Chrysin as a Bioactive Scaffold: Advances in Synthesis and Pharmacological Evaluation
Abstract
1. Introduction
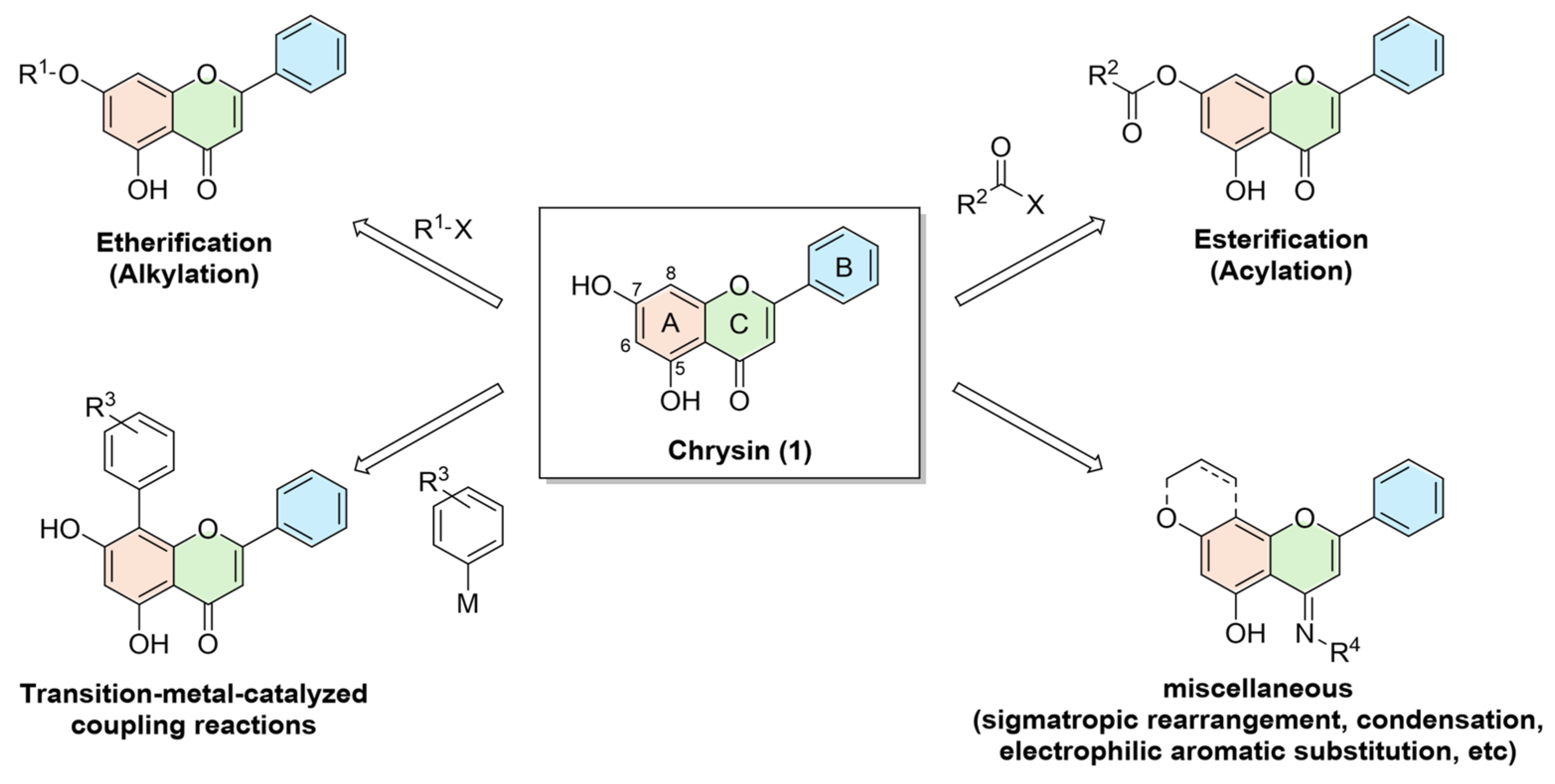
2. Synthetic Approaches Toward Structurally Diverse Chrysin Derivatives
2.1. Etherification (Alkylation)
2.2. Esterification (Acylation)
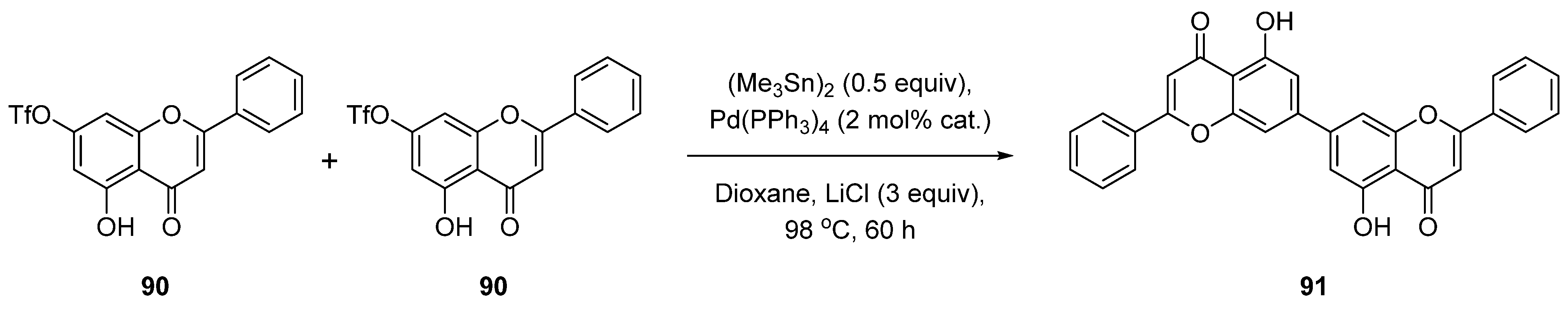
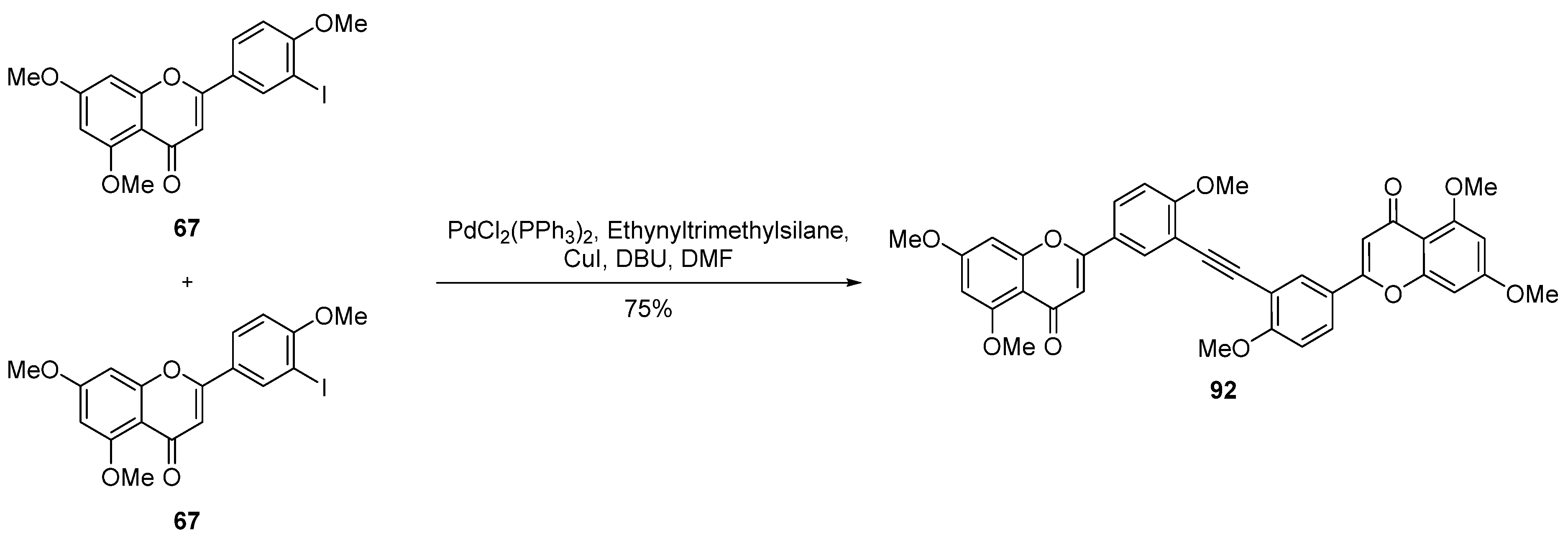
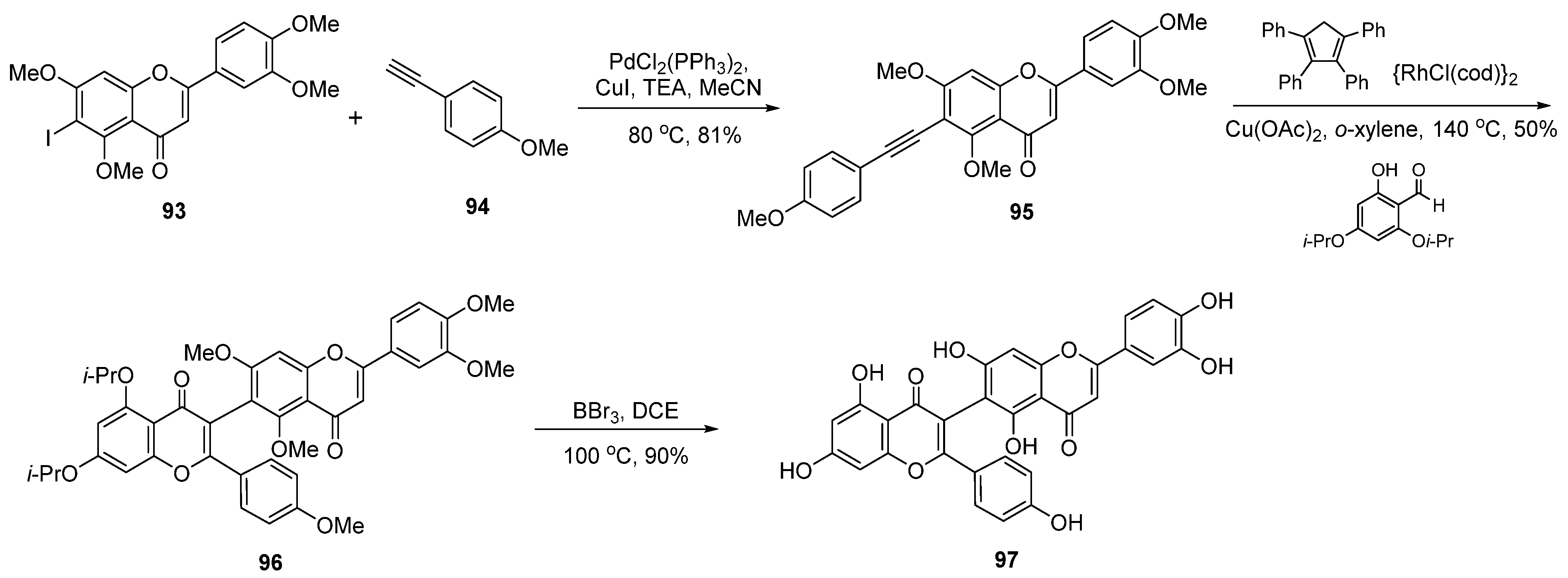
2.3. Transition-Metal-Mediated Coupling Reactions
2.4. Miscellaneous Transformations
3. Chrysin Derivatives and Their Biological Activities
3.1. Antitumor Activity
3.2. Anti-Inflammatory Activity
3.3. Anti-Microbial Activity
3.4. Antioxidant Activity
3.5. Anti-Diabetic Activity
3.6. Other Activities
3.7. Promising Chrysin Derivatives Across Therapeutic Domains
4. Research Progress on the Synthesis of Chrysin Derivatives
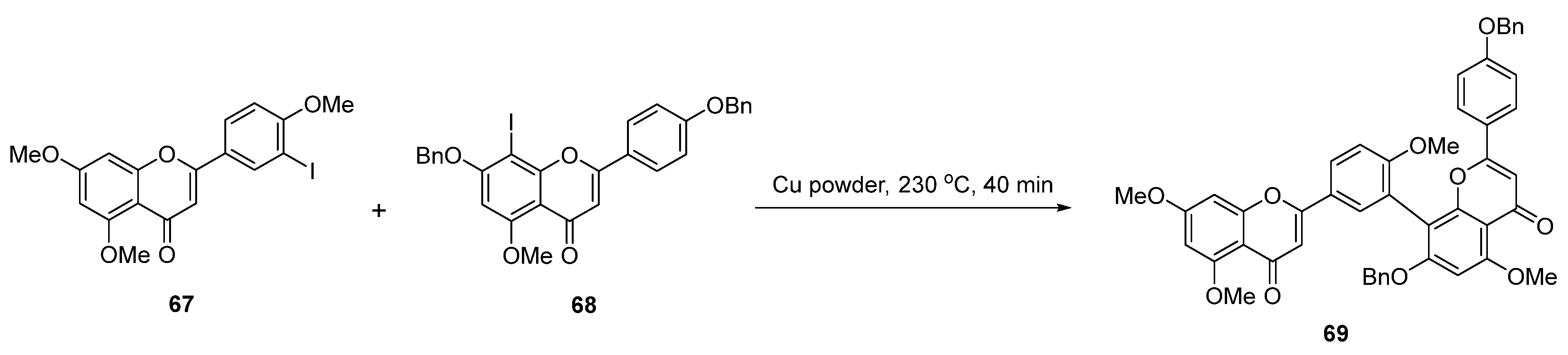
C-C Bond Formation via Transition-Metal-Mediated Coupling Reactions
5. Perspective
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| GI50 | 50% growth inhibition concentration |
| HDAC | Histone deacetylase |
| PARP | Poly(ADP-ribose) polymerase 1 |
| TLR4 | Toll-like receptor 4 |
| COX-2 | Cyclooxygenase-2 |
| LPS | Lipopolysaccharide |
| C. albicans | Candida albicans |
| E. coli | Escherichia coli |
| P. aeruginosa | Pseudomonas aeruginosa |
| S. aureus | Staphylococcus aureus |
| S. cerevisiae | Saccharomyces cerevisiae |
| S. pyogenes | Streptococcus pyogenes |
| ABTS | 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| AChE | Acetylcholinesterase |
| BuChE | Butyrylcholinesterase |
| K. pneumoniae | Klebsiella pneumoniae |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| FRAP | Ferric Reducing Antioxidant Power |
| NAFLD | Non-alcoholic fatty liver disease |
| PPARγ | Peroxisome Proliferator-Activated Receptor gamma |
| iNOS | Inducible nitric oxide synthase |
References
- Kılıç, C.S.; Kışla, M.M.; Amasya, G.; Şengel-Türk, C.T.; Ateş Alagöz, Z.; Gençler Özkan, A.M.; Ateş, İ.; Gümüşok, S.; Herrera-Bravo, J.; Sharifi-Rad, J.; et al. Rhoifolin: A Promising Flavonoid with Cytotoxic and Anticancer Properties—Molecular Mechanisms and Therapeutic Potential. EXCLI J. 2025, 24, 289–320. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Martinez, E.J.; Flores-Hernández, F.Y.; Salazar-Montes, A.M.; Nario-Chaidez, H.F.; Hernández-Ortega, L.D. Quercetin, a Flavonoid with Great Pharmacological Capacity. Molecules 2024, 29, 1000. [Google Scholar] [CrossRef] [PubMed]
- Rana, J.N.; Mumtaz, S. Prunin: An Emerging Anticancer Flavonoid. Int. J. Mol. Sci. 2025, 26, 2678. [Google Scholar] [CrossRef] [PubMed]
- Talebi, M.; Talebi, M.; Farkhondeh, T.; Simal-Gandara, J.; Kopustinskiene, D.M.; Bernatoniene, J.; Samarghandian, S. Emerging Cellular and Molecular Mechanisms Underlying Anticancer Indications of Chrysin. Cancer Cell Int. 2021, 21, 214. [Google Scholar] [CrossRef]
- Kurkiewicz, M.; Moździerz, A.; Rzepecka-Stojko, A.; Stojko, J. Chrysin: A Comprehensive Review of Its Pharmacological Properties and Therapeutic Potential. Pharmaceuticals 2025, 18, 1162. [Google Scholar] [CrossRef]
- Mani, R.; Natesan, V. Chrysin: Sources, Beneficial Pharmacological Activities, and Molecular Mechanism of Action. Phytochemistry 2018, 145, 187–196. [Google Scholar] [CrossRef]
- Stompor-Gorący, M.; Bajek-Bil, A.; Machaczka, M. Chrysin: Perspectives on Contemporary Status and Future Possibilities as Pro-Health Agent. Nutrients 2021, 13, 2038. [Google Scholar] [CrossRef]
- Kasala, E.R.; Bodduluru, L.N.; Madana, R.M.; Gogoi, R.; Barua, C.C. Chemopreventive and Therapeutic Potential of Chrysin in Cancer: Mechanistic Perspectives. Toxicol. Lett. 2015, 233, 214–225. [Google Scholar] [CrossRef]
- Naz, S.; Imran, M.; Rauf, A.; Orhan, I.E.; Shariati, M.A.; Iahtisham-Ul-Haq; IqraYasmin; Shahbaz, M.; Qaisrani, T.B.; Shah, Z.A.; et al. Chrysin: Pharmacological and Therapeutic Properties. Life Sci. 2019, 235, 116797. [Google Scholar] [CrossRef]
- Mishra, A.; Mishra, P.S.; Bandopadhyay, R.; Khurana, N.; Angelopoulou, E.; Paudel, Y.N.; Piperi, C. Neuroprotective Potential of Chrysin: Mechanistic Insights and Therapeutic Potential for Neurological Disorders. Molecules 2021, 26, 6456. [Google Scholar] [CrossRef]
- Shamsudin, N.F.; Ahmed, Q.U.; Mahmood, S.; Ali Shah, S.A.; Khatib, A.; Mukhtar, S.; Alsharif, M.A.; Parveen, H.; Zakaria, Z.A. Antibacterial Effects of Flavonoids and Their Structure-Activity Relationship Study: A Comparative Interpretation. Molecules 2022, 27, 1149. [Google Scholar] [CrossRef]
- Garg, A.; Chaturvedi, S. A Comprehensive Review on Chrysin: Emphasis on Molecular Targets, Pharmacological Actions and Bio-Pharmaceutical Aspects. Curr. Drug Targets 2022, 23, 420–436. [Google Scholar] [CrossRef]
- Shahbaz, M.; Naeem, H.; Imran, M.; Ul Hassan, H.; Alsagaby, S.A.; Al Abdulmonem, W.; Waqar, A.B.; Ghorab, A.H.; Abdelgawad, M.A.; Ghoneim, M.M.; et al. Chrysin a Promising Anticancer Agent: Recent Perspectives. Int. J. Food Prop. 2023, 26, 2294–2337. [Google Scholar] [CrossRef]
- Gao, S.; Siddiqui, N.; Etim, I.; Du, T.; Zhang, Y.; Liang, D. Developing Nutritional Component Chrysin as a Therapeutic Agent: Bioavailability and Pharmacokinetics Consideration, and ADME Mechanisms. Biomed. Pharmacother. 2021, 142, 112080. [Google Scholar] [CrossRef] [PubMed]
- Walle, T.; Otake, Y.; Brubaker, J.A.; Walle, U.K.; Halushka, P.V. Disposition and Metabolism of the Flavonoid Chrysin in Normal Volunteers. Br. J. Clin. Pharmacol. 2001, 51, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, X.; Zhao, R.; Yang, M.; Liu, W.; Dai, Q.; Bao, X.; Chen, Y.; Ma, J. The Amorphous Solid Dispersion of Chrysin in Plasdone® S630 Demonstrates Improved Oral Bioavailability and Antihyperlipidemic Performance in Rats. Pharmaceutics 2023, 15, 2378. [Google Scholar] [CrossRef]
- Yang, N.; Sun, R.; Liao, X.; Aa, J.; Wang, G. UDP-Glucuronosyltransferases (UGTs) and Their Related Metabolic Cross-Talk with Internal Homeostasis: A Systematic Review of UGT Isoforms for Precision Medicine. Pharmacol. Res. 2017, 121, 169–183. [Google Scholar] [CrossRef]
