Discovery of a Novel Anticoagulant Cystine Knot Peptide from Spider Venom Gland Transcriptome
Abstract
1. Introduction
2. Results
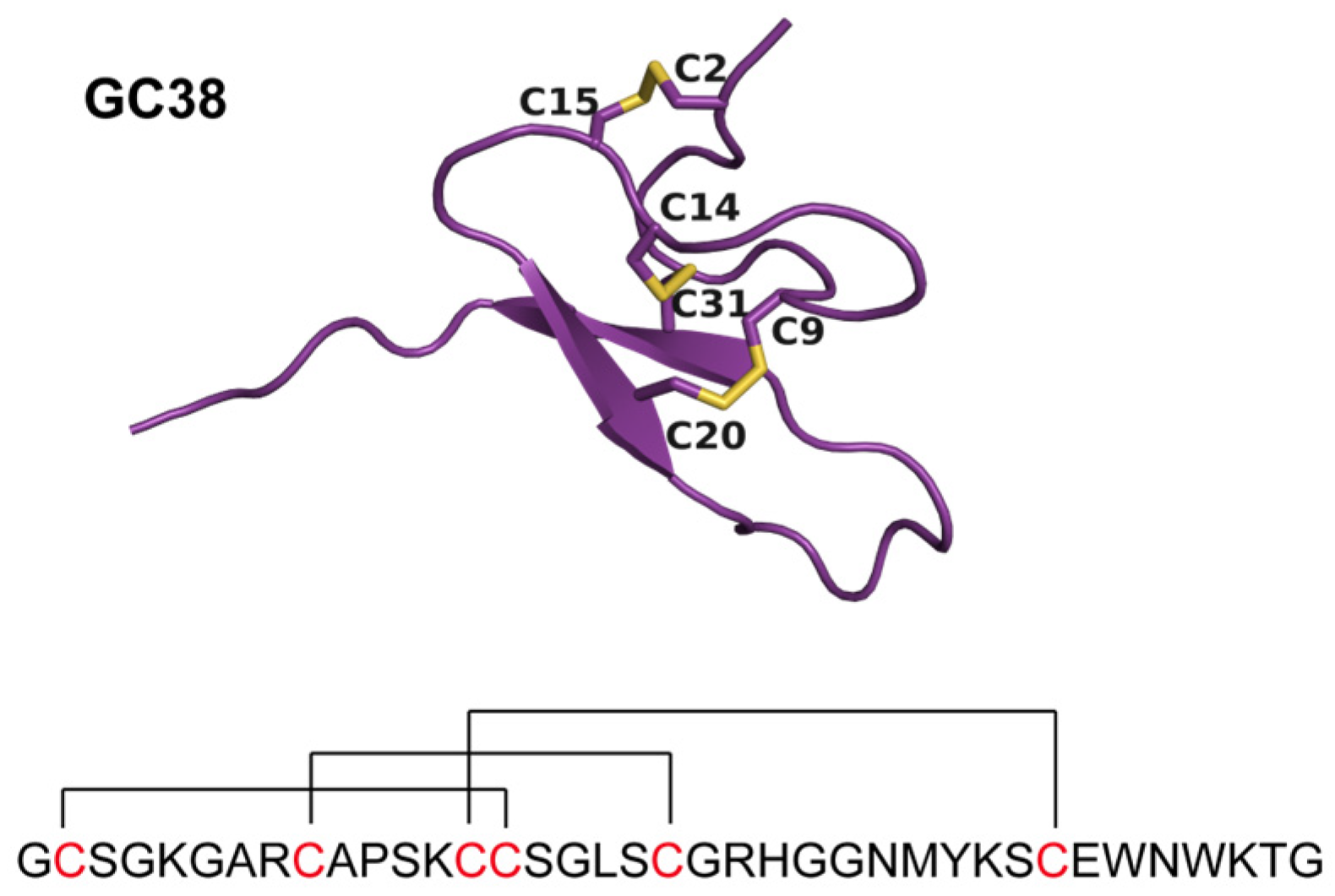
2.1. Identification and Characterization of the ICK Peptide GC38 from Macrothele sp. Venom
2.1.1. In Virtual Screening of GC38 Binding to the Anticoagulant Target APC
2.1.2. Identification of GC38 from Spider Venom Gland Transcriptome
2.1.3. Synthesis and Purification of Peptide GC38
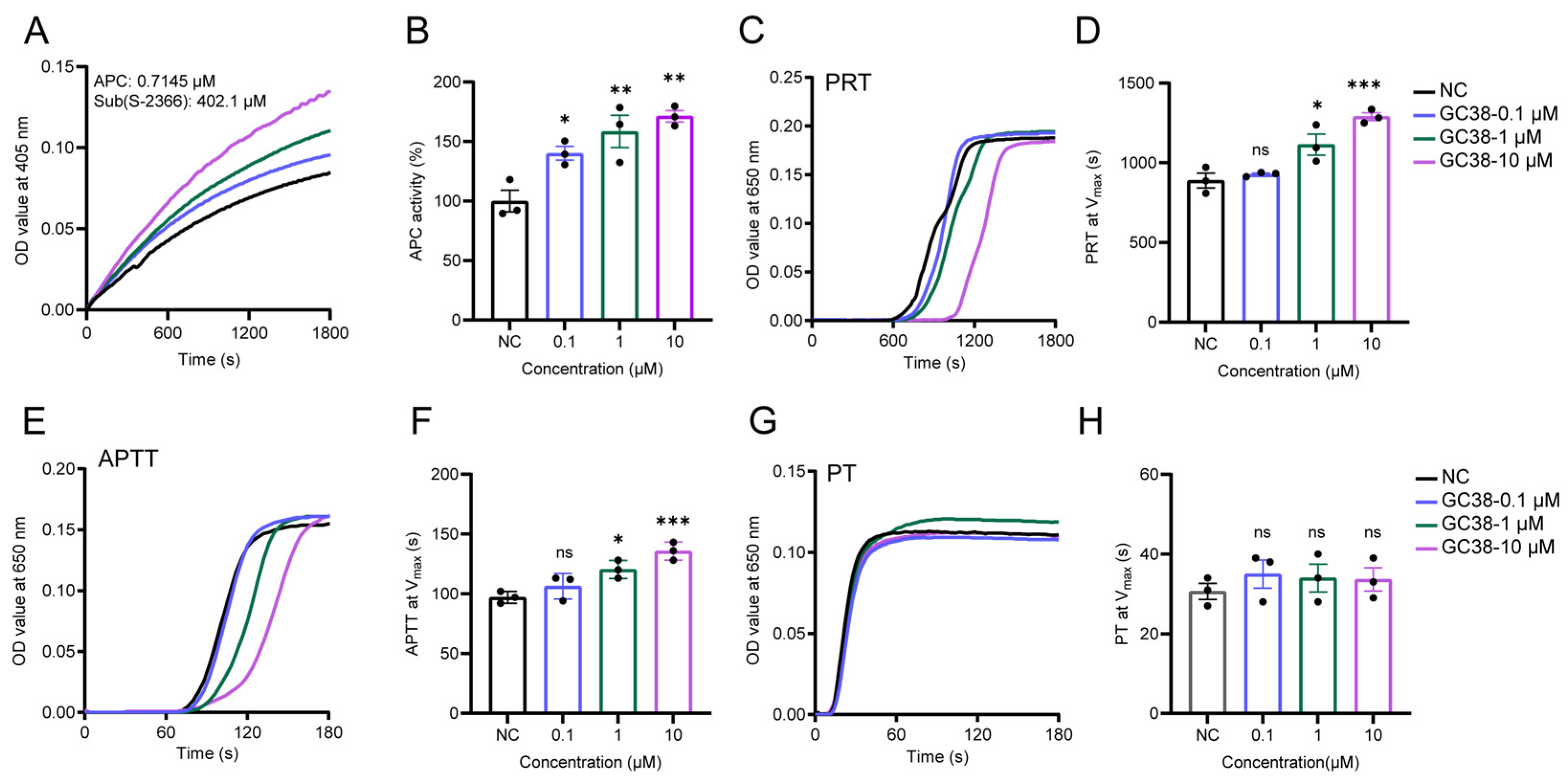
2.2. The Effect of GC38 Peptide on APC and Its In Vitro Anticoagulant Activity
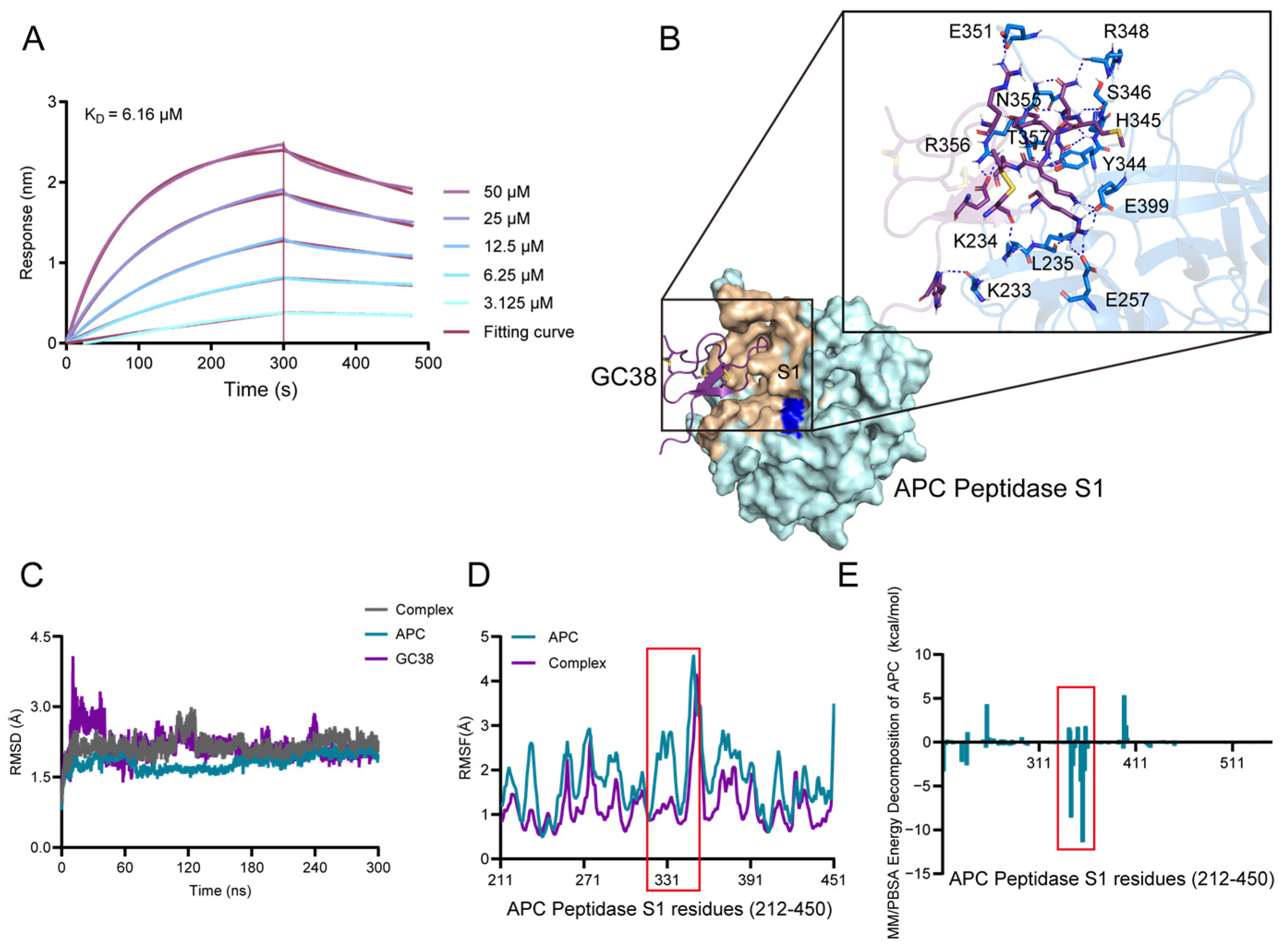
2.3. The Potential Binding Mechanism of GC38 and APC
2.3.1. Direct Binding of GC38 to APC
2.3.2. Molecular Docking and Binding Energy Analysis from MD Simulations
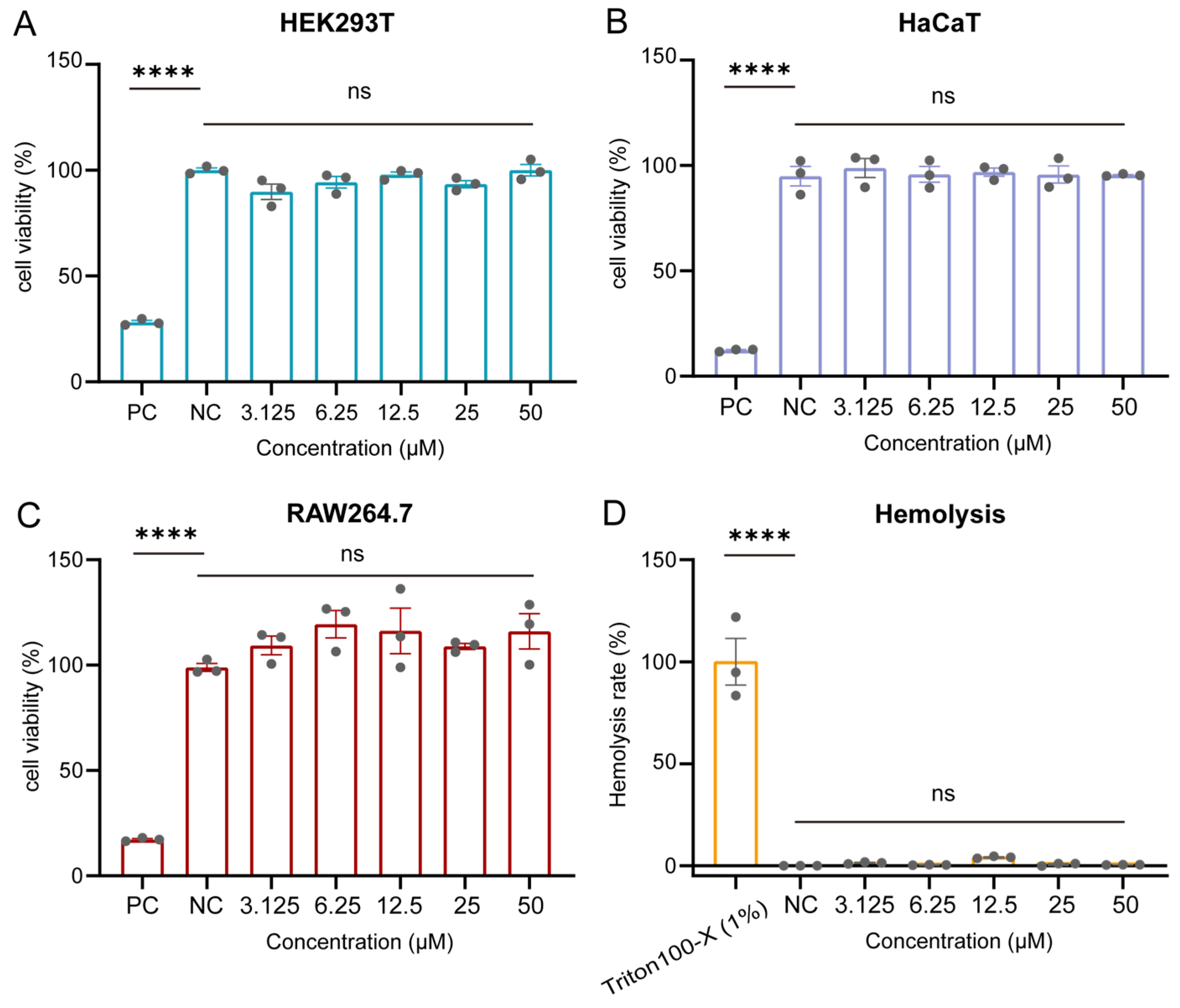
2.4. Cytotoxicity and Hemolysis Profiles of GC38
2.5. In Vivo Efficacy of the GC38 Peptide as an Antithrombotic, Anticoagulant, and Bleeding Treatment
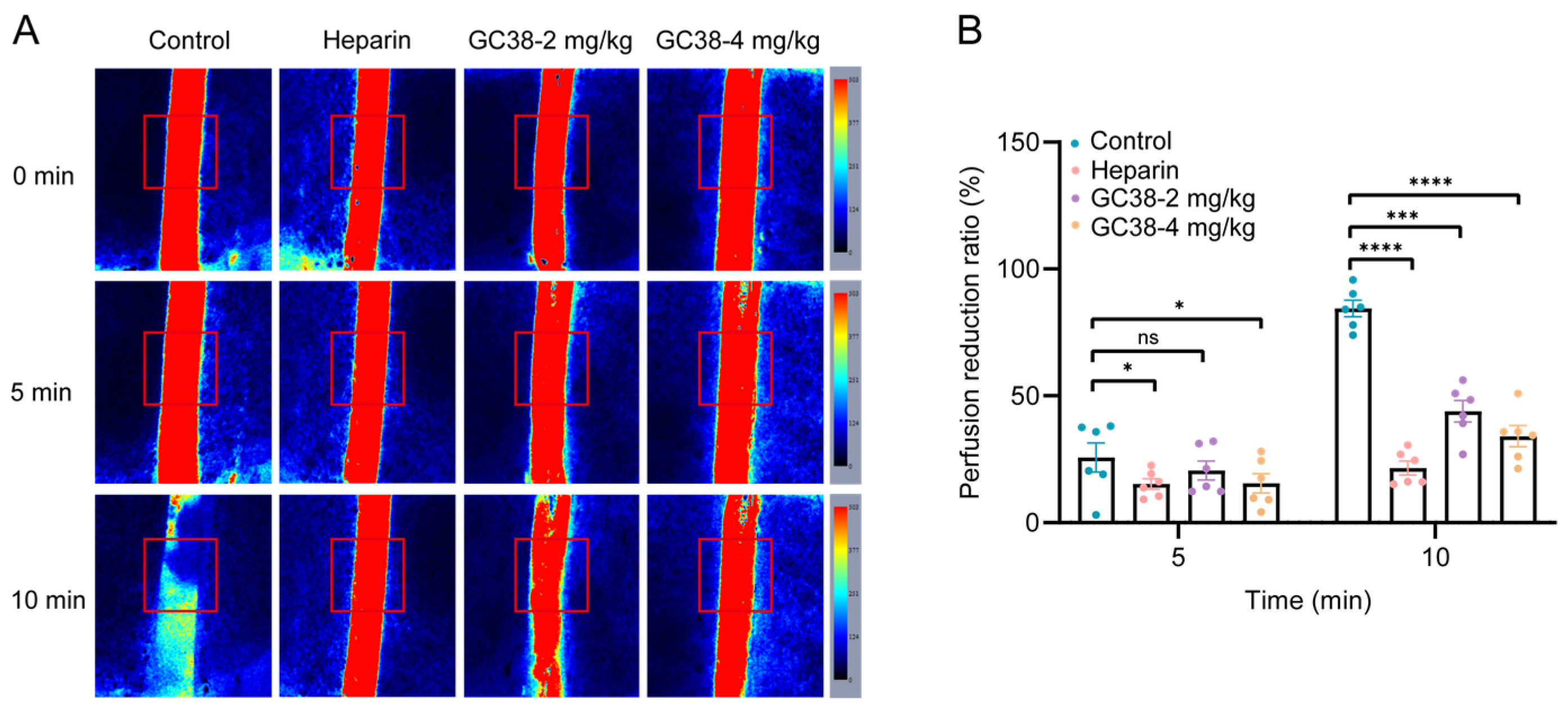
2.5.1. Antithrombotic and Anticoagulant Effects of GC38 in FeCl3-Induced Carotid Artery Thrombosis Model
2.5.2. Antithrombotic and Anticoagulant Efficacy of GC38 in Stasis-Induced Deep Vein Thrombosis (DVT) Model
2.5.3. Antithrombotic and Anticoagulant Efficacy of GC38 in Cortical Photothrombotic Stroke (CPS) Model
2.5.4. Bleeding Risk Assessment
3. Discussion
4. Materials and Methods
4.1. Chemicals and Materials
4.2. Identification and Characterization of the Peptide GC38 from Macrothele sp. Venom
4.2.1. Spider Collection, Venom Gland Dissection, and Venom Gland Transcriptome Sequencing and Assembly
4.2.2. Sequence Similarity Network Construction
4.2.3. GC38 Identification Based on Virtual Screening
4.2.4. Chemical Synthesis of GC38
4.3. In Vitro Assays for Evaluating GC38’s Effects on Coagulation
4.3.1. Measurement of APC Activity by Chromogenic Substrate Assay
4.3.2. Plasma Recalcification Assay
4.3.3. Activated Partial Thromboplastin Time (APTT) and Prothrombin Time (PT)
4.4. Potential Anticoagulant Mechanism of GC38
4.4.1. Bio-Layer Interferometry (BLI) Assay
