The Role of GABA Pathway Components in Pathogenesis of Neurodevelopmental Disorders
Abstract
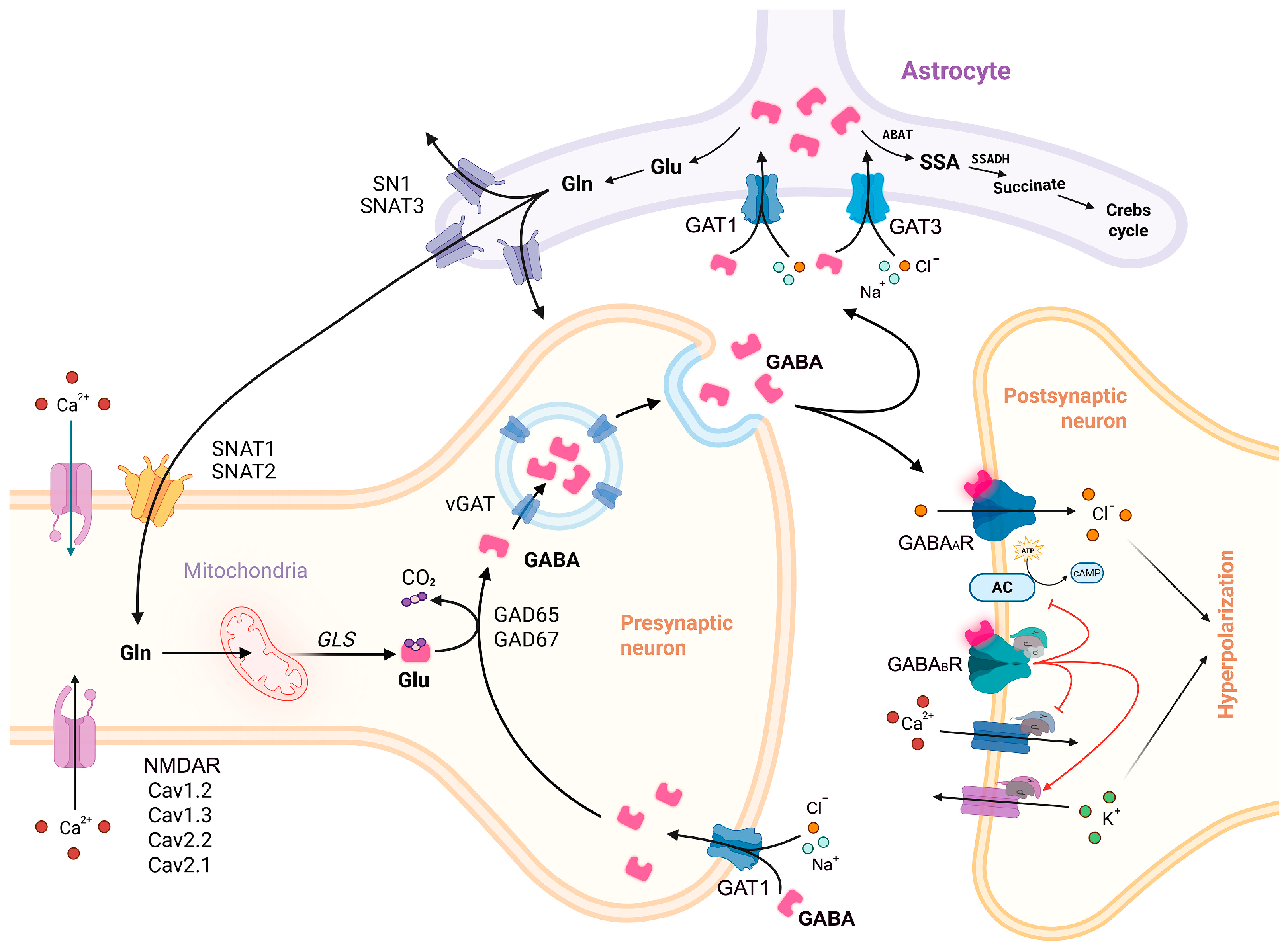
1. Introduction
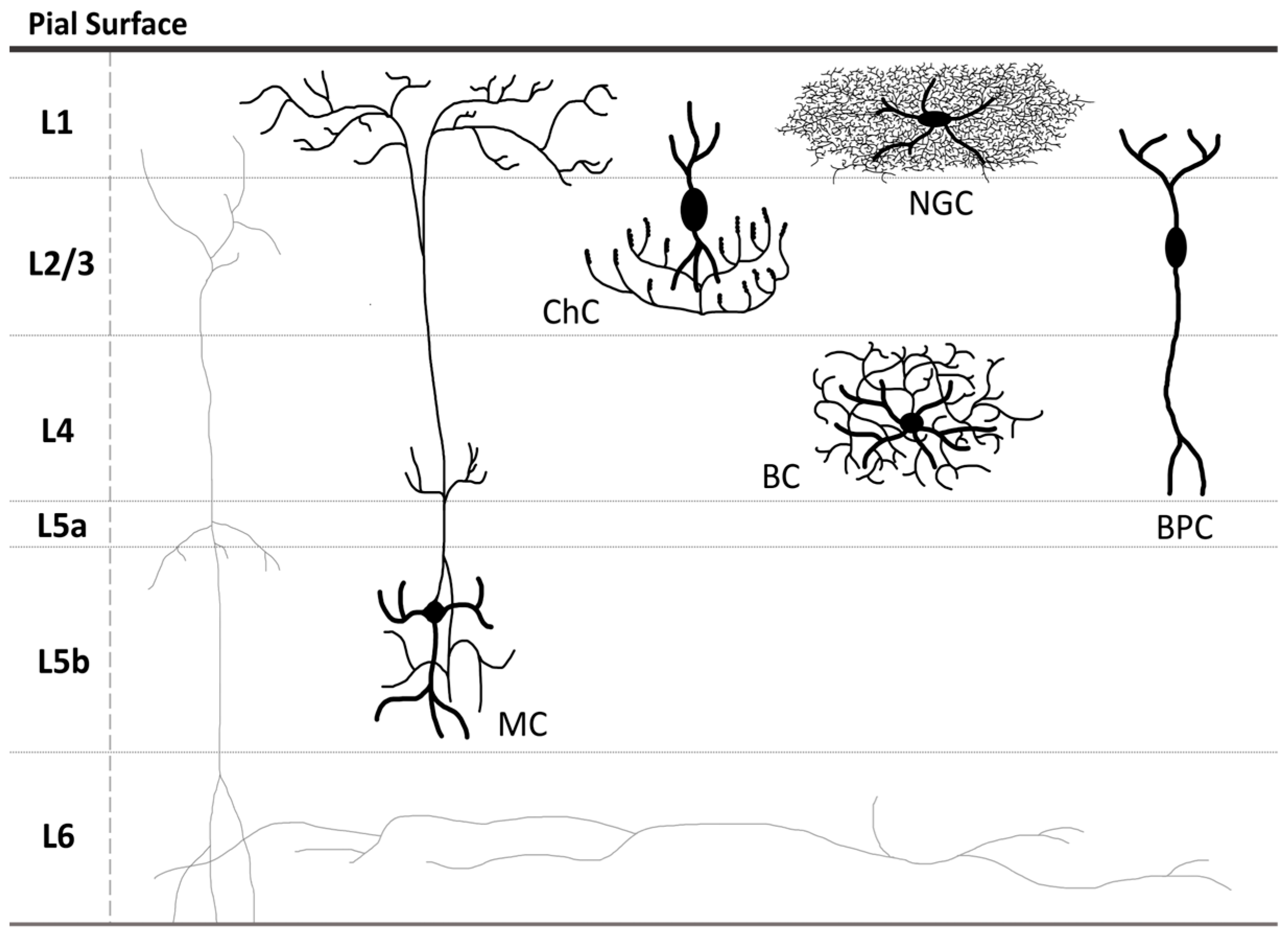
1.1. GABAergic Neurons: Classification, Functions, and Implication in Neurodevelopmental Disorders
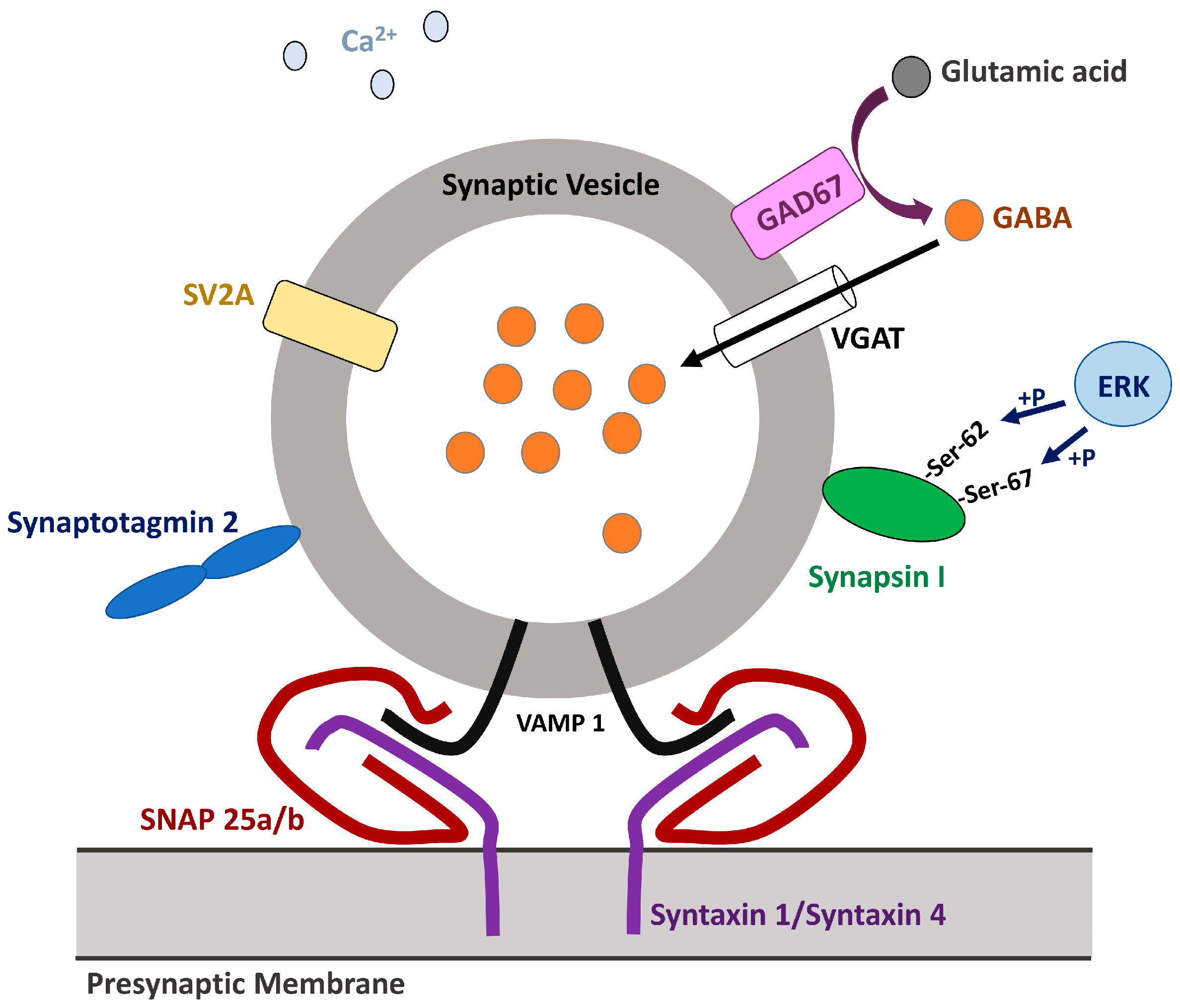
1.2. GABA Synthesis and Release
1.2.1. Sodium-Coupled Neutral Amino Acid Transporter 1
1.2.2. Phosphate-Activated Mitochondrial Glutaminases
1.2.3. Glutamic Acid Decarboxylases
1.2.4. Vesicular GABA Transporter/Vesicular Inhibitory Amino Acid Transporter
1.3. GABA Neurotransmission
1.3.1. GABAA Receptors
1.3.2. GABAB Receptors
1.4. GABA Reuptake
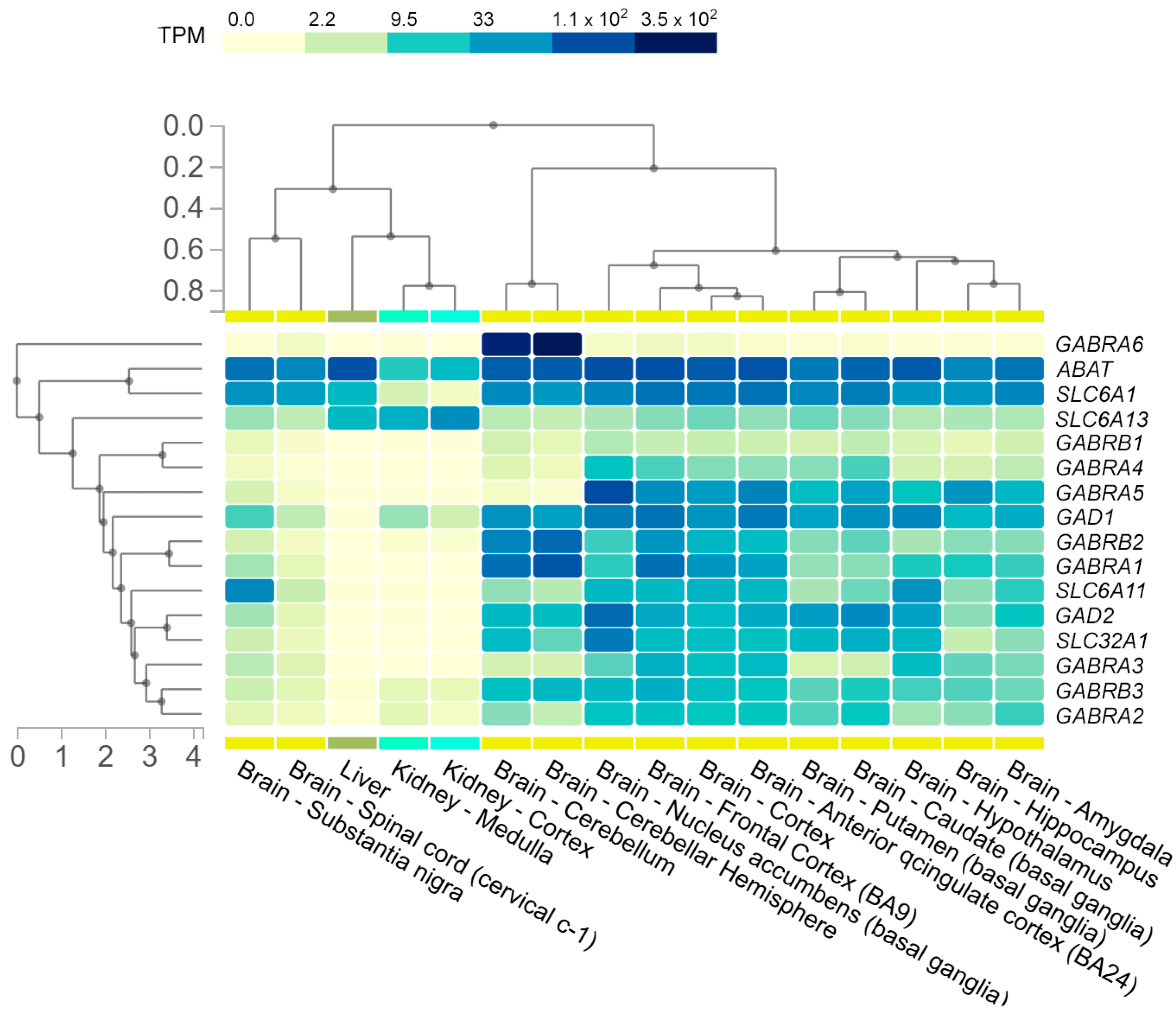
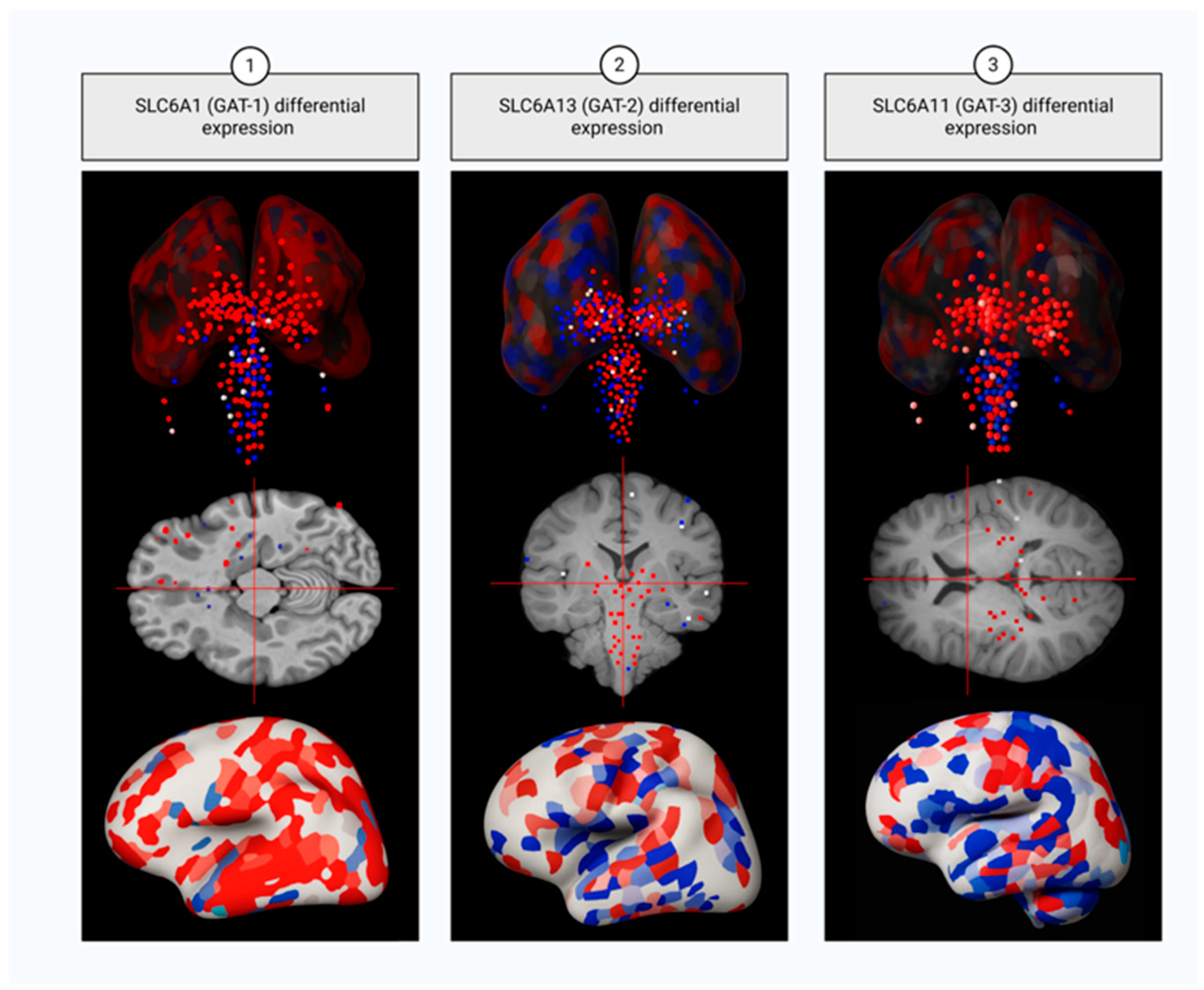
1.4.1. Sodium- and Chloride-Dependent GABA Transporter Type 1
1.4.2. Sodium- and Chloride-Dependent GABA Transporter Type 2
1.4.3. Sodium- and Chloride-Dependent GABA Transporter Type 3
1.4.4. Sodium- and Chloride-Dependent Betaine/GABA Transporter
1.5. GABA Catabolism
GABA Transaminase
1.6. Succinate-Semialdehyde Dehydrogenase
2. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5hmC | 5-Hydroxymethylcytosine |
| 5mC | 5-Methylcytosine |
| ASD | Autism Spectrum Disorder |
| BC(s) | Basket Cell(s) |
| BD | Bipolar Disorder |
| BPC(s) | Bipolar Cell(s) |
| BPND | Non-Displaceable Binding Potential |
| ChC(s) | Chandelier Cell(s) |
| CNS | Central Nervous System |
| CSF | Cerebrospinal Fluid |
| DBC(s) | Double Bouquet Cell(s) |
| DLPFC | Dorsolateral Prefrontal Cortex |
| DS | Down Syndrome |
| E/I balance | Excitatory/Inhibitory Neurotransmitter Balance |
| EMAS | Epilepsy with Myoclonic-Atonic Seizures |
| FXS | Fragile X Syndrome |
| GEFS+ | Febrile Seizures Plus |
| GABA | γ-aminobutyric acid |
| GWAS | Genome-Wide Association Studies |
| ID(s) | Intellectual Disability(-ies) |
| IGE | Idiopathic Generalized Epilepsy |
| LOF | Loss-of-Function |
| MC(s) | Martinotti Cell(s) |
| MDD | Major Depressive Disorder |
| NDD(s) | Neurodevelopmental Disorder(s) |
| NGC(s) | Small Button-type Neurogliaform Cell(s) |
| PET | Positron Emission Tomography |
| PFC | Prefrontal Cortex |
| PLP | Pyridoxal 5′-Phosphate |
| PWS | Prader–Willi Syndrome |
| PyC(s) | Pyramidal Cell(s) |
| sIPSCs | Spontaneous Inhibitory Postsynaptic Currents |
| SNP | Single Nucleotide Polymorphism |
| SSADHD | SSADH Deficiency |
| SV | Synaptic Vesicle |
| SZ | Schizophrenia |
References
- Groth, C.L.; Singh, A.; Zhang, Q.; Berman, B.D.; Narayanan, N.S. Gabaergic Modulation in Movement Related Oscillatory Activity: A Review of the Effect Pharmacologically and with Aging. Tremor Other Hyperkinet. Mov. 2021, 11, 48. [Google Scholar] [CrossRef]
- Ali, D.N.; Ali, H.M.; Lopez, M.R.; Kang, S.; Choi, D.S. Astrocytic GABAergic Regulation in Alcohol Use and Major Depressive Disorders. Cells 2024, 13, 318. [Google Scholar] [CrossRef]
- Benes, F.M.; Berretta, S. GABAergic Interneurons: Implications for Understanding Schizophrenia and Bipolar Disorder. Neuropsychopharmacology 2001, 25, 1–27, Erratum in Neuropsychopharmacol 2001, 25, 453. [Google Scholar] [CrossRef]
- Chen, Q.; Deister, C.A.; Gao, X.; Guo, B.; Lynn-Jones, T.; Chen, N.; Wells, M.F.; Liu, R.; Goard, M.J.; Dimidschstein, J.; et al. Dysfunction of Cortical GABAergic Neurons Leads to Sensory Hyper-Reactivity in a Shank3 Mouse Model of ASD. Nat. Neurosci. 2020, 23, 520–532. [Google Scholar] [CrossRef]
- Bandler, R.C.; Mayer, C. Deciphering Inhibitory Neuron Development: The Paths to Diversity. Curr. Opin. Neurobiol. 2023, 79, 102691. [Google Scholar] [CrossRef]
- Fekete, Z.; Weisz, F.; Karlócai, M.R.; Veres, J.M.; Andrási, T.; Hájos, N. Synaptic Communication within the Microcircuits of Pyramidal Neurons and Basket Cells in the Mouse Prefrontal Cortex. J. Physiol. 2024, 1–22. [Google Scholar] [CrossRef]
- Pieraut, S.; Gounko, N.; Sando, R.; Dang, W.; Rebboah, E.; Panda, S.; Madisen, L.; Zeng, H.; Maximov, A. Experience-Dependent Remodeling of Basket Cell Networks in the Dentate Gyrus. Neuron 2014, 84, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Veres, J.M.; Nagy, G.A.; Hájos, N. Perisomatic GABAergic Synapses of Basket Cells Effectively Control Principal Neuron Activity in Amygdala Networks. Elife 2017, 6, e20721. [Google Scholar] [CrossRef] [PubMed]
- Ribak, C.E.; Seress, L. Five Types of Basket Cell in the Hippocampal Dentate Gyrus: A Combined Golgi and Electron Microscopic Study. J. Neurocytol. 1983, 12, 577–597. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Brown, A.M.; Lackey, E.P.; Arancillo, M.; Lin, T.; Sillitoe, R.V. Purkinje Cell Neurotransmission Patterns Cerebellar Basket Cells into Zonal Modules Defined by Distinct Pinceau Sizes. Elife 2020, 9, e55569. [Google Scholar] [CrossRef]
- Senft, R.A.; Dymecki, S.M. Neuronal Pericellular Baskets: Neurotransmitter Convergence and Regulation of Network Excitability. Trends Neurosci. 2021, 44, 915–924. [Google Scholar] [CrossRef]
- Del Pino, I.; Brotons-Mas, J.R.; Marques-Smith, A.; Marighetto, A.; Frick, A.; Marín, O.; Rico, B. Abnormal Wiring of CCK+ Basket Cells Disrupts Spatial Information Coding. Nat. Neurosci. 2017, 20, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, Y.; Otsuka, T.; Morishima, M.; Ushimaru, M.; Kubota, Y. Control of Excitatory Hierarchical Circuits by Parvalbumin-FS Basket Cells in Layer 5 of the Frontal Cortex: Insights for Cortical Oscillations. J. Neurophysiol. 2019, 121, 2222–2236. [Google Scholar] [CrossRef] [PubMed]
- Markram, H.; Toledo-Rodriguez, M.; Wang, Y.; Gupta, A.; Silberberg, G.; Wu, C. Interneurons of the Neocortical Inhibitory System. Nat. Rev. Neurosci. 2004, 5, 793–807. [Google Scholar] [CrossRef]
- Pelkey, K.A.; Chittajallu, R.; Craig, M.T.; Tricoire, L.; Wester, J.C.; McBain, C.J. Hippocampal Gabaergic Inhibitory Interneurons. Physiol. Rev. 2017, 97, 1619–1747. [Google Scholar] [CrossRef]
- Druga, R.; Salaj, M.; Al-Redouan, A. Parvalbumin—Positive Neurons in the Neocortex: A Review. Physiol. Res. 2023, 72, S173–S191. [Google Scholar] [CrossRef]
- Seignette, K.; Jamann, N.; Papale, P.; Terra, H.; Porneso, R.O.; de Kraker, L.; van der Togt, C.; van der Aa, M.; Neering, P.; Ruimschotel, E.; et al. Experience Shapes Chandelier Cell Function and Structure in the Visual Cortex. Elife 2024, 12, RP91153. [Google Scholar] [CrossRef]
- Perumal, M.B.; Latimer, B.; Xu, L.; Stratton, P.; Nair, S.; Sah, P. Microcircuit Mechanisms for the Generation of Sharp-Wave Ripples in the Basolateral Amygdala: A Role for Chandelier Interneurons. Cell Rep. 2021, 35, 109106. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.; Sethares, C. The Organization of Double Bouquet Cells in Monkey Striate Cortex. J. Neurocytol. 1997, 26, 779–797. [Google Scholar] [CrossRef]
- Chrysanthidis, N.; Fiebig, F.; Lansner, A. Introducing Double Bouquet Cells into a Modular Cortical Associative Memory Model. J. Comput. Neurosci. 2019, 47, 223–230. [Google Scholar] [CrossRef]
- Lukacs, I.P.; Francavilla, R.; Field, M.; Hunter, E.; Howarth, M.; Horie, S.; Plaha, P.; Stacey, R.; Livermore, L.; Ansorge, O.; et al. Differential Effects of Group III Metabotropic Glutamate Receptors on Spontaneous Inhibitory Synaptic Currents in Spine-Innervating Double Bouquet and Parvalbumin-Expressing Dendrite-Targeting GABAergic Interneurons in Human Neocortex. Cereb. Cortex 2023, 33, 2101–2142. [Google Scholar] [CrossRef]