- Quan, E.; Wang, H.; Dong, D.; Zhang, X.; Wu, B. Characterization of Chrysin Glucuronidation in UGT1A1-Overexpressing HeLa Cells: Elucidating the Transporters Responsible for Efflux of Glucuronide. Drug Metab. Dispos. 2015, 43, 433–443. [Google Scholar] [CrossRef]
- Kumar, P.A. Simple Synthetic Processes for Chrysin, Norwogonin and Their Derivatives. Int. J. Res. Ayurveda Pharm. 2010, 1, 225–233. [Google Scholar]
- Zhu, Z.-Y.; Wang, W.-X.; Wang, Z.; Chen, L.-J.; Zhang, J.-Y.; Liu, X.; Wu, S.; Zhang, Y. Synthesis and Antitumor Activity Evaluation of Chrysin Derivatives. Eur. J. Med. Chem. 2014, 75, 297–300. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, X.; Qu, L.; Wang, J.; Yuan, J.; Chen, S.; Li, X.; Qu, C. Ultrasonic-Assisted Synthesis of Chrysin Derivatives Linked with 1,2,3-Triazoles by 1,3-Dipolar Cycloaddition Reaction. Ultrason. Sonochem. 2011, 18, 527–533. [Google Scholar] [CrossRef]
- Mayer, S.; Keglevich, P.; Ábrányi-Balogh, P.; Szigetvári, Á.; Dékány, M.; Szántay, C.; Hazai, L. Synthesis and In Vitro Anticancer Evaluation of Novel Chrysin and 7-Aminochrysin Derivatives. Molecules 2020, 25, 888. [Google Scholar] [CrossRef]
- Chen, N.; Wang, R.; Lu, L.-J.; Yan, L.-J.; Bai, L.-L.; Fu, Y.; Wang, Y.; Peng, D.-Q.; Chen, X.; Wang, C.-H.; et al. Synthesis of Chrysin Derivatives and Screening of Antitumor Activity. J. Asian Nat. Prod. Res. 2020, 22, 444–451. [Google Scholar] [CrossRef]
- Al-Oudat, B.A.; Alqudah, M.A.; Audat, S.A.; Al-Balas, Q.A.; El-Elimat, T.; Hassan, M.A.; Frhat, I.N.; Azaizeh, M.M. Design, Synthesis, and Biologic Evaluation of Novel Chrysin Derivatives as Cytotoxic Agents and Caspase-3/7 Activators. Drug Des. Devel. Ther. 2019, 13, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Talebi, M.; Talebi, M.; Farkhondeh, T.; Kopustinskiene, D.M.; Simal-Gandara, J.; Bernatoniene, J.; Samarghandian, S. An Updated Review on the Versatile Role of Chrysin in Neurological Diseases: Chemistry, Pharmacology, and Drug Delivery Approaches. Biomed. Pharmacother. 2021, 141, 111906. [Google Scholar] [CrossRef]
- Zou, P.; Otero, P.; Mejuto, J.C.; Simal-Gandara, J.; Xiao, J.; Cameselle, C.; Chen, S.; Lin, S.; Cao, H. Exploring the Mechanism of Flavonoids Modification by Dimerization Strategies and Their Potential to Enhance Biological Activity. Food Chem. 2025, 467, 142266. [Google Scholar] [CrossRef] [PubMed]
- Selepe, M.A.; Mthembu, S.T.; Sonopo, M.S. Total Synthesis of Isoflavonoids. Nat. Prod. Rep. 2025, 42, 540–591. [Google Scholar] [CrossRef] [PubMed]
- Caleffi, G.S.; Demidoff, F.C.; Nájera, C.; Costa, P.R.R. Asymmetric Hydrogenation and Transfer Hydrogenation in the Enantioselective Synthesis of Flavonoids. Org. Chem. Front. 2022, 9, 1165–1194. [Google Scholar] [CrossRef]
- Santos, C.M.M.; Silva, A.M.S. Transition Metal-Catalyzed Transformations of Chalcones. Chem. Rec. 2024, 24, e202400060. [Google Scholar] [CrossRef]
- Noor, R.; Zahoor, A.F.; Irfan, M.; Hussain, S.M.; Ahmad, S.; Irfan, A.; Kotwica-Mojzych, K.; Mojzych, M. Transition Metal Catalyzed Hiyama Cross-Coupling: Recent Methodology Developments and Synthetic Applications. Molecules 2022, 27, 5654. [Google Scholar] [CrossRef]
- Daskiewicz, J.-B.; Bayet, C.; Barron, D. Rearrangement of 5- O -Prenyl Flavones: A Regioselective Access to 6- C -(1,1-Dimethylallyl)- and 8- C -(3,3-Dimethylallyl)-Flavones. Tetrahedron Lett. 2001, 42, 7241–7244. [Google Scholar] [CrossRef]
- Kolling, D.; Stierhof, M.; Lasch, C.; Myronovskyi, M.; Luzhetskyy, A. A Promiscuous Halogenase for the Derivatization of Flavonoids. Molecules 2021, 26, 6220. [Google Scholar] [CrossRef] [PubMed]
- Marzec, E.; Świtalska, M.; Winiewska-Szajewska, M.; Wójcik, J.; Wietrzyk, J.; Maciejewska, A.M.; Poznański, J.; Mieczkowski, A. The Halogenation of Natural Flavonoids, Baicalein and Chrysin, Enhances Their Affinity to Human Protein Kinase CK2. IUBMB Life 2020, 72, 1250–1261. [Google Scholar] [CrossRef] [PubMed]
- Daskiewicz, J.-B.; Bayet, C.; Barron, D. Regioselective Syntheses of 6-(1,1-Dimethylallyl)- and 8-(3,3-Dimethylallyl) Chrysins. Tetrahedron 2002, 58, 3589–3595. [Google Scholar] [CrossRef]
- Walencik, P.K.; Choińska, R.; Gołębiewska, E.; Kalinowska, M. Metal–Flavonoid Interactions—From Simple Complexes to Advanced Systems. Molecules 2024, 29, 2573. [Google Scholar] [CrossRef]
- Mihajlović, L.E.; Trif, M.; Živković, M.B. Metal Complexes with Hydroxyflavones: A Study of Anticancer and Antimicrobial Activities. Inorganics 2025, 13, 250. [Google Scholar] [CrossRef]
- Somsakeesit, L.; Senawong, T.; Senawong, G.; Kumboonma, P.; Samankul, A.; Namwan, N.; Yenjai, C.; Phaosiri, C. Evaluation and Molecular Docking Study of Two Flavonoids from Oroxylum indicum (L.) Kurz and Their Semi-Synthetic Derivatives as Histone Deacetylase Inhibitors. J. Nat. Med. 2024, 78, 236–245. [Google Scholar] [CrossRef]
- Wang, H.-J.; Zhou, Y.-Y.; Liu, X.-L.; Zhang, W.-H.; Chen, S.; Liu, X.-W.; Zhou, Y. Regioselective Synthesis and Evaluation of 2-Amino 3-Cyano Chromene-Chrysin Hybrids as Potential Anticancer Agents. Bioorg. Med. Chem. Lett. 2020, 30, 127087. [Google Scholar] [CrossRef]
- Al-Oudat, B.A.; Ramapuram, H.; Malla, S.; Audat, S.A.; Hussein, N.; Len, J.M.; Kumari, S.; Bedi, M.F.; Ashby, C.R.; Tiwari, A.K. Novel Chrysin-De-Allyl PAC-1 Hybrid Analogues as Anticancer Compounds: Design, Synthesis, and Biological Evaluation. Molecules 2020, 25, 3063. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, W.; Liu, Z.; Li, H.; Liu, Y.; Liu, X.; Han, Z.; He, J.; Zeng, Y.; Guo, Y.; et al. Design, Synthesis, Antitumor Activity and Ct-DNA Binding Study of Photosensitive Drugs Based on Porphyrin Framework. Int. J. Biol. Macromol. 2023, 230, 123147. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, Q.; Zhang, L.; Yu, W.; Long, H.; He, J.; Liu, Y. Novel Photosensitizing Properties of Porphyrin–Chrysin Derivatives with Antitumor Activity in Vitro. J. Chem. Res. 2020, 44, 494–504. [Google Scholar] [CrossRef]
