Snake Toxins Affecting Blood Vessel Walls: Mode of Action and Biological Significance
Abstract
1. Introduction
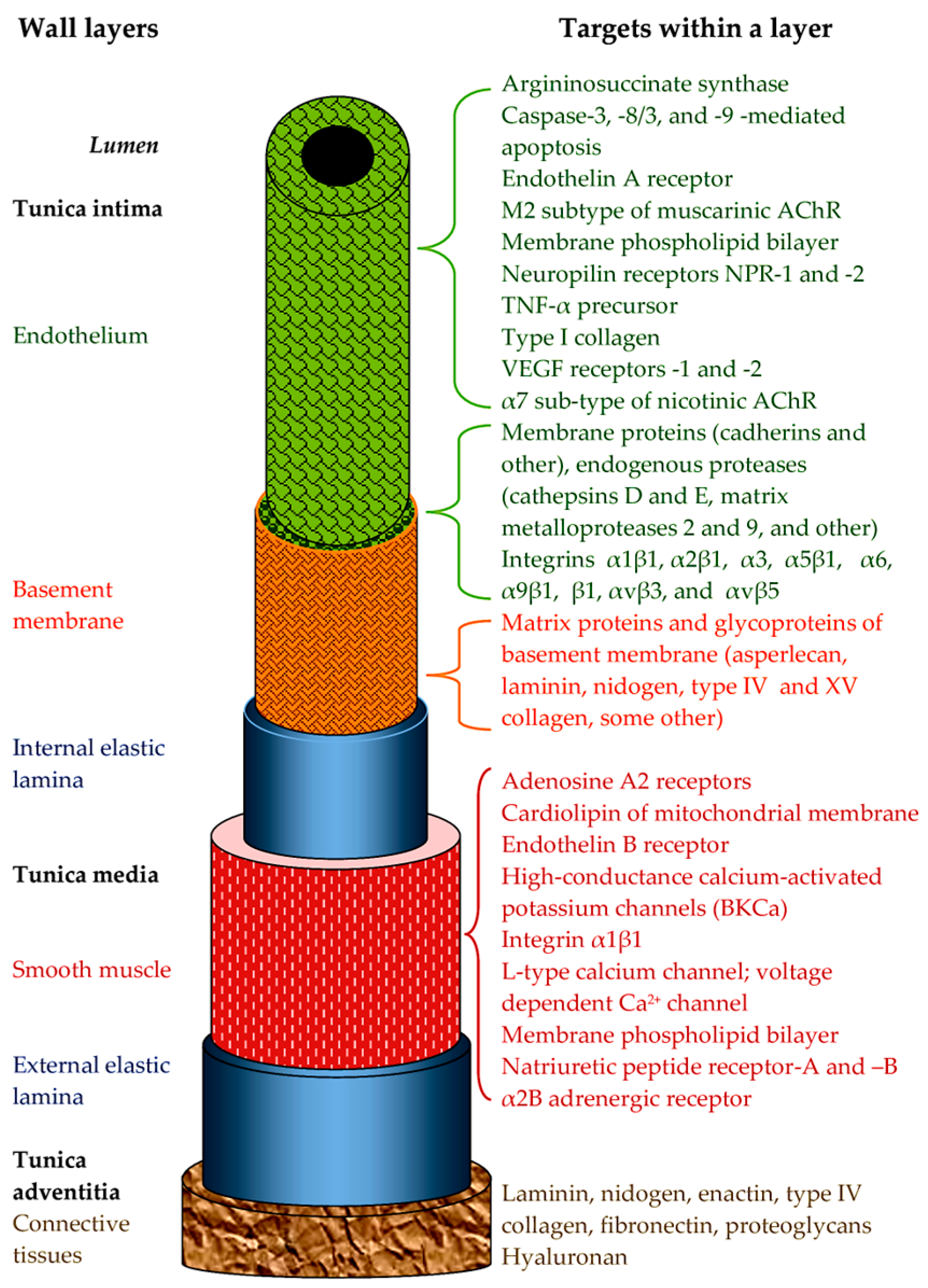
1.1. Snake Venom Damaging Strategies Within the CVS
- -
- -
- -
- By acting systemically on the tone of the vascular wall. For example, inhibition of the angiotensin-converting enzyme in the lung or kidney by toxins results in vessel relaxation throughout the bloodstream [5];
- -
- By acting directly on the walls of blood vessels.
- (i)
- The pharmacological activity of toxins aimed at changing vascular tone. Here, it is necessary to distinguish between direct effects on the cells of the vessel wall and indirect effects through nervous or humoral regulation. In this paper, we will only consider direct effects.
- (ii)
- The effects on the regenerative ability of vessel tissues. This includes effects on the growth and functioning of the vascular epithelium, as well as on neoangiogenesis.
- (iii)
- Direct damage to the structures of the vascular wall. Snake toxins can damage the plasma membranes of cells, disrupt intercellular interactions, and cleave the components of the extracellular matrix.
- (iv)
- Snake toxins, in addition to having damaging effects, can also have a protective effect on blood vessels.
1.2. Snake Venom Toxins Affecting the CVS
2. Toxins Affecting Vascular Tone
2.1. Bradykinin-Potentiating Peptides
2.2. Natriuretic Peptides
- -
- By endothelium-dependent vasorelaxation with increased NO production;
- -
- By lowering blood pressure by reducing vascular resistance (due to a decrease in the influx of Ca2+ ions into muscle cells);
- -
- By possibly also acting through K+ channels [3].
2.3. Sarafotoxins
2.4. Three-Finger Toxins
2.4.1. Cytotoxins
2.4.2. Muscarinic Toxins
2.4.3. Calciseptine and FS2
2.5. Vascular Endothelial Growth Factors
2.6. Phospholipases A2
2.7. Cysteine-Rich Secretory Proteins
2.8. Small Molecules and Nucleotidases
3. Toxins That Affect the Growth and Function of the Endothelium and the Regenerative Abilities of Blood Vessels
3.1. Vascular Endothelial Growth Factors
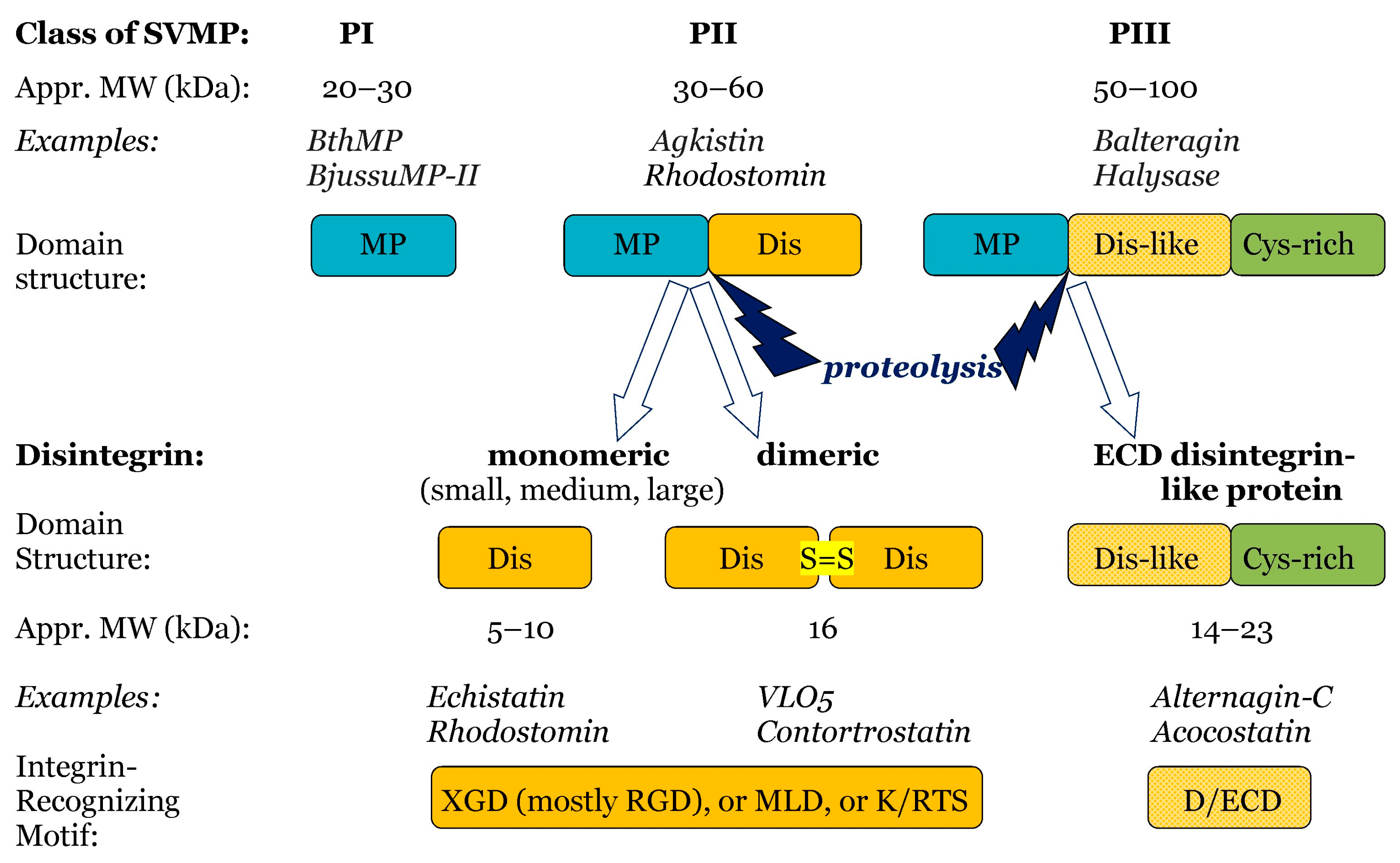
3.2. Disintegrins
- (i)
- By proteolysis of class P-II SVMPs, with cleavage between the catalytic domain and the disintegrin domain. Such disintegrins are referred to as RGD (Arg-Gly-Asp)-dependent, with the sequence XGD (X-Gly-Asp), MLD (Met-Leu-Asp), or K/RTS (Lys/Arg-Thr-Ser) on the exposed surface of the loop that specifically binds to integrins on target cells [53]. The group of RGD-dependent disintegrins can exist as monomers—small ones with four disulfides, medium ones with six disulfides, and large ones with seven disulfides. Moreover, there are two subgroups of disintegrins that form homo- or heterodimers, with 10 disulfides within each subunit [53]. According to their disulfide bond patterns, medium and large disintegrins can be further subdivided into two and three sub-groups, respectively [62].
