Evaluation of Potential Molecular Targets of the Alkaloid Epiisopiloturine, Involved in Cardioprotective Effects, Using Computational Molecular Docking in an Animal Model of Cardiac Ischemia and Reperfusion
Abstract
1. Introduction
2. Results
2.1. Computational Studies
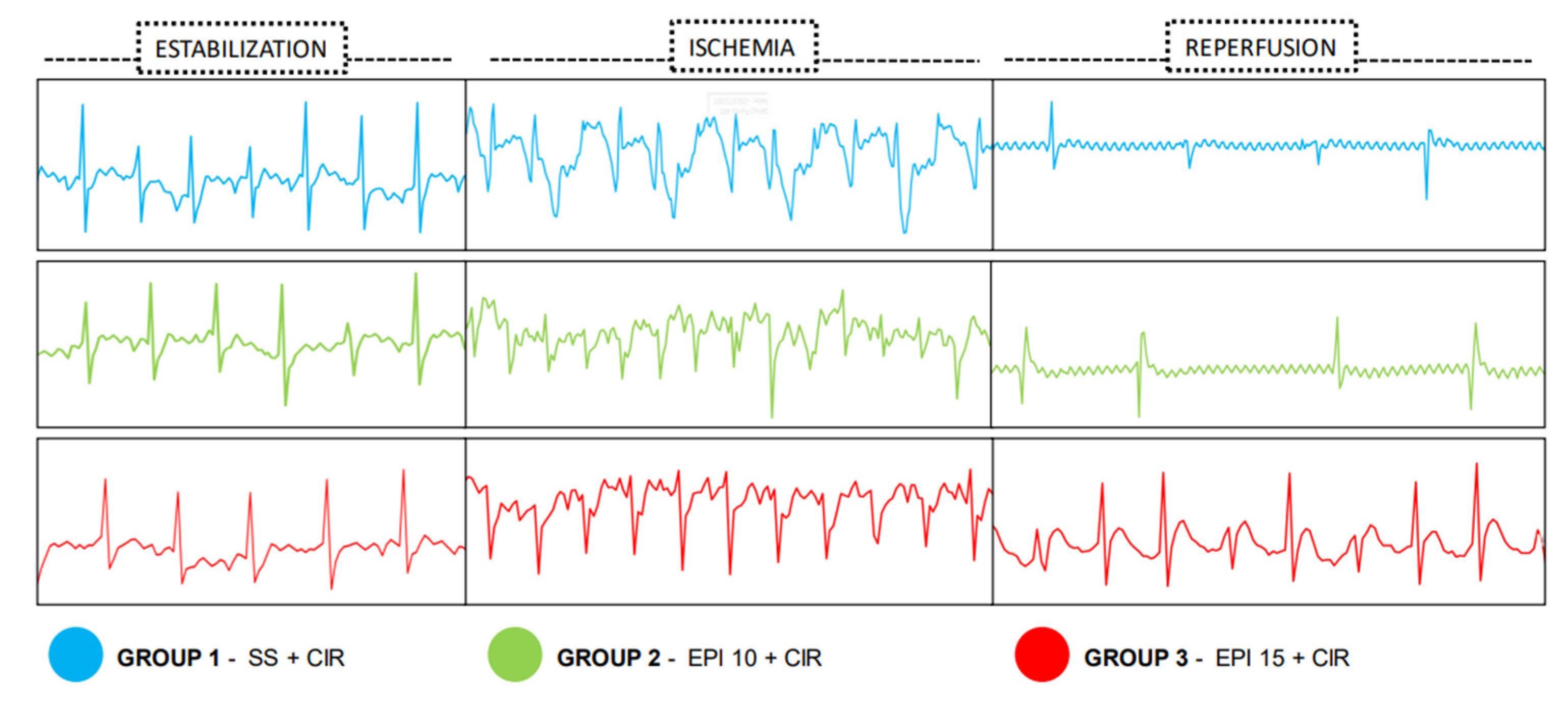
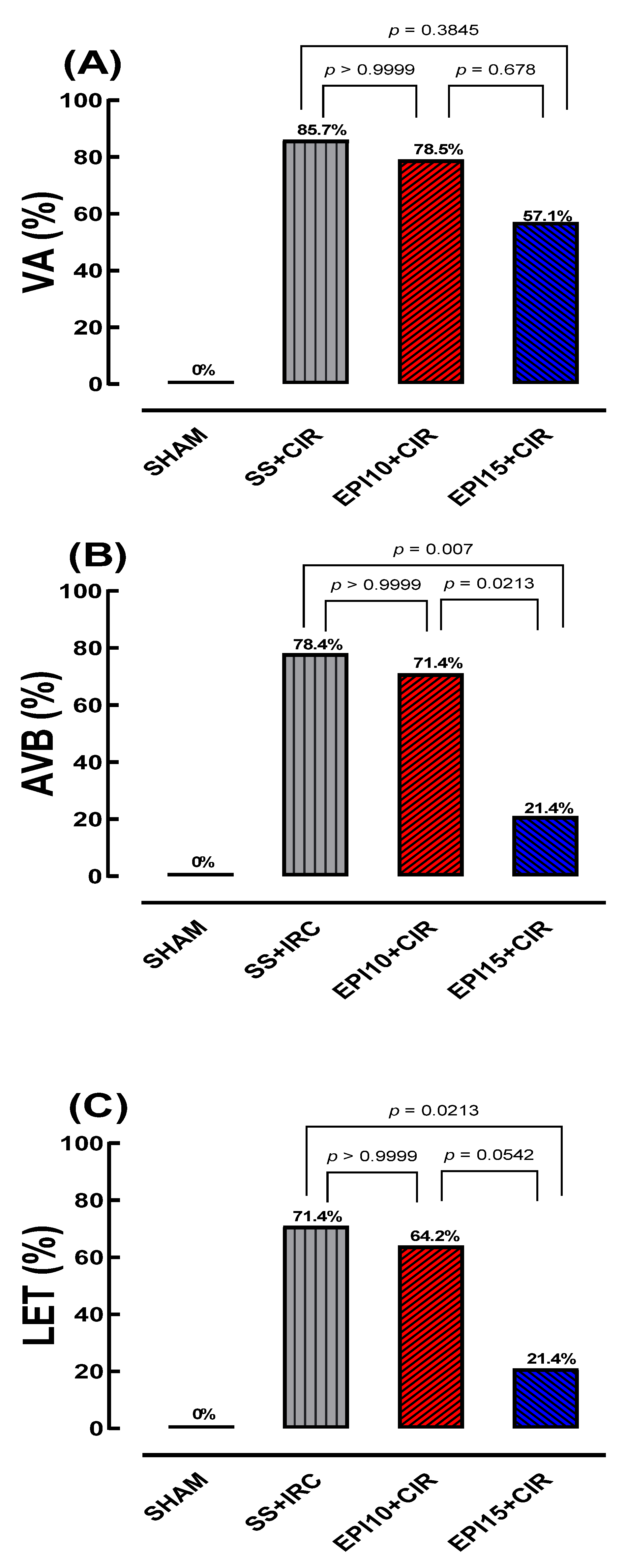
2.2. Incidence of VA, AVB and LET Induced by CIR
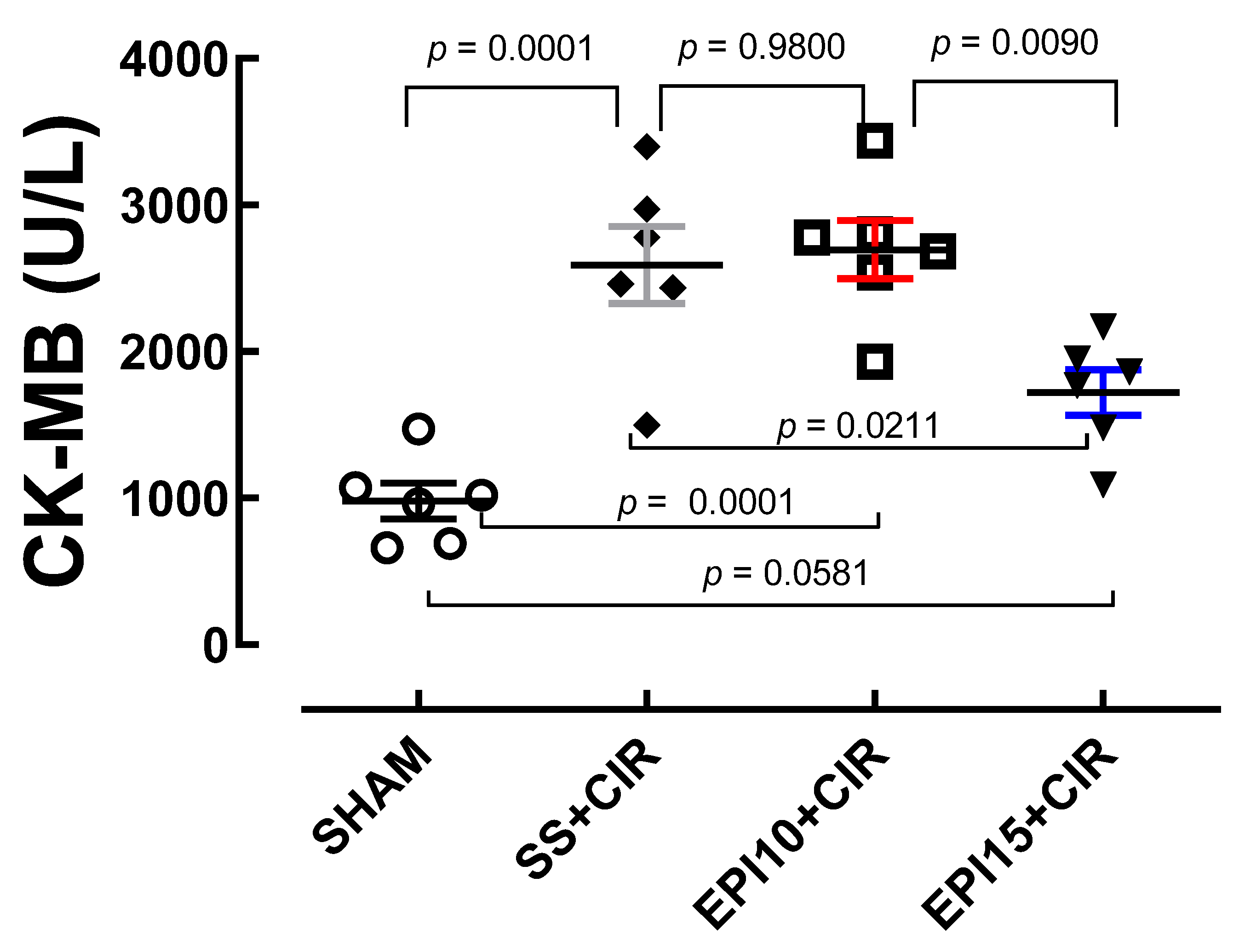
2.3. Effects of the EPI on CK-MB Levels in Rats Under CIR
2.4. Myocardial Histopathological Analysis
3. Discussion
4. Materials and Methods
4.1. Computational Studies
4.1.1. Drawing and Optimization of EPI
4.1.2. Molecular Docking
4.2. Animals
4.3. Cardiac Ischemia and Reperfusion (CIR) Induction
4.4. Assessment of Cardiac Activity During CIR
4.5. Biochemical Assessment of Heart Lesions’ Biomarkers
4.6. Histopathological Analysis of Left Ventricle Myocardial Tissue
4.7. Pharmacological Treatments
- (1)
- SHAM (sham-operated) group (n = 10): Rats were subjected to all CIR procedures, except for myocardial reperfusion and the use of a left descending coronary artery tourniquet. They were also subjected to ECG analysis to ascertain the incidence of VA, AVB, and LET.