- Tremblay, R.; Lee, S.; Rudy, B. GABAergic Interneurons in the Neocortex: From Cellular Properties to Circuits. Neuron 2016, 91, 260–292. [Google Scholar] [CrossRef]
- Warm, D.; Schroer, J.; Sinning, A. Gabaergic Interneurons in Early Brain Development: Conducting and Orchestrated by Cortical Network Activity. Front. Mol. Neurosci. 2022, 14, 807969. [Google Scholar] [CrossRef]
- Mancini, V.; Saleh, M.G.; Delavari, F.; Bagautdinova, J.; Eliez, S. Excitatory/Inhibitory Imbalance Underlies Hippocampal Atrophy in Individuals with 22q11.2 Deletion Syndrome with Psychotic Symptoms. Biol. Psychiatry 2023, 94, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Ragland, J.D.; Maddock, R.J.; Hurtado, M.Y.; Tanase, C.; Lesh, T.A.; Niendam, T.A.; Carter, C.S.; Ranganath, C. Disrupted GABAergic Facilitation of Working Memory Performance in People with Schizophrenia. Neuroimage Clin. 2020, 25, 102127. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kang, I.; Edden, R.A.E.; Namgung, E.; Kim, J.; Kim, J. Shorter Sleep Duration Is Associated with Lower GABA Levels in the Anterior Cingulate Cortex. Sleep Med. 2020, 71, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Filice, F.; Janickova, L.; Henzi, T.; Bilella, A.; Schwaller, B. The Parvalbumin Hypothesis of Autism Spectrum Disorder. Front. Cell. Neurosci. 2020, 14, 577525. [Google Scholar] [CrossRef]
- Gaus, R.; Popal, M.; Heinsen, H.; Schmitt, A.; Falkai, P.; Hof, P.R.; Schmitz, C.; Vollhardt, A. Reduced Cortical Neuron Number and Neuron Density in Schizophrenia with Focus on Area 24: A Post-Mortem Case–Control Study. Eur. Arch. Psychiatry Clin. Neurosci. 2023, 273, 1209–1223. [Google Scholar] [CrossRef]
- Rabinovitch, A.; Braunstein, D.; Rabinovitch, R.; Biton, Y. Possible Mechanism of Schizophrenia Origin by Excess GABA and Synaptic Pruning. IBRO Neurosci. Rep. 2023, 15, 126–130. [Google Scholar] [CrossRef]
- Qureshi, T.; Bjørkmo, M.; Nordengen, K.; Gundersen, V.; Utheim, T.P.; Watne, L.O.; Storm-Mathisen, J.; Hassel, B.; Chaudhry, F.A. SLC38A1 Conveys Astroglia-Derived Glutamine into GABAergic Interneurons for Neurotransmitter GABA Synthesis. Cells 2020, 9, 1686. [Google Scholar] [CrossRef]
- Mackenzie, B.; Schäfer, M.K.H.; Erickson, J.D.; Hedige, M.A.; Weihe, E.; Varoqui, H. Functional Properties and Cellular Distribution of the System A Glutamine Transporter SNAT1 Support Specialized Roles in Central Neurons. J. Biol. Chem. 2003, 278, 23720–23730. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, T.; Sørensen, C.; Berghuis, P.; Jensen, V.; Dobszay, M.B.; Farkas, T.; Dalen, K.T.; Guo, C.; Hassel, B.; Utheim, T.P.; et al. The Glutamine Transporter SLC38A1 Regulates GABAergic Neurotransmission and Synaptic Plasticity. Cereb. Cortex 2019, 29, 5166–5179. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lin, Y.; Liang, N.; Xue, Z.; Xu, J.; Lin, L.; Shen, Y.; Li, H.; Liu, J.; Lu, J. Methylome-Wide Association Study of Adolescent Depressive Episode with Psychotic Symptoms and Childhood Trauma. J. Affect Disord. 2025, 370, 439–448. [Google Scholar] [CrossRef]
- Jin, L.W.; Horiuchi, M.; Wulff, H.; Liu, X.B.; Cortopassi, G.A.; Erickson, J.D.; Maezawa, I. Dysregulation of Glutamine Transporter SNAT1 in Rett Syndrome Microglia: A Mechanism for Mitochondrial Dysfunction and Neurotoxicity. J. Neurosci. 2015, 35, 2516–2529. [Google Scholar] [CrossRef] [PubMed]
- Kawada, K.; Kuramoto, N.; Mimori, S. Possibility That the Onset of Autism Spectrum Disorder Is Induced by Failure of the Glutamine-Glutamate Cycle. Curr. Mol. Pharmacol. 2020, 14, 170–174. [Google Scholar] [CrossRef]
- Hidese, S.; Ota, M.; Wakabayashi, C.; Noda, T.; Ozawa, H.; Okubo, T.; Kunugi, H. Effects of Chronic L-Theanine Administration in Patients with Major Depressive Disorder: An Open-Label Study. Acta Neuropsychiatr. 2017, 29, 72–79. [Google Scholar] [CrossRef]
- Márquez, J.; Martín-Rufián, M.; Segura, J.A.; Matés, J.M.; Campos-Sandoval, J.A.; Alonso, F.J. Brain Glutaminases. Biomol. Concepts 2010, 1, 3–15. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.; Zhao, L.; Li, Y.; Zheng, J. Glutaminase 1 Is Essential for the Differentiation, Proliferation, and Survival of Human Neural Progenitor Cells. Stem Cells Dev. 2014, 23, 2782–2790. [Google Scholar] [CrossRef]
- Ji, C.; Tang, Y.; Zhang, Y.; Li, C.; Liang, H.; Ding, L.; Xia, X.; Xiong, L.; Qi, X.R.; Zheng, J.C. Microglial Glutaminase 1 Deficiency Mitigates Neuroinflammation Associated Depression. Brain Behav. Immun. 2022, 99, 231–245. [Google Scholar] [CrossRef]
- Ji, C.; Tang, Y.; Zhang, Y.; Huang, X.; Li, C.; Yang, Y.; Wu, Q.; Xia, X.; Cai, Q.; Qi, X.R.; et al. Glutaminase 1 Deficiency Confined in Forebrain Neurons Causes Autism Spectrum Disorder-like Behaviors. Cell Rep. 2023, 42, 112712. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Zhao, R.; Wu, B.; Lanoha, B.; Tong, Z.; Peer, J.; Liu, J.; Xiong, H.; Huang, Y.; et al. Glutaminase C Overexpression in the Brain Induces Learning Deficits, Synaptic Dysfunctions, and Neuroinflammation in Mice. Brain Behav. Immun. 2017, 66, 135–145. [Google Scholar] [CrossRef]
- Chen, H.; Fu, S.; Li, X.; Shi, M.; Qian, J.; Zhao, S.; Yuan, P.; Ding, L.; Xia, X.; Zheng, J.C. Microglial Glutaminase 1 Mediates Chronic Restraint Stress-Induced Depression-like Behaviors and Synaptic Damages. Signal Transduct. Target Ther. 2023, 8, 452. [Google Scholar] [CrossRef]
- Mingote, S.; Masson, J.; Gellman, C.; Thomsen, G.M.; Lin, C.S.; Merker, R.J.; Gaisler-Salomon, I.; Wang, Y.; Ernst, R.; Hen, R.; et al. Genetic Pharmacotherapy as an Early CNS Drug Development Strategy: Testing Glutaminase Inhibition for Schizophrenia Treatment in Adult Mice. Front. Syst. Neurosci. 2016, 9, 165. [Google Scholar] [CrossRef]
- Gaisler-Salomon, I.; Miller, G.M.; Chuhma, N.; Lee, S.; Zhang, H.; Ghoddoussi, F.; Lewandowski, N.; Fairhurst, S.; Wang, Y.; Conjard-Duplany, A.; et al. Glutaminase-Deficient Mice Display Hippocampal Hypoactivity, Insensitivity to Pro-Psychotic Drugs and Potentiated Latent Inhibition: Relevance to Schizophrenia. Neuropsychopharmacology 2009, 34, 2305–2322. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Ma, L.; Yuan, J.; Huang, Y.; Ban, Y.; Zhang, P.; Tan, D.; Liang, M.; Li, Z.; Gong, C.; et al. GLS2 Reduces the Occurrence of Epilepsy by Affecting Mitophagy Function in Mouse Hippocampal Neurons. CNS Neurosci. Ther. 2024, 30, e70036. [Google Scholar] [CrossRef] [PubMed]
- Trifonov, S.; Yamashita, Y.; Kase, M.; Maruyama, M.; Sugimoto, T. Glutamic Acid Decarboxylase 1 Alternative Splicing Isoforms: Characterization, Expression and Quantification in the Mouse Brain. BMC Neurosci. 2014, 15, 114. [Google Scholar] [CrossRef]
- Schwab, C.; Yu, S.; Wong, W.; McGeer, E.G.; McGeer, P.L. GAD65, GAD67, and GABAT Immunostaining in Human Brain and Apparent GAD65 Loss in Alzheimer’s Disease. J. Alzheimers Dis. 2013, 33, 1073–1088. [Google Scholar] [CrossRef]
- Mower, G.D.; Guo, Y. Comparison of the Expression of Two Forms of Glutamic Acid Decarboxylase (GAD67 and GAD65) in the Visual Cortex of Normal and Dark-Reared Cats. Dev. Brain Res. 2001, 126, 65–74. [Google Scholar] [CrossRef]
