Shared and Context-Specific Mechanisms of EMT and Cellular Plasticity in Cancer and Fibrotic Diseases
Abstract
1. Introduction
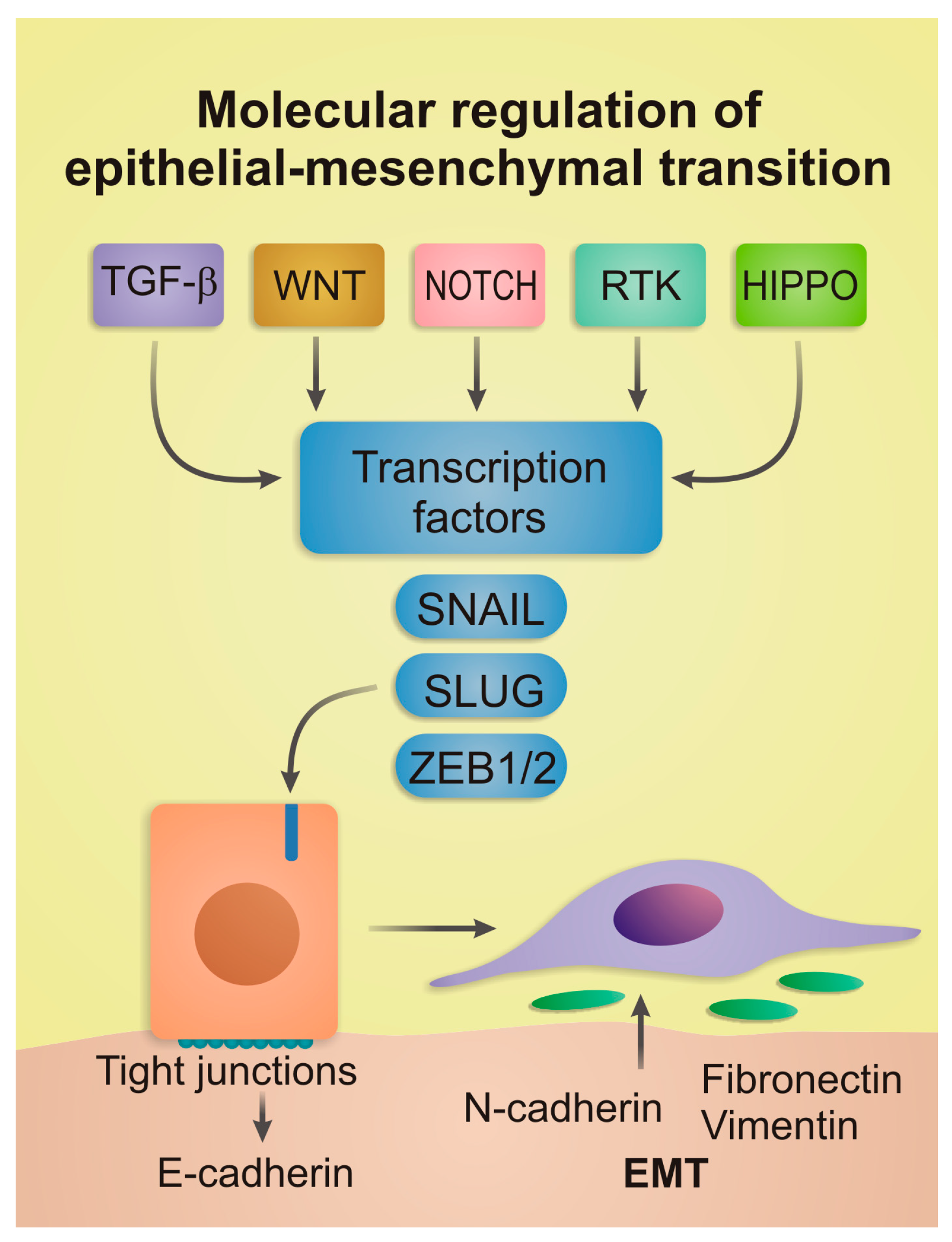
2. Core Molecular Mechanisms of EMT
2.1. Extracellular Signaling Pathways
2.2. Master Transcription Factors
2.3. MicroRNAs and Epigenetic Regulation
3. EMT and Cancer Plasticity
3.1. Partial and Hybrid EMT Phenotypes
3.2. EMT and Cancer Stem Cells
3.3. Functional Consequences: Invasion, Metastasis, and Therapy Resistance
4. EMT and Plasticity in Fibrotic Diseases
4.1. EMT and FMT: Concepts and Interactions
4.2. Inflammation–MSC Axis in Fibrotic Plasticity
4.3. Therapy Resistance and Plasticity
5. Shared and Distinct Features of EMT in Cancer and Fibrosis
5.1. Structural and Molecular Similarities
5.2. Functional Role of EMT in Fibrosis
5.3. Functional Role of EMT in Cancer
6. Potential Therapies and Future Perspectives
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cammareri, P.; Myant, K.B. Be Like Water, My Cells: Cell Plasticity and the Art of Transformation. Front. Cell Dev. Biol. 2023, 11, 1272730. [Google Scholar] [CrossRef]
- Qin, X.; Tape, C.J. Functional Analysis of Cell Plasticity Using Single-Cell Technologies. Trends Cell Biol. 2024, 34, 854–864. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The Basics of Epithelial-Mesenchymal Transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef]
- Yang, J.; Antin, P.; Berx, G.; Blanpain, C.; Brabletz, T.; Bronner, M.; Campbell, K.; Cano, A.; Casanova, J.; Christofori, G.; et al. Guidelines and Definitions for Research on Epithelial–Mesenchymal Transition. Nat. Rev. Mol. Cell Biol. 2020, 21, 341–352. [Google Scholar] [CrossRef]
- Li, M.; Luan, F.; Zhao, Y.; Hao, H.; Zhou, Y.; Han, W.; Fu, X. Epithelial-Mesenchymal Transition: An Emerging Target in Tissue Fibrosis. Exp. Biol. Med. 2015, 241, 1–13. [Google Scholar] [CrossRef]
- Ghafoor, S.; Garcia, E.; Jay, D.J.; Persad, S. Molecular Mechanisms Regulating Epithelial Mesenchymal Transition (EMT) to Promote Cancer Progression. Int. J. Mol. Sci. 2025, 26, 4364. [Google Scholar] [CrossRef]
- Stone, R.C.; Pastar, I.; Ojeh, N.; Chen, V.; Liu, S.; Garzon, K.I.; Tomic-Canic, M. Epithelial-Mesenchymal Transition in Tissue Repair and Fibrosis. Cell Tissue Res. 2016, 365, 495–506. [Google Scholar] [CrossRef]
- Yao, L.; Xu, Z.; Davies, D.E.; Jones, M.G.; Wang, Y. Dysregulated Bidirectional Epithelial–Mesenchymal Crosstalk: A Core Determinant of Lung Fibrosis Progression. Chin. Méd. J. Pulm. Crit. Care Med. 2024, 2, 27–33. [Google Scholar] [CrossRef]
- Jacobs, M.E.; de Vries, D.K.; Engelse, M.A.; Dumas, S.J.; Rabelink, T.J. Endothelial to Mesenchymal Transition in Kidney Fibrosis. Nephrol. Dial. Transplant. 2023, 39, 752–760. [Google Scholar] [CrossRef]
- Hadpech, S.; Thongboonkerd, V. Epithelial–Mesenchymal Plasticity in Kidney Fibrosis. Genesis 2024, 62, e23529. [Google Scholar] [CrossRef]
- Brown, M.S.; Abdollahi, B.; Wilkins, O.M.; Lu, H.; Chakraborty, P.; Ognjenovic, N.B.; Muller, K.E.; Jolly, M.K.; Christensen, B.C.; Hassanpour, S.; et al. Phenotypic Heterogeneity Driven by Plasticity of the Intermediate EMT State Governs Disease Progression and Metastasis in Breast Cancer. Sci. Adv. 2022, 8, eabj8002. [Google Scholar] [CrossRef]
- Akhmetkaliyev, A.; Alibrahim, N.; Shafiee, D.; Tulchinsky, E. EMT/MET Plasticity in Cancer and Go-or-Grow Decisions in Quiescence: The Two Sides of the Same Coin? Mol. Cancer 2023, 22, 90. [Google Scholar] [CrossRef]
- Perelli, L.; Zhang, L.; Mangiameli, S.; Giannese, F.; Mahadevan, K.K.; Peng, F.; Citron, F.; Khan, H.; Le, C.; Gurreri, E.; et al. Evolutionary Fingerprints of Epithelial-to-Mesenchymal Transition. Nature 2025, 640, 1083–1092. [Google Scholar] [CrossRef]
- Simeone, P.; Trerotola, M.; Franck, J.; Cardon, T.; Marchisio, M.; Fournier, I.; Salzet, M.; Maffia, M.; Vergara, D. The Multiverse Nature of Epithelial to Mesenchymal Transition. Semin. Cancer Biol. 2019, 58, 1–10. [Google Scholar] [CrossRef]
- Xue, C.; Chu, Q.; Shi, Q.; Zeng, Y.; Lu, J.; Li, L. Wnt Signaling Pathways in Biology and Disease: Mechanisms and Therapeutic Advances. Signal Transduct. Target. Ther. 2025, 10, 106. [Google Scholar] [CrossRef]
- Radhakrishnan, K.; Truong, L.; Carmichael, C.L. An “Unexpected” Role for EMT Transcription Factors in Hematological Development and Malignancy. Front. Immunol. 2023, 14, 1207360. [Google Scholar] [CrossRef]
- Papavassiliou, K.A.; Sofianidi, A.A.; Spiliopoulos, F.G.; Gogou, V.A.; Gargalionis, A.N.; Papavassiliou, A.G. YAP/TAZ Signaling in the Pathobiology of Pulmonary Fibrosis. Cells 2024, 13, 1519. [Google Scholar] [CrossRef]
- Piccolo, S.; Panciera, T.; Contessotto, P.; Cordenonsi, M. YAP/TAZ as Master Regulators in Cancer: Modulation, Function and Therapeutic Approaches. Nat. Cancer 2023, 4, 9–26. [Google Scholar] [CrossRef]
- Kong, F.; Ma, L.; Wang, X.; You, H.; Zheng, K.; Tang, R. Regulation of Epithelial-Mesenchymal Transition by Protein Lysine Acetylation. Cell Commun. Signal. 2022, 20, 57. [Google Scholar] [CrossRef]
- Bakalenko, N.; Kuznetsova, E.; Malashicheva, A. The Complex Interplay of TGF-β and Notch Signaling in the Pathogenesis of Fibrosis. Int. J. Mol. Sci. 2024, 25, 10803. [Google Scholar] [CrossRef]
- Nie, F.; Sun, X.; Sun, J.; Zhang, J.; Wang, Y. Epithelial-Mesenchymal Transition in Colorectal Cancer Metastasis and Progression: Molecular Mechanisms and Therapeutic Strategies. Cell Death Discov. 2025, 11, 336. [Google Scholar] [CrossRef]
- Nowak, E.; Bednarek, I. Aspects of the Epigenetic Regulation of EMT Related to Cancer Metastasis. Cells 2021, 10, 3435. [Google Scholar] [CrossRef]