- (ii)
3.3. Snake Venom Metalloproteinases
3.4. Snake C-Type Lectins
3.5. Kunitz-Type Serine Protease Inhibitor
3.6. Phospholipases A2
3.7. Cysteine-Rich Secretory Proteins
3.8. L-Amino Acid Oxidases
3.9. Inhibition of Neoangiogenesis as a Contributor to the Anti-Tumor Effect
3.10. Antiangiogenic Effect of α-Bungarotoxin
4. Toxins That Damage Vascular Wall Structures
4.1. Toxins That Damage the Lipid Bilayer of the Cell Membrane (Direct Cytotoxicity)
4.1.1. Cytotoxins
4.1.2. Phospholipases A2
4.2. Toxins Affecting the Cell Interaction with the Extracellular Matrix (ECM)
4.2.1. Disintegrins
4.2.2. Snake Venom Metalloproteinases
4.2.3. Vascular Apoptosis-Inducing Proteins and Disintegrins Evoke Endothelial Cell Apoptosis
4.2.4. Phospholipases A2
4.3. Toxins That Increase Vascular Permeability
4.3.1. Snake Venom Metalloproteinases
4.3.2. Disintegrins
4.3.3. Vascular Endothelial Growth Factors
4.3.4. Hyaluronidase
4.3.5. Crotaline Cysteine-Rich Secretory Proteins
4.3.6. γ-Bungarotoxin
4.3.7. Increased Vascular Permeability Due to Inflammation Caused by Snake Venom Toxins
5. Anti-Atherosclerotic Activity
6. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACE1 | Angiotensin-converting enzyme 1 |
| ADAM | A disintegrin and metalloproteinase |
| AR | Aortic ring |
| AsS | Argininosuccinate synthase |
| BCE | Bovine capillary endothelial |
| bFGF | Basic fibroblast growth factor |
| BM | Basement membrane |
| BKB2 | Bradykinin receptor B2 |
| BPP | Bradykinin-potentiating peptide |
| CAM | Chorioallantoic membrane |
| CRISP | Cysteine-rich secretory protein |
| CLLP | C-type lectin-like protein, or Snaclec |
| CTL | C-type lectin |
| CTX | Cytotoxin |
| ECM | Extracellular matrix |
| ET | Endothelin |
| FAK | Focal adhesion kinase |
| HBMEC | Human brain microvascular endothelial cell |
| HDMEC | Human dermal microvascular endothelial cell |
| HMEC-1 | Human microvascular endothelial cell line-1 |
| HUVEC | Human umbilical vein endothelial cell |
| ICPP | Increasing capillary permeability protein |
| MDLA | Methyl-D-L-aspartic acid |
| NP | Natriuretic peptide |
| NPR | Natriuretic peptide receptor |
| LAAO | L-amino acid oxidase |
| L-NAME | N(ω)-nitro-L-arginine methyl ester |
| NA | Noradrenaline |
| nAChR | Nicotinic acetyl choline receptor |
| NGF | Nerve growth factor |
| NOS | NO-synthase |
| PLA2 | Phospholipase A2 |
| ROS | Reactive oxygen species |
| SVMP | Snake venom metalloproteinase |
| TFT | Three-finger toxin |
| VAP | Vascular apoptosis-inducing protein |
| VEGF | Vascular endothelial growth factor |
| VEGFR | VEGF Receptor |
References
- Sajevic, T.; Leonardi, A.; Križaj, I. Haemostatically active proteins in snake venoms. Toxicon 2011, 57, 627–645. [Google Scholar] [CrossRef] [PubMed]
- Andrade, S.A.; de Souza, D.A.; Torres, A.L.; Oliveira, P.R.; Moreira, P.D.O.; da Silva, G.M.; Selistre-de-Araujo, H.S.; Chudzinski-Tavassi, A.M. Snake Envenomation: A component- based review on hemostatic alterations. Adv. Clin. Toxicol. 2022, 7, 236. [Google Scholar] [CrossRef]
- Messadi, E. Snake Venom Components as Therapeutic Drugs in Ischemic Heart Disease. Biomolecules 2023, 13, 1539. [Google Scholar] [CrossRef] [PubMed]
- Averin, A.S.; Utkin, Y.N. Cardiovascular Effects of Snake Toxins: Cardiotoxicity and Cardioprotection. Acta Naturae 2021, 13, 4–14. [Google Scholar] [CrossRef]
- Péterfi, O.; Boda, F.; Szabó, Z.; Ferencz, E.; Bába, L. Hypotensive Snake Venom Components—A Mini-Review. Molecules 2019, 24, 2778. [Google Scholar] [CrossRef]
- Averin, A.S.; Nenov, M.N.; Starkov, V.G.; Tsetlin, V.I.; Utkin, Y.N. Effects of Cardiotoxins from Naja oxiana Cobra Venom on Rat Heart Muscle and Aorta: A Comparative Study of Toxin-Induced Contraction Mechanisms. Toxins 2022, 14, 88. [Google Scholar] [CrossRef]
- Guerreiro, J.R.; Lameu, C.; Oliveira, E.F.; Klitzke, C.F.; Melo, R.L.; Linares, E.; Augusto, O.; Fox, J.W.; Lebrun, I.; Serrano, S.M.T.; et al. Argininosuccinate synthetase is a functional target for a snake venom anti-hypertensive peptide. Role in arginine and nitrix oxide production. J. Biol. Chem. 2009, 284, 20022–20033. [Google Scholar] [CrossRef]
- Morais, K.L.; Hayashi, M.A.; Bruni, F.M.; Lopes-Ferreira, M.; Camargo, A.C.; Ulrich, H.; Lameu, C. Bj-PRO-5a, a natural angiotensin-converting enzyme inhibitor, promotes vasodilatation mediated by both bradykinin B2 and M1 muscarinic acetylcholine receptors. Biochem. Pharmacol. 2011, 81, 736–742. [Google Scholar] [CrossRef]
- Ianzer, D.; Xavier, C.H.; Fraga, F.C.; Lautner, R.Q.; Guerreiro, J.R.; Machado, L.T.; Mendes, E.P.; de Camargo, A.C.; Santos, R.A. BPP-5a produces a potent and long-lasting NO-dependent antihypertensive effect. Ther. Adv. Cardiovasc. Dis. 2011, 5, 281–295. [Google Scholar] [CrossRef]
- Costa, S.R.; Vasconcelos, A.G.; Almeida, J.O.C.S.; Arcanjo, D.D.R.; Dematei, A.; Barbosa, E.A.; Silva, P.C.; Nascimento, T.; Santos, L.H.; Eaton, P.; et al. Structural Characterization and Rat Aortic Vascular Reactivity of Bradykinin-Potentiating Peptides (BPPs) from the Snake Venom of Bothrops moojeni from Delta do Parnaíba Region, Brazil. J. Nat. Prod. 2024, 87, 820–830. [Google Scholar] [CrossRef]
- Ang, W.F.; Koh, C.Y.; Kini, R.M. From Snake Venoms to Therapeutics: A Focus on Natriuretic Peptides. Pharmaceuticals 2022, 15, 1153. [Google Scholar] [CrossRef]
- Singh, G.; Maguire, J.J.; Kuc, R.E.; Skepper, J.N.; Fidock, M.; Davenport, A.P. Characterization of the snake venom ligand [125I]-DNP binding to natriuretic peptide receptor-A in human artery and potent DNP mediated vasodilatation. Br. J. Pharmacol. 2006, 149, 838–844. [Google Scholar] [CrossRef]
- Fry, B.G.; Wickramaratana, J.C.; Lemme, S.; Beuve, A.; Garbers, D.; Hodgson, W.C.; Alewood, P. Novel natriuretic peptides from the venom of the inland taipan (Oxyuranus microlepidotus): Isolation, chemical and biological characterisation. Biochem. Biophys. Res. Commun. 2005, 327, 1011–1015. [Google Scholar] [CrossRef]
- Sridharan, S.; Kini, R.M. Tail wags the dog: Activity of krait natriuretic peptide is determined by its C-terminal tail in a natriuretic peptide receptor-independent manner. Biochem. J. 2015, 469, 255–266. [Google Scholar] [CrossRef]
- Reeks, T.; Jones, A.; Brust, A.; Sridharan, S.; Corcilius, L.; Wilkinson, B.L.; Thaysen-Andersen, M.; Payne, R.J.; Kini, R.M.; Daly, N.L.; et al. A defined α-helix in the bifunctional O-glycosylated natriuretic peptide TcNPa from the venom of Tropidechis carinatus. Angew. Chem. Int. Ed. Engl. 2015, 54, 4828–4831. [Google Scholar] [CrossRef]
- Evangelista, J.S.; Martins, A.M.; Nascimento, N.R.; Sousa, C.M.; Alves, R.S.; Toyama, D.O.; Toyama, M.H.; Evangelista, J.J.; Menezes, D.B.; Fonteles, M.C.; et al. Renal and vascular effects of the natriuretic peptide isolated from Crotalus durissus cascavella venom. Toxicon 2008, 52, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, S.L.; Almeida, J.R.; Resende, L.M.; Martins, W.; Henriques, F.A.F.A.; Baldasso, P.A.; Soares, A.M.; Taranto, A.G.; Resende, R.R.; Marangoni, S.; et al. Isolation and Characterization of a Natriuretic Peptide from Crotalus oreganus abyssus (Grand Canyon Rattlesnake) and its Effects on Systemic Blood Pressure and Nitrite Levels. Int. J. Pept. Res. Ther. 2011, 17, 165–173. [Google Scholar] [CrossRef]
- Iglarz, M.; Steiner, P.; Wanner, D.; Rey, M.; Hess, P.; Clozel, M. Vascular Effects of Endothelin Receptor Antagonists Depends on Their Selectivity for ETA Versus ETB Receptors and on the Functionality of Endothelial ETB Receptors. J. Cardiovasc. Pharmacol. 2015, 66, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Maguire, J.J.; Kuc, R.E.; Pell, V.R.; Green, A.; Brown, M.; Kumar, S.; Wehrman, T.; Quinn, E.; Davenport, A.P. Comparison of human ETA and ETB receptor signalling via G-protein and β-arrestin pathways. Life Sci. 2012, 91, 544–549. [Google Scholar] [CrossRef]