- Grone, B.P.; Maruska, K.P. Three Distinct Glutamate Decarboxylase Genes in Vertebrates. Sci. Rep. 2016, 6, 30507. [Google Scholar] [CrossRef] [PubMed]
- Asada, H.; Kawamura, Y.; Maruyama, K.; Kume, H.; Ding, R.G.; Ji, F.Y.; Kanbara, N.; Kuzume, H.; Sanbo, M.; Yagi, T.; et al. Mice Lacking the 65 KDa Isoform of Glutamic Acid Decarboxylase (GAD65) Maintain Normal Levels of GAD67 and GABA in Their Brains but Are Susceptible to Seizures. Biochem. Biophys. Res. Commun. 1996, 229, 891–895. [Google Scholar] [CrossRef]
- Asada, H.; Kawamura, Y.; Maruyama, K.; Kume, H.; Ding, R.G.; Kanbara, N.; Kuzume, H.; Sanbo, M.; Yagi, T.; Obata, K. Cleft Palate and Decreased Brain γ-Aminobutyric Acid in Mice Lacking the 67-KDa Isoform of Glutamic Acid Decarboxylase. Proc. Natl. Acad. Sci. USA 1997, 94, 6496–6499. [Google Scholar] [CrossRef]
- Schwartz, H.L.; Chandonia, J.M.; Kash, S.F.; Kanaani, J.; Tunnell, E.; Domingo, A.; Cohen, F.E.; Banga, J.P.; Madec, A.M.; Richter, W.; et al. High-Resolution Autoreactive Epitope Mapping and Structural Modeling of the 65 KDa Form of Human Glutamic Acid Decarboxylase. J. Mol. Biol. 1999, 287, 983–999. [Google Scholar] [CrossRef]
- Shi, Y.; Veit, B.; Bækkeskov, S. Amino Acid Residues 24-31 but Not Palmitoylation of Cysteines 30 and 45 Are Required for Membrane Anchoring of Glutamic Acid Decarboxylase, GAD65. J. Cell Biol. 1994, 124, 927–934. [Google Scholar] [CrossRef]
- Wei, J.; Davis, K.M.; Wu, H.; Wu, J.Y. Protein Phosphorylation of Human Brain Glutamic Acid Decarboxylase (GAD)65 and GAD67 and Its Physiological Implications. Biochemistry 2004, 43, 6182–6189. [Google Scholar] [CrossRef] [PubMed]
- Magri, C.; Giacopuzzi, E.; La Via, L.; Bonini, D.; Ravasio, V.; Elhussiny, M.E.A.; Orizio, F.; Gangemi, F.; Valsecchi, P.; Bresciani, R.; et al. A Novel Homozygous Mutation in GAD1 Gene Described in a Schizophrenic Patient Impairs Activity and Dimerization of GAD67 Enzyme. Sci. Rep. 2018, 8, 15470, Erratum in Sci. Rep. 2020, 10, 4725. [Google Scholar] [CrossRef] [PubMed]
- Kanaani, J.; Diacovo, M.J.; El-Husseini, A.E.D.; Bredt, D.S.; Baekkeskov, S. Palmitoylation Controls Trafficking of GAD65 from Golgi Membranes to Axon-Specific Endosomes and a Rab5a-Dependent Pathway to Presynaptic Clusters. J. Cell Sci. 2004, 117, 2001–2013. [Google Scholar] [CrossRef]
- Su, F.; Pfundstein, G.; Sah, S.; Zhang, S.; Keable, R.; Hagan, D.W.; Sharpe, L.J.; Clemens, K.J.; Begg, D.; Phelps, E.A.; et al. Neuronal Growth Regulator 1 (NEGR1) Promotes the Synaptic Targeting of Glutamic Acid Decarboxylase 65 (GAD65). J. Neurochem. 2025, 169, e16279. [Google Scholar] [CrossRef]
- Gao, S.F.; Klomp, A.; Wu, J.L.; Swaab, D.F.; Bao, A.M. Reduced GAD65/67 Immunoreactivity in the Hypothalamic Paraventricular Nucleus in Depression: A Postmortem Study. J. Affect Disord. 2013, 149, 422–425. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Duan, S.; Wang, H.; Chen, W.; Zhao, X.; Zhang, A.; Wang, L.; Xuan, J.; Yu, L.; Wu, S.; et al. Comprehensive Analysis of Polymorphisms throughout GAD1 Gene: A Family-Based Association Study in Schizophrenia. J. Neural Transm. 2008, 115, 513–519. [Google Scholar] [CrossRef]
- Straub, R.E.; Lipska, B.K.; Egan, M.F.; Goldberg, T.E.; Callicott, J.H.; Mayhew, M.B.; Vakkalanka, R.K.; Kolachana, B.S.; Kleinman, J.E.; Weinberger, D.R. Allelic Variation in GAD1 (GAD67) Is Associated with Schizophrenia and Influences Cortical Function and Gene Expression. Mol. Psychiatry 2007, 12, 854–869. [Google Scholar] [CrossRef]
- Hyde, T.M.; Lipska, B.K.; Ali, T.; Mathew, S.V.; Law, A.J.; Metitiri, O.E.; Straub, R.E.; Ye, T.; Colantuoni, C.; Herman, M.M.; et al. Expression of GABA Signaling Molecules KCC2, NKCC1, and GAD1 in Cortical Development and Schizophrenia. J. Neurosci. 2011, 31, 11088–11095. [Google Scholar] [CrossRef]
- Tao, R.; Davis, K.N.; Li, C.; Shin, J.H.; Gao, Y.; Jaffe, A.E.; Gondré-Lewis, M.C.; Weinberger, D.R.; Kleinman, J.E.; Hyde, T.M. GAD1 Alternative Transcripts and DNA Methylation in Human Prefrontal Cortex and Hippocampus in Brain Development, Schizophrenia. Mol. Psychiatry 2018, 23, 1496–1505. [Google Scholar] [CrossRef]
- Storozheva, Z.I.; Kirenskaya, A.V.; Samylkin, D.V.; Gruden, M.A.; Sewell, R.D.E. Association of GAD1 Gene Polymorphism Rs3749034 with the Information Processing and Cognitive Event-Related Potentials in Schizophrenia Patients and Healthy Subjects. Clin. Neurophysiol. 2024, 166, 142–151. [Google Scholar] [CrossRef]
- Moyer, C.E.; Delevich, K.M.; Fish, K.N.; Asafu-Adjei, J.K.; Sampson, A.R.; Dorph-Petersen, K.A.; Lewis, D.A.; Sweet, R.A. Reduced Glutamate Decarboxylase 65 Protein within Primary Auditory Cortex Inhibitory Boutons in Schizophrenia. Biol. Psychiatry 2012, 72, 734–743. [Google Scholar] [CrossRef]
- Hansen, N.; Bartels, C.; Teegen, B.; Wiltfang, J.; Malchow, B. Catatonic Schizophrenia Associated with Cerebrospinal GAD65 Autoantibodies: Case Report and Literature Review. Front. Immunol. 2022, 13, 829058. [Google Scholar] [CrossRef]
- Yip, J.; Soghomonian, J.J.; Blatt, G.J. Decreased GAD67 MRNA Levels in Cerebellar Purkinje Cells in Autism: Pathophysiological Implications. Acta Neuropathol. 2007, 113, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Zhubi, A.; Chen, Y.; Dong, E.; Cook, E.H.; Guidotti, A.; Grayson, D.R. Increased Binding of MeCP2 to the GAD1 and RELN Promoters May Be Mediated by an Enrichment of 5-HmC in Autism Spectrum Disorder (ASD) Cerebellum. Transl. Psychiatry 2014, 4, e349. [Google Scholar] [CrossRef]
- Labouesse, M.A.; Dong, E.; Grayson, D.R.; Guidotti, A.; Meyer, U. Maternal Immune Activation Induces GAD1 and GAD2 Promoter Remodeling in the Offspring Prefrontal Cortex. Epigenetics 2015, 10, 1143–1155. [Google Scholar] [CrossRef]
- Neuray, C.; Maroofian, R.; Scala, M.; Sultan, T.; Pai, G.S.; Mojarrad, M.; Khashab, H.E.; Deholl, L.; Yue, W.; Alsaif, H.S.; et al. Early-Infantile Onset Epilepsy and Developmental Delay Caused by Bi-Allelic GAD1 Variants. Brain 2020, 143, 2388–2397. [Google Scholar] [CrossRef] [PubMed]
- Tröscher, A.R.; Mair, K.M.; Verdú De Juan, L.; Köck, U.; Steinmaurer, A.; Baier, H.; Becker, A.; Blümcke, I.; Finzel, M.; Geis, C.; et al. Temporal Lobe Epilepsy with GAD Antibodies: Neurons Killed by T Cells Not by Complement Membrane Attack Complex. Brain 2023, 146, 1436–1452. [Google Scholar] [CrossRef] [PubMed]
- Kajita, Y.; Ono, K.; Kaneda, S.; Mushiake, H. Pterostilben Upregulates GAD67-Mediated GABA Synthesis in Hippocampal Parvalbumin-Positive Cells. Neuroscience 2025, 573, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Nieoczym, D.; Socała, K.; Zelek-Molik, A.; Pieróg, M.; Przejczowska-Pomierny, K.; Szafarz, M.; Wyska, E.; Nalepa, I.; Wlaź, P. Anticonvulsant Effect of Pterostilbene and Its Influence on the Anxiety- and Depression-like Behavior in the Pentetrazol-Kindled Mice: Behavioral, Biochemical, and Molecular Studies. Psychopharmacology 2021, 238, 3167–3181. [Google Scholar] [CrossRef]
- Ku, T.H.; Lee, Y.J.; Wang, S.J.; Fan, C.H.; Tien, L.T. Effect of honokiol on activity of GAD(65) and GAD(67) in the cortex and hippocampus of mice. Phytomedicine 2011, 18, 1126–1129. [Google Scholar] [CrossRef]
