Distinct Intrinsic and Extrinsic Factors Differentially Regulate Skeletal Stem Cells in Calvaria Versus Long Bones During Bone Regeneration
Abstract
1. Introduction
2. Results
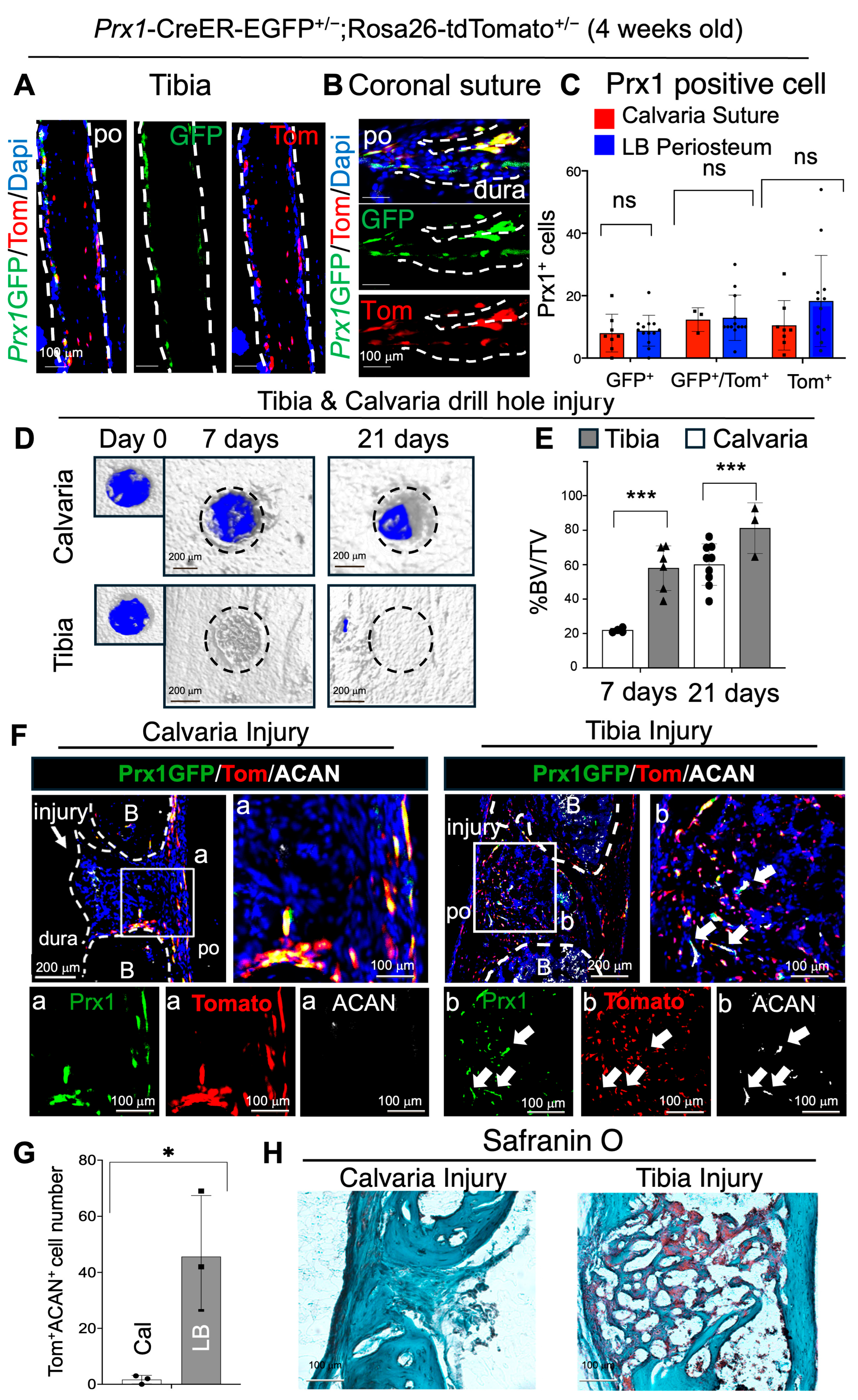
2.1. Endogenous Prx1+ Su-SSCs Display Direct Osteogenic Differentiation (Intramembranous) After Bone Injury, While Prx1+ LB-PSSCs Undergo Endochondral Ossifications During Bone Healing