- Izume, T.; Miyauchi, H.; Shihoya, W.; Nureki, O. Crystal structure of human endothelin ETB receptor in complex with sarafotoxin S6b. Biochem. Biophys. Res. Commun. 2020, 528, 383–388. [Google Scholar] [CrossRef]
- Utkin, Y.; Sunagar, K.; Jackson, T.N.; Reeks, T.; Fry, B.G. Three-finger toxins (3FTxs). In Venomous Reptiles and Their Toxins: Evolution, Pathophysiology and Biodiscovery; Fry, B.G., Ed.; Oxford University Press: New York, NY, USA, 2015; pp. 215–227. [Google Scholar]
- Ho, K.H.; Kwan, C.Y.; Huang, S.J.; Bourreau, J.P. Dual effect of cobra cardiotoxin on vascular smooth muscle and endothelium. Zhongguo Yao Li Xue Bao 1998, 19, 197–202. [Google Scholar]
- Chen, K.M.; Guan, Y.Y.; Sun, J.J. Effects of direct lytic factors from southern Chinese cobra venom on Ca2+ movement in rabbit aorta strip. Zhongguo Yao Li Xue Bao 1993, 14, 500–504. [Google Scholar] [PubMed]
- Kwan, C.Y.; Kwan, T.K.; Huang, S.J. Effect of calcium on the vascular contraction induced by cobra venom cardiotoxin. Clin. Exp. Pharmacol. Physiol. 2002, 29, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.J.; Leung, Y.M.; Huang, S.J.; Kwan, C.Y. Dual effects of extracellular Ca2+ on cardiotoxin-induced cytotoxicity and cytosolic Ca2+ changes in cultured single cells of rabbit aortic endothelium. Biochim. Biophys. Acta 1997, 1330, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Koivula, K.; Rondinelli, S.; Näsman, J. The Three-Finger Toxin MTα Is a Selective A2B-Adrenoceptor Antagonist. Toxicon 2010, 56, 440–447. [Google Scholar] [CrossRef]
- Ciolek, J.; Zoukimian, C.; Dhot, J.; Burban, M.; Triquigneaux, M.; Lauzier, B.; Guimbert, C.; Boturyn, D.; Ferron, M.; Ciccone, L.; et al. MT9, a natural peptide from black mamba venom antagonizes the muscarinic type 2 receptor and reverses the M2R-agonist-induced relaxation in rat and human arteries. Biomed. Pharmacother. 2022, 150, 113094. [Google Scholar] [CrossRef]
- Mesirca, P.; Chemin, J.; Barrère, C.; Torre, E.; Gallot, L.; Monteil, A.; Bidaud, I.; Diochot, S.; Lazdunski, M.; Soong, T.W.; et al. Selective blockade of Cav1.2 (α1C) versus Cav1.3 (α1D) L-type calcium channels by the black mamba toxin calciseptine. Nat. Commun. 2024, 15, 54. [Google Scholar] [CrossRef]
- de Weille, J.R.; Schweitz, H.; Maes, P.; Tartar, A.; Lazdunski, M. Calciseptine, a peptide isolated from black mamba venom, is a specific blocker of the L-type calcium channel. Proc. Natl. Acad. Sci. USA 1991, 88, 2437–2440. [Google Scholar] [CrossRef]
- Watanabe, T.X.; Itahara, Y.; Kuroda, H.; Chen, Y.N.; Kimura, T.; Sakakibara, S. Smooth muscle relaxing and hypotensive activities of synthetic calciseptine and the homologous snake venom peptide FS2. Jpn. J. Pharmacol. 1995, 68, 305–313. [Google Scholar] [CrossRef]
- Ferreira, I.G.; Pucca, M.B.; Oliveira, I.S.; Cerni, F.A.; Jacob, B.C.D.S.; Arantes, E.C. Snake venom vascular endothelial growth factors (svVEGFs): Unravelling their molecular structure, functions, and research potential. Cytokine Growth Factor Rev. 2021, 60, 133–143. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Matsunaga, Y.; Tokunaga, Y.; Obayashi, S.; Saito, M.; Morita, T. Snake venom Vascular Endothelial Growth Factors (VEGF-Fs) exclusively vary their structures and functions among species. J. Biol. Chem. 2009, 284, 9885–9891. [Google Scholar] [CrossRef]
- Komori, Y.; Nikai, T.; Taniguchi, K.; Masuda, K.; Sugihara, H. Vascular endothelial growth factor VEGF-like heparin-binding protein from the venom of Vipera aspis aspis (Aspic viper). Biochemistry 1999, 38, 11796–11803. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Takani, K.; Atoda, H.; Morita, T. Snake venom vascular endothelial growth factors (VEGFs) exhibit potent activity through their specific recognition of KDR (VEGF receptor 2). J. Biol. Chem. 2003, 278, 51985–51988. [Google Scholar] [CrossRef]
- ExplorEnz—The Enzyme Database. Available online: www.enzyme-database.org (accessed on 15 August 2025).
- Castro-Amorim, J.; Novo de Oliveira, A.; Da Silva, S.L.; Soares, A.M.; Mukherjee, A.K.; Ramos, M.J.; Fernandes, P.A. Catalytically Active Snake Venom PLA2 Enzymes: An Overview of Its Elusive Mechanisms of Reaction. J. Med. Chem. 2023, 66, 5364–5376. [Google Scholar] [CrossRef]
- Kini, R.M.; Evans, H.J. A model to explain the pharmacological effects of snake venom phospholipases A2. Toxicon 1989, 27, 613–635. [Google Scholar] [CrossRef] [PubMed]
- Vuong, N.T.; Jackson, T.N.W.; Wright, C.E. Role of Phospholipases A2 in Vascular Relaxation and Sympatholytic Effects of Five Australian Brown Snake, Pseudonaja spp., Venoms in Rat Isolated Tissues. Front. Pharmacol. 2021, 12, 754304. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Lee, C.Y. Relaxant effect of phospholipase A2 from Vipera russelli snake venom on rat aorta. Eur. J. Pharmacol. 1985, 118, 139–146. [Google Scholar] [CrossRef]
- Chaisakul, J.; Isbister, G.K.; Tare, M.; Parkington, H.C.; Hodgson, W.C. Hypotensive and vascular relaxant effects of phospholipase A2 toxins from Papuan taipan (Oxyuranus scutellatus) venom. Eur. J. Pharmacol. 2014, 723, 227–233. [Google Scholar] [CrossRef]
- Averin, A.; Starkov, V.; Tsetlin, V.; Utkin, Y. Effects of the Heterodimeric Neurotoxic Phospholipase A2 from the Venom of Vipera nikolskii on the Contractility of Rat Papillary Muscles and Thoracic Aortas. Toxins 2024, 16, 100. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, C.M.; Rey, F.M.; Cintra, A.C.O.; Sampaio, S.V.; Torqueti, M.R. Effects of crotoxin, a neurotoxin from Crotalus durissus terrificus snake venom, on human endothelial cells. Int. J. Biol. Macromol. 2019, 134, 613–621. [Google Scholar] [CrossRef]
- Zou, Z.; Zeng, F.; Zhang, L.; Niu, L.; Teng, M.; Li, X. Purification, crystallization and preliminary X-ray diffraction analysis of an acidic phospholipase A2 with vasoconstrictor activity from Agkistrodon halys pallas venom. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2012, 68, 1329–1332. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Zou, Z.; Niu, L.; Li, X.; Teng, M. AhV_aPA-induced vasoconstriction involves the IP3Rs-mediated Ca2+ releasing. Toxicon 2013, 70, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Pitari, G.M.; Giuliano, F.; Bianchi, A.; Corrado, A.P. Responses of rat aortic rings to crotoxin. Fundam. Clin. Pharmacol. 1996, 10, 180. [Google Scholar]
- Tadokoro, T.; Modahl, C.M.; Maenaka, K.; Aoki-Shioi, N. Cysteine-Rich Secretory Proteins (CRISPs) From Venomous Snakes: An Overview of the Functional Diversity in A Large and Underappreciated Superfamily. Toxins 2020, 12, 175. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Koike, H.; Sugiyama, Y.; Motoyoshi, K.; Wada, T.; Hishinuma, S.; Mita, M.; Morita, T. Cloning and Characterization of Novel Snake Venom Proteins That Block Smooth Muscle Contraction: Novel Proteins in Snake Venoms. Eur. J. Biochem. 2002, 269, 2708–2715. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Hyodo, F.; Morita, T. Wide Distribution of Cysteine-Rich Secretory Proteins in Snake Venoms: Isolation and Cloning of Novel Snake Venom Cysteine-Rich Secretory Proteins. Arch. Biochem. Biophys. 2003, 412, 133–141. [Google Scholar] [CrossRef]
- Wang, J.; Shen, B.; Guo, M.; Lou, X.; Duan, Y.; Cheng, X.P.; Teng, M.; Niu, L.; Liu, Q.; Huang, Q.; et al. Blocking effect and crystal structure of natrin toxin, a cysteine-rich secretory protein from Naja atra venom that targets the BKCa channel. Biochemistry 2005, 44, 10145–10152. [Google Scholar] [CrossRef]