- Deng, Z.; Fan, T.; Xiao, C.; Tian, H.; Zheng, Y.; Li, C.; He, J. TGF-β Signaling in Health, Disease and Therapeutics. Signal Transduct. Target. Ther. 2024, 9, 61. [Google Scholar] [CrossRef]
- Giarratana, A.O.; Prendergast, C.M.; Salvatore, M.M.; Capaccione, K.M. TGF-β Signaling: Critical Nexus of Fibrogenesis and Cancer. J. Transl. Med. 2024, 22, 594. [Google Scholar] [CrossRef]
- Peng, D.; Fu, M.; Wang, M.; Wei, Y.; Wei, X. Targeting TGF-β Signal Transduction for Fibrosis and Cancer Therapy. Mol. Cancer 2022, 21, 104. [Google Scholar] [CrossRef]
- Sun, L.; Xing, J.; Zhou, X.; Song, X.; Gao, S. Wnt/β-Catenin Signalling, Epithelial-Mesenchymal Transition and Crosslink Signalling in Colorectal Cancer Cells. Biomed. Pharmacother. 2024, 175, 116685. [Google Scholar] [CrossRef]
- Matsuno, Y.; Coelho, A.L.; Jarai, G.; Westwick, J.; Hogaboam, C.M. Notch Signaling Mediates TGF-Β1-Induced Epithelial–Mesenchymal Transition Through the Induction of Snai1. Int. J. Biochem. Cell Biol. 2012, 44, 776–789. [Google Scholar] [CrossRef]
- Johnson, R.; Halder, G. The Two Faces of Hippo: Targeting the Hippo Pathway for Regenerative Medicine and Cancer Treatment. Nat. Rev. Drug Discov. 2014, 13, 63–79. [Google Scholar] [CrossRef]
- Fu, M.; Hu, Y.; Lan, T.; Guan, K.-L.; Luo, T.; Luo, M. The Hippo Signalling Pathway and Its Implications in Human Health and Diseases. Signal Transduct. Target. Ther. 2022, 7, 376. [Google Scholar] [CrossRef]
- Kizawa, R.; Araya, J.; Fujita, Y. Divergent Roles of the Hippo Pathway in the Pathogenesis of Idiopathic Pulmonary Fibrosis: Tissue Homeostasis and Fibrosis. Inflamm. Regen. 2023, 43, 45. [Google Scholar] [CrossRef]
- Villarejo, A.; Cortés-Cabrera, Á.; Molina-Ortíz, P.; Portillo, F.; Cano, A. Differential Role of Snail1 and Snail2 Zinc Fingers in E-Cadherin Repression and Epithelial to Mesenchymal Transition. J. Biol. Chem. 2014, 289, 930–941. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Xu, H.; Hu, C.; Chen, K.; Tang, S.; Wu, X.; Zhang, Q.; Xiang, Q. HOPX Regulates the Invasion and Migration Abilities of Hepatocellular Carcinoma by Targeting SNAIL. Sci. Rep. 2025, 15, 29739. [Google Scholar] [CrossRef]
- Smith, B.N.; Burton, L.J.; Henderson, V.; Randle, D.D.; Morton, D.J.; Smith, B.A.; Taliaferro-Smith, L.; Nagappan, P.; Yates, C.; Zayzafoon, M.; et al. Snail Promotes Epithelial Mesenchymal Transition in Breast Cancer Cells in Part via Activation of Nuclear ERK2. PLoS ONE 2014, 9, e104987. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Sui, X.; Weng, L.; Liu, Y. SNAIL1: Linking Tumor Metastasis to Immune Evasion. Front. Immunol. 2021, 12, 724200. [Google Scholar] [CrossRef]
- Grande, M.T.; Sánchez-Laorden, B.; López-Blau, C.; Frutos, C.A.D.; Boutet, A.; Arévalo, M.; Rowe, R.G.; Weiss, S.J.; López-Novoa, J.M.; Nieto, M.A. Snail1-Induced Partial Epithelial-to-Mesenchymal Transition Drives Renal Fibrosis in Mice and Can Be Targeted to Reverse Established Disease. Nat. Med. 2015, 21, 989–997. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.; Zhao, J.; Li, Q.; Xu, C.; Wu, H.; Zhu, Z.; Tian, L. Snail-Mediated Partial Epithelial Mesenchymal Transition Augments the Differentiation of Local Lung Myofibroblast. Chemosphere 2021, 267, 128870. [Google Scholar] [CrossRef]
- Niayesh-Mehr, R.; Kalantar, M.; Bontempi, G.; Montaldo, C.; Ebrahimi, S.; Allameh, A.; Babaei, G.; Seif, F.; Strippoli, R. The Role of Epithelial-Mesenchymal Transition in Pulmonary Fibrosis: Lessons from Idiopathic Pulmonary Fibrosis and COVID-19. Cell Commun. Signal. 2024, 22, 542. [Google Scholar] [CrossRef]
- Reveles-Espinoza, A.M.; Roque, R.R.; Vallejo-Cardona, A.A. Role of ZEB1 in Immune Response, Inflammation and Membrane Remodeling During Neoplasia. Explor. Immunol. 2025, 5, 1003187. [Google Scholar] [CrossRef]
- Plaschka, M.; Benboubker, V.; Grimont, M.; Berthet, J.; Tonon, L.; Lopez, J.; Le-Bouar, M.; Balme, B.; Tondeur, G.; de la Fouchardière, A.; et al. ZEB1 Transcription Factor Promotes Immune Escape in Melanoma. J. Immunother. Cancer 2022, 10, e003484. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Zhang, L.; Sun, Y.; Ma, Y.; Zhang, Y. Alveolar Epithelial Cell Dysfunction and Epithelial-Mesenchymal Transition in Pulmonary Fibrosis Pathogenesis. Front. Mol. Biosci. 2025, 12, 1564176. [Google Scholar] [CrossRef]
- Jin, H.; Park, S.-Y.; Lee, J.E.; Park, H.; Jeong, M.; Lee, H.; Cho, J.; Lee, Y.-S. GTSE1-Driven ZEB1 Stabilization Promotes Pulmonary Fibrosis Through the Epithelial-to-Mesenchymal Transition. Mol. Ther. 2024, 32, 4138–4157. [Google Scholar] [CrossRef]
- Cheng, L.; Zhou, M.-Y.; Gu, Y.-J.; Chen, L.; Wang, Y. ZEB1: New Advances in Fibrosis and Cancer. Mol. Cell. Biochem. 2021, 476, 1643–1650. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; He, T.; Tong, Z.; Liao, L.; Huang, S.; Fakhouri, W.D.; Edwards, D.P.; Xu, J. Molecular Mechanisms of TWIST1-Regulated Transcription in EMT and Cancer Metastasis. EMBO Rep. 2023, 24, e56902. [Google Scholar] [CrossRef] [PubMed]
- Sepporta, M.-V.; Praz, V.; Bourloud, K.B.; Joseph, J.-M.; Jauquier, N.; Riggi, N.; Nardou-Auderset, K.; Petit, A.; Scoazec, J.-Y.; Sartelet, H.; et al. TWIST1 Expression Is Associated with High-Risk Neuroblastoma and Promotes Primary and Metastatic Tumor Growth. Commun. Biol. 2022, 5, 42. [Google Scholar] [CrossRef]
- Yang, J.; Mani, S.A.; Donaher, J.L.; Ramaswamy, S.; Itzykson, R.A.; Come, C.; Savagner, P.; Gitelman, I.; Richardson, A.; Weinberg, R.A. Twist, a Master Regulator of Morphogenesis, Plays an Essential Role in Tumor Metastasis. Cell 2004, 117, 927–939. [Google Scholar] [CrossRef]