4.4.2. Docking and Refining the Model of GC38 Binding to APC
4.4.3. Molecular Dynamics Simulation of the GC38-APC Complex
4.4.4. Binding Affinity Quantification Through MM/PBSA and Energy Decomposition
4.5. In Vitro Safety Assessment of GC38: Cytotoxicity and Hemolysis
4.5.1. Cytotoxicity Assay
4.5.2. Hemolysis Assay
4.6. Anticoagulant and Antithrombotic Efficacy of GC38 in Mouse Models and Tail Bleeding Time Assay
4.6.1. Animals and Ethics
4.6.2. FeCl3-Induced Carotid Artery Thrombosis Model
4.6.3. Stasis-Induced Deep Vein Thrombosis (DVT) Model
4.6.4. Rose Bengal-Mediated Cortical Photothrombosis Stroke (CPS) Model
4.6.5. Tail Bleeding Time
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APC | Activated protein C |
| MD | Molecular dynamics |
| PDB | Protein Data Bank |
| RMSD | Root mean square deviation |
| RMSF | Root mean square fluctuation |
| MM/PBSA | Mechanics/Poisson–Boltzmann surface area |
| HPLC | High-performance liquid chromatography |
| BLI | Biolayer interferometry |
| ICK | Inhibitor cystine knot |
| PRT | Plasma recalcification time |
| PT | Prothrombin time |
| APTT | Activated partial thromboplastin time |
| HE staining | Hematoxylin–eosin staining |
| NGS | Next-generation sequencing |
| LSP | Laser speckle perfusion |
| SPPS | Solid-phase peptide synthesis |
| TFA | Trifluoroacetic acid |
| CCK-8 | Cell Counting Kit 8 |
| CPS | Cortical photothrombosis stroke |
| IVC | Inferior vena cava |
| DVT | Deep vein thrombosis |
| ANOVA | One-way analysis of variance |
| SEM | Standard error of the mean |
References
- Wendelboe, A.M.; Raskob, G.E. Global burden of thrombosis: Epidemiologic aspects. Circ. Res. 2016, 118, 1340–1347. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J. Pathology of arterial thrombosis. Br. Med. Bull. 1994, 50, 789–802. [Google Scholar] [CrossRef]
- Germain, M.; Chasman, D.I.; de Haan, H.; Tang, W.; Lindström, S.; Weng, L.-C.; de Andrade, M.; de Visser, M.C.H.; Wiggins, K.L.; Suchon, P.; et al. Meta-analysis of 65,734 individuals identifies TSPAN15 and SLC44A2 as two susceptibility loci for venous thromboembolism. Am. J. Hum. Genet. 2015, 96, 532–542. [Google Scholar] [CrossRef]