- Vega-García, A.; Neri-Gómez, T.; Buzoianu-Anguiano, V.; Guerra-Araiza, C.; Segura-Uribe, J.; Feria-Romero, I.; Orozco-Suarez, S. Electroacupuncture Reduces Seizure Activity and Enhances GAD 67 and Glutamate Transporter Expression in Kainic Acid Induced Status Epilepticus in Infant Rats. Behav. Sci. 2019, 9, 68. [Google Scholar] [CrossRef]
- Huang, H.S.; Matevossian, A.; Whittle, C.; Se, Y.K.; Schumacher, A.; Baker, S.P.; Akbarian, S. Prefrontal Dysfunction in Schizophrenia Involves Mixed-Lineage Leukemia 1-Regulated Histone Methylation at GABAergic Gene Promoters. J. Neurosci. 2007, 27, 11254–11262. [Google Scholar] [CrossRef]
- Zaitsev, A.V.; Povysheva, N.V.; Lewis, D.A.; Krimer, L.S. P/Q-Type, but Not N-Type, Calcium Channels Mediate GABA Release from Fast-Spiking Interneurons to Pyramidal Cells in Rat Prefrontal Cortex. J. Neurophysiol. 2007, 97, 3567–3573. [Google Scholar] [CrossRef]
- Gopal, N.; Leitz, J.; Wang, C.; Esquivies, L.; Pfuetzner, R.A.; Brunger, A.T. A New Method for Isolation and Purification of Fusion-Competent Inhibitory Synaptic Vesicles. Curr. Res. Physiol. 2024, 7, 100121. [Google Scholar] [CrossRef]
- McIntire, S.L.; Reimer, R.J.; Schuske, K.; Edwards, R.H.; Jorgensen, E.M. Identification and Characterization of the Vesicular GABA Transporter. Nature 1997, 389, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, S.M.; Katsurabayashi, S.; Guillemin, I.; Friauf, E.; Rosenmund, C.; Brose, N.; Rhee, J.S. A Shared Vesicular Carrier Allows Synaptic Corelease of GABA and Glycine. Neuron 2006, 50, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Martens, H.; Weston, M.C.; Boulland, J.L.; Grønborg, M.; Grosche, J.; Kacza, J.; Hoffmann, A.; Matteoli, M.; Takamori, S.; Harkany, T.; et al. Unique Luminal Localization of VGAT-C Terminus Allows for Selective Labeling of Active Cortical GABAergic Synapses. J. Neurosci. 2008, 28, 13125–13131. [Google Scholar] [CrossRef]
- Chaudhry, F.A.; Reimer, R.J.; Bellocchio, E.E.; Danbolt, N.C.; Osen, K.K.; Edwards, R.H.; Storm-Mathisen, J. The Vesicular GABA Transporter, VGAT, Localizes to Synaptic Vesicles in Sets of Glycinergic as Well as GABAergic Neurons. J. Neurosci. 1998, 18, 9733–9750. [Google Scholar] [CrossRef] [PubMed]
- Fattorini, G.; Antonucci, F.; Menna, E.; Matteoli, M.; Conti, F. Co-Expression of VGLUT1 and VGAT Sustains Glutamate and GABA Co-Release and Is Regulated by Activity in Cortical Neurons. J. Cell Sci. 2015, 128, 1669–1673. [Google Scholar] [CrossRef]
- Xu, J.; Jo, A.; DeVries, R.P.; Deniz, S.; Cherian, S.; Sunmola, I.; Song, X.; Marshall, J.J.; Gruner, K.A.; Daigle, T.L.; et al. Intersectional Mapping of Multi-Transmitter Neurons and Other Cell Types in the Brain. Cell Rep. 2022, 40, 111036. [Google Scholar] [CrossRef] [PubMed]
- Heron, S.E.; Regan, B.M.; Harris, R.V.; Gardner, A.E.; Coleman, M.J.; Bennett, M.F.; Grinton, B.E.; Helbig, K.L.; Sperling, M.R.; Haut, S.; et al. Association of SLC32A1 Missense Variants with Genetic Epilepsy with Febrile Seizures Plus. Neurology 2021, 96, e2251–e2260. [Google Scholar] [CrossRef]
- Platzer, K.; Sticht, H.; Bupp, C.; Ganapathi, M.; Pereira, E.M.; Le Guyader, G.; Bilan, F.; Henderson, L.B.; Lemke, J.R.; Taschenberger, H.; et al. De Novo Missense Variants in SLC32A1 Cause a Developmental and Epileptic Encephalopathy Due to Impaired GABAergic Neurotransmission. Ann. Neurol. 2022, 92, 958–973. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Shi, J.; Liu, T.; Liu, J.; Liu, Y.; Li, J.; Luo, Y.; Luo, J.; Li, X.; Gong, H.; et al. Astragaloside IV Ameliorates Autism-like Behaviors in BTBR Mice by Modulating Camk2n2-Dependent OXPHOS and Neurotransmission in the MPFC. J. Adv. Res. 2025. [Google Scholar] [CrossRef]
- Alshammari, T.K.; Alshammari, M.A.; Nenov, M.N.; Hoxha, E.; Cambiaghi, M.; Marcinno, A.; James, T.F.; Singh, P.; Labate, D.; Li, J.; et al. Genetic Deletion of Fibroblast Growth Factor 14 Recapitulates Phenotypic Alterations Underlying Cognitive Impairment Associated with Schizophrenia. Transl. Psychiatry 2016, 6, e806. [Google Scholar] [CrossRef]
- San Martín, A.; Pagani, M.R. Understanding Intellectual Disability through RASopathies. J. Physiol. Paris 2014, 108, 232–239. [Google Scholar] [CrossRef]
- Ryu, H.H.; Kang, M.; Hwang, K.D.; Jang, H.B.; Kim, S.J.; Lee, Y.S. Neuron Type-Specific Expression of a Mutant KRAS Impairs Hippocampal-Dependent Learning and Memory. Sci. Rep. 2020, 10, 17730. [Google Scholar] [CrossRef]
- Cui, Y.; Costa, R.M.; Murphy, G.G.; Elgersma, Y.; Zhu, Y.; Gutmann, D.H.; Parada, L.F.; Mody, I.; Silva, A.J. Neurofibromin Regulation of ERK Signaling Modulates GABA Release and Learning. Cell 2008, 135, 549–560. [Google Scholar] [CrossRef]
- Young, A.B.; Chu, D. Distribution of GABAA and GABAB Receptors in Mammalian Brain: Potential Targets for Drug Development. Drug Dev. Res. 1990, 21, 161–167. [Google Scholar] [CrossRef]
- Mortensen, M.; Patel, B.; Smart, T.G. GABA Potency at GABA A Receptors Found in Synaptic and Extrasynaptic Zones. Front. Cell. Neurosci. 2012, 6, 1. [Google Scholar] [CrossRef]
- Petri, S.; Krampfl, K.; Dengler, R.; Bufler, J.; Weindl, A.; Arzberger, T. Human GABAA Receptors on Dopaminergic Neurons in the Pars Compacta of the Substantia Nigra. J. Comp. Neurol. 2002, 452, 360–366. [Google Scholar] [CrossRef]
- Gao, B.; Fritschy, J.M.; Benke, D.; Mohler, H. Neuron-Specific Expression of GABAA-Receptor Subtypes: Differential Association of the A1- and A3-Subunits with Serotonergic and Gabaergic Neurons. Neuroscience 1993, 54, 881–892. [Google Scholar] [CrossRef] [PubMed]
- Ghit, A.; Assal, D.; Al-Shami, A.S.; Hussein, D.E.E. GABAA Receptors: Structure, Function, Pharmacology, and Related Disorders. J. Genet. Eng. Biotechnol. 2021, 19, 123. [Google Scholar] [CrossRef]
- McKernan, R.M.; Whiting, P.J. Which GABAA-Receptor Subtypes Really Occur in the Brain? Trends Neurosci. 1996, 19, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Song, J.J.; Curtis, M.A.; Faull, R.L.M.; Waldvogel, H.J. The Regional and Cellular Distribution of GABAA Receptor Subunits in the Human Amygdala. J. Chem. Neuroanat. 2022, 126, 102185. [Google Scholar] [CrossRef]
- Yang, L.; Shen, H.; Merlin, L.R.; Smith, S.S. Pubertal Expression of A4βδ GABAA Receptors Reduces Seizure-Like Discharges in CA1 Hippocampus. Sci. Rep. 2016, 6, 31928. [Google Scholar] [CrossRef]
- Sur, C.; Quirk, K.; Dewar, D.; Atack, J.; Mckernan, R. Rat and Human Hippocampal A5 Subunit-Containing γ-Aminobutyric Acid(A) Receptors Have A5β3γ2 Pharmacological Characteristics. Mol. Pharmacol. 1998, 54, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Bahn, S.; Jones, A.; Wisden, W. Directing Gene Expression to Cerebellar Granule Cells Using γ-Aminobutyric Acid Type A Receptor A6 Subunit Transgenes. Proc. Natl. Acad. Sci. USA 1997, 94, 9417–9421. [Google Scholar] [CrossRef]
- Enz, R. GABAc Receptor p Subunits Are Heterogeneously Expressed in the Human CMS and Form Homo- And Heterooligomers with Distinct Physical Properties. Europ. J. Neurosci. 1999, 11, 41–50. [Google Scholar] [CrossRef]
- Nakamura, Y.; Darnieder, L.M.; Deeb, T.Z.; Moss, S.J. Regulation of GABAARs by Phosphorylation. Adv. Pharmacol. 2015, 72, 97–146. [Google Scholar] [CrossRef]
