Identification and Expression Analysis of CCCH Zinc Finger Proteins in Mulberry (Morus alba)
Abstract
1. Introduction
2. Results
2.1. Identification of the MaC3H Genes
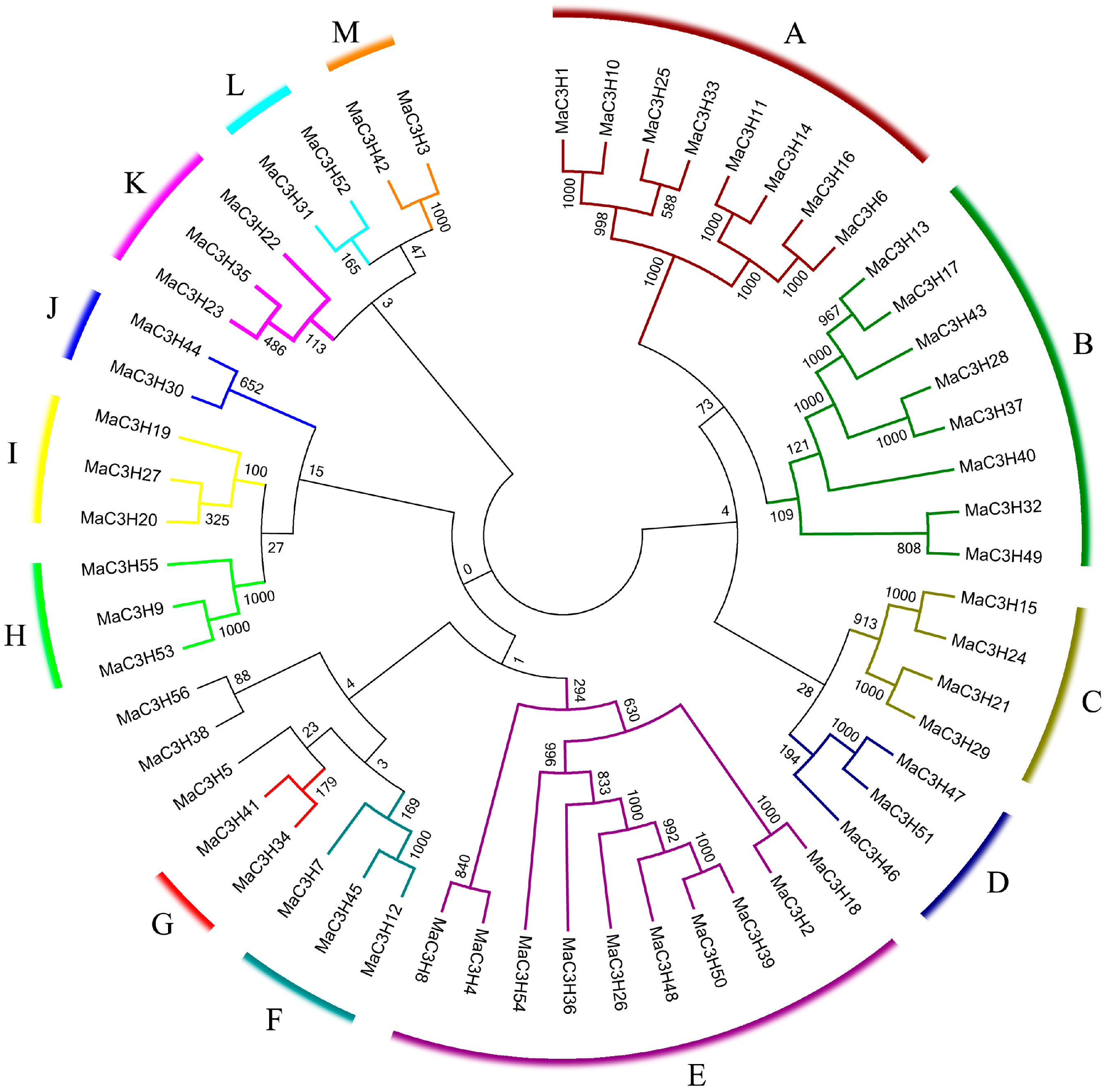
2.2. Phylogenetic Relationships
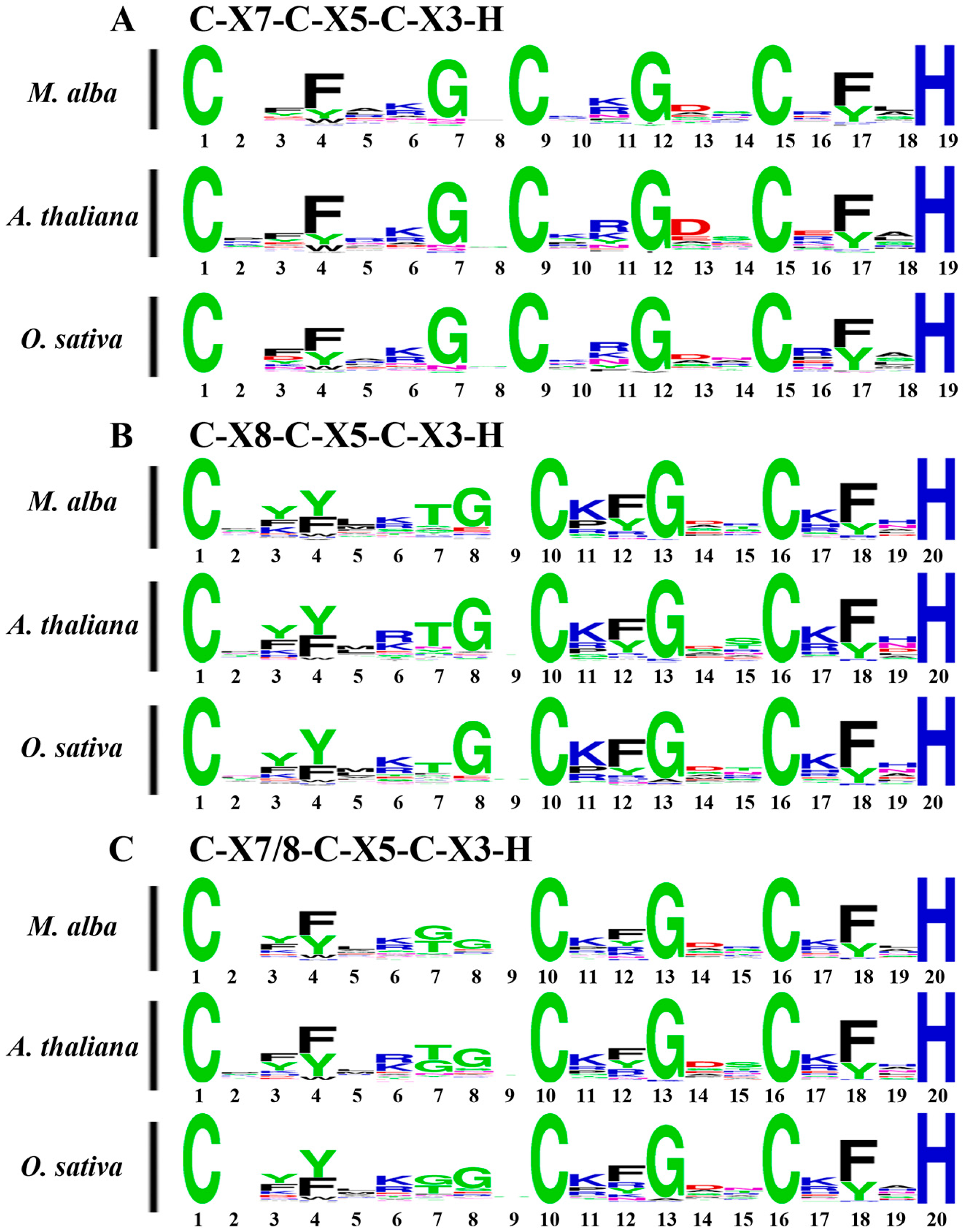
2.3. Motif and Gene Structure Analysis of MaC3Hs
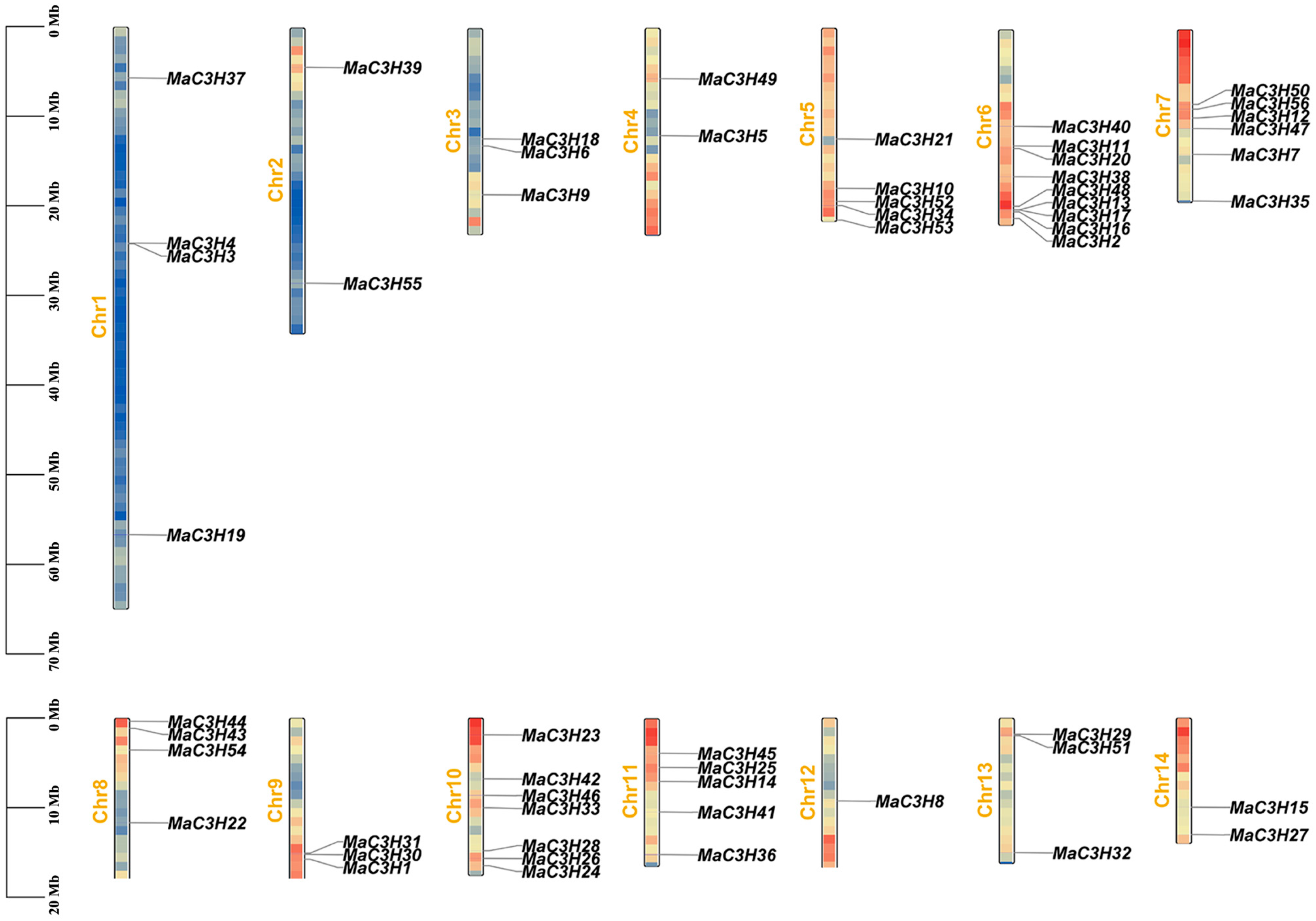
2.4. Synteny Analysis of MaC3Hs
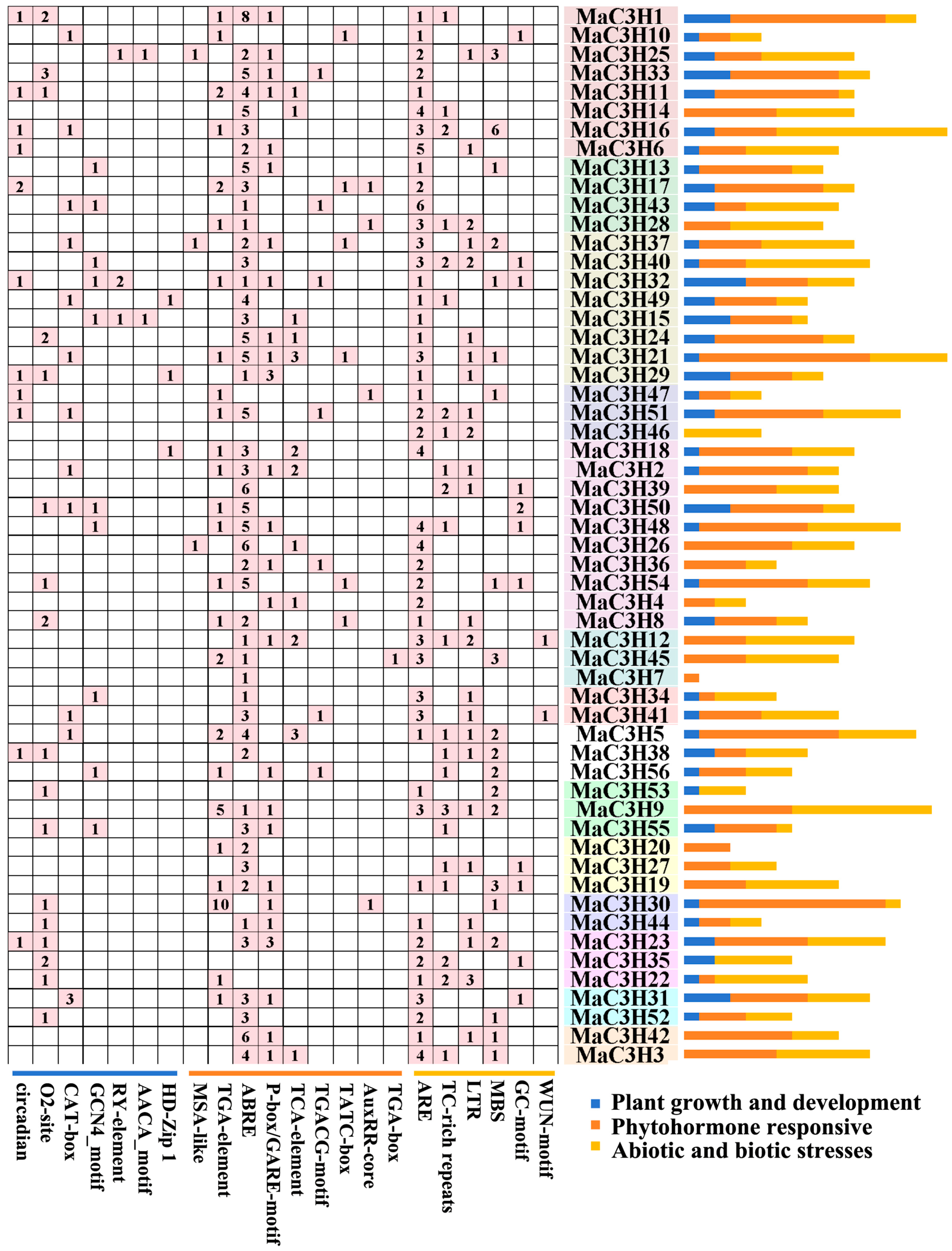
2.5. Cis-Acting Element Analysis of MaC3Hs
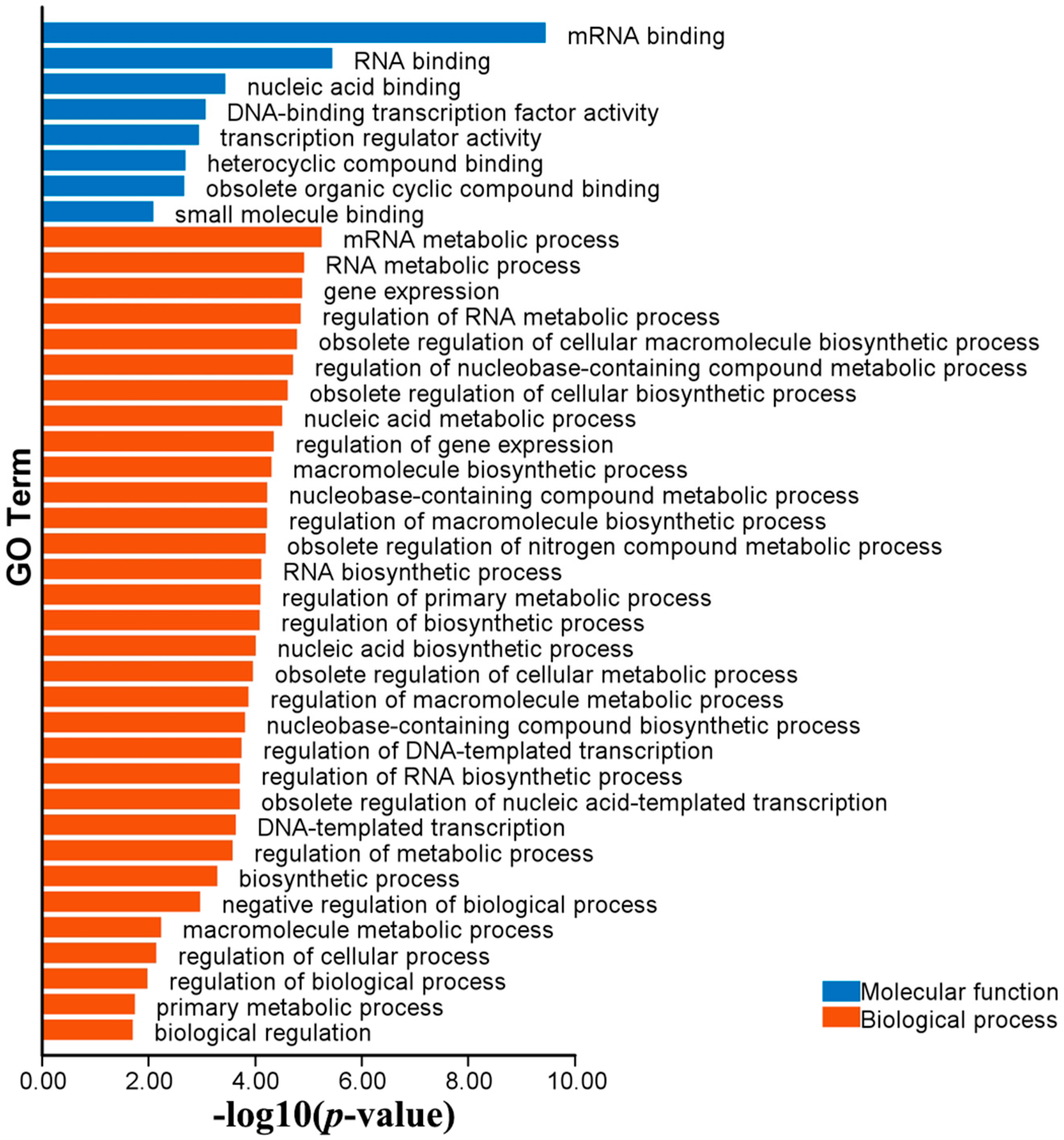
2.6. Functional Annotations of MaC3H Genes
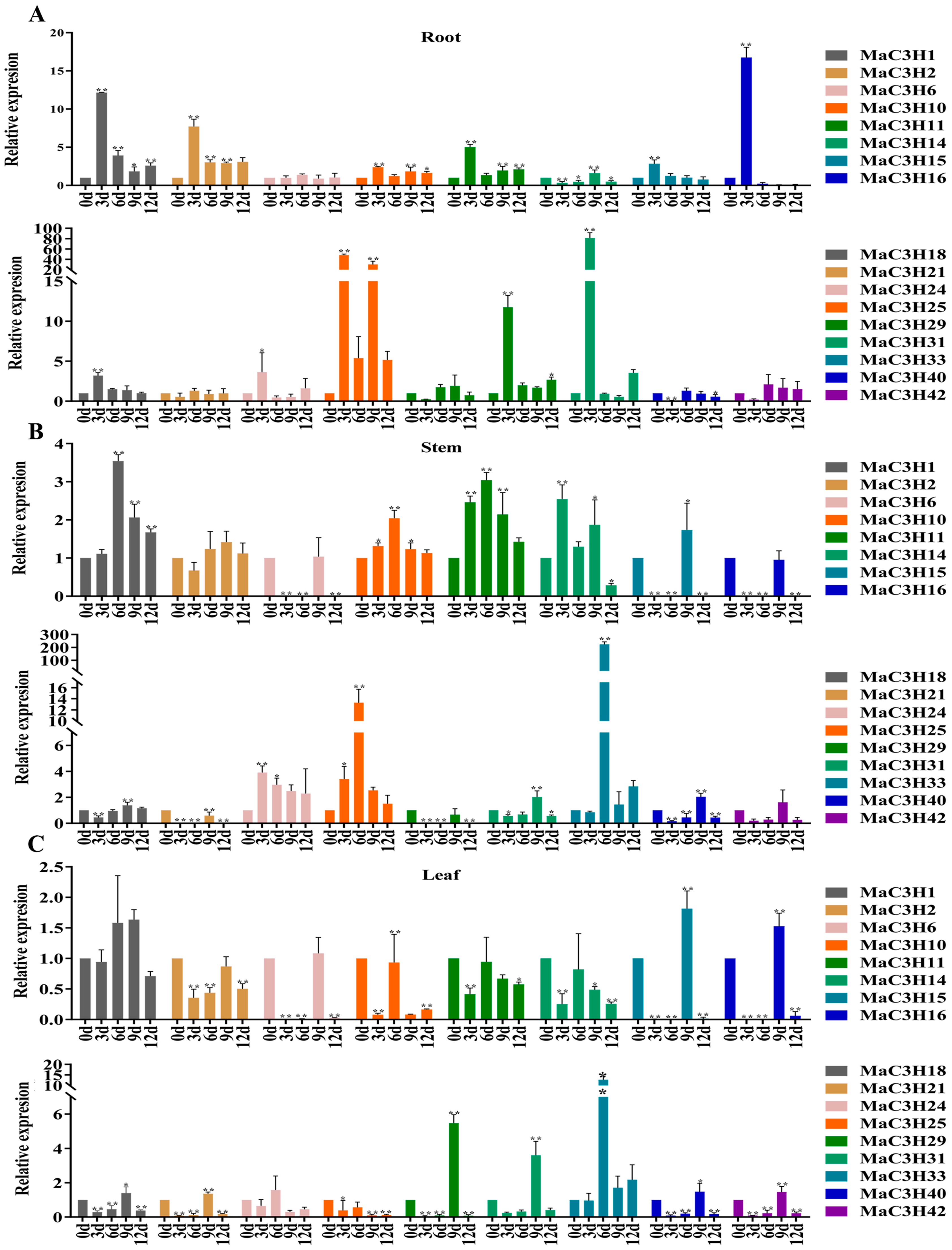
2.7. MaC3H Gene Expression Following Drought Treatment
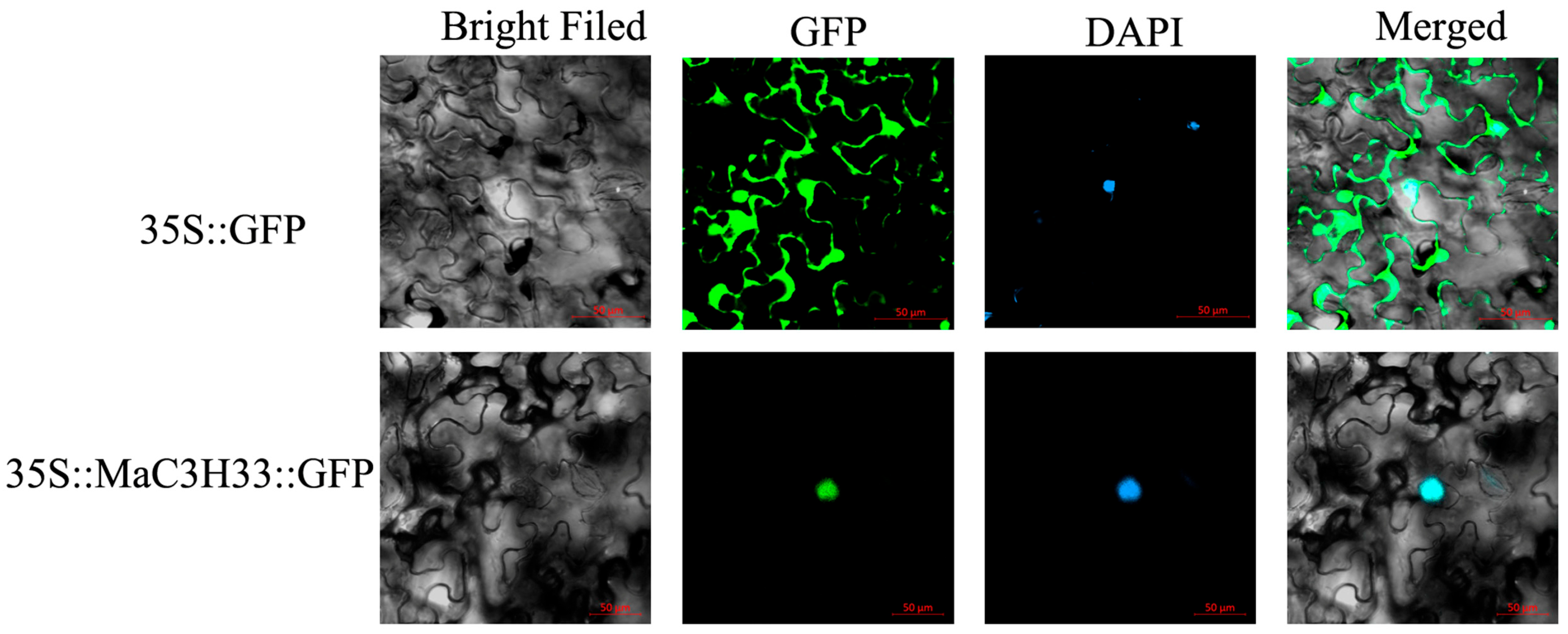
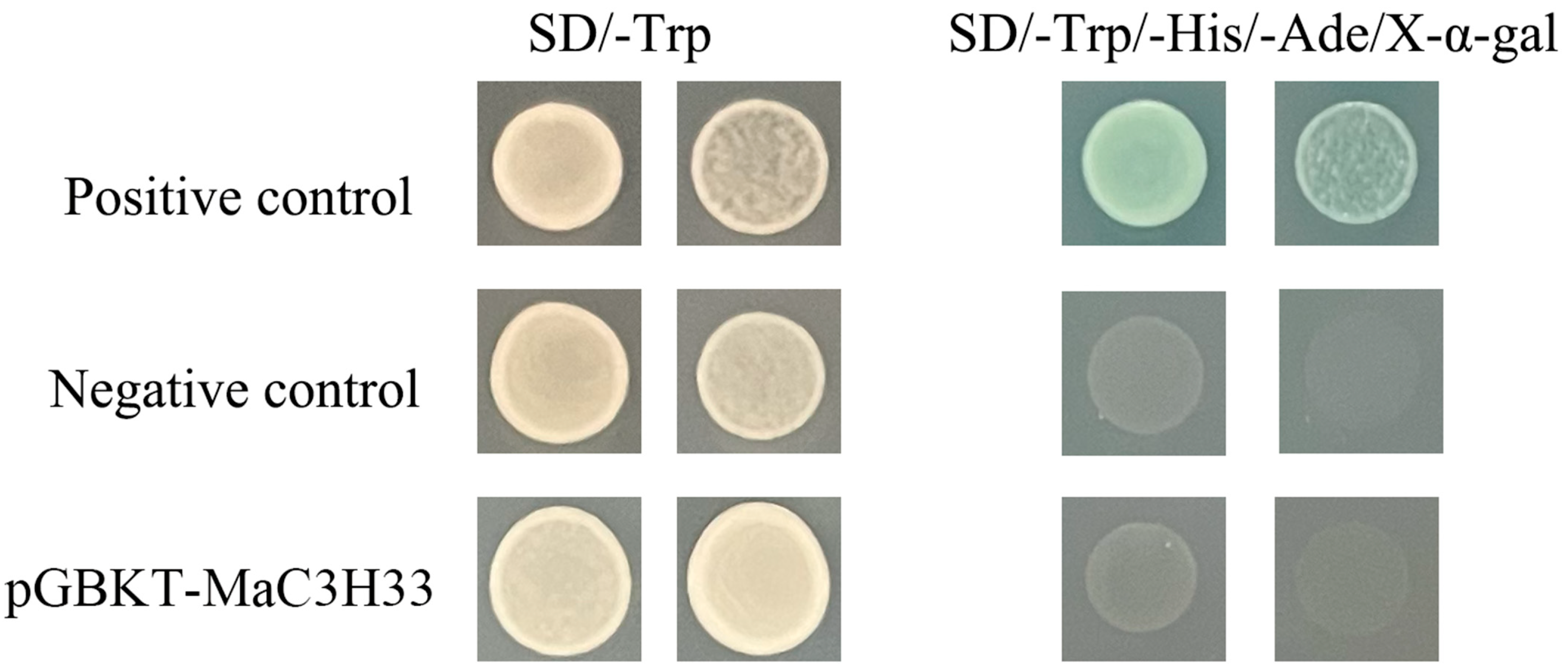
2.8. Subcellular Localization and Transcription Activation Assay
3. Discussion
4. Materials and Methods
4.1. Identification of CCCH Proteins in Mulberry Genome
4.2. Chromosomal Distribution Analysis
4.3. Phylogenetic Analysis and Gene Structure
4.4. Gene Duplication and Synteny Analysis
4.5. Cis-Element Analysis
4.6. Gene Ontology (GO) Annotation
4.7. Plant Materials and Drought Treatment
4.8. RNA Extraction and qRT-PCR
4.9. Subcellular Localization and Transactivation Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stege, J.T.; Guan, X.; Ho, T.; Beachy, R.N.; Barbas, C.F., III. Controlling gene expression in plants using synthetic zinc finger transcription factors. Plant J. 2002, 32, 1077–1086. [Google Scholar] [CrossRef]
- Blackshear, P. Tristetraprolin and other CCCH tandem zinc-finger proteins in the regulation of mRNA turnover. Biochem. Soc. Trans. 2002, 30, 945–952. [Google Scholar] [CrossRef]
- Wang, Q.; Song, S.; Lu, X.; Wang, Y.; Chen, Y.; Wu, X.; Tan, L.; Chai, G. Hormone regulation of CCCH zinc finger proteins in plants. Int. J. Mol. Sci. 2022, 23, 14288. [Google Scholar] [CrossRef]
- Takatsuji, H. Zinc-finger transcription factors in plants. Cell. Mol. Life Sci. CMLS 1998, 54, 582–596. [Google Scholar] [CrossRef]