- Pishko, A.M.; Lefler, D.S.; Gimotty, P.; Paydary, K.; Fardin, S.; Arepally, G.M.; Crowther, M.; Rice, L.; Vega, R.; Cines, D.B.; et al. The risk of major bleeding in patients with suspected heparin-induced thrombocytopenia. J. Thromb. Haemost. 2019, 17, 1956–1965. [Google Scholar] [CrossRef]
- Lindh, J.D.; Holm, L.; Dahl, M.-L.; Alfredsson, L.; Rane, A. Incidence and predictors of severe bleeding during warfarin treatment. J. Thromb. Thrombolysis 2008, 25, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Beckman, M.G.; Hooper, W.C.; Critchley, S.E.; Ortel, T.L. Venous thromboembolism: A public health concern. Am. J. Prev. Med. 2010, 38, S495–S501. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Yuan, T.; Shang, L. 3D printing of naturally derived adhesive hemostatic sponge. Research 2024, 7, 0446. [Google Scholar] [CrossRef]
- Xiong, W.; Chai, J.; Wu, J.; Li, J.; Lu, W.; Tian, M.; Jmel, M.A.; Ippel, J.H.; Kotsyfakis, M.; Dijkgraaf, I.; et al. Cathelicidin-HG alleviates sepsis-induced platelet dysfunction by inhibiting GPVI-mediated platelet activation. Research 2024, 7, 0381. [Google Scholar] [CrossRef]
- Zou, Y.; Shan, Z.; Han, Z.; Yang, J.; Lin, Y.; Gong, Z.; Xie, L.; Xu, J.; Xie, R.; Chen, Z.; et al. Regulating blood clot fibrin films to manipulate biomaterial-mediated foreign body responses. Research 2023, 6, 0225. [Google Scholar] [CrossRef]
- Liu, R.; Ding, Y.; Jiang, X.; Dong, R.; Zhang, Y.; Hua, Y.; Gai, C.; Wei, P. Anticoagulant peptides derived from animal-sourced traditional Chinese medicine and their pharmacological effects. Pharmacol. Res.-Mod. Chin. Med. 2024, 13, 100529. [Google Scholar] [CrossRef]
- Qadeer Yusuf, K.; Krittanawong, C.; Wang, Z.; Ahuja, T.; Alam, M.; Jneid, H. Bivalirudin versus heparin in patients undergoing percutaneous coronary intervention in acute coronary syndromes. J. Am. Coll. Cardiol. 2025, 85, 949. [Google Scholar] [CrossRef]
- Griffin, J.H.; Zlokovic, B.V.; Mosnier, L.O. Activated protein C: Biased for translation. Blood 2015, 125, 2898–2907. [Google Scholar] [CrossRef]
