Long-Term Effects of Stress During Adolescence on the Sex-Dependent Responses of Thyroid Axis and Target Tissues to Exercise in Male and Female Wistar Rats
Abstract
1. Introduction
2. Results
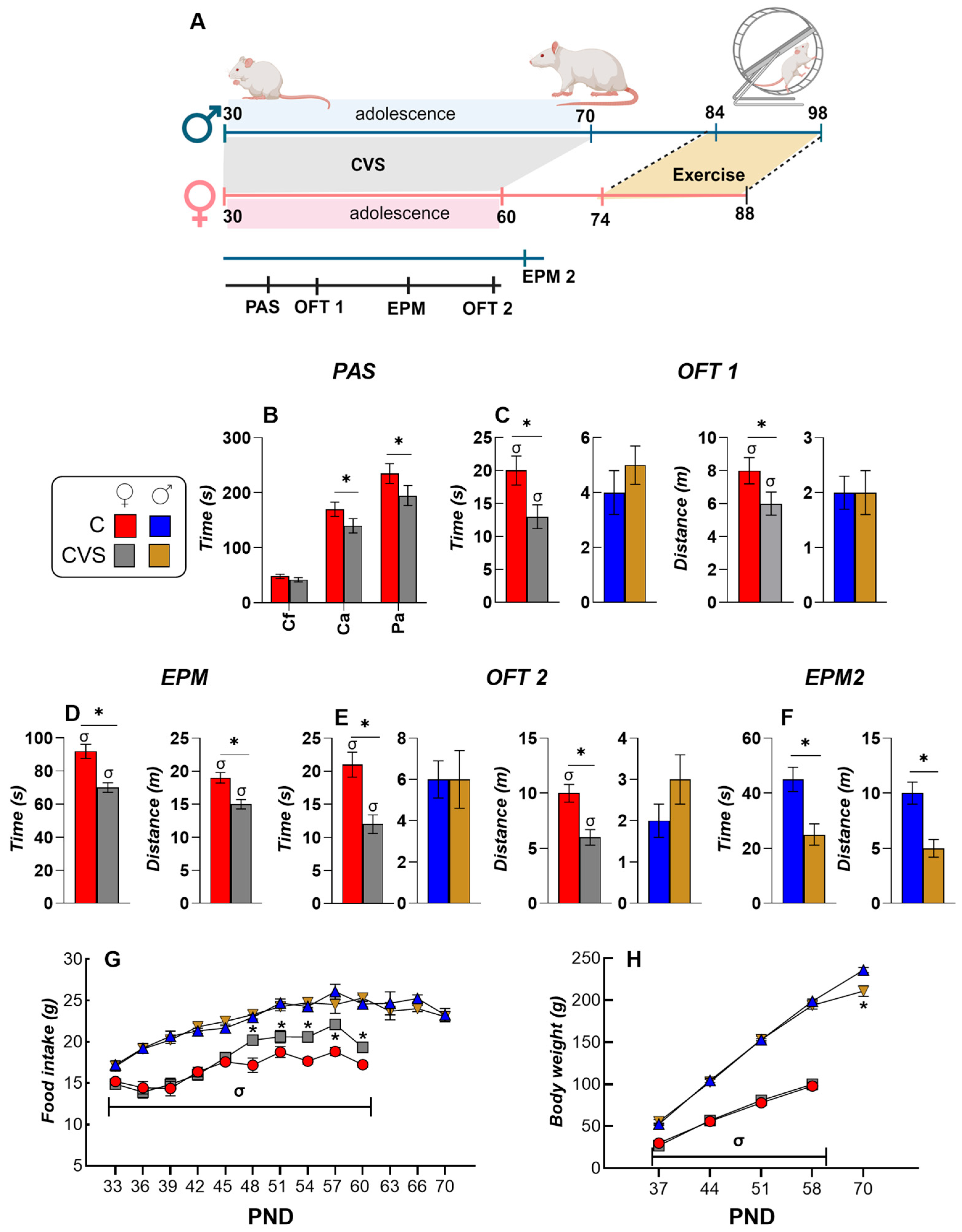
2.1. Behavioral and Ponderal Variables During Adolescence
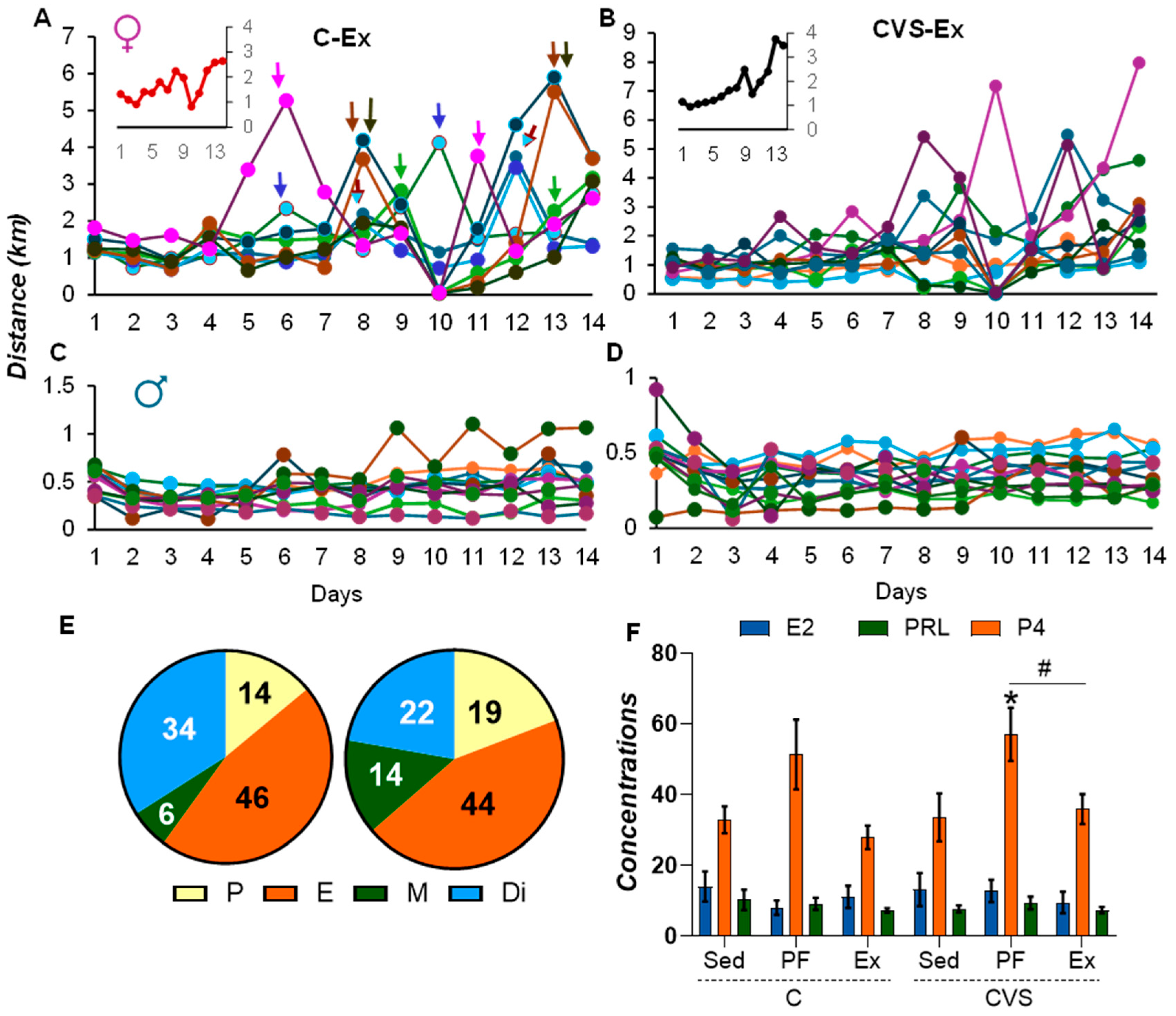
2.2. Long-Term Effects of CVS on Voluntary Exercise
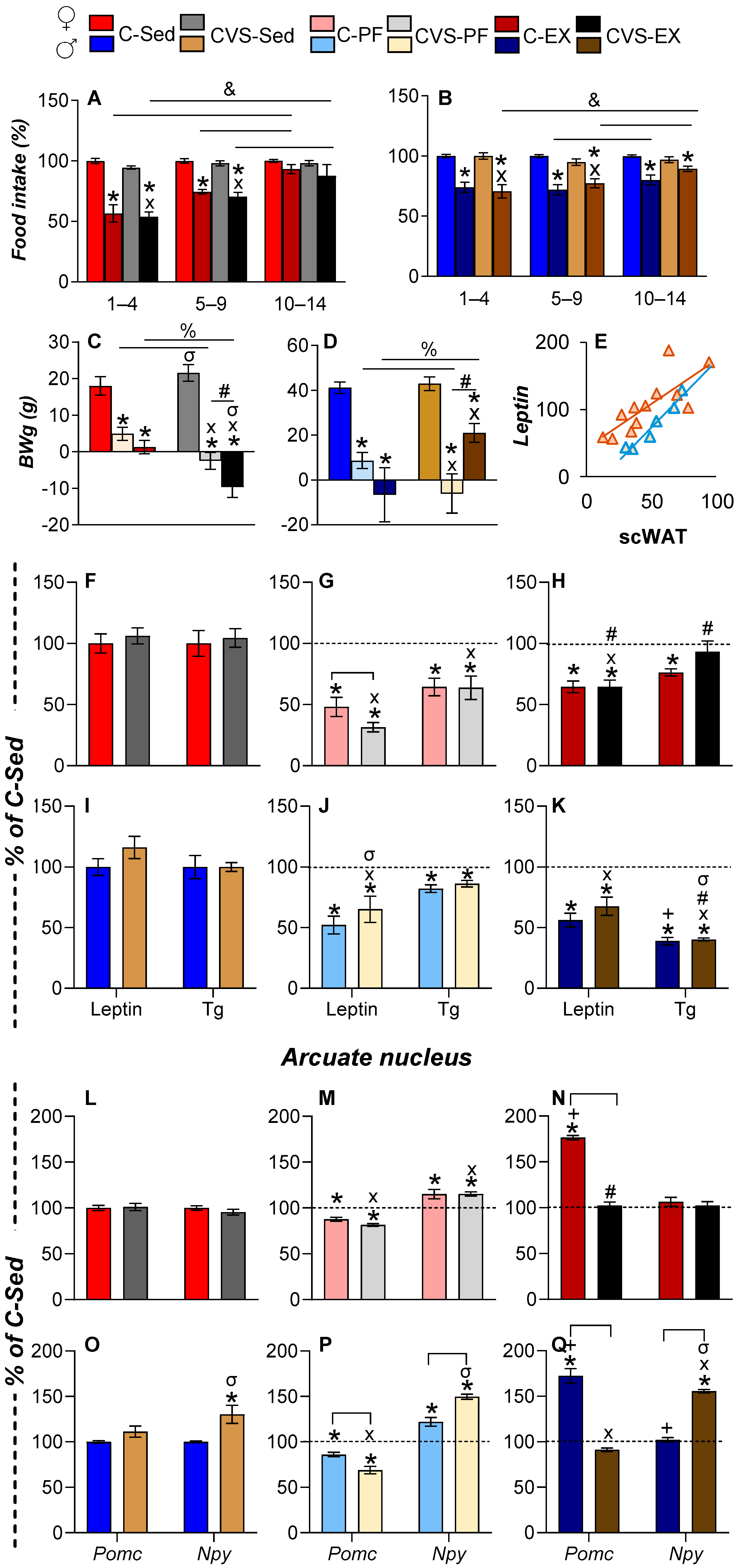
2.3. Voluntary Exercise Reduces Food Intake, Body Weight, and Fat Mass
2.4. Long-Term Effects of CVS on Serum Energy Markers and ARC Expression Under Mild Food Restriction or Exercise
2.5. CVS Alters HPA Axis Responses to Mild Food Restriction and Exercise
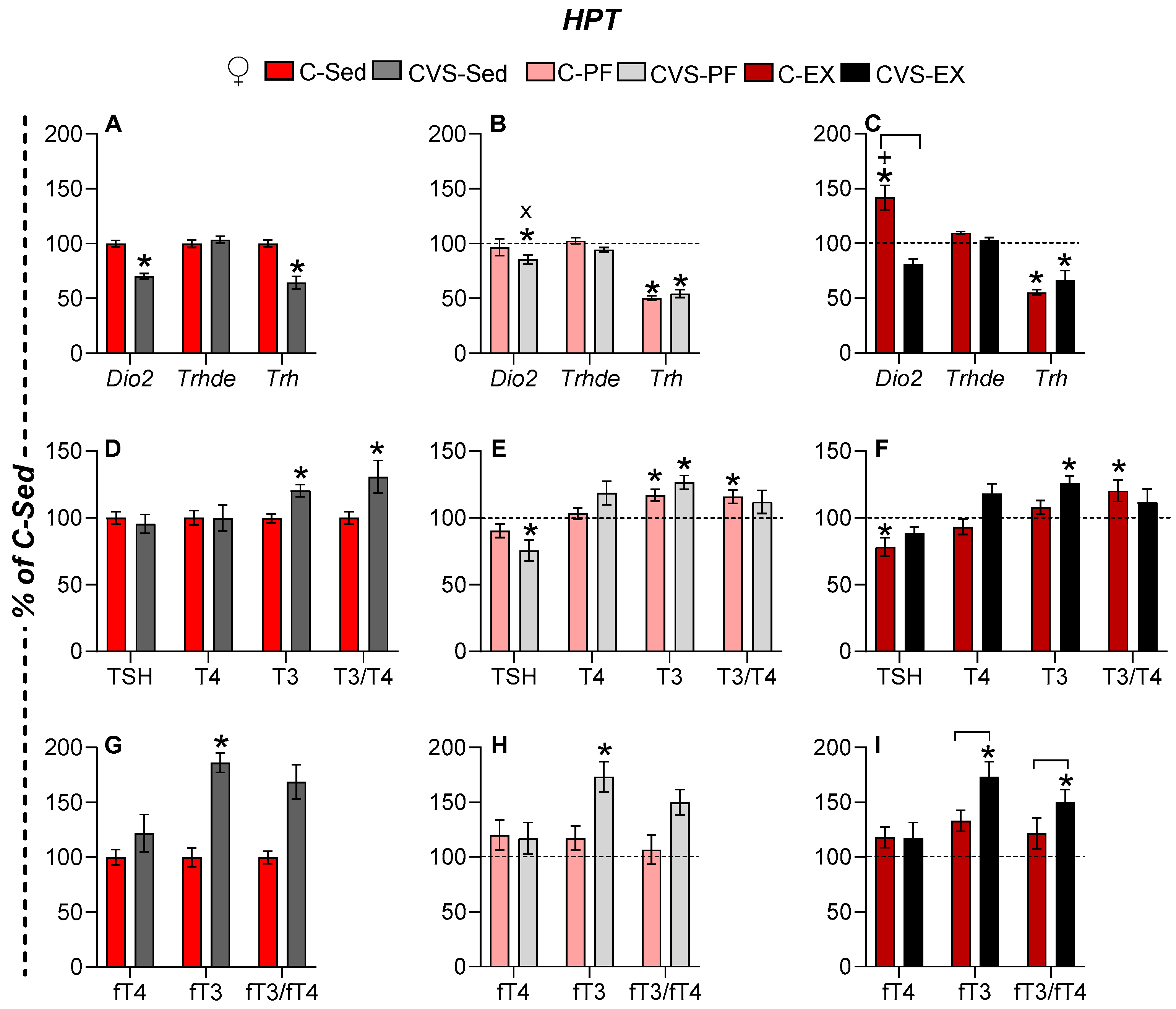
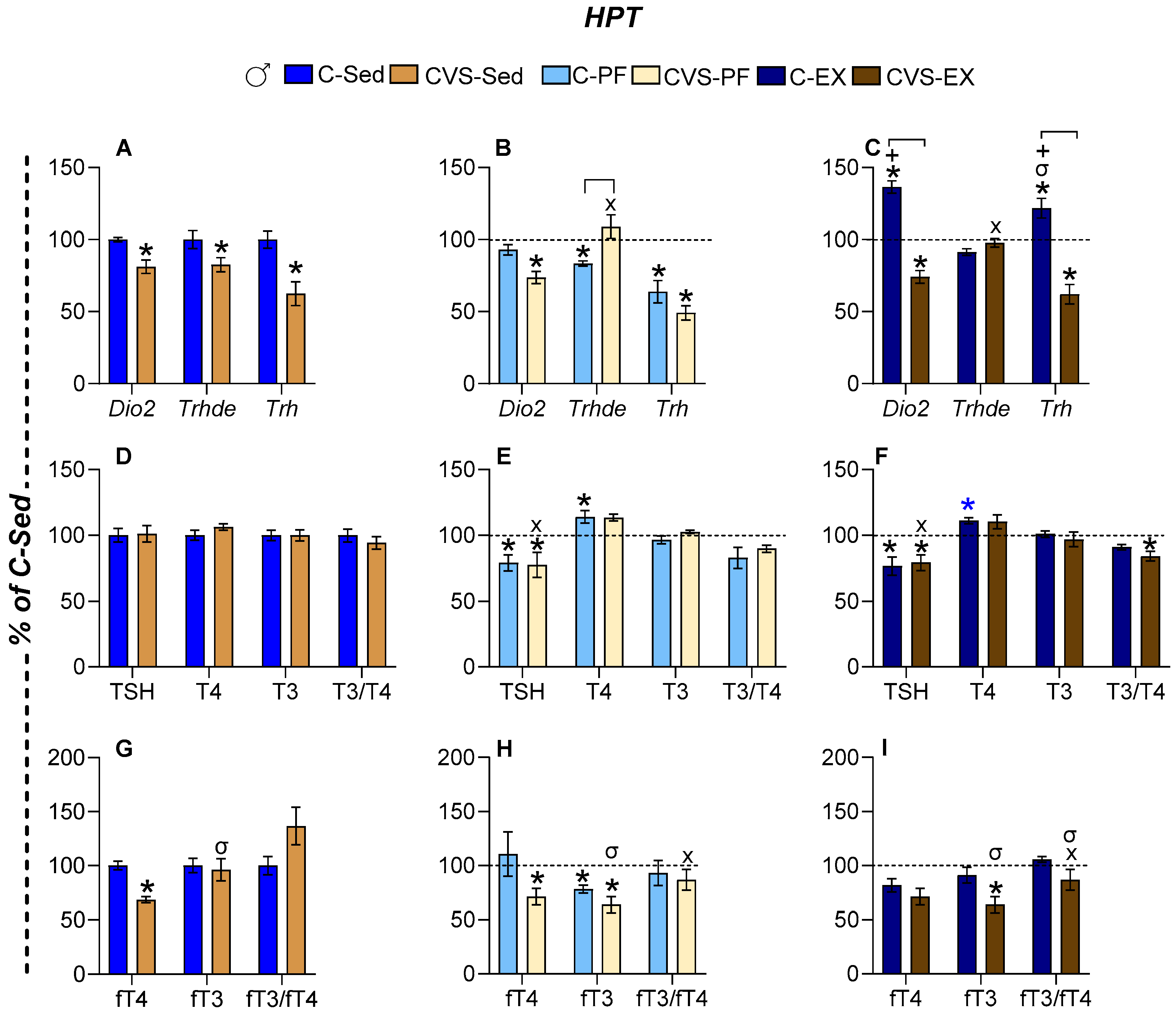
2.6. Long-Term Effects of CVS on HPT Axis Activity Under Mild Food Restriction and Exercise
2.6.1. Females
2.6.2. Males
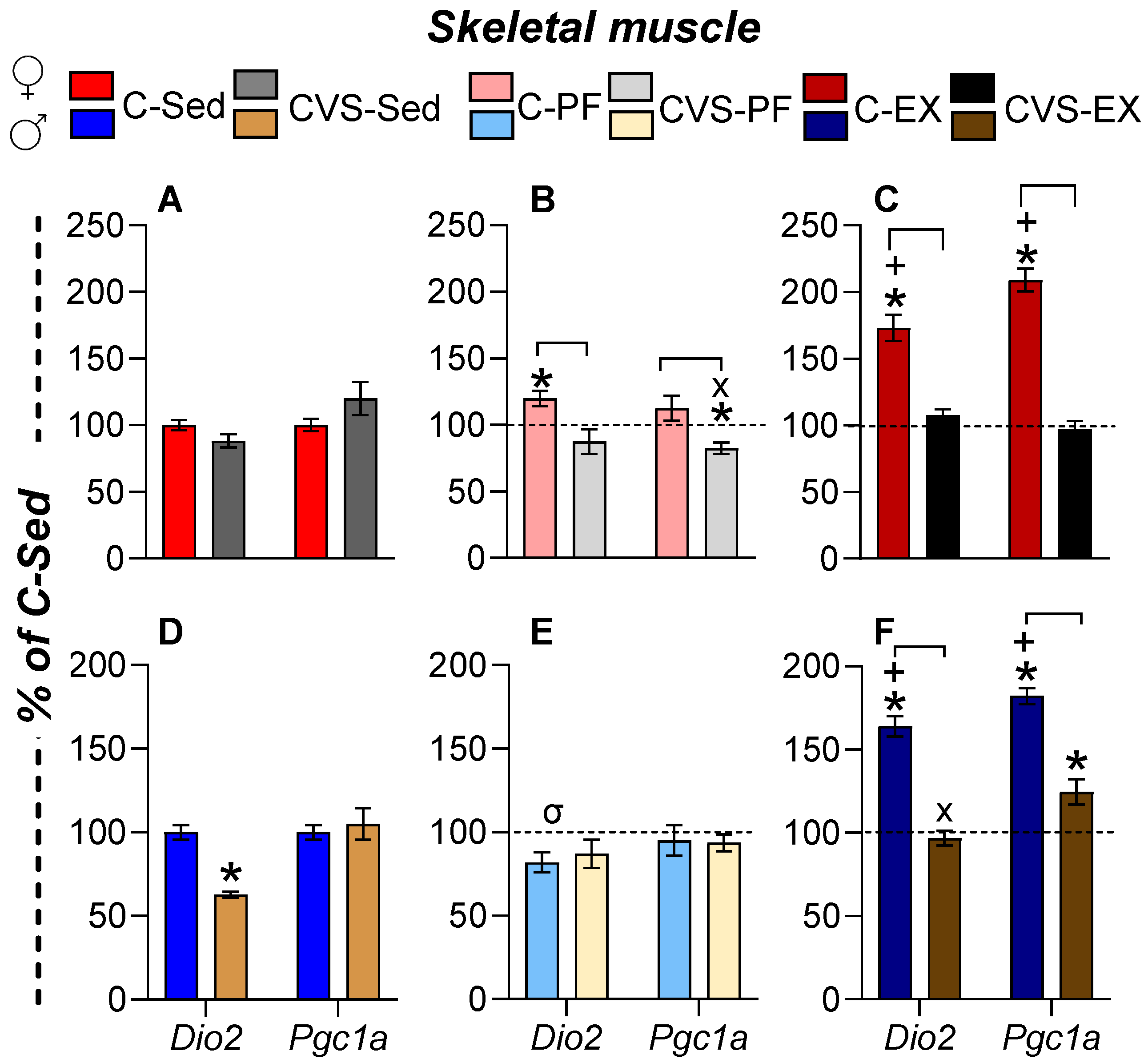
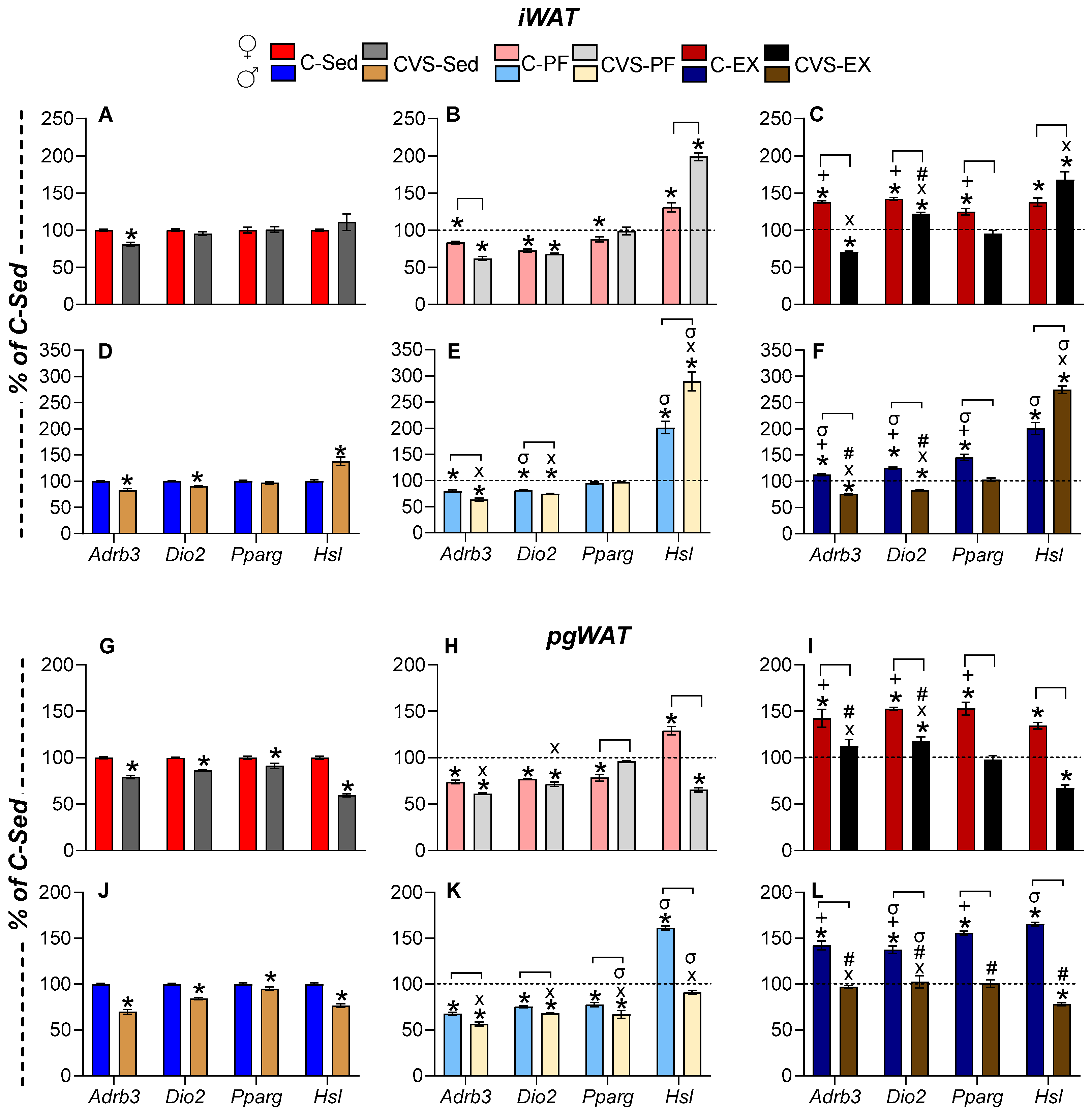
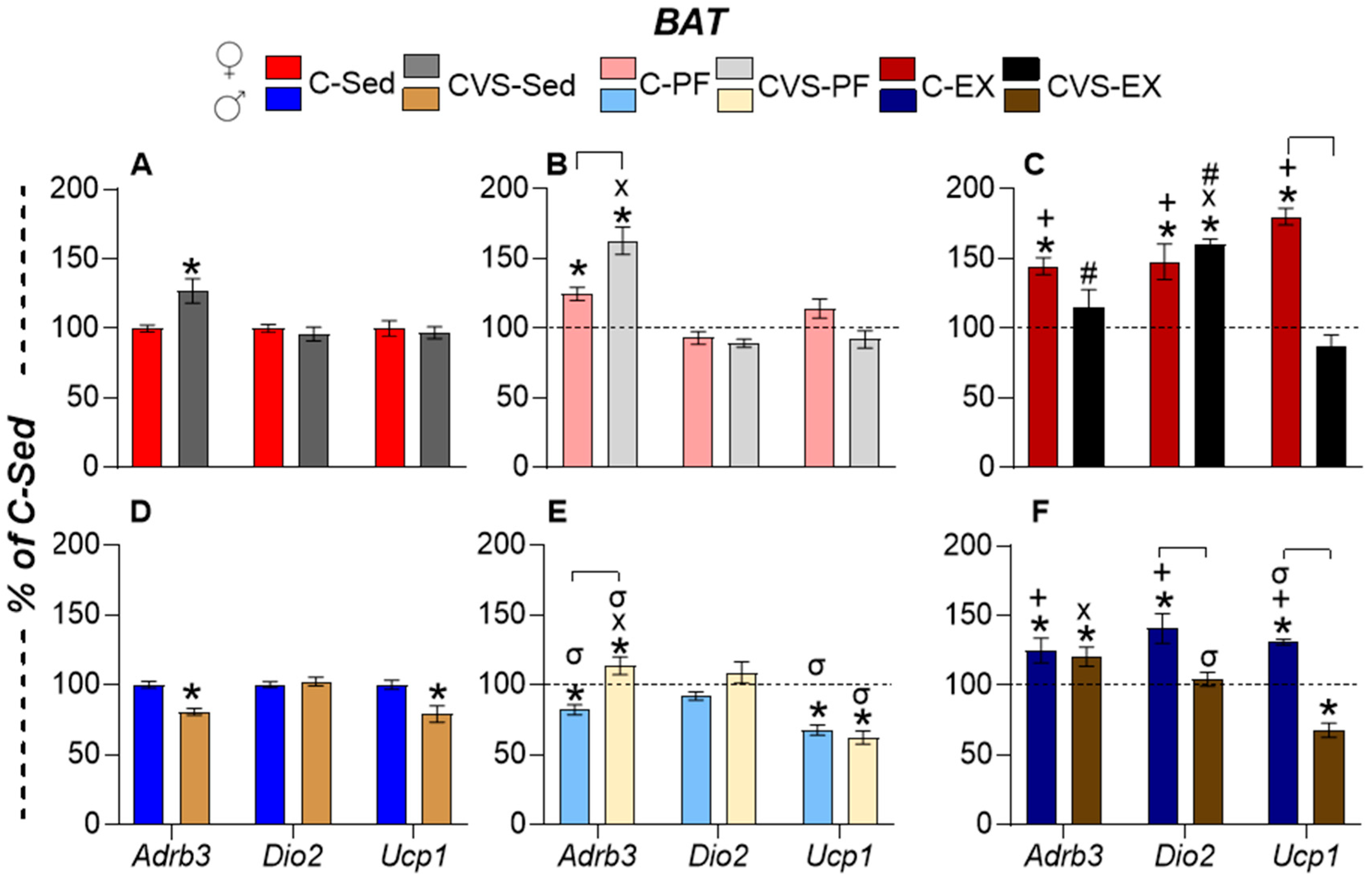
2.7. Long-Term Effects of CVS on Gene Expression in Target Organs
2.7.1. Skeletal Muscle (SKM)
2.7.2. White Adipose Tissue
2.7.3. Brown Adipose Tissue (BAT)
2.8. Correlation Analyses
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Groups
4.2. Behavioral Tests
4.3. Voluntary Exercise
4.4. Tissue Collection
4.5. Brain Dissections
4.6. Hormonal Measurements
4.7. RNA Extraction and mRNA Quantification
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACTH | Corticotrophin |
| Adrb3 | Adrenergic receptor β3 |
| AgRP | Agouti-related peptide |
| ARC | Arcuate nucleus |
| Avp | Arginine vasopressin |
| BAT | Brown adipose tissue |
| BW | Body weight |
| BWg | Body weight gain |
| C | Control |
| Cort | Corticosterone |
| CR | Calorie restriction |
| CRH | Corticotropin-releasing hormone |
| CVS | Chronic variable stress |
| Dio1 | Deiodinase 1 |
| Dio2 | Deiodinase 2 |
| Dio3 | Deiodinase 3 |
| E2 | Estradiol |
| EPM | Elevated plus maze |
| Ex | Exercised (voluntary wheel running) |
| FE | Food efficiency |
| FI | Food intake |
| fT3 | Free T3 |
| fT4 | Free T4 |
| Gr | Glucocorticoid receptor |
| HPA | Hypothalamus–pituitary–adrenal |
| Hprt | Hypoxanthine phosphoribosyl transferase 1 |
| HPT | Hypothalamic–pituitary–thyroid |
| HSL (Lipe) | Hormone-sensitive lipase |
| IL-16 | Interleukin-16 |
| iWAT | Inguinal WAT |
| MBH | Mediobasal hypothalamus |
| NPY | Neuropeptide Y |
| OFT | Open Field Test |
| P4 | Progesterone |
| PAS | Photobeam activity system |
| PF | Pair-fed |
| Pgc-1α | Peroxisome proliferator-activated receptor γ coactivator 1α |
| pgWAT | Perigonadal WAT |
| PND | Postnatal day |
| POMC | Pro-opiomelanocortin |
| Ppia | Cyclophilin A |
| Pparg | Peroxisome proliferator-activated receptor γ |
| PRL | Prolactin |
| PVN | Paraventricular nucleus of the hypothalamus |
| RFI | Relative food intake |
| Sed | Sedentary |
| SKM | Skeletal muscle |
| T3 | Triiodothyronine |
| T4 | Thyroxine |
| Tg | Triglycerides |
| TH | Thyroid hormones |
| TRH | Thyrotropin-releasing hormone |
| TRH-DE (Trhde) | TRH-degrading ectoenzyme |
| TSH | Thyrotropin |
| Ucp1 | Uncoupling protein-1 |
| WAT | White adipose tissue |
| WR | Wheel running |
References
- Kerr, N.R.; Booth, F.W. Contributions of physical inactivity and sedentary behavior to metabolic and endocrine diseases. Trends Endocrinol. Metab. 2022, 33, 817–827. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S.; Akil, H. Revisiting the Stress Concept: Implications for Affective Disorders. J. Neurosci. 2020, 40, 12–21. [Google Scholar] [CrossRef]
- Radley, J.J.; Herman, J.P. Preclinical Models of Chronic Stress: Adaptation or Pathology? Biol. Psychiatry 2023, 94, 194–202. [Google Scholar] [CrossRef]
- Agorastos, A.; Chrousos, G.P. The neuroendocrinology of stress: The stress-related continuum of chronic disease development. Mol. Psychiatry 2022, 27, 502–513. [Google Scholar] [CrossRef] [PubMed]
- Romeo, R.D. The metamorphosis of adolescent hormonal stress reactivity: A focus on animal models. Front. Neuroendocrinol. 2018, 49, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Dearing, C.; Handa, R.J.; Myers, B. Sex differences in autonomic responses to stress: Implications for cardiometabolic physiology. Am. J. Physiol. Endocrinol. Metab. 2022, 323, E281–E289. [Google Scholar] [CrossRef]
- Hird, E.J.; Slanina-Davies, A.; Lewis, G.; Hamer, M.; Roiser, J.P. From movement to motivation: A proposed framework to understand the antidepressant effect of exercise. Transl. Psychiatry 2024, 14, 273. [Google Scholar] [CrossRef]
- Esteves, J.V.; Stanford, K.I. Exercise as a tool to mitigate metabolic disease. Am. J. Physiol. Cell Physiol. 2024, 327, C587–C598. [Google Scholar] [CrossRef]
- Chow, L.S.; Gerszten, R.E.; Taylor, J.M.; Pedersen, B.K.; Van Praag, H.; Trappe, S.; Febbraio, M.A.; Galis, Z.S.; Gao, Y.; Haus, J.M.; et al. Exerkines in health, resilience and disease. Nat. Rev. Endocrinol. 2022, 18, 273–289. [Google Scholar] [CrossRef]
- Hwang, E.; Portillo, B.; Grose, K.; Fujikawa, T.; Williams, K.W. Exercise-induced hypothalamic neuroplasticity: Implications for energy and glucose metabolism. Mol. Metab. 2023, 73, 101745. [Google Scholar] [CrossRef]
- Quarta, C.; Claret, M.; Zeltser, L.M.; Williams, K.W.; Yeo, G.S.H.; Tschöp, M.H.; Diano, S.; Brüning, J.C.; Cota, D. POMC neuronal heterogeneity in energy balance and beyond: An integrated view. Nat. Metab. 2021, 3, 299–308. [Google Scholar] [CrossRef]