- Goldschen-Ohm, M.P. Benzodiazepine Modulation of GABAA Receptors: A Mechanistic Perspective. Biomolecules 2022, 12, 1784. [Google Scholar] [CrossRef]
- Ochoa-de la Paz, L.D.; Gulias-Cañizo, R.; D’Abril Ruíz-Leyja, E.; Sánchez-Castillo, H.; Parodí, J. The Role of GABA Neurotransmitter in the Human Central Nervous System, Physiology, and Pathophysiology. Rev. Mex. Neuroci. 2021, 22, 67–76. [Google Scholar] [CrossRef]
- Li, Y.; Yu, L.; Zhao, L.; Zeng, F.; Liu, Q.S. Resveratrol Modulates Cocaine-Induced Inhibitory Synaptic Plasticity in VTA Dopamine Neurons by Inhibiting Phosphodiesterases (PDEs). Sci. Rep. 2017, 7, 15657. [Google Scholar] [CrossRef]
- Jang, S.H.; Park, S.J.; Kim, K.A.; Han, S.K. Resveratrol Activates GABAA and/or Glycine Receptors on Substantia Gelatinosa Neurons of the Subnucleus Caudalis in Mice. Nat. Prod. Res. 2022, 36, 5788–5792. [Google Scholar] [CrossRef] [PubMed]
- Decui, L.; Garbinato, C.L.L.; Schneider, S.E.; Mazon, S.C.; Almeida, E.R.; Aguiar, G.P.S.; Müller, L.G.; Oliveira, J.V.; Siebel, A.M. Micronized Resveratrol Shows Promising Effects in a Seizure Model in Zebrafish and Signalizes an Important Advance in Epilepsy Treatment. Epilepsy Res. 2020, 159, 106243. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, S.H.; Folsom, T.D.; Rooney, R.J.; Thuras, P.D. Expression of GABAa A2-, B1-and e-Receptors Are Altered Significantly in the Lateral Cerebellum of Subjects with Schizophrenia, Major Depression and Bipolar Disorder. Transl. Psychiatry 2013, 3, e303. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, S.H.; Folsom, T.D.; Thuras, P.D. GABAA and GABAB Receptor Dysregulation in Superior Frontal Cortex of Subjects with Schizophrenia and Bipolar Disorder. Synapse 2017, 71. [Google Scholar] [CrossRef]
- Marques, T.R.; Ashok, A.H.; Angelescu, I.; Borgan, F.; Myers, J.; Lingford-Hughes, A.; Nutt, D.J.; Veronese, M.; Turkheimer, F.E.; Howes, O.D. GABA-A Receptor Differences in Schizophrenia: A Positron Emission Tomography Study Using [11C]Ro154513. Mol. Psychiatry 2021, 26, 2616–2625. [Google Scholar] [CrossRef]
- Mueller, T.M.; Haroutunian, V.; Meador-Woodruff, J.H. N-Glycosylation of GABAA Receptor Subunits Is Altered in Schizophrenia. Neuropsychopharmacology 2014, 39, 528–537. [Google Scholar] [CrossRef]
- Chiou, L.C.; Sieghart, W. IUPHAR Review: Alpha6-Containing GABAA Receptors—Novel Targets for the Treatment of Schizophrenia. Pharmacol. Res. 2025, 213, 107613. [Google Scholar] [CrossRef]
- Moradi, M.; Saidijam, M.; Yadegarazari, R.; Jahangard, L.; Seifi, M.; Matinnia, N.; Ghaleiha, A. Genes Encoding GABA-β and HT1D Receptors in Bipolar I (Manic Phase) Patients. Basic Clin. Neurosci. 2018, 9, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Maillard, P.Y.; Baer, S.; Schaefer, É.; Desnous, B.; Villeneuve, N.; Lépine, A.; Fabre, A.; Lacoste, C.; El Chehadeh, S.; Piton, A.; et al. Molecular and Clinical Descriptions of Patients with GABAA Receptor Gene Variants (GABRA1, GABRB2, GABRB3, GABRG2): A Cohort Study, Review of Literature, and Genotype–Phenotype Correlation. Epilepsia 2022, 63, 2519–2533. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Catron, M.; Zhou, C.; Janve, V.; Shen, W.; Howe, R.K.; Macdonald, R.L. GABAAreceptor Β3 Subunit Mutation D120N Causes Lennox-Gastaut Syndrome in Knock-in Mice. Brain Commun. 2020, 2, fcaa028. [Google Scholar] [CrossRef]
- Gonda, X.; Sarginson, J.; Eszlari, N.; Petschner, P.; Toth, Z.G.; Baksa, D.; Hullam, G.; Anderson, I.M.; Deakin, J.F.W.; Juhasz, G.; et al. A New Stress Sensor and Risk Factor for Suicide: The T Allele of the Functional Genetic Variant in the GABRA6 Gene. Sci. Rep. 2017, 7, 12887. [Google Scholar] [CrossRef]
- Zhang, T.; Li, J.; Yu, H.; Shi, Y.; Li, Z.; Wang, L.; Wang, Z.; Lu, T.; Wang, L.; Yue, W.; et al. Meta-Analysis of GABRB2 Polymorphisms and the Risk of Schizophrenia Combined with GWAS Data of the Han Chinese Population and Psychiatric Genomics Consortium. PLoS ONE 2018, 13, e0198690. [Google Scholar] [CrossRef] [PubMed]
- Roof, E.; Stone, W.; MacLean, W.; Feurer, I.D.; Thompson, T.; Butler, M.G. Intellectual Characteristics of Prader-Willi Syndrome: Comparison of Genetic Subtypes. J. Intellect. Disabil. Res. 2000, 44 Pt 1, 25–30. [Google Scholar] [CrossRef]
- Lucignani, G.; Panzacchi, A.; Bosio, L.; Moresco, R.M.; Ravasi, L.; Coppa, I.; Chiumello, G.; Frey, K.; Koeppe, R.; Fazio, F. GABAA Receptor Abnormalities in Prader-Willi Syndrome Assessed with Positron Emission Tomography and [11C]Flumazenil. Neuroimage 2004, 22, 22–28. [Google Scholar] [CrossRef]
- Ho, A.Y.; Dimitropoulos, A. Clinical Management of Behavioral Characteristics of Prader-Willi Syndrome. Neuropsychiatr. Dis. Treat. 2010, 6, 107–118. [Google Scholar] [CrossRef]
- Rice, L.J.; Lagopoulos, J.; Brammer, M.; Einfeld, S.L. Reduced Gamma-Aminobutyric Acid Is Associated with Emotional and Behavioral Problems in Prader-Willi Syndrome. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2016, 171, 1041–1048. [Google Scholar] [CrossRef]
- Paluszkiewicz, S.M.; Martin, B.S.; Huntsman, M.M. Fragile X Syndrome: The GABAergic System and Circuit Dysfunction. Dev. Neurosci. 2011, 33, 349–364. [Google Scholar] [CrossRef]
- D’Hulst, C.; De Geest, N.; Reeve, S.P.; Van Dam, D.; De Deyn, P.P.; Hassan, B.A.; Kooy, R.F. Decreased Expression of the GABAA Receptor in Fragile X Syndrome. Brain Res. 2006, 1121, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Peng, Z.; Tong, X.; Lindemeyer, A.K.; Cetina, Y.; Huang, C.S.; Olsen, R.W.; Otis, T.S.; Houser, C.R. Decreased Surface Expression of the δ Subunit of the GABAA Receptor Contributes to Reduced Tonic Inhibition in Dentate Granule Cells in a Mouse Model of Fragile X Syndrome. Exp. Neurol. 2017, 297, 168–178. [Google Scholar] [CrossRef]
- D’Hulst, C.; Heulens, I.; Van Der Aa, N.; Goffin, K.; Koole, M.; Porke, K.; Van De Velde, M.; Rooms, L.; Van Paesschen, W.; Van Esch, H.; et al. Positron Emission Tomography (PET) Quantification of GABAA Receptors in the Brain of Fragile X Patients. PLoS ONE 2015, 10, e0131486. [Google Scholar] [CrossRef]
- Hannan, S.B.; Lana-Elola, E.; Watson-Scales, S.; Fisher, E.M.C.; Tybulewicz, V.L.J.; Smart, T.G. Hippocampal Circuit-Specific Enhancement of GABA-Inhibition Caused by Discrete Gene Regions in a Down Syndrome Model. bioRxiv 2025. [Google Scholar] [CrossRef]
- Silbereis, J.C.; Nobuta, H.; Tsai, H.H.; Heine, V.M.; McKinsey, G.L.; Meijer, D.H.; Howard, M.A.; Petryniak, M.A.; Potter, G.B.; Alberta, J.A.; et al. Olig1 Function Is Required to Repress Dlx1/2 and Interneuron Production in Mammalian Brain. Neuron 2014, 81, 574–587. [Google Scholar] [CrossRef]
- Souchet, B.; Guedj, F.; Sahún, I.; Duchon, A.; Daubigney, F.; Badel, A.; Yanagawa, Y.; Barallobre, M.J.; Dierssen, M.; Yu, E.; et al. Excitation/Inhibition Balance and Learning Are Modified by Dyrk1a Gene Dosage. Neurobiol. Dis. 2014, 69, 65–75. [Google Scholar] [CrossRef]
- Goeldner, C.; Kishnani, P.S.; Skotko, B.G.; Casero, J.L.; Hipp, J.F.; Derks, M.; Hernandez, M.C.; Khwaja, O.; Lennon-Chrimes, S.; Noeldeke, J.; et al. A Randomized, Double-Blind, Placebo-Controlled Phase II Trial to Explore the Effects of a GABAA-A5 NAM (Basmisanil) on Intellectual Disability Associated with Down Syndrome. J. Neurodev. Disord. 2022, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Harashima, C.; Jacobowitz, D.M.; Witta, J.; Borke, R.C.; Best, T.K.; Siarey, R.J.; Galdzicki, Z. Abnormal Expression of the G-Protein-Activated Inwardly Rectifying Potassium Channel 2 (GIRK2) in Hippocampus, Frontal Cortex, and Substantia Nigra of Ts65Dn Mouse: A Model of Down Syndrome. J. Comp. Neurol. 2006, 494, 815–833. [Google Scholar] [CrossRef] [PubMed]