- Esmon, C.T. Protein C anticoagulant system—Anti-inflammatory effects. Semin. Immunopathol. 2012, 34, 127–132. [Google Scholar] [CrossRef]
- Marlar, R.A.; Kleiss, A.J.; Griffin, J.H. Mechanism of action of human activated protein C, a thrombin-dependent anticoagulant enzyme. Blood 1982, 59, 1067–1072. [Google Scholar] [CrossRef][Green Version]
- Polderdijk, S.G.I.; Adams, T.E.; Ivanciu, L.; Camire, R.M.; Baglin, T.P.; Huntington, J.A. Design and characterization of an APC-specific serpin for the treatment of hemophilia. Blood 2017, 129, 105–113. [Google Scholar] [CrossRef]
- Yan, M.; Wang, Y.; Niu, L.; Wang, S.; Yu, H.; Yin, Q.; Zhang, D.; Bai, Y.; Yin, Y.; Lin, S.; et al. Apolipoprotein A-IV regulates coagulation and ischemic stroke by potentiating activated protein C. J. Thromb. Haemost. 2025, 23, 2837–2848. [Google Scholar] [CrossRef] [PubMed]
- Heeb, M.; Fernández, J.; Yamashita, A.; McDowell, O.; Guo, Z.; Mosnier, L.; Deguchi, H.; Griffin, J. Activated protein Canticoagulant activity isenhanced by skeletal muscle myosin. Haematologica 2020, 105, e424–e427. [Google Scholar] [CrossRef] [PubMed]
- Fleming, H.; Preston, R.J.S. Mimicking activated protein C-progress by PARtnering peptides. J. Thromb. Haemost. 2024, 22, 2153–2155. [Google Scholar] [CrossRef] [PubMed]
- Healy, L.D.; Fernández, J.A.; Aiolfi, R.; Mosnier, L.O.; Griffin, J.H. An orthosteric/allosteric bivalent peptide agonist comprising covalently linked protease-activated receptor-derived peptides mimics in vitro and in vivo activities of activated protein C. J. Thromb. Haemost. 2024, 22, 2039–2051. [Google Scholar] [CrossRef]
- Saez, N.J.; Senff, S.; Jensen, J.E.; Er, S.Y.; Herzig, V.; Rash, L.D.; King, G.F. Spider-venom peptides as therapeutics. Toxins 2010, 2, 2851–2871. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, S.; Li, J.; Zhang, C.; Xiao, R.; Bai, X.; Xu, H.; Zhang, F. Unveiling diversity and function: Venom-associated microbes in two spiders, heteropoda venatoria and Chilobrachys guangxiensis. Microb. Ecol. 2024, 87, 156. [Google Scholar] [CrossRef]