- (2)
- SS + CIR group (n = 14): NWRs were pretreated with saline solution (SS) 0.9% intravenously and then submitted to cardiac ischemia and reperfusion (CIR). They were also subjected to ECG analysis to ascertain the incidence of VA, AVB, and LET.
- (3)
- EPI10 + CIR group (n = 14): NWRs were pretreated with 10 mg/kg EPI intravenously prior to cardiac ischemia and reperfusion (CIR). They were also subjected to ECG analysis to ascertain the incidence of VA, AVB, and LET.
- (4)
- EPI15 + CIR group (n = 14): NWRs were pretreated with 15 mg/kg EPI intravenously prior to cardiac ischemia and reperfusion (CIR). They were also subjected to ECG analysis to ascertain the incidence of VA, AVB, and LET.
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martin, S.S.; Aday, A.W.; Allen, N.B.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Bansal, N.; Beaton, A.Z.; et al. 2025 Heart Disease and Stroke Statistics: A Report of US and Global Data from the American Heart Association. Circulation 2025, 151, e41–e660. [Google Scholar] [CrossRef] [PubMed]
- Salari, N.; Morddarvanjoghi, F.; Abdolmaleki, A.; Rasoulpoor, S.; Khaleghi, A.A.; Hezarkhani, L.A.; Shohaimi, S.; Mohammadi, M. The Global Prevalence of Myocardial Infarction: A Systematic Review and Meta-Analysis. BMC Cardiovasc. Disord. 2023, 23, 206. [Google Scholar] [CrossRef] [PubMed]
- Vaduganathan, M.; Mensah, G.A.; Turco, J.V.; Fuster, V.; Roth, G.A. The Global Burden of Cardiovascular Diseases and Risk: A Compass for Future Health. J. Am. Coll. Cardiol. 2022, 80, 2361–2371. [Google Scholar] [CrossRef]
- Teo, K.K.; Rafiq, T. Cardiovascular Risk Factors and Prevention: A Perspective From Developing Countries. Can. J. Cardiol. 2021, 37, 733–743. [Google Scholar] [CrossRef]
- Naryzhnaya, N.V.; Maslov, L.N.; Derkachev, I.A.; Ma, H.; Zhang, Y.; Prasad, N.R.; Singh, N.; Fu, F.; Pei, J.; Sarybaev, A.; et al. The Effect of an Adaptation to Hypoxia on Cardiac Tolerance to Ischemia/Reperfusion. J. Biomed. Res. 2022, 37, 230–254. [Google Scholar] [CrossRef]
- Frampton, J.; Ortengren, A.R.; Zeitler, E.P. Arrhythmias After Acute Myocardial Infarction. Yale J. Biol. Med. 2023, 96, 83–94. [Google Scholar] [CrossRef]
- Panuccio, G.; Carabetta, N.; Torella, D.; de Rosa, S. Clinical Impact of Coronary Revascularization over Medical Treatment in Chronic Coronary Syndromes: A Systematic Review and Meta-Analysis. Hell. J. Cardiol. 2024, 78, 60–71. [Google Scholar] [CrossRef]
- Menezes-Rodrigues, F.S.; Tavares, J.G.P.; Vasques, E.R.; Errante, P.R.; de Araújo, E.A.; Pires-Oliveira, M.; Scorza, C.A.; Scorza, F.A.; Taha, M.O.; Caricati-Neto, A. Cardioprotective Effects of Pharmacological Blockade of the Mitochondrial Calcium Uniporter on Myocardial Ischemia-Reperfusion Injury. Acta Cir. Bras. 2020, 35, e202000306. [Google Scholar] [CrossRef]
- Zhou, M.; Yu, Y.; Luo, X.; Wang, J.; Lan, X.; Liu, P.; Feng, Y.; Jian, W. Myocardial Ischemia-Reperfusion Injury: Therapeutics from a Mitochondria-Centric Perspective. Cardiology 2021, 146, 781–792. [Google Scholar] [CrossRef]