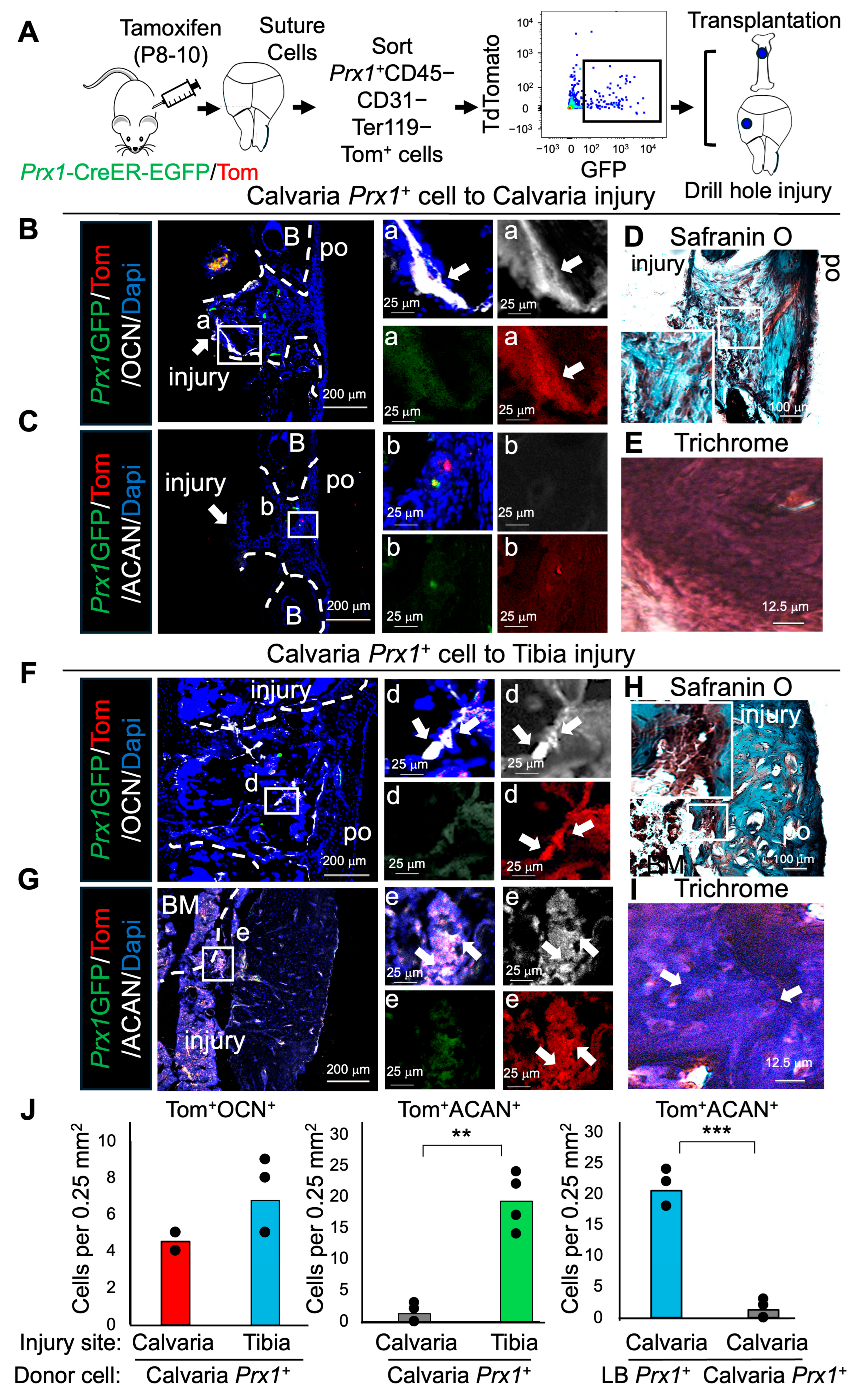
2.2. Prx1+ LB-PSSCs Preserve Endochondral Ossification After Orthotopic and Heterotopic Transplantation
2.3. Prx1+ Su-SSCs Acquire Osteochondrogenic Differentiation Plasticity After Heterotopic Transplantation into Tibial Injury
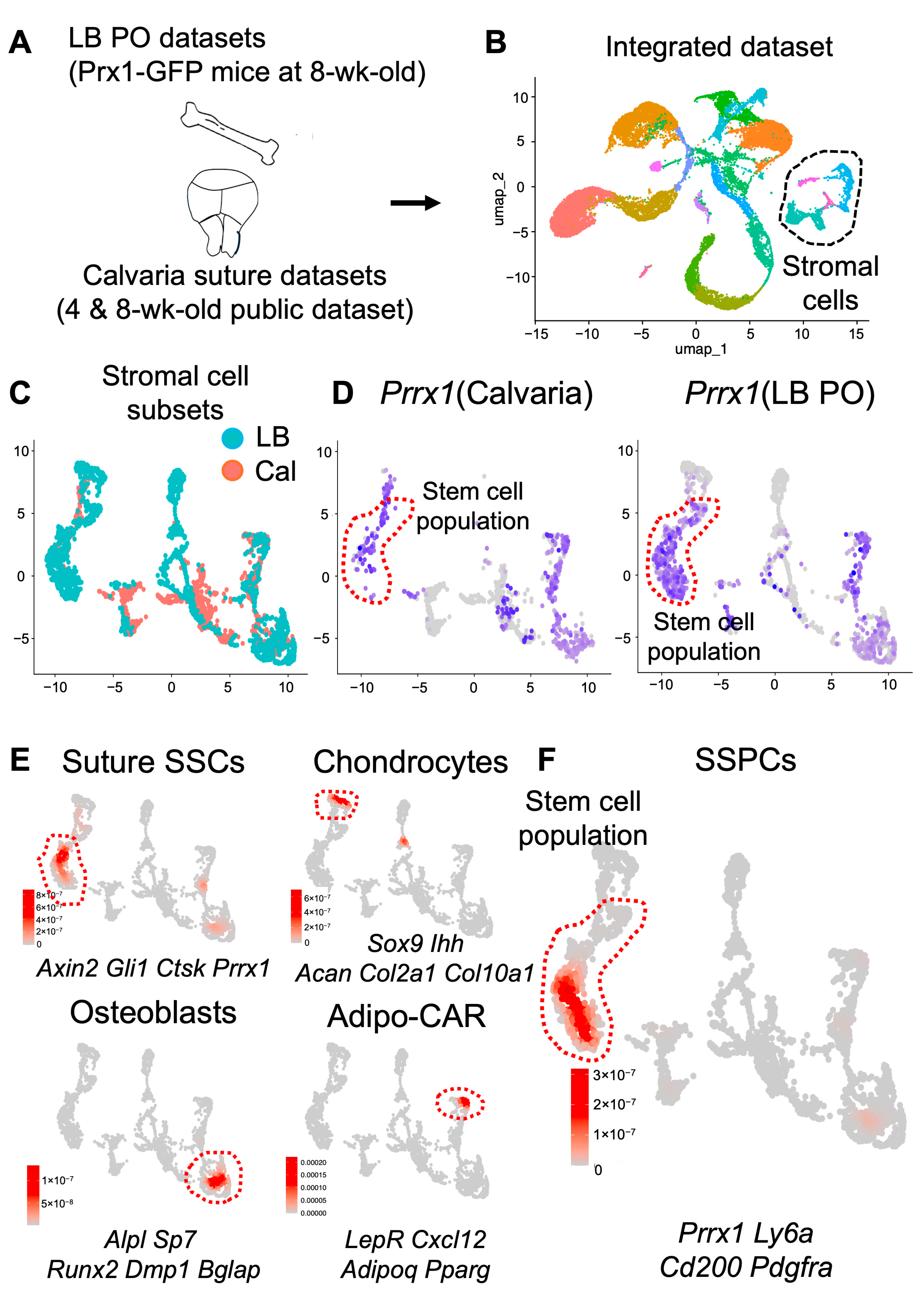
2.4. Calvaria and Long Bones Have Distinct Sub-Cellular Clusters