- Yan, X.; Song, J.; Yu, M.; Sun, H.-L.; Hao, H. Synthesis of Flavonoids Nitrogen Mustard Derivatives and Study on Their Antitumor Activity in Vitro. Bioorganic Chem. 2020, 96, 103613. [Google Scholar] [CrossRef]
- Németh-Rieder, A.; Keglevich, P.; Hunyadi, A.; Latif, A.D.; Zupkó, I.; Hazai, L. Synthesis and In Vitro Anticancer Evaluation of Flavone—1,2,3-Triazole Hybrids. Molecules 2023, 28, 626. [Google Scholar] [CrossRef] [PubMed]
- Noole, V.; Krishna, T.; Godeshala, S.; Meraji, S.; Rege, K.; Reddy, C.K.; Kedika, B. Synthesis and Biological Evaluation of New 1,2,3-Triazole Derivatives of the ChrysinFlavonoid as Anticancer Agents. Anticancer Agents Med. Chem. 2021, 22, 160–168. [Google Scholar] [CrossRef]
- Luan, T.; Quan, Z.; Fang, Y.; Yang, H. Design, Synthesis and Antiproliferative Activity of Chrysin Derivatives Bearing Triazole Moieties. Chin. J. Org. Chem. 2020, 40, 440. [Google Scholar] [CrossRef]
- Mayer, S.; Nagy, N.; Keglevich, P.; Szigetvári, Á.; Dékány, M.; Szántay Junior, C.; Hazai, L. Synthesis of Novel Vindoline-Chrysin Hybrids. Chem. Biodivers. 2022, 19, e202100725. [Google Scholar] [CrossRef]
- Xue, X.-Y.; He, J.-L.; Song, M.-W.; Yu, Z.; Shuang-Liu; Lin, M.-J.; Zhang, C.-P.; Bo-Ding; Hao-Wang; Ma, Z.-H.; et al. Design, Synthesis, Bioactivity, X-Ray Crystallography, and Molecular Docking Studies of Chrysin-1,3,5-Triazine Derivatives as Anticancer Agents. Bioorganic Chem. 2025, 161, 108486. [Google Scholar] [CrossRef]
- Yu, Q.; Huang, B.; Ling, Y. Design, Synthesis, and In Vitro Anticancer Activity of Novel ChrysinDerivatives. Lett. Drug Des. Discov. 2023, 20, 854–862. [Google Scholar] [CrossRef]
- Liu, Y.-M.; Li, Y.; Xiao, J.; Zhang, Q.-Z.; Song, J.-X. Design, Synthesis, and Preliminary Biological Evaluation of Chrysin Amino Acid Derivatives That Induce Apoptosis and Suppress Cell Migration. J. Asian Nat. Prod. Res. 2020, 22, 547–561. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Q.; He, J.; Yu, W.; Xiao, J.; Guo, Y.; Zhu, X.; Liu, Y. Synthesis and Biological Evaluation of Amino Acid Derivatives Containing Chrysin That Induce Apoptosis. Nat. Prod. Res. 2021, 35, 529–538. [Google Scholar] [CrossRef]
- Kamloon, T.; Senawong, T.; Senawong, G.; Namwan, N.; Kumboonma, P.; Somsakeesit, L.; Ritchumpon, P.; Nontakitticharoen, M.; Nasomjai, P.; Phaosiri, C. Exploring Putative Histone Deacetylase Inhibitors with Antiproliferative Activity of Chrysin Derivatives. Med. Chem. Res. 2025, 34, 1308–1320. [Google Scholar] [CrossRef]
- Yang, Y.; Tong, J.; Xie, X.; Cao, H.; Fu, Y.; Luo, Y.; Liu, S.; Chen, W.; Yang, N. Design, Synthesis, and Biological Evaluation of Novel Chrysin Derivatives as Poly(ADP-Ribose) Polymerase 1 (PARP1) Inhibitors for the Treatment of Breast Cancer. Chin. J. Nat. Med. 2024, 22, 455–465. [Google Scholar] [CrossRef]
- Oggero, J.; Gasser, F.B.; Zacarías, S.M.; Burns, P.; Baravalle, M.E.; Renna, M.S.; Ortega, H.H.; Vaillard, S.E.; Vaillard, V.A. PEGylation of Chrysin Improves Its Water Solubility While Preserving the In Vitro Biological Activity. J. Agric. Food Chem. 2023, 71, 19817–19831. [Google Scholar] [CrossRef] [PubMed]
- Jangid, A.K.; Solanki, R.; Patel, S.; Medicherla, K.; Pooja, D.; Kulhari, H. Improving Anticancer Activity of Chrysin Using Tumor Microenvironment pH-Responsive and Self-Assembled Nanoparticles. ACS Omega 2022, 7, 15919–15928. [Google Scholar] [CrossRef] [PubMed]
- Taldaev, A.; Svotin, A.A.; Obukhov, S.I.; Terekhov, R.P.; Selivanova, I.A. Modification of Biopharmaceutical Parameters of Flavonoids: A Review. Front. Chem. 2025, 13, 1602967. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, S.; Emamjomeh, A.; Sharifi, F.; Zarepour, A.; Aghaabbasi, K.; Dehshahri, A.; Sepahvand, A.M.; Zarrabi, A.; Beyzaei, H.; Zahedi, M.M.; et al. Lipid-Based Delivery Systems for Flavonoids and Flavonolignans: Liposomes, Nanoemulsions, and Solid Lipid Nanoparticles. Pharmaceutics 2023, 15, 1944. [Google Scholar] [CrossRef]
- Stevens Barrón, J.C.; Chapa González, C.; Álvarez Parrilla, E.; De La Rosa, L.A. Nanoparticle-Mediated Delivery of Flavonoids: Impact on Proinflammatory Cytokine Production: A Systematic Review. Biomolecules 2023, 13, 1158. [Google Scholar] [CrossRef]
- Rana, J.N.; Gul, K.; Mumtaz, S. Isorhamnetin: Reviewing Recent Developments in Anticancer Mechanisms and Nanoformulation-Driven Delivery. Int. J. Mol. Sci. 2025, 26, 7381. [Google Scholar] [CrossRef]
- Narendar, K.; Narsimha, K.; Sharma, N.; Siva, B.; Yaladanda, N.; Sridhar, B.; Mutheneni, S.R.; Balaji, A.S.; Babu, K.S. One-Pot Multi-Component Synthesis and Biological Evaluation of Spirooxindole Carbamate Derivatives of Major Flavonoids Isolated from Oroxylum indicum: Identification of Potent Anti-Proliferative Leads Active against Hepatocellular Carcinoma. Bioorganic Chem. 2025, 163, 108712. [Google Scholar] [CrossRef]
- Feng, X.; Yu, W.; Cao, L.; Meng, F.; Cong, M. A Novel Chrysin Thiazole Derivative Polarizes Macrophages to an M1 Phenotype via Targeting TLR4. Int. Immunopharmacol. 2020, 88, 106986. [Google Scholar] [CrossRef]
- Pouramiri, B.; Seyedhosseini, S.R.; Nematollahi, M.H.; Faramarz, S.; Seyedi, F.; Ayati, A. Green Synthesis and Anticancer Evaluation of Novel Chrysin Hydrazone Derivatives. Polycycl. Aromat. Compd. 2023, 43, 176–189. [Google Scholar] [CrossRef]
- Adhikari, S.; Nath, P.; Das, A.; Datta, A.; Baildya, N.; Duttaroy, A.K.; Pathak, S. A Review on Metal Complexes and Its Anti-Cancer Activities: Recent Updates from in Vivo Studies. Biomed. Pharmacother. 2024, 171, 116211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Y.; Fang, R.; Wei, W.; Wang, Y.; Jin, J.; Yang, F.; Chen, J. Organometallic Gold(I) and Gold(III) Complexes for Lung Cancer Treatment. Front. Pharmacol. 2022, 13, 979951. [Google Scholar] [CrossRef] [PubMed]
- Break, M.K.B.; Ansari, S.A.; Katamesh, A.A.; Albadari, N.; Alshammari, M.D.; Alkahtani, H.M. Synthesis, in Vitro and in Silico Studies of a Novel Chrysin-Ferrocene Schiff Base with Potent Anticancer Activity via G1 Arrest, Caspase-Dependent Apoptosis and Inhibition of Topoisomerase II. J. Enzym. Inhib. Med. Chem. 2025, 40, 2501377. [Google Scholar] [CrossRef] [PubMed]