- Kistamás, K.; Veress, R.; Horváth, B.; Bányász, T.; Nánási, P.P.; Eisner, D.A. Calcium Handling Defects and Cardiac Arrhythmia Syndromes. Front. Pharmacol. 2020, 11, 72. [Google Scholar] [CrossRef]
- de Araújo, E.A.; Tallo, F.S.; Oliveira, A.S.F.; Toghlobi, G.S.S.E.; Arantes, R.A.; Balsimelli, R.; Kehrwald-Balsimelli, B.; de Almeida Viana, B.L.; Matuda, F.S.; Nicolau, L.A.D.; et al. Cardiotoxic Effects Produced by Omeprazole and Methylene Blue in an Animal Model of Cardiac Ischemia and Reperfusion and Potential Implications for the Pharmacological Strategy for Vasoplegic Syndrome. Biomedicines 2024, 12, 582. [Google Scholar] [CrossRef] [PubMed]
- Tallo, F.S.; de Santana, P.O.; Pinto, S.A.G.; Lima, R.Y.; de Araújo, E.A.; Tavares, J.G.P.; Pires-Oliveira, M.; Nicolau, L.A.D.; Medeiros, J.V.R.; Taha, M.O.; et al. Pharmacological Modulation of the Ca2+/CAMP/Adenosine Signaling in Cardiac Cells as a New Cardioprotective Strategy to Reduce Severe Arrhythmias in Myocardial Infarction. J. Cardiovasc. Dev. Dis. 2023, 16, 1473. [Google Scholar] [CrossRef]
- Menezes-Rodrigues, F.S.; de Oliveira, M.P.; Araújo, E.A.; Ferraz, H.B.; Finsterer, J.; Olszewer, E.; Taha, M.O.; Scorza, C.A.; Caricati-Neto, A.; Scorza, F.A. Role of Cardiac Β1-Adrenergic and A1-Adenosine Receptors in Severe Arrhythmias Related to Parkinson’s Disease. Clinics 2023, 78, 100243. [Google Scholar] [CrossRef]
- Monteiro, W.P.; de Souza, E.B.; de Miranda, L.S.; Anjos, L.J.S.; Caldeira, C.F. Potential Distribution of Pilocarpus microphyllus in the Amazonia/Cerrado Biomes under Near-Future Climate Change Scenarios. Plants 2023, 12, 2106. [Google Scholar] [CrossRef]
- Langley, J.N. Preliminary Notice of Experiments on the Physiological Action of Jaborandi. Br. Med. J. 1875, 1, 241–242. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.P.; Moreno, P.R.H. Pilocarpus spp.: A Survey of Its Chemical Constituents and Biological Activities. Rev. Bras. Ciências Farm. 2004, 40, 116–137. [Google Scholar] [CrossRef]
- Pinheiro, C.U. Jaborandi (Pilocarpus sp., Rutaceae): A Wild Species. Econ. Bot. 1997, 51, 49–58. [Google Scholar] [CrossRef]
- Andrade-Neto, M.; Mendes, P.H.; Silveira, E.R. An Imidazole Alkaloid and Other Constituents from Pilocarpus trachyllophus. Phytochemistry 1996, 42, 885–887. [Google Scholar] [CrossRef]
- Veras, L.M.; Guimaraes, M.A.; Campelo, Y.D.; Vieira, M.M.; Nascimento, C.; Lima, D.F.; Vasconcelos, L.; Nakano, E.; Kuckelhaus, S.S.; Batista, M.C.; et al. Activity of Epiisopiloturine against Schistosoma mansoni. Curr. Med. Chem. 2012, 19, 2051–2058. [Google Scholar] [CrossRef]
- Silva, V.G.; Silva, R.O.; Damasceno, S.R.B.; Carvalho, N.S.; Prudêncio, R.S.; Aragão, K.S.; Guimarães, M.A.; Campos, S.A.; Véras, L.M.C.; Godejohann, M.; et al. Anti-Inflammatory and Antinociceptive Activity of Epiisopiloturine, an Imidazole Alkaloid Isolated from Pilocarpus microphyllus. J. Nat. Prod. 2013, 76, 1071–1077. [Google Scholar] [CrossRef]