- Lohner, K.; Prossnigg, F. Biological activity and structural aspects of PGLa interaction with membrane mimetic systems. Biochim Biophys Acta. 2009, 1788, 1656–1666. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Tang, Y.; Yin, W.; Liu, X.; Gao, P.; Zhang, C.; Tembrock, L.R.; Zhao, Y.; Yang, Z. From genome to proteome: Comprehensive identification of venom toxins from the Chinese funnel-web spider (Macrothelidae: Macrothele yani). Int. J. Biol. Macromol. 2024, 268, 131780. [Google Scholar] [CrossRef]
- Zhang, M.; Cai, W.; Yang, M.; Zhang, M.; Tembrock, L.R.; Yang, Z.; Liu, H.; Yang, Z. Transcriptomic and proteomic analyses reveal the diverse components in the venom of a recently described spider species Macrothele washanensis. Comp. Biochem. Physiol. Part D Genom. Proteom. 2025, 55, 101491. [Google Scholar] [CrossRef]
- Xiao, X.; Luo, X.; Huang, C.; Feng, X.; Wu, M.; Lu, M.; Kuang, J.; Peng, S.; Guo, Y.; Zhang, Z.; et al. Transcriptome analysis reveals the peptide toxins diversity of Macrothele palpator venom. Int. J. Biol. Macromol. 2023, 253, 126577. [Google Scholar] [CrossRef] [PubMed]
- Luan, N.; Zhou, C.; Li, P.; Ombati, R.; Yan, X.; Mo, G.; Rong, M.; Lai, R.; Duan, Z.; Zheng, R. Joannsin, a novel Kunitz-type FXa inhibitor from the venom of Prospirobolus joannsi. Thromb. Haemost. 2017, 117, 1031–1039. [Google Scholar] [CrossRef]
- Kong, Y.; Shao, Y.; Chen, H.; Ming, X.; Wang, J.-B.; Li, Z.-Y.; Wei, J.-F. A novel factor Xa-inhibiting peptide from centipedes venom. Int. J. Pept. Res. Ther. 2013, 19, 303–311. [Google Scholar] [CrossRef]
- Lüddecke, T.; Herzig, V.; von Reumont, B.M.; Vilcinskas, A. The biology and evolution of spider venoms. Biol. Rev. 2022, 97, 163–178. [Google Scholar] [CrossRef]
- Li, P.; Zhang, Z.; Liao, Q.; Meng, E.; Mwangi, J.; Lai, R.; Rong, M. LCTX-F2, a novel potentiator of coagulation factors from the spider venom of Lycosa singoriensis. Front. Pharmacol. 2020, 11, 896. [Google Scholar] [CrossRef] [PubMed]
- Usmanov, P.B.; Nuritova, F.A. The anticoagulant action of phospholipase A from Eresus niger spider venom. Toxicon 1994, 32, 625–628. [Google Scholar] [CrossRef]
