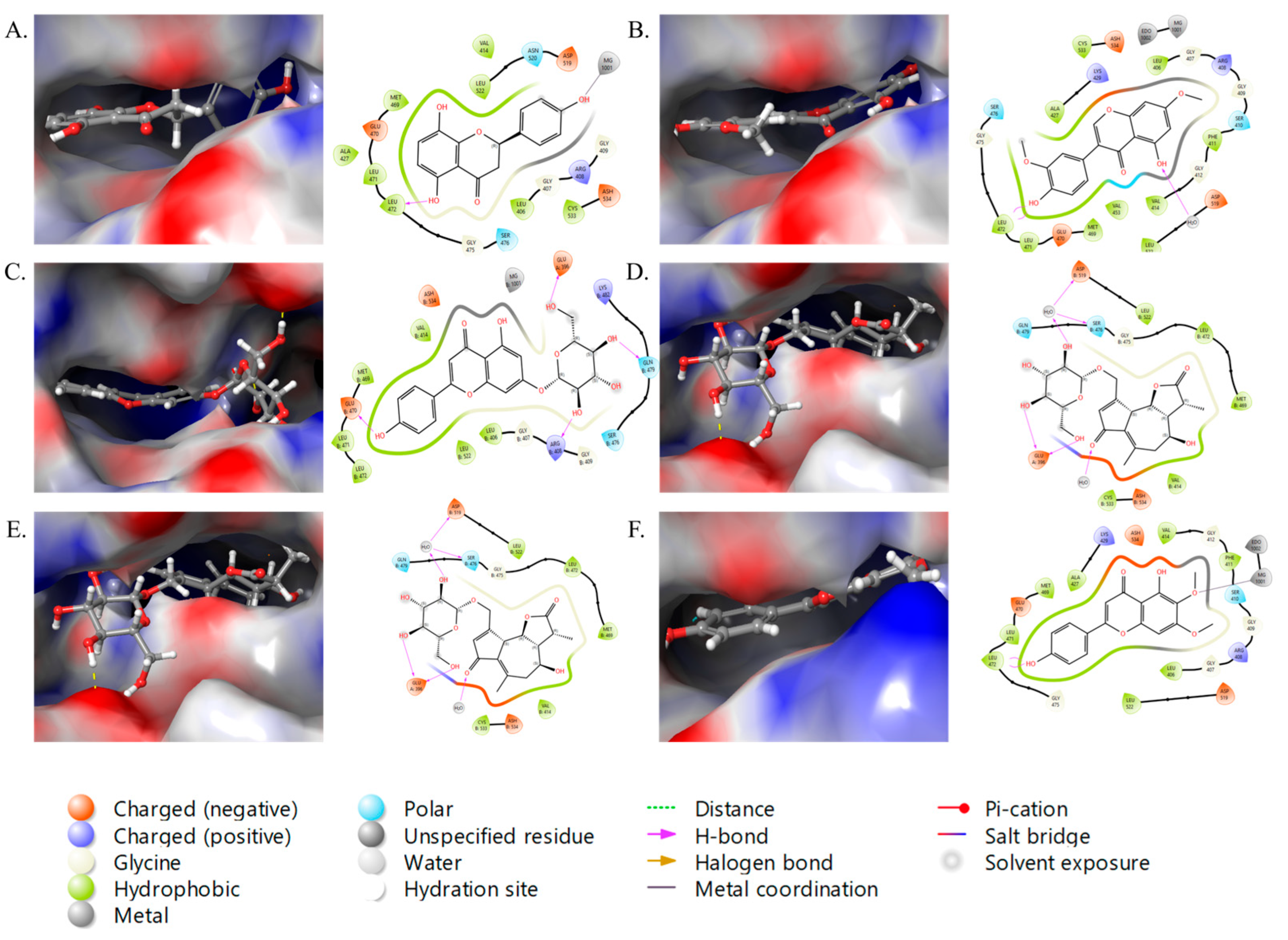
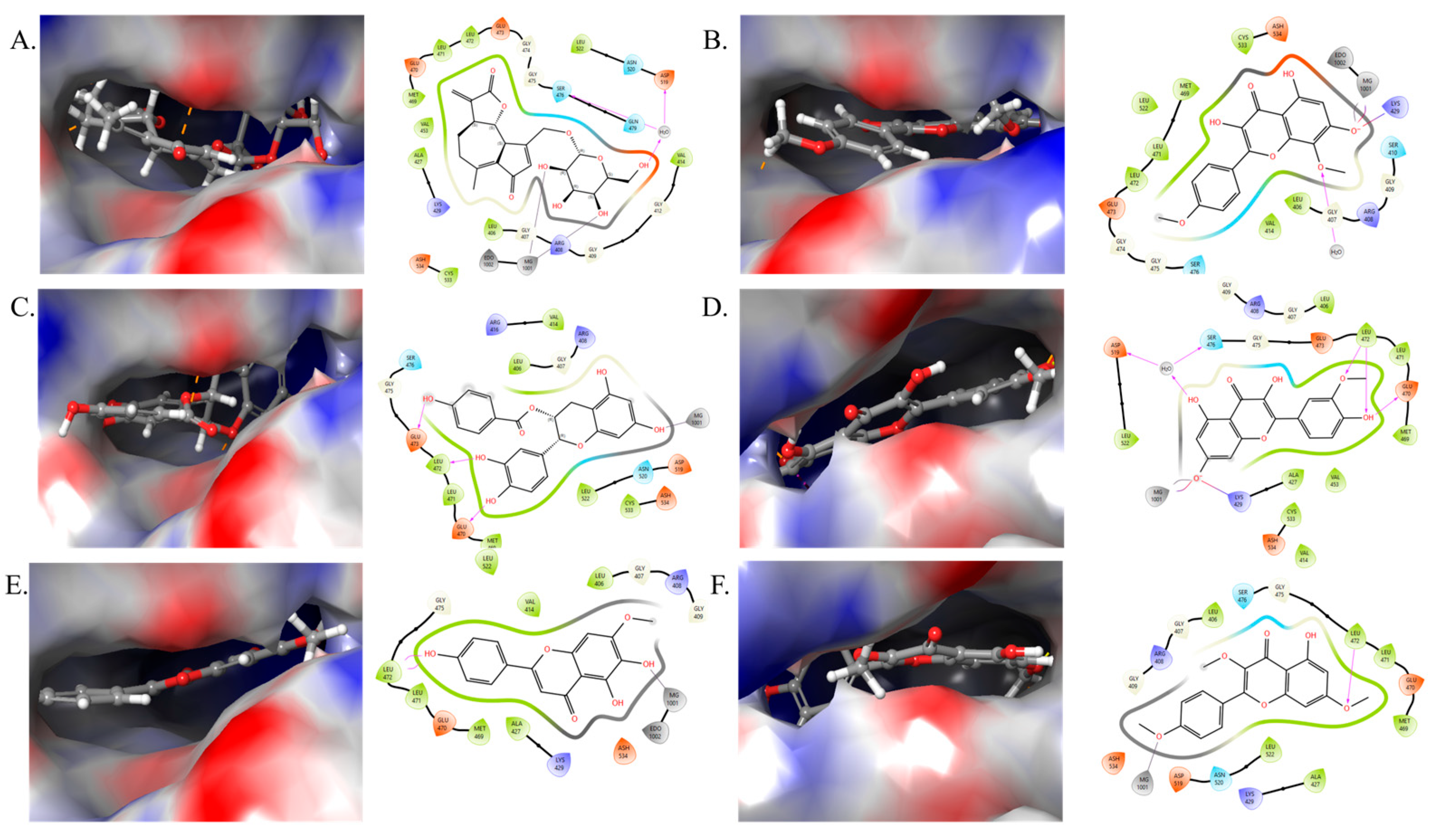
2.3. Molecular Docking Studies
3. Materials and Methods
3.1. Plant Material
3.2. α-Glucosidase Inhibitory Activity
3.3. Network Pharmacology
3.3.1. Screening of Compounds of Chicory
3.3.2. Identification of Selected Compound Targets and Disease Target Genes
3.3.3. Determination of Protein–Protein Interaction and Gene Ontology
3.3.4. Visualization by Cytoscape
3.4. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aisa, H.A.; Xin, X.; Tang, D. Chemical Constituents and Their Pharmacological Activities of Plants from Cichorium Genus. Chin. Herb. Med. 2020, 12, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Street, R.A.; Sidana, J.; Prinsloo, G. Cichorium Intybus: Traditional Uses, Phytochemistry, Pharmacology, and Toxicology. Evid. Based Complement. Altern. Med. 2013, 2013, 579319. [Google Scholar] [CrossRef]
- Cadalen, T.; Mörchen, M.; Blassiau, C.; Clabaut, A.; Scheer, I.; Hilbert, J.-L.; Hendriks, T.; Quillet, M.-C. Development of SSR Markers and Construction of a Consensus Genetic Map for Chicory (Cichorium intybus L.). Mol. Breed. 2010, 25, 699–722. [Google Scholar] [CrossRef]
- Šarić-Kundalić, B.; Dobeš, C.; Klatte-Asselmeyer, V.; Saukel, J. Ethnobotanical Survey of Traditionally Used Plants in Human Therapy of East, North and North-East Bosnia and Herzegovina. J. Ethnopharmacol. 2011, 133, 1051–1076. [Google Scholar] [CrossRef] [PubMed]
- Pushparaj, P.N.; Low, H.K.; Manikandan, J.; Tan, B.K.H.; Tan, C.H. Anti-Diabetic Effects of Cichorium Intybus in Streptozotocin-Induced Diabetic Rats. J. Ethnopharmacol. 2007, 111, 430–434. [Google Scholar] [CrossRef]
- Ghamarian, A.; Abdollahi, M.; Su, X.; Amiri, A.; Ahadi, A.; Nowrouzi, A. Effect of Chicory Seed Extract on Glucose Tolerance Test (GTT) and Metabolic Profile in Early and Late Stage Diabetic Rats. Daru 2012, 20, 56. [Google Scholar] [CrossRef]
- Hossain, M.J.; Al-Mamun, M.; Islam, M.R. Diabetes Mellitus, the Fastest Growing Global Public Health Concern: Early Detection Should Be Focused. Health Sci. Rep. 2024, 7, e2004. [Google Scholar] [CrossRef]
- Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2009, 32, S62–S67. [CrossRef] [PubMed]