- Rocha, T.M.; Machado, N.J.; de Sousa, J.A.C.; Araujo, E.V.O.; Guimaraes, M.A.; Lima, D.F.; Leite, J.R.D.S.D.A.; Leal, L.K.A.M. Imidazole Alkaloids Inhibit the Pro-Inflammatory Mechanisms of Human Neutrophil and Exhibit Anti-Inflammatory Properties in Vivo. J. Pharm. Pharmacol. 2019, 71, 849–859. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, J.A.C.; Azul, F.V.C.S.; de Araújo, A.B.; Tomé, R.C.; Silva, F.R.M.; de Vasconcelos, S.M.M.; Rios, F.J.; Leal, L.K.A.M. Epiisopiloturine, an Alkaloid from Pilocarpus microphyllus, Attenuates LPS-Induced Neuroinflammation by Interfering in the TLR4/NF-ΚB-MAPK Signaling Pathway in Microglial Cells. Oxid. Med. Cell. Longev. 2023, 2023, 4752502. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, L.R.; de Brito, T.V.; Júnior, J.S.D.C.; Júnior, G.J.D.; de Aguiar Magalhãres, D.; Sousa, S.G.; Silva, R.O.; da Silva, F.R.P.; Vasconcelos, D.F.P.; Véras, L.M.C.; et al. Epiisopiloturine, an Imidazole Alkaloid, Reverses Inflammation and Lipid Peroxidation Parameters in the Crohn Disease Model Induced by Trinitrobenzenosulfonic Acid in Wistar Rats. Biomed. Pharmacother. 2018, 102, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Peart, J.; Flood, A.; Linden, J.; Matherne, G.P.; Headrick, J.P. Adenosine-Mediated Cardioprotection in Ischemic-Reperfused Mouse Heart. J. Cardiovasc. Pharmacol. 2002, 39, 117–129. [Google Scholar] [CrossRef]
- Lasley, R.D.; Rhee, J.W.; Van Wylen, D.G.; Mentzer, R.M. Adenosine A1 Receptor Mediated Protection of the Globally Ischemic Isolated Rat Heart. J. Mol. Cell. Cardiol. 1990, 22, 39–47. [Google Scholar] [CrossRef]
- Yu, H.J.; Ma, H.; Green, R.D. Calcium Entry via L-Type Calcium Channels Acts as a Negative Regulator of Adenylyl Cyclase Activity and Cyclic AMP Levels in Cardiac Myocytes. Mol. Pharmacol. 1993, 44, 689–693. [Google Scholar] [CrossRef]
- Maghsoudi, S.; Shuaib, R.; van Bastelaere, B.; Dakshinamurti, S. Adenylyl Cyclase Isoforms 5 and 6 in the Cardiovascular System: Complex Regulation and Divergent Roles. Front. Pharmacol. 2024, 15, 1370506. [Google Scholar] [CrossRef]
- Maille, B.; Lalevée, N.; Marlinge, M.; Vahdat, J.; Mottola, G.; Degioanni, C.; de Maria, L.; Klein, V.; Thuny, F.; Franceschi, F.; et al. Adenosine and Adenosine Receptors: Advances in Atrial Fibrillation. Biomedicines 2022, 10, 2963. [Google Scholar] [CrossRef]
- Liao, Y.; Takashima, S.; Asano, Y.; Asakura, M.; Ogai, A.; Shintani, Y.; Minamino, T.; Asanuma, H.; Sanada, S.; Kim, J.; et al. Activation of Adenosine A1 Receptor Attenuates Cardiac Hypertrophy and Prevents Heart Failure in Murine Left Ventricular Pressure-Overload Model. Circ. Res. 2003, 93, 759–766. [Google Scholar] [CrossRef]
- Burnstock, G.; Pelleg, A. Cardiac Purinergic Signalling in Health and Disease. Purinergic Signal. 2015, 11, 1–46. [Google Scholar] [CrossRef]
- Burnstock, G. Purinergic Signaling in the Cardiovascular System. Circ. Res. 2017, 120, 207–228. [Google Scholar] [CrossRef]
- Safran, N.; Shneyvays, V.; Balas, N.; Jacobson, K.A.; Nawrath, H.; Shainberg, A. Cardioprotective Effects of Adenosine A1 and A3 Receptor Activation during Hypoxia in Isolated Rat Cardiac Myocytes. Mol. Cell. Biochem. 2001, 217, 143–152. [Google Scholar] [CrossRef]