- Ning, X.; Zhang, K.; Wu, Q.; Liu, M.; Sun, S. Emerging Role of Twist1 in Fibrotic Diseases. J. Cell. Mol. Med. 2018, 22, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Valenzi, E.; Bahudhanapati, H.; Tan, J.; Tabib, T.; Sullivan, D.I.; Nouraie, M.; Sembrat, J.; Fan, L.; Chen, K.; Liu, S.; et al. Single-Nucleus Chromatin Accessibility Identifies a Critical Role for TWIST1 in Idiopathic Pulmonary Fibrosis Myofibroblast Activity. Eur. Respir. J. 2023, 62, 2200474. [Google Scholar] [CrossRef]
- Shao, T.; Xue, Y.; Fang, M. Epigenetic Repression of Chloride Channel Accessory 2 Transcription in Cardiac Fibroblast: Implication in Cardiac Fibrosis. Front. Cell Dev. Biol. 2021, 9, 771466. [Google Scholar] [CrossRef]
- Xu, L.; Fu, M.; Chen, D.; Han, W.; Ostrowski, M.C.; Grossfeld, P.; Gao, P.; Ye, M. Endothelial-Specific Deletion of Ets-1 Attenuates Angiotensin II-Induced Cardiac Fibrosis via Suppression of Endothelial-to-Mesenchymal Transition. BMB Rep. 2019, 52, 595–600. [Google Scholar] [CrossRef]
- Bridges, R.S.; Kass, D.; Loh, K.; Glackin, C.; Borczuk, A.C.; Greenberg, S. Gene Expression Profiling of Pulmonary Fibrosis Identifies Twist1 as an Antiapoptotic Molecular “Rectifier” of Growth Factor Signaling. Am. J. Pathol. 2009, 175, 2351–2361. [Google Scholar] [CrossRef]
- Burgy, O.; Mailleux, A.A. ATAC-Ing Single Nucleus in Idiopathic Pulmonary Fibrosis: TWIST1 Strives Back for Myofibroblasts. Eur. Respir. J. 2023, 62, 2300881. [Google Scholar] [CrossRef] [PubMed]
- Sisto, M.; Lisi, S. Epigenetic Regulation of EMP/EMT-Dependent Fibrosis. Int. J. Mol. Sci. 2024, 25, 2775. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.; Turchinovich, A.; Feisst, M.; Riedel, F.; Haßdenteufel, K.; Scharli, P.; Hartkopf, A.D.; Brucker, S.Y.; Michel, L.; Burwinkel, B.; et al. Circulating miR-200 Family and CTCs in Metastatic Breast Cancer Before, During, and After a New Line of Systemic Treatment. Int. J. Mol. Sci. 2022, 23, 9535. [Google Scholar] [CrossRef]
- Garinet, S.; Didelot, A.; Denize, T.; Perrier, A.; Beinse, G.; Leclere, J.-B.; Oudart, J.-B.; Gibault, L.; Badoual, C.; Pimpec-Barthes, F.L.; et al. Clinical Assessment of the miR-34, miR-200, ZEB1 and SNAIL EMT Regulation Hub Underlines the Differential Prognostic Value of EMT miRs to Drive Mesenchymal Transition and Prognosis in Resected NSCLC. Br. J. Cancer 2021, 125, 1544–1551. [Google Scholar] [CrossRef]
- Liu, X.; Liu, G.; Tan, Y.; Liu, P.; Li, L. Upregulation of miR-200a Improves Ureteral Obstruction-Induced Renal Fibrosis via GAB1/Wnt/β-Catenin Signaling. Nefrologia (Engl. Ed.) 2023, 43, 21–31. [Google Scholar] [CrossRef]
- Wang, H.; Sun, K.; Peng, H.; Wang, Y.; Zhang, L. Emerging Roles of Noncoding RNAs in Idiopathic Pulmonary Fibrosis. Cell Death Discov. 2024, 10, 443. [Google Scholar] [CrossRef]
- Qi, Y.; Zhao, A.; Yang, P.; Jin, L.; Hao, C. miR-34a-5p Attenuates EMT Through Targeting SMAD4 in Silica-Induced Pulmonary Fibrosis. J. Cell. Mol. Med. 2020, 24, 12219–12224. [Google Scholar] [CrossRef] [PubMed]
- Li, W.J.; Wang, Y.; Liu, R.; Kasinski, A.L.; Shen, H.; Slack, F.J.; Tang, D.G. MicroRNA-34a: Potent Tumor Suppressor, Cancer Stem Cell Inhibitor, and Potential Anticancer Therapeutic. Front. Cell Dev. Biol. 2021, 9, 640587. [Google Scholar] [CrossRef]
- Guo, J.; Li, L.; Deng, N.; Xu, Y.; Wang, G.; Luo, H.; Xu, C.; Li, X. microRNA-203 Functions as a Natural Ras Inhibitor in Hepatocellular Carcinoma. Am. J. Cancer Res. 2022, 13, 1295–1309. [Google Scholar]
- Chu, L.; Xie, D.; Xu, D. Epigenetic Regulation of Fibroblasts and Crosstalk Between Cardiomyocytes and Non-Myocyte Cells in Cardiac Fibrosis. Biomolecules 2023, 13, 1382. [Google Scholar] [CrossRef]
- Dai, W.; Qiao, X.; Fang, Y.; Guo, R.; Bai, P.; Liu, S.; Li, T.; Jiang, Y.; Wei, S.; Na, Z.; et al. Epigenetics-Targeted Drugs: Current Paradigms and Future Challenges. Signal Transduct. Target. Ther. 2024, 9, 332. [Google Scholar] [CrossRef]
- Song, J.; Yang, P.; Chen, C.; Ding, W.; Tillement, O.; Bai, H.; Zhang, S. Targeting Epigenetic Regulators as a Promising Avenue to Overcome Cancer Therapy Resistance. Signal Transduct. Target. Ther. 2025, 10, 219. [Google Scholar] [CrossRef]
- Xiong, R.; Geng, B.; Jiang, W.; Hu, Y.; Hu, Z.; Hao, B.; Li, N.; Geng, Q. Histone Deacetylase 3 Deletion in Alveolar Type 2 Epithelial Cells Prevents Bleomycin-Induced Pulmonary Fibrosis. Clin. Epigenetics 2023, 15, 182. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.P.; Vanderhyden, B.C. Transcriptional Census of Epithelial-Mesenchymal Plasticity in Cancer. Sci. Adv. 2022, 8, eabi7640. [Google Scholar] [CrossRef] [PubMed]
- Tagliazucchi, G.M.; Wiecek, A.J.; Withnell, E.; Secrier, M. Genomic and Microenvironmental Heterogeneity Shaping Epithelial-to-Mesenchymal Trajectories in Cancer. Nat. Commun. 2023, 14, 789. [Google Scholar] [CrossRef]
- Arora, R.; Cao, C.; Kumar, M.; Sinha, S.; Chanda, A.; McNeil, R.; Samuel, D.; Arora, R.K.; Matthews, T.W.; Chandarana, S.; et al. Spatial Transcriptomics Reveals Distinct and Conserved Tumor Core and Edge Architectures That Predict Survival and Targeted Therapy Response. Nat. Commun. 2023, 14, 5029. [Google Scholar] [CrossRef] [PubMed]
- Dongre, A.; Weinberg, R.A. New Insights into the Mechanisms of Epithelial–Mesenchymal Transition and Implications for Cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef]
- Lovisa, S.; Zeisberg, M.; Kalluri, R. Partial Epithelial-to-Mesenchymal Transition and Other New Mechanisms of Kidney Fibrosis. Trends Endocrinol. Metab. 2016, 27, 681–695. [Google Scholar] [CrossRef]