- Antar, S.A.; Ashour, N.A.; Sharaky, M.; Khattab, M.; Ashour, N.A.; Zaid, R.T.; Roh, E.J.; Elkamhawy, A.; Al-Karmalawy, A.A. Diabetes Mellitus: Classification, Mediators, and Complications; A Gate to Identify Potential Targets for the Development of New Effective Treatments. Biomed. Pharmacother. 2023, 168, 115734. [Google Scholar] [CrossRef]
- Lu, X.; Xie, Q.; Pan, X.; Zhang, R.; Zhang, X.; Peng, G.; Zhang, Y.; Shen, S.; Tong, N. Type 2 Diabetes Mellitus in Adults: Pathogenesis, Prevention and Therapy. Sig. Transduct. Target. Ther. 2024, 9, 262. [Google Scholar] [CrossRef]
- Kay, A.M.; Simpson, C.L.; Stewart, J.A. The Role of AGE/RAGE Signaling in Diabetes-Mediated Vascular Calcification. J. Diabetes Res. 2016, 2016, 6809703. [Google Scholar] [CrossRef]
- Beale, E.G. Insulin Signaling and Insulin Resistance. J. Investig. Med. 2013, 61, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.; Sherman, A. Type 2 Diabetes: One Disease, Many Pathways. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E410–E426. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, Y.; Shi, L.; Li, L.; Zhang, D.; Gong, Z.; Wu, Q. Activation and Modulation of the AGEs-RAGE Axis: Implications for Inflammatory Pathologies and Therapeutic Interventions—A Review. Pharmacol. Res. 2024, 206, 107282. [Google Scholar] [CrossRef]
- Hopkins, A.L. Network Pharmacology: The next Paradigm in Drug Discovery. Nat. Chem. Biol. 2008, 4, 682–690. [Google Scholar] [CrossRef]
- Shah, A.B.; Lee, K.Y. Integrative Metabolomics and System Pharmacology Reveal the Antioxidant Blueprint of Psoralea Corylifolia. Sci. Rep. 2025, 15, 28632. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yang, L.; Yang, L.; He, C.; He, Y.; Chen, L.; Dong, Q.; Zhang, H.; Chen, S.; Li, P. Network Pharmacology: A Bright Guiding Light on the Way to Explore the Personalized Precise Medication of Traditional Chinese Medicine. Chin. Med. 2023, 18, 146. [Google Scholar] [CrossRef]
- Noor, F.; Tahir Ul Qamar, M.; Ashfaq, U.A.; Albutti, A.; Alwashmi, A.S.S.; Aljasir, M.A. Network Pharmacology Approach for Medicinal Plants: Review and Assessment. Pharmaceuticals 2022, 15, 572. [Google Scholar] [CrossRef]