- Urmaliya, V.B.; Church, J.E.; Coupar, I.M.; Rose’Meyer, R.B.; Pouton, C.W.; White, P.J. Cardioprotection Induced by Adenosine A1 Receptor Agonists in a Cardiac Cell Ischemia Model Involves Cooperative Activation of Adenosine A2A and A2B Receptors by Endogenous Adenosine. J. Cardiovasc. Pharmacol. 2009, 53, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Zhan, E.; McIntosh, V.J.; Lasley, R.D. Adenosine A2A and A2B Receptors Are Both Required for Adenosine A1 Receptor-Mediated Cardioprotection. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H1183-9. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Gao, Z.-G.; Nithipatikom, K.; Ijzerman, A.P.; van Veldhoven, J.P.D.; Jacobson, K.A.; Gross, G.J.; Auchampach, J.A. Protection from Myocardial Ischemia/Reperfusion Injury by a Positive Allosteric Modulator of the A3 Adenosine Receptor. J. Pharmacol. Exp. Ther. 2012, 340, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-ΚB in Biology and Targeted Therapy: New Insights and Translational Implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Y.; Zhou, H.; Hu, Y.; Chen, Y.; Guo, D. Signaling Pathways (TNF-α-NF-ΚB, TLR2-TLR4 as Well as ROS-MDA) and Cardiac Damages during Cardiac Surgeries (Coronary Stenting, Permanent Pacemaker Implantations, Radiofrequency Ablations). Curr. Top. Med. Chem. 2025, 25, 196–208. [Google Scholar] [CrossRef]
- Ismail, H.M.; Ahmed, S.A.; Alsaedi, A.M.; Almaramhy, W.H.; Alraddadi, M.K.; Albadrani, M.S.; Alhejaily, I.M.; Mohammad, F.A.; Ghaith, A.M.; Youssef, A.A. Reactive Oxygen and Nitrogen Species in Myocardial Infarction: Mechanistic Insights and Clinical Correlations. Med. Sci. 2025, 13, 152. [Google Scholar] [CrossRef]
- Szychowski, K.A. Current State of Knowledge on Amiodarone (AMD)-Induced Reactive Oxygen Species (ROS) Production in In Vitro and In Vivo Models. Oxygen 2025, 5, 16. [Google Scholar] [CrossRef]
- Yan, Q.; Liu, S.; Sun, Y.; Chen, C.; Yang, S.; Lin, M.; Long, J.; Yao, J.; Lin, Y.; Yi, F.; et al. Targeting Oxidative Stress as a Preventive and Therapeutic Approach for Cardiovascular Disease. J. Transl. Med. 2023, 21, 519. [Google Scholar] [CrossRef]
- Chassaing, C.; Dureng, G.; Baissat, J.; Duchêne-Marullaz, P. Pharmacological Evidence for Cardiac Muscarinic Receptor Subtypes. Life Sci. 1984, 35, 1739–1745. [Google Scholar] [CrossRef] [PubMed]
- Clague, R.U.; Eglen, R.M.; Strachan, A.C.; Whiting, R.L. Action of Agonists and Antagonists at Muscarinic Receptors Present on Ileum and Atria in Vitro. Br. J. Pharmacol. 1985, 86, 163–170. [Google Scholar] [CrossRef]
- Jaiswal, N.; Lambrecht, G.; Mutschler, E.; Malik, K.U. Effect of M2 Muscarinic Receptor Antagonist 4-DAMP, on Prostaglandin Synthesis and Mechanical Function in the Isolated Rabbit Heart. Gen. Pharmacol. 1989, 20, 497–502. [Google Scholar] [CrossRef]
- Kan, H.; Ruan, Y.; Malik, K.U. Localization and Characterization of the Subtypes(s) of Muscarinic Receptor Involved in Prostacyclin Synthesis in Rabbit Heart. J. Pharmacol. Exp. Ther. 1996, 276, 934–941. [Google Scholar] [CrossRef]
- Ford, A.P.; Eglen, R.M.; Whiting, R.L. Analysis of Muscarinic Cholinoceptors Mediating Phosphoinositide Hydrolysis in Guinea Pig Cardiac Muscle. Eur. J. Pharmacol. 1992, 225, 105–112. [Google Scholar] [CrossRef]