- Villar-Briones, A.; Aird, S.D. Organic and Peptidyl Constituents of Snake Venoms: The Picture Is Vastly More Complex Than We Imagined. Toxins 2018, 10, 392. [Google Scholar] [CrossRef]
- Caccin, P.; Pellegatti, P.; Fernandez, J.; Vono, M.; Cintra-Francischinelli, M.; Lomonte, B.; Gutiérrez, J.M.; Di Virgilio, F.; Montecucco, C. Why myotoxin-containing snake venoms possess powerful nucleotidases? Biochem. Biophys. Res. Commun. 2013, 430, 1289–1293. [Google Scholar] [CrossRef]
- Dhananjaya, B.L.; D’Souza, C.J. The pharmacological role of phosphatases (acid and alkaline phosphomonoesterases) in snake venoms related to release of purines—A multitoxin. Basic. Clin. Pharmacol. Toxicol. 2011, 108, 79–83. [Google Scholar] [CrossRef]
- Clissa, P.B.; Della-Casa, M.S.; Zychar, B.C.; Sanabani, S.S. The Role of Snake Venom Disintegrins in Angiogenesis. Toxins 2024, 16, 127. [Google Scholar] [CrossRef]
- Takahashi, H.; Hattori, S.; Iwamatsu, A.; Takizawa, H.; Shibuya, M. A novel snake venom vascular endothelial growth factor (VEGF) predominantly induces vascular permeability through preferential signaling via VEGF receptor-1. J. Biol. Chem. 2004, 279, 46304–46314. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.C.; Staniszewska, I.; Del Valle, L.; Tuszynski, G.P.; Marcinkiewicz, C. Angiostatic activity of obtustatin as α1β1 integrin inhibitor in experimental melanoma growth. Int. J. Cancer 2008, 123, 2195–2203. [Google Scholar] [CrossRef] [PubMed]
- Toivanen, P.I.; Nieminen, T.; Laakkonen, J.P.; Heikura, T.; Kaikkonen, M.U.; Ylä-Herttuala, S. Snake venom VEGF Vammin induces a highly efficient angiogenic response in skeletal muscle via VEGFR-2/NRP specific signaling. Sci. Rep. 2017, 7, 5525. [Google Scholar] [CrossRef] [PubMed]
- Gasmi, A.; Bourcier, C.; Aloui, Z.; Srairi, N.; Marchetti, S.; Gimond, C.; Wedge, S.R.; Hennequin, L.; Pouysségur, J. Complete structure of an increasing capillary permeability protein (ICPP) purified from Vipera lebetina venom. ICPP is angiogenic via vascular endothelial growth factor receptor signalling. J. Biol. Chem. 2002, 277, 29992–29998. [Google Scholar] [CrossRef]
- Chen, Y.L.; Tsai, I.H.; Hong, T.M.; Tsai, S.H. Crotalid venom vascular endothelial growth factors has preferential affinity for VEGFR-1. Characterization of Protobothrops mucrosquamatus venom VEGF. Thromb. Haemost. 2005, 93, 331–338. [Google Scholar] [CrossRef]
- Brown, M.C.; Calvete, J.J.; Staniszewska, I.; Walsh, E.M.; Perez-Liz, G.; Del Valle, L.; Lazarovici, P.; Marcinkiewicz, C. VEGF-related protein isolated from Vipera palestinae venom, promotes angiogenesis. Growth Factors 2007, 25, 108–117. [Google Scholar] [CrossRef]
- Ferreira, I.; Oliveira, I.; Bordon, K.; Reis, M.; Wiezel, G.; Sanchez, C.; Santos, L.; Santos-Filho, N.; Pucca, M.; Antunes, L.; et al. Beyond Angiogenesis: The Multitasking Approach of the First PEGylated Vascular Endothelial Growth Factor (CdtVEGF) from Brazilian Rattlesnake Venom. Toxins 2023, 15, 483. [Google Scholar] [CrossRef]
- Santana, H.M.; Ikenohuchi, Y.J.; Silva, M.D.S.; Farias, B.J.C.; Serrath, S.N.; Da Silva, C.P.; Magalhães, J.G.D.S.; Cruz, L.F.; Cardozo, D.G.; Ferreira, E.; et al. BjussuMP-II, a venom metalloproteinase, induces the release and cleavage of pro-inflammatory cytokines and disrupts human umbilical vein endothelial cells. Chem. Biol. Interact. 2024, 394, 110986. [Google Scholar] [CrossRef]
- Almeida, G.O.; de Oliveira, I.S.; Arantes, E.C.; Sampaio, S.V. Snake venom disintegrins update: Insights about new findings. J. Venom. Anim. Toxins Incl. Trop. Dis. 2023, 29, e20230039. [Google Scholar] [CrossRef]
- Marcinkiewicz, C. Applications of snake venom components to modulate integrin activities in cell-matrix interactions. Int. J. Biochem. Cell Biol. 2013, 45, 1974–1986. [Google Scholar] [CrossRef]
- Yeh, C.H.; Peng, H.C.; Huang, T.F. Accutin, a new disintegrin, inhibits angiogenesis in vitro and in vivo by acting as integrin alphavbeta3 antagonist and inducing apoptosis. Blood 1998, 92, 3268–3276. [Google Scholar] [CrossRef]
- Hong, S.Y.; Lee, H.; You, W.K.; Chung, K.H.; Kim, D.S.; Song, K. The snake venom disintegrin salmosin induces apoptosis by disassembly of focal adhesions in bovine capillary endothelial cells. Biochem. Biophys. Res. Commun. 2003, 302, 502–508. [Google Scholar] [CrossRef]
- Juliano, D.; Wang, Y.; Marcinkiewicz, C.; Rosenthal, L.A.; Stewart, G.J.; Niewiarowski, S. Disintegrin interaction with αvβ3 integrin on human umbilical vein endothelial cells: Expression of ligand-induced binding site on β3 subunit. Exp. Cell Res. 1996, 225, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.H.; Peng, H.C.; Yang, R.S.; Huang, T.F. Rhodostomin, a snake venom disintegrin, inhibits angiogenesis elicited by basic fibroblast growth factor and suppresses tumor growth by a selective αvβ3 blockade of endothelial cells. Mol. Pharmacol. 2001, 59, 1333–1342. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.Y.; Koh, Y.S.; Chung, K.H.; Kim, D.S. Snake venom disintegrin, saxatilin, inhibits platelet aggregation, human umbilical vein endothelial cell proliferation, and smooth muscle cell migration. Thromb. Res. 2002, 105, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Ramos, O.H.; Kauskot, A.; Cominetti, M.R.; Bechyne, I.; Salla Pontes, C.L.; Chareyre, F.; Manent, J.; Vassy, R.; Giovannini, M.; Legrand, C.; et al. A novel αvβ3-blocking disintegrin containing the RGD motive, DisBa-01, inhibits bFGF-induced angiogenesis and melanoma metastasis. Clin. Exp. Metastasis 2008, 25, 53–64. [Google Scholar] [CrossRef]
- Casali, B.C.; Baptista, M.P.; Pachane, B.C.; Cortez, A.A.; Altei, W.F.; Selistre-de-Araújo, H.S. Blockage of αvβ3 integrin in 3D culture of triple-negative breast cancer and endothelial cells inhibits migration and discourages endothelial-to-mesenchymal plasticity. Biochem. Biophys. Rep. 2024, 38, 101686. [Google Scholar] [CrossRef]
- Montenegro, C.F.; Salla-Pontes, C.L.; Ribeiro, J.U.; Machado, A.Z.; Ramos, R.F.; Figueiredo, C.C.; Morandi, V.; Selistre-de-Araujo, H.S. Blocking αvβ3 integrin by a recombinant RGD disintegrin impairs VEGF signaling in endothelial cells. Biochimie 2012, 94, 1812–1820. [Google Scholar] [CrossRef]
- Ferreira, B.A.; De Moura, F.B.R.; Tomiosso, T.C.; Correa, N.C.R.; Goulart, L.R.; Barcelos, L.S.; Clissa, P.B.; Araujo, F.A. Jararhagin-C, a disintegrin-like protein, improves wound healing in mice through stimulation of M2-like macrophage, angiogenesis and collagen deposition. Int. Immunopharmacol. 2021, 101, 108224. [Google Scholar] [CrossRef]
- Selistre-de-Araujo, H.S.; Cominetti, M.R.; Terruggi, C.H.; Mariano-Oliveira, A.; De Freitas, M.S.; Crepin, M.; Figueiredo, C.C.; Morandi, V. Alternagin-C, a disintegrin-like protein from the venom of Bothrops alternatus, modulates α2β1 integrin-mediated cell adhesion, migration and proliferation. Braz. J. Biol. Res. 2005, 38, 1505–1511. [Google Scholar] [CrossRef]
- Dos Santos, P.K.; Altei, W.F.; Danilucci, T.M.; Lino, R.L.B.; Pachane, B.C.; Nunes, A.C.C.; Selistre-de-Araujo, H.S. Alternagin-C (ALT-C), a disintegrin-like protein, attenuates α2β1 integrin and VEGF receptor 2 signaling resulting in angiogenesis inhibition. Biochimie 2020, 174, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Sant’Ana, E.M.; Gouvêa, C.M.; Nakaie, C.R.; Selistre-de-Araújo, H.S. Angiogenesis and growth factor modulation induced by alternagin C, a snake venom disintegrin-like, cysteine-rich protein on a rat skin wound model. Arch. Biochem. Biophys. 2008, 479, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Cominetti, M.R.; Terruggi, C.H.; Ramos, O.H.; Fox, J.W.; Mariano-Oliveira, A.; De Freitas, M.S.; Figueiredo, C.C.; Morandi, V.; Selistre-de-Araujo, H.S. Alternagin-C, a disintegrin-like protein, induces vascular endothelial cell growth factor (VEGF) expression and endothelial cell proliferation in vitro. J. Biol. Chem. 2004, 279, 18247–18255. [Google Scholar] [CrossRef]