- Jug, A.; Bratkovič, T.; Ilaš, J. Biolayer interferometry and its applications in drug discovery and development. TrAC Trends Anal. Chem. 2024, 176, 117741. [Google Scholar] [CrossRef]
- Rezaie, A.R. Exosite-dependent regulation of the protein C anticoagulant pathway. Trends Cardiovasc. Med. 2003, 13, 8–15. [Google Scholar] [CrossRef]
- Mesters, R.; Houghten, R.; Griffin, J. Identification of a sequence of human activated protein C (residues 390-404). J. Biol. Chem. 1991, 266, 24514–24519. [Google Scholar] [CrossRef] [PubMed]
- Greinacher, A.; Lubenow, N. Recombinant hirudin in clinical practice. Circulation 2001, 103, 1479–1484. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Gao, Q.; Chai, X.; Ren, W.; Zhang, G.; Kong, Y.; Zhang, Y.; Gao, J.; Lei, X.; Ma, L. A novel thrombin inhibitory peptide discovered from leech using affinity chromatography combined with ultra-high performance liquid chromatography-high resolution mass spectroscopy. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2020, 1151, 122153. [Google Scholar] [CrossRef]
- Shaikh, N.Y.; Sunagar, K. The deep-rooted origin of disulfide-rich spider venom toxins. eLife 2023, 12, e83761. [Google Scholar] [CrossRef] [PubMed]
- Pineda, S.S.; Undheim, E.A.; Rupasinghe, D.B.; Ikonomopoulou, M.P.; King, G.F. Spider Venomics: Implications for Drug Discovery. Future Med. Chem. 2014, 6, 1699–1714. [Google Scholar] [CrossRef]
- Shekh, S.; Moi, S.; Ch, V.; Govindu, P.; Gowd, K.H. Conformations of disulfides are conserved in inhibitory cystine knot (ICK) motif polypeptides. Toxicon 2022, 219, 106926. [Google Scholar] [CrossRef]
- Mathis, A.S.; Davé, N.; Shah, N.K.; Friedman, G.S. Bleeding and thrombosis in high-risk renal transplantation candidates using heparin. Ann. Pharmacother. 2004, 38, 537–543. [Google Scholar] [CrossRef]
- Nip, K.M.; Hafezqorani, S.; Gagalova, K.K.; Chiu, R.; Yang, C.; Warren, R.L.; Birol, I. Reference-free assembly of long-read transcriptome sequencing data with RNA-Bloom2. Nat. Commun. 2023, 14, 2940. [Google Scholar] [CrossRef]