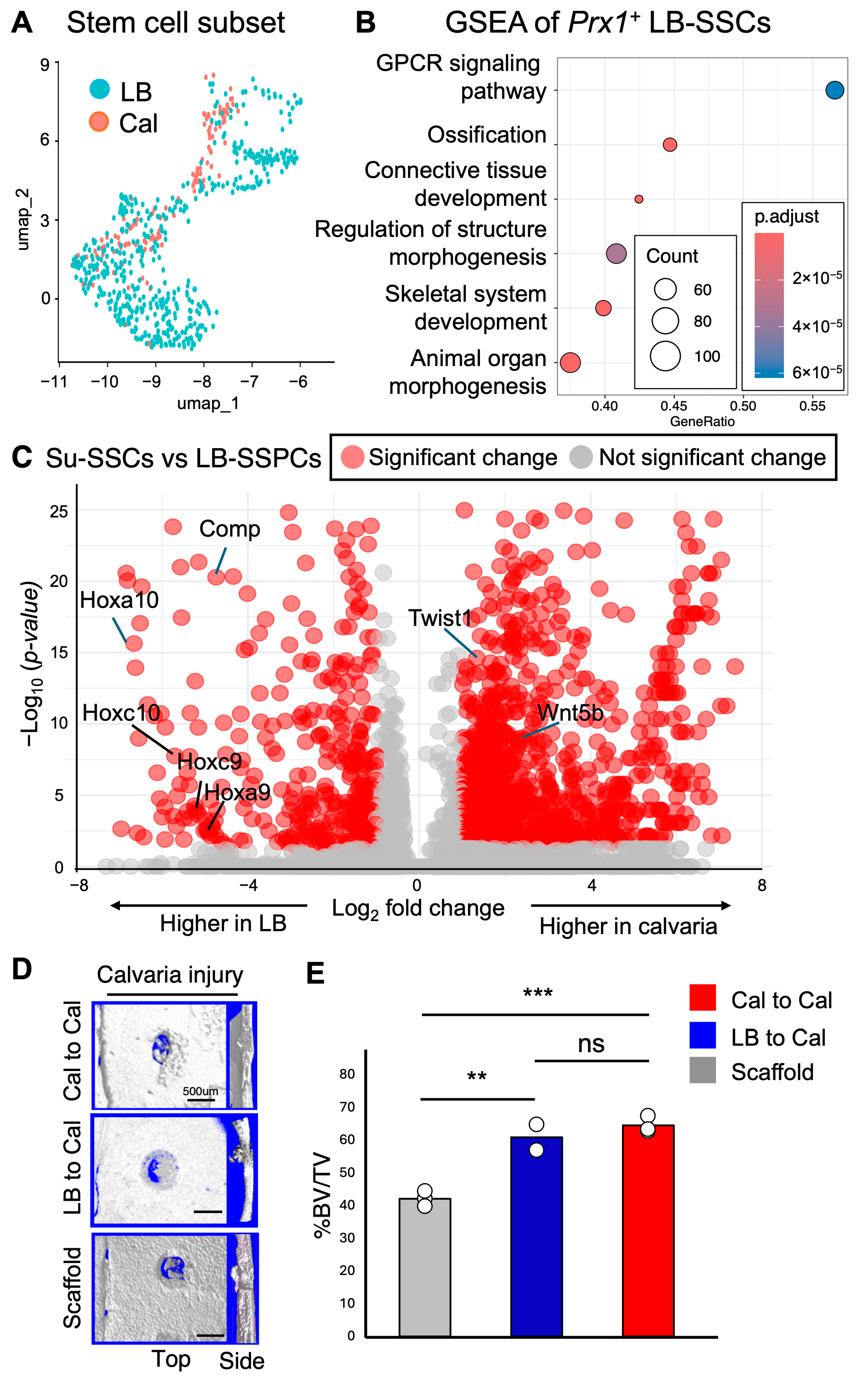
2.5. Differential Expression of Pro-Chondrogenic Genes in Prx1+ LB-PSSCs and Anti-Chondrogenic Genes in Prx1+ Su-SSCs
2.6. Transplantation of Prx1+ LB-PSSCs Induces Comparable Healing of Calvarial Bone Injury
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Histological Analysis
4.3. Safranin O Histological Staining
4.4. Immunofluorescence Staining
4.5. Micro-CT Analysis
4.6. Mouse Tibial and Calvarial Injury
4.7. Transplantation Experiment
4.8. Single-Cell RNA Sequence Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Society of Plastic Surgeons What Is Microsurgery? Microsurgery Microscope-Assisted Operations. Available online: https://www.plasticsurgery.org/reconstructive-procedures/microsurgery (accessed on 11 September 2025).