- Kim, D.H.; Yamaguchi, S.; Lim, S.; Oh, E.; Park, J.; Hanada, A.; Kamiya, Y.; Choi, G. SOMNUS, a CCCH-type zinc finger protein in Arabidopsis, negatively regulates light-dependent seed germination downstream of PIL5. Plant Cell 2008, 20, 1260–1277. [Google Scholar] [CrossRef] [PubMed]
- Seok, H.-Y.; Kim, T.; Lee, S.-Y.; Moon, Y.-H. Non-TZF transcriptional activator AtC3H12 negatively affects seed germination and seedling development in Arabidopsis. Int. J. Mol. Sci. 2022, 23, 1572. [Google Scholar] [CrossRef]
- Yan, Z.; Jia, J.; Yan, X.; Shi, H.; Han, Y. Arabidopsis KHZ1 and KHZ2, two novel non-tandem CCCH zinc-finger and K-homolog domain proteins, have redundant roles in the regulation of flowering and senescence. Plant Mol. Biol. 2017, 95, 549–565. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Xu, Z.; Cao, S.; Chen, K.; Li, S.; Liu, X.; Gao, C.; Zhang, B.; Zhou, Y. An uncanonical CCCH-tandem zinc-finger protein represses secondary wall synthesis and controls mechanical strength in rice. Mol. Plant 2018, 11, 163–174. [Google Scholar] [CrossRef]
- Seok, H.-Y.; Bae, H.; Kim, T.; Mehdi, S.M.M.; Nguyen, L.V.; Lee, S.-Y.; Moon, Y.-H. Non-TZF protein ATC3H59/ZFWD3 is involved in seed germination, seedling development, and seed development, interacting with PPPDE family protein Desi1 in Arabidopsis. Int. J. Mol. Sci. 2021, 22, 4738. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, R.; Cai, X.; Zheng, H.; Huang, Y.; Li, Y.; Cui, M.; Lin, M.; Tang, H. A loss-of-function mutation in OsTZF5 confers sensitivity to low temperature and effects the growth and development in rice. Plant Mol. Biol. 2024, 114, 116. [Google Scholar] [CrossRef]
- Liu, H.; Xiao, S.; Sui, S.; Huang, R.; Wang, X.; Wu, H.; Liu, X. A tandem CCCH type zinc finger protein gene CpC3H3 from Chimonanthus praecox promotes flowering and enhances drought tolerance in Arabidopsis. BMC Plant Biol. 2022, 22, 506. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Liu, H.-L.; Wang, K.; Gao, Y.-M.; Wu, M.; Xiang, Y. Identification of CCCH zinc finger proteins family in moso bamboo (Phyllostachys edulis), and PeC3H74 confers drought tolerance to transgenic plants. Front. Plant Sci. 2020, 11, 579255. [Google Scholar] [CrossRef]