- Sheu, J.R.; Yen, M.H.; Hung, W.C.; Lee, Y.M.; Su, C.H.; Huang, T.F. Triflavin inhibits platelet-induced vasoconstriction in de-endothelialized aorta. Arter. Thromb. Vasc. Biol. 1997, 17, 3461–3468. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.M.; Kim, R.; Del Valle, L.; Weaver, M.; Sheffield, J.; Lazarovici, P.; Marcinkiewicz, C. Importance of interaction between nerve growth factor and α9β1 integrin in glial tumor angiogenesis. Neuro Oncol. 2012, 14, 890–901. [Google Scholar] [CrossRef]
- Bolás, G.; de Rezende, F.F.; Lorente, C.; Sanz, L.; Eble, J.A.; Calvete, J.J. Inhibitory effects of recombinant RTS-jerdostatin on integrin α1β1 function during adhesion, migration and proliferation of rat aortic smooth muscle cells and angiogenesis. Toxicon 2014, 79, 45–54. [Google Scholar] [CrossRef]
- Takeda, S. ADAM and ADAMTS Family Proteins and Snake Venom Metalloproteinases: A Structural Overview. Toxins 2016, 8, 155. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Escalante, T.; Rucavado, A.; Herrera, C. Hemorrhage Caused by Snake Venom Metalloproteinases: A Journey of Discovery and Understanding. Toxins 2016, 8, 93. [Google Scholar] [CrossRef]
- Olaoba, O.T.; Karina Dos Santos, P.; Selistre-de-Araujo, H.S.; Ferreira de Souza, D.H. Snake Venom Metalloproteinases (SVMPs): A structure-function update. Toxicon X 2020, 7, 100052. [Google Scholar] [CrossRef]
- Bickler, P.E. Amplification of Snake Venom Toxicity by Endogenous Signaling Pathways. Toxins 2020, 12, 68. [Google Scholar] [CrossRef]
- Bhat, S.K.; Joshi, M.B.; Vasishta, S.; Jagadale, R.N.; Biligiri, S.G.; Coronado, M.A.; Arni, R.K.; Satyamoorthy, K. P-I metalloproteinases and L-amino acid oxidases from Bothrops species inhibit angiogenesis. J. Venom. Anim. Toxins Incl. Trop. Dis. 2021, 27, e20200180. [Google Scholar] [CrossRef]
- Oliveira, V.Q.; Santos, L.C.; Teixeira, S.C.; Correia, T.M.L.; Andrade, L.O.S.B.; Gimenes, S.N.C.; Colombini, M.; Marques, L.M.; Jiménez-Charris, E.; Freitas-de-Sousa, L.A.; et al. Antiangiogenic properties of BthMP, a P-I metalloproteinase from Bothrops moojeni snake venom by VEGF pathway in endothelial cells. Biochem. Biophys. Res. Commun. 2024, 706, 149748. [Google Scholar] [CrossRef]
- Eble, J.A. Structurally Robust and Functionally Highly Versatile-C-Type Lectin (-Related) Proteins in Snake Venoms. Toxins 2019, 11, 136. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.D.; Wong, W.Y.; Lee, M.M.; Yue, P.Y.; Dai, X.; Tsim, K.W.; Hsiao, W.W.; Li, M.; Li, X.Y.; Tai, W.C. Isolation and characterization of ZK002, a novel dual function snake venom protein from Deinagkistrodon acutus with anti-angiogenic and anti-inflammatory properties. Front. Pharmacol. 2023, 14, 1227962. [Google Scholar] [CrossRef] [PubMed]
- Pilorget, A.; Conesa, M.; Sarray, S.; Michaud-Levesque, J.; Daoud, S.; Kim, K.S.; Demeule, M.; Marvaldi, J.; El Ayeb, M.; Marrakchi, N.; et al. Lebectin, a Macrovipera lebetina venom-derived C-type lectin, inhibits angiogenesis both in vitro and in vivo. J. Cell. Physiol. 2007, 211, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Montassar, F.; Darche, M.; Blaizot, A.; Augustin, S.; Conart, J.B.; Millet, A.; Elayeb, M.; Sahel, J.A.; Réaux-Le Goazigo, A.; Sennlaub, F.; et al. Lebecetin, a C-type lectin, inhibits choroidal and retinal neovascularization. FASEB J. 2017, 31, 1107–1119. [Google Scholar] [CrossRef]
- Momic, T.; Cohen, G.; Reich, R.; Arlinghaus, F.T.; Eble, J.A.; Marcinkiewicz, C.; Lazarovici, P. Vixapatin (VP12), a c-type lectin-protein from Vipera xantina palestinae venom: Characterization as a novel anti-angiogenic compound. Toxins 2012, 4, 862–877. [Google Scholar] [CrossRef]
- Baudou, F.G.; Charó, N.L.; Scheidegger, M.A.; Stupirski, J.C.; Pérez Sáez, J.M.; Troncoso, M.F.; Massaro, M.; de Roodt, A.R.; De Marzi, M.C.; Schattner, M.; et al. A C-type lectin from Bothrops jararacussu venom reprograms endothelial cell biology. Angiogenesis 2024, 27, 583–586. [Google Scholar] [CrossRef]
- Mishra, M. Evolutionary Aspects of the Structural Convergence and Functional Diversification of Kunitz-Domain Inhibitors. J. Mol. Evol. 2020, 88, 537–548. [Google Scholar] [CrossRef]
- Morjen, M.; Honoré, S.; Bazaa, A.; Abdelkafi-Koubaa, Z.; Ellafi, A.; Mabrouk, K.; Kovacic, H.; El Ayeb, M.; Marrakchi, N.; Luis, J. PIVL, a snake venom Kunitz-type serine protease inhibitor, inhibits in vitro and in vivo angiogenesis. Microvasc. Res. 2014, 95, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Morjen, M.; Moslah, W.; Touihri-Baraketi, I.; Srairi-Abid, N.; Luis, J.; Marrakchi, N.; Jebali, J. Expression of the First Recombinant Anti-Tumoral Snake Venom Kunitz-Type Serine Protease Inhibitor. Toxins 2022, 14, 170. [Google Scholar] [CrossRef] [PubMed]
- Zouari-Kessentini, R.; Srairi-Abid, N.; Bazaa, A.; El Ayeb, M.; Luis, J.; Marrakchi, N. Antitumoral potential of Tunisian snake venoms secreted phospholipases A2. Biomed. Res. Int. 2013, 2013, 391389. [Google Scholar] [CrossRef] [PubMed]
- Bazaa, A.; Pasquier, E.; Defilles, C.; Limam, I.; Kessentini-Zouari, R.; Kallech-Ziri, O.; El Battari, A.; Braguer, D.; El Ayeb, M.; Marrakchi, N.; et al. MVL-PLA2, a snake venom phospholipase A2, inhibits angiogenesis through an increase in microtubule dynamics and disorganization of focal adhesions. PLoS ONE 2010, 5, e10124. [Google Scholar] [CrossRef]
- Sasovsky, D.J.; Angelina, E.; Leiva, L.C.; Bal de Kier Joffé, E.; Lomonte, B.; Bustillo, S. Comparative in vitro and in silico analysis of the ability of basic Asp49 phospholipase A2 and Lys49-phospholipase A2-like myotoxins from Bothrops diporus venom to inhibit the metastatic potential of murine mammary tumor cells and endothelial cell tubulogenesis: Asp49 vs Lys49 phospholipases A2: Inhibition of metastasis and angiogenesis. Chem. Biol. Interact. 2024, 402, 111217. [Google Scholar] [CrossRef]
- Fujisawa, D.; Yamazaki, Y.; Lomonte, B.; Morita, T. Catalytically inactive phospholipase A2 homologue binds to vascular endothelial growth factor receptor-2 via a C-terminal loop region. Biochem. J. 2008, 411, 515–522. [Google Scholar] [CrossRef]
- Polloni, L.; Azevedo, F.V.P.V.; Teixeira, S.C.; Moura, E.; Costa, T.R.; Gimenes, S.N.C.; Correia, L.I.V.; Freitas, V.; Yoneyama, K.A.G.; Rodrigues, R.S.; et al. Antiangiogenic effects of phospholipase A2 Lys49 BnSP-7 from Bothrops pauloensis snake venom on endothelial cells: An in vitro and ex vivo approach. Toxicol. Vitr. 2021, 72, 105099. [Google Scholar] [CrossRef]
- Van Petten de Vasconcelos Azevedo, F.; Lopes, D.S.; Zóia, M.A.P.; Correia, L.I.V.; Saito, N.; Fonseca, B.B.; Polloni, L.; Teixeira, S.C.; Goulart, L.R.; de Melo Rodrigues Ávila, V. A New Approach to Inhibiting Triple-Negative Breast Cancer: In Vitro, Ex Vivo and In Vivo Antiangiogenic Effect of BthTx-II, a PLA2-Asp-49 from Bothrops jararacussu Venom. Biomolecules 2022, 12, 258. [Google Scholar] [CrossRef]
- Santos, L.C.; Oliveira, V.Q.; Teixeira, S.C.; Correia, T.M.L.; Andrade, L.O.S.B.; Polloni, L.; Marques, L.M.; Clissa, P.B.; Baldo, C.; Ferro, E.A.V.; et al. PLA2-MjTX-II from Bothrops moojeni snake venom exhibits antimetastatic and antiangiogenic effects on human lung cancer cells. Toxicon 2024, 243, 107742. [Google Scholar] [CrossRef]