- Alem, M.B.; Desalegn, T.; Damena, T.; Bayle, E.A.; Koobotse, M.O.; Ngwira, K.J.; Ombito, J.O.; Zachariah, M.; Demissie, T.B. Organic–Inorganic Hybrid Salt and Mixed Ligand Cr(III) Complexes Containing the Natural Flavonoid Chrysin: Synthesis, Characterization, Computational, and Biological Studies. Front. Chem. 2023, 11, 1173604. [Google Scholar] [CrossRef]
- Rubio, A.R.; González, R.; Busto, N.; Vaquero, M.; Iglesias, A.L.; Jalón, F.A.; Espino, G.; Rodríguez, A.M.; García, B.; Manzano, B.R. Anticancer Activity of Half-Sandwich Ru, Rh and Ir Complexes with Chrysin Derived Ligands: Strong Effect of the Side Chain in the Ligand and Influence of the Metal. Pharmaceutics 2021, 13, 1540. [Google Scholar] [CrossRef]
- Huang, Y.; Zheng, D.; Bai, J.; Wang, W.; Yang, Z.; Tian, Y.; Fang, Y. The First Chrysin-Based Receptor for Anions Recognition: Experimental Investigations, DFT Studies and Its in Vitro Antitumor Activity. J. Mol. Struct. 2023, 1286, 135637. [Google Scholar] [CrossRef]
- Lee, Y.; Gu, E.J.; Song, H.-Y.; Yoo, B.-G.; Park, J.E.; Jeon, J.; Byun, E.-B. Exploring the Anti-Inflammatory Potential of Novel Chrysin Derivatives through Cyclooxygenase-2 Inhibition. ACS Omega 2024, 9, 50491–50503. [Google Scholar] [CrossRef]
- Zhao, X.; Du, C.; Zeng, Y.; Chen, Y.; Xu, J.; Yin, X.; Hu, C.; Mao, Z.; Lin, Y. Discovery of Novel Chrysin Derivatives as Potential Anti-Psoriasis Agents. Bioorganic Chem. 2024, 150, 107599. [Google Scholar] [CrossRef]
- Zhao, P.; Hou, Y.; Yan, T.; Kang, J.; Tian, Y.; Li, J.; Zeng, C.; Geng, F.; Liao, Q. Synthesis and Biological Evaluation of Chrysin Derivatives Containing α-Lipoic Acid for the Treatment of Inflammatory Bowel Disease. Front. Chem. 2024, 12, 1406051. [Google Scholar] [CrossRef]
- Ávila-Román, J.; Quevedo-Tinoco, L.; Oliveros-Ortiz, A.J.; García-Gil, S.; Rodríguez-García, G.; Motilva, V.; Gómez-Hurtado, M.A.; Talero, E. Synthesis and Bioevaluation of New Stable Derivatives of Chrysin-8-C-Glucoside That Modulate the Antioxidant Keap1/Nrf2/HO-1 Pathway in Human Macrophages. Pharmaceuticals 2024, 17, 1388. [Google Scholar] [CrossRef]
- Song, H.-Y.; Sik Kim, W.; Moo Han, J.; Yong Park, W.; Lim, S.-T.; Byun, E.-B. HMOC, a Chrysin Derivative, Induces Tolerogenic Properties in Lipopolysaccharide-Stimulated Dendritic Cells. Int. Immunopharmacol. 2021, 95, 107523. [Google Scholar] [CrossRef]
- Byun, E.-B.; Song, H.-Y.; Kim, W.S.; Han, J.M.; Seo, H.S.; Park, W.Y.; Kim, K.; Byun, E.-H. Chrysin Derivative CM1 and Exhibited Anti-Inflammatory Action by Upregulating Toll-Interacting Protein Expression in Lipopolysaccharide-Stimulated RAW264.7 Macrophage Cells. Molecules 2021, 26, 1532. [Google Scholar] [CrossRef]
- Lee, J.-H.; Ko, Y.-B.; Choi, Y.-M.; Kim, J.; Cho, H.-D.; Choi, H.; Song, H.-Y.; Han, J.-M.; Cha, G.-H.; Lee, Y.-H.; et al. CM1, a Chrysin Derivative, Protects from Endotoxin-Induced Lethal Shock by Regulating the Excessive Activation of Inflammatory Responses. Nutrients 2024, 16, 641. [Google Scholar] [CrossRef]
- Patel, K.B.; Rajani, D.; Ahmad, I.; Patel, H.; Patel, H.D.; Kumari, P. Chrysin Based Pyrimidine-Piperazine Hybrids: Design, Synthesis, in Vitro Antimicrobial and in Silico E. Coli Topoisomerase II DNA Gyrase Efficacy. Mol. Divers. 2024, 28, 1377–1392. [Google Scholar] [CrossRef]
- Bhowmik, S.; Anand, P.; Das, R.; Sen, T.; Akhter, Y.; Das, M.C.; De, U.C. Synthesis of New Chrysin Derivatives with Substantial Antibiofilm Activity. Mol. Divers. 2022, 26, 137–156. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Chen, J.; Wang, B.; Peng, A.-Y.; Mao, Z.-W.; Xia, W. Inhibition of Quorum-Sensing Regulator from Pseudomonas Aeruginosa Using a Flavone Derivative. Molecules 2022, 27, 2439. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, P.; Rao, V.S.; Reddy, P.M.; Babu, K.S.; Rao, M.S. Synthesis, Biological Evaluation and Molecular Modeling Studies of Novel C (7) Modified Analogues of Chrysin. Lett. Drug Des. Discov. 2020, 17, 873–883. [Google Scholar] [CrossRef]
- Patel, K.B.; Patel, R.V.; Ahmad, I.; Rajani, D.; Patel, H.; Mukherjee, S.; Kumari, P. Design, Synthesis, Molecular Docking, Molecular Dynamic Simulation, and MMGBSA Analysis of 7-O-Substituted 5-Hydroxy Flavone Derivatives. J. Biomol. Struct. Dyn. 2024, 42, 6378–6392. [Google Scholar] [CrossRef]
- Omonga, N.; Zia, Z.; Ghanbour, H.; Ragazzon-Smith, A.; Foster, H.; Hadfield, J.; Ragazzon, P. Facile Synthesis and Biological Evaluation of Chrysin Derivatives. J. Chem. Res. 2021, 45, 1083–1092. [Google Scholar] [CrossRef]
- Mutlu Gençkal, H. New Heteroleptic Cu(II) Complexes of Chrysin with 2,2′–Bipyridine and Substituted 1,10–Phenanthrolines: Synthesis, Characterization, Thermal Stability and Antioxidant Activity. J. Mol. Struct. 2020, 1209, 127917. [Google Scholar] [CrossRef]
- Yang, A.; Liu, C.; Zhang, H.; Wu, J.; Shen, R.; Kou, X. A Multifunctional Anti-AD Approach: Design, Synthesis, X-Ray Crystal Structure, Biological Evaluation and Molecular Docking of Chrysin Derivatives. Eur. J. Med. Chem. 2022, 233, 114216. [Google Scholar] [CrossRef]
- Liu, C.; Kou, X.; Wang, X.; Wu, J.; Yang, A.; Shen, R. Novel Chrysin Derivatives as Hidden Multifunctional Agents for Anti-Alzheimer’s Disease: Design, Synthesis and in Vitro Evaluation. Eur. J. Pharm. Sci. 2021, 166, 105976. [Google Scholar] [CrossRef]
- Liu, C.; Wu, J.; Hu, C.; Yang, A.; Shen, R.; Kou, X. Synthesis, Single Crystal Characterization and Anti-AD Activities of a Novel Complex of Cu(II) with in Situ Formed Protonated Chrysin Derivative Ligand. J. Inorg. Biochem. 2023, 239, 112086. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Song, H.-Y.; Byun, E.-B. Anti-Melanogenic Effects of Hydroxyethyl Chrysin through the Inhibition of Tyrosinase Activity: In Vitro and in Silico Approaches. Heliyon 2025, 11, e41718. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, Y.; Huang, G.; Xin, X.; Tang, L.; Li, H.; Lee, K.S.; Jin, B.R.; Gui, Z. Chemical Synthesis, Inhibitory Activity and Molecular Mechanism of 1-Deoxynojirimycin–Chrysin as a Potent α-Glucosidase Inhibitor. RSC Adv. 2021, 11, 38703–38711. [Google Scholar] [CrossRef] [PubMed]