- Lorsignol, A.; Rabiller, L.; Labit, E.; Casteilla, L.; Pénicaud, L. The nervous system and adipose tissues: A tale of dialogues. Am. J. Physiol. Endocrinol. Metab. 2023, 325, E480–E490. [Google Scholar] [CrossRef]
- Zhang, Z.; Su, J.; Tang, J.; Chung, L.; Page, J.C.; Winter, C.C.; Liu, Y.; Kegeles, E.; Conti, S.; Zhang, Y.; et al. Spinal projecting neurons in rostral ventromedial medulla co-regulate motor and sympathetic tone. Cell 2024, 187, 3427–3444.e21. [Google Scholar] [CrossRef]
- Joseph-Bravo, P.; Jaimes-Hoy, L.; Uribe, R.M.; Charli, J.L. 60 YEARS OF NEUROENDOCRINOLOGY: TRH, the first hypophysiotropic releasing hormone isolated: Control of the pituitary-thyroid axis. J. Endocrinol. 2015, 226, T85–T100, Erratum in: J. Endocrinol. 2015, 227, X3. https://doi.org/10.1530/JOE-15-0124e. [Google Scholar] [CrossRef]
- Costa-E.-Sousa, R.H.; Rorato, R.; Hollenberg, A.N.; Vella, K.R. Regulation of thyroid hormone levels by hypothalamic thyrotropin-releasing hormone neurons. Thyroid 2023, 33, 867–876. [Google Scholar] [CrossRef]
- Herman, J.P. The neuroendocrinology of stress: Glucocorticoid signaling mechanisms. Psychoneuroendocrinology 2021, 137, 105641. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.A.; Yen, P.M. Metabolic messengers: Thyroid hormones. Nat. Metab. 2024, 6, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Jimeno, B.; Verhulst, S. Meta-analysis reveals glucocorticoid levels reflect variation in metabolic rate, not ‘stress’. Elife 2023, 12, RP88205. [Google Scholar] [CrossRef]
- Russo, S.C.; Salas-Lucia, F.; Bianco, A.C. Deiodinases and the metabolic code for thyroid hormone action. Endocrinology 2021, 162, bqab059. [Google Scholar] [CrossRef] [PubMed]
- Köhrle, J.; Frädrich, C. Deiodinases control local cellular and systemic thyroid hormone availability. Free Radic. Biol. Med. 2022, 193, 59–79. [Google Scholar] [CrossRef]
- Silva, J.E.; Bianco, S.D. Thyroid-adrenergic interactions: Physiological; clinical implications. Thyroid 2008, 18, 157–165. [Google Scholar] [CrossRef]
- Mullur, R.; Liu, Y.Y.; Brent, G.A. Thyroid hormone regulation of metabolism. Physiol. Rev. 2014, 94, 355–382. [Google Scholar] [CrossRef]
- Guilherme, A.; Yenilmez, B.; Bedard, A.H.; Henriques, F.; Liu, D.; Lee, A.; Goldstein, L.; Kelly, M.; Nicoloro, S.M.; Chen, M.; et al. Control of adipocyte thermogenesis and lipogenesis through β3-adrenergic and thyroid hormone signal integration. Cell Rep. 2020, 31, 107598. [Google Scholar] [CrossRef] [PubMed]
- Bloise, F.F.; Cordeiro, A.; Ortiga-Carvalho, T.M. Role of thyroid hormone in skeletal muscle physiology. J. Endocrinol. 2018, 236, R57–R68. [Google Scholar] [CrossRef] [PubMed]
- Bocco, B.M.; Louzada, R.A.; Silvestre, D.H.; Santos, M.C.; Anne-Palmer, E.; Rangel, I.F.; Abdalla, S.; Ferreira, A.C.; Ribeiro, M.O.; Gereben, B.; et al. Thyroid hormone activation by type 2 deiodinase mediates exercise-induced peroxisome proliferator-activated receptor-γ coactivator-1α expression in skeletal muscle. J. Physiol. 2016, 594, 5255–5269. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Leow, M.K.S.; Dietrich, J.W. Minor perturbations of thyroid homeostasis and major cardiovascular endpoints—Physiological mechanisms and clinical evidence. Front. Cardiovasc. Med. 2022, 9, 942971. [Google Scholar] [CrossRef] [PubMed]
- Joseph-Bravo, P.; Jaimes-Hoy, L.; Charli, J.L. Regulation of TRH neurons and energy homeostasis-related signals under stress. J Endocrinol. 2015, 224, R139–R159, Erratum in J. Endocrinol. 2015, 227, X1. https://doi.org/10.1530/JOE-14-0593e. [Google Scholar] [CrossRef]
- Petrowski, K.; Kahaly, G.J. Stress & thyroid function—From bench to bedside. Endocr. Rev. 2025, bnaf015. [Google Scholar] [CrossRef]
- Uribe, R.M.; Redondo, J.L.; Charli, J.L.; Joseph-Bravo, P. Suckling and cold stress rapidly and transiently increase TRH mRNA in the paraventricular nucleus. Neuroendocrinology 1993, 58, 140–145. [Google Scholar] [CrossRef]
- Ruisseau, P.D.; Taché, Y.; Brazeau, P.; Collu, R. Pattern of adenohypophyseal hormone changes induced by various stressors in female and male rats. Neuroendocrinology 1978, 27, 257–271. [Google Scholar] [CrossRef]
- Fortunato, R.S.; Ignácio, D.L.; Padron, A.S.; Peçanha, R.; Marassi, M.P.; Rosenthal, D.; Werneck-de-Castro, J.P.S.; Carvalho, D.P. The effect of acute exercise session on thyroid hormone economy in rats. J. Endocrinol. 2008, 198, 347–353. [Google Scholar] [CrossRef]
- Hackney, A.C.; Saeidi, A. The thyroid axis, prolactin, and exercise in humans. Curr. Opin. Endocr. Metab. Res. 2019, 9, 45–50. [Google Scholar] [CrossRef]
- Weaver, I.C.; Diorio, J.; Seckl, J.R.; Szyf, M.; Meaney, M.J. Early environmental regulation of hippocampal glucocorticoid receptor gene expression: Characterization of intracellular mediators and potential genomic target sites. Ann. N. Y. Acad. Sci. 2004, 1024, 182–212. [Google Scholar] [CrossRef]
- Jaimes-Hoy, L.; Gutiérrez-Mariscal, M.; Vargas, Y.; Pérez-Maldonado, A.; Romero, F.; Sánchez-Jaramillo, E.; Charli, J.L.; Joseph-Bravo, P. Neonatal maternal separation alters, in a sex-specific manner, the expression of TRH, of TRH-degrading ectoenzyme in the rat hypothalamus, and the response of the thyroid axis to starvation. Endocrinology 2016, 157, 3253–3265. [Google Scholar] [CrossRef]