- Agu, P.C.; Afiukwa, C.A.; Orji, O.U.; Ezeh, E.M.; Ofoke, I.H.; Ogbu, C.O.; Ugwuja, E.I.; Aja, P.M. Molecular Docking as a Tool for the Discovery of Molecular Targets of Nutraceuticals in Diseases Management. Sci. Rep. 2023, 13, 13398. [Google Scholar] [CrossRef] [PubMed]
- Muhammed, M.T.; Aki-Yalcin, E. Molecular Docking: Principles, Advances, and Its Applications in Drug Discovery. Lett. Drug Des. Discov. 2024, 21, 480–495. [Google Scholar] [CrossRef]
- Sharma, M.; Afaque, A.; Dwivedi, S.; Jairajpuri, Z.S.; Shamsi, Y.; Khan, M.F.; Khan, M.I.; Ahmed, D. Cichorium Intybus Attenuates Streptozotocin Induced Diabetic Cardiomyopathy via Inhibition of Oxidative Stress and Inflammatory Response in Rats. Interdiscip. Toxicol. 2019, 12, 111–119. [Google Scholar] [CrossRef]
- Tian, S.; Wang, J.; Li, Y.; Li, D.; Xu, L.; Hou, T. The Application of in Silico Drug-Likeness Predictions in Pharmaceutical Research. Adv. Drug Deliv. Rev. 2015, 86, 2–10. [Google Scholar] [CrossRef]
- Wang, J.; Hou, T. Advances in Computationally Modeling Human Oral Bioavailability. Adv. Drug Deliv. Rev. 2015, 86, 11–16. [Google Scholar] [CrossRef]
- Romzova, M.; Hohenadel, D.; Kolostova, K.; Pinterova, D.; Fojtikova, M.; Ruzickova, S.; Dostal, C.; Bosak, V.; Rychlik, I.; Cerna, M. NFκB and Its Inhibitor IκB in Relation to Type 2 Diabetes and Its Microvascular and Atherosclerotic Complications. Hum. Immunol. 2006, 67, 706–713. [Google Scholar] [CrossRef]
- Baker, R.G.; Hayden, M.S.; Ghosh, S. NF-κB, Inflammation and Metabolic Disease. Cell Metab. 2011, 13, 11–22. [Google Scholar] [CrossRef]
- Fruman, D.A.; Chiu, H.; Hopkins, B.D.; Bagrodia, S.; Cantley, L.C.; Abraham, R.T. The PI3K Pathway in Human Disease. Cell 2017, 170, 605–635. [Google Scholar] [CrossRef] [PubMed]
- Tsay, A.; Wang, J.-C. The Role of PIK3R1 in Metabolic Function and Insulin Sensitivity. Int. J. Mol. Sci. 2023, 24, 12665. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yan, C.; Liu, J. Hyperinsulinemia-Induced KLF5 Mediates Endothelial Angiogenic Dysfunction in Diabetic Endothelial Cells. J. Mol. Hist. 2019, 50, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Kyriazis, I.D.; Hoffman, M.; Gaignebet, L.; Lucchese, A.M.; Markopoulou, E.; Palioura, D.; Wang, C.; Bannister, T.D.; Christofidou-Solomidou, M.; Oka, S.; et al. KLF5 Is Induced by FOXO1 and Causes Oxidative Stress and Diabetic Cardiomyopathy. Circ. Res. 2021, 128, 335–357. [Google Scholar] [CrossRef]