- Hairani, R.; Chavasiri, W. A New Series of Chrysin Derivatives as Potent Non-Saccharide α-Glucosidase Inhibitors. Fitoterapia 2022, 163, 105301. [Google Scholar] [CrossRef]
- An, S.; Ko, H.; Jang, H.; Park, I.G.; Ahn, S.; Hwang, S.Y.; Gong, J.; Oh, S.; Kwak, S.Y.; Lee, Y.; et al. Prenylated Chrysin Derivatives as Partial PPARγ Agonists with Adiponectin Secretion-Inducing Activity. ACS Med. Chem. Lett. 2023, 14, 425–431. [Google Scholar] [CrossRef]
- Poungcho, P.; Hairani, R.; Chaotham, C.; De-Eknamkul, W.; Chavasiri, W. Methoxylated Chrysin and Quercetin as Potent Stimulators of Melanogenesis. Int. J. Mol. Sci. 2025, 26, 3281. [Google Scholar] [CrossRef]
- Zhang, R.; Gao, C.; Hu, M.; Wang, X.; Li, S.; An, Z.; Yang, X.; Xie, Y. Synthesis and Biological Evaluation of the Novel Chrysin Prodrug for Non-Alcoholic Fatty Liver Disease Treatment. Front. Pharmacol. 2024, 15, 1336232. [Google Scholar] [CrossRef]
- Falbo, F.; Carullo, G.; Panti, A.; Spiga, O.; Gianibbi, B.; Ahmed, A.; Campiani, G.; Ramunno, A.; Aiello, F.; Fusi, F. Exploring the Chemical Space around Chrysin to Develop Novel Vascular CaV 1.2 Channel Blockers, Promising Vasorelaxant Agents. Arch. Pharm. 2024, 357, e2400536. [Google Scholar] [CrossRef]
- Suzuki, A. Organoboron Compounds in New Synthetic Reactions. Pure Appl. Chem. 1985, 57, 1749–1758. [Google Scholar] [CrossRef]
- Miyaura, N.; Suzuki, A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Ohe, T.; Miyaura, N.; Suzuki, A. Palladium-Catalyzed Cross-Coupling Reaction of Organoboron Compounds with Organic Triflates. J. Org. Chem. 1993, 58, 2201–2208. [Google Scholar] [CrossRef]
- Milstein, D.; Stille, J.K. A General, Selective, and Facile Method for Ketone Synthesis from Acid Chlorides and Organotin Compounds Catalyzed by Palladium. J. Am. Chem. Soc. 1978, 100, 3636–3638. [Google Scholar] [CrossRef]
- Chinchilla, R.; Nájera, C. The Sonogashira Reaction: A Booming Methodology in Synthetic Organic Chemistry. Chem. Rev. 2007, 107, 874–922. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; Ribeiro, D.; Silva, V.L.M.; Fernandes, E. Synthetic Flavonoid Dimers: Synthesis Strategies and Biological Activities. Eur. J. Med. Chem. 2025, 291, 117669. [Google Scholar] [CrossRef]
- Nakazawa, K.; Ito, M. Syntheses of Ring-Substituted Flavonoids and Allied Compounds. X. Synthesis of Ginkgetin. Chem. Pharm. Bull. 1963, 11, 283–288. [Google Scholar] [CrossRef]
- Lim, H.; Kim, S.B.; Park, H.; Chang, H.W.; Kim, H.P. New Anti-Inflammatory Synthetic Biflavonoid with C-C (6-6″) Linkage: Differential Effects on Cyclooxygenase-2 and Inducible Nitric Oxide Synthase. Arch. Pharm. Res. 2009, 32, 1525–1531. [Google Scholar] [CrossRef]
- Che, H.; Lim, H.; Kim, H.P.; Park, H. A Chrysin Analog Exhibited Strong Inhibitory Activities against Both PGE2 and NO Production. Eur. J. Med. Chem. 2011, 46, 4657–4660. [Google Scholar] [CrossRef]
- Lim, H.; Jin, J.H.; Park, H.; Kim, H.P. New Synthetic Anti-Inflammatory Chrysin Analog, 5,7-Dihydroxy-8-(Pyridine-4yl)Flavone. Eur. J. Pharmacol. 2011, 670, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Hurtová, M.; Biedermann, D.; Osifová, Z.; Cvačka, J.; Valentová, K.; Křen, V. Preparation of Synthetic and Natural Derivatives of Flavonoids Using Suzuki–Miyaura Cross-Coupling Reaction. Molecules 2022, 27, 967. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, E.; De Oliveira, N.C.; Pestana, Y.; Alves, M.A.; Da Silva, A.J.M. Regio- and Chemoselective Synthesis of Polyaryl Flavones by Combination of C-O/C-H Activation and Suzuki-Miyaura Cross Coupling Reactions. J. Mol. Struct. 2024, 1299, 137067. [Google Scholar] [CrossRef]
- Echavarren, A.M.; Stille, J.K. Palladium-Catalyzed Coupling of Aryl Triflates with Organostannanes. J. Am. Chem. Soc. 1987, 109, 5478–5486. [Google Scholar] [CrossRef]
- Lu, K.; Yang, K.; Jia, X.; Gao, X.; Zhao, X.; Pan, G.; Ma, Y.; Huang, Q.; Yu, P. Total Synthesis of I3,II8-Biapigenin and Ridiculuflavone A. Org. Chem. Front. 2017, 4, 578–586. [Google Scholar] [CrossRef]
- Santiago-de-la-Cruz, J.A.; Rivero-Segura, N.A.; Alvarez-Sánchez, M.E.; Gomez-Verjan, J.C. Network Pharmacology and Machine Learning Identify Flavonoids as Potential Senotherapeutics. Pharmaceuticals 2025, 18, 1176. [Google Scholar] [CrossRef]
- Taldaev, A.; Terekhov, R.; Nikitin, I.; Zhevlakova, A.; Selivanova, I. Insights into the Pharmacological Effects of Flavonoids: The Systematic Review of Computer Modeling. Int. J. Mol. Sci. 2022, 23, 6023. [Google Scholar] [CrossRef]
- Gangwal, A.; Lavecchia, A. Artificial Intelligence in Natural Product Drug Discovery: Current Applications and Future Perspectives. J. Med. Chem. 2025, 68, 3948–3969. [Google Scholar] [CrossRef]
- Park, J.E.; Ryu, S.-H.; Ito, S.; Shin, H.; Kim, Y.-H.; Jeon, J. Metabolite Analysis of 14C-Labeled Chloromethylisothiazolinone/Methylisothiazolinone for Toxicological Consideration of Inhaled Isothiazolinone Biocides in Lungs. Chemosphere 2024, 362, 142666. [Google Scholar] [CrossRef]
- Park, J.E.; Ryu, S.-H.; Ito, S.; Song, M.-K.; Gu, E.J.; Shin, H.; Kim, Y.-H.; Jeon, J. Bioaccumulation and in Vivo Fate of Toxic Benzylalkyldimethylammonium Chloride in Rats via the Radiotracer Analysis. Chemosphere 2023, 338, 139460. [Google Scholar] [CrossRef]
- Song, M.-K.; Eun Park, J.; Ryu, S.-H.; Baek, Y.-W.; Kim, Y.-H.; Im Kim, D.; Yoon, S.-H.; Shin, H.; Jeon, J.; Lee, K. Biodistribution and Respiratory Toxicity of Chloromethylisothiazolinone/Methylisothiazolinone Following Intranasal and Intratracheal Administration. Environ. Int. 2022, 170, 107643. [Google Scholar] [CrossRef]

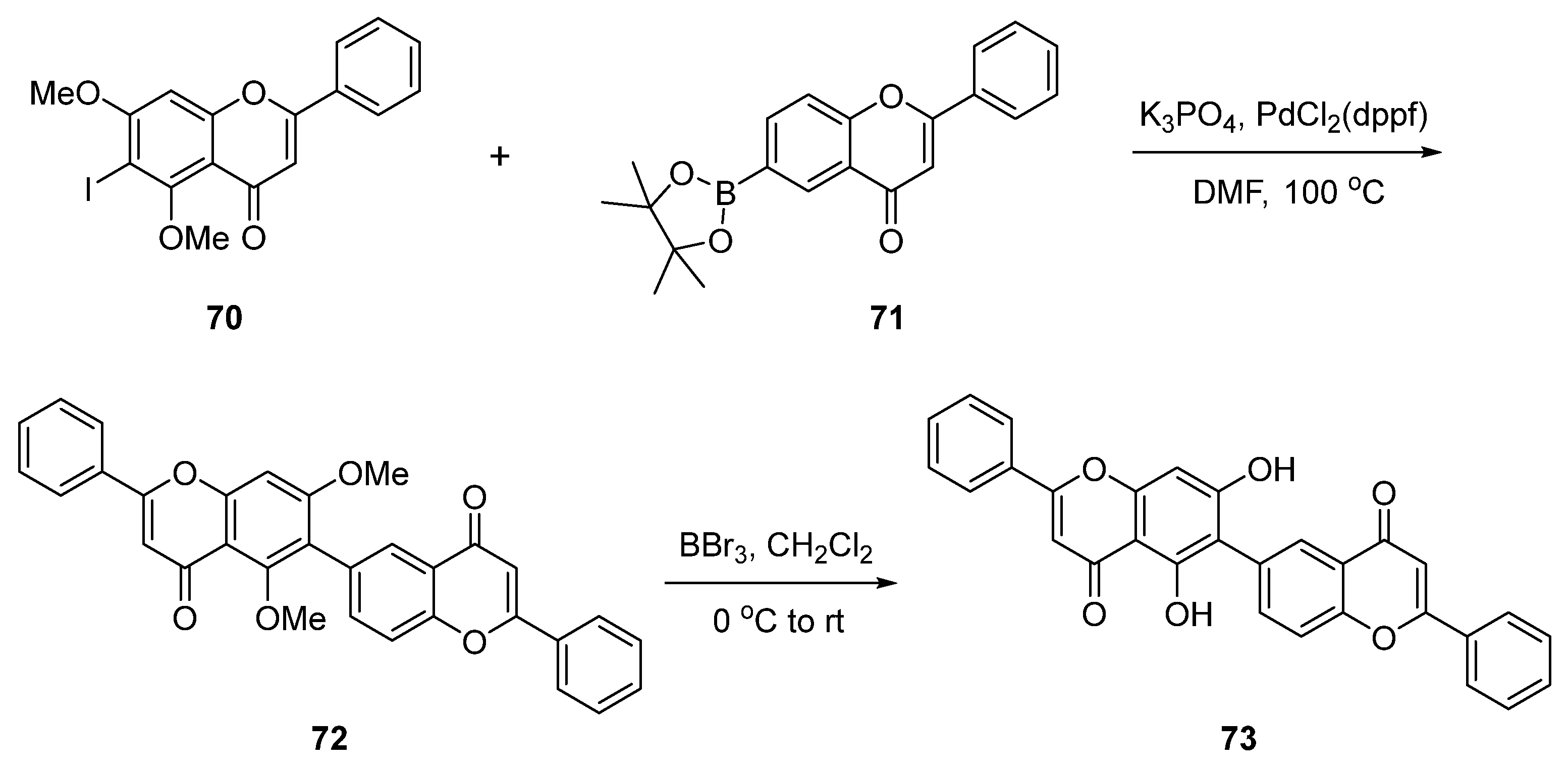
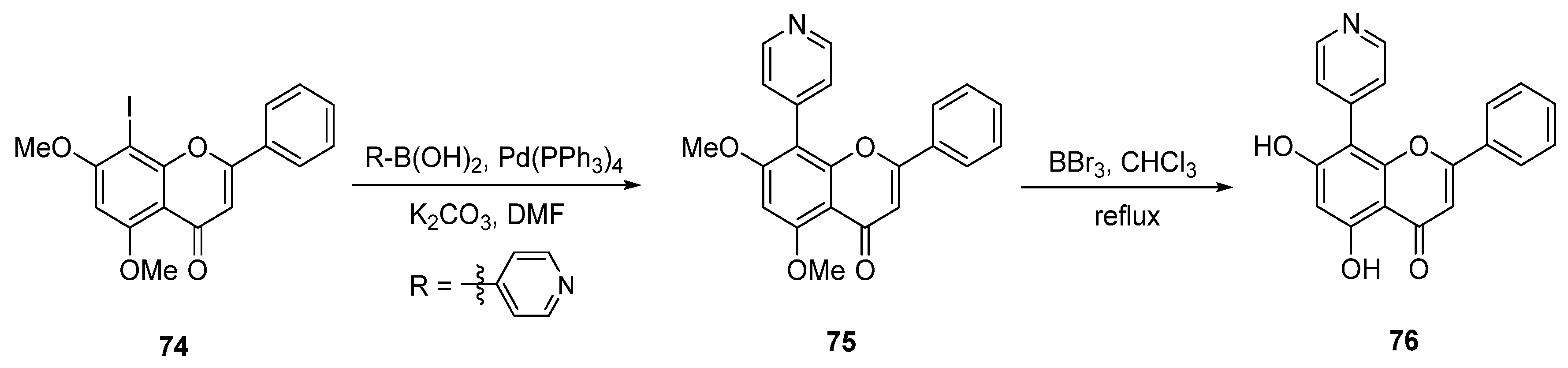
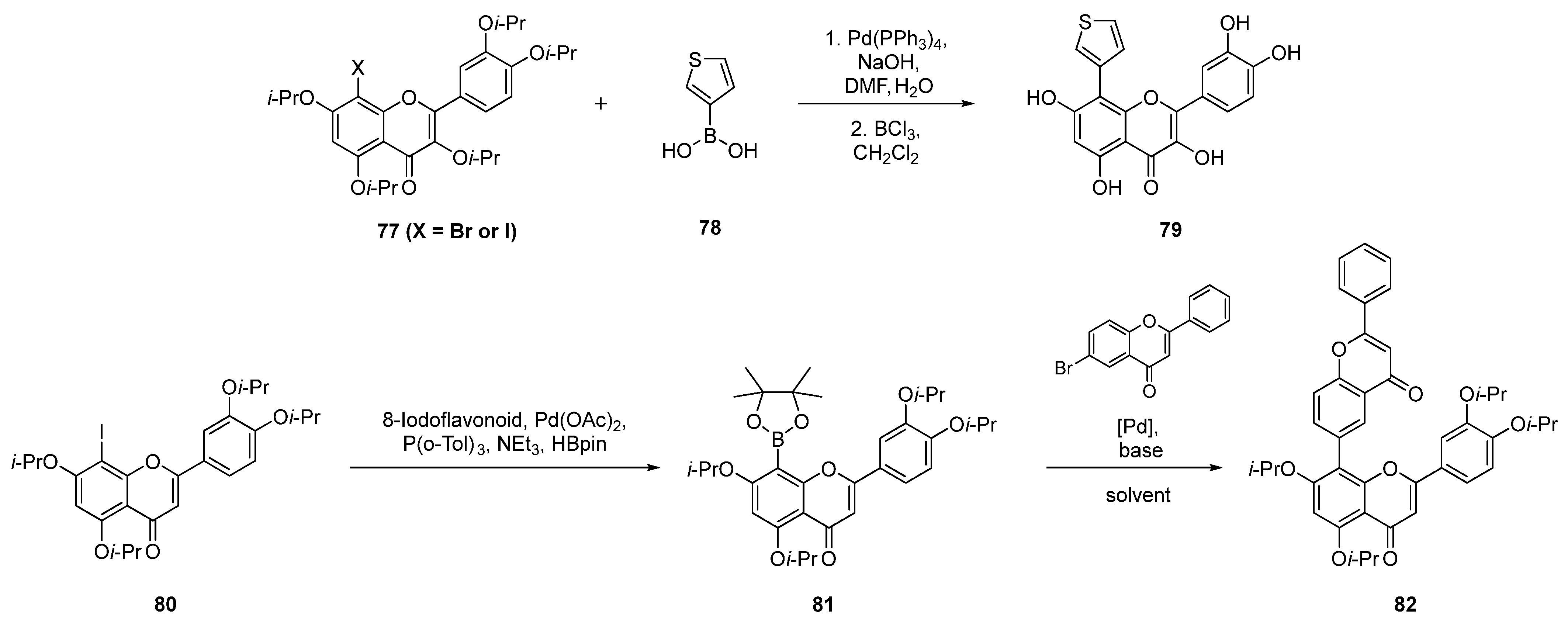
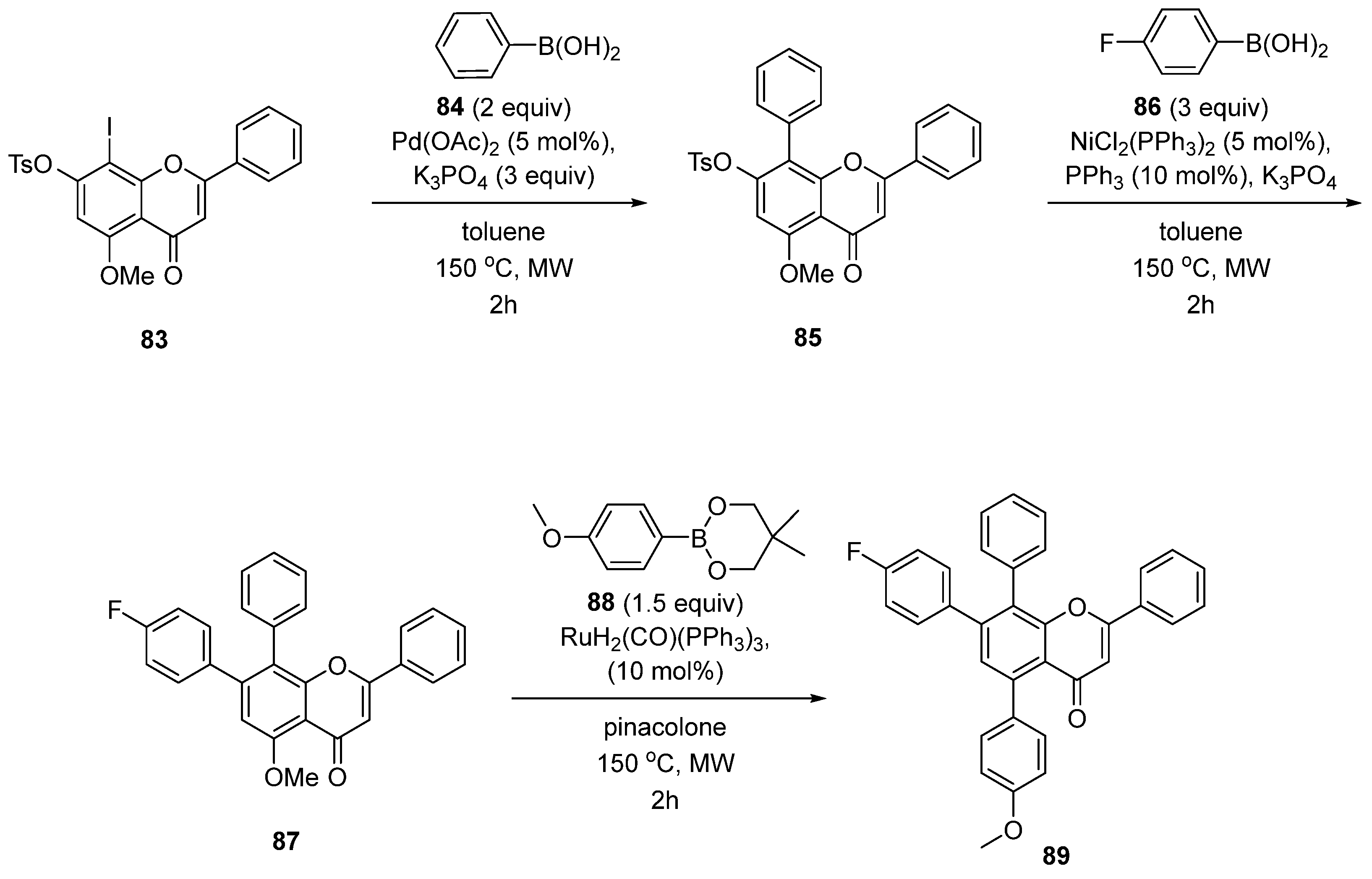
| Entry | Structure | Synthesis Method | Cell Lines (IC50) | Key Findings and Mechanisms | Ref |
|---|---|---|---|---|---|