- Kato, E.E.; Pimenta, L.A.; de Almeida, M.E.S.; Zambelli, V.O.; Dos Santos, M.F.; Sampaio, S.C. Crotoxin Inhibits Endothelial Cell Functions in Two- and Three-dimensional Tumor Microenvironment. Front. Pharmacol. 2021, 12, 713332. [Google Scholar] [CrossRef]
- Lecht, S.; Chiaverelli, R.A.; Gerstenhaber, J.; Calvete, J.J.; Lazarovici, P.; Casewell, N.R.; Harrison, R.; Lelkes, P.I.; Marcinkiewicz, C. Anti-angiogenic activities of snake venom CRISP isolated from Echis carinatus sochureki. Biochim. Biophys. Acta 2015, 1850, 1169–1179. [Google Scholar] [CrossRef]
- Wang, Y.L.; Kuo, J.H.; Lee, S.C.; Liu, J.S.; Hsieh, Y.C.; Shih, Y.T.; Chen, C.J.; Chiu, J.J.; Wu, W.G. Cobra CRISP functions as an inflammatory modulator via a novel Zn2+- and heparan sulfate-dependent transcriptional regulation of endothelial cell adhesion molecules. J. Biol. Chem. 2010, 285, 37872–37883. [Google Scholar] [CrossRef]
- Morais, I.C.O.; Pereira, G.J.S.; Orzáez, M.; Jorge, R.J.B.; Bincoletto, C.; Toyama, M.H.; Monteiro, H.A.S.; Smaili, S.S.; Pérez-Payá, E.; Martins, A.M.C. L-aminoacid oxidase from Bothrops leucurus venom induces nephrotoxicity via apoptosis and necrosis. PLoS ONE 2015, 10, e0132569. [Google Scholar] [CrossRef]
- Torii, S.; Naito, M.; Tsuruo, T. Apoxin I, a novel apoptosis-inducing factor with L-amino acid oxidase activity purified from Western diamondback rattlesnake venom. J. Biol. Chem. 1997, 272, 9539–9542. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.F.; Yang, H.W.; Wei, X.L.; Qiao, L.Y.; Wang, W.Y.; He, S.H. Purification, characterization and biological activities of the L-amino acid oxidase from Bungarus fasciatus snake venom. Toxicon 2009, 54, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.R.T.; Aissa, A.F.; Ribeiro, D.L.; Costa, T.R.; Ferreira, R.S., Jr.; Sampaio, S.V.; Antunes, L.M.G. Cytotoxic, genotoxic, and oxidative stress-inducing effect of an L-amino acid oxidase isolated from Bothrops jararacussu venom in a co-culture model of HepG2 and HUVEC cells. Int. J. Biol. Macromol. 2019, 127, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Fu, Y.; Peng, J.; Fu, Y.; Dong, S.; Ding, R.B.; Qi, X.; Bao, J. Emerging anticancer potential and mechanisms of snake venom toxins: A review. Int. J. Biol. Macromol. 2024, 269, 131990. [Google Scholar] [CrossRef]
- Almeida, T.C.; Ribeiro Silva, L.M.; Boaventura de Oliveira, A.M.; Lopes, F.S.R.; Sant’Anna, M.B.; Picolo, G. Cytotoxic effect of crotoxin on cancer cells and its antitumoral effects correlated to tumor microenvironment: A review. Int. J. Biol. Macromol. 2023, 242, 124892. [Google Scholar] [CrossRef]
- Wang, H.; Ke, M.; Tian, Y.; Wang, J.; Li, B.; Wang, Y.; Dou, J.; Zhou, C. BF-30 selectively inhibits melanoma cell proliferation via cytoplasmic membrane permeabilization and DNA-binding in vitro and in B16F10-bearing mice. Eur. J. Pharmacol. 2013, 707, 1–10. [Google Scholar] [CrossRef]
- Heeschen, C.; Weis, M.; Aicher, A.; Dimmeler, S.; Cooke, J.P. A novel angiogenic pathway mediated by non-neuronal nicotinic acetylcholine receptors. J. Clin. Investig. 2002, 110, 527–536. [Google Scholar] [CrossRef]
- Yu, J.; Huang, N.F.; Wilson, K.D.; Velotta, J.B.; Huang, M.; Li, Z.; Lee, A.; Robbins, R.C.; Cooke, J.P.; Wu, J.C. nAChRs mediate human em-bryonic stem cell-derived endothelial cells: Proliferation, apoptosis, and angiogenesis. PLoS ONE 2009, 4, e7040. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.J.; Lyzogubov, V.V.; Tytarenko, R.G.; Safar, A.N.; Bora, N.S.; Bora, P.S. The effect of nicotine on anti-vascular endothelial growth factor therapy in a mouse model of neovascular age-related macular degeneration. Retina 2012, 32, 1171–1180. [Google Scholar] [CrossRef] [PubMed]
- Kalita, B.; Utkin, Y.N.; Mukherjee, A.K. Current Insights in the Mechanisms of Cobra Venom Cytotoxins and Their Complexes in Inducing Toxicity: Implications in Antivenom Therapy. Toxins 2022, 14, 839. [Google Scholar] [CrossRef] [PubMed]
- Gasanoff, E.S.; Dagda, R.K. Cobra Venom Cytotoxins as a Tool for Probing Mechanisms of Mitochondrial Energetics and Understanding Mitochondrial Membrane Structure. Toxins 2024, 16, 287. [Google Scholar] [CrossRef]
- Hiu, J.J.; Yap, M.K.K. Cytotoxicity of snake venom enzymatic toxins: Phospholipase A2 and l-amino acid oxidase. Biochem. Soc. Trans. 2020, 48, 719–731. [Google Scholar] [CrossRef]
- Conlon, J.M.; Attoub, S.; Arafat, H.; Mechkarska, M.; Casewell, N.R.; Harrison, R.A.; Calvete, J.J. Cytotoxic activities of [Ser49]phospholipase A2 from the venom of the saw-scaled vipers Echis ocellatus, Echis pyramidum leakeyi, Echis carinatus sochureki, and Echis coloratus. Toxicon 2013, 71, 96–104. [Google Scholar] [CrossRef]
- Alonazi, M.; Krayem, N.; Alharbi, M.G.; Khayyat, A.I.A.; Alanazi, H.; Horchani, H.; Ben Bacha, A. Functional Characterization and Anti-Tumor Effect of a Novel Group II Secreted Phospholipase A2 from Snake Venom of Saudi Cerastes cerates gasperetti. Molecules 2023, 28, 6517. [Google Scholar] [CrossRef]
- Naves, M.P.C.; de Morais, C.R.; de Freitas, V.; Ribeiro, D.L.; Lopes, D.S.; Antunes, L.M.G.; de Melo Rodrigues, V.; de Rezende, A.A.A.; Spanó, M.A. Mutagenic and genotoxic activities of Phospholipase A2 Bothropstoxin-I from Bothrops jararacussu in Drosophila melanogaster and human cell lines. Int. J. Biol. Macromol. 2021, 182, 1602–1610. [Google Scholar] [CrossRef]
- Kolvekar, N.; Bhattacharya, N.; Sarkar, A.; Chakrabarty, D. How snake venom disintegrins affect platelet aggregation and cancer proliferation. Toxicon 2023, 221, 106982. [Google Scholar] [CrossRef]
- Silva, R.; D’Amico, G.; Hodivala-Dilke, K.M.; Reynolds, L.E. Integrins: The keys to unlocking angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1703–1713. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Rucavado, A.; Escalante, T.; Díaz, C. Hemorrhage induced by snake venom metalloproteinases: Biochemical and biophysical mechanisms involved in microvessel damage. Toxicon 2005, 45, 997–1011. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Escalante, T.; Rucavado, A.; Herrera, C.; Fox, J.W. A Comprehensive View of the Structural and Functional Alterations of Extracellular Matrix by Snake Venom Metalloproteinases (SVMPs): Novel Perspectives on the Pathophysiology of Envenoming. Toxins 2016, 8, 304. [Google Scholar] [CrossRef] [PubMed]
- Zelanis, A.; Oliveira, A.K.; Prudova, A.; Huesgen, P.F.; Tashima, A.K.; Kizhakkedathu, J.; Overall, C.M.; Serrano, S.M.T. Deep Profiling of the Cleavage Specificity and Human Substrates of Snake Venom Metalloprotease HF3 by Proteomic Identification of Cleavage Site Specificity (PICS) Using Proteome Derived Peptide Libraries and Terminal Amine Isotopic Labeling of Substrates (TAILS) N-Terminomics. J. Proteome Res. 2019, 18, 3419–3428. [Google Scholar] [CrossRef] [PubMed]
- Bustillo, S.; García-Denegri, M.E.; Gay, C.; Van de Velde, A.C.; Acosta, O.; Angulo, Y.; Lomonte, B.; Gutiérrez, J.M.; Leiva, L. Phospholipase A2 enhances the endothelial cell detachment effect of a snake venom metalloproteinase in the absence of catalysis. Chem. Biol. Interact. 2015, 240, 30–36. [Google Scholar] [CrossRef]
- Masuda, S.; Araki, S.; Yamamoto, T.; Kaji, K.; Hayashi, H. Purification of a vascular apoptosis-inducing factor from hemorrhagic snake venom. Biochem. Biophys. Res. Commun. 1997, 235, 59–63. [Google Scholar] [CrossRef]
- Wu, W.B.; Chang, S.C.; Liau, M.Y.; Huang, T.F. Purification, molecular cloning and mechanism of action of graminelysin I, a snake-venom-derived metalloproteinase that induces apoptosis of human endothelial cells. Biochem. J. 2001, 357, 719–728. [Google Scholar] [CrossRef]