- Jaimes-Hoy, L.; Pérez-Maldonado, A.; Narváez Bahena, E.; de la Cruz Guarneros, N.; Rodríguez-Rodríguez, A.; Charli, J.L.; Soberón, X.; Joseph-Bravo, P. Sex dimorphic changes in Trh gene methylation and thyroid-axis response to energy demands in maternally separated rats. Endocrinology 2021, 162, bqab110. [Google Scholar] [CrossRef] [PubMed]
- Osterlund, C.; Spencer, R.L. Corticosterone pretreatment suppresses stress-induced hypothalamic-pituitary-adrenal axis activity via multiple actions that vary with time, site of action, and de novo protein synthesis. J. Endocrinol. 2011, 208, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Sotelo-Rivera, I.; Jaimes-Hoy, L.; Cote-Vélez, A.; Espinoza-Ayala, C.; Charli, J.L.; Joseph-Bravo, P. An acute injection of corticosterone increases thyrotrophin-releasing hormone expression in the paraventricular nucleus of the hypothalamus but interferes with the rapid hypothalamus pituitary thyroid axis response to cold in male rats. J. Neuroendocrinol. 2014, 26, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Sotelo-Rivera, I.; Cote-Vélez, A.; Uribe, R.M.; Charli, J.L.; Joseph-Bravo, P. Glucocorticoids curtail stimuli-induced CREB phosphorylation in TRH neurons through interaction of the glucocorticoid receptor with the catalytic subunit of protein kinase A. Endocrine 2017, 55, 861–871. [Google Scholar] [CrossRef]
- Castillo-Campos, A.; Gutiérrez-Mata, A.; Charli, J.L.; Joseph-Bravo, P. Chronic stress inhibits hypothalamus-pituitary-thyroid axis; brown adipose tissue responses to acute cold exposure in male rats. J. Endocrinol. Investig. 2021, 44, 713–723. [Google Scholar] [CrossRef]
- Tanner, M.K.; Mellert, S.M.; Fallon, I.P.; Baratta, M.V.; Greenwood, B.N. Multiple Sex-; Circuit-Specific Mechanisms Underlie Exercise-Induced Stress Resistance. Curr. Top Behav. Neurosci. 2024, 67, 37–60. [Google Scholar] [CrossRef]
- Russell, V.A.; Zigmond, M.J.; Dimatelis, J.J.; Daniels, W.M.; Mabandla, M.V. The interaction between stress and exercise, and its impact on brain function. Metab. Brain Dis. 2014, 29, 255–260. [Google Scholar] [CrossRef]
- Parra-Montes de Oca, M.A.; Gutiérrez-Mariscal, M.; Salmerón-Jiménez, M.F.; Jaimes-Hoy, L.; Charli, J.L.; Joseph-Bravo, P. Voluntary exercise-induced activation of thyroid axis and reduction of white fat depots is attenuated by chronic stress in a sex dimorphic pattern in adult rats. Front. Endocrinol. 2019, 10, 418. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, H.J.; Lee, W.J.; Seong, J.K. A comparison of the metabolic effects of treadmill and wheel running exercise in mouse model. Lab Anim. Res. 2020, 36, 3. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Liu, Z.; Liu, Y.; Zhang, R.; Mo, H.; Gao, L.; Shi, Y. Physical exercise rectifies CUMS-induced aberrant regional homogeneity in mice accompanied by the adjustment of skeletal muscle PGC-1a/IDO1 signals and hippocampal function. Behav. Brain Res. 2020, 383, 112516. [Google Scholar] [CrossRef]
- Shen, H.; Li, X.; Zhai, J.; Zhang, X. Voluntary wheel-running exercise improvement of anxiety or depressive symptoms in different models of depression. Front. Behav. Neurosci. 2024, 18, 1435891. [Google Scholar] [CrossRef] [PubMed]
- Basso, J.C.; Morrell, J.I. Using wheel availability to shape running behavior of the rat towards improved behavioral and neurobiological outcomes. J. Neurosci. Methods. 2017, 290, 13–23. [Google Scholar] [CrossRef]
- Greenwood, B.N.; Fleshner, M. Voluntary wheel running: A useful rodent model for investigating the mechanisms of stress robustness and neural circuits of exercise motivation. Curr. Opin. Behav. Sci. 2019, 28, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Lundsgaard, A.M.; Fritzen, A.M.; Kiens, B. The Importance of Fatty Acids as Nutrients during Post-Exercise Recovery. Nutrients 2020, 12, 280. [Google Scholar] [CrossRef]
- Uribe, R.M.; Jaimes-Hoy, L.; Ramírez-Martínez, C.; García-Vázquez, A.; Romero, F.; Cisneros, M.; Cote-Vélez, A.; Charli, J.L.; Joseph-Bravo, P. Voluntary exercise adapts the hypothalamus-pituitary-thyroid axis in male rats. Endocrinology 2014, 155, 2020–2030. [Google Scholar] [CrossRef]
- Drzewiecki, C.M.; Juraska, J.M. The structural reorganization of the prefrontal cortex during adolescence as a framework for vulnerability to the environment. Pharmacol. Biochem. Behav. 2020, 199, 173044. [Google Scholar] [CrossRef]
- Schneider, M. Adolescence as a vulnerable period to alter rodent behavior. Cell Tissue Res. 2013, 354, 99–106. [Google Scholar] [CrossRef]
- Jankord, R.; Solomon, M.B.; Albertz, J.; Flak, J.N.; Zhang, R.; Herman, J.P. Stress vulnerability during adolescent development in rats. Endocrinology 2011, 152, 629–638. [Google Scholar] [CrossRef]
- Cotella, E.M.; Gómez, A.S.; Lemen, P.; Chen, C.; Fernández, G.; Hansen, C.; Herman, J.P.; Paglini, M.G. Long-term impact of chronic variable stress in adolescence versus adulthood. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 88, 303–310. [Google Scholar] [CrossRef]
- Bourke, C.H.; Neigh, G.N. Behavioral effects of chronic adolescent stress are sustained and sexually dimorphic. Horm. Behav. 2011, 60, 112–120. [Google Scholar] [CrossRef]