- Zhao, H.; Feng, J.; Ho, T.-V.; Grimes, W.; Urata, M.; Chai, Y. The Suture Provides a Niche for Mesenchymal Stem Cells of Craniofacial Bones. Nat. Cell Biol. 2015, 17, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Durham, E.L.; Howie, R.N.; Houck, R.; Oakes, B.; Grey, Z.; Hall, S.; Steed, M.; LaRue, A.; Muise-Helmericks, R.; Cray, J. Involvement of Calvarial Stem Cells in Healing: A Regional Analysis of Large Cranial Defects. Wound Rep. Reg. 2018, 26, 359–365. [Google Scholar] [CrossRef]
- Arthur, A.; Gronthos, S. Clinical Application of Bone Marrow Mesenchymal Stem/Stromal Cells to Repair Skeletal Tissue. Int. J. Mol. Sci. 2020, 21, 9759. [Google Scholar] [CrossRef]
- Donsante, S.; Palmisano, B.; Serafini, M.; Robey, P.G.; Corsi, A.; Riminucci, M. From Stem Cells to Bone-Forming Cells. Int. J. Mol. Sci. 2021, 22, 3989. [Google Scholar] [CrossRef]
- Sanz, M.; Dahlin, C.; Apatzidou, D.; Artzi, Z.; Bozic, D.; Calciolari, E.; De Bruyn, H.; Dommisch, H.; Donos, N.; Eickholz, P.; et al. Biomaterials and Regenerative Technologies Used in Bone Regeneration in the Craniomaxillofacial Region: Consensus Report of Group 2 of the 15th European Workshop on Periodontology on Bone Regeneration. J. Clin. Periodontol. 2019, 46, 82–91. [Google Scholar] [CrossRef]
- Borrelli, M.R.; Hu, M.S.; Longaker, M.T.; Lorenz, H.P. Tissue Engineering and Regenerative Medicine in Craniofacial Reconstruction and Facial Aesthetics. J. Craniofacial Surg. 2020, 31, 15–27. [Google Scholar] [CrossRef]
- Price, J.S.; Oyajobi, B.O.; Russell, R.G. The Cell Biology of Bone Growth. Eur. J. Clin. Nutr. 1994, 48 (Suppl. 1), S131–S149. [Google Scholar]
- Gilbert, S.F. Bones. In Developmental Biology, 6th ed.; Sinauer Associates: Sunderland, MA, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK10056/ (accessed on 11 September 2025).
- Chung, I.-H.; Yamaza, T.; Zhao, H.; Choung, P.-H.; Shi, S.; Chai, Y. Stem Cell Property of Postmigratory Cranial Neural Crest Cells and Their Utility in Alveolar Bone Regeneration and Tooth Development: Neural Crest Cells in Bone Regeneration. Stem Cells 2009, 27, 866–877. [Google Scholar] [CrossRef]
- Solidum, J.G.N.; Jeong, Y.; Heralde, F.; Park, D. Differential Regulation of Skeletal Stem/Progenitor Cells in Distinct Skeletal Compartments. Front. Physiol. 2023, 14, 1137063. [Google Scholar] [CrossRef] [PubMed]
- Lappin, T.R.J.; Grier, D.G.; Thompson, A.; Halliday, H.L. HOX Genes: Seductive Science, Mysterious Mechanisms. Ulst. Med. J. 2006, 75, 23–31. [Google Scholar]
- Leucht, P.; Kim, J.-B.; Amasha, R.; James, A.W.; Girod, S.; Helms, J.A. Embryonic Origin and Hox Status Determine Progenitor Cell Fate during Adult Bone Regeneration. Development 2008, 135, 2845–2854. [Google Scholar] [CrossRef]
- Bonaventure, J.; Skeletal Development in Human. Atlas of Genetics and Cytogenetics in Oncology and Haematology. 2001. Available online: https://atlasgeneticsoncology.org/teaching/30075/skeletal-development-in-human (accessed on 11 September 2025).