- Sahoo, S.; Ramu, S.; Nair, M.G.; Pillai, M.; Juan, B.P.S.; Milioli, H.Z.; Mandal, S.; Naidu, C.M.; Mavatkar, A.D.; Subramaniam, H.; et al. Increased Prevalence of Hybrid Epithelial/Mesenchymal State and Enhanced Phenotypic Heterogeneity in Basal Breast Cancer. iScience 2024, 27, 110116. [Google Scholar] [CrossRef]
- Joanito, I.; Wirapati, P.; Zhao, N.; Nawaz, Z.; Yeo, G.; Lee, F.; Eng, C.L.P.; Macalinao, D.C.; Kahraman, M.; Srinivasan, H.; et al. Single-Cell and Bulk Transcriptome Sequencing Identifies Two Epithelial Tumor Cell States and Refines the Consensus Molecular Classification of Colorectal Cancer. Nat. Genet. 2022, 54, 963–975. [Google Scholar] [CrossRef]
- Valdeolivas, A.; Amberg, B.; Giroud, N.; Richardson, M.; Gálvez, E.J.C.; Badillo, S.; Julien-Laferrière, A.; Túrós, D.; Voith von Voithenberg, L.; Wells, I.; et al. Profiling the Heterogeneity of Colorectal Cancer Consensus Molecular Subtypes Using Spatial Transcriptomics. npj Precis. Oncol. 2024, 8, 10. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, B.; Min, Q.; Yang, X.; Yan, S.; Ma, Y.; Li, S.; Fan, J.; Wang, Y.; Dong, B.; et al. Spatial Transcriptomics Delineates Molecular Features and Cellular Plasticity in Lung Adenocarcinoma Progression. Cell Discov. 2023, 9, 96. [Google Scholar] [CrossRef]
- Fontana, R.; Mestre-Farrera, A.; Yang, J. Update on Epithelial-Mesenchymal Plasticity in Cancer Progression. Annu. Rev. Pathol. Mech. Dis. 2023, 19, 133–156. [Google Scholar] [CrossRef]
- Lei, Z.-N.; Teng, Q.-X.; Koya, J.; Liu, Y.; Chen, Z.; Zeng, L.; Chen, Z.-S.; Fang, S.; Wang, J.; Liu, Y.; et al. The Correlation between Cancer Stem Cells and Epithelial-Mesenchymal Transition: Molecular Mechanisms and Significance in Cancer Theragnosis. Front. Immunol. 2024, 15, 1417201. [Google Scholar] [CrossRef] [PubMed]
- Batlle, E.; Clevers, H. Cancer Stem Cells Revisited. Nat. Med. 2017, 23, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- Kreso, A.; Dick, J.E. Evolution of the Cancer Stem Cell Model. Cell Stem. Cell 2014, 14, 275–291. [Google Scholar] [CrossRef]
- Mani, S.A.; Guo, W.; Liao, M.-J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M.; et al. The Epithelial-Mesenchymal Transition Generates Cells with Properties of Stem Cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef]
- den Hollander, P.; Maddela, J.J.; Mani, S.A. Spatial and Temporal Relationship Between Epithelial–Mesenchymal Transition (EMT) and Stem Cells in Cancer. Clin. Chem. 2024, 70, 190–205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, W.; Liu, S.; Chen, C. Targeting Breast Cancer Stem Cells. Int. J. Biol. Sci. 2023, 19, 552–570. [Google Scholar] [CrossRef]
- Yadav, M.; Sharma, A.; Patne, K.; Tabasum, S.; Suryavanshi, J.; Rawat, L.; Machaalani, M.; Eid, M.; Singh, R.P.; Choueiri, T.K.; et al. AXL Signaling in Cancer: From Molecular Insights to Targeted Therapies. Signal Transduct. Target. Ther. 2025, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Wang, J.; Li, Y.; Zhang, K.; Wang, T.; Bo, Z.; Lu, S.; Rodríguez, R.A.; Wei, R.; Zhu, M.; et al. EMT and Cancer Stem Cells: Drivers of Therapy Resistance and Promising Therapeutic Targets. Drug Resist. Updat. 2025, 83, 101276. [Google Scholar] [CrossRef]
- Lee, H.; Kim, B.; Park, J.; Park, S.; Yoo, G.; Yum, S.; Kang, W.; Lee, J.-M.; Youn, H.; Youn, B. Cancer Stem Cells: Landscape, Challenges and Emerging Therapeutic Innovations. Signal Transduct. Target. Ther. 2025, 10, 248. [Google Scholar] [CrossRef]
- Chu, X.; Tian, W.; Ning, J.; Xiao, G.; Zhou, Y.; Wang, Z.; Zhai, Z.; Tanzhu, G.; Yang, J.; Zhou, R. Cancer Stem Cells: Advances in Knowledge and Implications for Cancer Therapy. Signal Transduct. Target. Ther. 2024, 9, 170. [Google Scholar] [CrossRef]
- Stehbens, S.J.; Scarpa, E.; White, M.D. Perspectives in Collective Cell Migration—Moving Forward. J. Cell Sci. 2024, 137. [Google Scholar] [CrossRef] [PubMed]
- Lambert, A.W.; Pattabiraman, D.R.; Weinberg, R.A. Emerging Biological Principles of Metastasis. Cell 2017, 168, 670–691. [Google Scholar] [CrossRef]
- Brabletz, T.; Kalluri, R.; Nieto, M.A.; Weinberg, R.A. EMT in Cancer. Nat. Rev. Cancer 2018, 18, 128–134. [Google Scholar] [CrossRef]
- Saxena, K.; Jolly, M.K.; Balamurugan, K. Hypoxia, Partial EMT and Collective Migration: Emerging Culprits in Metastasis. Transl. Oncol. 2020, 13, 100845. [Google Scholar] [CrossRef]
- Guo, T.; Xu, J. Cancer-Associated Fibroblasts: A Versatile Mediator in Tumor Progression, Metastasis, and Targeted Therapy. Cancer Metastasis Rev. 2024, 43, 1095–1116. [Google Scholar] [CrossRef] [PubMed]
- Forsthuber, A.; Aschenbrenner, B.; Korosec, A.; Jacob, T.; Annusver, K.; Krajic, N.; Kholodniuk, D.; Frech, S.; Zhu, S.; Purkhauser, K.; et al. Cancer-Associated Fibroblast Subtypes Modulate the Tumor-Immune Microenvironment and Are Associated with Skin Cancer Malignancy. Nat. Commun. 2024, 15, 9678. [Google Scholar] [CrossRef]
- Mukherjee, A.; Bravo-Cordero, J.J. Regulation of Dormancy During Tumor Dissemination: The Role of the ECM. Cancer Metastasis Rev. 2023, 42, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Weston, W.A.; Barr, A.R. A Cell Cycle Centric View of Tumour Dormancy. Br. J. Cancer 2023, 129, 1535–1545. [Google Scholar] [CrossRef]
- Nieto, M.A.; Huang, R.Y.-J.; Jackson, R.A.; Thiery, J.P. EMT: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, J.; Wang, F.; Fang, Y.; Yang, Y.; Zhou, Q.; Yuan, W.; Gu, X.; Hu, J.; Yang, S. Pre-Metastatic Niche: Formation, Characteristics and Therapeutic Implication. Signal Transduct. Target. Ther. 2024, 9, 236. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, Y.; Tan, X.; Du, Y.; Wei, Y.; Liu, S. Extracellular Vesicle-Mediated Pre-Metastatic Niche Formation via Altering Host Microenvironments. Front. Immunol. 2024, 15, 1367373. [Google Scholar] [CrossRef]