- Guo, T.Y.; Liu, L.J.; Xu, L.Z.; Zhang, J.C.; Li, S.X.; Chen, C.; He, L.G.; Chen, Y.M.; Yang, H.D.; Lu, L.; et al. Alterations of the daily rhythms of HPT axis induced by chronic unpredicted mild stress in rats. Endocrine 2015, 48, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Wulsin, A.C.; Wick-Carlson, D.; Packard, B.; Morano, R.; Herman, J.P. Adolescent chronic stress causes hypothalamo-pituitary-adrenocortical hypo-responsiveness and depression-like behavior in adult female rats. Psychoneuroendocrinol 2016, 65, 109–117. [Google Scholar] [CrossRef]
- Mathis, V.; Wegman-Points, L.; Pope, B.; Lee, C.J.; Mohamed, M.; Rhodes, J.S.; Clark, P.J.; Clayton, S.; Yuan, L.L. Estrogen-mediated individual differences in female rat voluntary running behavior. J. Appl. Physiol. 2024, 136, 592–605. [Google Scholar] [CrossRef]
- Aguiar, A.S., Jr.; Speck, A.E.; Amaral, I.M.; Canas, P.M.; Cunha, R.A. The exercise sex gap and the impact of the estrous cycle on exercise performance in mice. Sci. Rep. 2018, 8, 10742. [Google Scholar] [CrossRef]
- Suchacki, K.J.; Thomas, B.J.; Ikushima, Y.M.; Chen, K.C.; Fyfe, C.; Tavares, A.A.S.; Sulston, R.J.; Lovdel, A.; Woodward, H.J.; Han, X.; et al. The effects of caloric restriction on adipose tissue and metabolic health are sex- and age-dependent. Elife 2023, 12, e88080. [Google Scholar] [CrossRef] [PubMed]
- Mauvais-Jarvis, F. Sex differences in energy metabolism: Natural selection, mechanisms and consequences. Nat. Rev. Nephrol. 2024, 20, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Münzberg, H.; Singh, P.; Heymsfield, S.B.; Yu, S.; Morrison, C.D. Recent advances in understanding the role of leptin in energy homeostasis. F1000Res 2020, 9, 451. [Google Scholar] [CrossRef]
- López López, A.L.; Escobar Villanueva, M.C.; Brianza Padilla, M.; Bonilla Jaime, H.; Alarcón Aguilar, F.J. Chronic unpredictable mild stress progressively disturbs glucose metabolism and appetite hormones in rats. Acta Endocrinol. 2018, 14, 16–23. [Google Scholar] [CrossRef]
- Sasse, S.K.; Nyhuis, T.J.; Masini, C.V.; Day, H.E.; Campeau, S. Central gene expression changes associated with enhanced neuroendocrine and autonomic response habituation to repeated noise stress after voluntary wheel running in rats. Front. Physiol. 2013, 4, 341. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, M.; Wilsgaard, T.; Grimsgaard, S.; Hopstock, L.A.; Hansson, P. Associations between postprandial triglyceride concentrations, sex, age and body mass index: Cross-sectional analyses from the Tromsø study 2015-2016. Front. Nutr. 2023, 10, 1158383. [Google Scholar] [CrossRef]
- Holcomb, L.E.; Rowe, P.; O’Neill, C.C.; DeWitt, E.A.; Kolwicz, S.C., Jr. Sex differences in endurance exercise capacity and skeletal muscle lipid metabolism in mice. Physiol. Rep. 2022, 10, e15174. [Google Scholar] [CrossRef]
- Cavalcanti-de-Albuquerque, J.P.; Donato, J. Rolling out physical exercise and energy homeostasis: Focus on hypothalamic circuitries. Front. Neuroendocrinol. 2021, 63, 100944. [Google Scholar] [CrossRef]
- Brady, L.S.; Smith, M.A.; Gold, P.W.; Herkenham, M. Altered expression of hypothalamic neuropeptide mRNAs in food-restricted and food-deprived rats. Neuroendocrinology 1990, 52, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Loh, K.; Herzog, H.; Shi, Y.C. Regulation of energy homeostasis by the NPY system. Trends Endocrinol. Metab. 2015, 26, 125–135. [Google Scholar] [CrossRef]
- He, Z.; Gao, Y.; Alhadeff, A.L.; Castorena, C.M.; Huang, Y.; Lieu, L.; Afrin, S.; Sun, J.; Betley, J.; Guo, H.; et al. Cellular and synaptic reorganization of arcuate NPY/AgRP and POMC neurons after exercise. Mol. Metab. 2018, 18, 107–119. [Google Scholar] [CrossRef]
- Urban, J.H.; Bauer-Dantoin, A.C.; Levine, J.E. Neuropeptide Y gene expression in the arcuate nucleus: Sexual dimorphism and modulation by testosterone. Endocrinology 1993, 132, 139–145. [Google Scholar] [CrossRef]
- Rebouças, E.C.; Leal, S.; Sá, S.I. Regulation of NPY and α-MSH expression by estradiol in the arcuate nucleus of Wistar female rats: A stereological study. Neurol. Res. 2016, 38, 740–747. [Google Scholar] [CrossRef]
- Heck, A.L.; Handa, R.J. Sex differences in the hypothalamic-pituitary-adrenal axis’ response to stress: An important role for gonadal hormones. Neuropsychopharmacology 2019, 44, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Lindblom, J.; Haitina, T.; Fredriksson, R.; Schiöth, H.B. Differential regulation of nuclear receptors, neuropeptides and peptide hormones in the hypothalamus and pituitary of food restricted rats. Brain Res. Mol. Brain Res. 2005, 133, 37–46. [Google Scholar] [CrossRef]
- Levay, E.A.; Tammer, A.H.; Penman, J.; Kent, S.; Paolini, A.G. Calorie restriction at increasing levels leads to augmented concentrations of corticosterone and decreasing concentrations of testosterone in rats. Nutr. Res. 2010, 30, 366–373. [Google Scholar] [CrossRef]
- Chen, C.; Nakagawa, S.; An, Y.; Ito, K.; Kitaichi, Y.; Kusumi, I. The exercise-glucocorticoid paradox: How exercise is beneficial to cognition, mood and the brain while increasing glucocorticoid levels. Front. Neuroendocrinol. 2017, 44, 83–102. [Google Scholar] [CrossRef]
- Fekete, C.; Lechan, R.M. Central regulation of hypothalamic-pituitary-thyroid axis under physiological; pathophysiological conditions. Endocr. Rev. 2014, 35, 159–194. [Google Scholar] [CrossRef]