- Lan, Y.; Chen, F.; Zhang, K.; Wang, L.; Zhang, S.; Wu, M.; Xiang, Y. The CCCH zinc finger protein PeC3H74 of Moso bamboo (Phyllostachys edulis) positively regulates drought and salinity tolerances in transgenic plants. Ind. Crops Prod. 2023, 206, 117683. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, J.; Wei, F.; Fu, X.; Wei, H.; Lu, J.; Ma, L.; Wang, H. The CCCH-type zinc-finger protein GhC3H20 enhances salt stress tolerance in Arabidopsis thaliana and cotton through ABA signal transduction pathway. Int. J. Mol. Sci. 2023, 24, 5057. [Google Scholar] [CrossRef]
- Bai, H.; Lin, P.; Li, X.; Liao, X.; Wan, L.; Yang, X.; Luo, Y.; Zhang, L.; Zhang, F.; Liu, S. DgC3H1, a CCCH zinc finger protein gene, confers cold tolerance in transgenic chrysanthemum. Sci. Hortic. 2021, 281, 109901. [Google Scholar] [CrossRef]
- Cai, J.; Wang, X.; Wang, Z.; Sheng, S.; Tang, F.; Zhang, Z. ZC3H13-mediated m6A modification ameliorates acute myocardial infarction through preventing inflammation, oxidative stress and ferroptosis by targeting lncRNA93358: ZC3H13 ameliorates AMI by inhibiting lncRNA93358 and ferroptosis. Inflammation 2025, 48, 1270–1284. [Google Scholar] [CrossRef]
- Selvaraj, M.G.; Jan, A.; Ishizaki, T.; Valencia, M.; Dedicova, B.; Maruyama, K.; Ogata, T.; Todaka, D.; Yamaguchi-Shinozaki, K.; Nakashima, K. Expression of the CCCH-tandem zinc finger protein gene OsTZF5 under a stress-inducible promoter mitigates the effect of drought stress on rice grain yield under field conditions. Plant Biotechnol. J. 2020, 18, 1711–1721. [Google Scholar] [CrossRef]