- van Nierop, F.S.; Scheltema, M.J.; Eggink, H.M.; Pols, T.W.; Sonne, D.P.; Knop, F.K.; Soeters, M.R. Clinical Relevance of the Bile Acid Receptor TGR5 in Metabolism. Lancet Diabetes Endocrinol. 2017, 5, 224–233. [Google Scholar] [CrossRef]
- Lund, M.L.; Sorrentino, G.; Egerod, K.L.; Kroone, C.; Mortensen, B.; Knop, F.K.; Reimann, F.; Gribble, F.M.; Drucker, D.J.; de Koning, E.J.P.; et al. L-Cell Differentiation Is Induced by Bile Acids Through GPBAR1 and Paracrine GLP-1 and Serotonin Signaling. Diabetes 2020, 69, 614–623. [Google Scholar] [CrossRef]
- Gunton, J.E. Hypoxia-Inducible Factors and Diabetes. J. Clin. Investig. 2020, 130, 5063–5073. [Google Scholar] [CrossRef]
- Cerychova, R.; Bohuslavova, R.; Papousek, F.; Sedmera, D.; Abaffy, P.; Benes, V.; Kolar, F.; Pavlinkova, G. Adverse Effects of HIF1A Mutation and Maternal Diabetes on the Offspring Heart. Cardiovasc. Diabetol. 2018, 17, 68. [Google Scholar] [CrossRef] [PubMed]
- Shahzadi, Z.; Yousaf, Z.; Anjum, I.; Bilal, M.; Yasin, H.; Aftab, A.; Booker, A.; Ullah, R.; Bari, A. Network Pharmacology and Molecular Docking: Combined Computational Approaches to Explore the Antihypertensive Potential of Fabaceae Species. Bioresour. Bioprocess. 2024, 11, 53. [Google Scholar] [CrossRef]
- Oguntibeju, O.O. Type 2 Diabetes Mellitus, Oxidative Stress and Inflammation: Examining the Links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45–63. [Google Scholar] [PubMed]
- Liu, J.; Li, K.; Yi, Z.; Saqirile; Wang, C.; Yang, R. Oxidative–Inflammatory Crosstalk and Multi-Target Natural Agents: Decoding Diabetic Vascular Complications. Curr. Issues Mol. Biol. 2025, 47, 614. [Google Scholar] [CrossRef] [PubMed]
- Ononamadu, C.J.; Ahmed, Z.B.; Seidel, V. Network Pharmacology, Molecular Docking and Molecular Dynamics Studies to Predict the Molecular Targets and Mechanisms of Action of Melissa Officinalis Phytoconstituents in Type-2 Diabetes Mellitus. Plants 2025, 14, 2828. [Google Scholar] [CrossRef]