- Sánchez, E.; Vargas, M.A.; Singru, P.S.; Pascual, I.; Romero, F.; Fekete, C.; Charli, J.L.; Lechan, R.M. Tanycyte pyroglutamyl peptidase II contributes to regulation of the hypothalamic-pituitary-thyroid axis through glial-axonal associations in the median eminence. Endocrinology 2009, 150, 2283–2291. [Google Scholar] [CrossRef] [PubMed]
- Araujo, R.L.; Andrade, B.M.; da Silva, M.L.; Ferreira, A.C.; Carvalho, D.P. Tissue-specific deiodinase regulation during food restriction and low replacement dose of leptin in rats. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E1157–E1163. [Google Scholar] [CrossRef]
- Joseph-Bravo, P.; Lazcano, I.; Jaimes-Hoy, L.; Gutierrez-Mariscal, M.; Sánchez-Jaramillo, E.; Uribe, R.M.; Charli, J.L. Sexually dimorphic dynamics of thyroid axis activity during fasting in rats. Front. Biosci. 2020, 25, 1305–1323. [Google Scholar] [CrossRef]
- van Haasteren, G.A.; Linkels, E.; van Toor, H.; Klootwijk, W.; Kaptein, E.; de Jong, F.H.; Reymond, M.J.; Visser, T.J.; de Greef, W.J. Effects of long-term food reduction on the hypothalamus-pituitary-thyroid axis in male and female rats. J. Endocrinol. 1996, 150, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.M.; Watt, M.J.; Febbraio, M.A. Metabolic communication during exercise. Nat. Metab. 2020, 2, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Cheng, Y.; Ge, R.; Chen, K.; Yang, L. Exercise-induced adaptation of neurons in the vertebrate locomotor system. J. Sport Health Sci. 2024, 13, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Weitzel, J.M.; Iwen, K.A. Coordination of mitochondrial biogenesis by thyroid hormone. Mol. Cell Endocrinol. 2011, 342, 1–7. [Google Scholar] [CrossRef]
- Nappi, A.; Moriello, C.; Morgante, M.; Fusco, F.; Crocetto, F.; Miro, C. Effects of thyroid hormones in skeletal muscle protein turnover. J. Basic Clin. Physiol. Pharmacol. 2024, 35, 253–264. [Google Scholar] [CrossRef]
- Baumgartner, A.; Hiedra, L.; Pinna, G.; Eravci, M.; Prengel, H.; Meinhold, H. Rat brain type II 5´-iodothyronine deiodinase activity is extremely sensitive to stress. J. Neurochem. 1998, 71, 817–826. [Google Scholar] [CrossRef]
- Toyoda, N.; Yasuzawa-Amano, S.; Nomura, E.; Yamauchi, A.; Nishimura, K.; Ukita, C.; Morimoto, S.; Kosaki, A.; Iwasaka, T.; Harney, J.W.; et al. Thyroid hormone activation in vascular smooth muscle cells is negatively regulated by glucocorticoid. Thyroid 2009, 19, 755–763. [Google Scholar] [CrossRef]
- Slocum, N.; Durrant, J.R.; Bailey, D.; Yoon, L.; Jordan, H.; Barton, J.; Brown, R.H.; Clifton, L.; Milliken, T.; Harrington, W.; et al. Responses of brown adipose tissue to diet-induced obesity, exercise, dietary restriction and ephedrine treatment. Exp. Toxicol. Pathol. 2013, 65, 549–557. [Google Scholar] [CrossRef]
- Pereira, V.H.; Marques, F.; Lages, V.; Pereira, F.G.; Patchev, A.; Almeida, O.F.; Almeida-Palha, J.; Sousa, N.; Cerqueira, J.J. Glucose intolerance after chronic stress is related with downregulated PPAR-γ in adipose tissue. Cardiovasc. Diabetol. 2016, 15, 114. [Google Scholar] [CrossRef] [PubMed]
- Holm, C.; Osterlund, T.; Laurell, H.; Contreras, J.A. Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Annu. Rev. Nutr. 2000, 20, 365–393. [Google Scholar] [CrossRef]
- Sun, C.; Mao, S.; Chen, S.; Zhang, W.; Liu, C. PPARs-orchestrated metabolic homeostasis in the adipose tissue. Int. J. Mol. Sci. 2021, 22, 8974. [Google Scholar] [CrossRef]
- Lehnig, A.C.; Stanford, K.I. Exercise-induced adaptations to white and brown adipose tissue. J. Exp. Biol. 2018, 221, jeb161570. [Google Scholar] [CrossRef]
- Slavin, B.G.; Ong, J.M.; Kern, P.A. Hormonal regulation of hormone-sensitive lipase activity and mRNA levels in isolated rat adipocytes. J. Lipid. Res. 1994, 35, 1535–1541. [Google Scholar] [CrossRef]
- Narita, T.; Kobayashi, M.; Itakura, K.; Itagawa, R.; Kabaya, R.; Sudo, Y.; Okita, N.; Higami, Y. Differential response to caloric restriction of retroperitoneal; epididymal and subcutaneous adipose tissue depots in rats. Exp. Gerontol. 2018, 104, 127–137. [Google Scholar] [CrossRef]
- Tsiloulis, T.; Watt, M.J. Exercise and the Regulation of Adipose Tissue Metabolism. Prog. Mol. Biol. Transl. Sci. 2015, 135, 175–201. [Google Scholar] [CrossRef]
- Granneman, J.G.; Lahners, K.N. Regulation of mouse beta 3-adrenergic receptor gene expression and mRNA splice variants in adipocytes. Am. J. Physiol. 1995, 268, C1040–C1044. [Google Scholar] [CrossRef]
- Fève, B.; Baude, B.; Krief, S.; Strosberg, A.D.; Pairault, J.; Emorine, L.J. Inhibition by dexamethasone down-regulates β3-adrenergic receptors in 3T3-F442A adipocytes. J. Biol. Chem. 1992, 267, 15909–15915. [Google Scholar] [CrossRef]
- Ayala-Sumuano, J.T.; Velez-del Valle, C.; Beltrán-Langarica, A.; Marsch-Moreno, M.; Hernandez-Mosqueira, C.; Kuri-Harcuch, W. Glucocorticoid paradoxically recruits adipose progenitors and impairs lipid homeostasis and glucose transport in mature adipocytes. Sci. Rep. 2013, 3, 2573. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Yamada, T. UCP1 Dependent and Independent Thermogenesis in Brown and Beige Adipocytes. Front. Endocrinol. 2020, 11, 498. [Google Scholar] [CrossRef] [PubMed]
- Dewal, R.S.; Stanford, K.I. Effects of exercise on brown and beige adipocytes. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2019, 1864, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Martinez-de Mena, R.; Anedda, A.; Cadenas, S.; Obregon, M.J. TSH effects on thermogenesis in rat brown adipocytes. Mol. Cell Endocrinol. 2015, 404, 151–158. [Google Scholar] [CrossRef]