- Frickey, T.; Lupas, A. CLANS: A Java application for visualizing protein families based on pairwise similarity. Bioinformatics 2004, 20, 3702–3704. [Google Scholar] [CrossRef]
- Teufel, F.; Almagro Armenteros, J.J.; Johansen, A.R.; Gíslason, M.H.; Pihl, S.I.; Tsirigos, K.D.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 6.0 predicts all five types of signal peptides using protein language models. Nat. Biotechnol. 2022, 40, 1023–1025. [Google Scholar] [CrossRef]
- Krogh, A.; Larsson, B.; von Heijne, G.; Sonnhammer, E.L.L. Predicting transmembrane protein topology with a hidden markov model: Application to complete genomes. J. Mol. Biol. 2001, 305, 567–580. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Rathore, A.S.; Kumar, N.; Choudhury, S.; Mehta, N.K.; Raghava, G.P.S. Prediction of hemolytic peptides and their hemolytic concentration. Commun. Biol. 2025, 8, 176. [Google Scholar] [CrossRef]
- Yan, Y.; Tao, H.; He, J.; Huang, S.-Y. The HDOCK server for integrated protein–protein docking. Nat. Protoc. 2020, 15, 1829–1852. [Google Scholar] [CrossRef] [PubMed]
- Mather, T.; Oganessyan, V.; Hof, P.; Huber, R.; Foundling, S.; Esmon, C.; Bode, W. The 2.8 A crystal structure of Gla-domainless activated protein C. EMBO J. 1996, 15, 6822–6831. [Google Scholar] [CrossRef]
- Kosugi, T.; Ohue, M. Design of cyclic peptides targeting protein–protein interactions using AlphaFold. Int. J. Mol. Sci. 2023, 24, 13257. [Google Scholar] [CrossRef]
- Lu, D.; Liu, Z. Protein refolding by size exclusion chromatography. J. Ind. Eng. Chem. 2002, 53, 1028–1033. [Google Scholar]
- Kitchen, S.; Geisen, U.; Kappelmayer, J.; Quehenberger, P.; Drieß, J.; Lowe, A.; Jones, R.; Boehm, J.G.; Miles, G.; Rozsnyai, G. Evaluating the analytical performance of five new coagulation assays for the measurement of prothrombin time and activated thromboplastin time. Clin. Lab. Haematol. 2018, 40, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Chaudhury, S.; Berrondo, M.; Weitzner, B.D.; Muthu, P.; Bergman, H.; Gray, J.J. Benchmarking and analysis of protein docking performance in Rosetta v3.2. PLoS ONE 2011, 6, e22477. [Google Scholar] [CrossRef]