| 1 |  2a: R = C2H5 2a: R = C2H52b: R = C3H7 2c: R = CH(CH3)CO2H | Etherification (7-OH) | For 2c: Vero (152.28 ± 3.82 µM), HeLa (13.91 ± 0.34 µM), A549 (147.38 ± 7.56 µM) | 7-OH substitution altered enzyme selectivity. Compound 2c inhibited HDAC8, increased H3 acetylation, and induced apoptosis. | [37] |
| 2 | 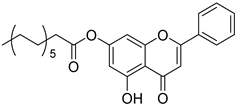 3 3 | Esterification (7-OH) | HepG2 (14.79 µM) | Long-chain myristoyl ester improved solubility. Enhanced membrane permeability translated into stronger cytotoxicity in HepG2 cells. | [23] |
| 3 |  4 4 | Etherification with N-(chloroacetyl) aniline analogs (7-OH) followed by Smiles rearrangement | GI50 values: MCF-7 (0.03 µM), HCT-15 (0.06 µM) | Smiles rearrangement gave a diphenylamine scaffold. Stronger π–π interactions yielded nanomolar potency in breast and colon cancer cells. | [22] |
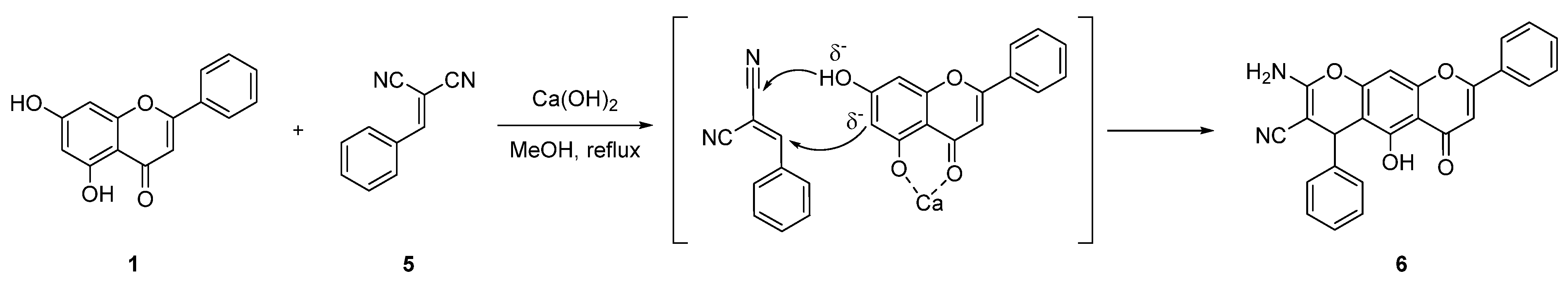
| 4 | 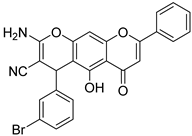 6 6 | Michael addition followed by cyclization (Scheme 1) | K562 (6.41 ± 0.49 µM) | Chromene–chrysin hybrid induced mitochondrial apoptosis. Regulated Bax/Bcl-2 balance and suppressed tumor growth in vivo. | [38] |
| 5 | 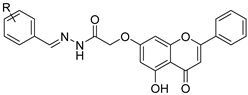 7a: R = 2,4-OH 7a: R = 2,4-OH7b: R = 2,3,4-OH | Etherification (7-OH), hydrazide formation, and hydrazone condensation | For 7a: MDA-MB-231 (5.98 µM) For 7b: MDA-MB-231 (9.40 µM) | PAC-1 hybrids induced G2 arrest and apoptosis. Triggered Bak upregulation, cytochrome c release, and caspase activation. | [39] |
| 6 |  8 8 | Etherification (7-OH) followed by coupling with porphyrin analogs | HeLa (6.26 ± 2.52 µM), A549 (23.37 ± 4.24 µM) | Porphyrin–chrysin hybrid acted as a photosensitizer. Positive charge and EWG groups enhanced phototoxicity under irradiation. | [40] |
| 7 | 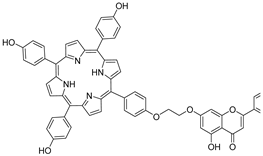 9 9 | Etherification (7-OH) followed by conjugation with porphyrin derivatives | MGC-803 (70.41 ± 2.15 µM), HeLa (26.51 ± 1.15 µM) | Porphyrin conjugates bound ct-DNA and generated ROS. Free-base derivatives outperformed metalated analogs in terms of photodynamic efficiency. | [41] |
| 8 | 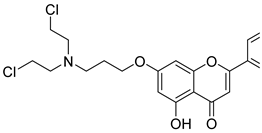 10 10 | Etherification (7-OH), substitution with diethanolamine, and chlorination | HeLa (1.43 µM), A549 (7.34 µM), HepG2 (11.31 µM), MCF-7 (4.90 µM), SH-SY5Y (7.86 µM), PC-3 (2.32 µM), DU145 (2.91 µM) | Nitrogen mustard–chrysin hybrids disrupted ΔΨm. Three-carbon linker produced the strongest cytotoxic effect. | [42] |
| 9 |  12 12 | Propargylation (5,7-OH) followed by click reaction | HeLa (0.7331 µM), SiHa (1.352 µM) | Bis-triazole substitution improved H-bonding. Compound 12 achieved submicromolar IC50 in HeLa cells. | [43] |
| 10 | 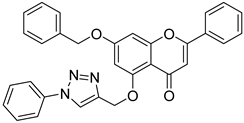 13 13 | Propargylation (5-OH) followed by click reaction | PC3 (10.8 ± 0.04 µM), MCF-7 (20.5 ± 0.2 µM) | Phenyl-triazole derivative enhanced π–π stacking. Showed potent activity in PC3 and MCF-7 cells. | [44] |
| 11 | 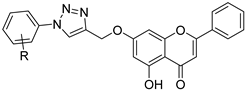 14a: R = 3-F 14a: R = 3-F14b: R = 2-Cl | Propargylation (7-OH) followed by click reaction | For 14a: MGC-803 (18.40 µM) For 14b: MGC-803 (5.92 µM) | Halogen-substituted triazoles improved activity. Chloro- and fluoroaryl groups boosted gastric cancer cell potency. | [45] |
| 12 | 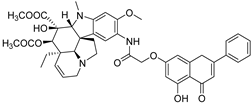 15 15 | Conjugation with a vindoline derivative (7-OH) followed by Smiles rearrangement | GI50 values: low micromolar levels for 60 human cancer cells | Vindoline–chrysin hybrids combined dual pharmacophores. Flexible linker design gave broad low-micromolar GI50 across the NCI-60 panel. | [46] |
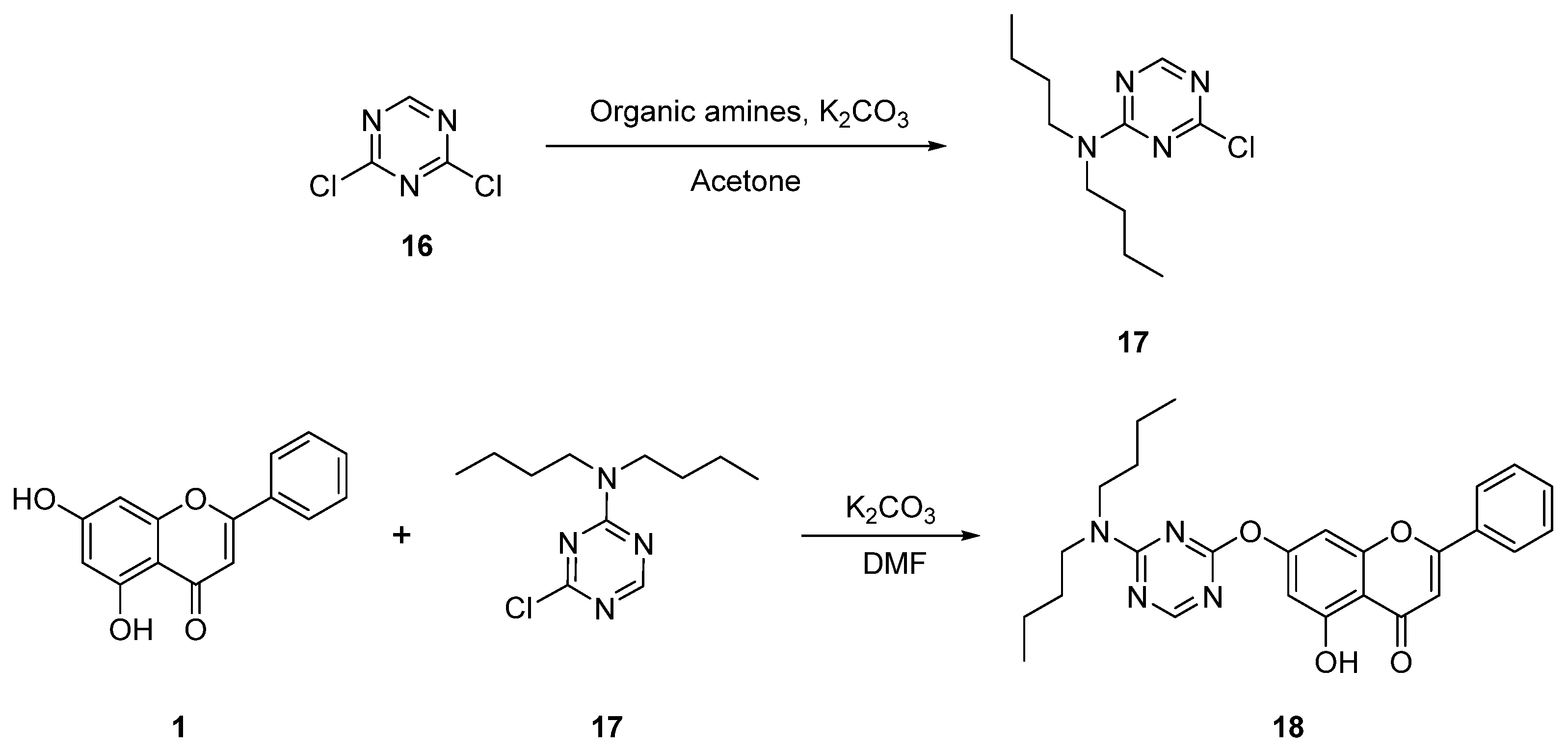
| 13 | 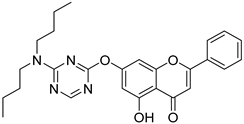 18 18 | Nucleophilic aromatic substitution with 1,3,5-triazine derivatives (7-OH) (Scheme 3) | HeLa (9.86 ± 0.37 µM), 293 T (37.34 ± 1.28 µM) | Triazine–chrysin hybrids introduced electron-deficient heteroaryl moieties. Compound 18 outperformed cisplatin in HeLa cells. | [47] |
| 14 | 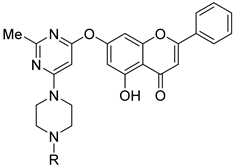 19a: (R =1-methyl piperazine) 19a: (R =1-methyl piperazine)19b: (R = methyl) | Nucleophilic aromatic substitution with 4,6-dichloropyrimidine derivatives (7-OH) | For 19a: A549 (30.30 µM), HepG2 (21.02 µM), MCF-7 (24.67 µM), PC-3 (22.130 µM) For 19b: HCT-116 (4.83 µM) | Pyrimidine–chrysin hybrids showed strong cytotoxicity. Compound 19b was especially potent against HCT-116 cells. | [48] |
| 15 | 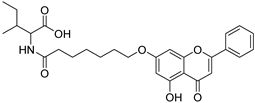 20 20 | Etherification (7-OH) followed by coupling with L-amino acids | MGC-803 (24.5 ± 3.4 µM), | Heptanoyl–L-amino acid conjugate increased solubility. Compound 20 showed improved efficacy in gastric cancer. | [49] |
| 16 | 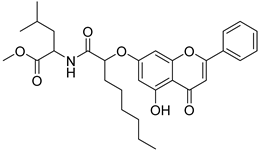 21 21 | Etherification (7-OH) followed by coupling with L-amino esters | MCF-7 (32.4 ± 1.8 µM), MDA-MB-231 (8.2 ± 1.6 µM) | Leucine methyl ester conjugate induced G2/M arrest. Activated mitochondrial apoptosis and inhibited Akt phosphorylation. | [50] |
| 17 | 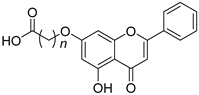 22a: n = 4 22a: n = 422b: n = 5 22c: n = 6 | Etherification (7-OH) | Vero (39.94~49.55 µM), HeLa (15.96~18.00 µM), HCT-116 (11.96~13.04 µM), A549 (40.0~41.48 µM), MCF-7 (21.82~37.28 µM) | Carboxylated derivatives targeted the HDAC active site. Zn2+ chelation supported inhibitory mechanism. | [51] |
| 18 † | 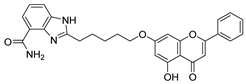 23 23 | Etherification (7-OH) followed by coupling with a benzimidazole derivative | MDA-MB-231 (6.2 ± 0.6 µM), MDA-MB-436 (4.5 ± 1.4 µM), HCC-1937 (3.9 ± 0.8 µM), MCF-7 (14.2 ± 1.1µM) | Benzimidazole pharmacophore enhanced PARP1 binding. Compound 23 inhibited BRCA-deficient breast cancer cells at nanomolar levels. | [52] |
| 19 | 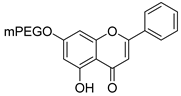 24a: (mPEG 350 g/mol) 24a: (mPEG 350 g/mol)24b: (mPEG 500 g/mol) 24c: (mPEG 750 g/mol) 24d: (mPEG 2 kDa) | Etherification (7-OH) | PC-3 (205.66 ± 2.60 µM), HepG2 (122.72 ± 9.92 µM) | PEGylation improved solubility and aggregation. mPEG-500 conjugate retained cytotoxic and redox activity. | [53] |