- Cramer, N.P.; Best, T.K.; Stoffel, M.; Siarey, R.J.; Galdzicki, Z. GABA B -GIRK2-Mediated Signaling in down Syndrome. Adv. Pharmacol. 2010, 58, 397–426. [Google Scholar] [CrossRef]
- Charles, K.J.; Deuchars, J.; Davies, C.H.; Pangalos, M.N. GABAB Receptor Subunit Expression in Glia. Mol. Cell. Neurosci. 2003, 24, 214–223. [Google Scholar] [CrossRef]
- Breton, J.D.; Stuart, G.J. GABAB Receptors in Neocortical and Hippocampal Pyramidal Neurons Are Coupled to Different Potassium Channels. Eur. J. Neurosci. 2017, 46, 2859–2866. [Google Scholar] [CrossRef]
- Takahashi, A.; Shimamoto, A.; Boyson, C.O.; DeBold, J.F.; Miczek, K.A. GABAB Receptor Modulation of Serotonin Neurons in the Dorsal Raphé Nucleus and Escalation of Aggression in Mice. J. Neurosci. 2010, 30, 11771–11780. [Google Scholar] [CrossRef] [PubMed]
- Waldvogel, H.J.; Baer, K.; Faull, R.L.M. The Localization of Inhibitory Neurotransmitter Receptors on Dopaminergic Neurons of the Human Substantia Nigra. J. Neural. Transm. Suppl. 2009, 73, 59–70. [Google Scholar] [CrossRef]
- Evenseth, L.S.M.; Gabrielsen, M.; Sylte, I. The GABAB Receptor—Structure, Ligand Binding and Drug Development. Molecules 2020, 25, 3093. [Google Scholar] [CrossRef]
- Fatemi, S.H.; Folsom, T.D.; Thuras, P.D. Deficits in GABAB Receptor System in Schizophrenia and Mood Disorders: A Postmortem Study. Schizophr. Res. 2011, 128, 37–43. [Google Scholar] [CrossRef]
- Gumerov, V.; Hegyi, H. MicroRNA-Derived Network Analysis of Differentially Methylated Genes in Schizophrenia, Implicating GABA Receptor B1 [GABBR1] and Protein Kinase B [AKT1]. Biol. Direct. 2015, 10, 59. [Google Scholar] [CrossRef]
- Miyazawa, A.; Kanahara, N.; Kogure, M.; Otsuka, I.; Okazaki, S.; Watanabe, Y.; Yamasaki, F.; Nakata, Y.; Oda, Y.; Hishimoto, A.; et al. A Preliminary Genetic Association Study of GAD1 and GABAB Receptor Genes in Patients with Treatment-Resistant Schizophrenia. Mol. Biol. Rep. 2022, 49, 2015–2024. [Google Scholar] [CrossRef] [PubMed]
- Zapata, J.; Moretto, E.; Hannan, S.; Murru, L.; Longatti, A.; Mazza, D.; Benedetti, L.; Fossati, M.; Heise, C.; Ponzoni, L.; et al. Epilepsy and Intellectual Disability Linked Protein Shrm4 Interaction with GABA B Rs Shapes Inhibitory Neurotransmission. Nat. Commun. 2017, 8, 14536. [Google Scholar] [CrossRef] [PubMed]
- Kolvenbach, C.M.; Felger, T.; Schierbaum, L.; Thiffault, I.; Pastinen, T.; Szczepańska, M.; Zaniew, M.; Adamczyk, P.; Bayat, A.; Yilmaz, Ö.; et al. X-Linked Variations in SHROOM4 Are Implicated in Congenital Anomalies of the Urinary Tract and the Anorectal, Cardiovascular and Central Nervous Systems. J. Med. Genet. 2023, 60, 587–596. [Google Scholar] [CrossRef]
- Kokkonen, H.; Siren, A.; Määttä, T.; Kamila Kadlubowska, M.; Acharya, A.; Nouel-Saied, L.M.; Leal, S.M.; Järvelä, I.; Schrauwen, I. Identification of Microduplications at Xp21.2 and Xq13.1 in Neurodevelopmental Disorders. Mol. Genet. Genomic. Med. 2021, 9, e1703. [Google Scholar] [CrossRef]
- Jomura, R.; Akanuma, S.I.; Tachikawa, M.; Hosoya, K.I. SLC6A and SLC16A Family of Transporters: Contribution to Transport of Creatine and Creatine Precursors in Creatine Biosynthesis and Distribution. Biochim. Biophys. Acta Biomembr. 2022, 1864, 183840. [Google Scholar] [CrossRef]
- Pramod, A.B.; Foster, J.; Carvelli, L.; Henry, L.K. SLC6 Transporters: Structure, Function, Regulation, Disease Association and Therapeutics. Mol. Aspects Med. 2013, 34, 197–219. [Google Scholar] [CrossRef]
- Yadav, R.; Han, G.W.; Gati, C. Molecular Basis of Human GABA Transporter 3 Inhibition. Nat. Commun. 2025, 16, 3830. [Google Scholar] [CrossRef]
- Damgaard, M.; Al-Khawaja, A.; Vogensen, S.B.; Jurik, A.; Sijm, M.; Lie, M.E.K.; Bæk, M.I.; Rosenthal, E.; Jensen, A.A.; Ecker, G.F.; et al. Identification of the First Highly Subtype-Selective Inhibitor of Human GABA Transporter GAT3. ACS Chem. Neurosci. 2015, 6, 1591–1599. [Google Scholar] [CrossRef]
- Motiwala, Z.; Aduri, N.G.; Shaye, H.; Han, G.W.; Lam, J.H.; Katritch, V.; Cherezov, V.; Gati, C. Structural Basis of GABA Reuptake Inhibition. Nature 2022, 606, 820–826, Erratum in Nature 2022, 608, E15. [Google Scholar] [CrossRef] [PubMed]
- Łątka, K.; Jończyk, J.; Bajda, M. Structure Modeling of γ-Aminobutyric Acid Transporters—Molecular Basics of Ligand Selectivity. Int. J. Biol. Macromol. 2020, 158, 1380–1389. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Kasture, A.S.; Fischer, F.P.; Sitte, H.H.; Hummel, T.; Sucic, S. A Transporter’s Doom or Destiny: SLC6A1 in Health and Disease, Novel Molecular Targets and Emerging Therapeutic Prospects. Front. Mol. Neurosci. 2024, 17, 1466694. [Google Scholar] [CrossRef] [PubMed]
- Fattorini, G.; Melone, M.; Conti, F. A Reappraisal of GAT-1 Localization in Neocortex. Front. Cell. Neurosci. 2020, 14, 9. [Google Scholar] [CrossRef]
- Melani, R.; Tritsch, N.X. Inhibitory Co-Transmission from Midbrain Dopamine Neurons Relies on Presynaptic GABA Uptake. Cell. Rep. 2022, 39, 110716. [Google Scholar] [CrossRef]
- Limani, F.; Srinivasan, S.; Hanzlova, M.; La Batide-Alanore, S.; Klotz, S.; Hnaskoxs, T.S.; Steinkellner, T. Evidence for Low Affinity of GABA at the Vesicular Monoamine Transporter VMAT2. Implications for Transmitter Co-Release from Dopamine Neurons. bioRxiv 2024. [Google Scholar] [CrossRef]
- Tritsch, N.X.; Ding, J.B.; Sabatini, B.L. Dopaminergic Neurons Inhibit Striatal Output through Non-Canonical Release of GABA. Nature 2012, 490, 262–266. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, W.; Ruan, Y.; Li, Z.; Yanjun; Ji, G.J.; Tian, Y.; Wang, K. Hyperactivity and Altered Functional Connectivity of the Ventral Striatum in Schizophrenia Compared with Bipolar Disorder: A Resting State FMRI Study. Psychiatry Res. Neuroimaging 2024, 345, 111881. [Google Scholar] [CrossRef] [PubMed]
- Imoukhuede, P.I.; Moss, F.J.; Michael, D.J.; Chow, R.H.; Lester, H.A. Ezrin Mediates Tethering of the γ-Aminobutyric Acid Transporter GAT1 to Actin Filaments via a C-Terminal PDZ-Interacting Domain. Biophys. J. 2009, 96, 2949–2960. [Google Scholar] [CrossRef] [PubMed]
- Bragina, L.; Marchionni, I.; Omrani, A.; Cozzi, A.; Pellegrini-Giampietro, D.E.; Cherubini, E.; Conti, F. GAT-1 Regulates Both Tonic and Phasic GABAA Receptor-Mediated Inhibition in the Cerebral Cortex. J. Neurochem. 2008, 105, 1781–1793. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.B.; Trinidad, M.; Ljungdahl, A.; Revalde, J.L.; Berguig, G.Y.; Wallace, W.; Patrick, C.S.; Bomba, L.; Arkin, M.; Dong, S.; et al. Haploinsufficiency Underlies the Neurodevelopmental Consequences of SLC6A1 Variants. Am. J. Hum. Genet. 2024, 111, 1222–1238. [Google Scholar] [CrossRef]
- Fischer, F.P.; Kasture, A.S.; Hummel, T.; Sucic, S. Molecular and Clinical Repercussions of GABA Transporter 1 Variants Gone Amiss: Links to Epilepsy and Developmental Spectrum Disorders. Front. Mol. Biosci. 2022, 9, 834498. [Google Scholar] [CrossRef]