- Gayathiri, E.; Prakash, P.; Chaudhari, S.Y.; Sabarathinam, S.; Priyadharshini, S.D.; Al-Sadoon, M.K.; Panneerselvam, J.; Chang, S.W.; Ravindran, B.; Mani, R.R. Interaction of Molecular Mechanisms of Plant-Derived Metabolites in Type 2 Diabetes Mellitus: A Network Pharmacology, Docking and Molecular Dynamics Approach on AKT1 Kinase. Energy Nexus 2025, 17, 100351. [Google Scholar] [CrossRef]
- Niu, Y.; Li, P.; Pang, Z. Comprehensive Studies on the Regulation of Type 2 Diabetes by Cucurbitane-Type Triterpenoids in Momordica charantia L.: Insights from Network Pharmacology and Molecular Docking and Dynamics. Pharmaceuticals 2025, 18, 474. [Google Scholar] [CrossRef]
- PIK3R1Cartwright, T.; Perkins, N.D.; Wilson, C.L. NFKB1: A Suppressor of Inflammation, Ageing and Cancer. FEBS J. 2016, 283, 1812–1822. [Google Scholar] [CrossRef]
- Skalski, B.; Lis, B.; Pecio, Ł.; Kontek, B.; Olas, B.; Żuchowski, J.; Stochmal, A. Isorhamnetin and Its New Derivatives Isolated from Sea Buckthorn Berries Prevent H2O2/Fe—Induced Oxidative Stress and Changes in Hemostasis. Food Chem. Toxicol. 2019, 125, 614–620. [Google Scholar] [CrossRef]
- Gong, G.; Guan, Y.-Y.; Zhang, Z.-L.; Rahman, K.; Wang, S.-J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A Review of Pharmacological Effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef]
- Nagarajan, G.R.; Seshadri, T.R. Flavonoid Components of the Heartwood of Prunus domestica Linn. Phytochemistry 1964, 3, 477–484. [Google Scholar] [CrossRef]
- Nooreen, Z.; Singh, S.; Singh, D.K.; Tandon, S.; Ahmad, A.; Luqman, S. Characterization and Evaluation of Bioactive Polyphenolic Constituents from Zanthoxylum armatum DC., a Traditionally Used Plant. Biomed. Pharmacother. 2017, 89, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurti, M.; Seshadri, T.R.; Shankaran, P.R. A Convenient Synthesis of 3,5,7-Trihydroxy-8-Methoxy Flavone, Prudomestin and Limocitrin. Tetrahedron 1966, 22, 941–948. [Google Scholar] [CrossRef]
- Hu, Y.-M.; Wang, H.; Ye, W.-C.; Zhao, S.-X. Flavones from flowers of Sesamum indicum. Zhongguo Zhong Yao Za Zhi 2007, 32, 603–605. [Google Scholar] [PubMed]
- Meng, Y.; Krzysiak, A.J.; Durako, M.J.; Kunzelman, J.I.; Wright, J.L.C. Flavones and Flavone Glycosides from Halophila johnsonii. Phytochemistry 2008, 69, 2603–2608. [Google Scholar] [CrossRef]