- Seok, H.-Y.; Lee, S.-Y.; Nguyen, L.V.; Bayzid, M.; Jang, Y.; Moon, Y.-H. AtC3H3, an Arabidopsis Non-TZF gene, enhances salt tolerance by increasing the expression of both ABA-dependent and-independent stress-responsive genes. Int. J. Mol. Sci. 2024, 25, 10943. [Google Scholar] [CrossRef]
- Zhu, J.-K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef]
- Zhu, J.-K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Grondin, A.; Natividad, M.A.; Ogata, T.; Jan, A.; Gaudin, A.C.; Trijatmiko, K.R.; Liwanag, E.; Maruyama, K.; Fujita, Y.; Yamaguchi-Shinozaki, K. A case study from the overexpression of OsTZF5, encoding a CCCH tandem zinc finger protein, in rice plants across nineteen yield trials. Rice 2024, 17, 25. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Han, B.; Chen, J.; Luo, D.; Zhou, Q.; Liu, Z. Multiomics analyses reveal MsC3H29 positively regulates flavonoid biosynthesis to improve drought resistance of autotetraploid cultivated alfalfa (Medicago sativa L.). J. Agric. Food Chem. 2024, 72, 14448–14465. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yang, J.; Pak, S.; Zeng, M.; Sun, J.; Yu, S.; He, Y.; Li, C. PuC3H35 confers drought tolerance by enhancing lignin and proanthocyanidin biosynthesis in the roots of Populus ussuriensis. New Phytol. 2022, 233, 390–408. [Google Scholar] [CrossRef]
- Guo, C.; Chen, L.; Cui, Y.; Tang, M.; Guo, Y.; Yi, Y.; Li, Y.; Liu, L.; Chen, L. RNA binding protein OsTZF7 traffics between the nucleus and processing bodies/stress granules and positively regulates drought stress in rice. Front. Plant Sci. 2022, 13, 802337. [Google Scholar] [CrossRef]
- Zhang, Z.-A.; Liu, J.-Y.; Tian, J.-L.; Khurshid, M.; Yan, C.-H.; Herman, R.A.; Gong, L.-C.; Wang, J. Chemical analysis of mulberry (Morus alba L.) leaves treated with jasmonates on nutrition composition and biological activity. Food Chem. 2025, 489, 144929. [Google Scholar] [CrossRef]
- Kewcharoenwong, P.; Sompornpailin, K. Novel hybrid spun silk yarn developed from Eri and mulberry silks under industrial level and its characterizations. J. Nat. Fibers 2025, 22, 2445567. [Google Scholar] [CrossRef]