- Mermer, F.; Poliquin, S.; Rigsby, K.; Rastogi, A.; Shen, W.; Romero-Morales, A.; Nwosu, G.; McGrath, P.; Demerast, S.; Aoto, J.; et al. Common Molecular Mechanisms of SLC6A1 Variant-Mediated Neurodevelopmental Disorders in Astrocytes and Neurons. Brain 2021, 144, 2499–2512. [Google Scholar] [CrossRef]
- Mikhailova, V.; Kondratyev, N.; Alfimova, M.; Kaleda, V.; Lezheiko, T.; Ublinsky, M.; Ushakov, V.; Lebedeva, I.; Galiakberova, A.; Artyuhov, A.; et al. The SLC6A1 Mutation Schizophrenia Case—A Comprehensive Case Study with IPSC Generation. Eur. Psychiatry 2024, 67, S764–S765. [Google Scholar] [CrossRef]
- Schleimer, S.B.; Hinton, T.; Dixon, G.; Johnston, G.A.R. GABA Transporters GAT-1 and GAT-3 in the Human Dorsolateral Prefrontal Cortex in Schizophrenia. Neuropsychobiology 2004, 50, 226–230. [Google Scholar] [CrossRef]
- Patel, A.B.; de Graaf, R.A.; Rothman, D.L.; Behar, K.L. Effects of γ-Aminobutyric Acid Transporter 1 Inhibition by Tiagabine on Brain Glutamate and γ-Aminobutyric Acid Metabolism in the Anesthetized Rat In Vivo. J. Neurosci. Res. 2015, 93, 1101–1108. [Google Scholar] [CrossRef]
- Bhatt, M.; Lazzarin, E.; Alberto-Silva, A.S.; Domingo, G.; Zerlotti, R.; Gradisch, R.; Bazzone, A.; Sitte, H.H.; Stockner, T.; Bossi, E. Unveiling the Crucial Role of Betaine: Modulation of GABA Homeostasis via SLC6A1 Transporter (GAT1). Cell. Mol. Life Sci. 2024, 81, 269. [Google Scholar] [CrossRef]
- DeLeeuw, M.B.; Shen, W.; Tian, X.; Ding, C.; Randhave, K.; Kang, J.Q. 4-Phenylbutyrate Restored GABA Uptake, Mitigated Seizures in SLC6A1 and SLC6A11 Microdeletions/3p- Syndrome: From Cellular Models to Human Patients. Epilepsy Res. 2025, 210, 107514. [Google Scholar] [CrossRef]
- Dikow, N.; Maas, B.; Karch, S.; Granzow, M.; Janssen, J.W.G.; Jauch, A.; Hinderhofer, K.; Sutter, C.; Schubert-Bast, S.; Anderlid, B.M.; et al. 3p25.3 Microdeletion of GABA Transporters SLC6A1 and SLC6A11 Results in Intellectual Disability, Epilepsy and Stereotypic Behavior. Am. J. Med. Genet. A 2014, 164A, 3061–3068. [Google Scholar] [CrossRef]
- Zhou, Y.; Holmseth, S.; Guo, C.; Hassel, B.; Höfner, G.; Huitfeldt, H.S.; Wanner, K.T.; Danbolt, N.C. Deletion of the γ-Aminobutyric Acid Transporter 2 (GAT2 and SLC6A13) Gene in Mice Leads to Changes in Liver and Brain Taurine Contents. J. Biol. Chem. 2012, 287, 35733–35746. [Google Scholar] [CrossRef] [PubMed]
- Kovalenko, I.L.; Galyamina, A.G.; Smagin, D.A.; Kudryavtseva, N.N. Influence of Chronic Social Stress on the Expression of Genes Associated with Neurotransmitter Systems in the Hypothalamus of Male Mice. Genetika 2024, 60, 44–54. [Google Scholar] [CrossRef]
- Minelli, A.; DeBiasi, S.; Brecha, N.C.; Zuccarello, L.V.; Conti, F. GAT-3, a High-Affinity GABA Plasma Membrane Transporter, Is Localized to Astrocytic Processes, and It Is Not Confined to the Vicinity of GABAergic Synapses in the Cerebral Cortex. J. Neurosci. 1996, 16, 6255–6264. [Google Scholar] [CrossRef]
- Melone, M.; Barbaresi, P.; Fattorini, G.; Conti, F. Neuronal Localization of the GABA Transporter GAT-3 in Human Cerebral Cortex: A Procedural Artifact? J. Chem. Neuroanat. 2005, 30, 45–54. [Google Scholar] [CrossRef]
- Beenhakker, M.P.; Huguenard, J.R. Astrocytes as Gatekeepers of GABAB Receptor Function. J. Neurosci. 2010, 30, 15262–15276. [Google Scholar] [CrossRef] [PubMed]
- Kersanté, F.; Rowley, S.C.S.; Pavlov, I.; Gutièrrez-Mecinas, M.; Semyanov, A.; Reul, J.M.H.M.; Walker, M.C.; Linthorst, A.C.E. A Functional Role for Both γ-Aminobutyric Acid (GABA) Transporter-1 and GABA Transporter-3 in the Modulation of Extracellular GABA and GABAergic Tonic Conductances in the Rat Hippocampus. J. Physiol. 2013, 591, 2429–2441. [Google Scholar] [CrossRef]
- Schijns, O.E.M.G.; Bisschop, J.; Rijkers, K.; Dings, J.; Vanherle, S.; Lindsey, P.; Smeets, H.J.M.; Hoogland, G. GAT-1 (Rs2697153) and GAT-3 (Rs2272400) Polymorphisms Are Associated with Febrile Seizures and Temporal Lobe Epilepsy. Epileptic Disord. 2020, 22, 176–182. [Google Scholar] [CrossRef]
- Zink, M.; Vollmayr, B.; Gebicke-Haerter, P.J.; Henn, F.A. Reduced Expression of GABA Transporter GAT3 in Helpless Rats, an Animal Model of Depression. Neurochem. Res. 2009, 34, 1584–1593. [Google Scholar] [CrossRef]
- Nentwig, T.B.; Obray, J.D.; Kruyer, A.; Wilkes, E.T.; Vaughan, D.T.; Scofield, M.D.; Chandler, L.J. Central Amygdala Astrocyte Plasticity Underlies GABAergic Dysregulation in Ethanol Dependence. Transl. Psychiatry 2025, 15, 132. [Google Scholar] [CrossRef]
- Kickinger, S.; Hellsberg, E.; Frølund, B.; Schousboe, A.; Ecker, G.F.; Wellendorph, P. Structural and Molecular Aspects of Betaine-GABA Transporter 1 (BGT1) and Its Relation to Brain Function. Neuropharmacology 2019, 161, 107644. [Google Scholar] [CrossRef]
- Li, J.; Lin, H.; Niu, F.; Zhu, X.; Shen, N.; Wang, X.; Li, L.; Liu, A.; Wu, X.; Sun, W.; et al. Combined Effect between Two Functional Polymorphisms of SLC6A12 Gene Is Associated with Temporal Lobe Epilepsy. J. Genet. 2015, 94, 637–642. [Google Scholar] [CrossRef]
- Ying, Y.; Liu, W.; Wang, H.; Shi, J.; Wang, Z.; Fei, J. GABA Transporter MGat4 Is Involved in Multiple Neural Functions in Mice. Biochim. Biophys. Acta Mol. Cell Res. 2024, 1871, 119740. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Kim, J.W.; Lee, S.K.; Kim, S.K.; Park, J.K.; Cho, A.R.; Chung, J.H.; Song, J.Y. Association between the SLC6A12 Gene and Negative Symptoms of Schizophrenia in a Korean Population. Psychiatry Res. 2011, 189, 478–479. [Google Scholar] [CrossRef]
- Storici, P.; Capitani, G.; De Biase, D.; Moser, M.; John, R.A.; Jansonius, J.N.; Schirmer, T. Crystal Structure of GABA-Aminotransferase, a Target for Antiepileptic Drug Therapy. Biochemistry 1999, 38, 8628–8634. [Google Scholar] [CrossRef] [PubMed]
- Clift, M.D.; Ji, H.; Deniau, G.P.; O’Hagan, D.; Silverman, R.B. Enantiomers of 4-Amino-3-Fluorobutanoic Acid as Substrates for γ-Aminobutyric Acid Aminotransferase. Conformational Probes for GABA Binding. Biochemistry 2007, 46, 13819–13828. [Google Scholar] [CrossRef] [PubMed]
- Besse, A.; Wu, P.; Bruni, F.; Donti, T.; Graham, B.H.; Craigen, W.J.; McFarland, R.; Moretti, P.; Lalani, S.; Scott, K.L.; et al. The GABA Transaminase, ABAT, Is Essential for Mitochondrial Nucleoside Metabolism. Cell Metab. 2015, 21, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.G.; Bahn, J.H.; Jang, J.S.; Park, J.; Kwon, O.S.; Cho, S.W.; Choi, S.Y. Human Brain GABA Transaminase Tissue Distribution and Molecular Expression. Eur. J. Biochem. 2000, 267, 5601–5607. [Google Scholar] [CrossRef]
- Park, M.G.; Lim, J.; Kim, D.; Lee, W.S.; Yoon, B.E.; Lee, C.J. Suppressing Astrocytic GABA Transaminase Enhances Tonic Inhibition and Weakens Hippocampal Spatial Memory. Exp. Mol. Med. 2025, 57, 379–389. [Google Scholar] [CrossRef]
- Koenig, M.K.; Hodgeman, R.; Riviello, J.J.; Chung, W.; Bain, J.; Chiriboga, C.A.; Ichikawa, K.; Osaka, H.; Tsuji, M.; Gibson, K.M.; et al. Phenotype of GABA-Transaminase Deficiency. Neurology 2017, 88, 1919–1924. [Google Scholar] [CrossRef]