- Zuo, C.; Cheng, J.; Yang, D.; Liu, Y.; Chen, H.; Lu, X.; Guo, M.; Yang, Z.; Wang, Z.; Wang, Y.; et al. Synergistic Effects of NF-κB1 and Inflammatory Pathway Polymorphisms on Hypertension and Dyslipidemia Susceptibility in Type 2 Diabetes. Diabetol. Metab. Syndr. 2025, 17, 269. [Google Scholar] [CrossRef]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-κB in Biology and Targeted Therapy: New Insights and Translational Implications. Sig. Transduct. Tabvcrget. Ther. 2024, 9, 53. [Google Scholar] [CrossRef]
- Zamanian, M.Y.; Alsaab, H.O.; Golmohammadi, M.; Yumashev, A.; Jabba, A.M.; Abid, M.K.; Joshi, A.; Alawadi, A.H.; Jafer, N.S.; Kianifar, F.; et al. NF-κB Pathway as a Molecular Target for Curcumin in Diabetes Mellitus Treatment: Focusing on Oxidative Stress and Inflammation. Cell Biochem. Funct. 2024, 42, e4030. [Google Scholar] [CrossRef]
- Fox, M.; Mott, H.R.; Owen, D. Class IA PI3K Regulatory Subunits: P110-Independent Roles and Structures. Biochem. Soc. Trans. 2020, 48, 1397–1417. [Google Scholar] [CrossRef]
- PIK3R1Takayoshi, T.; Hirota, Y.; Sugano, A.; Sugawara, K.; Takeuchi, T.; Ohta, M.; Yoshimura, K.; Nishikage, S.; Yamamoto, A.; Mimura, Y.; et al. PIK3R1 Mutations in Individuals with Insulin Resistance or Growth Retardation: Case Series and in Silico Functional Analysis. J. Diabetes Investig. 2025, 16, 1526–1534. [Google Scholar] [CrossRef]
- Shah, A.B.; Yoon, S.; Kim, J.H.; Zhumanova, K.; Ban, Y.J.; Lee, K.W.; Park, K.H. Effectiveness of Cyclohexyl Functionality in Ugonins from Helminthostachys zeylanica to PTP1B and α-Glucosidase Inhibitions. Int. J. Biol. Macromol. 2020, 165, 1822–1831. [Google Scholar] [CrossRef]
- Baiseitova, A.; Amzeyeva, U.; Tolepbergen, A.; Ulfanova, Y.; Shybyray, Y.; Dai, L.; Shang, X.; Shah, A.B.; Kim, J.Y.; Jenis, J. Response Surface Methodology Optimization on Ultrasonic-Assisted Extraction of Chicory (Cichorium intybus L.) and Their UPLC-ESI-QTOF-MS Analysis, Antioxidant and Hepatoprotective Activity. eFood 2025, 6, e70081. [Google Scholar] [CrossRef]