- Ren, Y.; Guo, G.; Wang, Z.; Zhu, L.; Geng, B. Response of yield and protein content of forage mulberry to irrigation in north china plain. Agronomy 2025, 15, 1016. [Google Scholar] [CrossRef]
- Wang, D.; Guo, Y.; Wu, C.; Yang, G.; Li, Y.; Zheng, C. Genome-wide analysis of CCCH zinc finger family in Arabidopsis and rice. BMC Genom. 2008, 9, 44. [Google Scholar] [CrossRef]
- Chai, G.; Hu, R.; Zhang, D.; Qi, G.; Zuo, R.; Cao, Y.; Chen, P.; Kong, Y.; Zhou, G. Comprehensive analysis of CCCH zinc finger family in poplar (Populus trichocarpa). BMC Genom. 2012, 13, 253. [Google Scholar] [CrossRef]
- Xu, G.; Guo, C.; Shan, H.; Kong, H. Divergence of duplicate genes in exon–intron structure. Proc. Natl. Acad. Sci. USA 2012, 109, 1187–1192. [Google Scholar] [CrossRef]
- Liu, H.; Gao, Y.; Wang, L.; Lan, Y.; Wu, M.; Yan, H.; Xiang, Y. Identification and expression analysis of AP2/ERF superfamily in pecan (Carya illinoensis). Sci. Hortic. 2022, 303, 111255. [Google Scholar] [CrossRef]
- Wei, W.; Lu, L.; Bian, X.H.; Li, Q.T.; Han, J.Q.; Tao, J.J.; Yin, C.C.; Lai, Y.C.; Li, W.; Bi, Y.D. Zinc-finger protein GmZF351 improves both salt and drought stress tolerance in soybean. J. Integr. Plant Biol. 2023, 65, 1636–1650. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, S.; Wu, H.; Huang, L.; Fu, L.; Zhan, C.; Lu, X.; Yang, L.; Dai, L.; Zeng, D. Identification and expression analysis of CCCH zinc finger family genes in Oryza sativa. Genes 2025, 16, 429. [Google Scholar] [CrossRef]
- Peng, X.; Zhao, Y.; Cao, J.; Zhang, W.; Jiang, H.; Li, X.; Ma, Q.; Zhu, S.; Cheng, B. CCCH-type zinc finger family in maize: Genome-wide identification, classification and expression profiling under abscisic acid and drought treatments. PLoS ONE 2012, 7, e40120. [Google Scholar] [CrossRef]
- Uddin, S.; Gull, S.; Hussain, H.A.; Mahmood, U.; Qasim, M.; Kamal, F.; Gaafar, A.-R.Z.; Aghayeva, S.; Iqbal, R.; Yang, X. Genome-wide identification, characterization and expression analysis of CsC3H gene family in cucumber (Cucumis sativus L.) under various abiotic stresses. Plant Sci. 2025, 359, 112631. [Google Scholar] [CrossRef]