- Sun, L.S.; Huber, F.; Robinson, R.B.; Bilezikian, J.P.; Steinberg, S.F.; Vulliemoz, Y. Muscarinic Receptor Heterogeneity in Neonatal Rat Ventricular Myocytes in Culture. J. Cardiovasc. Pharmacol. 1996, 27, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Pönicke, K.; Heinroth-Hoffmann, I.; Brodde, O.-E. Demonstration of Functional M3-Muscarinic Receptors in Ventricular Cardiomyocytes of Adult Rats. Br. J. Pharmacol. 2003, 138, 156–160. [Google Scholar] [CrossRef]
- Smith, C.M.; Wallis, R.M. Characterisation of [3H]-Darifenacin as a Novel Radioligand for the Study of Muscarinic M3 Receptors. J. Recept. Signal Transduct. Res. 1997, 17, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Nishimaru, K.; Tanaka, Y.; Tanaka, H.; Shigenobu, K. Positive and Negative Inotropic Effects of Muscarinic Receptor Stimulation in Mouse Left Atria. Life Sci. 2000, 66, 607–615. [Google Scholar] [CrossRef]
- Wang, Z.; Shi, H.; Wang, H. Functional M3 Muscarinic Acetylcholine Receptors in Mammalian Hearts. Br. J. Pharmacol. 2004, 142, 395–408. [Google Scholar] [CrossRef]
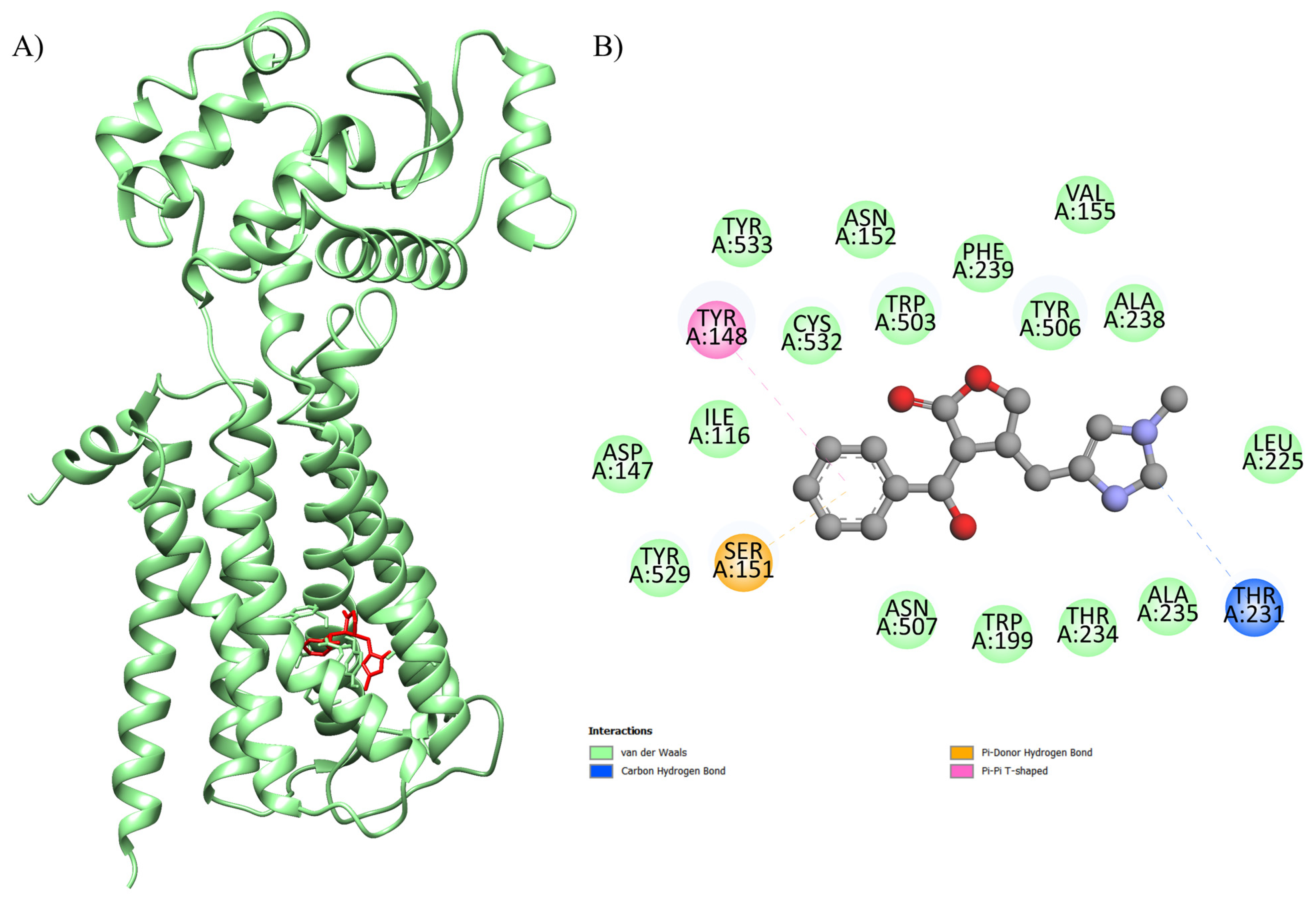
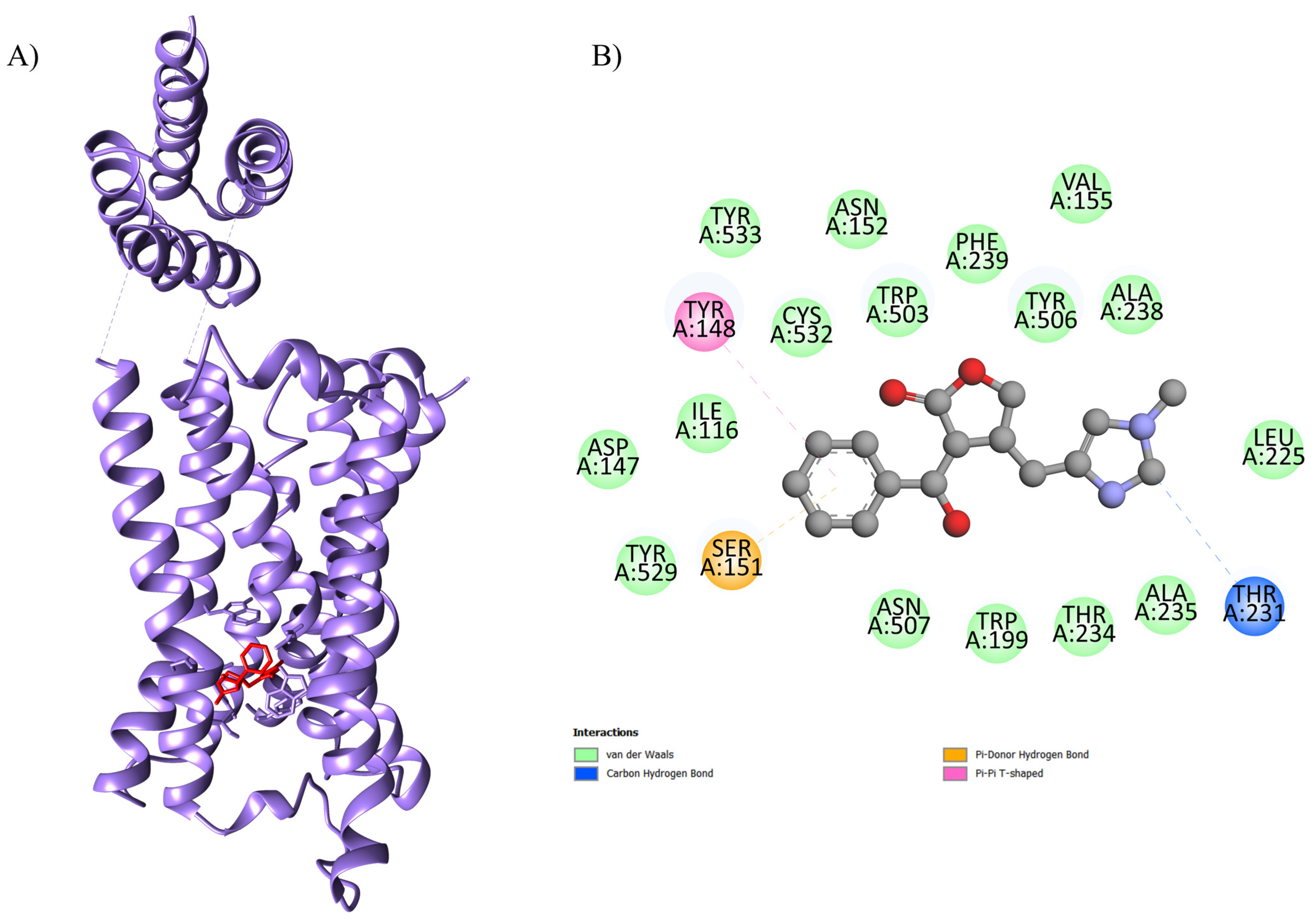

| Complex (Ligand-Protein) | ΔGbind a (kcal.mol−1) | Number of Runs | Standard Deviation | Ligands Interactions with Residues of Proteins b | Function of the Macromolecule |
|---|---|---|---|---|---|
| EPI-4U14 | −8.6 | 100 | 0.438 | Ser A: 151, Tyr A: 529, Asp A: 147, Ile A: 116, Tyr A: 148, Tyr A: 533, Cys A: 532, Asn A: 152, Trp A: 503, Phe A: 239, Tyr A: 506, Val A: 155, Ala A: 238, Leu A: 225, Thr A: 231, Ala A: 235, Thr A: 234, Trp A: 199, Asn A: 507 | Human GPCR involved in smooth muscle contraction, glandular secretions, vomiting induction, and cardiovascular regulation, as its activation in endothelial cells stimulates nitric oxide production, promoting vasodilation and blood pressure control. |
| EPI-5ZK8 | −8.4 | 100 | 0.443 | Ser A: 107, Thr A: 190, Tyr A: 104, Phe A: 195, Ala A: 191, Trp A: 400, Val A: 111, Ala A: 194, Asn A: 108, Trp A: 155, Thr A: 187, Val A: 407, Asn A: 404, Tyr A: 403, Tyr A: 426, Asp A: 103 and Tyr A: 430 | Human muscarinic acetylcholine receptor M2, a G protein-coupled receptor (GPCR) that plays a crucial role in regulating heart rate by slowing conduction in the sinoatrial and atrioventricular nodes. |
| EPI-6DT0 | −7.6 | 100 | 0.219 | Thr D: 361, Tyr D: 362, Glu D: 358, Glu C: 358, Ca A: 501, Glu B: 358, Glu A: 358, Thr A: 361, Tyr B: 362, Trp A: 354, Tyr C: 362, Trp C: 354, Tyr A: 362 | A transmembrane protein critical for cellular homeostasis, 6DT0 acts as the main channel allowing selective entry of calcium ions into the mitochondrial matrix, regulating essential processes such as ATP production, intracellular signaling, and activation of apoptotic pathways. In cardiac cells, it controls the energy production necessary for heart contraction, maintaining cardiac rhythm and function, and influencing apoptosis sensitivity under stress conditions, such as ischemia–reperfusion. |
| EPI-8E59 | −6.8 | 100 | 0.161 | MET A: 1091, GLN A: 1026, TYR A: 1448, PHE A: 1095, THR A: 1099, SER A: 1098, MET A: 1449, MET A: 1144, THR A: 1022, THR A: 1023, ILE A: 1445 | A membrane receptor is essential for regulating calcium influx in excitable cells, especially cardiomyocytes and neurons, where it controls muscle contraction, electrical conduction, and neurotransmitter release. |