- Nagappa, M.; Bindu, P.S.; Chiplunkar, S.; Govindaraj, P.; Narayanappa, G.; Krishnan, A.; Bharath, M.M.S.; Swaminathan, A.; Saini, J.; Arvinda, H.R.; et al. Hypersomnolence-Hyperkinetic Movement Disorder in a Child with Compound Heterozygous Mutation in 4-Aminobutyrate Aminotransferase (ABAT) Gene. Brain Dev. 2017, 39, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Oshi, A.; Alfaifi, A.; Seidahmed, M.Z.; Al Hussein, K.; Miqdad, A.; Samadi, A.; Abdelbasit, O. GABA Transaminase Deficiency. Case Report and Literature Review. Clin. Case Rep. 2021, 9, 1295–1298. [Google Scholar] [CrossRef]
- Hegde, A.U.; Karnavat, P.K.; Vyas, R.; DiBacco, M.L.; Grant, P.E.; Pearl, P.L. GABA Transaminase Deficiency with Survival Into Adulthood. J. Child Neurol. 2019, 34, 216–220. [Google Scholar] [CrossRef]
- Louro, P.; Ramos, L.; Robalo, C.; Cancelinha, C.; Dinis, A.; Veiga, R.; Pina, R.; Rebelo, O.; Pop, A.; Diogo, L.; et al. Phenotyping GABA Transaminase Deficiency: A Case Description and Literature Review. J. Inherit. Metab. Dis. 2016, 39, 743–747. [Google Scholar] [CrossRef]
- Chambliss, K.L.; Caudle, D.L.; Hinson, D.D.; Moomaw, C.R.; Slaughter, C.A.; Jakobs, C.; Gibson, K.M. Molecular Cloning of the Mature NAD+-Dependent Succinic Semialdehyde Dehydrogenase from Rat and Human: CDNA Isolation, Evolutionary Homology, and Tissue Expression. J. Biol. Chem. 1995, 270, 461–467. [Google Scholar] [CrossRef]
- Kim, Y.G.; Lee, S.; Kwon, O.S.; Park, S.Y.; Lee, S.J.; Park, B.J.; Kim, K.J. Redox-Switch Modulation of Human SSADH by Dynamic Catalytic Loop. EMBO J. 2009, 28, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Ryzlak, M.T.; Pietruszko, R. Human Brain “High Km” Aldehyde Dehydrogenase: Purification, Characterization, and Identification as NAD+-Dependent Succinic Semialdehyde Dehydrogenase. Arch. Biochem. Biophys. 1988, 266, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Pardi, D.J. Exploring the Relationships of Gamma-Hydroxybutyrate and Sleep on Metabolism, Physiology, and Behavior in Humans. Doctoral Thesis, Universiteit Leiden, Leiden, The Netherlands, 24 January 2019. [Google Scholar]
- Hoffman, P.L.; Wermuth, B.; Wartburg, J.V. Human Brain Aldehyde Reductases: Relationship to Succinic Semialdehyde Reductase and Aldose Reductase. J. Neurochem. 1980, 35, 354–366. [Google Scholar] [CrossRef]
- Colijn, M.A. The Characterization of Psychotic Symptoms in Succinic Semialdehyde Dehydrogenase Deficiency: A Review. Psychiatr. Genet. 2020, 30, 153–161. [Google Scholar] [CrossRef]
- Didiasova, M.; Cesaro, S.; Feldhoff, S.; Bettin, I.; Tiegel, N.; Füssgen, V.; Bertoldi, M.; Tikkanen, R. Functional Characterization of a Spectrum of Genetic Variants in a Family with Succinic Semialdehyde Dehydrogenase Deficiency. Int. J. Mol. Sci. 2024, 25, 5237. [Google Scholar] [CrossRef]
- Leo, S.; Capo, C.; Ciminelli, B.M.; Iacovelli, F.; Menduti, G.; Funghini, S.; Donati, M.A.; Falconi, M.; Rossi, L.; Malaspina, P. SSADH Deficiency in an Italian Family: A Novel ALDH5A1 Gene Mutation Affecting the Succinic Semialdehyde Substrate Binding Site. Metab. Brain Dis. 2017, 32, 1383–1388. [Google Scholar] [CrossRef]
- Fattahi, M.; Bushehri, A.; Alavi, A.; Asghariazar, V.; Nozari, A.; Ghasemi Firouzabadi, S.; Motamedian Dehkordi, P.; Javid, M.; Farajzadeh Valiliou, S.; Karimian, J.; et al. Bi-Allelic Mutations in ALDH5A1 Is Associated with Succinic Semialdehyde Dehydrogenase Deficiency and Severe Intellectual Disability. Gene 2020, 144918. [Google Scholar] [CrossRef]
- Menduti, G.; Biamino, E.; Vittorini, R.; Vesco, S.; Puccinelli, M.P.; Porta, F.; Capo, C.; Leo, S.; Ciminelli, B.M.; Iacovelli, F.; et al. Succinic Semialdehyde Dehydrogenase Deficiency: The Combination of a Novel ALDH5A1 Gene Mutation and a Missense SNP Strongly Affects SSADH Enzyme Activity and Stability. Mol. Genet. Metab. 2018, 124, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Casarano, M.; Alessandrì, M.G.; Salomons, G.S.; Moretti, E.; Jakobs, C.; Gibson, K.M.; Cioni, G.; Battini, R. Efficacy of Vigabatrin Intervention in a Mild Phenotypic Expression of Succinic Semialdehyde Dehydrogenase Deficiency. JIMD Rep. 2012, 2, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Vogel, K.R.; Ainslie, G.R.; McConnell, A.; Roullet, J.B.; Gibson, K.M. Toxicologic/Transport Properties of NCS-382, a γ-Hydroxybutyrate (GHB) Receptor Ligand, in Neuronal and Epithelial Cells: Therapeutic Implications for SSADH Deficiency, a GABA Metabolic Disorder. Toxicol. Vitr. 2018, 46, 203–212. [Google Scholar] [CrossRef]
- Vogel, K.R.; Ainslie, G.R.; Jansen, E.E.W.; Salomons, G.S.; Gibson, K.M. Therapeutic Relevance of MTOR Inhibition in Murine Succinate Semialdehyde Dehydrogenase Deficiency (SSADHD), a Disorder of GABA Metabolism. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 33–42. [Google Scholar] [CrossRef]
- Siegel, G.J. Basic Neurochemistry: Molecular, Cellular and Medical Aspects, 6th ed.; Agranoff, B.W., Albers, R.W., Fisher, S.K., Uhler, M.D., Eds.; Lippincott-Raven: Philadelphia, PA, USA, 1999. [Google Scholar]
- Chick, S.L.; Holmans, P.; Cameron, D.; Grozeva, D.; Sims, R.; Williams, J.; Bray, N.J.; Owen, M.J.; O’Donovan, M.C.; Walters, J.T.R.; et al. Whole-Exome Sequencing Analysis Identifies Risk Genes for Schizophrenia. Nat. Commun. 2025, 16, 7102. [Google Scholar] [CrossRef]
- Izquierdo-Altarejos, P.; Martínez-García, M.; Felipo, V. Extracellular Vesicles From Hyperammonemic Rats Induce Neuroinflammation in Cerebellum of Normal Rats: Role of Increased TNFα Content. Front. Immunol. 2022, 13, 921947. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Wang, Y.; Jiang, X.; Yuan, C. Mechanism of the Promotion of GEFS+ by the STAT3-Mediated Expression of Interleukin-6. Transl. Pediatr. 2022, 11, 1491–1501. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.V.; Grace, A.A. Adolescent Stress as a Driving Factor for Schizophrenia Development—A Basic Science Perspective. Schizophr. Bull. 2017, 43, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Hasler, G.; Van Der Veen, J.W.; Grillon, C.; Drevets, W.C.; Shen, J. Effect of Acute Psychological Stress on Prefrontal GABA Concentration Determined by Proton Magnetic Resonance Spectroscopy. Am. J. Psychiatry 2010, 167, 1226–1231. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marilovtseva, E.V.; Abdurazakov, A.; Kurishev, A.O.; Mikhailova, V.A.; Golimbet, V.E. The Role of GABA Pathway Components in Pathogenesis of Neurodevelopmental Disorders. Int. J. Mol. Sci. 2025, 26, 9492. https://doi.org/10.3390/ijms26199492
Marilovtseva EV, Abdurazakov A, Kurishev AO, Mikhailova VA, Golimbet VE. The Role of GABA Pathway Components in Pathogenesis of Neurodevelopmental Disorders. International Journal of Molecular Sciences. 2025; 26(19):9492. https://doi.org/10.3390/ijms26199492
Chicago/Turabian StyleMarilovtseva, Ekaterina V., Amal Abdurazakov, Artemiy O. Kurishev, Vera A. Mikhailova, and Vera E. Golimbet. 2025. "The Role of GABA Pathway Components in Pathogenesis of Neurodevelopmental Disorders" International Journal of Molecular Sciences 26, no. 19: 9492. https://doi.org/10.3390/ijms26199492
APA StyleMarilovtseva, E. V., Abdurazakov, A., Kurishev, A. O., Mikhailova, V. A., & Golimbet, V. E. (2025). The Role of GABA Pathway Components in Pathogenesis of Neurodevelopmental Disorders. International Journal of Molecular Sciences, 26(19), 9492. https://doi.org/10.3390/ijms26199492