- Jeffery, E.C.; Mann, T.L.A.; Pool, J.A.; Zhao, Z.; Morrison, S.J. Bone Marrow and Periosteal Skeletal Stem/Progenitor Cells Make Distinct Contributions to Bone Maintenance and Repair. Cell Stem Cell 2022, 29, 1547–1561.e6. [Google Scholar] [CrossRef]
- Ono, N. The Mechanism of Bone Repair: Stem Cells in the Periosteum Dedicated to Bridging a Large Gap. Cell Rep. Med. 2022, 3, 100807. [Google Scholar] [CrossRef]
- Jeong, Y.; Deveza, L.; Ortinau, L.; Lei, K.; Dawson, J.R.; Park, D. Identification of LRP1+CD13+ Human Periosteal Stem Cells That Require LRP1 for Bone Repair. JCI Insight 2024, 9, e173831. [Google Scholar] [CrossRef] [PubMed]
- Farmer, D.T.; Mlcochova, H.; Zhou, Y.; Koelling, N.; Wang, G.; Ashley, N.; Bugacov, H.; Chen, H.-J.; Parvez, R.; Tseng, K.-C.; et al. The Developing Mouse Coronal Suture at Single-Cell Resolution. Nat. Commun. 2021, 12, 4797. [Google Scholar] [CrossRef]
- Li, B.; Li, J.; Li, B.; Ouchi, T.; Li, L.; Li, Y.; Zhao, Z. A Single-Cell Transcriptomic Atlas Characterizes Age-Related Changes of Murine Cranial Stem Cell Niches. Aging Cell 2023, 22, e13980. [Google Scholar] [CrossRef]
- Wilk, K.; Yeh, S.-C.A.; Mortensen, L.J.; Ghaffarigarakani, S.; Lombardo, C.M.; Bassir, S.H.; Aldawood, Z.A.; Lin, C.P.; Intini, G. Postnatal Calvarial Skeletal Stem Cells Expressing PRX1 Reside Exclusively in the Calvarial Sutures and Are Required for Bone Regeneration. Stem Cell Rep. 2017, 8, 933–946. [Google Scholar] [CrossRef]
- Park, D.; Spencer, J.A.; Koh, B.I.; Kobayashi, T.; Fujisaki, J.; Clemens, T.L.; Lin, C.P.; Kronenberg, H.M.; Scadden, D.T. Endogenous Bone Marrow MSCs Are Dynamic, Fate-Restricted Participants in Bone Maintenance and Regeneration. Cell Stem Cell 2012, 10, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Debnath, S.; Yallowitz, A.R.; McCormick, J.; Lalani, S.; Zhang, T.; Xu, R.; Li, N.; Liu, Y.; Yang, Y.S.; Eiseman, M.; et al. Discovery of a Periosteal Stem Cell Mediating Intramembranous Bone Formation. Nature 2018, 562, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ilinski, A.; Gerstenfeld, L.C.; Bragdon, B. Prx1 Cell Subpopulations Identified in Various Tissues with Diverse Quiescence and Activation Ability Following Fracture and BMP2 Stimulation. Front. Physiol. 2023, 14, 1106474. [Google Scholar] [CrossRef]
- Maruyama, T.; Jeong, J.; Sheu, T.-J.; Hsu, W. Stem Cells of the Suture Mesenchyme in Craniofacial Bone Development, Repair and Regeneration. Nat. Commun. 2016, 7, 10526. [Google Scholar] [CrossRef] [PubMed]
- Pineault, K.M.; Song, J.Y.; Kozloff, K.M.; Lucas, D.; Wellik, D.M. Hox11 Expressing Regional Skeletal Stem Cells Are Progenitors for Osteoblasts, Chondrocytes and Adipocytes throughout Life. Nat. Commun. 2019, 10, 3168. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, J. Cartilage Oligomeric Matrix Protein, Diseases, and Therapeutic Opportunities. Int. J. Mol. Sci. 2022, 23, 9253. [Google Scholar] [CrossRef]