- Deng, Z.; Yang, Z.; Liu, X.; Dai, X.; Zhang, J.; Deng, K. Genome-wide identification and expression analysis of C3H zinc finger family in potato (Solanum tuberosum L.). Int. J. Mol. Sci. 2023, 24, 12888. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Feng, X.; Ding, B.; Huo, H.; Abdullah, M.; Hong, J.; Jiang, L.; Wang, H.; Li, R.; Cai, Y. Gap-free genome assemblies of two Pyrus bretschneideri cultivars and GWAS analyses identify a CCCH zinc finger protein as a key regulator of stone cell formation in pear fruit. Plant Commun. 2025, 6, 101238. [Google Scholar] [CrossRef]
- Zhang, H.; Dai, Z.; Zhang, X.; Shang, M.; Gao, X.; Ma, R.; Zhao, L.; Zhang, X.; Liu, Q.; Zhai, H. Natural allelic variations in IbCHYR1–IbZnFR complex regulate fusarium root rot resistance in sweet potato. Adv. Sci. 2025, 12, e15202. [Google Scholar] [CrossRef]
- Chai, G.; Liu, H.; Zhang, Y.; Wang, C.; Xu, H.; He, G.; Meng, J.; Tang, X.; Wang, D.; Zhou, G. Integration of C3H15-mediated transcriptional and post-transcriptional regulation confers plant thermotolerance in Arabidopsis. Plant J. 2024, 119, 1558–1569. [Google Scholar] [CrossRef]
- Frey, Y.; Goehring, L.; Haj, M.; Rona, G.; Fijen, C.; Pagano, M.; Huang, T.T.; Rothenberg, E.; Ziv, Y.; Shiloh, Y. ZC3H4 safeguards genome integrity by preventing transcription-replication conflicts at noncoding RNA loci. Sci. Adv. 2025, 11, eadt8346. [Google Scholar] [CrossRef]
- Yıldırım, B.Ş.; Öztürk, Z.N. Genome-wide in silico identification, classification, and evolutionary analysis of putative abiotic stress-related CCCH genes in carrot. Plant Mol. Biol. Report. 2025, 43, 1122–1143. [Google Scholar] [CrossRef]
- Zheng, L.; Dai, H.; Mu, Y.; Li, J.; Cheng, Y.; Han, J. Genome-wide identification and expression analysis of C3H gene family in melon. Front. Plant Sci. 2025, 16, 1500429. [Google Scholar] [CrossRef]