- Wang, C.; Bradley, P.; Baker, D. Protein–protein docking with backbone flexibility. J. Mol. Biol. 2007, 373, 503–519. [Google Scholar] [CrossRef] [PubMed]
- Eckly, A.; Hechler, B.; Freund, M.; Zerr, M.; Cazenave, J.P.; Lanza, F.; Mangin, P.H.; Gachet, C. Mechanisms underlying FeCl3-induced arterial thrombosis. J. Thromb. Haemost. 2011, 9, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, Y.; Fang, Y.; Gao, J.; Thuku, R.C.; Yang, J.; Na, C.; Lu, Q.; Fang, M. Targeting the contact-kinin system: A cyclopeptide with anti-thromboinflammatory properties against stroke. Eur. J. Pharmacol. 2025, 998, 177497. [Google Scholar] [CrossRef] [PubMed]
- Payne, H.; Brill, A. Stenosis of the inferior vena cava: A murine model of deep vein thrombosis. J. Vis. Exp. 2017, 130, e56697. [Google Scholar]
- Uzdensky, A.B. Photothrombotic stroke as a model of ischemic stroke. Transl. Stroke Res. 2018, 9, 437–451. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Yang, D.; Wang, W.; Huang, X.; Guo, R.; Cao, K.; Lu, Q.; Wang, Z.; Lai, R.; Li, J. Discovery of a Novel Anticoagulant Cystine Knot Peptide from Spider Venom Gland Transcriptome. Int. J. Mol. Sci. 2025, 26, 10154. https://doi.org/10.3390/ijms262010154
Gao J, Yang D, Wang W, Huang X, Guo R, Cao K, Lu Q, Wang Z, Lai R, Li J. Discovery of a Novel Anticoagulant Cystine Knot Peptide from Spider Venom Gland Transcriptome. International Journal of Molecular Sciences. 2025; 26(20):10154. https://doi.org/10.3390/ijms262010154
Chicago/Turabian StyleGao, Jinai, Di Yang, Wanting Wang, Xiaoshan Huang, Ruiyin Guo, Kaixun Cao, Qiumin Lu, Ziyi Wang, Ren Lai, and Juan Li. 2025. "Discovery of a Novel Anticoagulant Cystine Knot Peptide from Spider Venom Gland Transcriptome" International Journal of Molecular Sciences 26, no. 20: 10154. https://doi.org/10.3390/ijms262010154
APA StyleGao, J., Yang, D., Wang, W., Huang, X., Guo, R., Cao, K., Lu, Q., Wang, Z., Lai, R., & Li, J. (2025). Discovery of a Novel Anticoagulant Cystine Knot Peptide from Spider Venom Gland Transcriptome. International Journal of Molecular Sciences, 26(20), 10154. https://doi.org/10.3390/ijms262010154