- Murakami, M.; Kamiya, Y.; Morimura, T.; Araki, O.; Imamura, M.; Ogiwara, T.; Mizuma, H.; Mori, M. Thyrotropin receptors in brown adipose tissue: Thyrotropin stimulates type II iodothyronine deiodinase and uncoupling protein-1 in brown adipocytes. Endocrinology 2001, 142, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Foppen, E.; Fliers, E.; Kalsbeek, A. Effects of intracerebroventricular administration of neuropeptide Y on metabolic gene expression and energy metabolism in male rats. Endocrinology 2016, 157, 3070–3085. [Google Scholar] [CrossRef]
- Lundbäck, V.; Kulyté, A.; Dahlman, I.; Marcus, C. Adipose-specific inactivation of thyroid stimulating hormone receptors in mice modifies body weight, temperature and gene expression in adipocytes. Physiol. Rep. 2020, 8, e14538. [Google Scholar] [CrossRef] [PubMed]
- Martelli, D.; Brooks, V.L. Leptin increases: Physiological roles in the control of sympathetic nerve activity, energy balance, and the hypothalamic–pituitary–thyroid axis. Int. J. Mol. Sci 2023, 24, 2684. [Google Scholar] [CrossRef]
- Bianco, A.C.; Dumitrescu, A.; Gereben, B.; Ribeiro, M.O.; Fonseca, T.L.; Fernandes, G.W.; Bocco, B.M.L.C. Paradigms of Dynamic Control of Thyroid Hormone Signaling. Endocr. Rev. 2019, 40, 1000–1047. [Google Scholar] [CrossRef]
- McCormick, C.M. Methods and challenges in investigating sex-specific consequences of social stressors in adolescence in rats: Is it the stress or the social or the stage of development? Curr. Top Behav. Neurosci. 2022, 54, 23–58. [Google Scholar] [CrossRef]
- Sequeira-Cordero, A.; Salas-Bastos, A.; Fornaguera, J.; Brenes, J.C. Behavioural characterisation of chronic unpredictable stress based on ethologically relevant paradigms in rats. Sci. Rep. 2019, 9, 17403. [Google Scholar] [CrossRef] [PubMed]
- Stranahan, A.M.; Lee, K.; Mattson, M.P. Central mechanisms of HPA axis regulation by voluntary exercise. Neuromolecular. Med. 2008, 10, 118–227. [Google Scholar] [CrossRef] [PubMed]
- Ha, G.E.; Cheong, E. Chronic restraint stress decreases the excitability of hypothalamic POMC neuron and increases food intake. Exp. Neurobiol. 2021, 30, 375–386. [Google Scholar] [CrossRef]
- Yesmin, R.; Watanabe, M.; Sinha, A.S.; Ishibashi, M.; Wang, T.; Fukuda, A. A subpopulation of agouti-related peptide neurons exciting corticotropin-releasing hormone axon terminals in median eminence led to hypothalamic-pituitary-adrenal axis activation in response to food restriction. Front. Mol. Neurosci. 2022, 15, 990803. [Google Scholar] [CrossRef]
- Nair, B.B.; Khant, A.Z.; Porteous, R.; Prescott, M.; Glendining, K.A.; Jenkins, D.E.; Augustine, R.A.; Silva, M.S.B.; Yip, S.H.; Bouwer, G.T.; et al. Impact of chronic variable stress on neuroendocrine hypothalamus and pituitary in male and female C57BL/6J mice. J. Neuroendocrinol. 2021, 33, e12972. [Google Scholar] [CrossRef]
- Wray, J.R.; Davies, A.; Sefton, C.; Allen, T.J.; Adamson, A.; Chapman, P.; Lam, B.Y.H.; Yeo, G.S.H.; Coll, A.P.; Harno, E.; et al. Global transcriptomic analysis of the arcuate nucleus following chronic glucocorticoid treatment. Mol. Metab. 2019, 26, 5–17. [Google Scholar] [CrossRef]
- Coppola, A.; Meli, R.; Diano, S. Inverse shift in circulating corticosterone and leptin levels elevates hypothalamic deiodinase type 2 in fasted rats. Endocrinology 2005, 146, 2827–2833. [Google Scholar] [CrossRef]
- Giacco, A.; Cioffi, F.; Cuomo, A.; Simiele, R.; Senese, R.; Silvestri, E.; Amoresano, A.; Fontanarosa, C.; Petito, G.; Moreno, M.; et al. Mild endurance exercise during fasting increases gastrocnemius muscle and prefrontal cortex thyroid hormone levels through differential BHB and BCAA-mediated BDNF-mTOR signaling in rats. Nutrients 2022, 14, 1166. [Google Scholar] [CrossRef] [PubMed]
- Giacomello, E.; Nicoletti, C.; Canato, M.; Toniolo, L. Exercise mimetics in aging: Suggestions from a systematic review. Nutrients 2025, 17, 969. [Google Scholar] [CrossRef]
- Haque, N.; Tischkau, S.A. Sexual dimorphism in adipose-hypothalamic cross talk and the contribution of aryl hydrocarbon receptor to regulate energy homeostasis. Int. J. Mol. Sci. 2022, 23, 7679. [Google Scholar] [CrossRef]
- Queathem, E.D.; Welly, R.J.; Clart, L.M.; Rowles, C.C.; Timmons, H.; Fitzgerald, M.; Eichen, P.A.; Lubahn, D.B.; Vieira-Potter, V.J. White adipose tissue depots respond to chronic beta-3 adrenergic receptor activation in a sexually dimorphic and depot divergent manner. Cells 2021, 10, 3453. [Google Scholar] [CrossRef]
- Vidal, P.; Stanford, K.I. Exercise-Induced Adaptations to Adipose Tissue Thermogenesis. Front. Endocrinol. 2020, 11, 270. [Google Scholar] [CrossRef] [PubMed]
- Garritson, J.D.; Boudina, S. The effects of exercise on white and brown adipose tissue cellularity, metabolic activity and remodeling. Front. Physiol. 2021, 12, 772894. [Google Scholar] [CrossRef] [PubMed]
- Scheel, A.K.; Espelage, L.; Chadt, A. Many ways to rome: Exercise, cold exposure and diet-Do they all affect BAT activation and WAT browning in the same manner? Int. J. Mol. Sci. 2022, 23, 4759. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, Y.J.; Seong, J.K. AMP-activated protein kinase activation in skeletal muscle modulates exercise-induced uncoupled protein 1 expression in brown adipocyte in mouse model. J. Physiol. 2022, 600, 2359–2376. [Google Scholar] [CrossRef]