- Liu, Y.; Sinjab, A.; Min, J.; Han, G.; Paradiso, F.; Zhang, Y.; Wang, R.; Pei, G.; Dai, Y.; Liu, Y.; et al. Conserved Spatial Subtypes and Cellular Neighborhoods of Cancer-Associated Fibroblasts Revealed by Single-Cell Spatial Multi-Omics. Cancer Cell 2025, 43, 905–924.e6. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, J.; Ren, Y.; Liu, S.; Ba, Y.; Zuo, A.; Luo, P.; Cheng, Q.; Xu, H.; Han, X. Multi-Stage Mechanisms of Tumor Metastasis and Therapeutic Strategies. Signal Transduct. Target. Ther. 2024, 9, 270. [Google Scholar] [CrossRef]
- Lurje, I.; Gaisa, N.T.; Weiskirchen, R.; Tacke, F. Mechanisms of Organ Fibrosis: Emerging Concepts and Implications for Novel Treatment Strategies. Mol. Asp. Med. 2023, 92, 101191. [Google Scholar] [CrossRef] [PubMed]
- Ku, J.C.; Raiten, J.; Li, Y. Understanding Fibrosis: Mechanisms, Clinical Implications, Current Therapies, and Prospects for Future Interventions. Biomed. Eng. Adv. 2024, 7, 100118. [Google Scholar] [CrossRef]
- Mutsaers, H.A.M.; Merrild, C.; Nørregaard, R.; Plana-Ripoll, O. The Impact of Fibrotic Diseases on Global Mortality from 1990 to 2019. J. Transl. Med. 2023, 21, 818. [Google Scholar] [CrossRef]
- Francis, A.; Harhay, M.N.; Ong, A.C.M.; Tummalapalli, S.L.; Ortiz, A.; Fogo, A.B.; Fliser, D.; Roy-Chaudhury, P.; Fontana, M.; Nangaku, M.; et al. Chronic Kidney Disease and the Global Public Health Agenda: An International Consensus. Nat. Rev. Nephrol. 2024, 20, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Xie, W.; Zhang, Y.; Lei, L.; Pan, Y. Changing Epidemiology of Cirrhosis from 2010 to 2019: Results from the Global Burden Disease Study 2019. Ann. Med. 2023, 55, 2252326. [Google Scholar] [CrossRef]
- Ghazal, R.; Wang, M.; Liu, D.; Tschumperlin, D.J.; Pereira, N.L. Cardiac Fibrosis in the Multi-Omics Era: Implications for Heart Failure. Circ. Res. 2025, 136, 773–802. [Google Scholar] [CrossRef]
- Koudstaal, T.; Wijsenbeek, M.S. Idiopathic Pulmonary Fibrosis. La Press Médicale 2023, 52, 104166. [Google Scholar] [CrossRef] [PubMed]
- Podolanczuk, A.J.; Thomson, C.C.; Remy-Jardin, M.; Richeldi, L.; Martinez, F.J.; Kolb, M.; Raghu, G. Idiopathic Pulmonary Fibrosis: State of the Art for 2023. Eur. Respir. J. 2022, 61, 2200957. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Sun, Q.; Davis, F.; Mao, J.; Zhao, H.; Ma, D. Epithelial–Mesenchymal Transition in Organ Fibrosis Development: Current Understanding and Treatment Strategies. Burn. Trauma 2022, 10, tkac011. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Jin, D.; Zhang, Z.; Jin, D.; Xue, J.; Duan, L.; Zhang, Y.; Kang, X.; Lian, F. The Critical Role of the Hippo Signaling Pathway in Kidney Diseases. Front. Pharmacol. 2022, 13, 988175. [Google Scholar] [CrossRef]
- Huang, R.; Fu, P.; Ma, L. Kidney Fibrosis: From Mechanisms to Therapeutic Medicines. Signal Transduct. Target. Ther. 2023, 8, 129. [Google Scholar] [CrossRef]
- Wei, Q.; Gan, C.; Sun, M.; Xie, Y.; Liu, H.; Xue, T.; Deng, C.; Mo, C.; Ye, T. BRD4: An Effective Target for Organ Fibrosis. Biomark. Res. 2024, 12, 92. [Google Scholar] [CrossRef]
- O’Sullivan, E.D.; Mylonas, K.J.; Xin, C.; Baird, D.P.; Carvalho, C.; Docherty, M.-H.; Campbell, R.; Matchett, K.P.; Waddell, S.H.; Walker, A.D.; et al. Indian Hedgehog Release from TNF-Activated Renal Epithelia Drives Local and Remote Organ Fibrosis. Sci. Transl. Med. 2023, 15, eabn0736. [Google Scholar] [CrossRef]
- Jolly, M.K.; Ward, C.; Eapen, M.S.; Myers, S.; Hallgren, O.; Levine, H.; Sohal, S.S. Epithelial–Mesenchymal Transition, a Spectrum of States: Role in Lung Development, Homeostasis, and Disease. Dev. Dyn. 2018, 247, 346–358. [Google Scholar] [CrossRef]
- DiGiovanni, G.T.; Han, W.; Sherrill, T.P.; Taylor, C.J.; Nichols, D.S.; Geis, N.M.; Singha, U.K.; Calvi, C.L.; McCall, A.S.; Dixon, M.M.; et al. Epithelial Yap/Taz Are Required for Functional Alveolar Regeneration Following Acute Lung Injury. JCI Insight 2023, 8, e173374. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Cardiac Fibrosis. Cardiovasc. Res. 2020, 117, 1450–1488. [Google Scholar] [CrossRef]
- Kurose, H. Cardiac Fibrosis and Fibroblasts. Cells 2021, 10, 1716. [Google Scholar] [CrossRef]
- Xu, Y.; Kovacic, J.C. Endothelial to Mesenchymal Transition in Health and Disease. Annu. Rev. Physiol. 2022, 85, 245–267. [Google Scholar] [CrossRef]
- Acharya, P.; Chouhan, K.; Weiskirchen, S.; Weiskirchen, R. Cellular Mechanisms of Liver Fibrosis. Front. Pharmacol. 2021, 12, 671640. [Google Scholar] [CrossRef]
- Kisseleva, T.; Brenner, D. Molecular and Cellular Mechanisms of Liver Fibrosis and Its Regression. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 151–166. [Google Scholar] [CrossRef]
- Wang, Z.; Dong, S.; Zhou, W. Pancreatic Stellate Cells: Key Players in Pancreatic Health and Diseases (Review). Mol. Med. Rep. 2024, 30, 109. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Liu, N.; Bao, C.; Yang, D.; Ma, G.; Yi, W.; Xiao, G.; Cao, H. Mesenchymal Stem Cells in Fibrotic Diseases—The Two Sides of the Same Coin. Acta Pharmacol. Sin. 2023, 44, 268–287. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Cheng, H.; Dai, R.; Shang, L.; Zhang, X.; Wen, H. Macrophage Polarization in Tissue Fibrosis. PeerJ 2023, 11, e16092. [Google Scholar] [CrossRef]
- Li, C.; Wang, B. Mesenchymal Stem/Stromal Cells in Progressive Fibrogenic Involvement and Anti-Fibrosis Therapeutic Properties. Front. Cell Dev. Biol. 2022, 10, 902677. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, M.; Yang, J.; Chen, Y.; Wang, J. Engineerable Mesenchymal Stem Cell-Derived Extracellular Vesicles as Promising Therapeutic Strategies for Pulmonary Fibrosis. Stem Cell Res. Ther. 2025, 16, 367. [Google Scholar] [CrossRef]