- Araki, S.; Masuda, S.; Maeda, H.; Ying, M.J.; Hayashi, H. Involvement of specific integrins in apoptosis induced by vascular apoptosis-inducing protein 1. Toxicon 2002, 40, 535–542. [Google Scholar] [CrossRef]
- Komori, Y.; Sakai, K.; Masuda, K.; Nikai, A.T. Isolation and biochemical characterization of rubelase, a non-hemorrhagic elastase from Crotalus ruber ruber (Red Rattlesnake) venom. Toxins 2011, 3, 900–910. [Google Scholar] [CrossRef]
- Komori, Y.; Murakami, E.; Uchiya, K.; Nonogaki, T.; Nikai, T. Okinalysin, a novel P-I metalloproteinase from Ovophis okinavensis: Biological properties and effect on vascular endothelial cells. Toxins 2014, 6, 2594–2604. [Google Scholar] [CrossRef]
- You, W.K.; Seo, H.J.; Chung, K.H.; Kim, D.S. A novel metalloprotease from Gloydius halys venom induces endothelial cell apoptosis through its protease and disintegrin-like domains. J. Biochem. 2003, 134, 739–749. [Google Scholar] [CrossRef]
- Masuda, S.; Maeda, H.; Miao, J.Y.; Hayashi, H.; Araki, S. cDNA cloning and some additional peptide characterization of a single-chain vascular apoptosis-inducing protein, VAP2. Endothelium 2007, 14, 89–96. [Google Scholar] [CrossRef]
- Teklemariam, T.; Seoane, A.I.; Ramos, C.J.; Sanchez, E.E.; Lucena, S.E.; Perez, J.C.; Mandal, S.A.; Soto, J.G. Functional analysis of a recombinant PIII-SVMP, GST-acocostatin; an apoptotic inducer of HUVEC and HeLa, but not SK-Mel-28 cells. Toxicon 2011, 57, 646–656. [Google Scholar] [CrossRef]
- Saegusa, J.; Akakura, N.; Wu, C.Y.; Hoogland, C.; Ma, Z.; Lam, K.S.; Liu, F.T.; Takada, Y.K.; Takada, Y. Pro-inflammatory secretory phospholipase A2 type IIA binds to integrins αvβ3 and α4β1 and induces proliferation of monocytic cells in an integrin-dependent manner. J. Biol. Chem. 2008, 283, 26107–26115. [Google Scholar] [CrossRef] [PubMed]
- Kessentini-Zouari, R.; Jebali, J.; Taboubi, S.; Srairi-Abid, N.; Morjen, M.; Kallech-Ziri, O.; Bezzine, S.; Marvaldi, J.; El Ayeb, M.; Marrakchi, N.; et al. CC-PLA2-1 and CC-PLA2-2, two Cerastes cerastes venom-derived phospholipases A2, inhibit angiogenesis both in vitro and in vivo. Lab. Investig. 2010, 90, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Ownby, C.L.; Bjarnason, J.B.; Tu, A.T. Hemorrhagic toxins from rattlesnake (Crotalus atrox) venom. Pathogenesis of hemorrhage induced by three purified toxins. Am. J. Pathol. 1978, 93, 201–218. [Google Scholar]
- Baldo, C.; Lopes, D.S.; Faquim-Mauro, E.L.; Jacysyn, J.F.; Niland, S.; Eble, J.A.; Clissa, P.B.; Moura-da-Silva, A.M. Jararhagin disruption of endothelial cell anchorage is enhanced in collagen enriched matrices. Toxicon 2015, 108, 240–248. [Google Scholar] [CrossRef]
- Herrera, C.; Voisin, M.B.; Escalante, T.; Rucavado, A.; Nourshargh, S.; Gutiérrez, J.M. Effects of PI and PIII Snake Venom Haemorrhagic Metalloproteinases on the Microvasculature: A Confocal Microscopy Study on the Mouse Cremaster Muscle. PLoS ONE 2016, 11, e0168643. [Google Scholar] [CrossRef]
- Asega, A.F.; Menezes, M.C.; Trevisan-Silva, D.; Cajado-Carvalho, D.; Bertholim, L.; Oliveira, A.K.; Zelanis, A.; Serrano, S.M.T. Cleavage of proteoglycans, plasma proteins and the platelet-derived growth factor receptor in the hemorrhagic process induced by snake venom metalloproteinases. Sci. Rep. 2020, 10, 12912. [Google Scholar] [CrossRef]
- Escalante, T.; Ortiz, N.; Rucavado, A.; Sanchez, E.F.; Richardson, M.; Fox, J.W.; Gutiérrez, J.M. Role of collagens and perlecan in microvascular stability: Exploring the mechanism of capillary vessel damage by snake venom metalloproteinases. PLoS ONE 2011, 6, e28017. [Google Scholar] [CrossRef]
- Choi, H.J.; Kwon, I.; Kim, N.E.; Kim, J.; An, S.; Kang, S.; Hong, S.Y.; Nam, H.S.; Heo, J.H. Fc-saxatilin suppresses hypoxia-induced vascular leakage by regulating endothelial occludin expression. Thromb. Haemost. 2017, 117, 595–605. [Google Scholar] [CrossRef]
- Choi, H.J.; Kim, N.E.; Kwon, I.; Choi, D.; Kim, J.; Heo, J.H. Fc-saxatilin inhibits VEGF-induced permeability by regulating claudin-5 expression in human brain microvascular endothelial cells. Microvasc. Res. 2020, 128, 103953. [Google Scholar] [CrossRef]
- Zhong, S.; Wu, J.; Cui, Y.; Li, R.; Zhu, S.; Rong, M.; Lu, Q.; Lai, R. Vascular endothelial growth factor from Trimeresurus jerdonii venom specifically binds to VEGFR-2. Biochimie 2015, 116, 1–7. [Google Scholar] [CrossRef]
- Nakamura, H.; Murakami, T.; Imamura, T.; Toriba, M.; Chijiwa, T.; Ohno, M.; Oda-Ueda, N. Discovery of a novel vascular endothelial growth factor (VEGF) with no affinity to heparin in Gloydius tsushimaensis venom. Toxicon 2014, 86, 107–115. [Google Scholar] [CrossRef]
- Aloui, Z.; Hoos, S.; Geretti, E.; Kharmachi, H.; Haumont, P.Y.; Mejdoub, H.; Klagsbrun, M.; England, P.; Gasmi, A. Novel svVEGF isoforms from Macrovipera lebetina venom interact with neuropilins. Biochem. Biophys. Res. Commun. 2009, 389, 10–15. [Google Scholar] [CrossRef]
- Silva de França, F.; Tambourgi, D.V. Hyaluronan breakdown by snake venom hyaluronidases: From toxins delivery to immunopathology. Front. Immunol. 2023, 14, 1125899. [Google Scholar] [CrossRef]
- Suntravat, M.; Cromer, W.E.; Marquez, J.; Galan, J.A.; Zawieja, D.C.; Davies, P.; Salazar, E.; Sánchez, E.E. The isolation and characterization of a new snake venom cysteine-rich secretory protein (svCRiSP) from the venom of the Southern Pacific rattlesnake and its effect on vascular permeability. Toxicon 2019, 165, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Suntravat, M.; Sanchez, O.; Reyes, A.; Cirilo, A.; Ocheltree, J.S.; Galan, J.A.; Salazar, E.; Davies, P.; Sanchez, E.E. Evaluation of Signaling Pathways Profiling in Human Dermal Endothelial Cells Treated by Snake Venom Cysteine-Rich Secretory Proteins (svCRiSPs) from North American Snakes Using Reverse Phase Protein Array (RPPA). Toxins 2021, 13, 613. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yu, H.; Sun, C.; Dong, M.; Zhao, N.; Wang, Y.; Yu, K.; Zhang, J.; Xu, N.; Liu, W. γ-Bungarotoxin impairs the vascular endothelial barrier function by inhibiting integrin α5. Toxicol. Lett. 2023, 383, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.C.; Escalante, T.; Rucavado, A.; Gutiérrez, J.M. Basement membrane degradation and inflammation play a role in the pulmonary hemorrhage induced by a P-III snake venom metalloproteinase. Toxicon 2021, 197, 12–23. [Google Scholar] [CrossRef]
- Wei, Y.; Lu, Q.Y.; Zhong, X.J.; Guo, L.; Zeng, F.Y.; Sun, Q.Y. Cobra venom P-III class metalloproteinase atrase A induces inflammatory response and cell apoptosis in endothelial cells via its metalloproteinase domain. Toxicon 2023, 232, 107210. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, C.; Li, C.; Li, W. The effect of defibrase in prevention of experimental atherosclerosis in rabbits. Zhonghua Bing Li Xue Za Zhi 1999, 28, 112–114. [Google Scholar] [PubMed]
- Qin, W.; Zhang, L.; Li, Z.; Xiao, D.; Zhang, Y.; Zhang, H.; Mokembo, J.N.; Monayo, S.M.; Jha, N.K.; Kopylov, P.; et al. Endothelial to mesenchymal transition contributes to nicotine-induced atherosclerosis. Theranostics 2020, 10, 5276–5289. [Google Scholar] [CrossRef]
- Ichiki, T.; Dzhoyashvili, N.; Burnett, J.C., Jr. Natriuretic peptide based therapeutics for heart failure: Cenderitide: A novel first-in-class designer natriuretic peptide. Int. J. Cardiol. 2019, 281, 166–171. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Role of A Disintegrin and Metalloproteinase (ADAM) in Epithelial Dysfunction. Available online: https://clinicaltrials.gov/study/NCT00898859 (accessed on 15 August 2025).