| 20 | 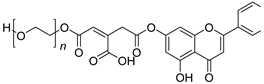 25 25 | Esterification (7-OH) with cis-aconityl functionalized PEG4000 | MDA-MB-231 (6.2 ± 2.3 µM), MCF-7 (26.1 ± 3.5 µM) | PEG4000–cis-aconityl conjugate enabled pH-sensitive release. Showed enhanced efficacy in breast cancer cells. | [54] |
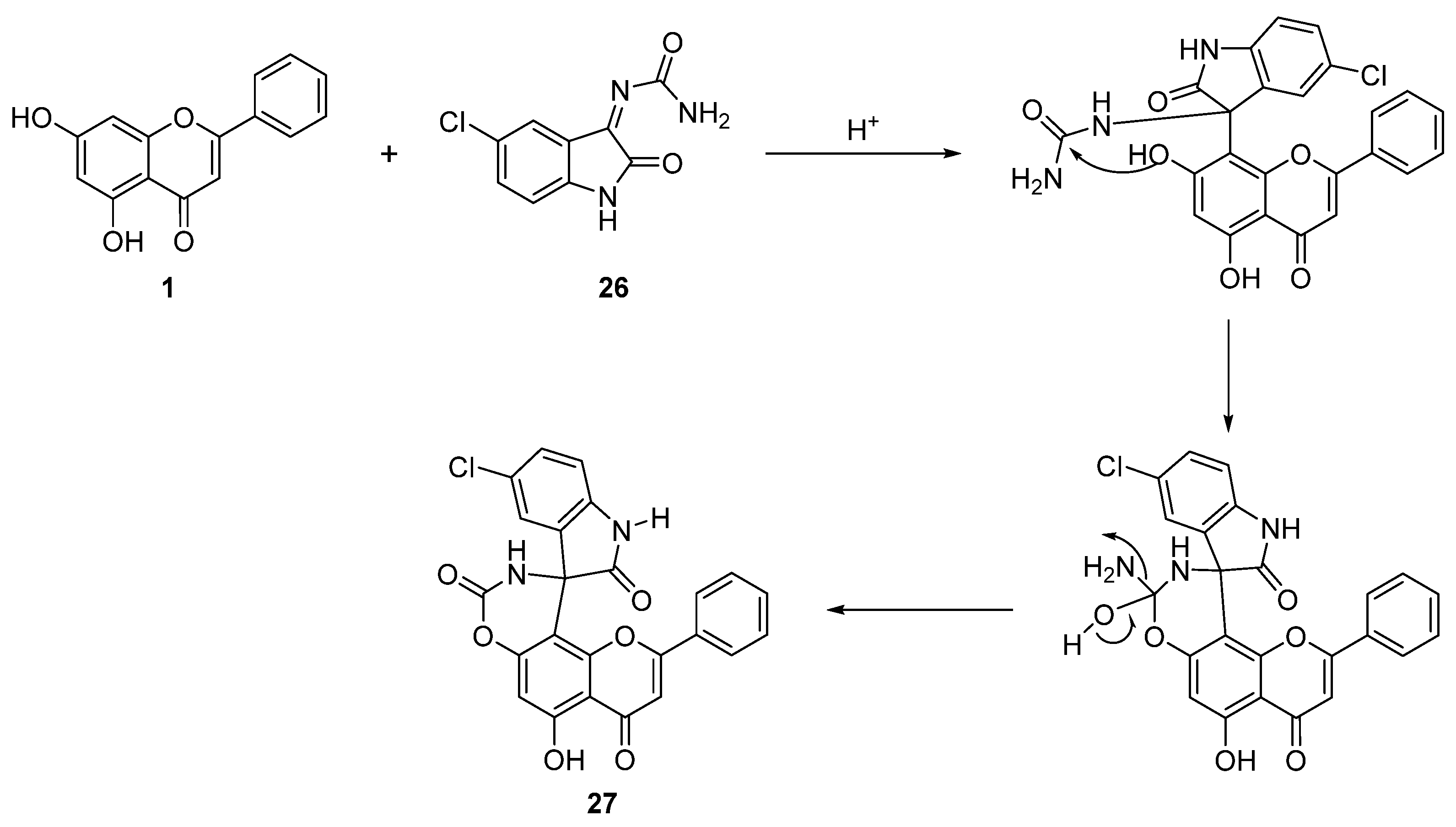
| 21 † | 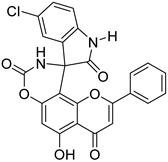 27 27 | Formation of spirooxindole carbamate derivatives (Scheme 4) | A549 (24.20 ± 2.60 µM), MDA MB-231 (24.93 ± 2.50 µM), HepG2 (2.50 ± 0.25 µM), HeLa (7.48 ± 1.23 µM), HEK-293 (14.25 ± 3.60 µM) | Spirooxindole carbamate bound to CD133 protein. Reduced CSC populations and inhibited tumorsphere formation. | [59] |
| 22 | 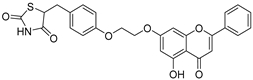 28 28 | Etherification (7-OH) followed by conjugation with a thiazolidine-2, 4-dione derivative | Anticancer agent as a TLR4 ligand | Thiazole derivative acted as a TLR4 ligand. Activated NF-κB signaling and reprogrammed TAMs to the M1 phenotype. | [60] |
| 23 | 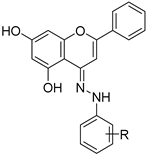 29a: R = 2-OMe 29a: R = 2-OMe29b: R = 4-OMe | Hydrazone formation (condensation) | for 29a: MCF-7 (179.28 µM), HepG2 (166.19 µM) for 29b: MCF-7 (101.44 µM), HepG2 (236.49 µM) | Hydrazone derivatives showed moderate cytotoxicity. Activity varied with methoxy substitution on phenylhydrazine. | [61] |
| 24 |  30 30 | Imine formation (condensation) | HepG2 (3.11 ± 0.41 µM), HCT-116 (3.47 ± 0.69 µM), A549 (3.35 ± 0.27 µM), MCF-7 (14.08 ± 0.96 µM) | Ferrocene Schiff base inhibited Topo II. Induced ROS-mediated apoptosis with ~5-fold selectivity over normal cells. | [64] |
| 25 | 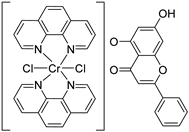 31 31 | Chelation with Cr(III) mixed-ligand complexes | MCF-7 (8.08 µM) | Cr(III)–phenanthroline–chrysin complex increased uptake. Showed higher cytotoxicity than free chrysin. | [65] |
| 26 | 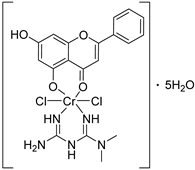 32 32 | Chelation with Cr(III) mixed-ligand complexes | MCF-7 (30.85 µM) | Cr(III)–metformin–chrysin complex altered polarity. Demonstrated improved activity but less than that of compound 31. | [65] |
| 27 | 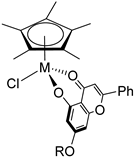 33a: (M = Ru), 33b: (M = Rh) 33a: (M = Ru), 33b: (M = Rh) 33c: (M = Ir) R = −(CH2)4-piperidine | Chelation with transition metal complexes | For 33a: SW480 (28.5 ± 1.3 µM), A549 (31.3 ± 1.6 µM) For 33b: SW480 (31.3 ± 1.3 µM), A549 (35.3 ± 1.0 µM) For 33c: SW480 (15.9 ± 1.3 µM), A549 (18.9 ± 1.1 µM) | Half-sandwich Ru, Rh, Ir complexes showed distinct profiles. Ir(III) complex induced ROS and mitochondrial apoptosis with high selectivity. | [66] |
| 28 | 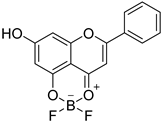 34 34 | Chelation with boron difluoride | MCF-7 (29.7 µM), SKOV3 (31.9 µM) | Difluoroboron complex modified electronic distribution. Showed >2-fold improved cytotoxicity versus parent chrysin. | [67] |
| Entry | Structure | Synthesis Methods | Biological Activity | Key Findings and Mechanisms | Ref |
|---|---|---|---|---|---|
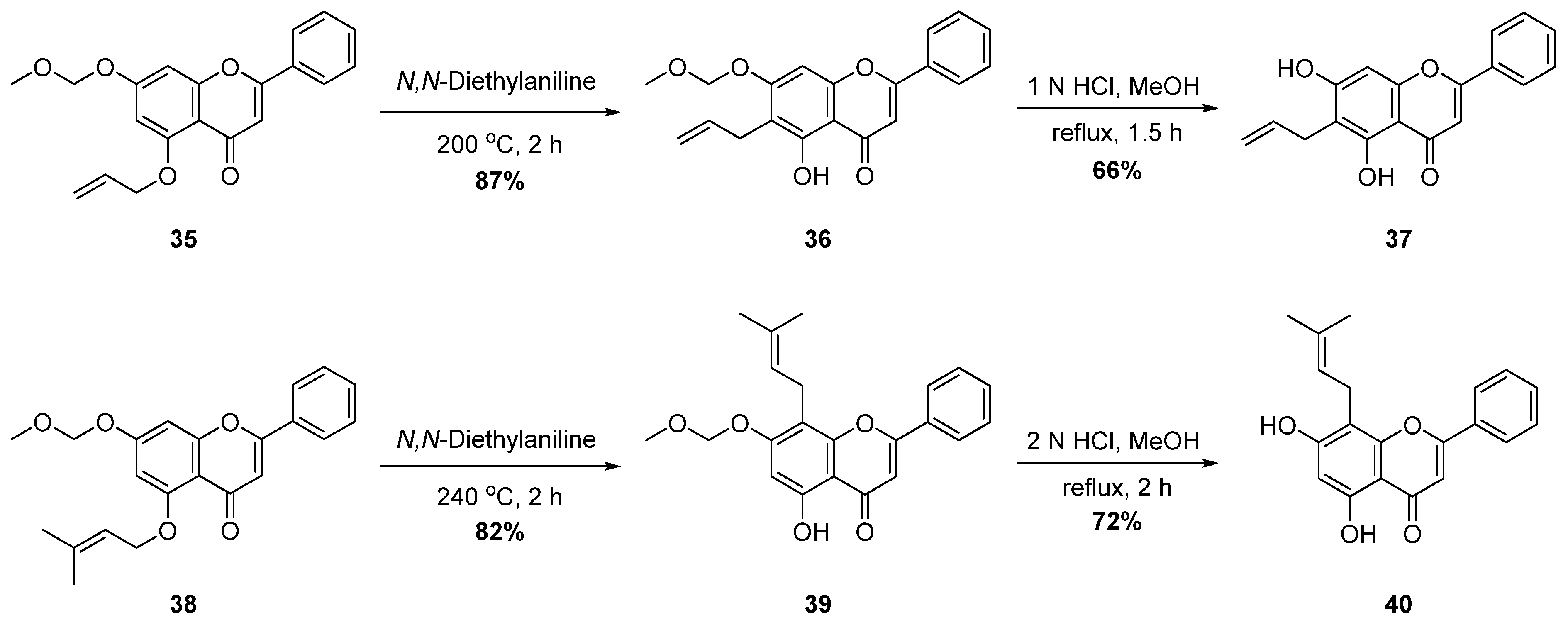
| 1 |  40 40 | Claisen rearrangement of prenyl group (5-OH) | Anti-inflammatory | Inhibited COX-2 (IC50 = 6.76, 9.63 μM vs. chrysin, 18.48 μM) in RAW264.7 cells, reducing IL-6, TNF-α, and PGE2. Docking showed strong COX-2 binding via H-bonds/hydrophobic contacts, confirming selective inhibition in vitro. | [68] |
| 2 | 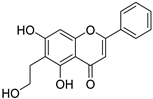 42 42 | Claisen rearrangement of allyl group (5-OH) | Anti-inflammatory | [68] | |
| 3 † | 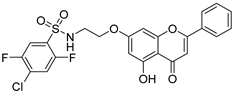 45 45 | Etherification (7-OH) followed by sulfonylation | Anti-inflammatory/ Anti-psoriasis | Suppressed NO, TNF-α, IL-6, and IL-17A (IC50 = 8.0 μM vs. dexamethasone, 19.5 μM) in RAW264.7 and HaCaT cells. Reduced epidermal thickness and cytokine levels in mice with imiquimod-induced psoriasis through NF-κB/STAT3 inhibition. | [69] |
| 4 † | 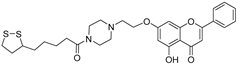 46 46 | Etherification (7-OH) followed by conjugation with α-lipoic acid | Anti-inflammatory | Inhibited monocyte adhesion to colon epithelial cells (IC50 = 4.71 μM vs. α-LA, 12.7 μM). Ameliorated TNBS-induced colitis in rats by suppressing ICAM-1/MCP-1 expression and JAK2/STAT3 signaling. | [70] |
| 5 | 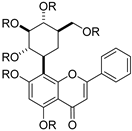 47a: R = Ac 47a: R = Ac47b: R = CO2Et | 47a: Acetylation using acetic anhydride 47b: Carbonylation using ethyl chloroformate | Anti-inflammatory/ Antioxidant | Reduced ROS, TNF-α, IL-1β, and COX-2 in LPS-stimulated THP-1 macrophages. Activated Keap1/Nrf2/HO-1; 47a showed the strongest effect, supporting antioxidant/anti-inflammatory dual action. | [71] |
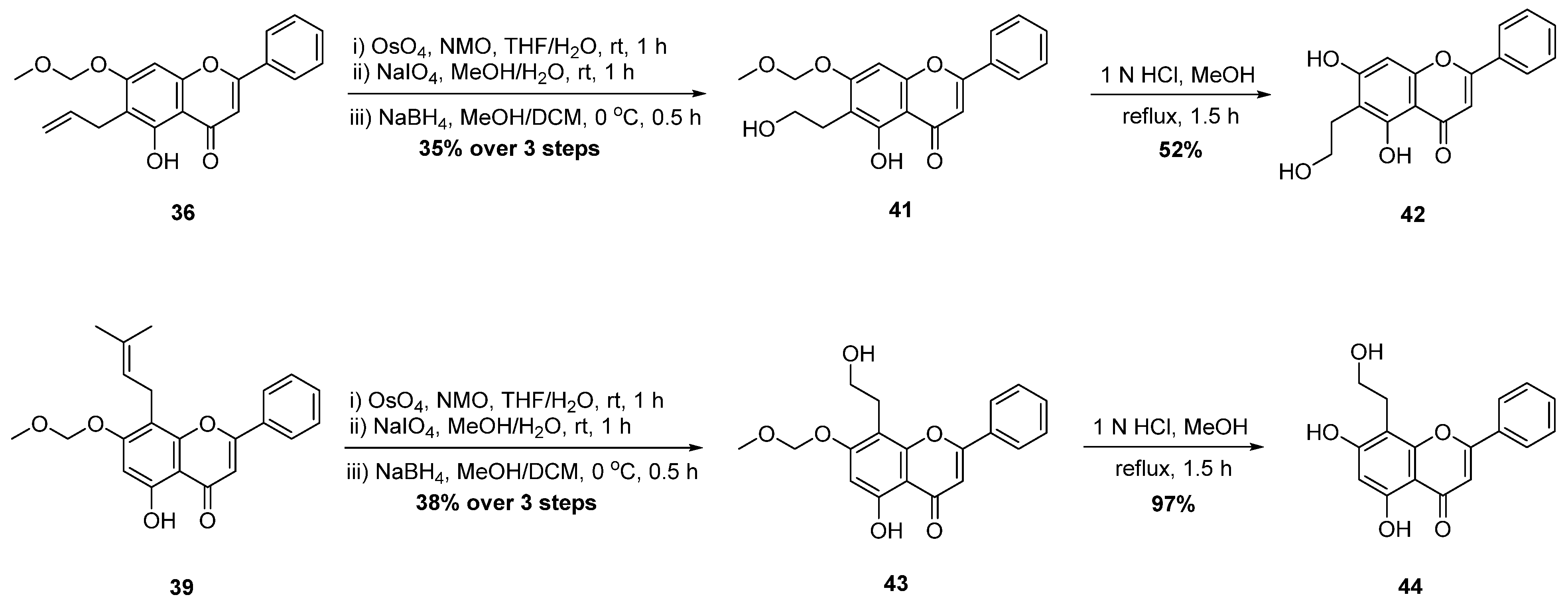
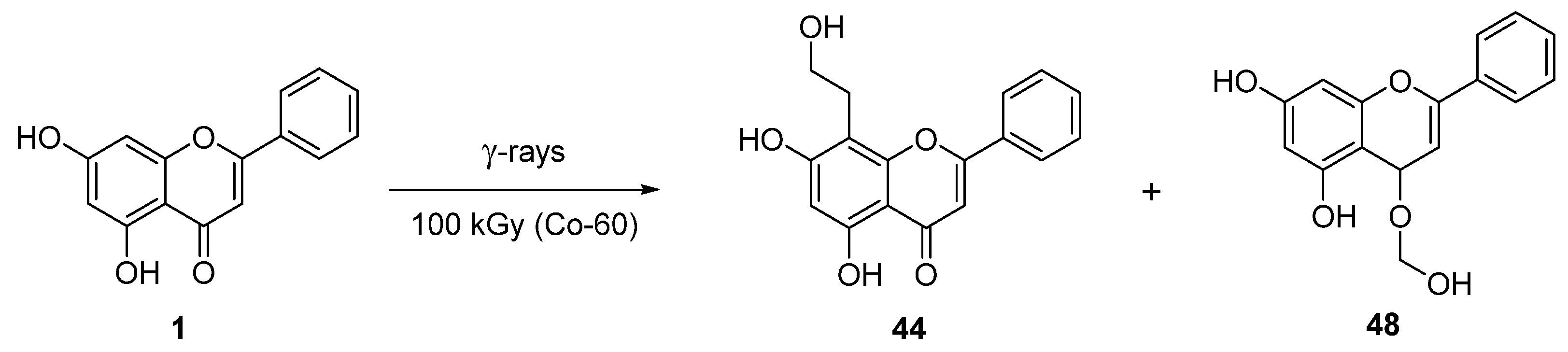
| 6 † | 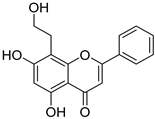 44 44 | γ-radiation | Anti-inflammatory | Reduced cytokine release (TNF-α, IL-6, NO) in RAW264.7 macrophages by upregulating Tollip. In vivo, alleviated dermatitis and endotoxin-induced shock in mice by suppressing NF-κB/MAPK pathways. | [72,73] |
| 7 † | 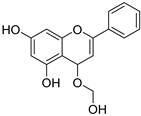 48 48 | γ-radiation | Anti-inflammatory | [74] | |
| 8 | 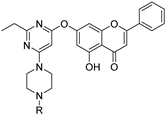 49a: 2,3-dichlorophenyl 49a: 2,3-dichlorophenyl49b: diphenyl methyl | Nucleophilic aromatic substitution with 4,6-dichloropyrimidine derivatives (7-OH) followed by substitution with piperazine analogs | Antimicrobial | Displayed strong antibacterial activity against E. coli (MIC 6.25–12.5 μg/mL) and antifungal effects against C. albicans. Docking confirmed binding to E. coli DNA gyrase and C. albicans CYP51, outperforming standard antibiotics. | [75] |
| 9 | 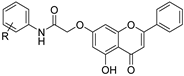 50a: R = 3-Cl 50a: R = 3-Cl50b: R = 4-F 50c: R = 2-OCH3 | Conjugation with N-(chloroacetyl) aniline analogs (7-OH) | Antimicrobial | Inhibited >92% biofilm formation in E. coli MTCC40 at sub-MIC levels (33.57% vs. chrysin). Suppressed motility and improved solubility/bioavailability, enhancing anti-biofilm efficacy. | [76] |