- Kou, M.; Huang, L.; Yang, J.; Chiang, Z.; Chen, S.; Liu, J.; Guo, L.; Zhang, X.; Zhou, X.; Xu, X.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles for Immunomodulation and Regeneration: A Next Generation Therapeutic Tool? Cell Death Dis. 2022, 13, 580. [Google Scholar] [CrossRef]
- Lee, J.H.; Massagué, J. TGF-β in Developmental and Fibrogenic EMTs. Semin. Cancer Biol. 2022, 86, 136–145. [Google Scholar] [CrossRef]
- Jiang, Y.; Cai, R.; Huang, Y.; Zhu, L.; Xiao, L.; Wang, C.; Wang, L. Macrophages in Organ Fibrosis: From Pathogenesis to Therapeutic Targets. Cell Death Discov. 2024, 10, 487. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Zhuang, S. New Insights Into the Role and Mechanism of Partial Epithelial-Mesenchymal Transition in Kidney Fibrosis. Front. Physiol. 2020, 11, 569322. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Wantono, C.; Tan, Y.; Deng, F.; Duan, T.; Liu, D. Regulators, Functions, and Mechanotransduction Pathways of Matrix Stiffness in Hepatic Disease. Front. Physiol. 2023, 14, 1098129. [Google Scholar] [CrossRef]
- Mai, Z.; Lin, Y.; Lin, P.; Zhao, X.; Cui, L. Modulating Extracellular Matrix Stiffness: A Strategic Approach to Boost Cancer Immunotherapy. Cell Death Dis. 2024, 15, 307. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Domkam, N.; Kabir, H.; Mansour, A.; Tsukamoto, S.; Yerima, G.; Adachi, T.; Mofrad, M.R.K. Emerging Mechanomedicines Informed by Mechanotransduction along the Integrin–Cytoskeleton–Nucleus Axis. APL Bioeng. 2025, 9, 021503. [Google Scholar] [CrossRef] [PubMed]
- Sharip, A.; Kunz, J. Mechanosignaling via Integrins: Pivotal Players in Liver Fibrosis Progression and Therapy. Cells 2025, 14, 266. [Google Scholar] [CrossRef]
- Di, X.; Gao, X.; Peng, L.; Ai, J.; Jin, X.; Qi, S.; Li, H.; Wang, K.; Luo, D. Cellular Mechanotransduction in Health and Diseases: From Molecular Mechanism to Therapeutic Targets. Signal Transduct. Target. Ther. 2023, 8, 282. [Google Scholar] [CrossRef]
- Gao, Y.; Li, J.; Cheng, W.; Diao, T.; Liu, H.; Bo, Y.; Liu, C.; Zhou, W.; Chen, M.; Zhang, Y.; et al. Cross-Tissue Human Fibroblast Atlas Reveals Myofibroblast Subtypes with Distinct Roles in Immune Modulation. Cancer Cell 2024, 42, 1764–1783.e10. [Google Scholar] [CrossRef] [PubMed]
- Olazaba, O.L.; Farrow, J.; Monkkonen, T. Fibroblast Heterogeneity and Functions: Insights from Single-Cell Sequencing in Wound Healing, Breast Cancer, Ovarian Cancer and Melanoma. Front. Genet. 2024, 15, 1304853. [Google Scholar] [CrossRef] [PubMed]
- Tsukui, T.; Sheppard, D. Stromal Heterogeneity in the Adult Lung Delineated by Single-Cell Genomics. Am. J. Physiol.-Cell Physiol. 2025, 328, C1964–C1972. [Google Scholar] [CrossRef]
- Liu, X.; Dai, K.; Zhang, X.; Huang, G.; Lynn, H.; Rabata, A.; Liang, J.; Noble, P.W.; Jiang, D. Multiple Fibroblast Subtypes Contribute to Matrix Deposition in Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2023, 69, 45–56. [Google Scholar] [CrossRef]
- Korsunsky, I.; Wei, K.; Pohin, M.; Kim, E.Y.; Barone, F.; Major, T.; Taylor, E.; Ravindran, R.; Kemble, S.; Watts, G.F.M.; et al. Cross-Tissue, Single-Cell Stromal Atlas Identifies Shared Pathological Fibroblast Phenotypes in Four Chronic Inflammatory Diseases. Med 2022, 3, 481–518.e14. [Google Scholar] [CrossRef]
- Chianese, M.; Screm, G.; Salton, F.; Confalonieri, P.; Trotta, L.; Barbieri, M.; Ruggero, L.; Mari, M.; Reccardini, N.; Geri, P.; et al. Pirfenidone and Nintedanib in Pulmonary Fibrosis: Lights and Shadows. Pharmaceuticals 2024, 17, 709. [Google Scholar] [CrossRef] [PubMed]
- Mackintosh, J.A.; Keir, G.; Troy, L.K.; Holland, A.E.; Grainge, C.; Chambers, D.C.; Sandford, D.; Jo, H.E.; Glaspole, I.; Wilsher, M.; et al. Treatment of Idiopathic Pulmonary Fibrosis and Progressive Pulmonary Fibrosis: A Position Statement from the Thoracic Society of Australia and New Zealand 2023 Revision. Respirology 2024, 29, 105–135. [Google Scholar] [CrossRef] [PubMed]
- Abedini, A.; Levinsohn, J.; Klötzer, K.A.; Dumoulin, B.; Ma, Z.; Frederick, J.; Dhillon, P.; Balzer, M.S.; Shrestha, R.; Liu, H.; et al. Single-Cell Multi-Omic and Spatial Profiling of Human Kidneys Implicates the Fibrotic Microenvironment in Kidney Disease Progression. Nat. Genet. 2024, 56, 1712–1724. [Google Scholar] [CrossRef]
- Kuppe, C.; Ibrahim, M.M.; Kranz, J.; Zhang, X.; Ziegler, S.; Perales-Patón, J.; Jansen, J.; Reimer, K.C.; Smith, J.R.; Dobie, R.; et al. Decoding Myofibroblast Origins in Human Kidney Fibrosis. Nature 2021, 589, 281–286. [Google Scholar] [CrossRef]
- Neelisetty, S.; Alford, C.; Reynolds, K.; Woodbury, L.; Nlandu-khodo, S.; Yang, H.; Fogo, A.B.; Hao, C.-M.; Harris, R.C.; Zent, R.; et al. Renal Fibrosis Is Not Reduced by Blocking Transforming Growth Factor-β Signaling in Matrix-Producing Interstitial Cells. Kidney Int. 2015, 88, 503–514. [Google Scholar] [CrossRef]
- Reiss, A.B.; Jacob, B.; Zubair, A.; Srivastava, A.; Johnson, M.; Leon, J.D. Fibrosis in Chronic Kidney Disease: Pathophysiology and Therapeutic Targets. J. Clin. Med. 2024, 13, 1881. [Google Scholar] [CrossRef]
- Morgado-Pascual, J.L.; Suarez-Alvarez, B.; Marchant, V.; Basantes, P.; Tharaux, P.-L.; Ortiz, A.; Lopez-Larrea, C.; Ruiz-Ortega, M.; Rayego-Mateos, S. Type IV Collagen and SOX9 Are Molecular Targets of BET Inhibition in Experimental Glomerulosclerosis. Int. J. Mol. Sci. 2022, 24, 486. [Google Scholar] [CrossRef]
- Mu, Y.; Liu, J.; Wu, Q.; Wang, B.; Hu, T.; Li, Y.; Yan, X.; Ma, L.; Tan, Z. A Dual Avβ1/Avβ6 Integrin Inhibitor Bexotegrast (PLN-74809) Ameliorates Organ Injury and Fibrogenesis in Fibrotic Kidney Disease. Eur. J. Pharmacol. 2024, 983, 176983. [Google Scholar] [CrossRef]