- Ko, M.; Kim, Y.; Kim, H.H.; Jeong, S.; Ahn, D.; Chung, S.J.; Kim, H. Network Pharmacology and Molecular Docking Approaches to Elucidate the Potential Compounds and Targets of Saeng-Ji-Hwang-Ko for Treatment of Type 2 Diabetes Mellitus. Comput. Biol. Med. 2022, 149, 106041. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Gallo, K.; Goede, A.; Preissner, R.; Gohlke, B.-O. SuperPred 3.0: Drug Classification and Target Prediction-a Machine Learning Approach. Nucleic Acids Res. 2022, 50, W726–W731. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING Database in 2023: Protein–Protein Association Networks and Functional Enrichment Analyses for Any Sequenced Genome of Interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Safran, M.; Dalah, I.; Alexander, J.; Rosen, N.; Iny Stein, T.; Shmoish, M.; Nativ, N.; Bahir, I.; Doniger, T.; Krug, H.; et al. GeneCards Version 3: The Human Gene Integrator. Database 2010, 2010, baq020. [Google Scholar] [CrossRef]
- Lopes, C.T.; Franz, M.; Kazi, F.; Donaldson, S.L.; Morris, Q.; Bader, G.D. Cytoscape Web: An Interactive Web-Based Network Browser. Bioinformatics 2010, 26, 2347–2348. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.-H.; Chen, S.-H.; Wu, H.-H.; Ho, C.-W.; Ko, M.-T.; Lin, C.-Y. cytoHubba: Identifying Hub Objects and Sub-Networks from Complex Interactome. BMC Syst. Biol. 2014, 8, S11. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.X.; Jung, D.; Yao, R. ShinyGO: A Graphical Gene-Set Enrichment Tool for Animals and Plants. Bioinformatics 2020, 36, 2628–2629. [Google Scholar] [CrossRef] [PubMed]






| S. No. | Compounds Name | Molecular Weight | Drug Likeness Score | Bioavailability |
|---|---|---|---|---|
| 1 | Ladanetin | 300.06 | 0.49 | 0.55 |
| 2 | 7,3′-dimethylorobol | 314.08 | 0.26 | 0.55 |
| 3 | Cirsimaritin | 314.08 | 0.47 | 0.55 |
| 4 | Kaempferol-3,7,4′-trimethyl ether | 328.09 | 0.21 | 0.55 |
| 5 | Apigenin-7-O-glucoside | 432.11 | 0.59 | 0.55 |
| 6 | Isorhamnetin | 316.06 | 0.39 | 0.55 |
| 7 | Prudomestin | 330.07 | 0.25 | 0.55 |
| 8 | Epicatechin 3-O-p-hydroxybenzoate | 410.10 | 0.90 | 0.55 |
| 9 | 4′,5,8-Tri-hydroxyflavanone | 272.07 | 0.30 | 0.55 |
| 10 | Crepidiaside A | 422.16 | 0.54 | 0.55 |
| 11 | Crepidiaside B | 424.17 | 0.79 | 0.55 |
| 12 | Cichorioside I | 440.17 | 0.72 | 0.55 |