| 10 | 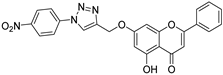 51 51 | Propargylation (7-OH) followed by click reaction | Antimicrobial | [76] | |
| 11 | 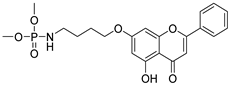 52 52 | Etherification (7-OH) | Antimicrobial | Inhibited LasR-regulated virulence factors (LasA, pyocyanin, elastase) and biofilm in P. aeruginosa. Docking showed phosphate group binding to Tyr47 in the LasR loop, destabilizing dimerization and DNA binding. | [77] |
| 12 |  53 53 | Etherification (7-OH) with epichlorohydrin, followed by epoxide opening | Antimicrobial | Exhibited broad antibacterial/antifungal activity (MIC 4.68–9.37 μg/mL; inhibition zones > 20 mm). Docking supported binding to E. coli FabH and S. cerevisiae target enzymes, aligning with experimental potency. | [78] |
| 13 |  54a: n = 3, R = 4-methoxyphenyl 54a: n = 3, R = 4-methoxyphenyl54b: n = 3, R = benzyl 54c: n = 4, R = chlorodiphenylmethyl | Etherification (7-OH) followed by nucleophilic substitution with piperazine analogs | Antimicrobial | Active against S. pyogenes and E. coli (MIC 12.5 μg/mL); weaker activity for 54c. Docking indicated strong affinity of 54c for S. aureus TyrRS and E. coli DNA GyrB despite higher MIC values. | [79] |
| 14 |  55 55 | Etherification (7-OH) under microwave irradiation | Antimicrobial | Showed potent antibacterial activity (MIC 25–62.5 μg/mL) against MRSA, P. aeruginosa, K. pneumoniae, and E. coli. Nitro substitution enhanced potency up to 20-fold versus chrysin, effective even on resistant strains. | [80] |
| 15 | 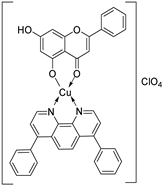 56 56 | Chelation with Cu(II)–1,10-phenanthroline complexes | Antioxidant | Demonstrated highest antioxidant capacity in ABTS assays among Cu–chrysin complexes. Improved H-atom transfer efficiency correlated with bulky phenanthroline ligand coordination. | [81] |
| 16 |  57 57 | Etherification (7-OH) | Antioxidant (Anti-Alzheimer) | Showed strong antioxidant and BuChE inhibition (sub-μM IC50) in vitro. Reduced Aβ1–42 aggregation and predicted BBB penetration, supporting AD therapeutic potential. | [82] |
| 17 | 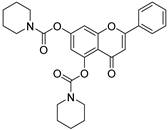 58 58 | Acylation with carbamoyl chloride derivatives (5,7-OH) | Antioxidant (Anti-Alzheimer) | Potent BuChE inhibitor (IC50 = 0.035 μM, >1000-fold more potent than AChE) with radical scavenging activity. Released free chrysin in vitro, chelating Cu2+/Fe2+ and blocking Aβ fibril formation. | [83] |
| 18 | 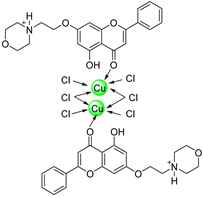 59 59 | Etherification with morphine derivative (7-OH) followed by chelation with Cu metal | Antioxidant (Anti-Alzheimer) | Enhanced antioxidant activity and cholinesterase inhibition in vitro. Suppressed Aβ aggregation with structural validation using X-ray crystallography. | [84] |
| 19 | 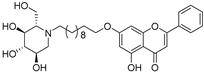 60 60 | Etherification (7-OH) followed by conjugation with 1-deoxynojirimycin | Antidiabetic | Strong α-glucosidase inhibition (IC50 = 0.51 μ M, 16× DNJ) via mixed-type binding. Docking revealed stable H-bonds and hydrophobic contacts with catalytic site. | [86] |
| 20 | 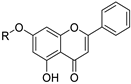 61a: R = (CH2)7CH3 61a: R = (CH2)7CH361b: R = CH2CHCH2 | Etherification (7-OH) | Antidiabetic | Showed superior α-glucosidase inhibition (IC50 = 0.08–3.47 μM) vs. acarbose. Compound 61a was competitive, while 61b and 62 acted as mixed inhibitors, demonstrating tunable inhibition modes. | [87] |
| 21 | 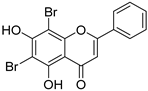 62 62 | Bromination | Antidiabetic | α-glucosidase inhibition (IC50 = 2.97 ± 0.03 μM) with mixed-type kinetics. | [87] |
| 22 | 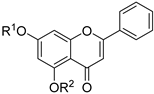 63a: R1 = prenyl, R2 = Bn 63a: R1 = prenyl, R2 = Bn63b: R1 = prenyl, R2 = prenyl | Etherification (5,7-OH) | Antidiabetic | Stimulated adiponectin secretion in hBMSCs and bound PPARγ. Compound 63b showed partial agonist activity comparable to that of pioglitazone, validated via docking and coactivator assays. | [88] |
| 23 | 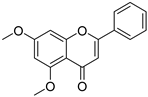 64 64 | Etherification (5,7-OH) | Melanogenesis stimulation | Enhanced melanogenesis: 2.7 times increase (1.9 times higher than chrysin). | [89] |
| 24 † | 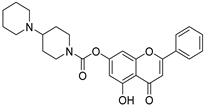 65 65 | Acylation with carbamoyl chloride derivatives (7-OH) | Anti-NAFLD | In vitro, it reduced lipid accumulation, oxidative stress, and hepatocellular injury in NAFLD model cells. In vivo, it alleviated hyperlipidemia, liver injury, body and liver weight gain, and insulin resistance in db/db mice. | [90] |
| 25 | 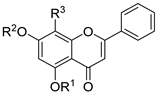 66a: R1 = H, R2 = CH3, R3 = H 66a: R1 = H, R2 = CH3, R3 = H66b: R1 = CH3, R2 = CH3, R3 = H 66c: R1 = H, R2 = H, R3 = NO2 66d: R1 = H, R2 = H, R3 = NH2 | 66a, b: Etherification (5,7-OH), 66c, d: Nitration followed by reduction | Vasorelaxant | 66a: reduced Ca2+ antagonism, weak vasorelaxant activity 66b: minimal Ca2+ blocking 66c: most potent vasorelaxant (pIC50 = 5.80, Emax ~99%), strong CaV1.2 inhibition 66d: similar efficacy to chrysin (Emax ~93–99%), vessel-selective vasorelaxation. | [91] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, C.Y.; Kim, C.-E.; Byun, E.-B.; Jeon, J. Chrysin as a Bioactive Scaffold: Advances in Synthesis and Pharmacological Evaluation. Int. J. Mol. Sci. 2025, 26, 9467. https://doi.org/10.3390/ijms26199467
Jeong CY, Kim C-E, Byun E-B, Jeon J. Chrysin as a Bioactive Scaffold: Advances in Synthesis and Pharmacological Evaluation. International Journal of Molecular Sciences. 2025; 26(19):9467. https://doi.org/10.3390/ijms26199467
Chicago/Turabian StyleJeong, Chae Yun, Chae-Eun Kim, Eui-Baek Byun, and Jongho Jeon. 2025. "Chrysin as a Bioactive Scaffold: Advances in Synthesis and Pharmacological Evaluation" International Journal of Molecular Sciences 26, no. 19: 9467. https://doi.org/10.3390/ijms26199467
APA StyleJeong, C. Y., Kim, C.-E., Byun, E.-B., & Jeon, J. (2025). Chrysin as a Bioactive Scaffold: Advances in Synthesis and Pharmacological Evaluation. International Journal of Molecular Sciences, 26(19), 9467. https://doi.org/10.3390/ijms26199467


_Kim.png)