- Bao, P.; Sun, J.; Qu, G.; Yan, M.; Cheng, S.; Ma, W.; Wang, J.; Hu, R. Identification and expression analysis of CCCH gene family and screening of key low temperature stress response gene CbuC3H24 and CbuC3H58 in Catalpa bungei. BMC Genom. 2024, 25, 779. [Google Scholar] [CrossRef]
- Tang, W.; Hao, Y.; Ma, X.; Shi, Y.; Dang, Y.; Dong, Z.; Zhao, Y.; Zhao, T.; Zhu, S.; Zhang, Z. Genome-wide analysis and identification of stress-responsive genes of the CCCH zinc finger family in Capsicum annuum L. Front. Plant Sci. 2023, 14, 1189038. [Google Scholar]
- Xu, W.; Jian, S.; Li, J.; Wang, Y.; Zhang, M.; Xia, K. Genomic identification of CCCH-Type zinc finger protein genes reveals the role of HuTZF3 in tolerance of heat and salt stress of pitaya (Hylocereus polyrhizus). Int. J. Mol. Sci. 2023, 24, 6359. [Google Scholar] [CrossRef]
- Jiao, F.; Luo, R.; Dai, X.; Liu, H.; Yu, G.; Han, S.; Lu, X.; Su, C.; Chen, Q.; Song, Q. Chromosome-level reference genome and population genomic analysis provide insights into the evolution and improvement of domesticated mulberry (Morus alba). Mol. Plant 2020, 13, 1001–1012. [Google Scholar] [CrossRef]
- Gasteiger, E.; Gattiker, A.; Hoogland, C.; Ivanyi, I.; Appel, R.D.; Bairoch, A. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003, 31, 3784–3788. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Crooks, G.E.; Hon, G.; Chandonia, J.-M.; Brenner, S.E. Weblogo: A sequence logo generator. Genome Res. 2004, 14, 1188–1190. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Jin, J.; Guo, A.-Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, Q.; Sun, R.; Xie, F.; Jones, D.C.; Zhang, B. Genome-wide identification and expression analysis of TCP transcription factors in Gossypium raimondii. Sci. Rep. 2014, 4, 6645. [Google Scholar] [CrossRef]