- Gao, C.; He, J.; Wang, Y.; Shao, G.; Lin, S.; Liu, J.; Ren, C.; Quan, Y.; Ying, Y.; Li, M.; et al. The Role of FAK in Renal Collecting Duct in the Progression from Acute Kidney Injury to Chronic Kidney Disease. Transl. Res. 2025, 282, 41–55. [Google Scholar] [CrossRef]
- Savary, G.; Dewaeles, E.; Diazzi, S.; Buscot, M.; Nottet, N.; Fassy, J.; Courcot, E.; Henaoui, I.-S.; Lemaire, J.; Martis, N.; et al. The Long Noncoding RNA DNM3OS Is a Reservoir of FibromiRs with Major Functions in Lung Fibroblast Response to TGF-β and Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2019, 200, 184–198. [Google Scholar] [CrossRef]
- Lv, H.; Qian, X.; Tao, Z.; Shu, J.; Shi, D.; Yu, J.; Fan, G.; Qian, Q.; Shen, L.; Lu, B. HOXA5-Induced lncRNA DNM3OS Promotes Human Embryo Lung Fibroblast Fibrosis via Recruiting EZH2 to Epigenetically Suppress TSC2 Expression. J. Thorac. Dis. 2024, 16, 1234–1246. [Google Scholar] [CrossRef] [PubMed]
- Bakir, B.; Chiarella, A.M.; Pitarresi, J.R.; Rustgi, A.K. EMT, MET, Plasticity, and Tumor Metastasis. Trends Cell Biol. 2020, 30, 764–776. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, Z.; Wang, C.; Miao, J.; Zhou, S.; Ren, Q.; Jia, N.; Zhou, L.; Liu, Y. Kidney Tubular Epithelial Cells Control Interstitial Fibroblast Fate by Releasing TNFAIP8-Encapsulated Exosomes. Cell Death Dis. 2023, 14, 672. [Google Scholar] [CrossRef] [PubMed]
- Strunz, M.; Simon, L.M.; Ansari, M.; Kathiriya, J.J.; Angelidis, I.; Mayr, C.H.; Tsidiridis, G.; Lange, M.; Mattner, L.F.; Yee, M.; et al. Alveolar Regeneration through a Krt8+ Transitional Stem Cell State That Persists in Human Lung Fibrosis. Nat. Commun. 2020, 11, 3559. [Google Scholar] [CrossRef]
- Rockey, D.C.; Friedman, S.L. Fibrosis Regression After Eradication of Hepatitis C Virus: From Bench to Bedside. Gastroenterology 2021, 160, 1502–1520.e1. [Google Scholar] [CrossRef]
- Kröger, N.; Zabelina, T.; Alchalby, H.; Stübig, T.; Wolschke, C.; Ayuk, F.; von Hünerbein, N.; Kvasnicka, H.-M.; Thiele, J.; Kreipe, H.-H.; et al. Dynamic of Bone Marrow Fibrosis Regression Predicts Survival After Allogeneic Stem Cell Transplantation for Myelofibrosis. Biol. Blood Marrow Transplant. 2014, 20, 812–815. [Google Scholar] [CrossRef] [PubMed]
- Pizzi, M.; Silver, R.T.; Barel, A.; Orazi, A. Recombinant Interferon-α in Myelofibrosis Reduces Bone Marrow Fibrosis, Improves Its Morphology and Is Associated with Clinical Response. Mod. Pathol. 2015, 28, 1315–1323. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.T.; Verstovsek, S.; Gupta, V.; Platzbecker, U.; Devos, T.; Kiladjian, J.; McLornan, D.P.; Perkins, A.; Fox, M.L.; McMullin, M.F.; et al. Changes in Bone Marrow Fibrosis During Momelotinib or Ruxolitinib Therapy Do Not Correlate with Efficacy Outcomes in Patients with Myelofibrosis. eJHaem 2024, 5, 105–116. [Google Scholar] [CrossRef]
- Wang, C.; Li, S.-W.; Zhong, X.; Liu, B.-C.; Lv, L.-L. An Update on Renal Fibrosis: From Mechanisms to Therapeutic Strategies with a Focus on Extracellular Vesicles. Kidney Res. Clin. Pract. 2023, 42, 174–187. [Google Scholar] [CrossRef]
- Habermann, A.C.; Gutierrez, A.J.; Bui, L.T.; Yahn, S.L.; Winters, N.I.; Calvi, C.L.; Peter, L.; Chung, M.-I.; Taylor, C.J.; Jetter, C.; et al. Single-Cell RNA Sequencing Reveals Profibrotic Roles of Distinct Epithelial and Mesenchymal Lineages in Pulmonary Fibrosis. Sci. Adv. 2020, 6, eaba1972. [Google Scholar] [CrossRef]
- Xu, Y.; Mizuno, T.; Sridharan, A.; Du, Y.; Guo, M.; Tang, J.; Wikenheiser-Brokamp, K.A.; Perl, A.-K.T.; Funari, V.A.; Gokey, J.J.; et al. Single-Cell RNA Sequencing Identifies Diverse Roles of Epithelial Cells in Idiopathic Pulmonary Fibrosis. JCI Insight 2016, 1, e90558. [Google Scholar] [CrossRef]
- Huang, Y.; Hong, W.; Wei, X. The Molecular Mechanisms and Therapeutic Strategies of EMT in Tumor Progression and Metastasis. J. Hematol. Oncol. 2022, 15, 129. [Google Scholar] [CrossRef]
- Zeisberg, M.; Neilson, E.G. Biomarkers for Epithelial-Mesenchymal Transitions. J. Clin. Investig. 2009, 119, 1429–1437. [Google Scholar] [CrossRef]
- Khalili-Tanha, G.; Radisky, E.S.; Radisky, D.C.; Shoari, A. Matrix Metalloproteinase-Driven Epithelial-Mesenchymal Transition: Implications in Health and Disease. J. Transl. Med. 2025, 23, 436. [Google Scholar] [CrossRef] [PubMed]
- Celià-Terrassa, T.; Kang, Y. How Important Is EMT for Cancer Metastasis? PLoS Biol. 2024, 22, e3002487. [Google Scholar] [CrossRef]
- Thompson, E.W.; Redfern, A.D.; Brabletz, S.; Berx, G.; Agarwal, V.; Ganesh, K.; Huang, R.Y.; Ishay-Ronen, D.; Savagner, P.; Sheng, G.; et al. EMT and Cancer: What Clinicians Should Know. Nat. Rev. Clin. Oncol. 2025, 22, 711–733. [Google Scholar] [CrossRef]
- Brabletz, S.; Schuhwerk, H.; Brabletz, T.; Stemmler, M.P. Dynamic EMT: A Multi-Tool for Tumor Progression. EMBO J. 2021, 40, EMBJ2021108647. [Google Scholar] [CrossRef]
- Shi, Z.-D.; Pang, K.; Wu, Z.-X.; Dong, Y.; Hao, L.; Qin, J.-X.; Wang, W.; Chen, Z.-S.; Han, C.-H. Tumor Cell Plasticity in Targeted Therapy-Induced Resistance: Mechanisms and New Strategies. Signal Transduct. Target. Ther. 2023, 8, 113. [Google Scholar] [CrossRef]
- Lloyd, S.M.; He, Y. Exploring Extracellular Matrix Crosslinking as a Therapeutic Approach to Fibrosis. Cells 2024, 13, 438. [Google Scholar] [CrossRef]