| EPI-7KL5 | −5.6 | 100 | 0.197 | Lys A: 14, Phe A: 66, Gly A: 24, Ser A: 18, Ala A: 11, Glu A: 15, Gly A: 26, Phe A: 17, Asp A: 21 | It regulates the activity of the cardiac ryanodine receptor RyR2, modulating calcium release from the sarcoplasmic reticulum during the excitation–contraction cycle in cardiomyocytes. This regulation is crucial for maintaining the frequency and strength of heart contractions, ensuring the synchronization of the heartbeat and efficient blood pumping. Additionally, the interaction with RyR2 is modulated by additional calcium ions, which stabilize the complex and allow fine-tuning of intracellular calcium signaling, contributing to cardiac homeostasis and preventing arrhythmias. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menezes-Rodrigues, F.S.; Andrade Costa, E.; De Marqui Moraes, P.I.; de Araújo, E.A.; Filho, C.E.B.; Véras, L.M.C.; de Araujo Sousa, P.S.; Rocha, J.A.; Junior, N.A.H.; Guizilini, S.; et al. Evaluation of Potential Molecular Targets of the Alkaloid Epiisopiloturine, Involved in Cardioprotective Effects, Using Computational Molecular Docking in an Animal Model of Cardiac Ischemia and Reperfusion. Int. J. Mol. Sci. 2025, 26, 9488. https://doi.org/10.3390/ijms26199488
Menezes-Rodrigues FS, Andrade Costa E, De Marqui Moraes PI, de Araújo EA, Filho CEB, Véras LMC, de Araujo Sousa PS, Rocha JA, Junior NAH, Guizilini S, et al. Evaluation of Potential Molecular Targets of the Alkaloid Epiisopiloturine, Involved in Cardioprotective Effects, Using Computational Molecular Docking in an Animal Model of Cardiac Ischemia and Reperfusion. International Journal of Molecular Sciences. 2025; 26(19):9488. https://doi.org/10.3390/ijms26199488
Chicago/Turabian StyleMenezes-Rodrigues, Francisco Sandro, Elisa Andrade Costa, Pedro Ivo De Marqui Moraes, Erisvaldo Amarante de Araújo, Carlos Eduardo Braga Filho, Leiz Maria Costa Véras, Paulo Sérgio de Araujo Sousa, Jefferson Almeida Rocha, Nelson Americo Hossne Junior, Solange Guizilini, and et al. 2025. "Evaluation of Potential Molecular Targets of the Alkaloid Epiisopiloturine, Involved in Cardioprotective Effects, Using Computational Molecular Docking in an Animal Model of Cardiac Ischemia and Reperfusion" International Journal of Molecular Sciences 26, no. 19: 9488. https://doi.org/10.3390/ijms26199488
APA StyleMenezes-Rodrigues, F. S., Andrade Costa, E., De Marqui Moraes, P. I., de Araújo, E. A., Filho, C. E. B., Véras, L. M. C., de Araujo Sousa, P. S., Rocha, J. A., Junior, N. A. H., Guizilini, S., Rocco, I. S., Gomes, W. J., Caricati-Neto, A., Pires-Oliveira, M., Silva, C. M. C., Wanderley, A. G., & Tallo, F. S. (2025). Evaluation of Potential Molecular Targets of the Alkaloid Epiisopiloturine, Involved in Cardioprotective Effects, Using Computational Molecular Docking in an Animal Model of Cardiac Ischemia and Reperfusion. International Journal of Molecular Sciences, 26(19), 9488. https://doi.org/10.3390/ijms26199488