- Blanc, G.; Wolfe, K.H. Widespread paleopolyploidy in model plant species inferred from age distributions of duplicate genes. Plant Cell 2004, 16, 1667–1678. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, H.; Zhang, Z.; Wu, J.; Feng, Y.; Zhu, Z. Divergence in function and expression of the NOD26-like intrinsic proteins in plants. BMC Genom. 2009, 10, 313. [Google Scholar] [CrossRef]
- Cantalapiedra, C.P.; Hernández-Plaza, A.; Letunic, I.; Bork, P.; Huerta-Cepas, J. eggNOG-mapper v2: Functional annotation, orthology assignments, and domain prediction at the metagenomic scale. Mol. Biol. Evol. 2021, 38, 5825–5829. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Zhao, M.; Zhou, R.; Xu, C.; Zhang, R.; Li, R.; Wang, T. The mulberry WRKY transcription factor MaWRKYIIc7 participates in regulating plant drought stress tolerance. Int. J. Mol. Sci. 2025, 26, 1714. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Xu, L.; Shi, C.; Wu, F.; Yang, S. Identification of the optimal quantitative RT-PCR reference gene for paper mulberry (Broussonetia papyrifera). Curr. Issues Mol. Biol. 2024, 46, 10779–10794. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, F.; Yu, J.; Han, Z.-H.; Deng, Y.-J. Identification and Expression Analysis of CCCH Zinc Finger Proteins in Mulberry (Morus alba). Int. J. Mol. Sci. 2025, 26, 9490. https://doi.org/10.3390/ijms26199490
Chen F, Yu J, Han Z-H, Deng Y-J. Identification and Expression Analysis of CCCH Zinc Finger Proteins in Mulberry (Morus alba). International Journal of Molecular Sciences. 2025; 26(19):9490. https://doi.org/10.3390/ijms26199490
Chicago/Turabian StyleChen, Feng, Jie Yu, Zhi-Hong Han, and Yong-Jin Deng. 2025. "Identification and Expression Analysis of CCCH Zinc Finger Proteins in Mulberry (Morus alba)" International Journal of Molecular Sciences 26, no. 19: 9490. https://doi.org/10.3390/ijms26199490
APA StyleChen, F., Yu, J., Han, Z.-H., & Deng, Y.-J. (2025). Identification and Expression Analysis of CCCH Zinc Finger Proteins in Mulberry (Morus alba). International Journal of Molecular Sciences, 26(19), 9490. https://doi.org/10.3390/ijms26199490