- Valle, A.; García-Palmer, F.J.; Oliver, J.; Roca, P. Sex differences in brown adipose tissue thermogenic features during caloric restriction. Cell Physiol. Biochem. 2007, 19, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Kaikaew, K.; Grefhorst, A.; Visser, J.A. Sex Differences in Brown Adipose Tissue Function: Sex Hormones, Glucocorticoids, and Their Crosstalk. Front. Endocrinol. 2021, 12, 652444. [Google Scholar] [CrossRef]
- Hellstrom, I.C.; Dhir, S.K.; Diorio, J.C.; Meaney, M.J. Maternal licking regulates hippocampal glucocorticoid receptor transcription through a thyroid hormone-serotonin-NGFI-A signalling cascade. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 2495–2510. [Google Scholar] [CrossRef] [PubMed]
- Louzada, R.A.; Santos, M.C.; Cavalcanti-de-Albuquerque, J.P.; Rangel, I.F.; Ferreira, A.C.; Galina, A.; Werneck-de-Castro, J.P.; Carvalho, D.P. Type 2 iodothyronine deiodinase is upregulated in rat slow- and fast-twitch skeletal muscle during cold exposure. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E1020–E1029. [Google Scholar] [CrossRef]
- Hernandez, A.; St Germain, D.L. Dexamethasone inhibits growth factor-induced type 3 deiodinase activity and mRNA expression in a cultured cell line derived from rat neonatal brown fat vascular-stromal cells. Endocrinology 2002, 143, 2652–2658. [Google Scholar] [CrossRef] [PubMed]
- Duanmu, Z.; Kocarek, T.A.; Runge-Morris, M. Transcriptional regulation of rat hepatic aryl sulfotransferase (SULT1A1) gene expression by glucocorticoids. Drug Metab. Dispos. 2001, 29, 1130–1135. [Google Scholar]
- Gutiérrez-Mariscal, M.; Sánchez, E.; García-Vázquez, A.; Rebolledo-Solleiro, D.; Charli, J.L.; Joseph-Bravo, P. Acute response of hypophysiotropic thyrotropin releasing hormone neurons and thyrotropin release to behavioral paradigms producing varying intensities of stress and physical activity. Regul. Pept. 2012, 179, 61–70. [Google Scholar] [CrossRef]
- Mason, J.; Southwick, S.; Yehuda, R.; Wang, S.; Riney, S.; Bremner, D.; Johnson, D.; Lubin, H.; Blake, D.; Zhou, G. Elevation of serum free triiodothyronine, total triiodothyronine, thyroxine-binding globulin, and total thyroxine levels in combat-related posttraumatic stress disorder. Arch. Gen. Psychiatry 1994, 51, 629–641. [Google Scholar] [CrossRef]
- Toloza, F.J.K.; Mao, Y.; Menon, L.P.; George, G.; Borikar, M.; Erwin, P.J.; Owen, R.R.; Maraka, S. Association of thyroid function with posttraumatic stress disorder: A systematic review and meta-analysis. Endocr. Pract. 2020, 26, 1173–1185. [Google Scholar] [CrossRef]
- Raise-Abdullahi, P.; Meamar, M.; Vafaei, A.A.; Alizadeh, M.; Dadkhah, M.; Shafia, S.; Ghalandari-Shamami, M.; Naderian, R.; Afshin Samaei, S.; Rashidy-Pour, A. Hypothalamus and post-traumatic stress disorder: A review. Brain Sci. 2023, 13, 1010. [Google Scholar] [CrossRef] [PubMed]
- Nowacka-Chmielewska, M.; Grabowska, K.; Grabowski, M.; Meybohm, P.; Burek, M.; Małecki, A. Running from stress: Neurobiological mechanisms of exercise-induced stress resilience. Int. J. Mol. Sci. 2022, 23, 13348. [Google Scholar] [CrossRef]
- Elias, E.; Zhang, A.I.; White, A.; Pyle, M.J.; Manners, M.T. Voluntary wheel running promotes resilience to the behavioral effects of unpredictable chronic mild stress in male and female mice. Stress 2023, 26, 2203769. [Google Scholar] [CrossRef]
- Reinoß, P.; Ciglieri, E.; Minére, M.; Bremser, S.; Klein, A.; Löhr, H.; Fuller, P.M.; Büschges, A.; Kloppenburg, P.; Fenselau, H.; et al. Hypothalamic Pomc neurons innervate the spinal cord and modulate the excitability of premotor circuits. Curr. Biol 2020, 30, 4579–4593.e7. [Google Scholar] [CrossRef]
- National Research Council (US) Committee for the Update of the Guide for the Care; Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press (US): Washington, DC, USA, 2011. [Google Scholar] [PubMed]
- Toth, E.; Gersner, R.; Wilf-Yarkoni, A.; Raizel, H.; Dar, D.E.; Richter-Levin, G.; Levit, O.; Zangen, A. Age-dependent effects of chronic stress on brain plasticity and depressive behavior. J. Neurochem. 2008, 107, 522–532. [Google Scholar] [CrossRef]
- Perelló, M.; Chacon, F.; Cardinali, D.P.; Esquifino, A.I.; Spinedi, E. Effect of social isolation on 24-h pattern of stress hormones and leptin in rats. Life Sci. 2006, 78, 1857–1862. [Google Scholar] [CrossRef]
- Cora, M.C.; Kooistra, L.; Travlos, G. Vaginal cytology of the laboratory rat and mouse: Review and criteria for the staging of the estrous cycle using stained vaginal smears. Toxicol. Pathol. 2015, 43, 776–793. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates–The New Coronal Set, 5th ed.; Academic Press: Cambridge, MA, USA, 2004. [Google Scholar]
- Chomczynski, P.; Sacchi, N. The single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction: Twenty-something years on. Nat. Protoc. 2006, 1, 581–585. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Krzywinski, M.; Birol, I.; Jones, S.; Marra, M. Hive plots—Rational approach to visualizing networks. Brief. Bioinform. 2012, 13, 627–644. [Google Scholar] [CrossRef] [PubMed]



| C | CVS | |||||
|---|---|---|---|---|---|---|
| Sed | PF | Ex | Sed | PF | Ex | |
| Females | ||||||