- Bradley, E.W.; Drissi, M.H. Wnt5b Regulates Mesenchymal Cell Aggregation and Chondrocyte Differentiation through the Planar Cell Polarity Pathway. J. Cell. Physiol. 2011, 226, 1683–1693. [Google Scholar] [CrossRef]
- Gu, S.; Boyer, T.G.; Naski, M.C. Basic Helix-Loop-Helix Transcription Factor Twist1 Inhibits Transactivator Function of Master Chondrogenic Regulator Sox9. J. Biol. Chem. 2012, 287, 21082–21092. [Google Scholar] [CrossRef]
- Iaquinta, M.R.; Mazzoni, E.; Bononi, I.; Rotondo, J.C.; Mazziotta, C.; Montesi, M.; Sprio, S.; Tampieri, A.; Tognon, M.; Martini, F. Adult Stem Cells for Bone Regeneration and Repair. Front. Cell Dev. Biol. 2019, 7, 268. [Google Scholar] [CrossRef]
- Ortinau, L.C.; Park, D. Do Adipogenic Stromal Cells Undergo Lineage Plasticity in Response to Bone Injury? BioEssays 2021, 43, 2000296. [Google Scholar] [CrossRef] [PubMed]
- Raines, A.M.; Magella, B.; Adam, M.; Potter, S.S. Key Pathways Regulated by HoxA9,10,11/HoxD9,10,11 during Limb Development. BMC Dev. Biol. 2015, 15, 28. [Google Scholar] [CrossRef]
- Feng, C.; Chan, W.C.W.; Lam, Y.; Wang, X.; Chen, P.; Niu, B.; Ng, V.C.W.; Yeo, J.C.; Stricker, S.; Cheah, K.S.E.; et al. Lgr5 and Col22a1 Mark Progenitor Cells in the Lineage toward Juvenile Articular Chondrocytes. Stem Cell Rep. 2019, 13, 713–729. [Google Scholar] [CrossRef]
- Park, D.; Spencer, J.A.; Lin, C.P.; Scadden, D.T. Sequential In Vivo Imaging of Osteogenic Stem/Progenitor Cells During Fracture Repair. J. Vis. Exp. 2014, 51289. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solidum, J.; Yamasaki, K.; Jeong, Y.; Ortinau, L.; Heralde, F., III; Park, D. Distinct Intrinsic and Extrinsic Factors Differentially Regulate Skeletal Stem Cells in Calvaria Versus Long Bones During Bone Regeneration. Int. J. Mol. Sci. 2025, 26, 9413. https://doi.org/10.3390/ijms26199413
Solidum J, Yamasaki K, Jeong Y, Ortinau L, Heralde F III, Park D. Distinct Intrinsic and Extrinsic Factors Differentially Regulate Skeletal Stem Cells in Calvaria Versus Long Bones During Bone Regeneration. International Journal of Molecular Sciences. 2025; 26(19):9413. https://doi.org/10.3390/ijms26199413
Chicago/Turabian StyleSolidum, Jea, Kohei Yamasaki, Youngjae Jeong, Laura Ortinau, Francisco Heralde, III, and Dongsu Park. 2025. "Distinct Intrinsic and Extrinsic Factors Differentially Regulate Skeletal Stem Cells in Calvaria Versus Long Bones During Bone Regeneration" International Journal of Molecular Sciences 26, no. 19: 9413. https://doi.org/10.3390/ijms26199413
APA StyleSolidum, J., Yamasaki, K., Jeong, Y., Ortinau, L., Heralde, F., III, & Park, D. (2025). Distinct Intrinsic and Extrinsic Factors Differentially Regulate Skeletal Stem Cells in Calvaria Versus Long Bones During Bone Regeneration. International Journal of Molecular Sciences, 26(19), 9413. https://doi.org/10.3390/ijms26199413