| 13 | Cichorioside B | 440.17 | 0.76 | 0.55 |
| S. No. | Compounds | NFKB1 (Glide Score) |
|---|---|---|
| 1 | 4′,5,8-tri-hydroxyflavanone | −7.53832 |
| 2 | 7,3′-dimethylorobol | −7.35084 |
| 3 | Apigenin-7-O-glucoside | −8.05496 |
| 4 | Cichorioside B | −6.10589 |
| 5 | Cichorioside I | −7.7162 |
| 6 | Cirsimaritin | −8.49519 |
| 7 | Crepidiaside A | −8.14023 |
| 8 | Prudomestin | −9.66427 |
| 9 | Epicatechin 3-O-p-hydroxybenzoate | −8.15487 |
| 10 | Isorhamnetin | −11.3776 |
| 11 | Ladanetin | −8.76043 |
| 12 | Kaempferol-3,7,4′-trimethyl ether | −6.48179 |
| S. No. | Compounds | PIK3R1 (Glide Score) |
|---|---|---|
| 1 | Cichorioside B | −7.16 |
| 2 | Cichorioside I | −8.62 |
| 3 | Crepidiaside B | −7.00 |
| 4 | Prudomestin | −7.57 |
| 5 | Isorhamnetin | −8.11 |
| 6 | Ladanetin | −9.36 |
| 7 | Kaempferol-3,7,4′-trimethyl ether | −7.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, A.B.; Baiseitova, A.; Amzeyeva, U.; Shang, X.; Jenis, J. α-Glucosidase Inhibition-Guided Network Pharmacology and Molecular Docking Reveal the Antidiabetic Potential of Cichorium intybus as a Functional Food. Int. J. Mol. Sci. 2025, 26, 9497. https://doi.org/10.3390/ijms26199497
Shah AB, Baiseitova A, Amzeyeva U, Shang X, Jenis J. α-Glucosidase Inhibition-Guided Network Pharmacology and Molecular Docking Reveal the Antidiabetic Potential of Cichorium intybus as a Functional Food. International Journal of Molecular Sciences. 2025; 26(19):9497. https://doi.org/10.3390/ijms26199497
Chicago/Turabian StyleShah, Abdul Bari, Aizhamal Baiseitova, Ulpan Amzeyeva, Xiaofei Shang, and Janar Jenis. 2025. "α-Glucosidase Inhibition-Guided Network Pharmacology and Molecular Docking Reveal the Antidiabetic Potential of Cichorium intybus as a Functional Food" International Journal of Molecular Sciences 26, no. 19: 9497. https://doi.org/10.3390/ijms26199497
APA StyleShah, A. B., Baiseitova, A., Amzeyeva, U., Shang, X., & Jenis, J. (2025). α-Glucosidase Inhibition-Guided Network Pharmacology and Molecular Docking Reveal the Antidiabetic Potential of Cichorium intybus as a Functional Food. International Journal of Molecular Sciences, 26(19), 9497. https://doi.org/10.3390/ijms26199497