- Scott, A.K.; Rafuse, M.; Neu, C.P. Mechanically Induced Alterations in Chromatin Architecture Guide the Balance between Cell Plasticity and Mechanical Memory. Front. Cell Dev. Biol. 2023, 11, 1084759. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Brown, K.K.; Collard, H.R.; Cottin, V.; Gibson, K.F.; Kaner, R.J.; Lederer, D.J.; Martinez, F.J.; Noble, P.W.; Song, J.W.; et al. Efficacy of Simtuzumab Versus Placebo in Patients with Idiopathic Pulmonary Fibrosis: A Randomised, Double-Blind, Controlled, Phase 2 Trial. Lancet Respir. Med. 2017, 5, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Abdelmalek, M.F.; Caldwell, S.; Shiffman, M.L.; Diehl, A.M.; Ghalib, R.; Lawitz, E.J.; Rockey, D.C.; Schall, R.A.; Jia, C.; et al. Simtuzumab Is Ineffective for Patients with Bridging Fibrosis or Compensated Cirrhosis Caused by Nonalcoholic Steatohepatitis. Gastroenterology 2018, 155, 1140–1153. [Google Scholar] [CrossRef]
- Harrison, S.A.; Bedossa, P.; Guy, C.D.; Schattenberg, J.M.; Loomba, R.; Taub, R.; Labriola, D.; Moussa, S.E.; Neff, G.W.; Rinella, M.E.; et al. A Phase 3, Randomized, Controlled Trial of Resmetirom in NASH with Liver Fibrosis. N. Engl. J. Med. 2024, 390, 497–509. [Google Scholar] [CrossRef]
- Wagner, D.E.; Alsafadi, H.N.; Mitash, N.; Justet, A.; Hu, Q.; Pineda, R.; Staab-Weijnitz, C.; Korfei, M.; Gvazava, N.; Wannemo, K.; et al. Inhibition of Epithelial Cell YAP-TEAD/LOX Signaling Attenuates Pulmonary Fibrosis in Preclinical Models. Nat. Commun. 2025, 16, 7099. [Google Scholar] [CrossRef]
- Tjin, G.; White, E.S.; Faiz, A.; Sicard, D.; Tschumperlin, D.J.; Mahar, A.; Kable, E.P.W.; Burgess, J.K. Lysyl Oxidases Regulate Fibrillar Collagen Remodelling in Idiopathic Pulmonary Fibrosis. Dis. Model. Mech. 2017, 10, 1301–1312. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Cui, Y.; Qiu, J.; Zhang, X.; Gao, Y.; Shang, Z.; Huang, L. Exploring the Therapeutic Potential of TGF-β Inhibitors for Liver Fibrosis: Targeting Multiple Signaling Pathways. J. Clin. Transl. Hepatol. 2025, 13, 588–598. [Google Scholar] [CrossRef]
- Bastos, V.A.F.; Fujimura, P.T.; de Souza, A.G.; Vaz, E.R.; Saito, N.; Sabino-Silva, R.; Goulart, L.R.; Cunha, T.M. Activin A Inhibitory Peptides Suppress Fibrotic Pathways by Targeting Epithelial–Mesenchymal Transition and Fibroblast–Myofibroblast Transformation in Idiopathic Pulmonary Fibrosis. Int. J. Mol. Sci. 2025, 26, 2705. [Google Scholar] [CrossRef] [PubMed]
- Kou, M.; Jiao, Y.; Li, Z.; Wei, B.; Li, Y.; Cai, Y.; Wei, W. Real-World Safety and Effectiveness of Pirfenidone and Nintedanib in the Treatment of Idiopathic Pulmonary Fibrosis: A Systematic Review and Meta-Analysis. Eur. J. Clin. Pharmacol. 2024, 80, 1445–1460. [Google Scholar] [CrossRef]
- Xu, M.; Xu, C.; Wang, R.; Tang, Q.; Zhou, Q.; Wu, W.; Wan, X.; Mo, H.; Pan, J.; Wang, S. Treating Human Cancer by Targeting EZH2. Genes Dis. 2025, 12, 101313. [Google Scholar] [CrossRef]
- Saiz, M.L.; Lozano-Chamizo, L.; Florez, A.B.; Marciello, M.; Diaz-Bulnes, P.; Corte-Iglesias, V.; Bernet, C.R.; Rodrigues-Diez, R.R.; Martin-Martin, C.; Rodriguez-Santamaria, M.; et al. BET Inhibitor Nanotherapy Halts Kidney Damage and Reduces Chronic Kidney Disease Progression After Ischemia-Reperfusion Injury. Biomed. Pharmacother. 2024, 174, 116492. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Huang, H.; Wei, X.; Li, H.; Li, J.; Xie, B.; Yang, Y.; Fang, X.; Wang, L.; Zhang, X.; et al. Clinical Investigation on Nebulized Human Umbilical Cord MSC-Derived Extracellular Vesicles for Pulmonary Fibrosis Treatment. Signal Transduct. Target. Ther. 2025, 10, 179. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Jin, J.; Fu, Z.; Wang, G.; Lei, X.; Xu, J.; Wang, J. Extracellular Vesicle-Based Drug Overview: Research Landscape, Quality Control and Nonclinical Evaluation Strategies. Signal Transduct. Target. Ther. 2025, 10, 255. [Google Scholar] [CrossRef]
- Wang, J.; Shi, Y.; Su, Y.; Pang, C.; Yang, Y.; Wang, W. Research Advances of Extracellular Vesicles in Lung Diseases. Cell Transplant. 2025, 34, 09636897251362031. [Google Scholar] [CrossRef]

| Aspect | Fibrosis | Cancer |
|---|---|---|
| Type of EMT | Type 2 | Type 3 |
| Stimulus | Chronic injury, persistent inflammation | Oncogenic signaling, tumor microenvironment |
| Phenotype | Myofibroblastic, ECM-secreting | Invasive, migratory, chemoresistant |
| State Stability | Relatively stable | Highly plastic and reversible |
| Functional Role | Pathological tissue remodeling and wound healing | Tumor progression, metastasis, and therapeutic escape |
| Reversibility (MET) | Limited | Frequent (especially during metastatic colonization) |
| Associated Stem Cells | Poorly characterized | EMT promotes CSC-like features |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bastos, V.A.F.; Souza, A.G.d.; Guedes, V.C.S.; Cunha, T.M. Shared and Context-Specific Mechanisms of EMT and Cellular Plasticity in Cancer and Fibrotic Diseases. Int. J. Mol. Sci. 2025, 26, 9476. https://doi.org/10.3390/ijms26199476
Bastos VAF, Souza AGd, Guedes VCS, Cunha TM. Shared and Context-Specific Mechanisms of EMT and Cellular Plasticity in Cancer and Fibrotic Diseases. International Journal of Molecular Sciences. 2025; 26(19):9476. https://doi.org/10.3390/ijms26199476
Chicago/Turabian StyleBastos, Victor Alexandre F., Aline Gomes de Souza, Virginia C. Silvestrini Guedes, and Thúlio M. Cunha. 2025. "Shared and Context-Specific Mechanisms of EMT and Cellular Plasticity in Cancer and Fibrotic Diseases" International Journal of Molecular Sciences 26, no. 19: 9476. https://doi.org/10.3390/ijms26199476
APA StyleBastos, V. A. F., Souza, A. G. d., Guedes, V. C. S., & Cunha, T. M. (2025). Shared and Context-Specific Mechanisms of EMT and Cellular Plasticity in Cancer and Fibrotic Diseases. International Journal of Molecular Sciences, 26(19), 9476. https://doi.org/10.3390/ijms26199476