- ClinicalTrials.gov. ADAMTS-13 and Von Willebrand Factor Levels and Activities in Children With Cirrhosis and/or Portal Hypertension. Available online: https://clinicaltrials.gov/study/NCT04267406 (accessed on 15 August 2025).
- ClinicalTrials.gov. ADAMTSL4 in Idiopathic Pulmonary Hypertension and CTEPH. Available online: https://clinicaltrials.gov/study/NCT05478226 (accessed on 15 August 2025).
- Cura, J.E.; Blanzaco, D.P.; Brisson, C.; Cura, M.A.; Cabrol, R.; Larrateguy, L.; Mendez, C.; Sechi, J.C.; Silveira, J.S.; Theiller, E.; et al. Phase I and pharmacokinetics study of crotoxin (cytotoxic PLA(2), NSC-624244) in patients with advanced cancer. Clin. Cancer Res. 2002, 8, 1033–1041. [Google Scholar]
- Medioni, J.; Brizard, M.; Elaidi, R.; Reid, P.F.; Benlhassan, K.; Bray, D. Innovative design for a phase 1 trial with intra-patient dose escalation: The Crotoxin study. Contemp. Clin. Trials Commun. 2017, 7, 186–188. [Google Scholar] [CrossRef]
- Sant’Anna, M.B.; Giardini, A.C.; Ribeiro, M.A.C.; Lopes, F.S.R.; Teixeira, N.B.; Kimura, L.F.; Bufalo, M.C.; Ribeiro, O.G.; Borrego, A.; Cabrera, W.H.K.; et al. The Crotoxin:SBA-15 Complex Down-Regulates the Incidence and Intensity of Experimental Autoimmune Encephalomyelitis Through Peripheral and Central Actions. Front. Immunol. 2020, 11, 591563. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, P.; Motti, E.F. 3-NAntC: A Potent Crotoxin B-Derived Peptide against the Triple-Negative MDA-MB-231 Breast Cancer Cell Line. Molecules 2024, 29, 1646. [Google Scholar] [CrossRef] [PubMed]

| Snake Toxin/A Typical Representative(s) | Main Effect(s) on Vessel Wall (and Key Target/Step of Mechanism) | |||||
|---|---|---|---|---|---|---|
| Vascular Tone | Angiogenesis | Cytotoxicity, Cell Death | Interaction with ECM | Capillary Permeability | ||
| PLA2 | AhV_aPA from Agkistrodon halys pallas or HDP-1 from Vipera nikolskii | Vasocontraction or vasorelaxation (by cyclooxygenase metabolites) | ||||
| MVL-PLA2 from Macrovipera lebetina or KDR-bp from Agkistrodon piscivorus piscivorus | Antiangiogenic (inhibition of cell adhesion and migration through integrins α5β1 or VEGFR-2) | |||||
| Bothropstoxin-I from Bothrops jararacussu | Necrosis; apoptosis (downregulation of anti-apoptotic proteins; DNA damage) | |||||
| CC-PLA2-1 and -2 from Cerastes cerastes | Loss of cell adhesion (αv (αvβ3), α2, α5β1 integrins) | |||||
| SVMP | BthMP from Bothrops moojeni | Antiangiogenic (inhibition of cell adhesion and migration; change in gene expression) | ||||
| VAPs (Crotalinae) | Caspase-3-dependent apoptosis (cell detachment through αvβ5, α3, α6, β1 integrins) | |||||
| BjussuMP-II from Bothrops jararacussu | Impedes adhesion and promotes cell detachment (degrades various ECM proteins; cleaves the TNF-α precursor) | |||||
| BaP1 from Bothrops asper | Hemorrhage per rhexis and per diapedesis (degrades the BM and adhesion proteins; interferes with collagen and integrins) | |||||
| LAAO | Bothrops spp. | Antiangiogenic (probably through cytotoxicity) | ||||
| All snakes | Necrosis; caspase-mediated apoptosis by ROS | |||||
| Hyaluronidase | All snakes | Digests hyaluronan | Increases | |||
| Snake Toxin (Source Venom) | Main Effect(s) on Vessel Wall (and Key Target/Step of Mechanism) | ||||||
|---|---|---|---|---|---|---|---|
| Vascular Tone | Angiogenesis | Cytotoxicity, Cell Death | Interaction with ECM | Capillary Permeability | Anti-Atherosclerotic | ||
| VEGF-F (Viperidae) |
Vasorelaxation
(increases NO production) | Promotes endothelial cell proliferation and migration (VEGFR-2) | Increases (VEGFR-1 and VEGFR-2) | ||||
| CRISP | (Australian and sea Elapidae) | Vasocontraction (BKCa) or Vasorelaxation (L-type Ca2+ channel) | |||||
| ES-CRISP from Echis carinatus sochureki | Antiangiogenic (growth factor signaling) | ||||||
| (Crotalinae) | Increases (through different pathways) | ||||||
| C-type lectins and snaclecs (Viperidae) | Antiangiogenic (cell adhesion and migration inhibition: integrins αvβ3, αvβ5, and α5β1, and VEGF signaling) | ||||||
| Disintegrin (Viperidae) | Inhibits angiospasm at thromboembolism (reduces the adhesion of platelets to the subendothelium) | Inhibition (integrins αvβ3, α5β1, α1β1, and α2β1) Promotion (growth factor signaling) | Caspase-3-dependent apoptosis (αvβ5 integrin; DNA fragmentation) | Detaches and rounds up cells (integrins vβ3 or α1β1) | Prevents vascular leakage (integrin αvβ3; VEGF signaling) | ||
| TFT | Cytotoxins (Naja genus) | Vasocontraction (Ca2+ channel) | Both necrotic and apoptotic (lysosomal damage, violation of energy metabolism) | ||||
| Muscarinic toxins MTα and MT9 from Dendroaspis polylepis | Vasorelaxation (α2B adrenoreceptor or M2 muscarinic AChR) | ||||||
| α-Bungarotoxin from Bungarus multicinctus | Inhibits nicotine-evoked endothelial network (abrogates upregulation of VEGF and bFGF gene expression) | Reduce atherosclerotic lesions (nicotine-induced EndMT) | |||||
| γ-Bungarotoxin from Bungarus multicinctus | Promotes (integrin α5) | ||||||
| Calciseptin and FS2 from Dendroaspis polylepis | Blocks contraction (L-type Ca2+ channels) | ||||||
| Kunitz-type ISP from Macrovipera lebetina transmediterranea | Antiangiogenic (inhibits cell adhesion and migration; integrin αvβ5) | ||||||
| Snake Toxin | Source Venoms | Main Effect on Vascular Tone (and Key Target/Step of Mechanism) | |
|---|---|---|---|
| Peptides | BPP | Bothrops spp. | Vasorelaxation (Releases NO from the endothelium) |
| NP | Different genera of Viperidae and Elapidae families | Vasorelaxation (Increases NO production; K+ channels) | |
| Sarafotoxins | Atractaspis spp. | Vasoconstriction or vasorelaxation (ET-A and ET-B receptor) | |
| Low-MW | Adenosine | All snakes | Vasorelaxation (adenosine A2 receptors) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osipov, A.V.; Utkin, Y.N. Snake Toxins Affecting Blood Vessel Walls: Mode of Action and Biological Significance. Int. J. Mol. Sci. 2025, 26, 9439. https://doi.org/10.3390/ijms26199439
Osipov AV, Utkin YN. Snake Toxins Affecting Blood Vessel Walls: Mode of Action and Biological Significance. International Journal of Molecular Sciences. 2025; 26(19):9439. https://doi.org/10.3390/ijms26199439
Chicago/Turabian StyleOsipov, Alexey V., and Yuri N. Utkin. 2025. "Snake Toxins Affecting Blood Vessel Walls: Mode of Action and Biological Significance" International Journal of Molecular Sciences 26, no. 19: 9439. https://doi.org/10.3390/ijms26199439
APA StyleOsipov, A. V., & Utkin, Y. N. (2025). Snake Toxins Affecting Blood Vessel Walls: Mode of Action and Biological Significance. International Journal of Molecular Sciences, 26(19), 9439. https://doi.org/10.3390/ijms26199439