| FI (g/d) | 18.7 ± 0.6 σ | 14 ± 0.01 * σ | 14.3 ± 0.5 * σ | 18 ± 0.6 σ | 13.4 ± 0.3 * X σ | 13.5 ± 0.7 * X σ |
| RFI (g/day/g BW) | 74 ± 4.1 σ | 56 ± 1.3 * σ | 58 ± 2.3 * | 77 ± 2.4 σ | 58 ± 1.3 X σ | 59 ± 6.9 X |
| FE (g BWg /100 g food) | 5 ± 1.3 σ | 2 ± 0.5 * | −1 ± 1.2 * | 7 ± 0.8 σ | 0.4 ± 1.0 Xσ | −7 ± 2.5 X & σ |
| %BWch | 107 ± 0.9 | 102 ± 0.6 * | 101 ± 0.6 * σ | 109 ± 0.9 | 99 ± 0.8 * X | 97 ± 1 * X σ |
| % pgWAT | 100 ± 4 | 78 ± 6.5 * | 61 ± 6 * | 115 ± 2 | 75 ± 4 * X σ | 73 ± 7.6 * X |
| % rWAT | 100 ± 3.7 | 90 ± 8.1 | 57 ± 7.2 * # | 113 ± 13.2 | 70 ± 5 * X | 72 ± 7.4 * X |
| % iscWAT | 100 ± 9.1 | 106 ± 11.9 | 74 ± 9.5 * # | 117 ± 12.7 | 96 ± 5.9 | 74 ± 3.4 * X # |
| Males | ||||||
| FI (g/day) | 25.3 ± 0.3 | 19 ± 0.48 * | 19 ± 0.9 * | 25 ± 0.7 | 21 ± 0.4 X | 20.3 ± 0.7 X |
| RFI (g/day/g BW) | 56 ± 2.0 | 48 ± 1.6 * | 51 ± 3.7 | 61 ± 1.1 | 52 ± 1.2 X | 51 ± 2.1 X |
| FE (g BWg /100 g food) | 11 ± 0.8 | 3 ± 2.2 * | 3 ± 2.2 * | 11 ± 1.3 | 6 ± 0.9 X | 4 ± 1.1 X |
| %BWch | 110 ± 0.7 | 103 ± 0.9 * | 92 ± 2.5 * # | 111 ± 0.68 | 98 ± 2 * X | 105 ± 1.2 X & |
| % pgWAT | 100 ± 4.4 | 84 ± 6.5 | 76 ± 4.1 * | 93 ± 6.8 | 92 ± 6.9 | 75 ± 5 * X & |
| % rWAT | 100 ± 5.9 | 85 ± 6.1 | 69 ± 4.2 * | 87 ± 7.3 | 87 ± 8.7 | 69 ± 4.5 * |
| % iscWAT | 100 ± 12.7 | 88 ± 13.6 | 69 ± 7.3 * | 90 ± 11.6 | 93 ± 8 | 68 ± 5.8 * X |
| Females | Males | |||||||||||
| C | CVS | C | CVS | |||||||||
| Sed | PF | Ex | Sed | PF | Ex | Sed | PF | Ex | Sed | PF | Ex | |
| Food intake | -- | ↓ | ↓ | -- | ↓ | ↓ | -- | ↓ | ↓ | -- | ↓ | ↓ |
| ΔBW | -- | ↓ | ↓↓ | -- | ↓ | ↓↓ | -- | ↓ | ↓↓ | -- | ↓↓ | ↓ |
| pgWAT | -- | ↓ | ↓ | -- | ↓ | ↓ | -- | -- | ↓ | -- | -- | ↓ |
| rWAT | -- | -- | ↓ | -- | ↓ | ↓ | -- | -- | ↓ | -- | -- | ↓ |
| iscWAT | -- | -- | ↓ | -- | -- | ↓ | -- | -- | ↓ | -- | -- | ↓ |
| Leptin | -- | ↓ | ↓ | -- | ↓↓ | ↓ | -- | ↓↓ | ↓ | -- | ↓ | ↓ |
| TG | -- | ↓ | ↓ | -- | ↓↓ | -- | -- | ↓ | ↓ | ↓ | ↓ | ↓↓ |
| Pomc | -- | ↓ | ↑ | -- | ↓ | -- | -- | ↓ | ↑↑ | -- | ↓ | -- |
| Npy | -- | ↑ | -- | -- | ↑ | -- | -- | ↑ | -- | ↑ | ↑↑ | ↑↑ |
| Crh | -- | ↓ | -- | -- | -- | -- | -- | -- | -- | -- | ↓ | -- |
| Avp | -- | ↓ | -- | -- | ↓↓ | ↓↓ | -- | -- | -- | -- | ↓↓ | -- |
| Gr | -- | -- | -- | -- | ↑ | -- | -- | ↑ | -- | ↑↑ | ↑↑ | ↑↑ |
| Cort | -- | ↑↑ | ↓↓ | -- | ↑↑ | ↑↑ | -- | ↑ | ↓ | ↑ | ↑↑ | ↑↑ |
| MBH Dio2 | -- | -- | ↑ | ↓ | ↓ | ↓ | -- | -- | ↑ | ↓ | ↓ | ↓ |
| Trhde | -- | -- | -- | -- | -- | -- | -- | ↓ | -- | ↓ | -- | -- |
| Trh | -- | ↓↓ | ↓↓ | ↓↓ | ↓↓ | ↓↓ | -- | ↓ | ↑ | ↓ | ↓↓ | ↓ |
| TSH | -- | -- | ↓ | -- | ↓ | -- | -- | ↓ | ↓ | -- | ↓ | ↓ |
| T4 | -- | -- | -- | -- | -- | -- | -- | ↑ | ↑ | -- | -- | -- |
| fT4 | -- | -- | -- | -- | -- | -- | -- | -- | -- | ↓ | ↓ | -- |
| T3 | -- | ↑ | -- | ↑ | ↑ | ↑ | -- | -- | -- | -- | -- | -- |
| T3/T4 | -- | ↑ | ↑ | ↑ | -- | -- | -- | -- | -- | -- | -- | ↓ |
| fT3 | -- | -- | -- | ↑↑ | ↑↑ | ↑↑ | -- | ↓ | -- | -- | ↓ | ↓ |
| fT3/fT4 | -- | -- | -- | -- | -- | ↑↑ | -- | -- | -- | -- | -- | -- |
| SM Dio2 | -- | ↑ | ↑↑ | -- | -- | -- | -- | -- | ↑↑ | ↓ | -- | -- |
| SM Pgc1a | -- | -- | ↑↑ | -- | ↓ | -- | -- | -- | ↑↑ | -- | -- | ↑↑ |
| iW Adrb3 | -- | ↓ | ↑ | ↓ | ↓ | ↓ | -- | ↓ | ↑ | ↓ | ↓ | ↓ |
| iW Dio2 | -- | ↓ | ↑ | -- | ↓ | ↑ | -- | ↓ | ↑ | ↓ | ↓ | ↓ |
| iW Pparg | -- | ↓ | ↑ | -- | -- | -- | -- | -- | ↑ | -- | -- | -- |
| iW Hsl | -- | ↑ | ↑ | -- | ↑↑ | ↑↑ | -- | ↑↑ | ↑↑ | ↑ | ↑↑ | ↑↑ |
| pgW Adrb3 | -- | ↓ | ↑ | ↓ | ↓ | -- | -- | ↓ | ↑ | ↓ | ↓↓ | -- |
| pgW Dio2 | -- | ↓ | ↑↑ | -- | ↓ | ↑ | -- | ↓ | ↑ | ↓ | ↓ | -- |
| pgW Pparg | -- | ↓ | ↑ | ↓ | -- | -- | -- | ↓ | ↑↑ | ↓ | ↓ | -- |
| pgW Hsl | -- | ↑ | ↑ | ↓ | ↓ | ↓ | -- | ↑↑ | ↑↑ | ↓ | -- | ↓ |
| BAT Adrb3 | -- | ↑ | ↑↑ | ↑ | ↑ | -- | -- | ↓ | ↑ | ↓ | ↑ | ↑ |
| BAT Dio2 | -- | -- | ↑↑ | -- | -- | ↑ | -- | -- | ↑↑ | -- | -- | -- |
| BAT Ucp1 | -- | -- | ↑↑ | -- | -- | -- | -- | ↓ | ↑ | ↓ | ↓ | ↓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parra-Montes de Oca, M.; Jaimes-Hoy, L.; Garduño, K.; García-Herrera, R.; Charli, J.-L.; Joseph-Bravo, P. Long-Term Effects of Stress During Adolescence on the Sex-Dependent Responses of Thyroid Axis and Target Tissues to Exercise in Male and Female Wistar Rats. Int. J. Mol. Sci. 2025, 26, 9425. https://doi.org/10.3390/ijms26199425
Parra-Montes de Oca M, Jaimes-Hoy L, Garduño K, García-Herrera R, Charli J-L, Joseph-Bravo P. Long-Term Effects of Stress During Adolescence on the Sex-Dependent Responses of Thyroid Axis and Target Tissues to Exercise in Male and Female Wistar Rats. International Journal of Molecular Sciences. 2025; 26(19):9425. https://doi.org/10.3390/ijms26199425
Chicago/Turabian StyleParra-Montes de Oca, Marco, Lorraine Jaimes-Hoy, Karen Garduño, Rodrigo García-Herrera, Jean-Louis Charli, and Patricia Joseph-Bravo. 2025. "Long-Term Effects of Stress During Adolescence on the Sex-Dependent Responses of Thyroid Axis and Target Tissues to Exercise in Male and Female Wistar Rats" International Journal of Molecular Sciences 26, no. 19: 9425. https://doi.org/10.3390/ijms26199425
APA StyleParra-Montes de Oca, M., Jaimes-Hoy, L., Garduño, K., García-Herrera, R., Charli, J.-L., & Joseph-Bravo, P. (2025). Long-Term Effects of Stress During Adolescence on the Sex-Dependent Responses of Thyroid Axis and Target Tissues to Exercise in Male and Female Wistar Rats. International Journal of Molecular Sciences, 26(19), 9425. https://doi.org/10.3390/ijms26199425








